Abstract
Mood disorders/psychosis have been associated with dysfunctions in the default mode network (DMN). However, the relative contributions of DMN regions to state and trait disturbances in pediatric bipolar disorder (PBD) remain unclear. The aim of this study was to investigate the possible mechanisms of PBD through brain imaging and explore the influence of psychotic symptoms on functional alterations in PBD patients. Twenty-nine psychotic and 26 non-psychotic PBD patients, as well as 19 age- and sex-matched healthy controls underwent a resting-state functional MRI scan and the data were analyzed by independent component analysis. The DMN component from the fMRI data was extracted for each participant. Spearman’s rank correlation analysis was performed between aberrant connectivity and clinical measurements. The results demonstrated that psychotic PBD was characterized by aberrant DMN connectivity in the anterior cingulate cortex/medial prefrontal cortex, bilateral caudate nucleus, bilateral angular gyri, and left middle temporal gyrus, while non-psychotic PBD was not, suggesting further impairment with the development of psychosis. In summary, we demonstrated unique impairment in DMN functional connectivity in the psychotic PBD group. These specific neuroanatomical abnormalities may shed light on the underlying pathophysiology and presentation of PBD.
Keywords: Pediatric bipolar disorder, Default mode, Resting-state fMRI, Functional connectivity, Psychotic symptom
Introduction
Pediatric bipolar disorder (PBD) is an episodic mood disorder characterized by discrete episodes of depression and mania [1]. Approximately 1% of children and adolescents are diagnosed with PBD [2]. PBD is distinguished from adult BD by more atypical symptoms and comorbidities [3]. Moreover, at least 50% of those with PBD have experienced psychosis during their lifetime. PBD patients with psychotic symptoms are characterized by longer disease duration, more recurrences, greater symptom severity, longer hospitalization, increased impairment, and greater difficulty achieving remission [4], which suggest that the psychosis domain represents a useful starting point for comparing the differences and commonalities between PBD groups with and without these symptoms.
To date, many studies have been directed towards the etiology, neurobiology, and pathogenesis of BD [5]. The pathophysiological substrate in PBD patients, however, has not yet been fully elucidated, and the neuroimaging patterns of PBD patients, especially those with psychotic symptoms, remain unclear. As functional magnetic resonance imaging (fMRI) is established as a technique for the unbiased analysis of functional abnormalities in the brain, many studies have used it to study adult BD, mostly focusing on changes in the cortico-limbic pathways that are responsible for emotion regulation [6]. Although individuals with adult BD have been shown to have altered prefrontal activation and synchronous co-activation across cognitive tasks and in resting-state fMRI (rs-fMRI) [6, 7], relatively little is known about the disturbances in the patterns of brain function in psychotic PBD patients.
A growing body of evidence shows that distributed neural networks exhibit spontaneous activity at rest [8]. Prior research using rs-fMRI techniques has revealed abnormalities in the intrinsic connectivity networks in several psychiatric disorders [9–11]. Complex neuropsychiatric diseases like BD may result from disrupted neural computations across networks of regions [12]. To our knowledge, only a few rs-fMRI studies have focused on PBD patients. Wu et al. found that individuals with pediatric mania have altered intrinsic resting-state networks compared to healthy controls [13]. Our previous work also found aberrant resting-state neuronal activity in PBD patients with mania, but in the regions of the ventral-affective and dorsal-cognitive circuits [14–16].
Connectivity within the resting-state default mode network (DMN) has been shown to be compromised in many neuropsychiatric disorders [14, 17–19]. The DMN is relevant to the origin and experience of mood and psychotic symptoms [11], and is distributed largely across regions comprising the medial prefrontal cortex (mPFC), precuneus, posterior cingulate cortex (PCC), medial and lateral parietal cortex (angular gyrus), middle temporal gyrus (MTG), and parts of the basal ganglia that are thought to characterize basal neural activity [20]. Despite the influence of the DMN in neuropsychiatric disorders, few studies have examined connectivity within the DMN in psychotic BD [5, 21]. Meda et al. [21] found that an overrepresentation of genes influences the altered DMN connectivity in psychoses, and Ongür et al. [22] reported reduced mPFC connectivity in the DMN in patients with BD, although reports are inconsistent as to whether hypo- or hyper-connectivity in the DMN is associated with the risk of psychosis.
The main objective of the present study was to assess whether the distinction between psychotic PBD and non-psychotic PBD can improve the identification of neurobiological markers for this complex illness. We sought to clarify the similarities and differences between psychotic and non-psychotic PBD using DMN connectivity as a quantitative disease marker. We hypothesized that strongly altered (both increased and decreased) DMN connectivity would persist in psychotic PBD and non-psychotic PBD groups compared with healthy controls during the resting state, especially in the PCC/precuneus, the anterior cingulate cortex (ACC)/mPFC, and the middle temporal cortex.
