Abstract
Hyperhomocysteinemia (Hhcy) is an independent risk factor for Alzheimer’s disease (AD), and insulin-resistance is commonly seen in patients with Hhcy. Liraglutide (Lir), a glucagon-like peptide that increases the secretion and sensitivity of insulin, has a neurotrophic or neuroprotective effect. However, it is not known whether Lir ameliorates the AD-like pathology and memory deficit induced by Hhcy. By vena caudalis injection of homocysteine to produce the Hhcy model in rats, we found here that simultaneous administration of Lir for 2 weeks ameliorated the Hhcy-induced memory deficit, along with increased density of dendritic spines and up-regulation of synaptic proteins. Lir also attenuated the Hhcy-induced tau hyperphosphorylation and Aβ overproduction, and the molecular mechanisms involved the restoration of protein phosphatase-2A activity and inhibition of β- and γ-secretases. Phosphorylated insulin receptor substrate-1 also decreased after treatment with Lir. Our data reveal that Lir improves the Hhcy-induced AD-like spatial memory deficit and the mechanisms involve the modulation of insulin-resistance and the pathways generating abnormal tau and Aβ.
Keywords: Liraglutide, Hyperhomocysteinemia, Glucagon-like peptide-1 receptor, Tau, β-Amyloid
Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative disorder characterized by the accumulation of senile plaques composed of amyloid beta (Aβ) peptide and neurofibrillary tangles of hyperphosphorylated tau proteins [1, 2]. A symptom of AD is progressive memory loss [3]. A growing body of clinical and basic studies have demonstrated that elevation of plasma homocysteine (Hcy) leading to hyperhomocysteinemia (Hhcy) is an independent and high risk factor for AD [4, 5]. Hhcy induces AD-like β-amyloid peptide accumulation and increases tau hyperphosphorylation in rats [6]. Further, Hhcy impairs DNA repair in hippocampal neurons and makes them more vulnerable to amyloid toxicity [7]. In addition, Hhcy impairs synaptic plasticity by reducing synapse-associated proteins and long-term potentiation [8].
Glucagon-like peptide-1 (GLP-1) is produced by a discrete population of hindbrain neurons [9, 10]. It acts through a G-protein–coupled receptor glucagon-like peptide-1 receptor (GLP-1R) expressed in central and peripheral neurons [11]. Previous studies have reported that GLP-1R-knockout mice are deficient in spatial learning and show impairments in synaptic plasticity and cognitive performance, while overexpression of GLR-1R in the rat hippocampus improves learning and memory [12, 13]. In addition, compared to young animals, GLP-1R immunoreactivity and its protein level are lower in adult and aged gerbils [14]. These data suggest an association of GLP-1R activity or expression with cognitive capacities.
Liraglutide (Lir), a novel long-lasting incretin mimetic, is a GLP-1 analog that has been used to treat type 2 diabetes mellitus [15, 16]. Accumulating evidence indicates that GLP-1 is neurotrophic and neuroprotective, and its analogs protect neurons from glutamate toxicity in vitro, reduce the level of Aβ, and decrease the hyperphosphorylation level of tau protein in the brain [17, 18]. Previous reports suggest that Lir reverses the memory impairment and synaptic loss and reduces the plaque loading in the hippocampus and cortex of aged amyloid precursor protein/presenilin 1 (APP/PS1) mice [19]. GLP-1 appears to improve insulin sensitivity and glucose uptake in both human and rat adipose tissue and skeletal muscle [20]. Currently, the effect of liraglutide on pathology induced by Hhcy has not been reported.
Therefore, we designed experiments to investigate the effects of liraglutide on the AD-like symptoms and underlying mechanisms in a rat model of Hhcy.
Materials and Methods
Animals and Drug Administration
Male Sprague-Dawley rats (250 ± 20 g) supplied by the Experimental Animal Center of Tongji Medical College, Huazhong University of Science and Technology, were housed with free access to food and water under a 12:12 h reversed light:dark cycle. The rats were injected via the vena caudalis with hcy (400 μg/kg per day) or normal saline (Ctrl) for 14 days; in the Hcy groups, 150 μg/kg, 300 μg/kg, or 450 μg/kg per 12 h of Lir, or saline was simultaneously administered via subcutaneous injection for 14 days. The Hcy was injected each day between 09:00 and 11:30, and Lir was injected twice (08:00 and 20:00). The animals were sacrificed after being anesthetized by 6% chloral hydrate 24 h after assessment of spatial memory. All animal experiments were performed according to the ‘Policies on the Use of Animals and Humans in Neuroscience Research’ revised and approved by the Society for Neuroscience in 1995, and approved by the Academic Review Board of Tongji Medical College, Huazhong University of Science and Technology.
Morris Water Maze Test
The standard Morris water maze procedure was used with minor modifications [21]. Briefly, the rats (n = 10/group) received hcy and simultaneous Lir injection for 14 days and were trained to find a hidden platform in water maze for five consecutive days, with four trials/day at 30-min intervals. The rats were allowed to search for the platform for up to 60 s, after which they were guided to the platform and allowed to stay on it for 20 s. In each trial, the swimming pathway and latency to locate the platform were recorded using a video tracking system (Zhenghua, Anhui, China). In the probe trials measured on day 7, rats were allowed to swim for 60 s after the hidden platform had been removed. The number of times the platform was crossed, the time to first reach the former location of the platform (latency), and the swimming path were recorded.
Oral Glucose Tolerance Test (OGTT)
The OGTT was performed on rats that had fasted for 12 h on day 15 (the last day of the Hcy injections). The rats (10/group) were given by gavage a 40% glucose solution (2 g/kg body weight). Blood was collected from the tail vein 0, 60, and 120 min after glucose loading, and the glucose level was measured.
