Abstract
Background:
Recent clinical trials have shown that adjunctive glucocorticoids is associated with inhibiting excessive inflammatory response and modulating cytokines release offering several advantages over conventional therapy on relieving clinical symptoms, reducing mortality, and improving prognosis. However, given the severe complications triggered by glucocorticosteroid, whether similar benefits may be achieved by patients undergoing glucocorticosteroid intervention remains controversial. Our meta-analysis aimed to investigate the efficacy and safety of adjunctive glucocorticoids in the treatment of severe community acquired pneumonia.
Methods:
A search of PubMed, EMBASE, Cochrane Library, EBASO, Medline, Google Scholar, Science Dicet, CBM, and CNKI databases was performed to analyze all relevant randomized controlled trials (RCTs) of corticosteroids in patients with severe community acquired pneumonia (CAP) up to January 2018. All-cause mortality, C-reactive protein (CRP) level, incidence of septic shock, and requirement of mechanical ventilation were selected as efficacy outcomes. Major adverse events involving super infection, upper gastrointestinal bleeding, and hyperglycemia were safety outcomes. Meta-analysis was conducted with RevMan 5.3 software.
Results:
A total of 10 RCTs comprising 665 patients were included for analysis. Regarding efficacy outcomes, adjunctive corticosteroid seemed to be superior compared with conventional treatment in terms of all-cause mortality (relative risk [RR]: 0.47, 95% confidence interval [CI], 0.3–0.74, P = .001), CRP level on day 8 after administration (standard mean difference [SMD]: −0.8, 95% CI, −1.11 to −0.5, P < .001), incidence of septic shock (odds ratio [OR] 0.15, 95% CI, 0.07–0.29, P < .001) and requirement for mechanical ventilation (OR: 0.32, 95% CI, 0.20–0.52, P < .001). Meanwhile, we found that low dose (≤86 mg) (RR: 0.41, 95% CI, 0.21–0.82, P = .01) and prolonged (>5 days) (RR: 0.35, 95% CI, 0.15–0.81, P = .01) use of corticosteroids in dosage modus of a maintenance dose after a bolus (RR: 0.28, 95% CI, 0.14–0.55, P = .002) obtained better results in death through subgroup analysis. Regarding safety outcomes, no difference was observed between 2 groups in terms of upper gastrointestinal bleeding (OR: 0.83, 95% CI, 0.27–2.52, P = .74), hyperglycemia (OR: 1.3, 95% CI, 0.68–2.49, P = .42), and super infection (OR: 1.11, 95% CI, 0.14–9.13, P = .92).
Conclusion:
Adjunctive corticosteroid yielded favorable outcomes in the treatment of severe community acquired pneumonia (SCAP) as evidenced by decreased all-cause mortality, incidence of septic shock, and requirement for mechanical ventilation without increasing risk of adverse events. Low dose (≤86 mg/d), prolonged use (>5 days) of corticosteroid in dosage modus of a maintenance dose after a bolus can be recommended as preferred regimen to guard against SCAP.
Keywords: glucocorticoids, meta-analysis, severe community acquired pneumonia
1. Introduction
Community acquired pneumonia (CAP) is infectious pulmonary parenchymal inflammation occurred in the community environment (outside the hospital), including pneumonia with a clearly latent pathogen infection that develops during the average incubation period after admission.[1] Mainly caused by bacterial infection, severe community acquired pneumonia (SCAP) has the characteristics of rapid progress, critical illness, high morbidity, and high mortality. In recent years, SCAP has even become the first generally fatal infectious disease in developed countries. [2] In 2012, the mortality rate of pneumonia in China was about 1,746/100,000, among which the mortality rate of SCAP could reach 20% to 50%.[3] In Europe, health investors spend about $11.8 billion a year on the treatment of pneumonia.[4] Severe community-acquired pneumonia has caused great financial burden on the current medical system. It has been reported that no matter what the pathogenic microorganism was, the inability of the host to comprehensively downregulate systemic inflammation is the main cause of high mortality in patients with SCAP.[5]Therefore, it is of great importance for SCAP treatment to prevent the inflammatory from deteriorating actively and effectively.
