Abstract
The present study was designed to examine the therapeutic effects of Botulinum neurotoxin A (BoNT/A) on depression-like behaviors in mice and to explore the potential mechanisms. These results revealed that a single facial injection of BoNT/A induced a rapid and prolonged improvement of depression-like behaviors in naïve and space-restriction-stressed (SRS) mice, reflected by a decreased duration of immobility in behavioral despair tests. BoNT/A significantly increased the 5-hydroxytryptamine (5-HT) levels in several brain regions, including the hippocampus and hypothalamus, in SRS mice. BoNT/A increased the expression of the N-methyl-D-aspartate receptor subunits NR1 and NR2B in the hippocampus, which were significantly decreased in SRS mice. Furthermore, BoNT/A significantly increased the expression of brain-derived neurotrophic factor (BDNF) in the hippocampus, hypothalamus, prefrontal cortex, and amygdala, which were decreased in SRS mice. Finally, BoNT/A transiently increased the levels of phosphorylated extracellular signal-regulated kinase (p-ERK) and cAMP-response element binding protein (p-CREB), which were suppressed in the hippocampus of SRS mice. Collectively, these results demonstrated that BoNT/A treatment has anti-depressant-like activity in mice, and this is associated with increased 5-HT levels and the activation of BDNF/ERK/CREB pathways in the hippocampus, supporting further investigation of BoNT/A therapy in depression.
Keywords: Botulinum neurotoxin, Depression, 5-HT, BDNF, Hippocampus
Introduction
Depression is a prevalent psychiatric disorder, characterized by depressed mood, anhedonia, irritability, fatigue, attention deficits, and abnormalities in appetite and sleep [1, 2]. Depression affects ~16% of the world’s population and is a leading cause of suicide, substantially contributing to the global burden of public health [3]. Unfortunately, clinically-used antidepressants are often accompanied by undesirable side-effects [3], and it has been estimated that 30–50% of patients are not responsive to current antidepressant treatment, which may reflect the complex etiology of depression [2, 4]. Recently, it has been shown that the anesthetic drug ketamine has rapid and sustained antidepressant actions in major depression patients after a single injection [5, 6], supporting the possibility of designing novel therapeutics with rapid-acting antidepressants [7–9]. Therefore, the development of new antidepressant medications with limited side-effects is essential for the management of depression.
Botulinum neurotoxins (BoNTs) were first used clinically to treat strabismus in 1977 [10]. Currently, they are widely used to treat excessive muscle stiffness, spasticity, and dystonia [11]. BoNTs are bacterial proteases produced by Clostridium botulinum and related species [12] and comprise seven serotypes, termed BoNT/A–G. BoNTs (150 kDa) consist of a heavy chain (HC, 100 kDa) and a light chain (LC, 50 kDa) [13]. The C-terminal region (50 kDa) of the HC is a receptor-binding domain, which acts on specific cell surface molecules on neurons, while the N-terminal domain (50 kDa) of the HC is the translocation domain, which interacts with the active site of the LC and is a metalloprotease [13]. As a result, BoNTs inhibit the exocytosis of synaptic vesicles containing neurotransmitters, such as acetylcholine [14]. Interestingly, it has been shown that BoNT/A undergoes long-distance retrograde axonal transport to enter the central nervous system after local peripheral injection and produce central effects, such as analgesia [15]. Thus, these results indicate that peripheral application of BoNT/A can have central effects. Recently, many potential clinical application of BoNTs for treating a variety of neurological conditions have been suggested, including post-herpetic neuralgia, trigeminal neuralgia [16], Parkinson’s disease [17], migraine [18], and neuropathic pain [19]. Intriguingly, several clinical case reports and a few randomized controlled trials have demonstrated that facial glabellar injection of BoNT/A may be beneficial in the treatment of depression [20–22], possibly independent of its esthetic effects. However, the precise mechanisms underlying the anti-depressant effects of BoNT/A remain largely unknown.
Therefore, in the present study, we aimed to investigate the therapeutic effects of BoNT/A in preclinical mouse models and to explore the potential mechanisms.
Materials and Methods
Animals
Male adult ICR mice (6 weeks–8 weeks, 20g–25g) were purchased from the Shanghai Laboratory Animal Center (Shanghai, China). All animals were maintained under a 12-h light/dark cycle with food and water ad libitum, and the room was kept at 22 ± 2 °C and 40%–60% humidity. We performed all mouse experiments between 09:00 and 16:00. Four mice were housed in each cage. All animal experiments were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee of Soochow University.
Drugs and Administration
BoNT/A was from The Lanzhou Institute of Biological Products (Lanzhou, China); fluoxetine hydrochloride was from Aladdin Biochemical Polytron Technologies Inc. (Shanghai, China); and imipramine hydrochloride was from the TCI Chemical Industry Development Co., Ltd (Shanghai, China). The reagents were dissolved in sterile saline. BoNT/A was injected intramuscularly at three points on both cheeks at the indicated doses. Imipramine (10 mg/kg) and fluoxetine (10 mg/kg) were injected intraperitoneally (i.p.) daily.
Spatial Restraint Stress
We used spatial restraint stress (SRS) to induce a mouse model of depression as previously reported [23, 24]. Each mouse was gently placed into a modified well-ventilated 50 mL centrifuge tube where it remained for 2.5 h (09:00 to 11:30) daily for 2 weeks. Mice were restricted to moving forward or backward in the tubes. Mice in the control group remained undisturbed in their home cages without access to water and food, like the SRS mice. After SRS, the mice were returned to their home cages.
Forced Swimming Test
The forced swimming test (FST) was performed as previously described [25]. Briefly, mice were placed in a 2-L beaker filled with 1.5 L water at 24 °C–26 °C for 6 min. Immobility time was recorded as the lack of all movement except that required to keep the mouse afloat. Immobility times were recorded during the last 4 min. Finally, mice were returned to their home cages after careful drying and warming with a hair-dryer.
Tail Suspension Test
For the tail suspension test (TST) we used a method as described previously [26]. Briefly, mice were suspended 50 cm above the floor for 6 min using adhesive tape placed ~1 cm from the tip of the tail. The immobility time was recorded during the last 4 min.
Sucrose Preference Test
As previously reported [27], mice were housed individually and trained for 24 h to drink water from two bottles. The next day, one bottle of water was replaced with a bottle of 1% sucrose. After 24 h, the positions of the two bottles were exchanged. On day 4, mice were deprived of water and food for 24 h. Before water and food were supplied, the bottles were weighed. After 8 h and 24 h, the bottles were weighed again to calculate fluid consumption. The intake and percentage of consumed sucrose to total amount consumed was also calculated.
Body Weight
Body weight was measured by electronic balance and recorded at the indicated times.
Rotarod Test
We used the Rotarod test to assess motor function after BoNT/A injection. The test was performed as previously reported. For training, mice were placed on the Rotarod at 17 rpm for 20 min until they could stay on it without falling. After training, mice were tested three times at 17 rpm. The latency to falling was recorded and analyzed as previously described [28].
Open Field Test
Locomotor activity was measured by the open field test in an acrylic box (40 cm × 40 cm × 40 cm). The intensity of illumination was 100–300 lux at 3 m above the floor and the apparatus was black. Each mouse was placed in the same corner of the box, and locomotion was recorded for 10 min. The movement distance and number of crossings were recorded by the software of the open field instrument (XR-XZ301, Shanghai Xinruan Information Technology Co. Ltd, Shanghai, China).
