Abstract
Activation of inflammatory responses regulates the transmission of pain pathways through an integrated network in the peripheral and central nervous systems. The immunopotentiator thymosin alpha-1 (Tα1) has recently been reported to have anti-inflammatory and neuroprotective functions in rodents. However, how Tα1 affects inflammatory pain remains unclear. In the present study, intraperitoneal injection of Tα1 attenuated complete Freund’s adjuvant (CFA)-induced pain hypersensitivity, and decreased the up-regulation of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) in inflamed skin and the spinal cord. We found that CFA-induced peripheral inflammation evoked strong microglial activation, but the effect was reversed by Tα1. Notably, Tα1 reversed the CFA-induced up-regulation of vesicular glutamate transporter (VGLUT) and down-regulated the vesicular γ-aminobutyric acid transporter (VGAT) in the spinal cord. Taken together, these results suggest that Tα1 plays a therapeutic role in inflammatory pain and in the modulation of microglia-induced pro-inflammatory cytokine production in addition to mediation of VGLUT and VGAT expression in the spinal cord.
Electronic supplementary material
The online version of this article (10.1007/s12264-019-00346-z) contains supplementary material, which is available to authorized users.
Keywords: Thymosin alpha-1, Cytokine, Microglia, Vesicular glutamate transporter type 2, Vesicular γ-aminobutyric acid transporter
Introduction
Up to 10% of adults suffer from chronic pain [1], which not only compromises the quality of life but also imposes medical and financial burdens on society. Inflammation, a necessary process that protects against tissue injury and disease, involves, in part, circulating and resident immune cells and mediators [2]. The inflammatory state is characterized by the production of pain initiated by a normally innocuous stimulus, and is referred to as inflammatory pain [3]. Inflammation is also a driving force for pathogenic mechanisms of chronic pain. Inflammatory pain caused by inflamed tissues may occur in the absence of external stimuli. Moreover, noxious triggers enhance pain responses (hyperalgesia) while innocuous stimuli may induce pain (allodynia). The pathological mechanism of inflammatory pain involves both the central and peripheral nervous systems [4] and arises from the sensitization of primary nociceptors and central sensitization by the release of direct-acting inflammatory mediators contributing to pain hypersensitivity [5, 6]. Multiple inflammatory factors such as pro-inflammatory cytokines (e.g., TNF-α and IL-1β) and chemokines sensitize peripheral nociceptors in inflamed or damaged tissues [7, 8]. The pain signal induced by peripheral sensitivity is transmitted upstream to the spinal cord and brain regions through a cascade of electrical and chemical signals [11]. Pain and chronic pain involve the central nervous system (CNS), the immune system, and glia [9, 10]. Some evidence suggests that activation of an immune response can regulate the transmission of pain signals in an integrated network of immune cells, glia, and neurons [10, 11]. Inflammatory pain models usually employ the intraplantar injection of chemical agents, such as complete Freund’s adjuvant (CFA) or carrageenan [12].
Thymosin alpha 1 (Tα1) is a naturally-occurring polypeptide of 28 amino-acids, that is primarily expressed in the thymus [13]. Tα1 is a type of widely-used immunopotentiator in infectious diseases, immunodeficiency disorders, chronic lung inflammation, and malignancies. The drug serves as an immune manipulator through a mechanism that functions by immune-associated modulation via innate immune receptors [14]. Previous reports have suggested that specific cell populations in the rat CNS can express and respond to stimulation by Tα1 [15]. In a preliminary in vitro study using whole-cell recordings, Tα1 has been shown to regulate synaptic transmission in hippocampal neurons [16], but the route by which Tα1 influences neuronal function remains unclear. Our preliminary findings revealed that Tα1 plays beneficial roles in cognitive function and hippocampal neurogenesis associated with immune modulation after peripheral administration [17].
Glial activation commonly occurs in CNS diseases, and is regarded as a feature of the tissue response to infectious disease or inflammation [18]. Glia, including microglia and astrocytes, are able to switch from a quiescent or resting state to a reactive state depending on the triggers, and serve as immune cells to coordinate neuronal activity [19]. The activated microglia transform their morphology by producing various mediators, including pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukin (IL)-1β, and IL-6 [20]. Glia-associated neuro-inflammation has been reported to be a pathogenic mechanism of nervous disorders, and inhibition of pro-inflammatory cytokines attenuates the disease processes [21, 22]. Recent research has revealed the potential impact of spinal astrocytes and microglia on the initiation and development of pain induced by peripheral inflammation [23, 24]. Upon triggering activation, microglia secrete a large amount of algesic substances, including TNF-α, IL-1β, and IL-6 into the spinal cord [25]. The pro-inflammatory cytokines released by spinal glia have been consistently shown to boost and maintain allodynia and hyperalgesia [26, 27]. In addition, direct intrathecal administration of TNF-α, IL-1β, and IL-6 peptides promotes mechanical allodynia and heat hyperalgesia while blockade of the pro-inflammatory cytokines abates inflammatory pain [28, 29]. Therefore, active signals from glia are relayed to terminal brain regions by way of peripheral immune activation and through afferent nerve inputs, and glia are regarded as the probable signal-relay station [30, 31]. The anti-inflammatory cytokine IL-4 has analgesic effects that are possibly related to blockade of the pro-inflammatory cytokines TNF-α and IL-1β released by a peripheral mechanism [32].
The vesicular glutamate transporter (VGLUT) is one of the transporters that package glutamate (a dominant, fast excitatory transmitter) into synaptic vesicles, followed by fast excitatory synaptic transmission in the CNS [33]. VGLUT type 2 (VGLUT2) is abundantly distributed in the spinal cord [34], a finding that suggests that it is involved in the transmission of pain signals [35]. In contrast, the vesicular γ-aminobutyric acid transporter (VGAT), which belongs to the family of vesicular amino-acid transporters, functions in the uptake of both γ-aminobutyric acid (GABA) and glycine, which are dominant, fast inhibitory transmitters, into synaptic vesicles in the spinal cord [36–38]. Both GABAergic and glycinergic inhibitory control of superficial neurons is reduced in the dorsal horn of the spinal cord by peripheral stimuli [37, 38]. Disruption of this inhibitory action commonly induces inflammatory pain hypersensitivity [39]. Importantly, enhanced excitatory and weakened inhibitory synaptic transmission evoke central sensitization, which is critical for the development and maintenance of persistent pain [40, 41]. In this study, we used a CFA-induced inflammatory pain model to investigate the influence of Tα1 on inflammatory pain, and explored the potential mechanisms by which the peripheral and central systems affect Tα1-mediated inflammatory pain.
Materials and Methods
Animals
C57BL/6 J mice (male, 8 weeks–10 weeks old) were obtained from Sun Yat-Sen University Laboratory Animal Center (Guangzhou, China). The mice had free access to water and food with a 12-h light/12-h dark cycle, and were individually housed under constant humidity (55%) and temperature (22 °C ± 2 °C) in a specific pathogen-free facility. All experiments were approved by the Ethics Committee of Guangzhou University of Chinese Medicine, and were carried out according to the guidelines of the International Association for the Study of Pain.
