Abstract
ZNF804A rs1344706 has been identified as one of the risk genes for schizophrenia. However, the neural mechanisms underlying this association are unknown. Given that ZNF804A upregulates the expression of COMT, we hypothesized that ZNF804A may influence brain activity by interacting with COMT. Here, we genotyped ZNF804A rs1344706 and COMT rs4680 in 218 healthy Chinese participants. Amplitudes of low-frequency fluctuations (ALFFs) were applied to analyze the main and interaction effects of ZNF804A rs1344706 and COMT rs4680. The ALFFs of the bilateral dorsolateral prefrontal cortex showed a significant ZNF804A rs1344706 × COMT rs4680 interaction, manifesting as a U-shaped modulation, presumably by dopamine signaling. Significant main effects were also found. These findings suggest that ZNF804A affects the resting-state functional activation by interacting with COMT, and may improve our understanding of the neurobiological effects of ZNF804A and its association with schizophrenia.
Electronic supplementary material
The online version of this article (10.1007/s12264-019-00357-w) contains supplementary material, which is available to authorized users.
Keywords: Zinc finger protein 804A, Catechol-O-methyltransferase, Amplitudes of low-frequency fluctuations, fMRI
Introduction
Schizophrenia (SZ) is a severe and complex psychiatric disorder with high polygenic heredity [1–3]. Many studies have shown that the dopamine (DA) system is of fundamental importance to the etiology of SZ, and its dysfunction may lead to the neurobiological abnormalities in SZ [4]. Catechol-O-methyltransferase (COMT) is thought to be the most important modulator of synaptic DA concentration in the brain [5, 6]. COMT participates in DA degradation at the biochemical level, as well as complex cognitive functions such as cognitive control and working memory at the psychological level [7]. DA is vital for neuronal development and an appropriate DA level promotes neuronal growth and differentiation [8]. Compared with Val/Val homozygotes, COMT rs4680 Met-allele carriers have decreased enzymatic activity and increased synaptic DA concentration [2, 6]. The modulation of cognitive function and imaging phenotypes by extracellular DA is nonlinear. That is to say, both the lowest and highest DA levels may have an unfavorable effect [5, 9–11].
Zinc finger protein 804A (ZNF804A) is one of the candidate genes for schizophrenia and some association studies have been conducted for ZNF804A rs1344706 [12–14]. Overexpression of ZNF804A mRNA has been suggested to regulate the expression of schizophrenia-associated genes, including COMT [15]. Expressed ZNF804A can interact with the promoter region of COMT and increase the transcript level of COMT [15], suggesting that a potential genotypic interaction causes different phenotypes.
The resting-state spontaneous brain activity was selected because of the functional properties of the DA system, which has a complex release pattern, dynamically influencing the neuronal activity [16–18]. Based on the upregulating effect of ZNF804A on COMT [15] and the modulatory effect of COMT on DA signaling [5, 6, 19], we can roughly estimate the extracellular DA levels in the human brain. For instance, the COMT Met-allele-ZNF804A CC carriers would have the highest DA level, while the COMT Val/Val-ZNF804A CA carriers would have the lowest DA level.
The amplitude of low-frequency fluctuations (ALFF) is considered a reliable and sensitive measure in both healthy and clinical populations [20–22]. The spontaneous LFFs of blood oxygenation level-dependent (BOLD) signals at rest have been identified as a biological measure of baseline spontaneous activity in the brain [21, 23–26]. An abnormal ALFF can reflect a pathophysiological state in a brain area and help locate specific impaired brain regions during the resting state [21]. As an additional advantage, ALFF can be used to assess neuronal activity within the entire brain [21]. Most recent studies have suggested that ALFF might be a measure of the spontaneous neuronal activity in brain regions [21] associated with the low-frequency BOLD fluctuations obtained by resting fMRI [27]. In particular, using rs-fMRI, many previous studies have reported the dysfunctional regulation in schizophrenia patients [28–30].
In this study, we measured resting-state spontaneous activity in the brains of healthy participants to test the hypothesis that ZNF804A affects brain function by indirectly modulating the expression level of COMT.
Materials and Methods
A total of 218 right-handed Chinese participants (18–60 years, mean 29.0 years; 85 males and 133 females) were recruited from the community. There was no personal history of a DSM-IV Axis I Disorder (SCID) [31] in these individuals or their first-degree family members, as assessed by the Family History Screen for Epidemiologic Studies [32]. None had a history of medical or neurological disorders or took medications with a potential effect on the central nervous system or had a loss of consciousness for ≥ 5 min. The participants did not use substances the week prior to scanning, and all had negative results for urine toxicology screens on the day of scanning and had no contraindications for MRI examination. After a full explanation of the study, all participants provided written informed consent. This research was approved by the Medical Research Ethics Committee of China Medical University and were in accord with the Declaration of Helsinki.