Golgi-Cox Staining
Golgi-Cox staining was carried out as previously reported [22]. In brief, the rats (2/group) were killed by overdose of chloral hydrate (1 g/kg), and then the whole brain was immersed in Golgi-Cox solution (5% potassium chromate, 5% potassium dichromate, and 5% chloral hydrate) in the dark for 10 days. The brains were transferred to tissue-protectant solution for another 7 days. Coronal sections through the hippocampus were cut at 75 μm on a vibrating microtome (VT1000 S; Leica, Germany). All the sections were developed using ammonia, then dehydrated and mounted. Images were captured on a microscope (Olympus BX60, Tokyo, Japan)
Enzyme-Linked Immune-Sorbent Assay (ELISA)
Plasma Hcy, insulin, and Aβ were analyzed as previously described [7], and six hippocampi per group were used for ELISA. To assess the concentration of Aβ in hippocampal lysates, they were homogenized in buffer (phosphate-buffered saline with 1% phenylmethylsulfonyl fluoride [PMSF], supplemented with protease inhibitor cocktail), and centrifuged at 12,000 g for 10 min. To measure the concentration of plasma Hcy, blood samples were drawn by heart puncture and promptly centrifuged at 2000 g for 15 min at 4 °C, and the supernatant was stored at −80 °C. Aβ40, Aβ42, and insulin were quantified using ELISA kits for rat insulin, Aβ1-40, Aβ1-42 (Elabscience, Wuhan, China), and rat Hcy (Cusabio, Wuhan, China) in accordance with the manufacturers’ instructions. The Aβ and Hcy concentrations were determined by comparison with standard curves.
Immunohistochemical Staining
For immunohistochemical studies, the rats (2/group) were sacrificed by overdose of chloral hydrate (1 g/kg) and perfused through the aorta with 100 mL 0.9% NaCl followed by 400 mL phosphate buffer containing 4% paraformaldehyde. The brain was removed and post-fixed in 4% paraformaldehyde overnight. After embedding in wax, it was sectioned at 3 μm on a microtome (Leica, Nussloch, Germany). After dewaxing and antigen retrieval, the sections were blocked with 0.3% H2O2 in phosphate buffered saline for 30 min and nonspecific sites were blocked with bovine serum albumin for 30 min at room temperature. Sections were then incubated with primary antibodies overnight at 4 °C. The immunoreaction was developed using horseradish peroxidase kits and visualized with diaminobenzidine (brown). Images were captured on a light microscope (Olympus BX60, Tokyo, Japan).
Western Blotting
After sacrifice, the brain was rapidly removed and the hippocampi were separated and stored in −80 °C. The hippocampus tissue (n = 6/group) was homogenized with RIPA buffer (50 mmol/L Tris-HCl [pH 7.5], 150 mmol/L NaCl, 1% Na-deoxycholate, 500 mmol/L EDTA [pH 8.0], 0.4% NaF, and 1% Nonidet P40, supplemented with protease inhibitor cocktail and 1% PMSF) and the protein concentrations measured by the BCA method. Samples of equal amounts of protein were loaded and separated on 10% SDS-PAGE gel and transferred to nitrocellulose membranes. The membranes were blocked with 5% nonfat milk in Tris-buffered saline (pH 7.5) for 1 h at room temperature, and incubated with primary antibodies overnight at 4 °C. The blots were then incubated with secondary antibodies (1:10000, IRDyeTM 800/IRDyeTM 800CW) for 2 h. Signals were detected using a Li-Cor Odyssey Infrared Imaging System, and the bands were analyzed with ImageJ. The primary antibodies were rabbit anti-PSD95, Synapsin I GluA1, GluA2, GluN2A, GluN2B, pS668 APP, and GLP-1R (1:1000, Abcam, UK) and rabbit-anti-pTau Thr231, Ser396, Ser404, Thr205, rabbit-anti-pS312 insulin receptor substrate (IRS), IRS (1:500, Signalway Antibody, College Park, MD), rabbit-anti-pPP2Ac(307) (catalytic subunit of protein phosphatase 2A), demethylated PP2Ac (Dm PP2Ac), PP2Ac (1:1000, Millipore, Billerica, MA), rabbit-anti-APP, β-secretase 1 (BACE1), ADAM10, and presenilin 1 (PSEN1) (1:1000, Proteintech, Chicago, IL).
Statistical Analysis
All results were obtained from repeated experiments and are presented as mean ± SEM. Two-way ANOVA was used for comparisons. ImageJ, SPSS 15.0, and GraphPad Prism 5.0 were used for data analysis. The results were considered to have significant differences when P < 0.05.