Animal Model and Drug Treatment
The inflammatory pain model was created by intraplantar injection of CFA (Sigma, St. Louis, MO) into the plantar surface of the left hindpaw (30 μL per mouse) under brief anesthesia with 3% isoflurane. The control group was injected with saline. Tα1 (Zadaxin) in lyophilized powder form was reconstituted in sterile distilled water. Mice were treated intraperitoneally (i.p.) with Tα1 (200 μg/kg, 100 μL saline) [42] daily for 3 consecutive days in CFA-injected mice beginning 1 day after intraplantar injection of CFA. The vehicle group was treated with saline at the same time points. In addition, another group of mice was given with Tα1 i.p. (50 μg/kg, 100 μg/kg, 200 μg/kg, or 500 μg/kg, 100 μL saline) daily for 3 consecutive days beginning 1 day after intraplantar injection of CFA.
Study Design
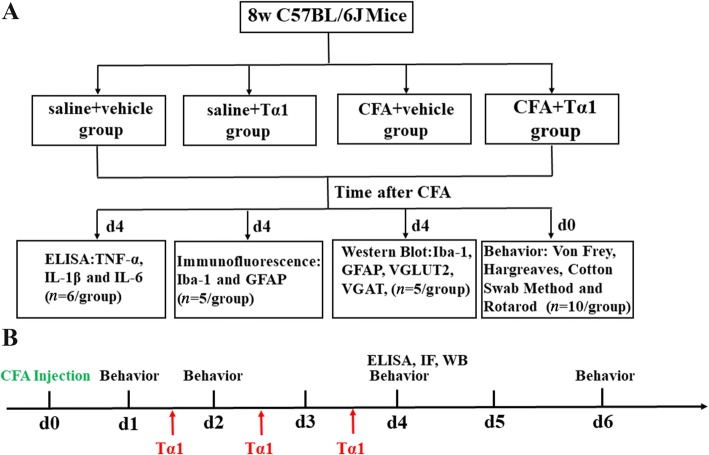
C57BL/6 J mice were randomly divided into four groups (saline + vehicle, saline + Tα1, CFA + vehicle, and CFA + Tα1 groups) for pain behavior tests, immunofluorescence, and enzyme-linked immunosorbent assay (ELISA), as well as western blot analysis. The study design and time points of all manipulations are shown in Fig. 1.
Fig. 1.
Schematic and timeline of the experimental design. ELISA, enzyme-linked immunosorbent assay; IF, immunofluorescence; WB, Western blot.
Behavioral Analysis
Baseline thresholds were tested prior to intraplantar CFA injection, and the nociceptive thresholds were tested in the ipsilateral hind paw at 1 day (d1), d2, d4, and d6 after CFA injection.
Thermal hyperalgesia was assessed by the latency of paw withdrawal using a radiant heat apparatus (Ugo Basile, Gemonio, Italy) and the Hargreaves method [43]. Each mouse was put in an individual restrainer on a glass plate and allowed to adapt for 30 min before testing. The plantar surface of the hind paw was exposed to a noxious thermal beam from the radiant heat apparatus until the paw was withdrawn. A cutoff latency of 20 s was set to avoid potential injury. Each test was applied at 5-min intervals, and an average of three values from each mouse was calculated for the latency.
Each mouse was acclimated to a Plexiglas chamber on an elevated mesh floor for 30 min prior to measurements using the “up-down” method to assess mechanical allodynia [44]. Successive calibrated von Frey monofilaments (Ugo Basile) were applied vertically to the plantar surface of the ipsilateral hind paw with enough force to bend the monofilament over 6 s. The minimum filament force evoking at least 3 responses (such as sharp paw withdrawal, shaking, or licking/biting the stimulated hind paw) over 5 stimulations in total during a round of testing was defined as the mechanical threshold. The manipulation was repeated twice, and the mean value was defined as the threshold.
The cotton swab method was performed using a cotton swab as described previously to assess dynamic mechanical allodynia [45]. Each mouse was habituated to a Plexiglas chamber on an elevated mesh floor for 1 h prior to testing. The tip of a cotton swab was trimmed. The swab was lightly stroked across the surface of the injured hind paw from heel to toe, and a positive response (such as lifting, shaking, or licking the paw) was recorded; negative responses were recorded in the absence of such behavior. The application was repeated 10 times, and the percentage of positive response was used to indicate the degree of paw withdrawal.
To avoid the effect of sensorimotor coordination on pain behavior, an accelerating rotarod (Shanghai Ji-liang Technology Co., Ltd, Shanghai, China) was used according to a published protocol 5 days after CFA injection [46]. Mice were initially trained to stay on the rotarod at 5 rpm for 5 min. If mice fell off, they were repositioned on the rotarod until they remained in position for 5 min. This training was carried out over two consecutive days. After the initial training period, the rotarod test was performed. The rotarod rotation rate was increased from 4 rpm to 40 rpm over 5 min. The mice were tested twice at 20-min intervals, and the time of falling was recorded; the average time was defined as the latency of the rotarod test.
ELISA
Mice were transcardially perfused with 0.9% sterile saline after anesthesia with 10% chloral hydrate 4 days after CFA injection. The L4–6 spinal segments and hind paws (including skin and underlying muscle) were immediately dissected and homogenized on ice in radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotine Biotechnology, Shanghai, China) containing a protease inhibitor cocktail (Sigma) and 1 mmol/L phenylmethane sulfonyl fluoride before centrifugation for 15 min at 12,000 rpm (4 °C). The total protein concentration in the supernatant was determined using the Enhanced BCA Protein Assay kit (Beyotine Biotechnology), and the concentrations were adjusted to 4.0 mg/mL according to the manufacturers protocol. The concentrations of cytokines (TNF-α, IL-1β, and IL-6) in hind paws and spinal cord were determined with an ELISA kit (Beyotine Biotechnology) according to the product protocols. The absorbance at 450 nm was quantified with a microreader (Biotek Elx800, Winooski, VT).
Western Blot
Western blotting was performed using a published protocol [47]. Briefly, mice were euthanized after deep anesthesia with 10% chloral hydrate. The L3–5 spinal segments were removed and homogenized in RIPA lysis buffer 4 days after CFA injection. The protein content was quantified using the Enhanced BCA Protein Assay Kit (Beyotine Biotechnology), and all samples were adjusted to 4.0 mg/mL. The extracted protein was boiled for 5 min at 95 °C with 5 × loading buffer (Beyotine Biotechnology), and an equal volume of the protein mixture was loaded onto an SDS-PAGE gel and transferred onto PVDF membranes in a Western blot system (Bio-Rad, Hercules, CA) at an appropriate voltage and duration. The membranes were blocked with 5% nonfat milk for 1 h at room temperature (RT) before being probed with the primary antibodies rabbit anti-VGULT1 (1:3000, Invitrogen, Carlsbad, CA), rabbit anti-VGULT2 (1:3000, Synaptic System, Goettingen, Germany), rabbit anti-VGAT (1:3000, Millipore, Darmstadt, Germany), mouse anti-GFAP (1:2000, Sigma-Aldrich, Darmstadt, Germany), rabbit anti-Iba1 (1:2000, Abcam), and rabbit anti-β-actin (1:5000, Abbkine, San Diego, CA) at 4 °C overnight followed by incubation with HRP-conjugated secondary antibodies (1:4000, goat anti-rabbit, goat anti-mouse; Abbkine) for 1 h at RT. Immunoblots were visualized with a chemiluminescence system (Peiqing Science and Technology Co., Ltd, Shanghai, China). Densitometry of the selected bands was determined using ImageJ software (NIH, Bethesda, MD).