Materials and Methods
Participants
This study was carried out by a senior psychiatrist of the Child and Adolescent Psychiatric Clinic of the Second Xiangya Hospital of Central South University, China, who has 40 years of experience in the diagnosis and treatment of pediatric mood disorders. A total of 55 children and adolescents were recruited from the clinic, including 29 bipolar patients with psychotic symptoms and 26 bipolar patients without psychotic symptoms (age, sex, and ethnicity matched to controls). As bipolar I and bipolar II disorders may be variants of the same disorder and not separate entities, both types of patients were recruited. Simultaneously, 19 age- and gender-matched healthy controls were recruited through advertisement. Inclusion and exclusion criteria for PBD patients were provided in our previous work [14–16]. This study was approved by the Ethics Committee of the Second Xiangya Hospital of Central South University, and written informed consent was given by all participants prior to participation.
Demographics and Clinical Assessments
The PBD patients were required to meet the DSM-IV-TR diagnostic criteria [23] for BD, including at least one episode of mania, depression, or remission. There were two established subtypes of BD: type I and type II. The subtype BD II was distinguished from BD I mainly by the absence of full-blown manic episodes. Participants were classified as psychotic PBD if they had a psychotic event (hallucinations and/or delusions) over the course of their lifetime, as assessed by the consensus of two board-certified child/adolescent psychiatrists using the Child Schedule for Affective Disorders and Schizophrenia Present and Lifetime Version [24]. All participants completed the Wechsler Abbreviated Scale of Intelligence as an overall measure of cognitive ability [25]. The severity of the current mood symptoms of each BD participant was determined using the Young Mania Rating Scale (YMRS) [26], Mood and Feelings Questionnaire (MFQ) [27], Pittsburgh Sleep Quality Index (PSQI) [28], and Beck Suicide Ideation Scale (BSIS) [29].
MRI Acquisition
All MRI data were acquired on a 3.0-Tesla scanner (Allegra, Siemens Medical System, Erlangen, Germany) with a standard head coil at the Magnetic Resonance Center of Hunan Provincial People’s Hospital. Functional image acquisition was done using a single-shot gradient echo-echo imaging sequence [repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, slices = 30, slice thickness = 4 mm, gap = 0.4 mm, field of view (FOV) = 240 × 240 mm2, matrix = 64 × 64, flip angle = 90°]. A total of 250 volumes were recorded in 500 s. Axial anatomical images were acquired using a 3-dimensional T1-weighted sequence (TR/TE = 2300 ms/2.03 ms, matrix = 256 × 256, FOV = 256 × 256 mm2, slices = 176, flip angle = 9°, slice thickness = 1.0 mm, without gap). During the recordings, all participants were told to relax, hold still, keep their eyes closed without falling asleep, and think of nothing in particular. Foam padding was used to minimize head motion.
Data Preprocessing
Data were preprocessed using Statistical Parametric Mapping software (SPM8; www.fil.ion.ucl.ac.uk/spm/software/spm8/). After slice timing and realignment for head motion correction, any participant whose head motion exceeded 1.0 mm or rotation exceeded 1.0° (scan by scan) was excluded. Eight participants were excluded due to excessive head motion. Twenty-five PBD patients with psychosis, 23 PBD patients without psychosis, and 18 healthy controls remained for further independent component analysis (ICA). Group differences in translational and rotational head motion were also evaluated according to the following formula:
where L is the length of the time series (L = 250 in this study), xi, yi, and zi are translations/rotations at the ith time point in the x, y, and z directions. The results showed that the three groups did not significantly differ in image quality (one-way ANOVA, F = 0.92, P = 0.40 for rotational motion, and F = 1.15, P = 0.32 for translational motion). The standard Montreal Neurological Institute (MNI) template provided by SPM was used in normalization with resampling to a voxel size of 3 mm × 3 mm × 3 mm. The normalized images were then smoothed by convolution with an isotropic Gaussian kernel of 8 mm full-width at half-maximum to decrease spatial noise. At each stage the output was validated visually, and scans were discarded if they did not meet quality control standards.
Group ICA and Identification of the DMN
Spatial ICA was performed using the Group ICA in the fMRI Toolbox (GIFT, version 3.0a, http://mialab.mrn.org/software/gift) to identify independent components (ICs) for all 74 participants. Dimension estimation on the datasets of the three groups was performed to determine the number of ICs using the minimum description length criterion [30]. The numbers of ICs in the three groups (control, psychotic PBD and non-psychotic PBD) were 41, 45, and 44 respectively, ensuring that the resting-state networks had a similar spatial pattern in the three groups. We employed standard methods of rejecting artefactual ICA networks. Network components were examined visually to eliminate those clearly representing artifacts. Then the DMN components of the three groups were selected based on the largest spatial correlation with a specific DMN template, which was derived from a previous study [31].