Genotyping
Genomic DNA was extracted from peripheral blood samples using the Qiagen QIAamp DNA Mini Kit (Hilden, Germany). The ZNF804A rs1344706 (C/A) and COMT rs4680 (Val158Met) genotypes were determined using the Sanger sequencing method. The participants were divided into six genotypic subgroups: Val/Val/AA, Val/Val/CA, Val/Val/CC, Met-allele/AA, Met-allele/CA, and Met-allele/CC according to the COMT rs4680 and ZNF804A rs1344706 genotypes.
MRI Data Acquisition
MRI scans were performed on a 3-Tesla GE Signa HDX MR scanner (General Electric, Boston, MA) in the Department of Radiology of the First Hospital of China Medical University. Three-dimensional T1-weighted images were obtained using a fast spoiled gradient echo sequence: repetition time (TR) = 7.1 ms, echo time (TE) = 3.2 ms, field of view (FOV) = 24 cm × 24 cm, matrix = 240 × 240, flip angle = 15°, slice thickness = 1 mm, no gap. Functional imaging data were acquired using gradient echo planar imaging: TR = 2000 ms, TE = 30 ms, FOV = 24 cm × 24 cm, matrix = 64 × 64, flip angle = 90°, 35 3-mm slices without gap. Earplugs and soft pads were used during scanning to reduce scanner noise and restrict head motion. Participants were instructed to relax with their eyes closed but remain awake and think of nothing in particular throughout the resting-state scan.
MRI Data Preprocessing
Data preprocessing was conducted using the Data Processing & Analysis of Brain Imaging software package (http://rfmri.org/dpabi) [33], running on MatLab. The first 10 time points were discarded to allow adaptation to the scanning noise and spin saturation. Slice timing and realignment for head motion correction were performed. Participants with head motion > 3.0 mm of maximum displacement in any dimension (x, y, or z) or 3° of maximum angular motion were excluded. The images were normalized into standard Montreal Neurological Institute (MNI) space, and re-sampled to 3 mm × 3 mm × 3 mm voxels. Then, spatial smoothing was conducted with a 6-mm full-width at half-maximum Gaussian kernel. Removal of linear trends and bandpass filtering (0.01–0.08 Hz) were performed to reduce very low-frequency drift and high-frequency noise [34]. Nuisance covariates (head motion parameters, cerebrospinal fluid signal, white matter signal, and global mean signal) generated in the above steps were regressed out.
ALFF Calculation
ALFF calculations were performed as described in previous studies [35]. The filtered time series were first converted to the frequency domain using a Fast Fourier transform, and the power spectrum was then determined. The square root was calculated at each frequency of the power spectrum and averaged between 0.01 and 0.08 Hz. This averaged square root was termed the ALFF [22]. For standardization, the ALFF of each voxel was divided by the global mean ALFF value. The global mean ALFF was calculated only within the brain, excluding the background and tissues outside of the brain.
Statistical Analysis
A two-way (COMT and ZNF804A) analysis of variance (ANOVA) was used to compare demographic data (age and education) with SPSS 13.0 software (SPSS Inc., Chicago, IL). Chi-square tests were used to compare gender between subgroups (P < 0.05). Two schemes were used to determine the order of the six genotypic groups based on DA signaling from low to high under different hypotheses for the effects of ZNF804A rs1344706 and COMT rs4680. If the COMT rs4680 has a stronger effect than ZNF804A rs1344706, the presumed order would be: Val/Val/CA < Val/Val/AA < Val/Val/CC < Met-allele/CA < Met-allele/AA < Met-allele/CC. Conversely, if the ZNF804A rs1344706 has a stronger effect, the presumed order would be: Val/Val/CA < Met-allele/CA < Val/Val/AA < Met-allele/AA < Val/Val/CC < Met-allele/CC. We used curve fitting analysis to determine which scheme was more plausible, based on the U-shaped modulation of DA.
Two-way ANOVA was performed in Statistical Parametric Mapping (SPM) software (SPM8; Wellcome Department of Cognitive Neurology, London, UK) with ZNF804A rs1344706 (AA, CA, and CC) and COMT rs4680 (Val/Val, Met-allele) as two independent variables. The main and interaction effects between genotype groups were modeled. Age, gender, and education were considered to be covariates. Significant genotype interactions were interpreted using graphic displays and by performing post-hoc exploratory analyses separately.
Voxels with a P value < 0.01 and cluster size > 837 mm3 (31 voxels) were considered indicative of significant differences; this was equal to a corrected threshold of P < 0.05 as determined by the Monte Carlo simulation (AlphaSim by B.D. Ward in AFNI software, http://afni.nimh.nih.gov/ [36]).