Immunofluorescence
Immunofluorescence staining was performed as previously described [48]. Briefly, anesthetized mice were transcardially perfused with normal saline followed by 4% paraformaldehyde 4 days after CFA injection. Intact lumbar spinal cords were quickly removed and post-fixed in the same fixative. The spinal cords were then cryoprotected in 0.1 mol/L phosphate buffer containing 30% sucrose at 4 °C. Sections were cut at 20 μm on a freezing microtome. The sections were washed 3 times (10 min per wash) in PBS, then blocked in 5% goat serum containing 0.3% Triton X-100 for 1 h at RT. After incubation in the primary antibody overnight at 4 °C, sections were incubated with the secondary antibodies Alexa Fluor 488 goat anti-rabbit (1:500, Abways Technology, Shanghai, China) and Alexa Fluor 488 goat anti-mouse (1:500, Abways Technology). The primary antibodies were rabbit anti-IBa1 (1:2000, Abcam), mouse anti-GFAP (1:4000, Sigma-Aldrich, Darmstadt, Germany), mouse anti-NeuN (1:500, Millipore), rabbit anti-VGULT2 (1:400, Synaptic System). Rabbit anti-VGAT (1:500, Millipore), and mouse anti-Calretinin (CR) (1:500, Millipore). The sections were counterstained with DAPI to observe nuclei, and then cover-slipped. The images were acquired under a laser scanning confocal microscope (Nikon A1 Confocal System, Tokyo, Japan).
Statistical Analysis
Data are presented as the mean ± SEM. Data were statistically analyzed by one-way or two-way ANOVA of the LSD post-hoc test or Tukey’s test with the Statistical Package for Social Sciences 21.0. The statistical significance was determined as P < 0.05.
Results
Tα1 Alleviates CFA-Induced Pain Hypersensitivity
The CFA-induced pain model is widely used to investigate chronic inflammatory pain [49]. To explore whether Tα1 is able to alleviate such pain, we injected 30 μL CFA into the plantar surface of the left hind paw of mice followed by administration of Tα1. Initially, we performed dose-response studies for Tα1. When given Tα1, CFA-treated mice displayed a dramatic increase in mechanical withdrawal threshold in a dose-dependent manner from 50 μg/kg to 500 μg/kg (Fig. S1A). A significant increase in mechanical withdrawal threshold was detected at 200 μg/kg Tα1. Consistent with the Tα1 dose-dependence of mechanical withdrawal threshold, there was a significant decrease in mechanical response to the cotton swab and an increase in thermal latency at 200 μg/kg Tα1 (Fig. S1B and C), indicating that pain behavior is sensitive to the 200 μg/kg dose of Tα1. No differences in falling latencies measured by the rotarod test were found (Fig. S1D).
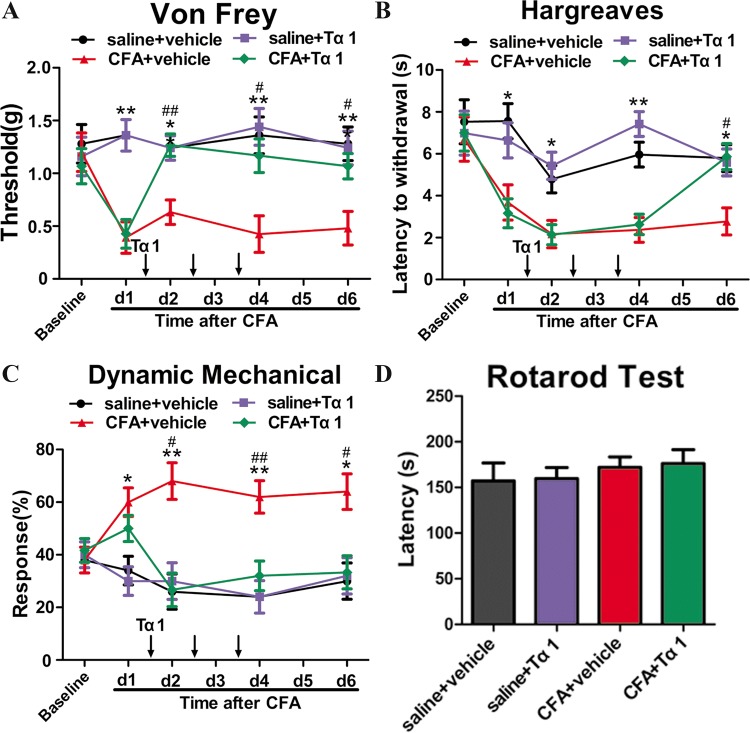
The first dose of Tα1 was given 1 day after CFA injection, followed by daily administration for 3 consecutive days. The pain behavior was assessed on days 1, 2, 4, and 6 after CFA injection. The mechanical allodynia was assessed by the “up-down” method and the cotton swab method. The mechanical withdrawal threshold showed a decrease after the first CFA injection while Tα1 reversed the change in threshold induced by CFA (P < 0.05, Fig. 2A) after the first Tα1 administration. However, Tα1 had no significant effect on the saline group (Fig. 2A). Likewise, CFA increased the mechanical response to the cotton swab, while Tα1 reduced the CFA-induced response (P < 0.05, Fig. 2C). Nonetheless, significant differences in responses between the saline + Tα1 group and the saline group were found (Fig. 2C).
Fig. 2.
Effect of i.p. injection of Tα1 on CFA-induced mechanical allodynia and thermal hyperalgesia in mice. A The mechanical withdrawal threshold in response to von Frey filaments in mice treated with saline + vehicle, saline + Tα1, CFA + vehicle, and CFA + Tα1. B The paw withdrawal latency to a noxious thermal beam in mice treated with saline + vehicle, saline + Tα1, CFA + vehicle, and CFA + Tα1. C The percentage of positive paw withdrawal in response to a cotton swab in mice treated with saline + vehicle, saline + Tα1, CFA + vehicle, and CFA + Tα1. D Falling latency in the rotarod test. Data are expressed as mean ± SEM (n = 10/group); *P < 0.05, **P < 0.01, CFA + vehicle vs saline + vehicle; #P < 0.05, ##P < 0.01, CFA + Tα1 vs CFA + vehicle.
To evaluate heat hyperalgesia, we measured thermal latency using the Hargreaves method. The thermal latency in the CFA group was lower than that in the saline group at 6 days after CFA injection (P < 0.05, Fig. 2B). Tα1 significantly increased the thermal latency only on the third day of treatment (P < 0.05, Fig. 2B). Tα1 had no influence on thermal latency in the saline group (Fig. 2B). The four groups showed no differences in falling latencies (Fig. 2D). These findings suggest that Tα1 is effective against chronic inflammatory pain, but does not significantly impact sensorimotor coordination in mice.