Statistical Analysis
Statistical analysis was calculated using SPSS version 22.0 for the demographics and clinical datasets, and SPM8 for fMRI images. The spatial maps of each DMN were extracted from the data of all participants, then random-effects analysis using one-sample t-tests [P < 0.001, with family-wise error (FWE) rate correction] was performed within each group. To explore the DMN differences among the three groups, one-way analysis of variance (ANOVA) was then performed on the individual DMN maps in a voxel-by-voxel manner [P < 0.001, correction for multiple comparisons using the false discovery rate (FDR) criterion]. Head motion parameters, age, and gender were included as covariates in the statistical analyses of the present and following functional data. Lastly, post-hoc t-tests were performed to assess the inter-group differences among DMN regions, with thresholds set at P < 0.001 (FDR correction for multiple comparisons). In addition, Spearman’s rank correlation was calculated between variations in DMN z values and clinical scores separately within PBD groups. Correlation analysis was performed using SPSS 22.0, and the threshold was set at a significance level of P < 0.05 (uncorrected).
Results
Demographics and Clinical Characteristics
The demographics and clinical characteristics of the PBD patients and healthy controls are summarized in Table 1. There were no significant differences in age (P = 0.111), gender (P = 0.686), education (P = 0.441), BSIS (P = 0. 228), or IQ (P = 0.322) among the three groups (control, psychotic PBD, and non-psychotic PBD). The PBD sample consisted of 29 type I BD and 19 type II BD. As expected, there were significant differences in the mean MFQ (P = 0.015), PSQI (P = 0.017), and YMRS scores (P = 0.003). Thirteen (27.1%) patients were free of medication at the time of MRI.
Table 1.
Demographics and clinical characteristics of all participants.
| Psychotic PBD Mean (SD) |
Non-psychotic PBD Mean (SD) |
Healthy controls Mean (SD) |
t/F/χ 2 | P value | |
|---|---|---|---|---|---|
| Age | 15.28 (1.57) | 14.7 (2.10) | 14.11 (1.61) | 2.277 | 0.111 |
| Gender (male/female) | 11/14 | 12/11 | 7/11 | 0.753 | 0.686 |
| Education (years) | 8.16 (1.43) | 7.96 (2.23) | 7.39 (2.25) | 0.829 | 0.441 |
| IQ | 100.56 (14.9) | 103.78 (12.77) | 106.28 (6.40) | 1.155 | 0.322 |
| Illness duration (months) | 16.20 (11.89) | 14.96 (13.01) | 0.346 | 0.731 | |
| Onset age (years) | 13.96 (1.46) | 13.39 (1.92) | 1.16 | 0.252 | |
| Episode times (years) | 3.40 (1.53) | 3.17 (2.42) | 0.390 | 0.698 | |
| BD-I/BD-II | 14/11 | 15/8 | 0.426 | 0.514 | |
| YMRS | 16.16 (14.47)a | 15.96 (14.79)a | 3.67 (2.11) | 6.394 | 0.003 |
| MFQ | 16.56 (14.36)a | 13.30 (11.60)b | 6.11 (3.43) | 4.510 | 0.015 |
| PSQI | 5.48 (3.66) | 7.26 (4.07)a | 4.00 (2.45) | 4.364 | 0.017 |
| BSIS | 3.20 (4.95) | 2.87 (3.46) | 1.22 (2.16) | 1.513 | 0.228 |
| PBD-manic | 7 (28.0%) | 7 (34.6%) | |||
| PBD-depression | 8 (32.0%) | 7 (30.8%) | |||
| PBD-remitted | 10 (40.0%) | 9 (34.6%) | 0.036 | 0.982 | |
| Family history (yes/no) | 4/21 | 7/16 | 1.413 | 0.235 | |
| Medication, n (%) | 4 (16.0%) | 9 (39.1%) | 3.245 | 0.072 |
PBD, pediatric bipolar disorder; IQ, intelligence quotient; YMRS, Young Mania Rating Scale; MFQ, Mood and Feelings Questionnaire; PSQI, Pittsburgh Sleep Quality Index; BSIS, Beck Suicide Ideation Scale.
aP < 0.01 compared to healthy controls.
bP < 0.05 compared to healthy controls.
Within-Group ICA Analyses
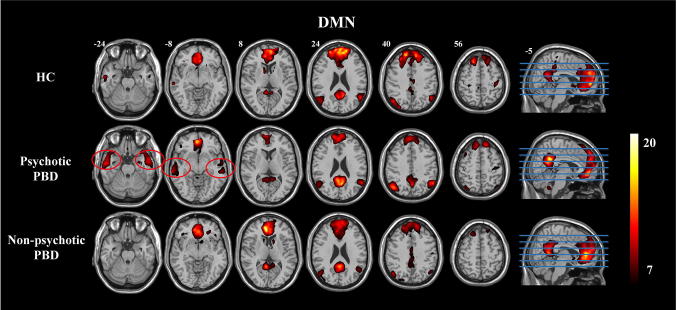
The one-sample t-test revealed a typical spatial pattern in the DMN in each group (Fig. 1). The voxels consistent with spatial connectivity were bilaterally distributed within a few mesial and lateral brain structures: the ACC/mPFC, PCC/precuneus, bilateral angular gyri, bilateral MTG, and bilateral caudate nucleus. Interestingly, significantly higher connectivity scores in the bilateral MTG were shown in the PBD patients with psychosis than in controls and non-psychotic PBD patients, substantiating the view that the MTG plays a key role in PBD patients with psychosis (Fig. 1).