Results
Demographics and genotypic results
There was no significant difference in age (P = 0.317), gender (P = 0.738) or education (P = 0.714) among the six genotypic subgroups (Table 1). The genotype frequencies of both COMT rs4680 and ZNF804A rs1344706 were consistent with Hardy-Weinberg equilibrium (COMT: χ2 = 0.41, P = 0.52; ZNF804A: χ2 = 2.22, P = 0.13).
Table 1.
Demographics of different genotypic groups
| Genotypic groups | n = 218 | Age (years) | Education (years) | Gender (male/female) |
|---|---|---|---|---|
| COMT Met-allele/ZNF804A AA | 18 | 27.28 (9.492) | 12.11 (3.984) | 8/10 |
| COMT Met-allele/ZNF804A CA | 58 | 28.43 (13.462) | 12.11 (3.663) | 26/32 |
| COMT Met-allele/ZNF804A CC | 22 | 26.50 (11.999) | 12.05 (3.798) | 8/14 |
| COMT Val/Val/ZNF804A AA | 31 | 27.65 (11.803) | 12.16 (3.417) | 10/21 |
| COMT Val/Val/ZNF804A CA | 62 | 29.05 (12.768) | 11.81 (3.625) | 25/37 |
| COMT Val/Val/ZNF804A CC | 27 | 34.07 (14.301) | 13.27 (4.153) | 8/19 |
| Statistics | 218 | F = 1.186 | F = 0.581 | χ2 = 2.751 |
| P | 0.317 | 0.714 | 0.738 |
Data are shown as the mean (SD)
ALFF Results
Interactions Involving COMT and ZNF804A Genotypes
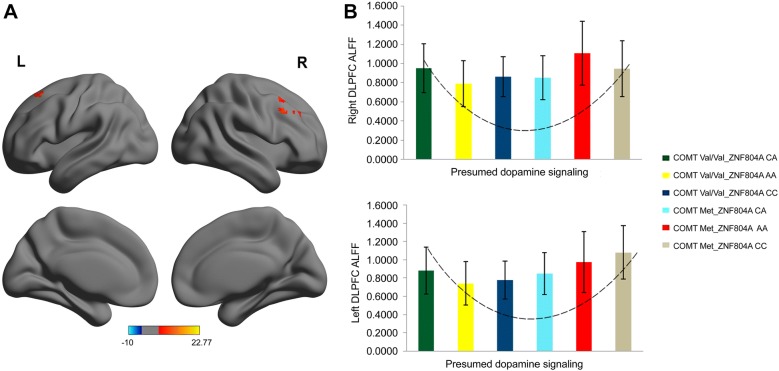
The significant interaction and main effects between the two SNPs are listed in Table 2. We found a significant COMT rs4680 × ZNF804A rs1344706 interaction (Right: = 23.583, P < 0.001, corrected; Left: F = 9.689, P < 0.001, corrected) in the ALFF values for the bilateral dorsolateral prefrontal cortex (DLPFC) (Left: Peak MNI coordinates: x = − 21, y = 21, z = 54; cluster size = 31 voxels; peak F = 9.2002; Right: Peak MNI coordinates: x = 30, y = 33, z = 33; cluster size = 116 voxels; peak F = 11.028; Fig. 1A). The ALFF values with a significant COMT rs4680 × ZNF804A rs1344706 interaction were extracted from the data of each participant. The distributions of ALFFs of the two clusters in the genotypic subgroups (which reflected the presumed DA signaling) displayed a U-shape (Fig. 1B). By sorting according to COMT, the quadratic regression was significant (Right: r2 = 0.068, P = 0.001; Left: r2 = 0.115, P < 0.001) (Fig. S1).
Table 2.
Clusters exhibiting the influence of COMT and ZNF804A polymorphism on ALFF
| Brain areas | BA | Cluster size (Voxel) | Peak MNI coordinates | Peak F value | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Main effect of COMT polymorphism | ||||||
| Right orbitofrontal cortex | 11 | 33 | 12 | 51 | − 24 | 22.761 |
| Left paracentral lobule/middle cingulate gyrus | 6/31 | 47 | 0 | − 15 | 45 | 22.554 |
| Left dorsolateral prefrontal cortex | 8 | 69 | − 21 | 21 | 54 | 23.802 |
| Right parietal cortex | 7/5/40 | 352 | 6 | − 51 | 69 | 40.151 |
| Main effect of ZNF804A polymorphism | ||||||
| Left calcarine gyrus/posterior cingulate gyrus/cuneus | 18 | 42 | − 18 | − 69 | 12 | 11.550 |
| Interaction effect of COMT × ZNF804A | ||||||
| Right dorsolateral prefrontal cortex | 8/9 | 116 | 30 | 33 | 33 | 23.583 |
| Left dorsolateral prefrontal cortex | 8 | 31 | − 21 | 21 | 54 | 9.689 |
These findings correspond to a corrected P < 0.01 by AlphaSim correction.
