Dear Editor,
Becoming a mother is one of the most monumental experiences in a woman’s lifetime. Women typically bear the primary caregiving responsibility for their infants, and they undergo numerous changes both mentally and physically, including behavioral, emotional, and hormonal changes, during the postpartum period. Studies have indicated that hormonal, experiential, and temporal factors significantly regulate emotional and cognitive brain functions during the postpartum period [1]. The determination of the neural basis of a maternal brain is critically important for understanding mother-infant attachments and thus perpetuating the human species. Until recently, limited investigations of the neuroanatomical and functional status of mothers have contributed to understanding the processing of maternal behaviors.
Previous studies using magnetic resonance imaging (MRI) technology have indicated that the brains of mothers undergo both structural and functional changes during the postpartum period. Structural MRI has indicated that brain structures in mothers show dynamic plasticity, including alterations in grey matter volume. These longitudinal voxel-based morphometry studies have confirmed that pregnancy induces substantial structural changes, such as alterations in the grey matter volume in regions associated with social cognition and maternal motivation and behavior [2]. Longitudinal task-related functional MRI (fMRI) studies have shown that emotional reactivity is increased 4–6 weeks postpartum in healthy women [3]. Activity in prefrontal areas during response inhibition tasks decreases over time during the postpartum period [4]. The results of these studies indicate that the neural mechanisms of cognitive and emotional processing likely vary in healthy mothers, which suggests that structurally and functionally important adaptations occur during the postpartum period.
Researchers have found that the risk of postpartum mental disorders, such as depression, anxiety, and mood swings, is increased for several months after delivery among primiparous mothers [5]. A resting-state fMRI (rs-fMRI) study investigated postpartum depression and discovered that depressed mothers show significant increases in regional homogeneity (ReHo) in the posterior cingulate gyrus, parietal lobe, and frontal lobe [6]. These studies revealed that the resting-state activity of the default mode network might be associated with impaired empathic and self-other relational processing in women with postpartum depression [7]. However, most published studies have focused on postpartum depression, while few have explored the patterns of spontaneous brain activity in healthy mothers. Recently, a study of postpartum women without depression indicated that spontaneous neural activity is lower in the posterior cingulate cortex and prefrontal cortex than in nulliparous women [8]. Therefore, additional correlative studies using rs-fMRI are needed to examine the intrinsic neural activity in postpartum women to further understand the neural mechanisms of maternal behaviors.
Previous neuroimaging studies in mothers have concentrated mainly on exploring functional brain activity in response to infant stimuli using task-related fMRI and alterations in brain structure using voxel-based morphometry. However, resting-state brain activation in mothers may also change over time while nurturing infants. Rs-fMRI can be used to investigate the spontaneous neural activity in mothers. In the current study, we set out to explore the spontaneous neuronal activity relevant to maternal behaviors in mothers during the infant-rearing years without the confounding effects of task performance using cross-sectional and longitudinal rs-fMRI together with the above methods. Thus, we conducted a longitudinal rs-fMRI study in a group of mothers within one year of giving birth to explore changes in the amplitude of low-frequency fluctuation (ALFF) and ReHo between baseline and after approximately two years of child-rearing. A cross-sectional rs-fMRI study in two groups (mothers and non-mothers) was also carried out to investigate whether the spontaneous brain activity differs between mothers and non-mothers. We recruited a total of 47 new mothers and 26 non-mothers to obtain rs-fMRI and 3D T1-weighted structural images at baseline. Ultimately, 23 mothers were included in the longitudinal evaluation for ~2 years (Fig. S1). The demographic data for the mother and non-mother groups are listed in Table S1.