HPLC Analysis of 5-Hydroxytryptamine (5-HT) Level
We used HPLC analysis to determine the levels of 5-HT in different brain regions, as in our previous report [29]. The brain was dissected, weighed, and then homogenized with an ultrasonic homogenizer (Microsonic, Dortmund, Germany) in 400 μL perchloric acid (0.4 mol/L). Samples were then centrifuged at 20,000 rpm at 4 °C for 25 min, filtered through a 0.22 μm syringe filter, and stored at −80 °C until chemical analysis. The concentrations of 5-HT were measured by applying reverse-phase HPLC with electrochemical detection (LC-6A, Shimadzu Corp., Kyoto, Japan). The reverse-phase column was a C18 column (TC-C18, Agilent, Middelburg, Netherlands) perfused for analysis with a mobile phase composed of 0.1 mol/L NaAc (with 0.1 mol/L EDTA-Na2) and 10% methanol at pH 5.1. The flow rate was 1 mL/min. The data were quantified using the area under the peaks and external standards. The results are presented as nanograms per gram wet tissue (ng/g).
Quantitative RT-PCR
We used TRIzol reagent (Invitrogen, Carlsbad, CA) to extract total RNA according to the manufacturer’s protocol. One microgram of total RNA was reverse-transcribed to synthesize cDNA using a Revert Aid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA). Q-PCR was performed with SYBR Green PCR Master Mix (Roche, Basle, Switzerland). Reactions were amplified and analyzed with an ABI 7500 Real-Time PCR system. The primers were as follows: BNDF-forward: GGTCACAGCGGCAGATAAAAAGAC; BDNF-reverse: TTGGGTAGTTCGGCATTGCGAG; GAPDH-forward: CAAGGTCATCCATGACAACTTTG; GAPDH-reverse: GTCCACCACCCTGTTGCTGTAG. All data were normalized to GAPDH control, and relative expression levels between conditions were calculated by the comparative CT (2-ΔΔCT) method.
Western Blotting
The brain was rapidly removed and homogenized in lysis buffer containing phosphatase inhibitors and cocktail of protease inhibitors for total protein extraction. The protein concentrations were determined by BCA Protein Assay (Pierce, Rockford, IL). SDS-PAGE was performed on 10% polyacrylamide gels at 80 V for 30 min and 120 V for 2 h. After transfer onto a PVDF membrane, the blots were blocked with 5% nonfat milk in TBST and incubated overnight at 4 °C with primary antibodies, followed by secondary antibodies. The following primary antibodies were used: mouse anti-tubulin (1:1000; Cat# Ab102-02; Vazyme), mouse anti-GAPDH (1:1000; Cat#: man1002 Mesgen), rabbit anti-BDNF (1:2000; Cat# ab108319; Abcam), rabbit anti-SNAP25 (1:1000; Cat# ab5666; Abcam), rabbit anti-NR1 (1:1000; Cat# ab109182; Abcam), rabbit anti-NR2A (1:1000; Cat# ab4205; Abcam), rabbit anti-NR2B (1:1000; Cat# ab4212; Abcam), mouse anti-p-ERK (1:1000; Cat# sc-7383; SanTa), rabbit anti-p-CREB (1:1000; Cat# 9198; CST]) and secondary antibodies (1:2000, Mesgen). Protein bands were visualized using an enhanced chemiluminescence detection kit (Pierce). The band densities were imaged and analyzed using Tanon 5200 Multi (Tanon, Shanghai).
Statistical Analysis
All data are presented as mean ± SEM. Differences between two groups were determined with Student’s t-test. One-way ANOVA with the Bonferroni post-test was used for multiple group comparisons. Two-way repeated-measured ANOVA with the Bonferroni post-test was used to analyze data with multiple time points. P <0.05 was considered statistically significant.
Results
BoNT/A Treatment Improves Depressive-Like Behaviors in Naïve Mice
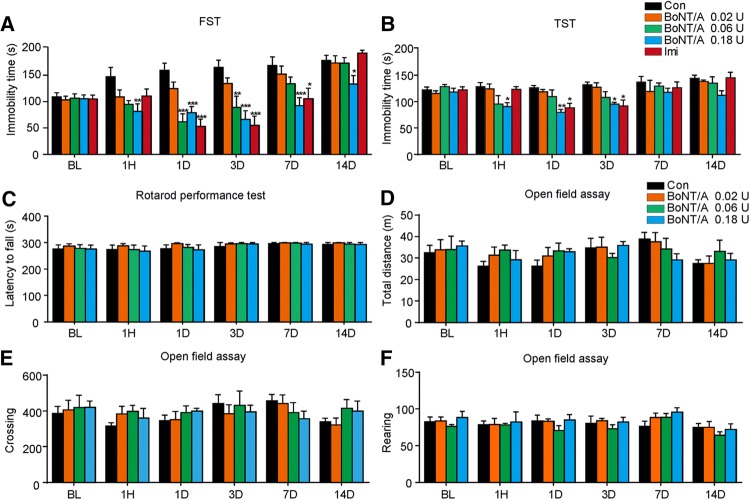
To explore the potential anti-depressant effects of BoNT/A therapy, we first examined the behavioral outcomes of facial injection BoNT/A in naïve mice using the forced swimming test (FST) and the tail suspension test (TST), behavioral tests of despair in mice. For FST, single facial injection of BoNT/A at 0.06 U and 0.18 U significantly decreased the immobility time compared with the control group, and this decrease occurred during 14 days after injection of 0.18 U BoNT/A (Fig. 1A; for 0.02 U: Ftreatment(1, 13)= 3.560, P = 0.0817; Ftime(5, 65) = 9.302, P <0.0001; Ftreatment×time(5, 65) = 0.8647, p = 0.5097; for 0.06 U: Ftreatment(1, 15)= 11.77, P = 0.0037; Ftime(5, 75) = 11.62, P <0.0001; Ftreatment×time(5, 75) = 6.163, P <0.0001; for 0.18 U: Ftreatment(1, 16) = 20.69, P = 0.0003; Ftime(5, 80) = 4.808, P = 0.0007; Ftreatment×time(5, 80) = 4.543, P = 0.0011). As a positive control, administration of imipramine (10 mg/kg) also significantly decreased the immobility time during 1–7 days compared with the vehicle group (Fig. 1A; Ftreatment(1, 15) = 17.12, p = 0.0009; Ftime(5, 75) = 13.26, P <0.0001; Ftreatment×time(5, 75) = 9.758, P <0.0001). In the TST, single facial injection of BoNT/A at 0.06 U and 0.18 U significantly decreased the immobility time from 1 h to 3 days after injection of BoNT/A (Fig. 1B; for 0.06 U: Ftreatment(1, 12) = 3.725, P = 0.0776; Ftime (5, 60) = 3.088, P = 0.0152; Ftreatment×time(5, 60) = 1.375, P = 0.2464; for 0.18 U: Ftreatment(1, 12) = 46.30, P <0.0001; Ftime(5, 60) = 4.597, P = 0.0013; Ftreatment×time(5, 60) = 2.594, P = 0.0344). As a positive control, imipramine (10 mg/kg) also significantly decreased the immobility time during 1–3 days compared with the vehicle group (Fig. 1B; Ftreatment(1, 12) = 8.946, P = 0.0113; Ftime (5, 60) = 6.074, P = 0.0001; Ftreatment×time(5, 60) = 2.848, P = 0.0226). In addition, we found that the motor coordination and locomotor activity was not altered by treatment with BoNT/A on the Rotarod test (Fig. 1C) and the open field test (Fig. 1D–F). Together, these data indicated that BoNT/A has anti-depressive effects in native mice without affecting motor function.
Fig. 1.