BA Brodmann’s area.
Fig. 1.
The significant COMT rs4680 × ZNF804A rs1344706 interaction. A Clusters with significance for the interaction effect in the bilateral DLPFC ( < 0.001 by AlphaSim correction, and 31 voxels minimum). Color bar represents the range of values. B Interaction graph showing the distribution of ALFFs of the two clusters in these genotypic subgroups (which reflected presumed DA signaling) displayed as a U-shape.
Effect of COMT Genotype on ALFF
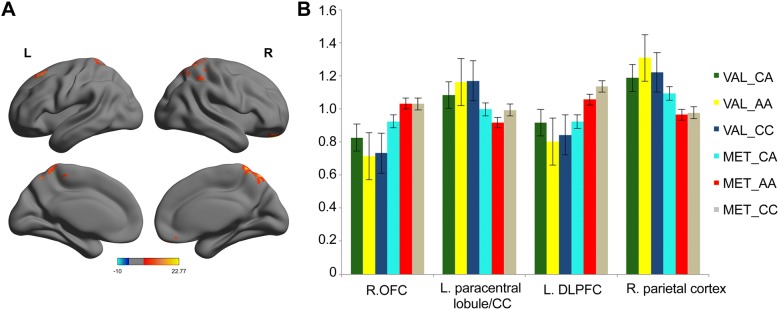
After performing a two-way ANOVA on the ALFF maps, significant main effects of COMT were found (all P < 0.001, corrected). COMT Met carriers showed increased ALFF in the left DLPFC and right orbitofrontal cortex (OFC), as well as decreased ALFF in the right parietal cortex, left paracentral lobule, and middle cingulate gyrus (Fig. 2).
Fig. 2.
The main effect of COMT genotype on ALFF. A Clusters with a significant main effect of COMT genotype in the left DLPFC, right OFC, right parietal cortex, left paracentral lobule, and middle cingulated gyrus. Color bar represents the range of values. BCOMT Met carriers showed increased ALFF in the left DLPFC and right OFC, and decreased ALFF in the right parietal cortex, left paracentral lobule, and middle cingulated gyrus compared to COMT Val/Val carriers at rest (all P < 0.001, corrected).
Effect of ZNF804A Genotype on ALFF
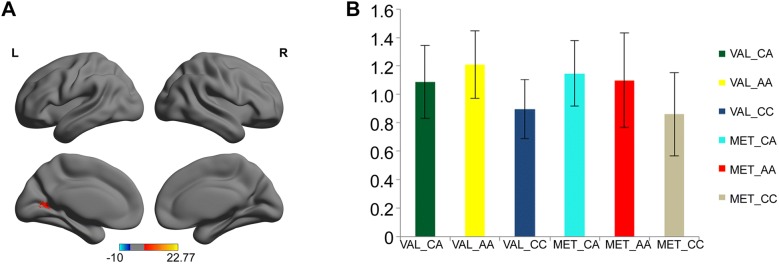
There was also a significant main effect of ZNF804A (F = 11.550, P < 0.001, corrected). ZNF804A CC homozygotes exhibited decreased ALFF in the left calcarine gyrus, posterior cingulate gyrus, and cuneus than the CA (P < 0.001, corrected) and AA genotypes (P < 0.001, corrected). There was no significant difference in ALFF between CA and AA carriers (P = 0.534, corrected) (Fig. 3).
Fig. 3.
The main effect of ZNF804A genotype on ALFF. A A cluster (red) with significant main effect of ZNF804A genotype, including the left calcarine gyrus, posterior cingulate gyrus, and cuneus. Color bar represents the range of values. BZNF804A CC homozygotes exhibited decreased ALFF in the cluster containing the left calcarine gyrus, posterior cingulate gyrus, and cuneus than CA (P < 0.001, corrected) and AA genotypes (P < 0.001, corrected).
Discussion
In the present study, we have demonstrated for the first time that two SNPs (COMT rs4680 and ZNF804A rs1344706) influence spontaneous regional brain activity in healthy individuals. We found a significant COMT rs4680 × ZNF804A rs1344706 interaction in the ALFF in the bilateral DLPFC, suggesting a U-shaped modulation by presumed DA signaling. These measurable alterations in brain activity may serve as useful hallmarks to help reflect different expression levels of DA.