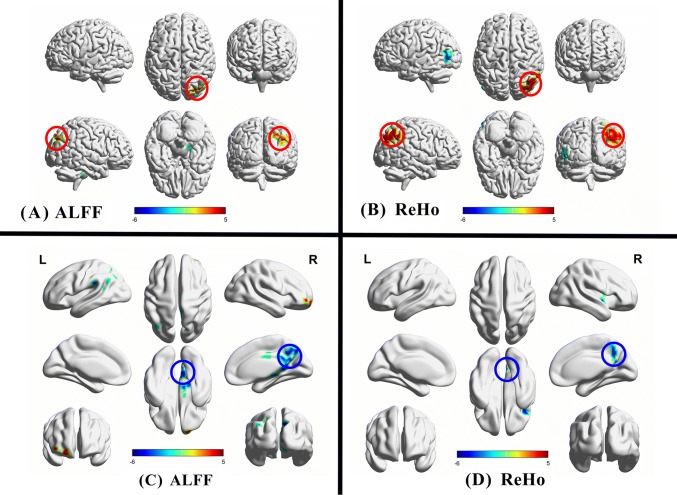
Compared with the non-mothers, the mothers had increased ALFF in the right precuneus, right angular gyrus, and right superior occipital gyrus and decreased ALFF in the right cerebellar anterior lobe, right brainstem, and right parahippocampal gyrus (Table S2 and Fig. 1). The ReHo analysis revealed significant differences between mothers and non-mothers. Compared with non-mothers, new mothers showed significantly increased ReHo in the right inferior parietal lobe, right superior parietal lobe, right angular gyrus, right precuneus, and right supramarginal gyrus and decreased ReHo in the left middle and inferior temporal gyri (Table S2 and Fig. 1). In addition, we identified significant longitudinal alterations in the ALFF and ReHo values in mothers (scan 1 versus scan 2). Significantly lower ALFF values were identified in the bilateral precuneus, left inferior parietal lobe, left angular gyrus, left supramarginal gyrus, left superior and middle temporal gyri, right middle and posterior cingulate gyri, right thalamus, right cerebellar posterior lobe, right brainstem, and right hippocampus of mothers; the ALFF was increased in the right superior, middle, and medial frontal gyri (Table S3 and Fig. 1). Compared with scan 1, mothers (scan 2) showed decreased ReHo values in the right insula, right inferior frontal gyrus, right precentral gyrus, right precuneus, and right posterior cingulate cortex, whereas an increased ReHo value was identified in the left cerebellar posterior lobe (Table S3 and Fig. 1).
Fig. 1.
A, B Regions in which the ALFF (A) and ReHo (B) values exhibited significant differences between mothers and non-mothers. C, D Regions in which the ALFF (C) and ReHo (D) values showed longitudinal alterations in mothers between the two scans (scan 1 vs scan 2). Color bars, display window for t-values.
To the best of our knowledge, the present investigation is the first to use both a cross-sectional and a longitudinal study to ascertain whether differences in spontaneous brain activity exist between mothers and non-mothers and to detect altered resting-state spontaneous neural activity over time in mothers during the postpartum period. First, cross-sectional analyses demonstrated that the baseline images from mothers showed larger ALFF/ReHo values in the superior and inferior parietal lobe and precuneus, and smaller ALFF/ReHo values in the cerebellum, brainstem, and temporal lobe than non-mothers. Second, longitudinal analyses at 2-year follow-up showed lower ALFF/ReHo values in the parietal lobe, cingulate cortex, precuneus, and thalamus, and higher ALFF/ReHo values in the frontal lobe and cerebellum at the second scan in mothers.