Fig. 1.
One-sample t-test results for group-level DMNs in the control (HC), psychotic PBD, and non-psychotic PBD groups. Connectivity scores in the bilateral MTG (red circles) were higher in the psychotic PBD group. The color scale represents t values in each DMN (maps thresholded at P < 0.001, FWE corrected).
Between-Group ICA Analyses
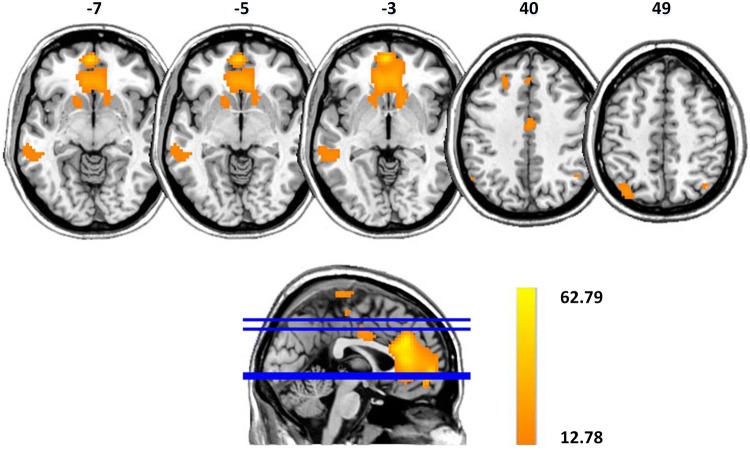
The results of one-way ANOVA showed significant DMN differences among the three groups (P < 0.001, FDR corrected). The connectivity differences were demonstrated in the ACC/mPFC, bilateral caudate nucleus, bilateral MTG, and bilateral angular gyri (Fig. 2). Table 2 provides a list of the regions displaying DMN connectivity differences, along with the MNI coordinates of the peak foci.
Fig. 2.
DMN differences among control, psychotic PBD, and non-psychotic PBD groups (maps thresholded at P < 0.001, FDR corrected). Altered connectivity shows differences in the ACC/mPFC, bilateral caudate nucleus, bilateral MTG, and bilateral angular gyri. The color scale represents F values of ANOVA.
Table 2.
Regions showing DMN connectivity differences among the three groups (control, psychotic PBD, and non-psychotic PBD).
| Brain regions | MNI coordinates x, y, z |
Peak F value |
Volume |
|---|---|---|---|
| ACC/mPFC (L/R) | −6 33 12/6 33 15 | 58.7/61.97 | 321/318 |
| Caudate nucleus (L/R) | −15 18 0/12 15 0 | 17.74/22.58 | 20/62 |
| Angular gyrus (L/R) | −36 −69 48/42 −69 51 | 18.91/15.48 | 31/11 |
| MTG (L) | −51 −36 0 | 14.9 | 76 |
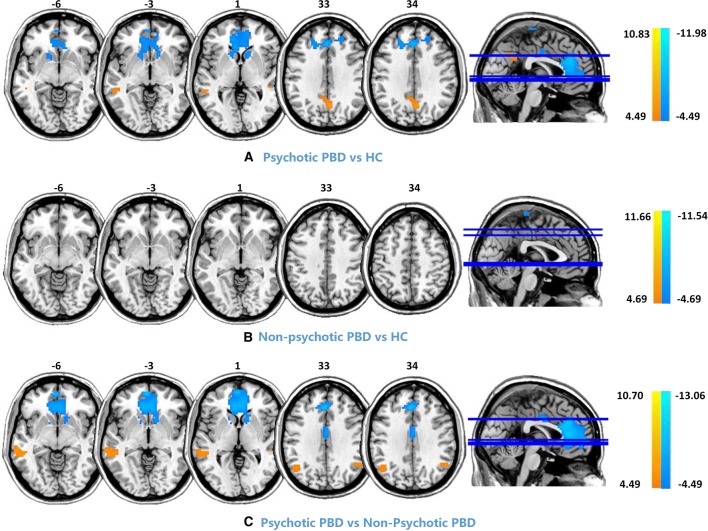
Post-hoc comparisons of intra-network connectivity among the three groups are shown in Fig. 3 and Table 3 (P < 0.001, FDR corrected). Compared with controls, PBD patients with psychosis showed decreased functional connectivity (FC) in the ACC, mPFC, and bilateral caudate nucleus, as well as increased FC in the PCC and left middle temporal cortex. Compared to non-psychotic PBD patients, those with psychosis exhibited decreased FC in the ACC, mPFC, and bilateral caudate nucleus, as well as increased FC in the bilateral angular gyrus and left MTG. No significant differences were found between PBD patients without psychosis and controls.
Fig. 3.