It is well known that brain DA may closely affect neurobiological and behavioral phenotypes such as working memory, emotional response, and cognitive control, especially in prefrontal cortex (PFC) [37]. DA modulates the structural and functional properties of the brain in a nonlinear manner [38]. As a result, both lower and higher extracellular DA levels may impair neuronal integrity and survival. An optimal extracellular DA level may induce the generation of brain-derived neurotropic factor (BDNF) and facilitate neuronal growth. An excessive DA level can reduce BDNF gene expression [39]. In contrast, reduced DA signaling can impair the expression of DA-mediated behavioral responses by affecting the neurochemical architecture of the striatum [40]. This neural mechanism may be explained by the DA level-dependent neurotrophic and neurotoxic effects [5, 41–43]. Val-allele carriers have relatively high COMT activity and presumably low baseline DA; conversely, Met-allele carriers have relatively low COMT activity and presumably high baseline DA.
Although it is known that COMT rs4680 affects brain function by modulating the DA concentration in brain regions especially the PFC, the effect of ZNF804A rs1344706 on DA signaling is rather complex [12, 44, 45]. A-allele carriers may have a higher expression of ZNF804A than CC homozygotes [46, 47]. The ZNF804A CA heterozygote in the other SNP (rs12476147) can further result in the over-expression of ZNF804A in the human brain [48, 49]. Consequently, this presumably results in a significant increase in the transcript levels of COMT [15]. Different genotypic combinations of ZNF804A rs1344706 and COMT rs4680 can generate multiple subgroups with different levels of DA. We proposed two sorting schemes according to the two genotypes. Based on the inverted U-shape hypothesis of DA signaling on the PFC [9], quadratic curve-fitting analysis suggested that a more reasonable sorting scheme is Val/Val/ CA < Val/Val/AA < Val/Val/CC < Met-allele/CA < Met-allele/AA < Met-allele/CC in the presumed DA signaling from low to high. In addition, our presumed sorting scheme is consistent with other findings of rs-fMRI studies examining prefrontal volume [50].
Here, we found U-shaped modulation of the presumed DA signaling in the ALFF of the bilateral DLPFC, which can be well explained by DA-dependent neurotrophic and neurotoxic effects. The PFC contains a large number of DA receptors and is highly sensitive to its dopaminergic environment. The DLPFC is the major component of heteromodal cortex [51]. Human and non-human primate studies indicate that heteromodal cortex contains interconnected higher-order neural circuits mediating complex cognitive tasks, such as language, focused attention, and working memory. More evidence indicates that SZ patients have deficits in cognitive function, especially for functions subserved by heteromodal cortex. DLPFC involvement in SZ is well documented [52]. Increased ALFF values in the bilateral DLPFC indicated abnormities in brain activity [53, 54]. Individuals with the COMT Val/Val_ZNF804A CC or the COMT Met-allele_ZNF804A CA genotype have optimal DA levels, which correspond with a normal ALFF on the bilateral DLPFC. In contrast, individuals with the COMT Val/Val_ZNF804A CA or the COMT Met-allele_ZNF804A CC genotype have a DA level that is either too high or too low, which may impair neuronal survival and show an increased ALFF [50]. The U-shaped modulation of function may indicate a compensatory mechanism through which healthy young adults could maintain normal behavioral.
Although we focused on the significant interaction effect between the two SNPs, there were also significant main effects of COMT and ZNF804A on the ALFF. It is possible that variation in COMT and ZNF804A may contribute to the abnormal ALFF values in different brain regions separately in individuals. Both COMT and ZNF804A have their respective effects on brain activities, and our results are consistent with the previous findings of rs-fMRI studies [55, 56].
A number of limitations of this study should be discussed. First, the present research did not include a sample of SZ patients. Nevertheless, our results may be applicable to SZ because the two SNPs have been separately shown with effects between healthy individuals and SZ patients. Therefore, our findings in healthy individuals could provide some indications for patients. Second, although we suggest that the effects of genetic variation on corticolimbic circuitry may be implicated in neuropathophysiology, it is likely that the development of symptoms also depends on other interacting factors, such as genetic or environmental factors. More studies are needed to clarify these processes and directly assess the effects on SZ patients. A larger sample is needed to verify our results.
Conclusions
In conclusion, our findings imply that ZNF804A rs1344706 and COMT rs4680 are associated with brain activity of corticolimbic neural circuitry. We first investigated the epistatic interaction between ZNF804A rs1344706 and COMT rs4680 in the functional activation of the bilateral DLPFC, which may improve our further understanding on the pathophysiological mechanism underlying functional abnormalities in the prefrontal-corticolimbic circuitry in schizophrenia.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Basic Research Development Program of China (2016YFC0906400, 2016YFC1306900 and 2016YFC0904300), the National Natural Science Foundation of China (81571311 and 81571331), the National Science Fund for Distinguished Young Scholars of China (81725005), the National High Tech Development Project (863 Project) of China (2015AA020513), the Science and Technology Project of Liaoning Province, China (2015225018), and the Educational Foundation (Pandeng Scholarship) of Liaoning Province, China. We are grateful for the support of Department of Radiology and Psychiatry, First Affiliated Hospital of China Medical University. We thank all the participants for their cooperation.