BoNT/A treatment decreased the immobility time during FST and TST in naive mice. ICR mice received a single facial injection of BoNT/A (0.02, 0.06, and 0.18 U), and the FST (A) and TST (B) were performed at different time points. Motor function and locomotion activity were assessed by the Rotarod test (C) and open field test (D–F), respectively. *P <0.05, **P <0.01, ***P <0.001 vs. control mice (two-way ANOVA with post-hoc Bonferroni test). The data are presented as mean ± SEM, n = 5–10/group. Con, control; BL, baseline; Imi, imipramine.
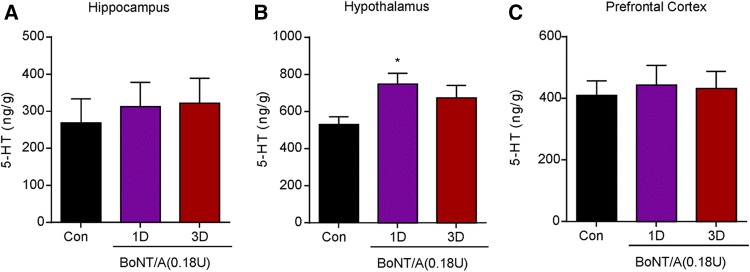
BoNT/A Treatment Increases 5-HT Levels in the Hypothalamus of Naive Mice
To explore the mechanisms underlying the anti-depressive activity of BoNT/A in naïve mice, we subsequently determined the 5-HT levels in brain regions using HPLC. We found that treatment with BoNT/A significantly increased the 5-HT levels in the hypothalamus (Fig. 2; F(2,15) = 3.811, P = 0.0459), but not in the hippocampus and prefrontal cortex (Fig. 2). These data indicated that augmented 5-HT levels in the hypothalamus may be involved in the anti-depressive activity of BoNT/A in naïve mice, although the mechanisms underlying this up-regulation remain to be determined.
Fig. 2.
BoNT/A treatment increased the level of 5-HT in the hypothalamus of naïve mice. 5-HT levels in the hippocampus (A), hypothalamus (B), and prefrontal cortex (C) were analyzed by HPLC. *P <0.05 vs. control mice (one-way ANOVA with post-hoc Bonferroni test). Each column is presented as mean ± SEM, n = 6–7.
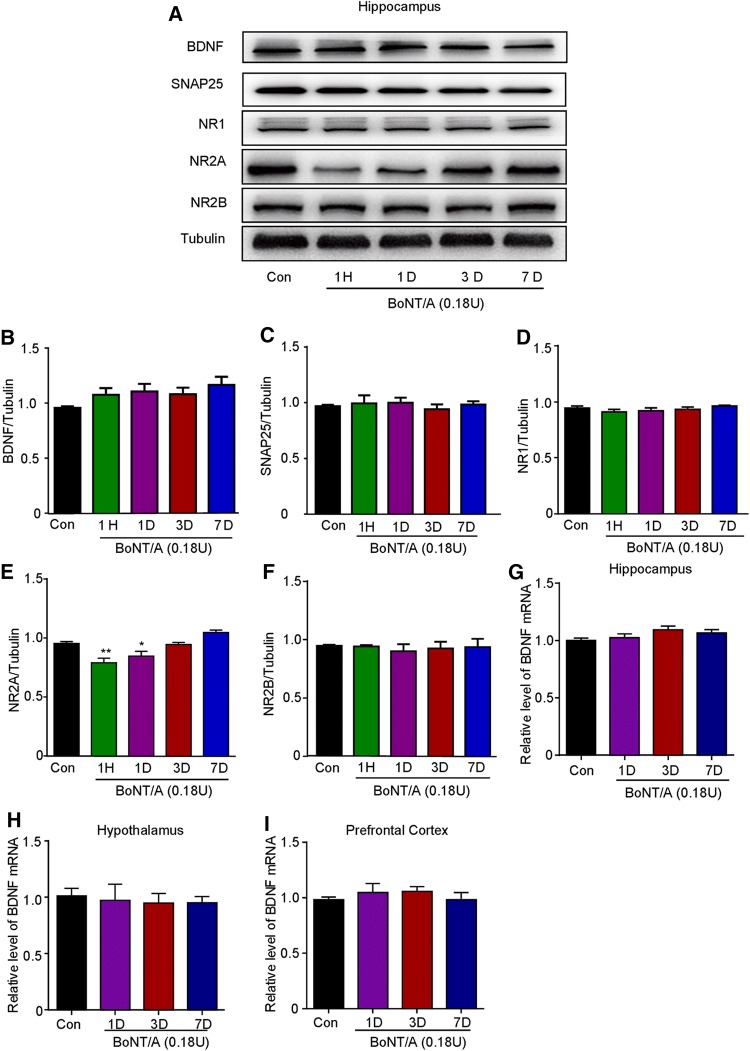
In naive mice, we further found that the protein expression of BDNF in the hippocampus did not change after BoNT/A treatment (Fig. 3A, B). The expression of synaptosomal-associated protein 25 (SNAP25) in the hippocampus was not altered after BoNT/A treatment (Fig. 3C), suggesting that peripherally-administered BoNT/A may be not able to reach the hippocampus. A role of N-methyl-D-aspartate receptor (NMDAR) subunits, such as NR1, NR2A and NR2B, has been implicated in the pathogenesis of depression [30, 31], so we further investigated the involvement of NMDAR subunits in the antidepressant-like effects induced by BoNT/A in naïve mice. The expression of NR1 and NR2B in the hippocampus did not change (Fig. 3D–F), but the expression of NR2A in the hippocampus was significantly decreased from 1 h to 1 day after BoNT/A treatment (Fig. 3E; F(4, 25) = 11.74, P <0.0001). In addition, we found that the mRNA expression of BDNF was not altered in the hippocampus, hypothalamus, and prefrontal cortex after BoNT/A treatment (Fig. 3G–I). Thus, these results indicated that decreased expression of the NMDAR subunit NR2A in the hippocampus is likely involved in the anti-depressive effects of BoNT/A treatment in naïve mice.
Fig. 3.
Effects of BoNT/A treatment on the expression of BDNF, SNAP25, and NMDA receptors in the brain of naïve mice. A Representative western blots showing the protein expression of BDNF, NMDA receptors, and SNAP25 in the hippocampus of naïve mice at different time points after BoNT/A treatment. B–F Semi-quantitative analysis showing the effects of BoNT/A on the expression of BDNF (B), NR1 (C), NR2A (D), NR2B (E), and SNAP25 (F) in naive mice. *P <0.05, **P <0.01 compared with controls (one-way ANOVA with post-hoc Bonferroni test). The data are presented as mean ± SEM, n = 6. G–I mRNA expression of BDNF determined by q-PCR in the hippocampus (G), hypothalamus (H), and prefrontal cortex (I). The data are presented as mean ± SEM, n = 5–6.