We used ALFF and ReHo to quantify the neural activity in mothers. In the cross-sectional study, consistently increased ALFF and ReHo values were found in the precuneus and angular gurus; inconsistent alterations in ALFF and ReHo were found in the middle and inferior temporal gyri, cerebellar anterior lobe, brainstem, and parahippocampal gyrus. In the longitudinal study, we found consistently decreased ALFF and ReHo values in the posterior cingulate gyrus and precuneus, and inconsistent changes in ALFF and ReHo in the inferior parietal lobe, superior and middle temporal gyri, superior and middle frontal gyri, brainstem, thalamus, and cerebellar posterior lobe in mothers over time, either decreases or increases. The ALFF and ReHo values showed a strong positive correlation [9]. Hence, this close relationship can support our consistent findings in ALFF and ReHo in mothers. Meanwhile, they also reflect different aspects of the spontaneous neural activity according to the analysis method used for ALFF and ReHo, which may explain the differential findings in ALFF and ReHo. Li et al. showed that ALFF can be complementary to ReHo in measuring local spontaneous activity [10]. Therefore, combination of the two indexes can reveal more comprehensive and interesting information about spontaneous brain activity in mothers than either method alone [10].
Zheng et al. reported that, relative to nulliparous women, postpartum women have reduced ALFF and ReHo values primarily in the posterior cingulate cortex and prefrontal cortex and increased ALFF values in the cerebellar posterior lobe, and the ALFF/ReHo values in the posterior cingulate cortex are positively associated with impaired cognitive functioning [8]. Their results are not completely consistent with our study. In our study, the mothers were mainly in the late postpartum period, and the study was extended until two years after delivery. One possible explanation is that differences in the analyzed postpartum stages of the mothers resulted in findings that differed from those reported in the study by Zheng et al. [8]. Importantly, here, we used both cross-sectional and longitudinal analyses, and comprehensively demonstrated the altered activation pattern of the spontaneous neural activity in mothers over time. In addition, our rs-fMRI results are in line with our previous task-related fMRI study, our previous study with Bornstein et al. found that these mothers in the first scan exhibited enhanced neural activity in the superior medial frontal gyri, superior temporal gyrus, insula, precuneus, precentral gyrus, and anterior cingulate cortex when they heard infants crying [11]. Rocchetti et al. also reported that many of these brain areas, including the thalamus, parietal lobe, insula, and prefrontal cortex, exhibit significantly increased activation in mothers in response to baby-related stimuli during the postpartum period, supporting the role of these brain regions in the development and expression of maternal behaviors [12]. The altered functioning is accompanied by structural changes in the brain. Previous structural brain studies have shown that grey matter volume is decreased in areas such as the prefrontal and temporal cortex and anterior and posterior cortical midline during pregnancy and the postpartum period, and notably these grey matter reductions endure for at least two years post-pregnancy [2]. Grey matter volume has a significant impact on the patterns of decreased brain activity [13]. Our research further suggested that the brain function of new mothers changed even under resting-state conditions, and these structural and functional changes may be due to specific neural plasticity.
Due to the common hemodynamic response, the regional synchrony of blood oxygenation level-dependent activity is correlated with task activation, and the ALFF and ReHo values can explain the variance of task activation. Hence, we found higher ALFF and ReHo values in mothers than in non-mothers, consistent with the increased neural activation when mothers respond to infant-related stimuli. Importantly, the ALFF/ReHo values of some brain regions increased in the cross-sectional study, while they decreased in the longitudinal study. These findings suggest that these alterations in mothers had recovered toward initial levels at 2-year follow-up during the postpartum period. The increased spontaneous neural activity indicated that additional neural resources are recruited to compensate for cognitive and emotive functions in other brain regions. Hence, these specific brain regions may support the critical ability of women to develop the behaviors and thoughts needed to successfully care for newborn infants. We also found decreased spontaneous neural activity in the cerebellum, brainstem, and temporal lobe in mothers, which may be consistent with the reduced grey matter volume, and the findings suggested that this decreased spontaneous activity is correlated with the specific reorganization of cognitive function.