Tα1 Reduces CFA-Induced Upregulation of Pro-inflammatory Cytokines in the Spinal Cord and Inflamed Paw
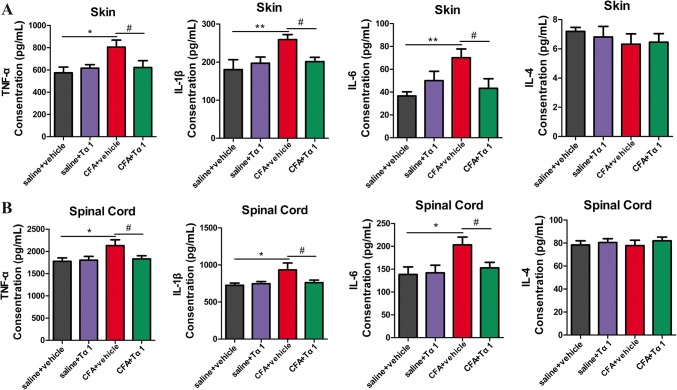
Microglia release multifarious pro-inflammatory cytokines that contribute to chronic pain [20, 26]. The protein expression of cytokines in the spinal cord and inflamed paw of CFA-induced inflammatory pain model mice were determined. Our data showed that the protein levels of TNF-α, IL-1β, and IL-6 were significantly up-regulated in the spinal cord and inflamed paw of mice in the CFA group compared with the saline group (paw: TNF-α, P < 0.05; IL-1β, P < 0.01; and IL-6, P < 0.01; spinal cord: TNF-α, P < 0.05; IL-1β, P < 0.05; and IL-6, P < 0.05, Fig. 3A and B). By contrast, the levels of TNF-α, IL-1β, and IL-6 in the CFA + Tα1 group were significantly decreased with respect to those in the CFA group after the last treatment with Tα1 (Fig. 3A, B). No differences in the expression of TNF-α, IL-1β, and IL-6 in the spinal cord and inflamed paw of mice were found between the saline alone and the saline + Tα1 groups (Fig. 3A, B). There was no difference in the expression level of IL-4 in the spinal cord and inflamed paw among the four groups (Fig. 3A, B). These results confirmed the anti-neuroinflammatory effect of Tα1 in vivo.
Fig. 3.
Effects of Ta1 on pro-inflammatory cytokines in skin and spinal cord. A Expression levels of TNF-α, IL-1β, IL-6, and IL-4 in inflamed skin tissue by ELISA. B Expression levels of TNF-α, IL-1β, IL-6, and IL-4 in spinal cord by ELISA. Data are expressed as the mean ± SEM (n = 6/group); *P < 0.05, **P < 0.01, CFA + vehicle vs saline + vehicle; #P < 0.05, CFA + Tα1 vs CFA + vehicle.
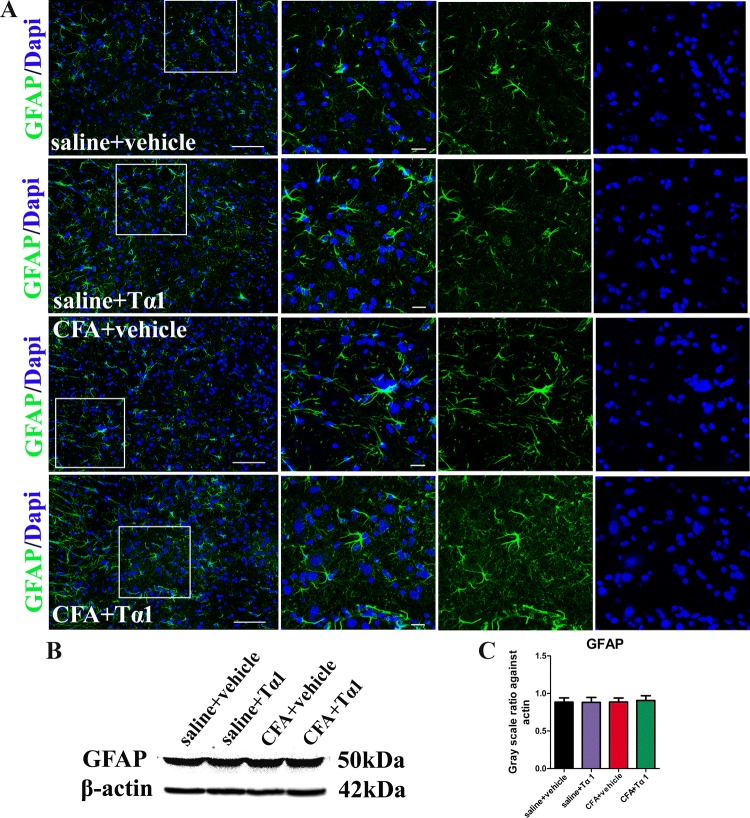
Tα1 Suppresses CFA-Induced Activation of Microglia, but Not GFAP
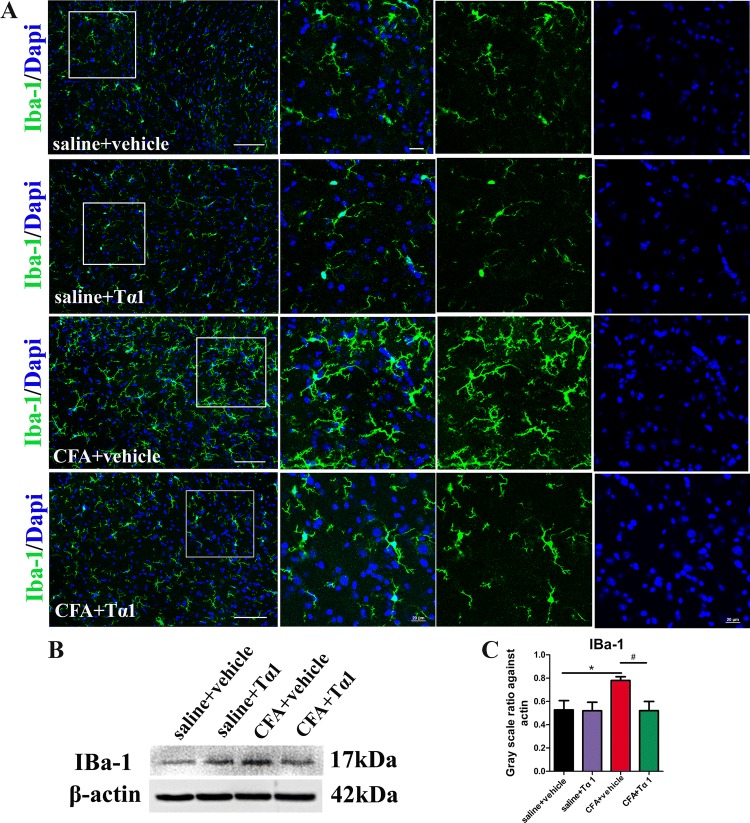
To determine whether the role of Tα1 in CFA-induced inflammatory pain is regulated via glial activation, the expression and morphology of astrocytes and microglia were studied in the spinal cord after the last Tα1 treatment. Astrocytes and microglia were labelled with immunofluorescence markers for GFAP and IBA-1. Immunostaining showed that microglia (Fig. 4A) and astrocytes (Fig. 5A) normally expressed fine, long processes in the saline group. In the CFA group, the microglia appeared to have extensively branched processes and hypertrophy of the cell body (Fig. 4A). However, the morphology of astrocytes was not changed by CFA (Fig. 5A). After treatment for 3 days, Tα1 inhibited the reactive state of microglia (Fig. 4A). There were no morphological differences for microglia (Fig. 4A) and astrocytes (Fig. 5A) between the saline and saline + Tα1 groups. Moreover, the protein expression level of IBA-1 (Fig. 4B, C; P < 0.05), but not GFAP (Fig. 5B, C), was significantly increased after CFA treatment. However, Tα1 treatment decreased IBA-1 expression (Fig. 4B, C; P < 0.05), but not GFAP expression (Fig. 5B, C), compared with the CFA group after the last treatment with Tα1. The results revealed that the CFA-induced inflammatory pain is closely associated with microglial activation [50], and that consecutive Tα1 treatment relieves microglial activation in the spinal cord.
Fig. 4.
Effect of i.p. injection of Tα1 on microglial activation induced by CFA. A IBa-1-immunoreactive cells in the spinal cord of mice treated with saline + vehicle, saline + Tα1, CFA + vehicle, and CFA + Tα1 (scale bars, 100 μm and 50 μm). B Representative gel images of IBa-1 in the spinal cord. C IBa-1 protein expression in the spinal cord. Data are expressed as mean ± SEM (n = 5/group); *P < 0.05, CFA + vehicle vs saline + vehicle; #P < 0.05, CFA + Tα1 vs CFA + vehicle.