On account of the powerful effects on nonspecific anti-inflammatory and immunomodulation, glucocorticoids can regulate the sophisticated balance of cytokines network. In this manner, it has been the recent consensus that adjunctive corticosteroids and antibiotic treatment are the logical strategies for the critical infectious disease in the medical department.[6] A study by Tagami[7] revealed that in a case of 2524 patients with severe CAP, 28 days mortality in the hormone group of 631 patients was significantly declined compared with the placebo group; another experiment demonstrated that pigs treated with corticosteroid and antibiotic had lower pulmonary bacterial load and lighter histological pneumonia in the pseudomonas aeruginosa model of mechanic ventilation pigs.[8] In addition, Salluh found that most of the patients with severe CAP, in particular the patients complicated by septic shock and ARDS, had immune functional dysfunction and adrenal insufficiency following the infection, resulting in the occurrence of the critical illness-related corticosteroid insufficiency (CIRCI). Besides, the decreased baseline cortisol level was closely related to the severity and outcome of the SCAP patients.[7,9] Therefore, corticosteroid substitution therapy was gradually applied to the treatment of severe CAP patients. Some guidelines pointed out that low dose of glucocorticoids showed a decrease in mortality caused by shock and could effectively improve oxygenation index.[10] However, at the same time, related complications such as hyperglycemia, triggered by strong negative side effect of glucocorticoids, forced clinicians to be cautious in their application. Based on the above discrepancy, this paper evaluated the efficacy and safety of systemic adjunctive glucocorticoids therapy in adult patients with severe CAP by means of meta-analysis, so as to provide certain guidance for clinical work.
2. Materials and methods
We conducted this study in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.
2.1. Criteria for considering study
2.1.1. Type of studies
A study considered eligible for this review had to meet the following inclusion criteria: clinical randomized controlled trials published at home and abroad in English and Chinese which compared antimicrobial therapy and adjunctive systematic glucocorticoids therapy with antimicrobial therapy alone in patients with severe community acquired pneumonia.
2.1.2. Type of participants
Patients aged >18 years old who were diagnosed with SCAP that met the definition and diagnostic criteria formulated by the American Association of Infectious Diseases and American Thoracic Society (IDSA/ATS) in 2007, British Thoracic Society (BTS) or Chinese Medical Association in 2016.
Diagnostic criteria for severe CAP in IDSA/ATS: At least 1 major or 3 minor criteria were satisfied. Major criteria: necessity of tracheal intubation and mechanical ventilation; occurrence of septic shock or still requiring vasoactive drugs after active fluid resuscitation. Minor criteria: respiratory rate≥30 bpm; oxygenation index≤250 mm Hg; radiological multilobar involvement; disturbance of consciousness or disorientation; blood urea nitrogen ≥7.14 mmol/L; systolic blood pressure <90 mm Hg, requiring active fluid resuscitation.
Diagnosis criteria of severe CAP in BTS: The CURB-65 score in conjunction with clinical judgment was recommended as the severity assessment strategy in hospital for CAP. Patients with CURB-65 score ≥3 are considered as severe CAP. The CURB-65 score: confusion (1 point): new mental confusion; urea (1 point): raised >7 mmol/L; respiratory rate (1 point): raised ≥30/min; blood pressure (1 point): systolic≤90 mm Hg or/and diastolic≤60 mm Hg; age (1 point) ≥65 years.
2.1.3. Type of Interventions
Glucocorticosteroid was used as exposure factor. Both the experiment group and the control group were given conventional anti-infection treatment. Patients in the experiment group were given adjunctive systematic glucocorticosteroid on the basis of anti-infection treatment, whereas patients in the control group were given placebo with equal dose. The classification, dose, time, and frequency of glucocorticoids were not limited, but the data were clear.
2.1.4. Type of outcome measurement
The major outcome indicator was all-cause mortality. The secondary outcome indicators were considered: inflammatory index outcome (CRP), the risk of septic shock (which need vasopressors), need for mechanical ventilation (invasive or not invasive), and occurrence of adverse reaction, including the risk of super infection, hyperglycemia, and upper gastrointestinal bleeding.
2.1.5. Exclusion criteria
Articles were excluded according to the following criteria: original studies were nonclinical randomized controlled trials; studies with inaccurate data of glucocorticoids (e.g., therapeutic dose, time, frequency, efficacy, and so on) applied to the treatment in SCAP and studies with unclear state of the illness (e.g., lack of risk estimates and relevant outcomes); studies involved SCAP patients suffered from immune hypofunction (e.g., immunosuppression) or complicated by influenza, SARS, malignancy, pregnant, lactation, or those with nosocomial pneumonia, viral pneumonia, and pediatric patients; studies with insufficient data, literature review, case reports, meeting minutes, and so on; republished literature; patients with the same material used in multiple articles, take the latest published articles for data extraction; gastrointestinal bleeding within 3 months of hospitalization; and conditions requiring prednisone (acute asthma and COPD) over 0.5 mg/kg/d.