BoNT/A Treatment Improves Depressive-Like Behaviors in Mice Undergoing Spatial Restraint Stress (SRS)
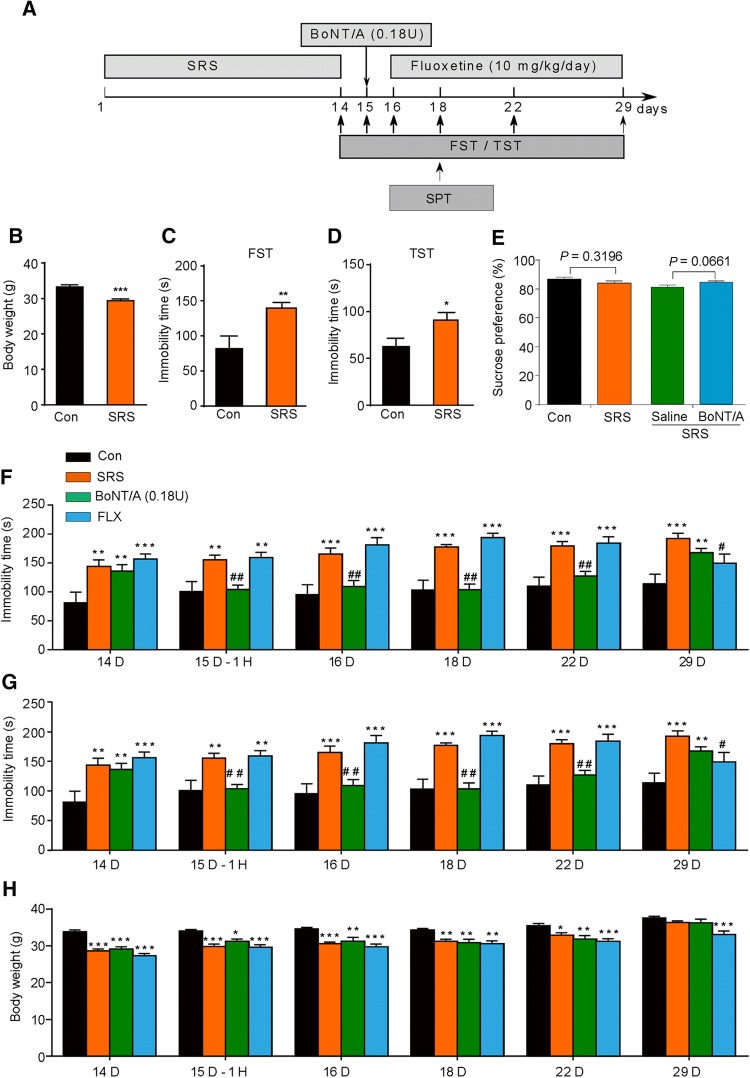
Previous reports have demonstrated that repeated SRS causes depression-like behaviors in rodents, and it has been widely used as a preclinical model of depression [23] (Fig. 4A). Body weight was significantly lower in SRS mice than in controls (Fig. 4B; t = 3.972, P = 0.0004). Compared to controls, the SRS mice showed depressive-like behaviors, reflected by significantly increased immobility time in the FST (Fig. 4C; t = 3.435, P = 0.002) and TST (Fig. 4D; t = 2.093, P = 0.0449). In the sucrose preference test, there was no significant difference between SRS-treated mice and controls (Fig. 4E), consistent with a previous report [24]. Together, our data suggested that SRS mice display robust depression-like behaviors.
Fig. 4.
BoNT/A treatment attenuates depression-like behaviors in SRS mice. A The experimental procedure. B Body weight. C, D Immobility time in the FST (C) and TST (D) were increased in SRS-treated mice. *P <0.05, **P <0.01, ***P <0.001 vs. controls. Student’s t-test; n = 10–22/group. E There was no significant different in sucrose preference between SRS and control mice. Student’s t-test; n = 7–10/group. F, G Immobility time in the FST (F) and TST (G) at different time points after BoNT/A injection in SRS mice. H Body weight at different time points after BoNT/A injection in SRS mice. *P <0.05, **P <0.01, ***P <0.001 vs. controls (two-way ANOVA with post-hoc Bonferroni test). #P <0.05, ##P <0.01 vs. SRS mice (two-way ANOVA with post-hoc Bonferroni test). The data are presented as mean ± SEM, n = 7–10/group. Con, control; FLX, fluoxetine; SRS, space restriction stressed.
To further investigate the antidepressant-like effects of BoNT/A treatment on SRS-treated mice, a single facial injection of 0.18 U BoNT/A was given to SRS mice (Fig. 4) and the immobility times in the FST and TST were recorded at different time points. We found that SRS mice displayed depression-like behaviors lasting at least 14 days in the FST and TST. We found that the immobility time in the FST was significantly higher in SRS mice than in controls (Fig. 4F; Fstress(1, 16) = 2.261, P = 0.1521; Ftime(5, 80) = 8.069, P <0.0001; Fstress×time(5, 80) = 0.7005, P = 0.6247). Intriguingly, BoNT/A treatment significantly decreased the immobility time in the FST from 1 h to 14 days after injection (Fig. 4F; Ftreatment(3, 32) = 13.43, P <0.0001, Ftime(5, 160) = 8.300, P <0.0001; Ftreatment×time(15, 160) = 5.504, P <0.0001). Similarly, the immobility time in the TST was also higher in SRS mice than in controls (Fig. 4G; Fstress(1,15) = 15.99, P = 0.0012; Ftime(5, 75) = 0.3418, P = 0.8859; Fstress×time(5, 75) = 0.2495, P = 0.9388). BoNT/A treatment significantly decreased the immobility time in the TST from 1 h to 14 days after injection (Fig. 4G; Ftreatment(1, 18) = 9.534, P = 0.0063; Ftime (5, 90) = 0.9089, P = 0.4790; Ftreatment×time(5, 90) = 0.5871, P = 0.7098). Meanwhile, fluoxetine, a common clinically-used antidepressant, was used as a positive control. We found that fluoxetine significantly decreased the immobility time in the FST only on day 14 after the first injection (Fig. 4F; Ftreatment(1, 16) = 0.04011, P = 0.8438; Ftime(5, 80) = 6.020, P <0.0001; Ftreatment×time(5, 80) = 4.113, P = 0.0022) and the TST (Fig. 4G; Ftreatment(1, 16) = 1.542, P = 0.2323; Ftime(5, 80) = 2.106, P = 0.0732; Ftreatment×time(5, 80) = 2.288, P = 0.0536). In addition, body weight was lower in SRS mice than in controls (Fig. 4H; Fstress(1, 14) =37.78, P <0.0001; Ftime(5, 70) = 60.46, P <0.0001; Fstress×time(5, 70) = 6.823, P <0.0001). BoNT/A treatment did not change the body weight of SRS mice (Fig. 4H; Ftreatment(1, 14) = 0.05242, P = 0.8222; Ftime(5, 70) = 49.67, P <0.0001; Ftreatment×time(5, 70) = 1.415, P = 0.2297). In contrast, fluoxetine further reduced the body weight of SRS mice after treatment for 14 days (Fig. 4H; Ftreatment(1, 14) = 2.621, P = 0.1277; Ftime(5, 70) = 73.56, P <0.0001; Ftreatment×time(5, 70) = 4.178, P = 0.0022). In the sucrose preference test, BoNT/A treatment showed a tendency to increase the sucrose preference but did not reach statistical significance (Fig. 4E). Together, our data suggested that single facial injection of BoNT/A has significant antidepressant-like effects in SRS mice.
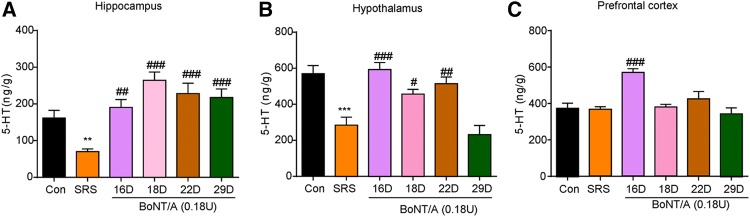
BoNT/A Treatment Increases 5-HT Levels in the Brain of SRS Mice
Previous reports have shown that reduced 5-HT levels in the brain contribute to the pathogenesis of depression in both animal models and patients [32]. In the present study, HPLC analysis showed that 5-HT levels were significantly decreased in the hippocampus (Fig. 5A; t = 3.383, P = 0.0277) and hypothalamus (Fig. 5B; t = 6.596, P = 0.0027), but not in the prefrontal cortex (Fig. 5C) in SRS mice. Interestingly, BoNT/A treatment significantly increased the 5-HT level in the hippocampus (Fig. 5A; F(4, 10) = 21.46, P <0.0001), hypothalamus (Fig. 5B; F(4, 10) = 12.29, P = 0.0007), and prefrontal cortex (Fig. 5C; F(4, 10) = 7.101, P = 0.0056). Thus, these data indicated that elevated 5-HT levels in distinct brain regions may be involved in the antidepressant-like effects of BoNT/A therapy in SRS mice.