Fig. 5.
Results for GFAP activation treated with a combination of Tα1 and CFA. A GFAP immunoreactivity in the spinal cord of mice treated with saline + vehicle, saline + Tα1, CFA + vehicle, and CFA + Tα1 (scale bars, 100 μm for the left panels and 50 μm for the right three-column panels). B Representative gel images of GFAP in the spinal cord. C GFAP expression in the spinal cord. Data are expressed as mean ± SEM (n = 5/group).
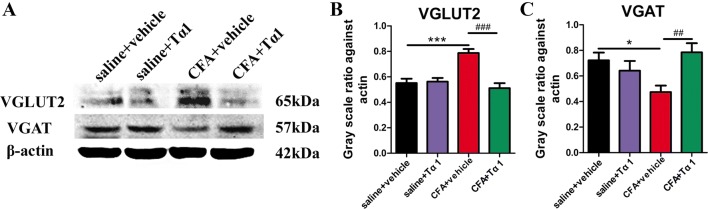
Tα1 Reverses CFA-Induced Expression of VGLUT2 and VGAT in Spinal Cord
There are findings that cytokines are involved in the modulation of excitatory and inhibitory synaptic transmission and activity in dorsal horn neurons [28]. VGLUT2 and VGAT (excitatory and inhibitory neuronal markers, respectively) are located in the spinal cord [51]. We co-stained for VGLUT2/NeuN and VGAT/CR to determine their localization. Immunofluorescence analysis showed co-expression of VGLUT2/NeuN and VGAT/CR in the dorsal horn (Fig. S2).
To further explore the effect of Tα1 on synaptic transmission in the spinal cord, the expression of VGLUT2 and VGAT after the last Tα1 treatment was determined by western blot analysis. VGLUT2 protein expression in the CFA group was increased relative to the saline group (Fig. 6A, B; P < 0.001), while this up-regulation was decreased by Tα1 treatment (Fig. 6A, B; P < 0.001). There was no significant difference between the saline + Tα1 group and the saline group. Western blot analysis demonstrated that the CFA group had lower expression of VGAT than the saline group (Fig. 6A, C; P < 0.05). VGAT expression was higher in the CFA + Tα1 group than in the CFA group (Fig. 6A, C; P < 0.01), and there was no difference in VGAT expression between the saline + Tα1 group and the saline group. Moreover, there was no difference in VGLUT1 expression among the four groups (Fig. S3).
Fig. 6.
Tα1 modulates the expression of VGLUT2 and VGAT induced by CFA in the mouse spinal cord. A Representative gel images of VGLUT2 and VGAT. B VGLUT2 protein levels. C VGAT protein levels. Data are expressed as mean ± SEM (n = 5/group); *P < 0.05, ***P < 0.001, CFA + vehicle vs saline + vehicle; ##P < 0.01, ###P < 0.001, CFA + Tα1 vs CFA + vehicle.
Discussion
We set out to investigate whether repetitive treatment with Tα1 contributes to CFA-induced inflammatory pain and explore the mechanisms involved. Our results demonstrated that consecutive treatment with Tα1 had an anti-nociceptive and anti-inflammatory effect in mice after CFA injection. Tα1 treatment attenuated pain hypersensitivity and the spinal activation of microglia, but not of astrocytes, induced by CFA. Tα1 decreased CFA-induced pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) in the inflamed hind paw and the spinal cord. Moreover, Tα1 inhibited the expression of VGLUT2 in the spinal cord, but reversed the decrease of VGAT expression induced by CFA. These data suggest that Tα1 might be effective in easing chronic inflammatory pain via non-neuronal and neuronal pathways associated with possible suppression of the inflammation response in mice.
Intraplantar injection of CFA is commonly used to investigate inflammatory pain because of the intense inflammation, fine reproducibility, and generally-accepted pain phenotype [2]. The location of CFA injection is primarily characterized by a local immune response [52] leading to a granulomatous type of inflammation [53]. Previous reports suggest that Tα1 plays an essential role in the diagnosis and cure of immune diseases [54]. Tα1 has also been used to enhance cognition via immune regulation [17]. In this study, repetitive Tα1 treatment at 200 μg/kg alleviated mechanical allodynia and heat hyperalgesia in the inflammatory pain model. It should be noted that repetitive, but not single, treatment with Tα1 suppressed chronic inflammatory pain. Intriguingly, when Tα1 was administered 1 day after CFA injection, an analgesic role for Tα1 appeared, and the effect persisted until 6 days after CFA injection with daily treatment for 3 consecutive days. However, the analgesic effect of Tα1 on heat hyperalgesia appeared on day 3, the last Tα1 treatment. These data suggest that Tα1 has a delayed effect on heat hyperalgesia.
Inflammatory pain arises from the activation and sensitization of nociceptors through inflammatory mediators [5]. The mediators are secreted by infiltrating and activating leukocytes in response to inflammatory stimuli such as viruses, parasites, and bacteria [55]. Cytokines may also directly activate nociceptors [56, 57]. Intraplantar injection of small doses of IL-1β induces a strong mechanical sensitization of nociceptors [5, 58]. TNF-α is recognized as an effective pro-inflammatory factor, and is also rapidly produced by macrophages in response to a variety of inflammatory stimuli. Moreover, TNF-α-induced inflammatory hyper-nociception is attenuated by deletion of the TNFR1 gene, and blockade of IL-6 is also an alternative route to control the inflammatory sensitization of nociceptors [5]. In addition, IL-1β has been implicated in mediating the TNF-α-induced thermal and mechanical sensitization of nociceptors induced by intraplantar administration of CFA [59]. Our data showed that TNF-α, IL-1β, and IL-6 expression increased in the inflamed skin after intraplantar injection of CFA. This result is consistent with previous findings that showed the expression levels of these cytokines are increased in the paws of rats subsequently given the antigen [60]. Meanwhile, consecutive treatment with Tα1 suppressed the up-regulation of these pro-inflammatory cytokines induced by CFA.
Glial activity is involved in multiple pain-associated pathways in response to peripheral injury [53, 61, 62]. Microglia are activated in response to pathological events in the CNS [18, 63]. Activated microglia demonstrate amoeboid morphology, increased expression of cell markers, and functional changes (including production or release of pro-inflammatory cytokines) [27]. In CFA-injected rats, elevated expression of Mac-1 mRNA and enhanced immunoreactivity of OX-42 at various phases of inflammation have been reported in the spinal cord during the acute, subacute, and chronic phases of CFA-induced peripheral inflammation [50]. Accumulating evidence suggests that spinal microglia play a crucial role in the development and maintenance of inflammatory pain and neuropathic pain [64, 65]. An antagonist of microglial activation, minocycline is significantly more potent in delaying the initiation of mechanical allodynia [66]. Our results are consistent with studies showing that the protein expression level of IBA-1 is up-regulated by CFA. And Tα1 not only attenuated pain hypersensitivity and the release of pro-inflammatory cytokines in skin, but also suppressed the IBA-1 protein expression and progression of amoeboid morphology induced by CFA.
