Dear Editor,
Bipolar disorder (BD) is a chronic and disabling disorder characterized by manic, hypomanic, or depressive episodes, with an estimated lifetime prevalence ranging from 1% to 5%. Globally, it is a leading cause of disability and a socioeconomic burden. Gene-environment interactions are thought to be involved in the neurobiology of BD, with genetic factors contributing to 60%–85% of BD patients. Twin studies have shown a heritability of 59%, and an increased risk of BD has been found in first-degree relatives of probands [1]. Currently, many genes have been reported as possible risk factors for BD, including PBRM1, CACNA1C, ANK3, ODZ4, SYNE1, ITIH1, GABRB1, DAOA, NCAN, and TRANK1. Environmental factors such as maternal stress, prenatal malnutrition, preterm birth, childhood abuse, stressful life events, and cannabis use can also contribute to the development of BD.
Early recognition and treatment of BD can improve treatment responses and the prognosis [2]. However, there is always a long delay from the onset of disease to a definite diagnosis. Nearly half of BD patients develop their first mood episode at puberty. Kataoka et al. have found a significant association of early disease onset in BD probands with de novo protein-altering mutations when compared with non-carriers [3]. Therefore, age at onset (AAO) may be a potential clinical marker for genetic susceptibility to BD. The relationship between risk genes for BD and AAO, however, has not been fully investigated.
In this study, we aimed to explore the correlation between high-risk single-nucleotide polymorphism (SNP) loci and AAO in Chinese patients with BD. A total of 224 Han Chinese participants with BD were consecutively recruited from the First Affiliated Hospital of Zhejiang University School of Medicine from August 2011 to March 2016. The diagnosis was made according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) and further confirmed by the Structural Clinical Interview for DSM-IV. All patients were diagnosed with either type I or II BD. The exclusion criteria included prior or current comorbidity of other psychiatric diseases, alcohol or other psychoactive substance abuse, poisoning, or other physical conditions that could cause emotional lability. After a search of gene linkage analysis or genome-wide association studies in databases (PubMed, EMBASE, and the Cochrane Library) up to February 2016, 35 SNP loci were screened as candidates. The genotypes were determined by the MassArray system (Agena iPLEX assay, San Diego, CA), with a 384-element SpectroCHIP gene array. Given our small sample size, the minor allele frequency of each SNP locus was required to be > 0.05, and 26 SNP loci were finally included in the analysis. To determine the relationship between SNP loci and AAO of BD, Plink and Haploview statistical software was used and logistic regression analysis was performed. All study procedures were approved by the Hospital Ethics Committee of the First Affiliated Hospital of Zhejiang University School of Medicine and were in accordance with the Helsinki Declaration of 1975. Informed consent was given by all participants.
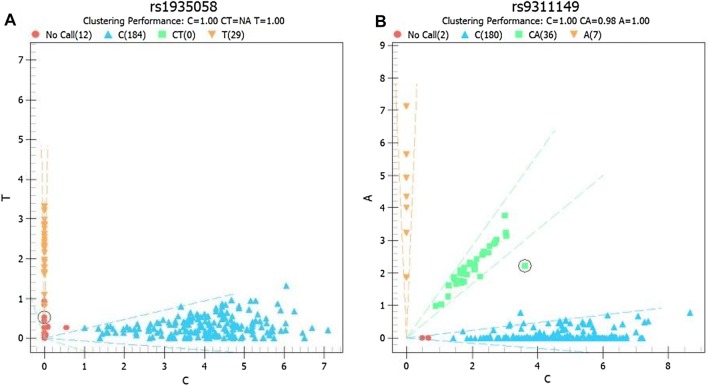
The participants were predominantly female (59.8%, 134/224), BD type II (62.1%, 139/224), and the mean AAO was 23.5 ± 11.2 years. Genotyping was carried out in all participants, and distribution vector maps were drawn for each SNP. The detection rate of rs1935058 was 94.7%, and the genotypic vector maps of rs1935058 and rs9311149 showed unsatisfactory clustering performance (Fig. 1). No significant correlation was found between SNP loci and AAO, except for a positive correlation for the rs12899449 locus (P = 0.026; BETA = 8.024) (Table 1). In subgroup analysis, the impact of gender and type of BD on AAO was further analyzed. The results showed no significant association between SNP loci and female or BD type I. rs12899449 was a significant locus in males (P = 0.018), and rs12899449, rs1064395, and rs6046396 were significant loci in BD type II (P = 0.002, 0.018, and 0.040, respectively) (Table 1). For rs12899449, however, the numbers of AA, GA, and GG genotypes were 210, 6, and 1, respectively, in all participants; and 133, 2, and 1 in the BD type II group. Similarly, the numbers of AA, GA, and GG genotypes for rs1064395 were 110, 26, and 1 respectively in the BD type II group. Therefore, due to the genotype distribution bias, no firm conclusion could be drawn for rs12899449 and rs1064395, so the findings in this study only suggested a significant correlation between rs6046396 and AAO in individuals with BD type II. The ages of patients (mean ± standard deviation) with the rs6046396 AA, GA, and GG genotypes were 20.2 ± 10.5, 23.6 ± 11.1, and 26.0 ± 14.2, respectively. Therefore, the A allele was considered to be the risk allele type.