Fig. 5.
BoNT/A treatment increased the levels of 5-HT in the brain of SRS mice. A–C 5-HT levels in the hippocampus (A), hypothalamus (B), and prefrontal cortex (C) analyzed by HPLC. **P <0.01, ***P <0.001 vs. control mice (Student’ s t-test); #P <0.05, ##P <0.01, ###P <0.001 vs. SRS mice (one-way ANOVA with post-hoc Bonferroni test). The data are presented as mean ± SEM, n = 6–7/group. SRS, space restriction stressed.
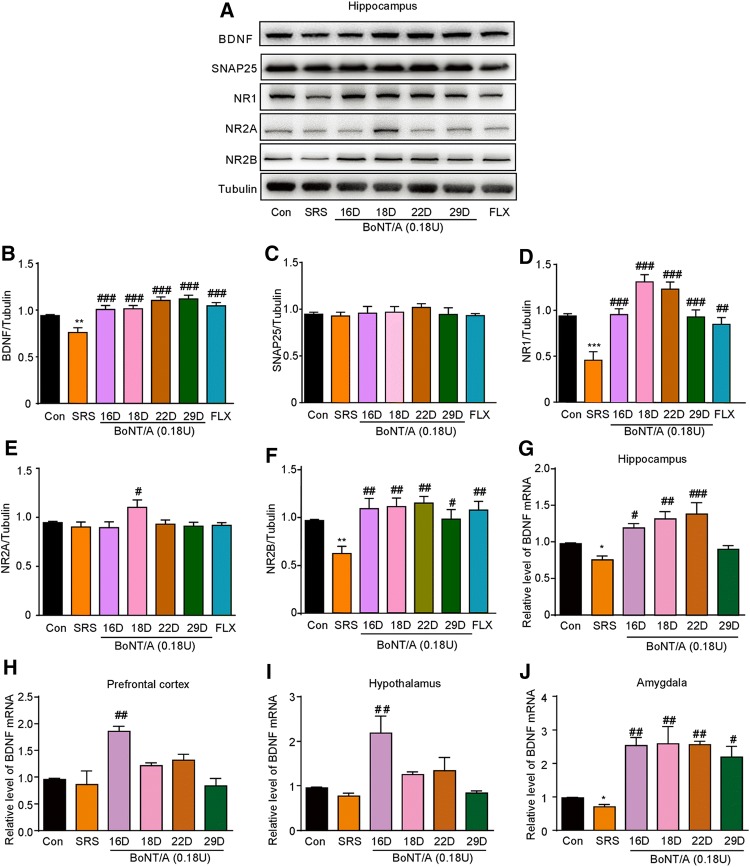
BoNT/A Treatment Causes Up-Regulation of BDNF Expression in the Brain of SRS Mice
Series of studies have pointed out that a decreased BDNF level is involved in the pathogenesis of depression [33] and antidepressants increase the production of BDNF in the hippocampus [34]. Thus, we further investigated the involvement of BDNF in the anti-depression induced by BoNT/A treatment in SRS mice. Western blotting analysis showed that the protein expression of BDNF in the hippocampus was decreased in SRS mice (Fig. 6A and B; t = 3.500, P = 0.0057), while BoNT/A treatment significantly increased the protein expression of BDNF (Fig. 6A-B; F(5, 30) = 10.37, P <0.0001).
Fig. 6.
Effects of BoNT/A treatment on the expression of BDNF, NMDA receptors and SNAP25 in SRS mice. A Representative western blots showing the protein expression of BDNF, NMDA receptors, and SNAP25 in the hippocampus of SRS mice after BoNT/A treatment at different time points. B–F Semi-quantitative analysis showing the expression changes of BDNF (B), SNAP25 (C), NR1 (D), NR2A (E), and NR2B (F). **P <0.01, ***P <0.001 vs. controls (Student’ s t-test). ##P <0.01, ###P <0.001 vs. SRS mice (one-way ANOVA with post-hoc Bonferroni test). n = 6. G–H mRNA expression of BDNF determined by qPCR in the hippocampus (G), prefrontal cortex (H), hypothalamus (I), and amygdala (J). *P <0.05 vs. controls (Student’s t-test). #P <0.05, ##P <0.01, ###P <0.001 vs. SRS mice (one-way ANOVA with post-hoc Bonferroni test). The data are presented as mean ± SEM, n = 6. Con, control; FLX, fluoxetine; SRS, space restriction stressed.
We further found that BoNT/A treatment did not alter the expression of SNAP25 in control and SRS mice (Fig. 6C). The NMDAR subunits NR1 (Fig. 6D; t = 5.102, P = 0.0005) and NR2B (Fig. 6F; t = 4.529, P = 0.0011) were decreased, while NR2A did not change in the hippocampus in SRS-treated mice vs. controls (Fig. 6E; t = 0.8309, P = 0.4254). Intriguingly, BoNT/A treatment remarkably increased the expression of NR1 (Fig. 6D; F(5, 30) = 15.50, P <0.0001) and NR2B (Fig. 6F; F(5, 30) = 4.758, P = 0.0026) in the hippocampus in SRS mice. Together, these results indicated distinct NMDAR subunits, NR1 and NR2B, are involved in the antidepressant-like effects of BoNT/A treatment. In addition, the mRNA expression of BDNF was decreased in the hippocampus (Fig. 6G; t = 4.117, P = 0.0146) and amygdala (Fig. 6J; t = 4.129, P = 0.0145) in SRS-treated mice. BoNT/A treatment significantly increased the mRNA expression of BDNF in the hippocampus during 1–7 days post-injection of BoNT/A (Fig. 6G; F(4, 10) = 8.700, P = 0.0027) and during 1–14 days post-injection in the amygdala (Fig. 6J; F(4, 10) = 7.423, P = 0.0048). Although the mRNA expression of BDNF did not change in the prefrontal cortex (Fig. 6H) and hypothalamus (Fig. 6I) in SRS mice vs. controls, BoNT/A treatment transiently increased the mRNA expression of BDNF in these brain regions in SRS mice (Fig. 6H; F(4, 10) = 7.727, P = 0.0042; Fig. 6I; F(4, 10) = 6.459, P = 0.0078). Thus, these data suggested that up-regulation of BDNF expression in the brain may contribute to the antidepressant-like effects of BoNT/A treatment.