Results
Lir Ameliorates Hhcy-Induced Memory Deficits
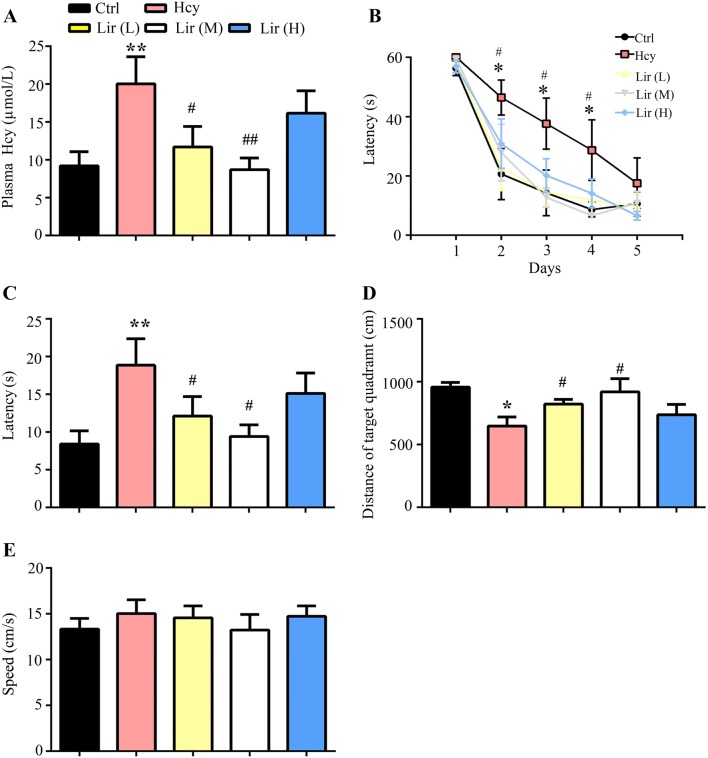
To produce an animal model with AD-like pathology, rats were injected with Hcy or saline for 14 days with or without simultaneous supplementation of low Lir (L) (150 μg/kg per day), medium Lir (M) (300 μg/kg per day) or high Lir (H) (450 μg/kg per day) dose of Lir. The plasma Hcy level and spatial memory were examined on day 15 after the injections and, as expected, Hcy level was increased after 14 days of injection, while simultaneous supplementation with the low or medium dose but not the high dose of Lir reduced it (Fig. 1A; Hhcy effect, F(4,25) = 13.08, P < 0.01). A spatial learning deficit was found in the Hcy group on days 2, 3, and 4 of spatial acquisition, while supplementation with low or medium Lir attenuated the Hhcy-induced learning deficit (Fig. 1B). In the probe trial, we also found that rats supplemented with a low or medium dose of Lir spent more time in the target quadrant (Fig. 1C) and took less time to reach the location of the formerly hidden platform (Fig. 1D), suggesting that Lir improves the Hhcy-induced spatial memory deficit. The motor activity did not change (Fig. 1E). These results together suggested that Lir reduces plasma Hcy, and ameliorates the Hhcy-induced learning and memory deficits.
Fig. 1.
Liraglutide (Lir) decreases plasma Hcy level and ameliorates memory deficits in Hhcy rats. A Administration of Lir restored plasma Hcy level as measured by ELISA. B Administration of Lir improves spatial learning, as shown by a decreased escape latency to find the hidden platform in the Morris water maze test. C, D Administration of Lir improves spatial memory as shown by the decreased time to reach the platform location (latency) and the increased total distance in the target quadrant after removal of the platform on day 7. E Administration of Lir did not affect the swimming speed of the mice, suggesting no changes in motor function. L, low Lir (150 μg/kg per 12 h); M, medium Lir (300 μg/kg per 12 h); H, high Lir (450 μg/kg per 12 h). The data are expressed as mean ± SEM (n = 10/group). *P < 0.05, **P < 0.01 vs Ctrl; #P < 0.05, ##P < 0.01 vs Hcy.
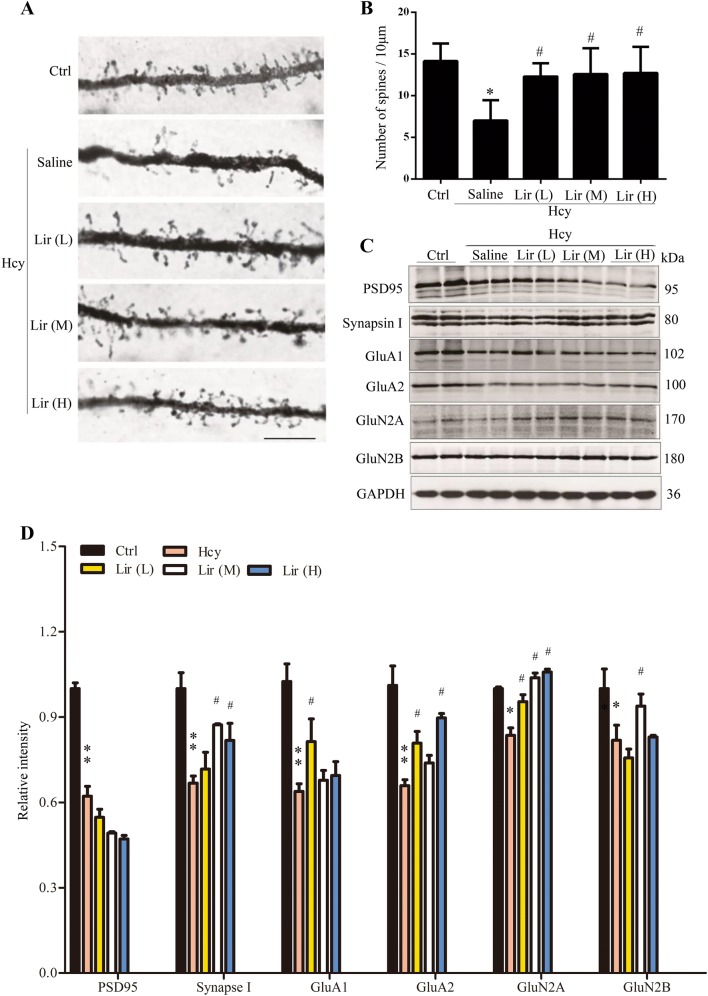
Lir Rescues Hhcy-Inhibited Spine Formation and Expression of Synapse-Associated Proteins in Hippocampus
A previous study has shown that Hcy impairs dendritogenesis [8]. To investigate whether Lir preserves spine density and dendritic complexity, we analyzed neurons in rat cortex. The average number of dendritic spines was decreased in Hhcy rats, and supplementation with a low or medium dose of Lir restored spine numbers (Fig. 2A, B). To clarify the mechanisms underlying Lir-ameliorated memory deficits and spine reduction in Hhcy rats, we examined the expression levels of several pre- and post-synaptic proteins. We found that the levels of PSD95 (67% ± 6%), Synapsin I (68% ± 3%), GluA1 (63% ± 3%), GluA2 (65% ± 3%), GluN2A (80% ± 4%), and GluN2B (75% ± 7%) were decreased in the hippocampus of Hhcy rats, whereas simultaneous supplementation with Lir partially restored the levels of these proteins (Fig. 2C, D). These data revealed the molecular mechanisms underlying the improvement of learning and memory by Lir.