Conflicts of interest
The authors have no conflicts of interest to declare.
Contributor Information
Yanqing Tang, Email: yanqingtang@163.com.
Xiaohong Gong, Email: gongxh@fudan.edu.cn.
Ke Xu, Email: kxu@cmu.edu.cn.
References
- 1.International Schizophrenia Consortium. Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kauppi K, Westlye LT, Tesli M, Bettella F, Brandt CL, Mattingsdal M, et al. Polygenic risk for schizophrenia associated with working memory-related prefrontal brain activation in patients with schizophrenia and healthy controls. Schizophr Bull. 2015;41:736–743. doi: 10.1093/schbul/sbu152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walton E, Turner J, Gollub RL, Manoach DS, Yendiki A, Ho BC, et al. Cumulative genetic risk and prefrontal activity in patients with schizophrenia. Schizophr Bull. 2013;39:703–711. doi: 10.1093/schbul/sbr190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y, Yu H, Li W, Liu B, Zhang H, Ding S, et al. Association between cerebral dopamine neurotrophic factor (CDNF) 2 polymorphisms and schizophrenia susceptibility and symptoms in the Han Chinese population. Behav Brain Funct. 2018;14:1. doi: 10.1186/s12993-017-0133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Mannisto PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev. 1999;51:593–628. [PubMed] [Google Scholar]
- 7.Elton A, Smith CT, Parrish MH, Boettiger CA. COMT Val(158)Met polymorphism exerts sex-dependent effects on fMRI measures of brain function. Front Hum Neurosci. 2017;11:578. doi: 10.3389/fnhum.2017.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ojala KE, Janssen LK, Hashemi MM, Timmer MHM, Geurts DEM, Ter Huurne NP, et al. Dopaminergic drug effects on probability weighting during risky decision making. eNeuro 2018, 5. [DOI] [PMC free article] [PubMed]
- 9.Tian T, Qin W, Liu B, Wang D, Wang J, Jiang T, et al. Catechol-O-methyltransferase Val158Met polymorphism modulates gray matter volume and functional connectivity of the default mode network. PLoS One. 2013;8:e78697. doi: 10.1371/journal.pone.0078697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao F, Zhang X, Qin W, Liu F, Wang Q, Xu Q, et al. Network-dependent modulation of COMT and DRD2 polymorphisms in healthy young adults. Sci Rep. 2015;5:17996. doi: 10.1038/srep17996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cools R, D’Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 2011;69:e113–e125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esslinger C, Walter H, Kirsch P, Erk S, Schnell K, Arnold C, et al. Neural mechanisms of a genome-wide supported psychosis variant. Science. 2009;324:605. doi: 10.1126/science.1167768. [DOI] [PubMed] [Google Scholar]
- 13.O’Donovan MC, Craddock N, Norton N, Williams H, Peirce T, Moskvina V, et al. Identification of loci associated with schizophrenia by genome-wide association and follow-up. Nat Genet. 2008;40:1053–1055. doi: 10.1038/ng.201. [DOI] [PubMed] [Google Scholar]
- 14.Xiao X, Luo XJ, Chang H, Liu Z, Li M. Evaluation of European schizophrenia GWAS loci in Asian populations via comprehensive meta-analyses. Mol Neurobiol. 2017;54:4071–4080. doi: 10.1007/s12035-016-9990-3. [DOI] [PubMed] [Google Scholar]
- 15.Girgenti MJ, LoTurco JJ, Maher BJ. ZNF804a regulates expression of the schizophrenia-associated genes PRSS16, COMT, PDE4B, and DRD2. PLoS One. 2012;7:e32404. doi: 10.1371/journal.pone.0032404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owesson-White CA, Ariansen J, Stuber GD, Cleaveland NA, Cheer JF, Wightman RM, et al. Neural encoding of cocaine-seeking behavior is coincident with phasic dopamine release in the accumbens core and shell. Eur J Neurosci. 2009;30:1117–1127. doi: 10.1111/j.1460-9568.2009.06916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci. 2016;17:524–532. doi: 10.1038/nrn.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh Y, Park C, Kim DH, Shin H, Kang YM, DeWaele M, et al. Monitoring in vivo changes in tonic extracellular dopamine level by charge-balancing multiple waveform fast-scan cyclic voltammetry. Anal Chem. 2016;88:10962–10970. doi: 10.1021/acs.analchem.6b02605. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang XQ, Lui S, Deng W, Chan RC, Wu QZ, Jiang LJ, et al. Localization of cerebral functional deficits in treatment-naive, first-episode schizophrenia using resting-state fMRI. Neuroimage. 2010;49:2901–2906. doi: 10.1016/j.neuroimage.2009.11.072. [DOI] [PubMed] [Google Scholar]