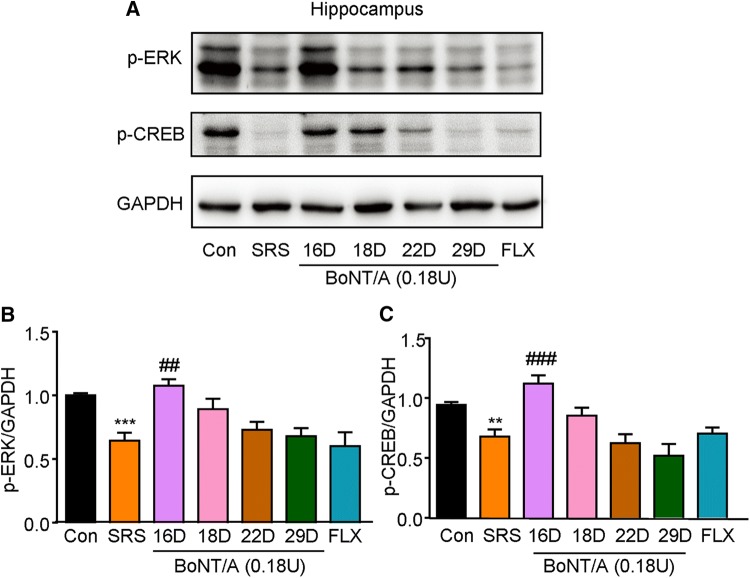
BoNT/A Treatment Activates the Intracellular ERK-CREB Pathway in the Hippocampus of Mice
The intracellular ERK-CREB pathway has been demonstrated to play important roles in neurotrophin signaling and neurogenesis, which are involved in the pathogenesis of depression [35]. As shown in Fig. 7, the expression of phosphorylated ERK (p-ERK) and p-CREB was suppressed in the hippocampus of SRS mice (Fig. 7A, B; t = 5.350, P = 0.0003; Fig. 7C; t = 4.034, P = 0.0024). Interestingly, BoNT/A treatment increased the expression of p-ERK and p-CREB one day after BoNT/A treatment (Fig. 7A, B; F(5, 30) = 5.810, P = 0.0007; Fig. 7C; F(5, 30) = 8.619, P < 0.0001). Thus, these data suggested that transient up-regulation of p-ERK and p-CREB may be involved in the antidepressant-like effects of BoNT/A treatment in mice.
Fig. 7.
BoNT/A treatment activates ERK-CREB signaling pathway in the hippocampus of SRS mice. A Representative western blots showing the effects of BoNT/A on the expression of p-ERK and p-CREB. B–C Semi-quantitative analysis of the expression of p-ERK (B) and p-CREB (C) after BoNT/A treatment. **P <0.01, ***P <0.001 vs. controls (Student’s t-test); ##P <0.01, ###p <0.001 vs. SRS mice (one-way ANOVA with post-hoc Bonferroni test). The data are presented as mean ± SEM, n = 6. Con, control; FLX, fluoxetine; SRS, space restriction stressed.
Discussion
BoNT/A has already been approved by the U.S. Food and Drug Administration for the treatment of several neurological disorders, including strabismus, blepharospasm, and hemifacial spasm [11]. Recently, it was reported that BoNT/A has been used to treat many neurological diseases, including migraine, Parkinson’s disease, and neuropathic pain [16, 17, 19]. Intriguingly, several clinical case reports have shown that BoNT/A can relieve the symptoms of depression, but the underlying mechanisms are largely unknown. In the present study, we demonstrated the antidepressant-like effects of BoNT/A on models of depression induced by SRS in mice using the FST and TST. Mechanistically, BoNT/A clearly increased the 5-HT levels in the brain and the expression of NMDAR subunits in SRS mice. Further, we found that BoNT/A increased the mRNA and protein levels of BDNF and activated intracellular downstream ERK-CREB signaling pathways, which are important in neurotrophin signaling and neurogenesis in the hippocampus. Collectively, our results provide strong preclinical evidence to support the idea that BoNT/A therapy attenuates the depressive-like behaviors in SRS mice, indicating that BoNT/A may be a promising therapeutic drug for depression.
Currently, several theories explain the anti-depressive effects of BoNT/A therapy [20–22, 36]. The first is related to the esthetic effects of BoNT/A; a more pleasant facial expression improves social interactions, leading to improvement in self-esteem and mood. The second is based on the facial feedback hypothesis, which states that facial expressions influence emotional states. Injection of BoNT/A into the glabellar area interferes with emotional responses because paralysis of the muscle does not allow the expression of certain negative emotions, such as sadness, anger, and fear, which are frequently experienced by patients suffering from depression. Recent functional MRI studies have shown that, after glabellar injections of BoNT/A, the amygdala becomes less responsive to negative stimuli [37]. Our present study showed that peripheral administration of BoNT/A did not alter the expression of SNAP25, indicating that BoNT/A is not transported to the hippocampus. Peripheral administration of BoNT/A in naïve mice also significantly increased the 5-HT levels in the distinct brain regions, including the hypothalamus. Thus, these findings suggested that peripheral administration of BoNT/A may indirectly cause neurochemical changes in the brain, possible by peripheral alteration of facial movement.
The complexity of the pathogenesis of major depression is reflected in the variety of causal factors, including genetic and environmental factors and their interactions [2, 38, 39]. Mounting numbers of studies have demonstrated that monoamine neurotransmitters, particularly noradrenaline and 5-HT, in the brain are reduced in depressed patients and in preclinical animal models with depressive-like behaviors [32]. Antidepressants, such as serotonin-selective reuptake inhibitors (SSRIs), increase the synaptic availability of 5-HT [40]. In the present study, decreased levels of 5-HT in the brain were demonstrated in SRS mice, consistent with previous reports [23]. Importantly, we found that BoNT/A treatment significantly increased the 5-HT levels in the hippocampus, prefrontal cortex, hypothalamus, and amygdala. Thus, these data suggested that the anti-depressive effects of BoNT/A treatment may partially be due to increasing 5-HT levels in brain regions in an animal model of depression.
Emerging evidence has also shown that glutamatergic neurotransmission dysfunction is involved in the pathogenesis of depression, indicating modulators of glutamate signaling as novel potential drugs for depression [41]. Human post-mortem studies have further implicated the NMDAR subunits NR1, NR2A, and NR2B in depression [42]. However, the distinct roles of these subunits in depression are inconsistent [43–45]. It has been reported that NR2A and NR2B expression are reduced in the prefrontal cortex in major depression [43]. However, it has also been reported that NR1, but not NR2A/B, are increased in an animal model of depression induced by chronic mild stress [44]. Thus, these results suggested that the involvement of distinct NMDAR subunits in depression may depend on the animal models used. In the present study, we found that the expression of NR1 and NR2B were significantly decreased in the hippocampus of SRS mice, while BoNT/A treatment increased the expression of NR1 and NR2B in the hippocampus of SRS mice. However, the precise mechanisms underlying effect of BoNT/A on the expression of NMDARs in depression warrant further investigation.
In the past decades, BDNF, a member of the neurotrophin family, has been documented to play essential roles in neurotrophic support during neurodevelopment and neurogenesis in the hippocampus [46]. BDNF plays a critical role in synaptic plasticity mainly via activation of NMDARs in the hippocampus [47], and in many neurological diseases, including learning and memory impairment and schizophrenia [48, 49]. Intriguingly, BDNF is decreased in animal models of depression and depressed patients [34, 50, 51] and several commonly-used antidepressants (e.g. SSRIs) increase the production of BDNF in the hippocampus and prefrontal cortex to exert anti-depression effects [52, 53]. Direct administration of BDNF activates ERK signaling that results in rapid synaptic protein synthesis [54, 55]. In addition, BDNF promotes CREB phosphorylation through ERK activation [56]. CREB activation is important for neurogenesis and attenuates depressive-like behaviors in mice [57]. In the present study, we found that BoNT/A up-regulated the expression of BDNF in the hippocampus at both the mRNA and protein levels, and this was decreased in SRS mice. Furthermore, we found that BoNT/A therapy activated the downstream ERK-CREB signaling pathways of BDNF in the hippocampus of mice. Thus, we postulated that BoNT/A treatment attenuates the depression-like behaviors possibly through activation of the BDNF-ERK-CREB signaling pathways in the hippocampus of mice.
The forced swimming and tail suspension tests are widely used to test models of depression and antidepressant-like effects in rodents [26, 58], although opinions differ [58, 59]. In the present study, we found that BoNT/A improved SRS-induced depressive-like behaviors in mice, reflected by decreased immobility time in the FST and TST, although BoNT/A had no evident effects on the sucrose preference test in SRS mice. Thus, the chronic unpredictable mild stress depression model may be suitable for further investigation of the antidepressant-like effects of BoNT/A.