2.2. Retrieval strategy for identification of studies
According to retrieval purpose and requirement, this study would be divided into 5 parts: patient (P), intervention (I), comparison (C), outcome (O), and experimental design (S), namely, PICOS principle in the evidence-based medicine, of which we retrieved the subject words by computer. Literature searches for all RCTs on the efficacy and prognosis index of adjunctive glucocorticoids therapy in the treatment of severe community acquired pneumonia were conducted in the Embase, PubMed, Cochrane Library, EBASO, Medline, Google Scholar, Science Dictionary, China Biomedical Literature Database (CBM), China National Knowledge Infrastructure (CNKI), Chinese Science and Technology Journal Database (VIP), Wan Fang Knowledge Service Platform, and other Chinese and English database dated from inception to January 2018. Furthermore, we had searched the Clinical Trials for qualification test that had not yet published, and manually consulted the reference list included in the study, the existing meta-analysis, and articles incorporated into Springer Link, Nature, OVID, JAMA (American medical association) to extend the search. The key words used for retrieval were including the following terms: “severe community acquired pneumonia,” “severe pneumonia,” “severe CAP’, ‘community acquired pneumonia”; “adrenal cortex hormone,” “hormones,” “glucocorticoids,” “steroids,” “corticosteroids,” “corticoid,” “hydrocortisone,” “prednisone,” “methylprednisolone,” “dexamethasone”; “randomized controlled trials” in English and Chinese. Combining the above subject words with logical relation words and truncated character constituted the search formula which we used for pre-retrieval, and adjust the retrieval strategy depending on the retrieval result.
2.3. Selection of studies
Two investigators independently assessed and screened all identified literature in accordance with the inclusion and exclusion criteria, with the divergences resolved by discussion or adjudicated with the third investigator when necessary.
2.4. Data Extraction and management
Data from the selected studies were extracted and crosschecked by the 2 above investigators making use of a standardized data extraction form. Disagreements were resolved by consensus. The following information was extracted from each selected literature: basic information: authors, year of publication, study design, and so on; baseline characteristics of participants: age, number of participants, gender, diagnosis criteria of the disease, location of research and treatment, and so on; interventions: simple size and number of incidents in experimental group and control group, the specific implementation method of intervention measure which in this study consisted of delivery time, dose, frequency and ways of glucocorticoids; outcome index: measure unit, upper and lower limits; results: grouping, data type, statistical data of each included study. Information related to the outcome indicators, which included all-cause mortality, the level of CRP on 8 days after treatment, the occurrence of sepsis shock, need for mechanical ventilation, and adverse events (upper gastrointestinal bleeding and secondary infection), was extracted directly from the data table of included studies.
2.5. Assessment of risk of bias in included studies
In this study, the quality assessment of each included studies was based on the Cochrane Collaboration's tool to evaluate the risk of bias which considered the following methodological item: selection bias (random sequence generation and allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting), and other bias[11]. Each item was classified into 3 types: low risk, high risk, and unclear risk.
2.6. Statistical Analysis
All of statistical analysis was performed with the Review Manager Version 5.3 software (Cochrane Collaboration). Meta-analysis was a secondary analysis that incorporated the statistics of multiple identical studies; consequently, the heterogeneity test was required before combining the result of studies to determine whether the statistics could be incorporated. Heterogeneity of the results across studies could be assessed by means of Cochrane Q test and I2 statistics. The interpretation of the former was made by assigning attributes of low and high in case of P > .10 and P < .10, whereas the latter was considered indicative of low, moderate, and high heterogeneity in case of I2≤25%, 25% to 50%, and >50%, respectively. We used different effect models to analysis effect quantity based on the degree of heterogeneity. If substantial heterogeneity were observed, the random-effect model would be performed; otherwise the fixed-effect model was employed to analysis consolidated statistics if there was no or little statistical and clinical heterogeneity. When heterogeneity was too significant to combine statistics, the sensitivity analysis and subgroup analysis could be carried out to investigate the origin of potential heterogeneity by removing a trial at a time, with contribution of individual study to the overall estimate evaluated, so as to value and judge the stability and reliability of the analysis results. In addition, the different effect quantities were applied for calculation depending on the data classification. Throughout the meta-analysis, pooled risk ratios (RRs) and 95% confidence interval (CI) were calculated to estimate dichotomous variables using Mantel–Hænzel model, whereas pooled standardized mean difference (SMD) and 95% CI for continuous variables. All the results were examined by 2-tailed test with a statistical significance level limit of 0.05 (P < .05). To evaluate the potential publication bias across studies, funnel plots were constructed plotting each trial result against their precision graphically. We determine the degree of publication bias by visual inspection of funnel plot asymmetry.