Fig. 2.
Liraglutide (Lir) rescues Hhcy-inhibited spine formation and expression of synapse-associated proteins in rat hippocampus. A, B Representative Golgi-Cox images and statistics showing that administration of Lir preserves spine density in cortical neurons (scale bar, 10 μm; n = 2/group). C, D Western blots and statistics showing that Lir restores the levels of synapse-associated proteins in hippocampus (GAPDH was used as loading control; n = 6/group). Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01 vs Ctrl; #P < 0.05, ##P < 0.01 vs Hcy.
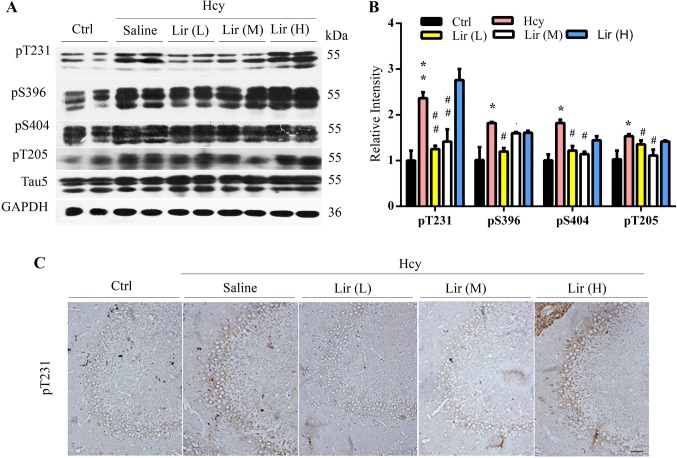
Lir Attenuates Hhcy-Induced Tau Hyperphosphorylation
As hyperphosphorylation and the accumulation of tau proteins also contribute to the learning and memory deficits, we measured the levels of phosphorylated tau. The results showed that the level of tau phosphorylated at Thr205 (pT205), Thr231 (pT231), Ser396 (pS396), and Ser404 (pS404) in the hippocampal extracts of Hhcy rats increased, while simultaneous supplementation with Lir attenuated the Hhcy-induced tau hyperphosphorylation at the above epitopes (Fig. 3A–C). The level of total tau did not differ among groups (Fig. 3A).
Fig. 3.
Liraglutide (Lir) attenuates Hhcy-induced tau hyperphosphorylation. A–C Administration of Lir attenuates Hhcy-induced tau hyperphosphorylation at several AD-associated epitopes as measured by Western blotting (A and B, n = 6/group) and immunohistochemistry (n = 2/group; scale bar, 100 μm). Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01 vs Ctrl; #P < 0.05; ##P < 0.01 vs Hcy.
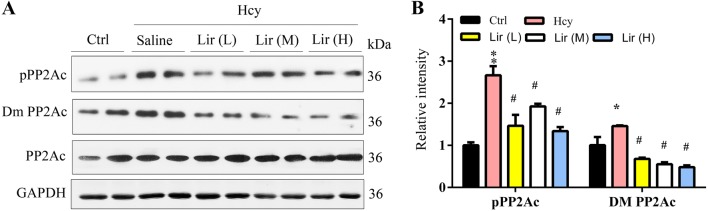
Lir Activates Protein Phosphatase 2A by Decreasing its Inhibitory Phosphorylated and Demethylated Catalytic Subunit (PP2Ac)
To explore the mechanisms underlying the altered tau phosphorylation induced by Lir, we investigated the activity-associated modifications of PP2Ac. We found that the inhibitory phosphorylation of PP2Ac at Tyr307 (pPP2Ac) and demethylation at Leu309 (Dm PP2Ac) increased in Hhcy rats, and supplementation with Lir restored the phosphorylation and methylation levels (Fig. 4A, B). These data together suggested that Lir attenuates Hhcy-induced tau hyperphosphorylation at several AD-associated sites, and the mechanism involves the activation of PP2Ac.
Fig. 4.
Liraglutide (Lir) activates PP2A by decreasing the inhibitory phosphorylated and demethylated PP2Ac. A, B Lir restores Hhcy-induced PP2A inhibition shown by the reduced phosphorylation (pPP2A) and de-methylation (Dm-PP2A) measured by Western blotting. Data are expressed as mean ± SEM (n = 4/group; *P < 0.05, **P < 0.01 vs Ctrl; #P < 0.05; ##P < 0.01 vs Hcy).
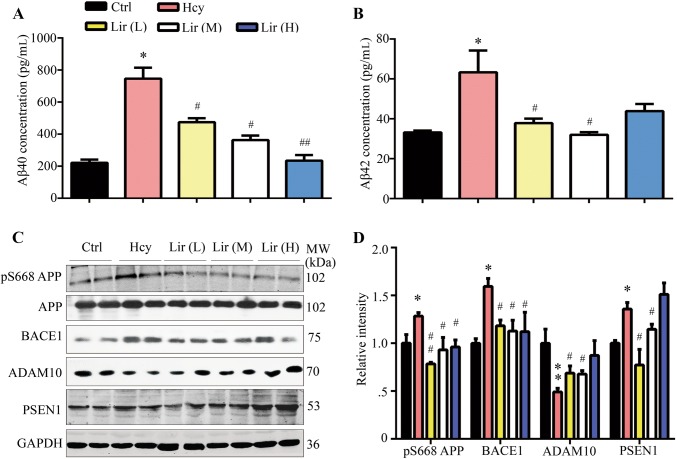
Lir Attenuates Hhcy-Induced Aβ Overproduction with Attenuation of APP Phosphorylation and Secretase Activity
It has been reported that tail vein injection of Hcy for 14 days induces Aβ accumulation in rats [7], so we used this to investigate the effect of Lir on Aβ. The ELISA results showed that the Aβ1-40 and Aβ1-42 levels both increased in the hippocampus of Hhcy rats. However, while Lir treatment decreased the Aβ1-40 level in a dose-dependent manner, the Aβ1-42 level after a high dose of Lir did not significantly change relative to Hhcy rats (Fig. 5A, B). To explore the possible mechanisms underlying the effects of Hhcy and Lir on Aβ production, we assessed the phosphorylation level of APP at Ser688 (pS668APP), and the expression levels of BACE1 (β-secretase), PSEN1 (γ-secretase), and ADAM10 (α-secretase). We found that the levels of pS668APP, BACE1, and PSEN1 increased and ADAM10 decreased in Hhcy rats, while simultaneous supplementation with Lir restored the levels of all four to normal (Fig. 5C, D). These data suggested that Lir attenuates Aβ overproduction via mechanisms involving inhibiting APP phosphorylation and modulating α-, β-, and γ-secretases.