In summary, our present work provided strong preclinical evidence that BoNT/A treatment attenuates depression-like behaviors in mouse models. Clinically, BoNT/A is a relative safe therapy in neurological practice with limited side-effects. Thereby, our studies support further investigation of the possibility of BoNT/A therapy as a novel strategy for the treatment of depression.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81870874, 31371179, 81300968, and 81671270) and the Natural Science Foundation of Jiangsu Province, China (BK20170004, 2015-JY-029, and BK20140372), Jiangsu Key Laboratory of Neuropsychiatric Diseases (BM2013003), the Second Affiliated Hospital of Soochow University Preponderant Clinic Discipline Group Project Funding (XKQ2015002), the Postgraduate Research and Practice Innovation Program of Jiangsu Province, China (KYCX17-2000), Suzhou Science and Technology For People’s Livelihood (SYS201706), the Postgraduate Research and Practice Innovation Program of Jiangsu Province, China (KYCX17_2034).
Conflict of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Weifeng Luo, Email: lwfwxx@126.com.
Tong Liu, Email: liutong80@suda.edu.cn.
References
- 1.Scifo E, Pabba M, Kapadia F, Ma T, Lewis DA, Tseng GC, et al. Sustained Molecular Pathology Across Episodes and Remission in Major Depressive Disorder. Biol Psychiatry. 2017;83:81–89. doi: 10.1016/j.biopsych.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belmaker RH, Agam G. Major depressive disorder. N Engl J Med. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- 3.Kupfer DJ, Frank E, Phillips ML. Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet. 2012;379:1045–1055. doi: 10.1016/S0140-6736(11)60602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mann JJ. The medical management of depression. N Engl J Med. 2005;353:1819–1834. doi: 10.1056/NEJMra050730. [DOI] [PubMed] [Google Scholar]
- 5.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Y, Cui Y, Sang K, Dong Y, Ni Z, Ma S, et al. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature. 2018;554:317–322. doi: 10.1038/nature25509. [DOI] [PubMed] [Google Scholar]
- 7.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zanos P, Thompson SM, Duman RS, Zarate CA, Jr, Gould TD. Convergent mechanisms underlying rapid antidepressant action. CNS Drugs. 2018;32:197–227. doi: 10.1007/s40263-018-0492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akhtar H, Bukhari F, Nazir M, Anwar MN, Shahzad A. Therapeutic efficacy of neurostimulation for depression: techniques, current modalities, and future challenges. Neurosci Bull. 2016;32:115–126. doi: 10.1007/s12264-015-0009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lacy BE, Weiser K, Kennedy A. Botulinum toxin and gastrointestinal tract disorders: panacea, placebo, or pathway to the future? Gastroenterol Hepatol (N Y) 2008;4:283–295. [PMC free article] [PubMed] [Google Scholar]
- 11.Cote TR, Mohan AK, Polder JA, Walton MK, Braun MM. Botulinum toxin type A injections: adverse events reported to the US Food and Drug Administration in therapeutic and cosmetic cases. J Am Acad Dermatol. 2005;53:407–415. doi: 10.1016/j.jaad.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Carter AT, Peck MW. Genomes, neurotoxins and biology of Clostridium botulinum Group I and Group II. Res Microbiol. 2015;166:303–317. doi: 10.1016/j.resmic.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ayyar BV, Atassi MZ. Effects of membrane properties on the binding activities of the HN and HC heavy-chain domains of botulinum neurotoxin A. Biochim Biophys Acta. 2016;1864:1678–1685. doi: 10.1016/j.bbapap.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Rossetto O, Pirazzini M, Montecucco C. Botulinum neurotoxins: genetic, structural and mechanistic insights. Nat Rev Microbiol. 2014;12:535–549. doi: 10.1038/nrmicro3295. [DOI] [PubMed] [Google Scholar]
- 15.Papagiannopoulou D, Vardouli L, Dimitriadis F, Apostolidis A. Retrograde transport of radiolabelled botulinum neurotoxin type A to the CNS after intradetrusor injection in rats. BJU Int. 2016;117:697–704. doi: 10.1111/bju.13163. [DOI] [PubMed] [Google Scholar]
- 16.Chen YW, Chuang SK. Botulinum Toxin A might be an alternative or adjunct therapy for the treatment of trigeminal and postherpetic neuralgia. J Evid Based Dent Pract. 2017;17:259–261. doi: 10.1016/j.jebdp.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Mills R, Bahroo L, Pagan F. An update on the use of botulinum toxin therapy in Parkinson’s disease. Curr Neurol Neurosci Rep. 2015;15:511. doi: 10.1007/s11910-014-0511-3. [DOI] [PubMed] [Google Scholar]
- 18.Ramachandran R, Yaksh TL. Therapeutic use of botulinum toxin in migraine: mechanisms of action. Br J Pharmacol. 2014;171:4177–4192. doi: 10.1111/bph.12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Attal N, de Andrade DC, Adam F, Ranoux D, Teixeira MJ, Galhardoni R, et al. Safety and efficacy of repeated injections of botulinum toxin A in peripheral neuropathic pain (BOTNEP): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2016;15:555–565. doi: 10.1016/S1474-4422(16)00017-X. [DOI] [PubMed] [Google Scholar]
- 20.Magid M, Reichenberg JS, Poth PE, Robertson HT, LaViolette AK, Kruger TH, et al. Treatment of major depressive disorder using botulinum toxin A: a 24-week randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75:837–844. doi: 10.4088/JCP.13m08845. [DOI] [PubMed] [Google Scholar]
- 21.Magid M, Finzi E, Kruger TH, Robertson HT, Keeling BH, Jung S, et al. Treating depression with botulinum toxin: a pooled analysis of randomized controlled trials. Pharmacopsychiatry. 2015;48:205–210. doi: 10.1055/s-0035-1559621. [DOI] [PubMed] [Google Scholar]
- 22.Finzi E, Rosenthal NE. Treatment of depression with onabotulinumtoxinA: a randomized, double-blind, placebo controlled trial. J Psychiatr Res. 2014;52:1–6. doi: 10.1016/j.jpsychires.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Xu Z, Guo X, Yang Y, Tucker D, Lu Y, Xin N, et al. Low-level laser irradiation improves depression-like behaviors in mice. Mol Neurobiol. 2017;54:4551–4559. doi: 10.1007/s12035-016-9983-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang G, Chen L, Yang L, Hua X, Zhou B, Miao Z, et al. Combined use of spatial restraint stress and middle cerebral artery occlusion is a novel model of post-stroke depression in mice. Sci Rep. 2015;5:16751. doi: 10.1038/srep16751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- 26.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 27.Liu MY, Yin CY, Zhu LJ, Zhu XH, Xu C, Luo CX, et al. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat Protoc. 2018;13:1686–1698. doi: 10.1038/s41596-018-0011-z. [DOI] [PubMed] [Google Scholar]