Astrocytes perform a variety of functions in the modulation of nearby neuronal activity [67]. When lesions are produced by multiple types of stimuli, astrocytes undergo modification with massive hyperplasia of the cell bodies and cytoplasmic branches, and the biochemical marker of astrogliosis exhibits over-expression of GFAP. Our data showed that the protein expression level of GFAP was not increased by CFA, and Tα1 did not affect astrocyte morphology or GFAP expression. Previous research has established that astrocyte activation is initiated just after the subacute phase, while microglia activation occurs after the acute phase of peripheral inflammation, which indicates that astrocytes are activated after microglia activation following peripheral inflammation in the CNS [53]. In our study, astrocytes might not be activated in the early stage of CFA-induced pain. Moreover, the dose of intraplantar CFA injection in this study differed from that of previous studies [50, 68], which may result in different GFAP expression after CFA. Our data suggest that the anti-nociceptive influence of Tα1 probably derives from the blockade of microglial activation.
Glia are activated to induce pain hypersensitivity possibly through the production of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 [27]. Inhibiting TNF-α, IL-1β, and IL-6 by gene-knockout or intrathecal injection of neutralizing antibody alleviates inflammatory pain [29, 66, 69]. Furthermore, intrathecal delivery of exogenous TNF-α, IL-1β, and IL-6 induces the development and maintenance of mechanical allodynia and thermal hyperalgesia [28, 69, 70]. Ligustilide has been reported to exert anti-nociceptive effects by inhibition of microglial activation and the production of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) [71]. In line with these findings, we also found that the expression of pro-inflammatory cytokines and microglial activation paralleled the pain hypersensitivity following intraplantar injection of CFA. The results indicate that pro-inflammatory cytokines derived from activated microglia may initiate and maintain pain behavior following the peripheral stimuli of inflammation, but the effect is attenuated by treatment with Tα1. Surprisingly, neither Tα1 nor CFA affected IL-4 expression, suggesting that IL-4 is not associated with the analgesic and anti-inflammatory effects of Tα1 on CFA-induced inflammatory pain. We speculate that Tα1 suppresses inflammatory pain through a decrease of pro-inflammatory cytokines.
Central sensitization, defined as enhanced synaptic transmission in dorsal horn neurons, has been demonstrated to contribute to chronic pain [40, 41, 72]. Both increased excitatory and reduced inhibitory synaptic transmission induce central sensitization. In addition, failure of inhibitory synaptic transmission, or dis-inhibition, has been suggested to be a critical mechanism for neuropathic pain sensitization [37, 73]. Patch-clamp recordings in spinal neurons have shown that the frequency of spontaneous excitatory postsynaptic currents (sEPSCs) is enhanced by TNF-α and that IL-6 decreases the frequency of spontaneous inhibitory postsynaptic currents (sIPSCs), but IL-1β increases the frequency and amplitude of sEPSCs and reduces the frequency and amplitude of sIPSCs [28]. The results demonstrated that pro-inflammatory cytokines give rise to central sensitization via enhanced excitatory or attenuated inhibitory synaptic transmission in dorsal horn neurons. The frequency of sEPSCs is strongly enhanced in lamina II neurons after TNF-α treatment because of increasing glutamate release from presynaptic terminals [8], and TNF-α may induce central sensitization by increasing glutamate release from presynaptic terminals [41]. In addition, GABA and glycine, acting as fast inhibitory neurotransmitters, exert inhibitory control over spinal neurons through both presynaptic and postsynaptic mechanisms [74]. VGLUT2 and VGAT act as transporters of packaged glutamate and GABA and glycine, respectively. The expression and localization patterns of VGLUT2 and VGAT in the spinal cord are consistent with the nociceptive pathway, suggesting that they are associated with the transmission of pain signals [35, 74–76]. In the present study, western blot analysis showed increased VGLUT2 and VGAT expression in the CFA group that was paralleled by up-regulation of pro-inflammatory cytokines in the spinal cord and the initiation of pain-related behavior. Nevertheless, these effects were attenuated by Tα1. In addition, co-expression of VGLUT2/NeuN and VGAT/CR was shown by immunofluorescence, demonstrating the expression of VGLUT2 and VGAT in neurons of the dorsal horn. These data suggest that Tα1 reverses the CFA-induced mechanical allodynia and thermal hyperalgesia, possibly via VGLUT2 and VGAT expression indirectly mediated by pro-inflammatory cytokines released by microglia.
In summary, the present study demonstrated that systematic delivery of Tα1 significantly attenuated the mechanical allodynia and thermal hyperalgesia induced by CFA. The analgesic role of Tα1 may involve the blockade of microglial activation and the production of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) as well as the modulation of VGLUT2 and VGAT expression. These findings provide support for the idea that alleviation of Tα1-induced inflammatory pain may be related to the down-regulation of pro-inflammatory cytokines in the inflamed skin and the spinal cord and the blockade of microglial activation in addition to modulation of VGLUT2 and VGAT expression in the spinal cord.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the Foundation for Distinguished Young Talents in Higher Education of Guangdong Province, China (2016KQNCX019 and 2016KQNCX027), the National Natural Science Foundation of China (31571041), the Guangdong Provincial Department of Education Innovating Strong National Engineering Major Project (2014GKXM031), and Guangdong Provincial Universities and Colleges Pearl River Scholar Funded Scheme (2016).
Conflict of interest
The authors claim that there are no conflicts of interest.
Contributor Information
Yunlong Xu, Email: yunlongxu69@outlook.com.
Yongjun Chen, Email: ychen@gzucm.edu.cn.
References
- 1.Goldberg DS, McGee SJ. Pain as a global public health priority. BMC Public Health. 2011;11:770. doi: 10.1186/1471-2458-11-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghasemlou N, Chiu IM, Julien JP, Woolf CJ. CD11b + Ly6G- myeloid cells mediate mechanical inflammatory pain hypersensitivity. Proc Natl Acad Sci U S A. 2015;112:E6808–E6817. doi: 10.1073/pnas.1501372112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kidd BL, Urban LA. Mechanisms of inflammatory pain. Br J Anaesth. 2001;87:3–11. doi: 10.1093/bja/87.1.3. [DOI] [PubMed] [Google Scholar]
- 4.Mifflin KA, Kerr BJ. The transition from acute to chronic pain: understanding how different biological systems interact. Can J Anaesth. 2014;61:112–122. doi: 10.1007/s12630-013-0087-4. [DOI] [PubMed] [Google Scholar]
- 5.Verri WA Jr, Cunha TM, Parada CA, Poole S, Cunha FQ, Ferreira SH. Hypernociceptive role of cytokines and chemokines: targets for analgesic drug development? Pharmacol Ther 2006, 112: 116–138. [DOI] [PubMed]