Fig. 5.
Liraglutide (Lir) attenuates Hhcy-induced Aβ overproduction with modulated APP phosphorylation and the activity of secretases. A, B Administration of Lir arrests the Hhcy-induced Aβ1-40 and Aβ1-42 elevation in hippocampal extracts measured by ELISA (n = 6/group). C, D Lir attenuates Hhcy-induced elevation of pS668-APP, BACE1, and PSEN1 with restoration of ADAM10 assessed by Western blotting and the quantitative analysis (n = 4/group). Data are expressed as mean ± SEM (*P < 0.05, **P < 0.01 vs Ctrl; #P < 0.05, ##P < 0.01 vs Hcy).
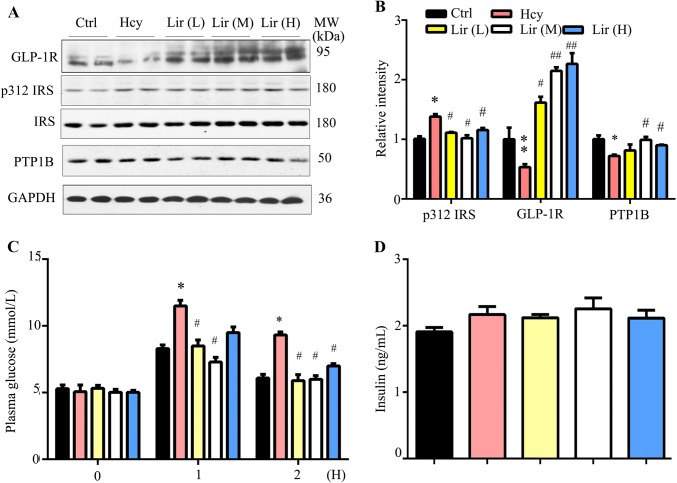
Lir Restores Hhcy-Induced Impairment of Insulin Sensitivity
Hhcy patients have a high risk of insulin resistance, which can cause memory deficits and AD-like pathology [23, 24], and a recognized action of Lir is to stimulate insulin secretion. We measured the plasma glucose level on day 15 after 14 days of Hcy exposure, but no change was detected (Ctrl vs Hhcy, 5.8 ± 0.2 mmol/L vs 6.0 ± 0.1 mmol/L). However, the protein levels of phosphorylated insulin receptor substrate-1 (IRS-1) at site 312 were up-regulated and GLP-1R was decreased in the Hhcy group. Supplementation with Lir decreased the pIRS-1 to normal level, and increased GLP-1R to a level higher than the normal control (Fig. 6A, B). OGTT assays revealed that the fasting plasma glucose level was increased 1 h and 2 h after glucose gavage in the Hhcy group, while Lir supplementation attenuated the glucose level without changing the insulin level (Fig. 6C, D). These data suggested that simultaneous peripheral Lir treatment improves Hhcy-induced peripheral and brain insulin resistance.
Fig. 6.
Liraglutide (Lir) restores Hhcy-induced impairment of insulin sensitivity. A, B Administration of Lir at the middle (M) and high (H) doses increases the expression level of insulin receptor (IRS) and glucagon-like peptide-1 receptor (GLP-1R) as assessed by Western blotting (n = 4/group). C, D Administration of Lir attenuated Hhcy-induced plasma glucose elevation measured at 1 h and 2 h after glucose gavage (C) without significantly affecting the plasma insulin level (D). Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01 vs Ctrl; #P < 0.05, ##P < 0.01 vs Hcy.
Discussion
To date, AD cannot be definitely diagnosed in the early stage, while a later stage diagnosis provides a very limited capacity for developing efficient therapies. Therefore, treating the high-risk factors, such as a decreasing plasma Hcy level and its downstream effects, is an important strategy for decreasing the prevalence of AD. Besides the classical hallmark pathology of the AD brain, such as accumulation of the hyperphosphorylated tau and aggregated Aβ, brain insulin-resistance provides a potential target for delaying the pathological process of AD. Emerging studies have shown that brain insulin resistance can cause or at least contribute to the full spectrum of AD pathology and symptoms. Insulin-regulated functions are disrupted in the AD brain, such as regulation of cerebral blood flow, inflammatory responses, oxidative stress, Aβ clearance, tau phosphorylation, apoptosis, lipid metabolism, transmitter receptor trafficking, synaptic plasticity, and memory formation. Thus, improving brain insulin resistance could be a promising strategy for the treatment of AD [25]. Lir is a GLP-1 analog that enhances the secretion of insulin and restores insulin sensitivity [26]. In the present study, we showed that insulin resistance changed both in the periphery and in the central nervous system after induction by Hcy. Then, supplementation with Lir effectively decreased the plasma Hcy level and improved brain insulin resistance with amelioration of tau hyperphosphorylation, Aβ aggregation, and the memory deficit in rats. This suggests that Lir is a promising agent for the treatment of AD-like pathology.