- 28.Wang B, Su CJ, Liu TT, Zhou Y, Feng Y, Huang Y, et al. The neuroprotection of low-dose morphine in cellular and animal models of Parkinson’s disease through ameliorating endoplasmic reticulum (ER) stress and activating autophagy. Front Mol Neurosci. 2018;11:120. doi: 10.3389/fnmol.2018.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian B, Wang XL, Huang Y, Chen LH, Cheng RX, Zhou FM, et al. Peripheral and spinal 5-HT receptors participate in cholestatic itch and antinociception induced by bile duct ligation in rats. Sci Rep. 2016;6:36286. doi: 10.1038/srep36286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghasemi M, Phillips C, Trillo L, De Miguel Z, Das D, Salehi A. The role of NMDA receptors in the pathophysiology and treatment of mood disorders. Neurosci Biobehav Rev. 2014;47:336–358. doi: 10.1016/j.neubiorev.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 31.Dang YH, Ma XC, Zhang JC, Ren Q, Wu J, Gao CG, et al. Targeting of NMDA receptors in the treatment of major depression. Curr Pharm Des. 2014;20:5151–5159. doi: 10.2174/1381612819666140110120435. [DOI] [PubMed] [Google Scholar]
- 32.Blier P. Neurotransmitter targeting in the treatment of depression. J Clin Psychiatry. 2013;74(Suppl 2):19–24. doi: 10.4088/JCP.12084su1c.04. [DOI] [PubMed] [Google Scholar]
- 33.Kishi T, Yoshimura R, Ikuta T, Iwata N. Brain-derived neurotrophic factor and major depressive disorder: evidence from meta-analyses. Front Psychiatry. 2017;8:308. doi: 10.3389/fpsyt.2017.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molendijk ML, Spinhoven P, Polak M, Bus BA, Penninx BW, Elzinga BM. Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (n=9484) Mol Psychiatry. 2014;19:791–800. doi: 10.1038/mp.2013.105. [DOI] [PubMed] [Google Scholar]
- 35.Yi LT, Li J, Liu BB, Luo L, Liu Q, Geng D. BDNF-ERK-CREB signalling mediates the role of miR-132 in the regulation of the effects of oleanolic acid in male mice. J Psychiatry Neurosci. 2014;39:348–359. doi: 10.1503/jpn.130169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reichenberg JS, Hauptman AJ, Robertson HT, Finzi E, Kruger TH, Wollmer MA, et al. Botulinum toxin for depression: Does patient appearance matter? J Am Acad Dermatol. 2016;74(171–173):e171. doi: 10.1016/j.jaad.2015.08.051. [DOI] [PubMed] [Google Scholar]
- 37.Kim MJ, Neta M, Davis FC, Ruberry EJ, Dinescu D, Heatherton TF, et al. Botulinum toxin-induced facial muscle paralysis affects amygdala responses to the perception of emotional expressions: preliminary findings from an A-B-A design. Biol Mood Anxiety Disord. 2014;4:11. doi: 10.1186/2045-5380-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jia J, Le W. Molecular network of neuronal autophagy in the pathophysiology and treatment of depression. Neurosci Bull. 2015;31:427–434. doi: 10.1007/s12264-015-1548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Godlewska BR, Browning M, Norbury R, Cowen PJ, Harmer CJ. Early changes in emotional processing as a marker of clinical response to SSRI treatment in depression. Transl Psychiatry. 2016;6:e957. doi: 10.1038/tp.2016.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duman RS. Pathophysiology of depression and innovative treatments: remodeling glutamatergic synaptic connections. Dialogues Clin Neurosci. 2014;16:11–27. doi: 10.31887/DCNS.2014.16.1/rduman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murrough JW, Abdallah CG, Mathew SJ. Targeting glutamate signalling in depression: progress and prospects. Nat Rev Drug Discov. 2017;16:472–486. doi: 10.1038/nrd.2017.16. [DOI] [PubMed] [Google Scholar]
- 43.Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:70–75. doi: 10.1016/j.pnpbp.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang J, Xue W, Xia B, Ren L, Tao W, Chen C, et al. Involvement of normalized NMDA receptor and mTOR-related signaling in rapid antidepressant effects of Yueju and ketamine on chronically stressed mice. Sci Rep. 2015;5:13573. doi: 10.1038/srep13573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee YA, Goto Y. Chronic stress modulation of prefrontal cortical NMDA receptor expression disrupts limbic structure-prefrontal cortex interaction. Eur J Neurosci. 2011;34:426–436. doi: 10.1111/j.1460-9568.2011.07750.x. [DOI] [PubMed] [Google Scholar]
- 46.Linker RA, Lee DH, Demir S, Wiese S, Kruse N, Siglienti I, et al. Functional role of brain-derived neurotrophic factor in neuroprotective autoimmunity: therapeutic implications in a model of multiple sclerosis. Brain. 2010;133:2248–2263. doi: 10.1093/brain/awq179. [DOI] [PubMed] [Google Scholar]
- 47.Maqsood R, Stone TW. The Gut-Brain Axis, BDNF, NMDA and CNS Disorders. Neurochem Res. 2016;41:2819–2835. doi: 10.1007/s11064-016-2039-1. [DOI] [PubMed] [Google Scholar]
- 48.Leal G, Bramham CR, Duarte CB. BDNF and hippocampal synaptic plasticity. Vitam Horm. 2017;104:153–195. doi: 10.1016/bs.vh.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 49.Bekinschtein P, Cammarota M, Medina JH. BDNF and memory processing. Neuropharmacology 2014, 76 Pt C: 677–683. [DOI] [PubMed]
- 50.Guo F, Zhang Q, Zhang B, Fu Z, Wu B, Huang C, et al. Burst-firing patterns in the prefrontal cortex underlying the neuronal mechanisms of depression probed by antidepressants. Eur J Neurosci. 2014;40:3538–3547. doi: 10.1111/ejn.12725. [DOI] [PubMed] [Google Scholar]
- 51.Pilar-Cuellar F, Vidal R, Diaz A, Castro E, dos Anjos S, Pascual-Brazo J, et al. Neural plasticity and proliferation in the generation of antidepressant effects: hippocampal implication. Neural Plast. 2013;2013:537265. doi: 10.1155/2013/537265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghosal S, Bang E, Yue W, Hare BD, Lepack AE, Girgenti MJ, et al. Activity-dependent brain-derived neurotrophic factor release is required for the rapid antidepressant actions of scopolamine. Biol Psychiatry. 2017;83:29–37. doi: 10.1016/j.biopsych.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li K, Shen S, Ji YT, Li XY, Zhang LS, Wang XD. Melatonin augments the effects of fluoxetine on depression-like behavior and hippocampal BDNF-TrkB signaling. Neurosci Bull. 2018;34:303–311. doi: 10.1007/s12264-017-0189-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ohta KI, Suzuki S, Warita K, Kaji T, Kusaka T, Miki T. Prolonged maternal separation attenuates BDNF-ERK signaling correlated with spine formation in the hippocampus during early brain development. J Neurochem. 2017;141:179–194. doi: 10.1111/jnc.13977. [DOI] [PubMed] [Google Scholar]
- 55.Lee S, Yang M, Kim J, Son Y, Kim J, Kang S, et al. Involvement of BDNF/ERK signaling in spontaneous recovery from trimethyltin-induced hippocampal neurotoxicity in mice. Brain Res Bull. 2016;121:48–58. doi: 10.1016/j.brainresbull.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Einoch R, Weinreb O, Mandiuk N, Youdim MBH, Bilker W, Silver H. The involvement of BDNF-CREB signaling pathways in the pharmacological mechanism of combined SSRI- antipsychotic treatment in schizophrenia. Eur Neuropsychopharmacol. 2017;27:470–483. doi: 10.1016/j.euroneuro.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 57.Gascon S, Ortega F, Gotz M. Transient CREB-mediated transcription is key in direct neuronal reprogramming. Neurogenesis (Austin) 2017;4:e1285383. doi: 10.1080/23262133.2017.1285383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Molendijk ML, de Kloet ER. Immobility in the forced swim test is adaptive and does not reflect depression. Psychoneuroendocrinology. 2015;62:389–391. doi: 10.1016/j.psyneuen.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 59.de Kloet ER, Molendijk ML. Coping with the forced swim stressor: towards understanding an adaptive mechanism. Neural Plast. 2016;2016:6503162. doi: 10.1155/2016/6503162. [DOI] [PMC free article] [PubMed] [Google Scholar]