Fig. 1.
Genotype vector maps for rs1935058 and rs9311149.
Table 1.
Genotype frequencies of rs12899449, rs1064395, and rs6046396, and their relationships with age of onset.
| SNP | Genotype | Total | Male | Female | Bipolar type I | Bipolar type II | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | BETA | SE | P* | N (%) | BETA | SE | P | N (%) | BETA | SE | P | N (%) | BETA | SE | P | N (%) | BETA | SE | P | ||
| rs12899449 | AA | 210 (93.8) | 8.024 | 3.575 | 0.026 | 82 (91.1) | 13.780 | 5.686 | 0.018 | 128 (95.5) | 2.521 | 4.610 | 0.585 | 77 (90.6) | −2.276 | 5.482 | 0.679 | 133 (95.7) | 14.09 | 4.562 | 0.002 |
| GA | 6 (2.7) | 5 (5.6) | 1 (0.7) | 4 (4.7) | 2 (1.4) | ||||||||||||||||
| GG | 1 (0.4) | 0 (0.0) | 1 (0.7) | 0 (0.0) | 1 (0.7) | ||||||||||||||||
| Missing | 7 (3.1) | 3 (3.3) | 4 (3.0) | 4 (4.7) | 3 (2.2) | ||||||||||||||||
| rs1064395 | GG | 176 (78.6) | 2.183 | 1.716 | 0.205 | 73 (81.1) | 3.218 | 3.565 | 0.369 | 103 (76.9) | 2.058 | 1.773 | 0.248 | 66 (77.6) | −1.552 | 2.267 | 0.496 | 110 (79.1) | 5.439 | 2.27 | 0.018 |
| GA | 41 (18.3) | 15 (16.7) | 26 (19.4) | 15 (17.6) | 26 (18.7) | ||||||||||||||||
| AA | 4 (1.8) | 0 (0.0) | 4 (3.0) | 3 (3.5) | 1 (0.7) | ||||||||||||||||
| Missing | 3 (1.3) | 2 (2.2) | 1 (0.7) | 1 (1.2) | 2 (1.4) | ||||||||||||||||
| rs6046396 | AA | 102 (45.5) | 1.303 | 1.188 | 0.274 | 43 (47.8) | 3.392 | 1.932 | 0.083 | 59 (44.0) | −0.558 | 1.463 | 0.704 | 35 (41.2) | −2.282 | 1.801 | 0.209 | 67 (48.2) | 3.122 | 1.502 | 0.040 |
| GA | 103 (46.0) | 37 (41.1) | 66 (49.3) | 42 (49.4) | 61 (43.9) | ||||||||||||||||
| GG | 19 (8.5) | 10 (11.1) | 9 (6.7) | 8 (9.4) | 11 (7.9) | ||||||||||||||||
*P < 0.05 (two-tailed).
Here, we explored the correlation between high-risk SNP loci and AAO in Han Chinese BD patients. We identified a significant association between rs6046396 and AAO in BD type II individuals. Although rs6046396 has been reported to be associated with BD in previous studies [4], its relationship with AAO in BD patients had not yet been investigated. Therefore, the above finding is new and worth further verification.
The rs6046396 A/G single-nucleotide variation is upstream of RIN2 (Ras and Rab interactor 2) at 20p11.23 [4]. However, the mechanism by which this SNP locus functions to facilitate the onset of BD is still unknown. The protein encoded by RIN2 binds preferentially to the GTP-bound form of RAB5 protein over the GDP-bound form, acting as a guanine nucleotide exchange factor and activating RAB5 [5, 6]. RAB5 protein is a small GTPase involved in membrane trafficking and is a crucial regulatory component of the endocytic pathway and cellular signaling cascades [7, 8]. In the presynaptic terminal, RAB5 is required for maintaining endosome integrity [9]. At synapses, endocytosed vesicular membrane may be reused for a new round of neurotransmitter release [9]. Therefore, RAB5 dysfunction impairs the release efficacy of neurotransmitters such as serotonin, noradrenaline, dopamine, and gamma-aminobutyric acid, which are involved in the pathogenesis of affective disorders.
In addition, RAB5 protein has been reported to participate in various immune processes, including T cell migration, phagolysosome formation, and cytokine secretion [10–12]. The role of immune dysfunction in affective disorders has emerged as a popular research focus in recent years [13, 14]. The immune system plays an important role in brain development during early life [15]. Therefore, the impact of the rs6046396 genotype on AAO in the BD population may be affected by RAB5-associated immune activity.