The concept of brain insulin resistance in AD was first proposed by Siegfried Hoyer and colleagues, who hypothesized that desensitization of neuronal insulin receptors may explain the reduced brain glucose metabolism in this disorder [27, 28]. Hhcy could cause insulin resistance. Clinical studies have shown that insulin-resistance in patients with polycystic ovary syndrome is associated with elevated plasma Hcy [29]. Hhcy clearly exacerbates insulin-resistance in H-type hypertensive patients. Furthermore, 50 days of injection of Hcy induces peripheral insulin-resistance in Sprague-Dawley rats, and this may be mediated by affecting the hepatic trans-sulfuration pathway [30]. Consistent with the above results, we found that Hcy injection for two weeks induced insulin-resistance, which was demonstrated by the increased fasting plasma glucose level after intragastric administration of glucose in the OGTT test. Furthermore, increased protein levels of phosphorylated IRS-1 in the Hhcy-induced rat hippocampus were found when compared with normal rats, indicating the occurrence of brain insulin-resistance after induction by Hcy. IRS-1 functions as the effector for the insulin receptor. Elevation in the phosphorylation pattern of IRS-1 resulting in impaired insulin signaling has long been established as a pathological marker of insulin-resistance in peripheral tissues [31, 32]. Differentially-phosphorylated forms of IRS-1 have been introduced as pathological markers of brain insulin resistance in postmortem AD brains, with profoundly impaired insulin and IRS-1 signaling [33, 34]. This suggests that brain insulin resistance occurs after injection of Hcy.
It has been suggested that GLP-1 analogs, such as Lir, are promising therapeutic agents in AD at an early clinical stage before extensive, irreversible neurodegeneration occurs. After exposure to Lir, brain tissue from amnestic mild cognitive impairment (aMCI) patients who had indications of brain insulin resistance, was found to be more responsive to insulin [35]. Furthermore, Lir restores brain insulin sensitivity in APP/PS1 mice via IRS-1 [36]. Otherwise, Lir exerts its antidiabetic function via PTP1B, which is upstream of IRS-1, in the periphery [37]. In the present study, we found that p-IRS-1 at the 312 site was upregulated in Hcy rats with decreased PTP1B, while supplementation with Lir decreased the phosphorylated IRS-1 level and enhanced PTP1B protein expression. These results suggest that the Lir not only improves insulin resistance in the periphery, but also ameliorates brain insulin resistance induced by Hcy in the hippocampus. However, the underlying mechanism of how Lir or GLP-1R regulates the expression of PTP1B needs further exploration.
Accumulation of hyperphosphorylated tau forming neurofibrillary tangles is positively correlated with memory loss in AD patients; therefore reducing tau phosphorylation may prevent AD progression. Previous studies have shown that high plasma hcy promotes tau hyperphosphorylation via inhibition of the activity of PP2A; the mechanisms involve increased inhibitory demethylation/phosphorylation of PP2AC [5, 6, 38]. In the present study, we also found tau hyperphosphorylation at multiple AD-associated sites (pT205, pT231, pS396, and pS404) in Hhcy rats, while Lir supplementation remarkably attenuated tau hyperphosphorylation with reduced demethylation and phosphorylation of PP2A, indicating restoration of PP2A activity by Lir. These data suggest that Lir attenuates AD-like tau hyperphosphorylation via a down-regulated Hcy plasma level, thus preserving PP2A. It has been reported that Lir decreases methylglyoxal-induced tau hyperphosphorylation via inhibition of glycogen synthase kinase-3β (GSK-3β) [39]. In the present study, we did not detect a change of GSK-3β activity, consistent with the previous report.
APP undergoes amyloidogenic processing by the β-secretase BACE1 to generate the extracellular fragment sAPPβ and a C-terminal transmembrane fragment (C99), and non-amyloidogenic processing by the α-secretase ADAM10 to generate sAPPα. The C99 is further cleaved by the γ-secretase complex, which causes generation of the toxic Aβ peptide [40, 41]. Although Aβ1-40 predominates, in vivo studies have revealed that Aβ1-42 is a major constituent of amyloid plaques, and suggest that Aβ1-42 aggregation plays a key role in the initiation of plaque formation and AD pathogenesis [42, 43]. We found that the levels of both Aβ1-40 and Aβ1-42 were increased in Hhcy-induced rat hippocampus. Though the Aβ1-42 level was also downregulated by the low or medium doses of Lir, the decrease was not statistically significant with the high dose, perhaps because of the upregulation of PSEN1 (Fig. 5C). Consistent with previous findings [7], we found an increased PSEN1 protein level induced by Hcy. And this enhancement was restored to normal by the low and medium doses of Lir, suggesting that it has an impact on PSEN1 expression. Phosphorylation of APP at 688 facilitates Aβ production by increasing BACE1 cleavage [44]. We also found upregulation of APP688 phosphorylation and BACE1 expression as well as downregulation of ADAM10 induced by Hhcy, and Lir differentially attenuated BACE1 and ADAM10 at different doses.
In summary, we demonstrated here that Lir efficiently ameliorates Hhcy-induced spatial memory deficits via several mechanisms, such as improving insulin resistance and restoring the expression of synaptic proteins. Lir also attenuates Hhcy-induced tau hyperphosphorylation and Aβ overproduction, and the mechanisms involve the restoration of PP2A activity and the modulation of APP phosphorylation, as well as BACE1, PSEN1, and ADAM10 activity.
Acknowledgements
This work was supported by the National Key R&D Program of China, National Basic Research Development Program of the Ministry of Science and Technology of China (2016YFC1305800), the National Natural Science Foundation of China (31730035, 91632305, and 81721005), and the Integrated Innovation Team for Major Human Disease Program of Tongji Medical College, Huazhong University of Science and Technology, China.
Conflict of interest
The authors claim that there are no conflicts of interest.