- 21.Yang H, Long XY, Yang Y, Yan H, Zhu CZ, Zhou XP, et al. Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. Neuroimage. 2007;36:144–152. doi: 10.1016/j.neuroimage.2007.01.054. [DOI] [PubMed] [Google Scholar]
- 22.Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. doi: 10.1016/j.braindev.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- 24.Di X, Kannurpatti SS, Rypma B, Biswal BB. Calibrating BOLD fMRI activations with neurovascular and anatomical constraints. Cereb Cortex. 2013;23:255–263. doi: 10.1093/cercor/bhs001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palacios EM, Sala-Llonch R, Junque C, Roig T, Tormos JM, Bargallo N, et al. Resting-state functional magnetic resonance imaging activity and connectivity and cognitive outcome in traumatic brain injury. JAMA Neurol. 2013;70:845–851. doi: 10.1001/jamaneurol.2013.38. [DOI] [PubMed] [Google Scholar]
- 26.Salvador R, Vega D, Pascual JC, Marco J, Canales-Rodriguez EJ, Aguilar S, et al. Converging medial frontal resting state and diffusion-based abnormalities in borderline personality disorder. Biol Psychiatry. 2016;79:107–116. doi: 10.1016/j.biopsych.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 27.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 28.Fryer SL, Roach BJ, Ford JM, Turner JA, van Erp TG, Voyvodic J, et al. Relating intrinsic low-frequency BOLD cortical oscillations to cognition in schizophrenia. Neuropsychopharmacology. 2015;40:2705–2714. doi: 10.1038/npp.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hare SM, Ford JM, Ahmadi A, Damaraju E, Belger A, Bustillo J, et al. Modality-dependent impact of hallucinations on low-frequency fluctuations in schizophrenia. Schizophr Bull. 2017;43:389–396. doi: 10.1093/schbul/sbx021.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu ML, Zong XF, Mann JJ, Zheng JJ, Liao YH, Li ZC, et al. A review of the functional and anatomical default mode network in schizophrenia. Neurosci Bull. 2017;33:73–84. doi: 10.1007/s12264-016-0090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.First MB, SpitzerRL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I & II Disorders (Version 2.0). New York: New York State Psychiatric Institute, 1995.
- 32.Lish JD, Weissman MM, Adams PB, Hoven CW, Bird H. Family psychiatric screening instruments for epidemiologic studies: pilot testing and validation. Psychiatry Res. 1995;57:169–180. doi: 10.1016/0165-1781(95)02632-7. [DOI] [PubMed] [Google Scholar]
- 33.Yan CG, Wang XD, Zuo XN, Zang YF. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics. 2016;14:339–351. doi: 10.1007/s12021-016-9299-4. [DOI] [PubMed] [Google Scholar]
- 34.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 35.Yin Y, Li L, Jin C, Hu X, Duan L, Eyler LT, et al. Abnormal baseline brain activity in posttraumatic stress disorder: a resting-state functional magnetic resonance imaging study. Neurosci Lett. 2011;498:185–189. doi: 10.1016/j.neulet.2011.02.069. [DOI] [PubMed] [Google Scholar]
- 36.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 37.Cinque S, Zoratto F, Poleggi A, Leo D, Cerniglia L, Cimino S, et al. Behavioral phenotyping of dopamine transporter knockout rats: compulsive traits, motor stereotypies, and anhedonia. Front Psychiatry. 2018;9:43. doi: 10.3389/fpsyt.2018.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuppers E, Beyer C. Dopamine regulates brain-derived neurotrophic factor (BDNF) expression in cultured embryonic mouse striatal cells. Neuroreport. 2001;12:1175–1179. doi: 10.1097/00001756-200105080-00025. [DOI] [PubMed] [Google Scholar]
- 39.Fumagalli F, Racagni G, Colombo E, Riva MA. BDNF gene expression is reduced in the frontal cortex of dopamine transporter knockout mice. Mol Psychiatry. 2003;8:898–899. doi: 10.1038/sj.mp.4001370. [DOI] [PubMed] [Google Scholar]
- 40.Xu M, Moratalla R, Gold LH, Hiroi N, Koob GF, Graybiel AM, et al. Dopamine D1 receptor mutant mice are deficient in striatal expression of dynorphin and in dopamine-mediated behavioral responses. Cell. 1994;79:729–742. doi: 10.1016/0092-8674(94)90557-6. [DOI] [PubMed] [Google Scholar]