3. Results
3.1. Search results and Basic characteristic of included studies
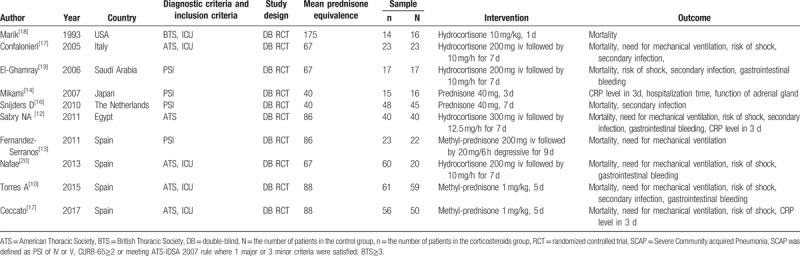
Based on the aforementioned search strategy, a total of 2075 potentially eligible studies (89 through PubMed, 165 through Embase, 1084 through Cochrane Library, 4 through Wan Fang, 22 through VIP, 42 through CNKI, 24 through CBM, 88 through EBSO and Medline, 417 through Google Scholar, 100 through Science Dicet) were identified in the initial search of the database, of which 456 duplicate studies were excluded using a software named EndNote. After removing duplicates, 1619 studies were remained. Among them, 1602 irrelevant articles including reviews, meeting reports, cases, and repeated study reports were eliminated by screening of title and abstract. Full-text articles assessed for eligibility were performed in the remaining 17 studies and 7 studies were discarded after full review and in-depth information evaluation for the following reasons: nonrandomized controlled trials (n = 4); nonsevere CAP (n = 2); and not found in search (n = 1). Ultimately, 10 RCTs [12–21] covering a total of 665 participants (357 randomly assigned to corticoid group and 308 randomly assigned to placebo group) were enrolled in the meta-analysis. A flow diagram was presented in Figure 1, demonstrating the search strategy and selection process. The different type of corticosteroid including methyl-prednisone, prednisone, and hydrocortisone could be converted to prednisone based on dose conversion equation. Treatment duration ranged from 1 to 9 days. The detailed baseline characteristics of included studies were summarized in Table 1.
Figure 1.
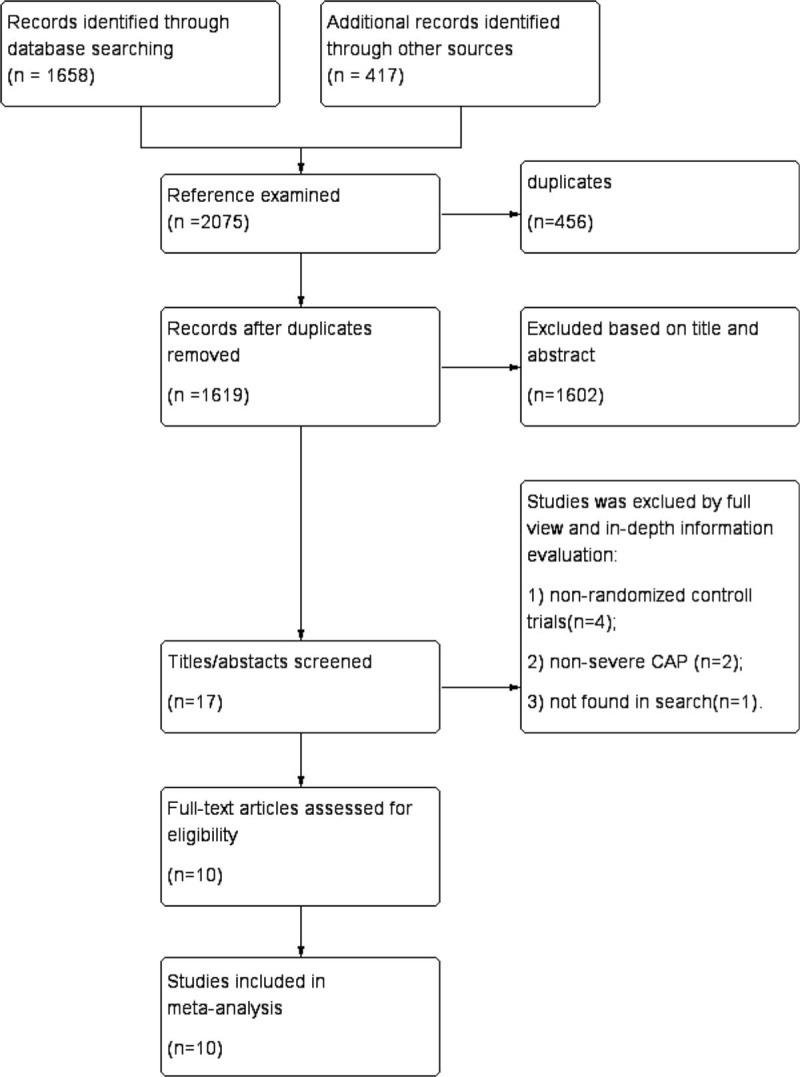
PRISMA flow diagram of search strategy and study selection process.
Table 1.
Baseline characteristics of included studies in the meta analysis.