- 6.Cunha TM, Verri WA, Jr, Silva JS, Poole S, Cunha FQ, Ferreira SH. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc Natl Acad Sci U S A. 2005;102:1755–1760. doi: 10.1073/pnas.0409225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: Peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361:184–187. doi: 10.1016/j.neulet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Xu ZZ, Zhang L, Liu T, Park JY, Berta T, Yang R, et al. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 2010;16:592–597. doi: 10.1038/nm.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Leo JA, Tanga FY, Tawfk VL. Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist 2004, 10: 40–52. [DOI] [PubMed]
- 10.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 11.Xpudala L. Interactions between the immune and nervous systems in pain. Nat Med. 2010;16:1267–1276. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang CP, Chen HN, Su HL, Hsieh CL, Chen WH, Lai ZR, et al. Electroacupuncture reduces carrageenan- and CFA-induced inflammatory pain accompanied by changing the expression of Nav1.7 and Nav1.8, rather than Nav1.9, in mice dorsal root ganglia. Evid Based Complement Alternat Med 2013, 2013: 312184. [DOI] [PMC free article] [PubMed]
- 13.Goldstein AL, Goldstein AL. From lab to bedside: emerging clinical applications of thymosin alpha 1. Expert Opin Biol Ther. 2009;9:593–608. doi: 10.1517/14712590902911412. [DOI] [PubMed] [Google Scholar]
- 14.Romani L, Bistoni F, Perruccio K, Montagnoli C, Gaziano R, Bozza S, et al. Thymosin alpha1 activates dendritic cell tryptophan catabolism and establishes a regulatory environment for balance of inflammation and tolerance. Blood. 2006;108:2265–2274. doi: 10.1182/blood-2006-02-004762. [DOI] [PubMed] [Google Scholar]
- 15.Turrini P, Aloe L. Evidence that endogenous thymosin alpha-1 is present in the rat central nervous system. Neurochem Int. 1999;35:463–470. doi: 10.1016/S0197-0186(99)00084-4. [DOI] [PubMed] [Google Scholar]
- 16.Yang S, Liu ZW, Zhou WX, Zhang YX. Thymosin alpha-1 modulates excitatory synaptic transmission in cultured hippocampal neurons in rats. Neurosci Lett. 2003;350:81–84. doi: 10.1016/S0304-3940(03)00862-0. [DOI] [PubMed] [Google Scholar]
- 17.Wang G, He F, Xu Y, Zhang Y, Wang X, Zhou C, et al. Immunopotentiator thymosin alpha-1 promotes neurogenesis and cognition in the developing mouse via a systemic Th1 bias. Neurosci Bull. 2017;33:675–684. doi: 10.1007/s12264-017-0162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 19.Hansson E. Could chronic pain and spread of pain sensation be induced and maintained by glial activation? Acta Physiol (Oxf) 2006;187:321–327. doi: 10.1111/j.1748-1716.2006.01568.x. [DOI] [PubMed] [Google Scholar]
- 20.Cunningham C. Microglia and neurodegeneration: the role of systemic inflammation. Glia. 2012;61:71–90. doi: 10.1002/glia.22350. [DOI] [PubMed] [Google Scholar]
- 21.Ellrichmann G, Reick C, Saft C, Linker RA. The role of the immune system in Huntington’s disease. Clin Dev Immunol. 2013;2013:541259. doi: 10.1155/2013/541259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rizzo F, Riboldi G, Salani S, Nizzardo M, Simone C, Corti S, et al. Cellular therapy to target neuroinflammation in amyotrophic lateral sclerosis. Cell Mol Life Sci. 2014;71:999–1015. doi: 10.1007/s00018-013-1480-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raghavendra V, Tanga FY, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. 2003;306:624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- 24.Raghavendra V, Tanga F, Rutkowski MD, DeLeo JA. Antihyperalgesic and morphine sparing actions of propentofylline following nerve injury in rats: mechanistic implications of spinal glia and proinflammatory cytokines. Pain. 2003;104:655–664. doi: 10.1016/S0304-3959(03)00138-6. [DOI] [PubMed] [Google Scholar]
- 25.Mika J, Zychowska M, Popiolek-Barczyk K, Rojewska E, Przewlocka B. Importance of glial activation in neuropathic pain. Eur J Pharmacol. 2013;716:106–119. doi: 10.1016/j.ejphar.2013.01.072. [DOI] [PubMed] [Google Scholar]
- 26.Watkins LR, Maier SF. Glia: a novel drug discovery target for clinical pain. Nat Rev Drug Discov. 2003;2:973–985. doi: 10.1038/nrd1251. [DOI] [PubMed] [Google Scholar]
- 27.Sweitzer SM, Colburn RW, Rutkowski M, DeLeo JA. Acute peripheral inflammation induces moderate glial activation and spinal IL-1β expression that correlates with pain behavior in the rat. Brain Res. 1999;829:209–221. doi: 10.1016/S0006-8993(99)01326-8. [DOI] [PubMed] [Google Scholar]
- 28.Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28:5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Berta T, Xu ZZ, Liu T, Park JY, Ji RR. TNF-alpha contributes to spinal cord synaptic plasticity and inflammatory pain: distinct role of TNF receptor subtypes 1 and 2. Pain. 2011;152:419–427. doi: 10.1016/j.pain.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo W, Wang H, Watanabe M, Shimizu K, Zou S, LaGraize SC, et al. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J Neurosci. 2007;27:6006–6018. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie W, Strong JA, Zhang JM. Early blockade of injured primary sensory afferents reduces glial cell activation in two rat neuropathic pain models. Neuroscience 2009, 160: 847–857. [DOI] [PMC free article] [PubMed]
- 32.Vale ML, Marques JB, Moreira CA, Rocha FA, Ferreira SH, Poole S, et al. Antinociceptive effects of interleukin-4, -10, and -13 on the writhing response in mice and zymosan-induced knee joint incapacitation in rats. J Pharmacol Exp Ther. 2003;304:102–108. doi: 10.1124/jpet.102.038703. [DOI] [PubMed] [Google Scholar]
- 33.Fremeau RTJ, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Brumovsky P, Watanabe M, Hökfelt T. Expression of the vesicular glutamate transporters-1 and -2 in adult mouse dorsal root ganglia and spinal cord and their regulation by nerve injury. Neuroscience. 2007;147:469–490. doi: 10.1016/j.neuroscience.2007.02.068. [DOI] [PubMed] [Google Scholar]
- 35.Landry M, Bouali-Benazzouz R, El Mestikawy S, Ravassard P, Nagy F. Expression of vesicular glutamate transporters in rat lumbar spinal cord, with a note on dorsal root ganglia. J Comp Neurol. 2004;468:380–394. doi: 10.1002/cne.10988. [DOI] [PubMed] [Google Scholar]
- 36.Aubrey KR. Presynaptic control of inhibitory neurotransmitter content in VIAAT containing synaptic vesicles. Neurochem Int. 2016;98:94–102. doi: 10.1016/j.neuint.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002;22:6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muller F, Heinke B, Sandkuhler J. Reduction of glycine receptor mediated miniature inhibitory postsynaptic currents in rat spinal lamina I neurons after peripheral inflammation. Neuroscience. 2003;122:799–805. doi: 10.1016/j.neuroscience.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 39.Harvey RJ, Depner UB, Wassle H, Ahmadi S, Heindl C, Reinold H, et al. GlyR alpha3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science. 2004;304:884–887. doi: 10.1126/science.1094925. [DOI] [PubMed] [Google Scholar]