As suggested in previous neuroimaging studies, we found altered brain regions, including the temporo-parietal junction [14], precuneus [15], and medial prefrontal cortex [16], that are involved in theory of mind (ToM) processing. Meanwhile, Hoekzema et al. have also shown that grey matter volume in the ToM network is reduced during pregnancy and the postpartum period [2]. ToM is the cognitive ability to identify and understand other individuals’ mental states, such as beliefs, feelings, or desires [17]. Our results showed that increased or decreased ALFF and ReHo values in these regions occurred in mothers; this might represent regional functional recombination in the ToM during the postpartum period. Furthermore, we discovered that altered regional spontaneous neural activity overlapped with maternal empathy-related regions. Maternal empathy and related social behaviors play key roles in positive parenting [18]. Studies have shown that maternal empathy appears to depend on distinct neural systems, such as the thalamo-cingulate circuit as a neural alarm system [19], the mirror-neuron system, the somatosensory and insular cortices, and limbic areas [20]. Combined with our findings of altered regional spontaneous activity in specific regions in mothers (e.g., the cingulate cortex, thalamus, insula, and inferior parietal lobe), we have determined for the first time that mothers may also exhibit a gradual and functional reorganization of the empathy network. These altered empathy-related regions comprise the neural bases of human parental care. For example, when a mother hears an infant crying, areas related to he altered mirror-neuron system may enable her to efficiently understand and simulate her child’s feelings internally and thus adapt action responses to the crying infant. These changes in mothers may occur because of a series of evolutionary needs in their ability to perceive and respond adaptively to new-born signals. Moreover, these alterations are required for a prolonged postnatal period.
In conclusion, our study shows that changes in resting-state spontaneous brain activity occur over time in women after delivery and that significant differences exist between mothers and non-mothers. The alterations in the ALFF and ReHo values were predominantly located in the frontal, parietal, and limbic regions, which include regions corresponding to the empathy and ToM networks. These results demonstrate that spontaneous neuronal activity in mothers exhibits adaptive changes during the process of nurturing infants (i.e., women require physiologically important adaptation during the postpartum period). These adaptive alterations may allow mothers to better recognize and respond to the affective states and needs of their infants. These findings improve our understanding of and provide novel insights into the behavior, cognition, and affection of mothers from the perspective of resting-state brain function.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81571658 and U1632274) and the Social Science Foundation of China (15ZDB016). We thank Xiaonan Liu for proposing modifications and making suggestions during the preparation of the manuscript. We thank all the volunteers for their participation in the study.
Conflicts of interest
All authors declared that they have no conflict of interest with the content of this article.
Contributor Information
Xiaoxia Du, Email: xxdu@phy.ecnu.edu.cn.
Zhong Chen, Email: chenz@xmu.edu.cn.
References
- 1.Albin-Brooks C, Nealer C, Sabihi S, Haim A, Leuner B. The influence of offspring, parity, and oxytocin on cognitive flexibility during the postpartum period. Horm Behav. 2017;89:130–136. doi: 10.1016/j.yhbeh.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoekzema E, Barba-Muller E, Pozzobon C, Picado M, Lucco F, Garcia-Garcia D, et al. Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci. 2017;20:287–296. doi: 10.1038/nn.4458. [DOI] [PubMed] [Google Scholar]