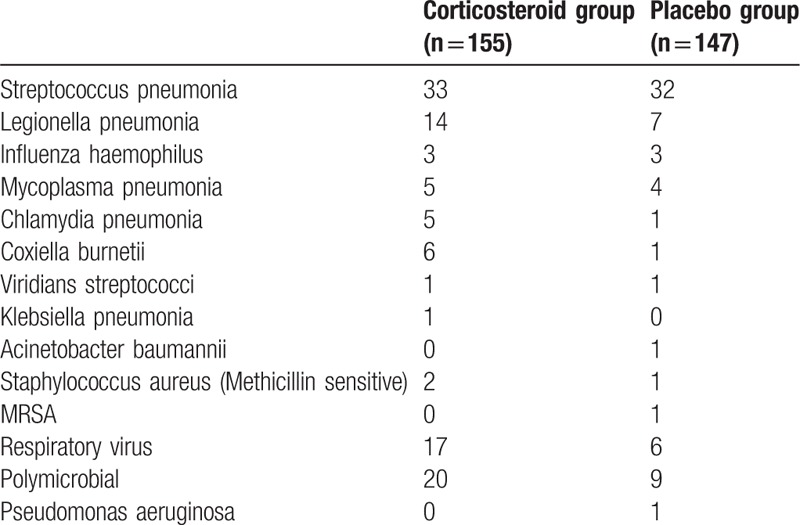
3.2. Microbiology
Data concerning the distribution of pathogen were shown in Table 2. Only 4 out of 10 RCTs assessed the etiological classification of SCAP patients with circumstance, involving a total of 302 patients. In the summary data table of microbiology, it could be found that streptococcus pneumonia, legionella pneumonia, and polymicrobial infection were susceptible pathogens for patients with SCAP. No statistically significant difference in etiology was observed between 2 groups. A definitive etiological diagnosis was obtained in 139 (89.6%) patients of corticosteroid group as well as 107 (72.7%) patients of placebo group.
Table 2.
Microbiologic identification.
3.3. Assessment of risk of bias
As illustrated in Figures 2 and 3, a summary table of review author's judgments about each risk of bias item was presented as percentages across all studies. All bias had relatively detailed description in most of included studies but other index of bias.
Figure 2.
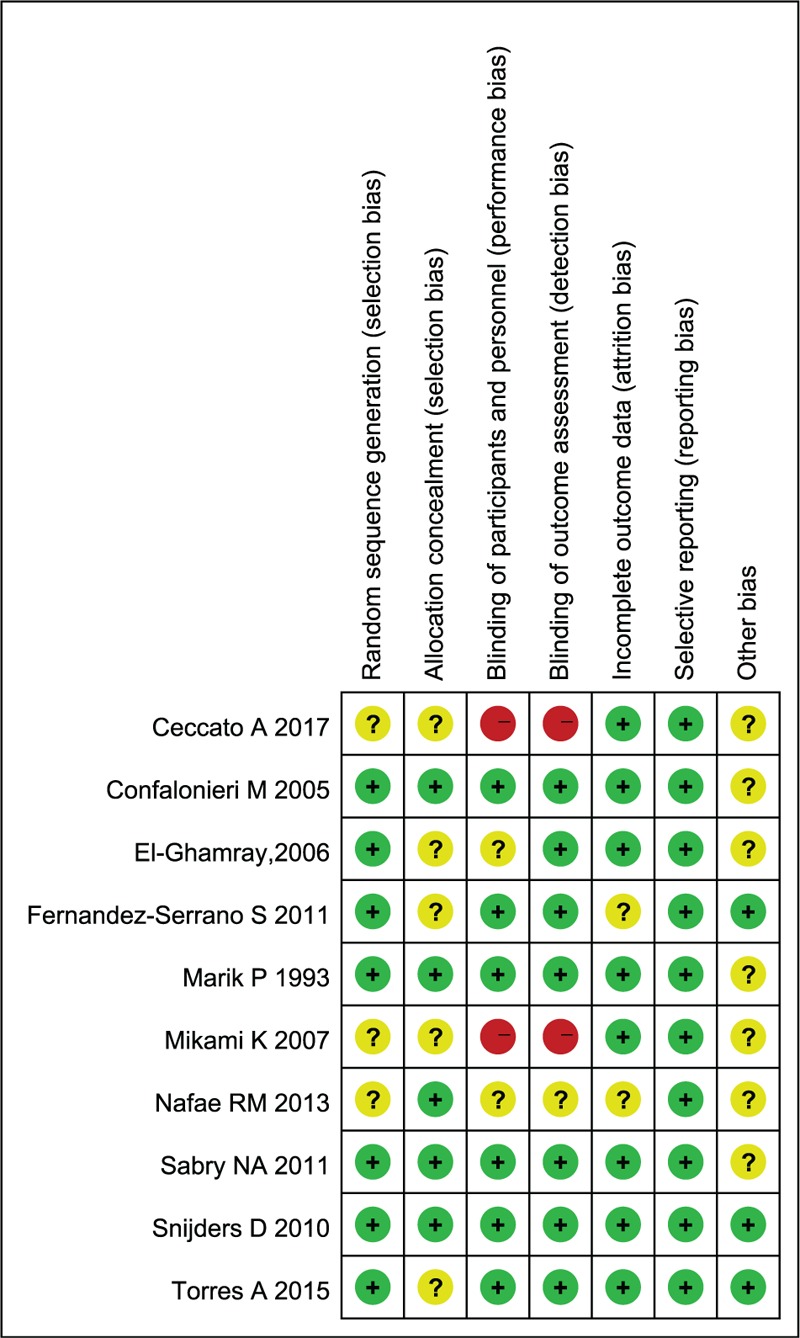
Risk of bias summary.