Post-hoc comparison (P < 0.001, FDR corrected) of intra-network connectivity among the three groups [control (HC), psychotic PBD, and non-psychotic PBD]. Compared with the controls, patients with psychotic PBD displayed significantly increased connectivity in the left MTG and PCC, as well as decreased connectivity in the ACC/mPFC and bilateral caudate nucleus. Compared with the non-psychotic PBD patients, the psychotic PBD patients showed increased connectivity in the left MTG and bilateral angular gyri, as well as decreased connectivity in the ACC and bilateral caudate nucleus. The color scale represents t values.
Table 3.
Regions showing DMN connectivity differences between psychotic PBD patients and controls (HC), and between psychotic PBD and non-psychotic PBD patients.
| Psychotic PBD vs HC | Psychotic PBD vs non-psychotic PBD | ||||||
|---|---|---|---|---|---|---|---|
| Brain regions | MNI x, y, z |
Peak t value |
Volume | Brain regions | MNI x, y, z |
Peak t value |
Volume |
| Increased connectivity | |||||||
| MTG (L) | −51 −39 −3 | 5.43 | 58 | MTG (L) | −45 −36 −6 | 5.46 | 176 |
| PCC (L/R) | −6 −48 27/9 −48 30 | 5.20/4.92.0 | 18/11 | Angular gyrus (L/R) | −42 −60 39/60 −60 36 | 5.61/8.18 | 129/102 |
| Decreased connectivity | |||||||
| ACC/mPFC (L/R) | −6 36 12/3 36 12 | −8.63/−7.76 | 532/341 | ACC/mPFC (L/R) | 0 45 6/9 33 6 | −9.0/−7.6 | 304/292 |
| Caudate nucleus (L/R) | −12 6 12/12 0 12 | −5.17/−5.70 | 47/59 | Caudate nucleus (L/R) | −15 15 6/12 12 6 | −4.81/−5.87 | 22/100 |
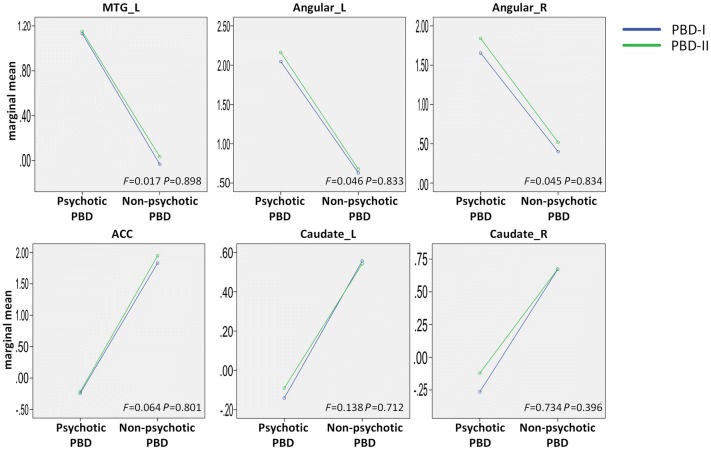
Interactions of PBD group (psychotic and non-psychotic) × PBD type (I and II) were analyzed to determine whether the different subtypes mediate the abnormal FC in the DMN. The results showed no significant interactions between group and type in the areas of the left MTG, bilateral angular gyri, ACC, and bilateral caudate nucleus (Fig. 4, uncorrected P < 0.05 for all interactions), suggesting that the abnormal FC in the DMN is not mediated by different subtypes.
Fig. 4.
Interaction between PBD group (psychotic and non-psychotic) and PBD subtype (I and II) in the abnormal functional connectivity regions of the DMN. F values from ANOVA showed no significant interactions in the areas of the left MTG, bilateral angular gyri, ACC, and bilateral caudate nucleus (uncorrected P < 0.05 for all interactions).
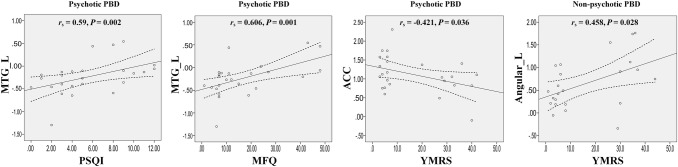
In addition, the psychotic PBD group showed a positive relationship between the left MTG and PSQI (r = 0.590; P = 0.002) and MPQ (r = 0.606; P = 0.001) scores, and a negative relationship between the ACC and YMRS (r = −0.421; P = 0.036). The non-psychotic PBD group showed a positive relationship between the left angular and YMRS (r = 0.458; P = 0.028); P values unadjusted α = 0.05 (Fig. 5).
Fig. 5.
Correlations between clinical symptoms and aberrant functional connectivity in the DMN of psychotic and non-psychotic PBD groups. The threshold was set at a significance level of P < 0.05 (uncorrected).