References
- 1.Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. doi: 10.1016/S0006-291X(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 2.Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopera F, Ardilla A, Martinez A, Madrigal L, Arango-Viana JC, Lemere CA, et al. Clinical features of early-onset Alzheimer disease in a large kindred with an E280A presenilin-1 mutation. JAMA. 1997;277:793–799. doi: 10.1001/jama.1997.03540340027028. [DOI] [PubMed] [Google Scholar]
- 4.Zhou SJ, Zhang LG, Chen HM, Li JY, Li R, Zhang XM, et al. Prevalence and clinical-demographic correlates of hyperhomocysteinemia in inpatients with bipolar disorder in a Han Chinese population. Psychiatry Res. 2018;259:364–369. doi: 10.1016/j.psychres.2017.08.063. [DOI] [PubMed] [Google Scholar]
- 5.Zhang CE, Tian Q, Wei W, Peng JH, Liu GP, Zhou XW, et al. Homocysteine induces tau phosphorylation by inactivating protein phosphatase 2A in rat hippocampus. Neurobiol Aging. 2008;29:1654–1665. doi: 10.1016/j.neurobiolaging.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 6.Wei W, Liu YH, Zhang CE, Wang Q, Wei Z, Mousseau DD, et al. Folate/vitamin-B12 prevents chronic hyperhomocysteinemia-induced tau hyperphosphorylation and memory deficits in aged rats. J Alzheimers Dis. 2011;27:639–650. doi: 10.3233/JAD-2011-110770. [DOI] [PubMed] [Google Scholar]
- 7.Zhang CE, Wei W, Liu YH, Peng JH, Tian Q, Liu GP, et al. Hyperhomocysteinemia increases beta-amyloid by enhancing expression of gamma-secretase and phosphorylation of amyloid precursor protein in rat brain. Am J Pathol. 2009;174:1481–1491. doi: 10.2353/ajpath.2009.081036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chai GS, Jiang X, Ni ZF, Ma ZW, Xie AJ, Cheng XS, et al. Betaine attenuates Alzheimer-like pathological changes and memory deficits induced by homocysteine. J Neurochem. 2013;124:388–396. doi: 10.1111/jnc.12094. [DOI] [PubMed] [Google Scholar]
- 9.Li B, Xi X, Roane DS, Ryan DH, Martin RJ. Distribution of glucokinase, glucose transporter GLUT2, sulfonylurea receptor-1, glucagon-like peptide-1 receptor and neuropeptide Y messenger RNAs in rat brain by quantitative real time RT-PCR. Brain Res Mol Brain Res. 2003;113:139–142. doi: 10.1016/S0169-328X(03)00125-6. [DOI] [PubMed] [Google Scholar]
- 10.Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience. 1997;77:257–270. doi: 10.1016/S0306-4522(96)00434-4. [DOI] [PubMed] [Google Scholar]
- 11.Nakade Y, Tsukamoto K, Pappas TN, Takahashi T. Central glucagon like peptide-1 delays solid gastric emptying via central CRF and peripheral sympathetic pathway in rats. Brain Res. 2006;1111:117–121. doi: 10.1016/j.brainres.2006.06.090. [DOI] [PubMed] [Google Scholar]
- 12.Abbas T, Faivre E, Holscher C. Impairment of synaptic plasticity and memory formation in GLP-1 receptor KO mice: interaction between type 2 diabetes and Alzheimer’s disease. Behav Brain Res. 2009;205:265–271. doi: 10.1016/j.bbr.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 13.During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, et al. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med. 2003;9:1173–1179. doi: 10.1038/nm919. [DOI] [PubMed] [Google Scholar]
- 14.Lee CH, Yoo KY, Ryu PD, Park JH, Choi JH, Kim S, et al. Decreased glucagon-like peptide-1 receptor immunoreactivity in the dentate granule cell layer from adult in the gerbil hippocampus. Cell Mol Neurobiol. 2011;31:345–350. doi: 10.1007/s10571-010-9632-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juhl CB, Hollingdal M, Sturis J, Jakobsen G, Agerso H, Veldhuis J, et al. Bedtime administration of NN2211, a long-acting GLP-1 derivative, substantially reduces fasting and postprandial glycemia in type 2 diabetes. Diabetes. 2002;51:424–429. doi: 10.2337/diabetes.51.2.424. [DOI] [PubMed] [Google Scholar]
- 16.Chang AM, Jakobsen G, Sturis J, Smith MJ, Bloem CJ, An B, et al. The GLP-1 derivative NN2211 restores beta-cell sensitivity to glucose in type 2 diabetic patients after a single dose. Diabetes. 2003;52:1786–1791. doi: 10.2337/diabetes.52.7.1786. [DOI] [PubMed] [Google Scholar]
- 17.Li L, Zhang ZF, Holscher C, Gao C, Jiang YH, Liu YZ. (Val(8)) glucagon-like peptide-1 prevents tau hyperphosphorylation, impairment of spatial learning and ultra-structural cellular damage induced by streptozotocin in rat brains. Eur J Pharmacol. 2012;674:280–286. doi: 10.1016/j.ejphar.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Gault VA, Holscher C. GLP-1 agonists facilitate hippocampal LTP and reverse the impairment of LTP induced by beta-amyloid. Eur J Pharmacol. 2008;587:112–117. doi: 10.1016/j.ejphar.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 19.McClean PL, Holscher C. Liraglutide can reverse memory impairment, synaptic loss and reduce plaque load in aged APP/PS1 mice, a model of Alzheimer’s disease. Neuropharmacology. 2014;76(Pt A):57–67. doi: 10.1016/j.neuropharm.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Ahren B, Pacini G. Dose-related effects of GLP-1 on insulin secretion, insulin sensitivity, and glucose effectiveness in mice. Am J Physiol. 1999;277:E996–E1004. doi: 10.1152/ajpendo.1999.277.6.E996. [DOI] [PubMed] [Google Scholar]