Figure 3.
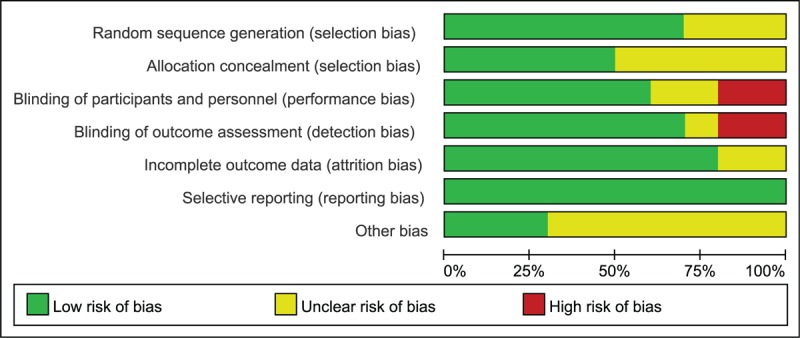
Risk of bias graph.
3.4. Meta analysis
3.4.1. All-cause mortality
As far as all-cause mortality was concerned, 9 articles covering a total of 634 patients contributed to the overall analysis of mortality comparing patients treated with conventional antimicrobial plus adjunctive corticosteroid: 7.6% (26/342) receiving died, versus patients treated with conventional antimicrobial plus placebo: 16% (47/292) receiving died. No heterogeneity was observed among these trials (I2 = 0%). Based on the pooled result applying fixed-effects model, corticosteroid treatment was associated with a significant reduction in all-cause mortality of patients with SCAP (relative ratio [RR]: 0.47, 95% confidence interval [CI], 0.3–0.74, P = .001; Fig. 4A).
Figure 4.
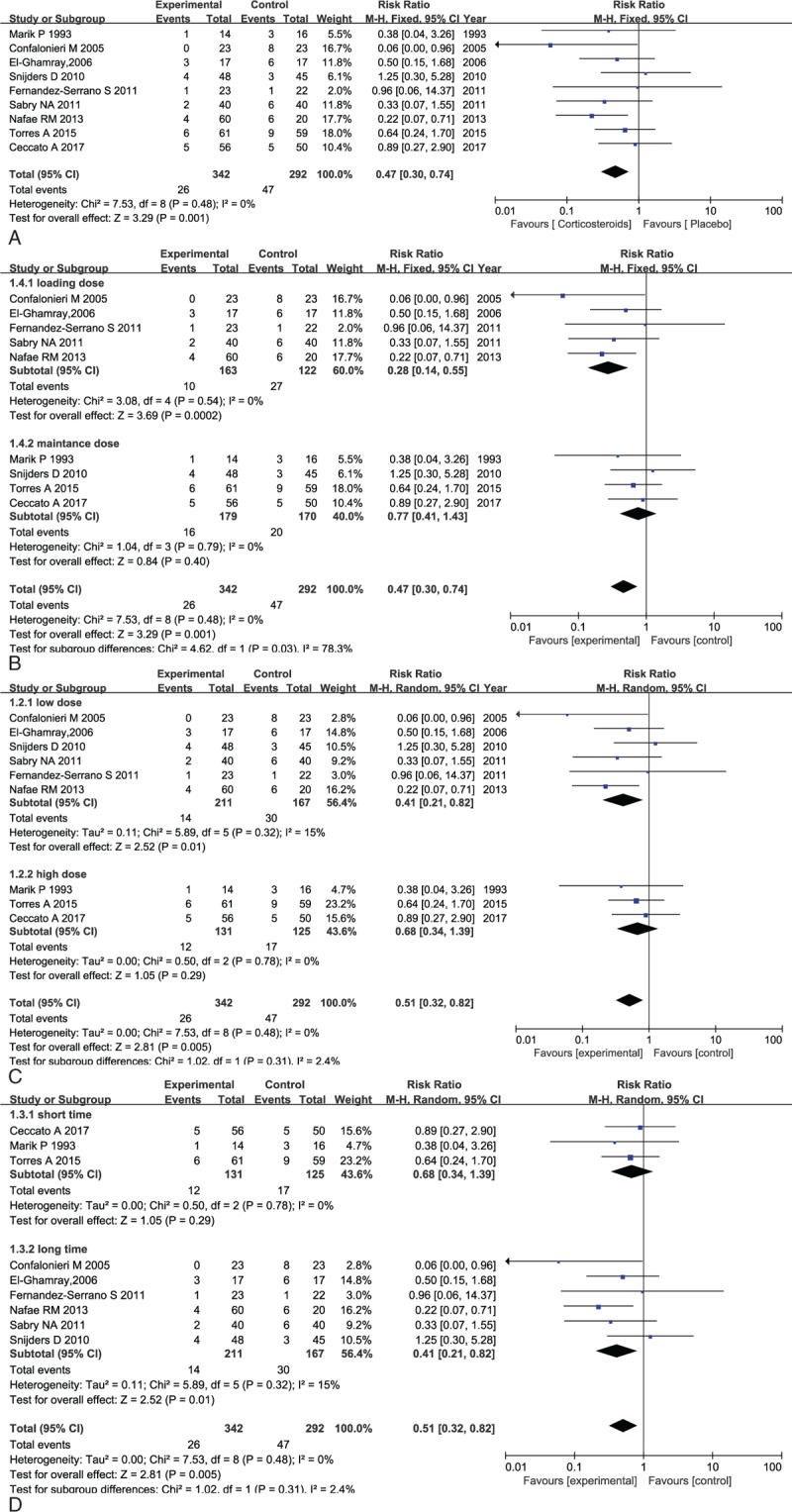
(A) Forest plot comparing all-cause mortality in patients with corticosteroid versus placebo. (B) Forest plot for the subgroup analysis of death on drug delivery of corticosteroid applied in patients with severe CAP. (C) Forest plot for the subgroup analysis of death on therapeutic dose of corticosteroid applied in patients with severe CAP. (D) Forest plot for the subgroup analysis of death on duration of corticosteroid applied in patients with severe CAP.
However, the optimal dose and duration of corticosteroid therapy for patients with severe CAP were still unclear, which is why subgroup analysis was conducted on the therapeutic dose and duration. As can be seen in Figure 2, corticosteroid was given in 2 different ways: one was a intravenous bolus followed by a maintenance intravenous dose for a few days, whereas the other one was a infusion of a maintenance dose merely. Besides, the median equivalent on an average day and treatment duration was 86 mg prednisone and 5 days and then we defined 0 to 5 days, 5 to 9 days, ≤86 mg/d and >86 mg/d as short time, long time, low dose, and high dose, respectively, and conducted subgroup analysis of included data. Subgroup analysis