Discussion
Using ICA methodology, we demonstrated altered resting-state DMNs in patients with psychotic PBD, non-psychotic PBD, and healthy controls. Also, we revealed a dissociation between anterior and posterior FC within the DMN. Compared to healthy controls, PBD patients with psychosis showed more widespread changes in FC values in some cortical and subcortical regions than patients without psychosis, suggesting that the development of psychosis causes further impairment. Compared to the non-psychotic PBD group, the psychotic PBD group showed greater FC in the left temporal gyrus and bilateral angular gyrus, as well as decreased FC in the ACC/mPFC and bilateral caudate nucleus. In addition, this was the first study, to our knowledge, to assess the connectivity differences of the DMN in PBD patients in the context of psychosis.
The results from ANOVA, which showed that PBD patients had altered FC in the DMN in the regions of the ACC/mPFC, bilateral caudate nucleus, bilateral MTG, and bilateral angular gyrus, align with prior studies that have uncovered cognitive deficits in PBD [32]. For example, PBD patients with working memory deficits have increased mPFC and temporal fMRI activation [33]. Similar fronto-temporal changes have been found in PBD patients performing picture and face tasks with varying emotional valence [34, 35], perhaps because an interaction between the prefrontal cortex and temporal cortex is required in the cognitive processes of top-down control [36]. Also, since the altered regions are known to fully interact with other prefrontal cortical areas and are likely involved in emotional regulation and reward-related feedback, lesions in these regions would affect decision-making and emotional control in patients with PBD [37].
The ACC/mPFC and bilateral caudate nucleus displayed lower FC in the psychotic than in the non-psychotic and control groups. One possibility is that there are differences in underlying neurotransmitter dysfunctions that could plausibly affect the resting-state activity via modulating the default mode [38]. Several recent papers have suggested possible roles for the anterior DMN (mPFC-ACC-caudate nucleus). These regions show mood-state-dependent activity changes in BD and are linked to emotion generation and appraisal [6, 7]. Meda et al. [21] revealed genetic associations of the resting-state DMN in psychotic BD and schizophrenia (SZ), and dissected the underlying biological/molecular pathways that might mediate the genetic risk of psychosis via the DMN. Ongür et al. [22] reported reduced mPFC resting-state connectivity within the DMN in patients with BP and SZ. Anticevic et al. [39] found aberrant global prefrontal and fronto-limbic connectivity in BD patients with a history of psychosis. Our findings substantially extend prior work on BD with the detection of global disruptions in prefrontal connectivity, and highlight that the aberrant anterior DMN connectivity might represent a potential trait characteristic or risk factor for PBD patients with psychosis.
The PCC and angular gyrus are key DMN hubs that have been shown repeatedly to be aberrant in both adult [40] and pediatric BD [41]. In the current work, the PCC displayed lower connectivity in PBD patients with psychosis than in controls, while the angular gyrus displayed higher connectivity in psychotic than in non-psychotic patients. In our previous studies, abnormal resting-state neuronal activity in the parietal cortex was found in PBD patients with mania [14]. Jafri et al. [42] examined the FC in SZ patients and reported abnormally high connections in the fronto-parietal network as well. PBD patients with psychosis had significantly higher DMN connectivity overall, suggesting abnormal functional organization of the key resting-state network hubs. The left MTG demonstrated a strong trend toward aberrance, but only in the PBD patients with psychosis, which corroborates the evidence from numerous structural studies showing that BD is associated with alterations in the temporal lobe [43]. Another rs-fMRI study of adult BD patients with depression noted that the amplitude of low-frequency fluctuations was increased in the bilateral temporal gyri [40] and our results suggest that the MTG is associated with the generation of psychotic activity in patients with PBD. The correlation results suggest that the increased connectivity of the MTG in psychotic versus non-psychotic PBD patients might be an important marker for the expression of the disease state of PBD.
Some aspects of this study could be improved. First, our sample size was relatively small. If confirmed in larger samples, our results might serve as a biomarker in the diagnosis of PBD with and without psychotic symptoms. Second, nearly a quarter of the PBD patients were taking medication. Previous studies have suggested that drug treatment mediates the abnormal FC [44]. Although we demonstrated network alterations in psychotic compared with non-psychotic PBD patients, it remains to be seen how antipsychotic medication affects the brain connectivity in PBD patients during the resting state.
In conclusion, compared with healthy controls, our results demonstrated that PBD patients with psychosis are characterized by aberrant DMN connectivity in the ACC/mPFC, caudate nucleus, angular gyrus, and MTG, whereas PBD patients without psychosis are not, suggesting further impairment with the development of psychosis. Meanwhile, associations between clinical symptoms and aberrant FC in the DMN were also found in PBD patients with psychosis. Our findings, from the first study to evaluate FC in pediatric BD patients with and without psychosis, highlight the possibility that aberrant DMN connectivity might represent a potential trait characteristic for psychotic PBD.
Acknowledgements
This work was supported by National Natural Science Foundation of China (81171291, 81371531, 81571344, 81871344), the Natural Science Foundation of Jiangsu Province, China (BK20161109), the Key Program for Guangming Lu (BWS11J063, and 10z026), the Natural Science Foundation of the Higher Education Institutions of Jiangsu Province, China (18KJB190003), and the Postdoctoral Science Foundation of China (2014M552700). We would like to thank the experts at the Magnetic Resonance Center of Hunan Provincial People’s Hospital for providing scan time and technical assistant.