- 41.Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- 42.Qin S, Cousijn H, Rijpkema M, Luo J, Franke B, Hermans EJ, et al. The effect of moderate acute psychological stress on working memory-related neural activity is modulated by a genetic variation in catecholaminergic function in humans. Front Integr Neurosci. 2012;6:16. doi: 10.3389/fnint.2012.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bertolino A, Fazio L, Caforio G, Blasi G, Rampino A, Romano R, et al. Functional variants of the dopamine receptor D2 gene modulate prefronto-striatal phenotypes in schizophrenia. Brain. 2009;132:417–425. doi: 10.1093/brain/awn248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walter H, Schnell K, Erk S, Arnold C, Kirsch P, Esslinger C, et al. Effects of a genome-wide supported psychosis risk variant on neural activation during a theory-of-mind task. Mol Psychiatry. 2011;16:462–470. doi: 10.1038/mp.2010.18. [DOI] [PubMed] [Google Scholar]
- 45.Mallas E, Carletti F, Chaddock CA, Shergill S, Woolley J, Picchioni MM, et al. The impact of CACNA1C gene, and its epistasis with ZNF804A, on white matter microstructure in health, schizophrenia and bipolar disorder(1) Genes Brain Behav. 2017;16:479–488. doi: 10.1111/gbb.12355. [DOI] [PubMed] [Google Scholar]
- 46.Schultz CC, Nenadic I, Riley B, Vladimirov VI, Wagner G, Koch K, et al. ZNF804A and cortical structure in schizophrenia: in vivo and postmortem studies. Schizophr Bull. 2014;40:532–541. doi: 10.1093/schbul/sbt123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams HJ, Norton N, Dwyer S, Moskvina V, Nikolov I, Carroll L, et al. Fine mapping of ZNF804A and genome-wide significant evidence for its involvement in schizophrenia and bipolar disorder. Mol Psychiatry. 2011;16:429–441. doi: 10.1038/mp.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guella I, Vawter MP. Allelic imbalance associated with the schizophrenia risk SNP rs1344706 indicates a cis-acting variant in ZNF804A. Schizophr Res. 2014;153:243–245. doi: 10.1016/j.schres.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guella I, Sequeira A, Rollins B, Morgan L, Myers RM, Watson SJ, et al. Evidence of allelic imbalance in the schizophrenia susceptibility gene ZNF804A in human dorsolateral prefrontal cortex. Schizophr Res. 2014;152:111–116. doi: 10.1016/j.schres.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu Q, Xiong Y, Yuan C, Liu F, Zhao F, Shen J, et al. ZNF804A rs1344706 interacts with COMT rs4680 to affect prefrontal volume in healthy adults. Brain Imaging Behav. 2018;12:13–19. doi: 10.1007/s11682-016-9671-x. [DOI] [PubMed] [Google Scholar]
- 51.Pearlson GD, Petty RG, Ross CA, Tien AY. Schizophrenia: a disease of heteromodal association cortex? Neuropsychopharmacology. 1996;14:1–17. doi: 10.1016/S0893-133X(96)80054-6. [DOI] [PubMed] [Google Scholar]
- 52.Weinberger DR, Berman KF, Suddath R, Torrey EF. Evidence of dysfunction of a prefrontal-limbic network in schizophrenia: a magnetic resonance imaging and regional cerebral blood flow study of discordant monozygotic twins. Am J Psychiatry. 1992;149:890–897. doi: 10.1176/ajp.149.7.890. [DOI] [PubMed] [Google Scholar]
- 53.Turner JA, Damaraju E, van Erp TG, Mathalon DH, Ford JM, Voyvodic J, et al. A multi-site resting state fMRI study on the amplitude of low frequency fluctuations in schizophrenia. Front Neurosci. 2013;7:137. doi: 10.3389/fnins.2013.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cui L, Gong X, Tang Y, Kong L, Chang M, Geng H, et al. Relationship between the LHPP gene polymorphism and resting-state brain activity in major depressive disorder. Neural Plast. 2016;2016:9162590. doi: 10.1155/2016/9162590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bearden CE, Jawad AF, Lynch DR, Sokol S, Kanes SJ, McDonald-McGinn DM, et al. Effects of a functional COMT polymorphism on prefrontal cognitive function in patients with 22q11.2 deletion syndrome. Am J Psychiatry 2004, 161: 1700–1702. [DOI] [PubMed]
- 56.Zhang Y, Yan H, Liao J, Yu H, Jiang S, Liu Q, et al. ZNF804A variation may affect hippocampal-prefrontal resting-state functional connectivity in schizophrenic and healthy individuals. Neurosci Bull. 2018;34:507–516. doi: 10.1007/s12264-018-0221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.