- 21.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaqout S, Kaindl AM. Golgi-Cox staining step by step. Front Neuroanat. 2016;10:38. doi: 10.3389/fnana.2016.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giltay EJ, Hoogeveen EK, Elbers JM, Gooren LJ, Asscheman H, Stehouwer CD. Insulin resistance is associated with elevated plasma total homocysteine levels in healthy, non-obese subjects. Atherosclerosis. 1998;139:197–198. doi: 10.1016/S0021-9150(98)00067-7. [DOI] [PubMed] [Google Scholar]
- 24.Luo L, Ma J, Li Y, Hu Z, Jiang C, Cai H, et al. Cystatin C induces insulin resistance in hippocampal neurons and promotes cognitive dysfunction in rodents. Neurosci Bull. 2018;34:543–545. doi: 10.1007/s12264-018-0226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnold SE, Arvanitakis Z, Macauley-Rambach SL, Koenig AM, Wang HY, Ahima RS, et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol. 2018;14:168–181. doi: 10.1038/nrneurol.2017.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacDonald PE, El-Kholy W, Riedel MJ, Salapatek AM, Light PE, Wheeler MB. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes. 2002;51(Suppl 3):S434–S442. doi: 10.2337/diabetes.51.2007.S434. [DOI] [PubMed] [Google Scholar]
- 27.Hoyer S, Muller D, Plaschke K. Desensitization of brain insulin receptor. Effect on glucose/energy and related metabolism. J Neural Transm Suppl. 1994;44:259–268. doi: 10.1007/978-3-7091-9350-1_20. [DOI] [PubMed] [Google Scholar]
- 28.Henneberg N, Hoyer S. Desensitization of the neuronal insulin receptor: a new approach in the etiopathogenesis of late-onset sporadic dementia of the Alzheimer type (SDAT)? Arch Gerontol Geriatr. 1995;21:63–74. doi: 10.1016/0167-4943(95)00646-3. [DOI] [PubMed] [Google Scholar]
- 29.Schachter M, Raziel A, Friedler S, Strassburger D, Bern O, Ron-El R. Insulin resistance in patients with polycystic ovary syndrome is associated with elevated plasma homocysteine. Hum Reprod. 2003;18:721–727. doi: 10.1093/humrep/deg190. [DOI] [PubMed] [Google Scholar]
- 30.Golbahar J, Aminzadeh MA, Kassab SE, Omrani GR. Hyperhomocysteinemia induces insulin resistance in male Sprague-Dawley rats. Diabetes Res Clin Pract. 2007;76:1–5. doi: 10.1016/j.diabres.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 31.Copps KD, White MF. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia. 2012;55:2565–2582. doi: 10.1007/s00125-012-2644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoehn KL, Hohnen-Behrens C, Cederberg A, Wu LE, Turner N, Yuasa T, et al. IRS1-independent defects define major nodes of insulin resistance. Cell Metab. 2008;7:421–433. doi: 10.1016/j.cmet.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talbot K, Wang HY. The nature, significance, and glucagon-like peptide-1 analog treatment of brain insulin resistance in Alzheimer’s disease. Alzheimers Dement. 2014;10:S12–S25. doi: 10.1016/j.jalz.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122:1316–1338. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang HY, Bakshi K, Frankfurt M, Stucky A, Goberdhan M, Shah SM, et al. Reducing amyloid-related Alzheimer’s disease pathogenesis by a small molecule targeting filamin A. J Neurosci. 2012;32:9773–9784. doi: 10.1523/JNEUROSCI.0354-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bomfim TR, Forny-Germano L, Sathler LB, Brito-Moreira J, Houzel JC, Decker H, et al. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease- associated Abeta oligomers. J Clin Invest. 2012;122:1339–1353. doi: 10.1172/JCI57256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ji W, Chen X, Lv J, Wang M, Ren S, Yuan B, et al. Liraglutide exerts antidiabetic effect via PTP1B and PI3K/Akt2 signaling pathway in skeletal muscle of KKAy mice. Int J Endocrinol. 2014;2014:312452. doi: 10.1155/2014/312452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sontag E, Nunbhakdi-Craig V, Sontag JM, Diaz-Arrastia R, Ogris E, Dayal S, et al. Protein phosphatase 2A methyltransferase links homocysteine metabolism with tau and amyloid precursor protein regulation. J Neurosci. 2007;27:2751–2759. doi: 10.1523/JNEUROSCI.3316-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qi L, Chen Z, Wang Y, Liu X, Liu X, Ke L, et al. Subcutaneous liraglutide ameliorates methylglyoxal-induced Alzheimer-like tau pathology and cognitive impairment by modulating tau hyperphosphorylation and glycogen synthase kinase-3beta. Am J Transl Res. 2017;9:247–260. [PMC free article] [PubMed] [Google Scholar]
- 40.Kojro E, Gimpl G, Lammich S, Marz W, Fahrenholz F. Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the alpha -secretase ADAM 10. Proc Natl Acad Sci U S A. 2001;98:5815–5820. doi: 10.1073/pnas.081612998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cai H, Wang Y, McCarthy D, Wen H, Borchelt DR, Price DL, et al. BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat Neurosci. 2001;4:233–234. doi: 10.1038/85064. [DOI] [PubMed] [Google Scholar]
- 42.McGowan E, Pickford F, Kim J, Onstead L, Eriksen J, Yu C, et al. Abeta42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron. 2005;47:191–199. doi: 10.1016/j.neuron.2005.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43) Neuron. 1994;13:45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- 44.Lee MS, Kao SC, Lemere CA, Xia W, Tseng HC, Zhou Y, et al. APP processing is regulated by cytoplasmic phosphorylation. J Cell Biol. 2003;163:83–95. doi: 10.1083/jcb.200301115. [DOI] [PMC free article] [PubMed] [Google Scholar]