performed on drug delivery, dose, and duration were illustrated as below (Fig. 4B–D). No heterogeneity was reported among the studies in the subgroup analysis on drug delivery (I2 = 0%) and meta-analysis calculated with fix-effects model, indicating that the mode delivering a maintenance dose after a bolus (RR: 0.28, 95% CI, 0.14–0.55, P = .002) was associated with more significant reduction in death as compared with the mode delivering maintenance dose merely (RR: 0.77, 95% CI, 0.41–0.55, P = .40) (Fig. 4B).
Moreover, as depicted in Figure 4C, the consistence test indicated low heterogeneity among the studies in the subgroup analysis conducted by optimal therapeutic dose (I2 = 15%, 0%). According to fixed-effects model, low dose (≤86 mg/d) administration proved to be more effective in reducing mortality of patients with severe CAP (RR: 0.41, 95% CI, 0.21–0.82, P = .01) compared with high dose (>86 mg/d) administration (RR: 0.77, 95% CI, 0.41–1.43, P = .4).
As for intervention duration, with a relatively low heterogeneity across these RCTs, the further subgroup analysis carried out revealed that significant differences were observed in the outcomes where a lower incidence of death was shown in the overall comparison (RR: 0.46, 95% CI, 0.27–0.80, P = .006, I2 = 4%) as well as in the prolonged use of corticosteroid (>5 days, RR: 0.35, 95% CI, 0.15–0.81, P = .01, I2 = 23%). However, there was no statistically significant difference detected reducing all-cause mortality in short course subgroup analysis (≤5 days, RR: 0.65, 95% CI, 0.29–1.44, P = .29, I2 = 0%) (Fig. 4D).
Visual inspection of funnel plot depicted on the basis of meta-analysis outcomes did not reveal a skewed distribution for death, suggesting that no significant evidence of publication bias was obtained for mortality. Moreover, all trails located in 95% confidence interval was considerate an indicative of low heterogeneity, as illustrated in Figure 5. However, the distribution was relatively dispersed due to the small simple size.
Figure 5.