- 40.Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 41.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 42.Romani L, Oikonomou V, Moretti S, Iannitti RG, D’Adamo MC, Villella VR. Thymosin α1 represents a potential potent single-molecule-based therapy for cystic fibrosis. Nat Med. 2017;23:590–600. doi: 10.1038/nm.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang W, Gu J, Li YQ, Tao YX. Are voltage-gated sodium channels on the dorsal root ganglion involved in the development of neuropathic pain? Mol Pain. 2011;7:16. doi: 10.1186/1744-8069-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.La Porta C, Bura SA, Aracil-Fernández A, Manzanares J, Maldonado R. Role of CB1 and CB2 cannabinoid receptors in the development of joint pain induced by monosodium iodoacetate. Pain. 2013;154:160–174. doi: 10.1016/j.pain.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 45.Garrison SR, Dietrich A, Stucky CL. TRPC1 contributes to light-touch sensation and mechanical responses in low-threshold cutaneous sensory neurons. J Neurophysiol. 2011;107:913–922. doi: 10.1152/jn.00658.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duan B, Cheng L, Bourane S, Britz O, Padilla C, Garcia-Campmany L, et al. Identification of spinal circuits transmitting and gating mechanical pain. Cell. 2014;159:1417–1432. doi: 10.1016/j.cell.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao F, Xiang HC, Li HP, Jia M, Pan XL, Pan HL, et al. Electroacupuncture inhibits NLRP3 inflammasome activation through CB2 receptors in inflammatory pain. Brain Behav Immun. 2018;67:91–100. doi: 10.1016/j.bbi.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 48.Cui L, Miao X, Liang L, Abdus-Saboor I, Olson W, Fleming MS, et al. Identification of early RET+ deep dorsal spinal cord interneurons in gating pain. Neuron. 2016;91:1413. doi: 10.1016/j.neuron.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 49.Du JY, Fang JQ, Liang Y, Fang JF. Electroacupuncture attenuates mechanical allodynia by suppressing the spinal JNK1/2 pathway in a rat model of inflammatory pain. Brain Res Bull. 2014;108:27–36. doi: 10.1016/j.brainresbull.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 50.Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glialactivation and proinflammatory cytokine expression in the CNS. Eur J Neurosci. 2004;20:467–473. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- 51.Cui L, Miao X, Liang L, Abdus-Saboor I, Olson W, Fleming MS, et al. Identification of early RET+ deep dorsal spinal cord interneurons in gating pain. Neuron. 2016;91:1413. doi: 10.1016/j.neuron.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 52.Wiedemann F1, Link R, Pumpe K, Jacobshagen U, Schaefer HE, Wiesmüller KH, et al. Histopathological studies on the local reactions induced by complete Freund’s adjuvant (CFA), bacterial lipopolysaccharide (LPS), and synthetic lipopeptide (P3C) conjugates. J Pathol 1991, 164: 265–271. [DOI] [PubMed]
- 53.Shah NM, Mangat GK, Balakrishnan C, Buch VI, Joshi VR. Accidental selfinjection with Freund’s complete adjuvant. J Assoc Physicians India. 2001;49:366–368. [PubMed] [Google Scholar]
- 54.Ancell CD, Phipps J, Young L. Thymosin alpha-1. Am J Health Syst Pharm. 2001;58:879–885. doi: 10.1093/ajhp/58.10.879. [DOI] [PubMed] [Google Scholar]
- 55.Miller RJ, Jung H, Bhangoo SK, White FA. Cytokine and chemokine regulation of sensory neuron function. Handbook Exp Pharmacol. 2009;194:417–449. doi: 10.1007/978-3-540-79090-7_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parada CA, Yeh JJ, Joseph EK, Levine JD. Tumor necrosis factor receptor type-1 in sensory neurons contributes to induction of chronic enhancement of inflammatory hyperalgesia in rat. Eur J Neurosci. 2003;17:1847–1852. doi: 10.1046/j.1460-9568.2003.02626.x. [DOI] [PubMed] [Google Scholar]
- 57.Obreja O, Rathee PK, Lips KS, Distler C, Kress M. IL-1 beta potentiates heat-activated currents in rat sensory neurons: involvement of IL-1RI, tyrosine kinase, and protein kinase C. FASEB J. 2002;16:1497–1503. doi: 10.1096/fj.02-0101com. [DOI] [PubMed] [Google Scholar]
- 58.Ferreira SH, Lorenzetti BB, Bristow AF, Poole S. Interleukin-1 beta as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature. 1988;334:698–700. doi: 10.1038/334698a0. [DOI] [PubMed] [Google Scholar]
- 59.Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. Br J Pharmacol. 1997;121:417–424. doi: 10.1038/sj.bjp.0701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cunha JM, Sachs D, Canetti CA, Poole S, Ferreira SH, Cunha FQ. The critical role of leukotriene B4 in antigen-induced mechanical hyperalgesia in immunised rats. Br J Pharmacol. 2003;139:1135–1145. doi: 10.1038/sj.bjp.0705346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wei F, Guo W, Zou S, Ren K, Dubner R. Supraspinal glial-neuronal interactions contribute to descending pain facilitation. J Neurosci. 2008;28:10482–10495. doi: 10.1523/JNEUROSCI.3593-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsuda M. Modulation of pain and itch by spinal glia. Neurosci Bull. 2018;34:178–185. doi: 10.1007/s12264-017-0129-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vila M, Jackson-Lewis V, Guegan C, Wu D, Teismann P, Choi DK, et al. The role of glial cells in Parkinsons’s disease. Curr Opin Neurol. 2001;14:483–489. doi: 10.1097/00019052-200108000-00009. [DOI] [PubMed] [Google Scholar]
- 64.Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther. 2010;126:56–68. doi: 10.1016/j.pharmthera.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schomberg D, Olson JK. Immune responses of microglia in the spinal cord: contribution to pain states. Exp Neurol. 2012;234:262–270. doi: 10.1016/j.expneurol.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 66.Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, et al. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115:71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 67.Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- 68.Liao HY, Hsieh CL, Huang CP, Lin YW. Electroacupuncture attenuates CFA-induced inflammatory pain by suppressing Nav1.8 through S100B, TRPV1, opioid, and adenosine pathways in mice. Sci Rep 2017, 7: 42531. [DOI] [PMC free article] [PubMed]
- 69.Reeve AJ, Patel S, Fox A, Walker K, Urban L. Intrathecally administered endotoxin or cytokines produce allodynia, hyperalgesia and changes in spinal cord neuronal responses to nociceptive stimuli in the rat. Eur J Pain 2000, 4: 247–257. [DOI] [PubMed]
- 70.Sung CS, Wen ZH, Chang WK, Ho ST, Tsai SK, Chang YC, et al. Intrathecal interleukin-1beta administration induces thermal hyperalgesia by activating inducible nitric oxide synthase expression in the rat spinal cord. Brain Res. 2004;1015:145–153. doi: 10.1016/j.brainres.2004.04.068. [DOI] [PubMed] [Google Scholar]
- 71.Zhu MD, Zhao LX, Wang XT, Gao YJ, Zhang ZJ. Ligustilide inhibits microglia-mediated proinflammatory cytokines production and inflammatory pain. Brain Res Bull. 2014;109:54–60. doi: 10.1016/j.brainresbull.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 72.Duan B, Cheng L, Ma Q. Spinal circuits transmitting mechanical pain and itch. Neurosci Bull. 2018;34:186–193. doi: 10.1007/s12264-017-0136-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zeilhofer HU. The glycinergic control of spinal pain processing. Cell Mol Life Sci. 2005;62:2027–2035. doi: 10.1007/s00018-005-5107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Torsney C, Macdermott AB. Disinhibition opens the gate to pathological pain signaling in superficial neurokinin 1 receptor-expressing neurons in rat spinal cord. J Neurosci. 2006;26:1833–1843. doi: 10.1523/JNEUROSCI.4584-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Malet M, Vieytes CA, Lundgren KH, Seal RP, Tomasella E, Seroogy KB, et al. Transcript expression of vesicular glutamate transporters in lumbar dorsal root ganglia and the spinal cord of mice-effects of peripheral axotomy or hindpaw inflammation. Neuroscience. 2013;248:95–111. doi: 10.1016/j.neuroscience.2013.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang ZT, Yu G, Wang HS, Yi SP, Su RB, Gong ZH. Changes in VGLUT2 expression and function in pain-related supraspinal regions correlate with the pathogenesis of neuropathic pain in a mouse spared nerve injury model. Brain Res. 2015;1624:515–524. doi: 10.1016/j.brainres.2015.08.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.