Conflict of interest
The authors claim that there are no conflicts of interest.
Contributor Information
Linyan Su, Email: su-linyan@hotmail.com.
Guangming Lu, Email: cjr.luguangming@vip.163.com.
References
- 1.Goodwin FK, Jamison KR. Manic-Depressive Illness. New York: Oxford University Press; 2007. pp. 54–59. [Google Scholar]
- 2.Johnson JG, Cohen P, Brook JS. Associations between bipolar disorder and other psychiatric disorders during adolescence and early adulthood: a community-based longitudinal investigation. Am J Psychiatry. 2000;157:1679–1681. doi: 10.1176/appi.ajp.157.10.1679. [DOI] [PubMed] [Google Scholar]
- 3.Perlis RH, Miyahara S, Marangell LB, Wisniewski SR, Ostacher M, Delbello MP, et al. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the systematic treatment enhancement program for bipolar disorder (step-bd) Biol Psychiatry. 2004;55:875–881. doi: 10.1016/j.biopsych.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 4.Coryell W, Leon AC, Turvey C, Akiskal HS, Mueller T, Endicott J. The significance of psychotic features in manic episodes: a report from the NIMH collaborative study. J Affect Disord. 2001;67:79–88. doi: 10.1016/S0165-0327(99)00024-5. [DOI] [PubMed] [Google Scholar]
- 5.Meda SA, Gill A, Stevens MC, Lorenzoni RP, Glahn DC, Calhoun VD, et al. Differences in resting-state fMRI functional network connectivity between schizophrenia and psychotic bipolar probands and their unaffected first-degree relatives. Biol Psychiatry. 2012;71:881–889. doi: 10.1016/j.biopsych.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strakowski SM, Delbello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10:105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- 7.Strakowski SM, Adler CM, Almeida J, Altshuler LL, Blumberg HP, Chang KD, et al. The functional neuroanatomy of bipolar disorder: a consensus model. Bipolar Disord. 2012;14:313–325. doi: 10.1111/j.1399-5618.2012.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 9.Uddin LQ, Kelly AM, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp. 2009;30:625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Wang C, Zhu X, Tan Y, Zhong Y. Aberrant connectivity within the default mode network in first-episode, treatment-naïve major depressive disorder. J Affect Disord. 2015;183:49–56. doi: 10.1016/j.jad.2015.04.052. [DOI] [PubMed] [Google Scholar]
- 11.Vargas C, López-Jaramillo C, Vieta E. A systematic literature review of resting state network–functional MRI in bipolar disorder. J Affect Disord. 2013;150:727–735. doi: 10.1016/j.jad.2013.05.083. [DOI] [PubMed] [Google Scholar]
- 12.Anticevic A, Brumbaugh MS, Winkler AM, Lombardo LE, Barrett J, Corlett PR, et al. Global prefrontal and fronto-amygdala dysconnectivity in bipolar I disorder with psychosis history. Biol Psychiatry. 2013;73:565–573. doi: 10.1016/j.biopsych.2012.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu M, Lu LH, Passarotti AM, Wegbreit E, Fitzgerald J, Pavuluri MN. Altered affective, executive and sensorimotor resting state networks in patients with pediatric mania. J Psychiatry Neurosci. 2013;38:232–240. doi: 10.1503/jpn.120073. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Lu D, Jiao Q, Zhong Y, Gao W, Xiao Q, Liu X, et al. Altered baseline brain activity in children with bipolar disorder during mania state: a resting-state study. Neuropsychiatr Dis Treat. 2014;10:317–323. doi: 10.2147/NDT.S54663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao Q, Zhong Y, Lu D, Gao W, Jiao Q, Lu G, et al. Altered regional homogeneity in pediatric bipolar disorder during manic state: a resting-state fMRI study. PLoS One. 2013;8:e57978. doi: 10.1371/journal.pone.0057978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao W, Jiao Q, Lu S, Yuan Z, Qi R, Lu D, et al. Alterations of regional homogeneity in pediatric bipolar depression: a resting-state fmri study. BMC Psychiatry. 2014;14:222. doi: 10.1186/s12888-014-0222-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao X, Zhang W, Yao L, Xiao Y, Liu L, Liu J, et al. Association between structural and functional brain alterations in drug-free patients with schizophrenia: a multimodal meta-analysis. J Psychiatry Neurosci. 2018;43:131–142. doi: 10.1503/jpn.160219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang YM, Zou LQ, Xie WL, Yang ZY, Zhu XZ, Cheung EFC, et al. Altered functional connectivity of the default mode network in patients with schizo-obsessive comorbidity: a comparison between schizophrenia and obsessive-compulsive disorder. Schizophr Bull. 2018 doi: 10.1093/schbul/sbx194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu ML, Zong XF, Mann JJ, Zheng JJ, Liao YH, Li ZC, et al. A review of the functional and anatomical default mode network in schizophrenia. Neurosci Bull. 2017;33:1–12. doi: 10.1007/s12264-016-0090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 21.Meda SA, Ruaño G, Windemuth A, O’Neil K, Berwise C, Dunn SM, et al. Multivariate analysis reveals genetic associations of the resting default mode network in psychotic bipolar disorder and schizophrenia. Proc Natl Acad Sci U S A. 2014;111:E2066–E2075. doi: 10.1073/pnas.1313093111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ongür D, Lundy M, Greenhouse I, Shinn AK, Menon V, Cohen BM, et al. Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res. 2010;183:59–68. doi: 10.1016/j.pscychresns.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mittal VA, Walker EF. Diagnostic and statistical manual of mental disorders. Psychiatry Res. 2011;189:158–159. doi: 10.1016/j.psychres.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (k-sads-pl): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 25.Irby SM, Floyd RG. Test review: wechsler abbreviated scale of intelligence, second edition. Can J Sch Psychol. 2013;28:295–299. doi: 10.1177/0829573513493982. [DOI] [Google Scholar]
- 26.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 27.Wood A, Kroll L, Moore A, Harrington R. Properties of the mood and feelings questionnaire in adolescent psychiatric outpatients: a research note. J Child Psychol Psychiatry. 1995;36:327–334. doi: 10.1111/j.1469-7610.1995.tb01828.x. [DOI] [PubMed] [Google Scholar]
- 28.Smyth C. The pittsburgh sleep quality index (PSQI) Insight. 2003;25:97–98. doi: 10.1067/min.2000.107649. [DOI] [PubMed] [Google Scholar]
- 29.Beck AT, Kovacs M, Weissman A. Assessment of suicidal ideation: the scale for suicide ideation. J Consult Clin Psychol. 1979;47:343–352. doi: 10.1037/0022-006X.47.2.343. [DOI] [PubMed] [Google Scholar]
- 30.Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39:1666–1681. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao W, Chen H, Feng Y, Mantini D, Gentili C, Pan Z, et al. Selective aberrant functional connectivity of resting state networks in social anxiety disorder. Neuroimage. 2010;52:1549–1558. doi: 10.1016/j.neuroimage.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Delbello MP, Adler CM, Strakowski SM. The neurophysiology of childhood and adolescent bipolar disorder. CNS Spectr. 2006;11:298–311. doi: 10.1017/S1092852900020794. [DOI] [PubMed] [Google Scholar]
- 33.Adler CM, Holland SK, Schmithorst V, Tuchfarber MJ, Strakowski SM. Changes in neuronal activation in patients with bipolar disorder during performance of a working memory task. Bipolar Disord. 2004;6:540–549. doi: 10.1111/j.1399-5618.2004.00117.x. [DOI] [PubMed] [Google Scholar]
- 34.Kalmar JH, Wang F, Chepenik LG, Womer FY, Jones MM, Pittman B, et al. Relation between amygdala structure and function in adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:636–642. doi: 10.1097/CHI.0b013e31819f6fbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: a functional magnetic resonance imaging investigation. Arch Gen Psychiatry. 2004;61:781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- 36.Dickstein DP, Gorrostieta C, Ombao H, Goldberg LD, Brazel AC, Gable CJ, et al. Fronto-temporal spontaneous resting state functional connectivity in pediatric bipolar disorder. Biol Psychiatry. 2010;68:839–846. doi: 10.1016/j.biopsych.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adler CM, DelBello MP, Strakowski SM. Brain network dysfunction in bipolar disorder. CNS Spectr. 2006;11:312–320. doi: 10.1017/S1092852900020800. [DOI] [PubMed] [Google Scholar]
- 38.Minzenberg MJ, Yoon JH, Carter CS. Modafinil modulation of the default mode network. Psychopharmacology (Berl) 2011;215:23–31. doi: 10.1007/s00213-010-2111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anticevic A, Brumbaugh MS, Winkler AM, Lombardo LE, Barrett J, Corlett PR, et al. Global prefrontal and fronto-amygdala dysconnectivity in bipolar i disorder with psychosis history. Biol Psychiatry. 2013;73:565–573. doi: 10.1016/j.biopsych.2012.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu CH, Li F, Li SF, Wang YJ, Tie CL, Wu HY, et al. Abnormal baseline brain activity in bipolar depression: a resting state functional magnetic resonance imaging study. Psychiatry Res. 2012;203:175–179. doi: 10.1016/j.pscychresns.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 41.Pavuluri MN, Sweeney JA. Integrating functional brain neuroimaging and developmental cognitive neuroscience in child psychiatry research. J Am Acad Child Adolesc Psychiatry. 2008;47:1273–1288. doi: 10.1097/CHI.0b013e318185d2d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39:1666–1681. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen X, Wen W, Malhi GS, Ivanovski B, Sachdev PS. Regional gray matter changes in bipolar disorder: a voxel-based morphometric study. Aust N Z J Psychiatry. 2007;41:327–336. doi: 10.1080/00048670701213229. [DOI] [PubMed] [Google Scholar]
- 44.Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry. 2008;165:313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]