- 3.Gingnell M, Toffoletto S, Wikstrom J, Engman J, Bannbers E, Comasco E, et al. Emotional anticipation after delivery - a longitudinal neuroimaging study of the postpartum period. Sci Rep. 2017;7:114. doi: 10.1038/s41598-017-00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bannbers E, Gingnell M, Engman J, Morell A, Sylven S, Skalkidou A, et al. Prefrontal activity during response inhibition decreases over time in the postpartum period. Behav Brain Res. 2013;241:132–138. doi: 10.1016/j.bbr.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Munk-Olsen T, Laursen TM, Pedersen CB, Mors O, Mortensen PB. New parents and mental disorders - A population-based register study. J Am Med Assoc. 2006;296:2582–2589. doi: 10.1001/jama.296.21.2582. [DOI] [PubMed] [Google Scholar]
- 6.Wang XJ, Wang J, Liu ZH, Ming Y, Zhang SW. Increased posterior cingulate, medial frontal and decreased temporal regional homogeneity in depressed mothers: a resting-state functional magnetic resonance study. Procedia Environ Sci. 2011;8:737–743. doi: 10.1016/j.proeng.2011.08.1149. [DOI] [Google Scholar]
- 7.Chase HW, Moses-Kolko EL, Zevallos C, Wisner KL, Phillips ML. Disrupted posterior cingulate-amygdala connectivity in postpartum depressed women as measured with resting BOLD fMRI. Soc Cogn Affect Neurosci. 2014;9:1069–1075. doi: 10.1093/scan/nst083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng JX, Chen YC, Chen H, Jiang L, Bo F, Feng Y, et al. Disrupted spontaneous neural activity related to cognitive impairment in postpartum women. Front Psychol. 2018;9:624. doi: 10.3389/fpsyg.2018.00624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan R, Di X, Kim EH, Barik S, Rypma B, Biswal BB. Regional homogeneity of resting-state fMRI contributes to both neurovascular and task activation variations. Magn Reson Imaging. 2013;31:1492–1500. doi: 10.1016/j.mri.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.An L, Cao QJ, Sui MQ, Sun L, Zou QH, Zang YF, et al. Local synchronization and amplitude of the fluctuation of spontaneous brain activity in attention-deficit/hyperactivity disorder: a resting-state fMRI study. Neurosci Bull. 2013;29:603–613. doi: 10.1007/s12264-013-1353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bornstein MH, Putnick DL, Rigo P, Esposito G, Swain JE, Suwalsky JTD, et al. Neurobiology of culturally common maternal responses to infant cry. Proc Natl Acad Sci U S A. 2017;114:E9465–E9473. doi: 10.1073/pnas.1712022114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rocchetti M, Radua J, Paloyelis Y, Xenaki LA, Frascarelli M, Caverzasi E, et al. Neurofunctional maps of the ‘maternal brain’ and the effects of oxytocin: A multimodal voxel-based meta-analysis. Psychiatry Clin Neurosci. 2014;68:733–751. doi: 10.1111/pcn.12185. [DOI] [PubMed] [Google Scholar]
- 13.Sheppard JP, Wang JP, Wong PC. Large-scale cortical functional organization and speech perception across the lifespan. PLoS One. 2011;6:e16510. doi: 10.1371/journal.pone.0016510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schurz M, Tholen MG, Perner J, Mars RB, Sallet J. Specifying the brain anatomy underlying temporo-parietal junction activations for theory of mind: a review using probabilistic atlases from different imaging modalities. Hum Brain Mapp. 2017;38:4788–4805. doi: 10.1002/hbm.23675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 16.Sabihi S, Dong SM, Durosko NE, Leuner B. Oxytocin in the medial prefrontal cortex regulates maternal care, maternal aggression and anxiety during the postpartum period. Front Behav Neurosci. 2014;8:258. doi: 10.3389/fnbeh.2014.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaafsma SM, Pfaff DW, Spunt RP, Adolphs R. Deconstructing and reconstructing theory of mind. Trends Cogn Sci. 2015;19:65–72. doi: 10.1016/j.tics.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atzil S, Hendler T, Zagoory-Sharon O, Winetraub Y, Feldman R. Synchrony and specificity in the maternal and the paternal brain: relations to oxytocin and vasopressin. J Am Acad Child Adolesc Psychiatry. 2012;51:798–811. doi: 10.1016/j.jaac.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hamner MB, et al. A potential role for thalamocingulate circuitry in human maternal behavior. Biol Psychiat. 2002;51:431–445. doi: 10.1016/S0006-3223(01)01284-7. [DOI] [PubMed] [Google Scholar]
- 20.Doucet L, Shao B, Wang L, Oldham GR. I know how you feel, but it does not always help Integrating emotion recognition, agreeableness, and cognitive ability in a compensatory model of service performance. J Serve Manage. 2016;27:320–338. doi: 10.1108/JOSM-11-2014-0307. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.