Given the study limitations, the results should be prudently interpreted. First, the sample size was small, which may weaken the statistical power. Second, we did not analyze the effects of environment on AAO, which could also result in the early onset of BD. Third, no healthy controls were recruited. The correlation between the high-risk SNPs included and Han Chinese should be determined before further investigations. Fourth, multiple testing correction was not carried out in the analysis, and this may lead to false-positives. However, the findings in this study were predominantly negative.
In summary, we investigated the relationship between high-risk SNPs and AAO in Han Chinese individuals with BD and revealed that the AA genotype of rs6046396 was associated with the early onset of BD type II. This finding needs to be further verified in large samples.
Acknowledgements
We thank all the patients who participated in this study. This work was supported by the National Key Basic Research Program (2016YFC1307100 and 2016YFC1307102); the National Clinical Research Center for Mental Health Disorders (2015BAI13B02); and a Key Research Project of Zhejiang Province (2015C03040).
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Lichtenstein P, Yip BH, Björk C, Pawitan Y, Cannon TD, Sullivan PF, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: A population-based study. Lancet. 2009;373:234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joyce K, Thompson A, Marwaha S. Is treatment for bipolar disorder more effective earlier in illness course? A comprehensive literature review. Int J Bipolar Disord. 2016;4:19. doi: 10.1186/s40345-016-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kataoka M, Matoba N, Sawada T, Kazuno AA, Ishiwata M, Fujii K, et al. Exome sequencing for bipolar disorder points to roles of de novo loss-of-function and protein-altering mutations. Molecular Psychiatry. 2016;21:885–893. doi: 10.1038/mp.2016.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith EN, Bloss CS, Badner JA, Barrett T, Belmonte PL, Berrettini W, et al. Genome-wide association study of bipolar disorder in European American and African American individuals. Mol Psychiatry. 2009;14:755–763. doi: 10.1038/mp.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimura T, Sakisaka T, Baba T, Yamada T, Takai Y. Involvement of the Ras-Ras-activated Rab5 guanine nucleotide exchange factor RIN2-Rab5 pathway in the hepatocyte growth factor-induced endocytosis of E-cadherin. J Biol Chem. 2006;281:10598–10609. doi: 10.1074/jbc.M510531200. [DOI] [PubMed] [Google Scholar]
- 6.Saito K, Murai J, Kajiho H, Kontani K, Kurosu H, Katada T. A novel binding protein composed of homophilic tetramer exhibits unique properties for the small GTPase Rab5. J Biol Chem. 2002;277:3412–3418. doi: 10.1074/jbc.M106276200. [DOI] [PubMed] [Google Scholar]
- 7.Fukuda M. Regulation of secretory vesicle traffic by Rab small GTPases. Cell Mol Life Sci. 2008;65:2801–2813. doi: 10.1007/s00018-008-8351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carney DS, Davies BA, Horazdovsky BF. Vps9 domain-containing proteins: activators of Rab5 GTPases from yeast to neurons. Trends Cell Biol. 2006;16:27–35. doi: 10.1016/j.tcb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Wucherpfennig T, Wilsch-Bräuninger M, González-Gaitán M. Role of Drosophila Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J Cell Biol. 2003;161:609–624. doi: 10.1083/jcb.200211087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prashar A, Schnettger L, Bernard EM, Gutierrez MG. Rab GTPases in immunity and inflammation. Front Cell Infect Microbiol. 2017;7:435. doi: 10.3389/fcimb.2017.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ong ST, Freeley M, Skubis-Zegadło J, Fazil MH, Kelleher D, Fresser F, et al. Phosphorylation of Rab5a Protein by Protein Kinase Cϵ Is crucial for T-cell Migration. J Biol Chem. 2014;289:19420–19434. doi: 10.1074/jbc.M113.545863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vieira OV, Bucci C, Harrison RE, Trimble WS, Lanzetti L, Gruenberg J, et al. Modulation of Rab5 and Rab7 recruitment to phagosomes by phosphatidylinositol 3-kinase. Mol Cell Biol. 2003;23:2501–2514. doi: 10.1128/MCB.23.7.2501-2514.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldsmith DR, Rapaport MH. Miller BJ A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21:1696–1709. doi: 10.1038/mp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu W, Zheng YL, Tian LP, Lai JB, Hu CC, Zhang P. Circulating T lymphocyte subsets, cytokines, and immune checkpoint inhibitors in patients with bipolar II or major depression: a preliminary study. Sci Rep. 2017;7:40530. doi: 10.1038/srep40530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang G, He F, Xu Y, Zhang Y, Wang X, Zhou C, et al. Immunopotentiator Thymosin Alpha-1 Promotes Neurogenesis and Cognition in the Developing Mouse via a Systemic Th1 Bias. Neurosci Bull. 2017;33:675–684. doi: 10.1007/s12264-017-0162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]