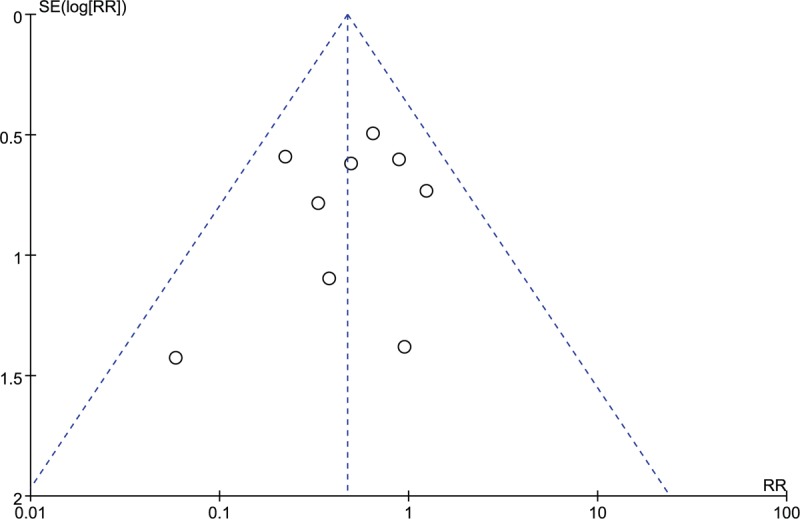
Funnel plot for the assessment of publication bias on all-cause mortality.
3.4.2. C-reactive protein
Three published RCTs comprising 206 patients were eligible for the CRP level by day 8 after the administration therapy in patients with severe CAP. As reported in Figure 6, there was significant evidence of high heterogeneity (I2 = 59%) across these studies. The overall analysis pooled with random-effects model, indicating that the CRP level on day 8 after administration corticosteroid was significantly lower than conventional treatment (SMD: −0.8, 95% CI, −1.11 to −0.5, P < .001), suggesting that corticosteroid administration proved to be superior in decreasing CRP level.
Figure 6.
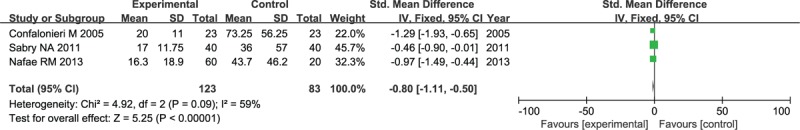
Forest plot of CRP level on study 8 after administration therapy in patients with severe CAP.
3.4.3. Incidence of septic shock
Septic shock outcomes were provided in 6 RCTs covering a total of 466 patients with 257 (5.4%) assigned randomly to corticosteroid group versus 209 (22.4%) assigned randomly to placebo group. The consistence test indicated no heterogeneity (I2 = 0%) among these studies; hence, the fixed-effects model was selected to carry out meta-analysis, which demonstrated that compared with patients in placebo group, corticosteroid was associated with significant decreased risks of the incidence on septic shock (odds ratio [OR]: 0.15, 95% CI, 0.07–0.29, P < .001; Fig. 7).
Figure 7.
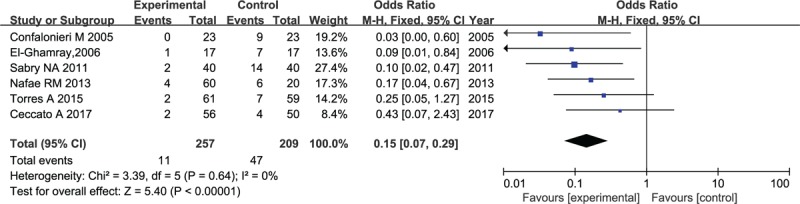
Forest plot for the incidence of septic shock in patients with corticosteroid versus placebo.
3.4.4. Incidence of mechanical ventilation
The incidence of mechanical ventilation was reported in a total of 507 patients with severe CAP. Without heterogeneity (I2 = 0%) among the 7 included studies, there was a significant difference detected in the outcome where a high number of patients who received placebo required mechanical ventilation compared to patients who received corticosteroid (OR: 0.32, 95% CI, 0.20–0.52, P < .001, Fig. 8), providing sufficient evidence of a benefit of corticosteroid for the reduction of lung injury and oxygenation improvement.
Figure 8.
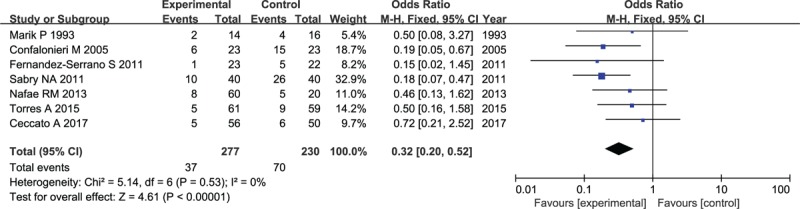
Forest plot for the need of mechanical ventilation in patients with corticosteroid versus placebo.
3.4.5. Incidence of adverse events
3.4.5.1. Incidence of upper gastrointestinal bleeding
Six out of 10 studies that eventually included in the meta-analysis were applicable for the incidence of upper gastrointestinal bleeding. As indicated in Figure 9, no heterogeneity was found across trials (I2 = 0%) and the pooled outcome performed by the fixed-effects model implied that there was no difference in the endpoint of upper gastrointestinal bleeding between patients treated with corticosteroid and those who received antimicrobial therapy alone (OR: 0.83, 95% CI, 0.27–2.52, P = .74).
Figure 9.
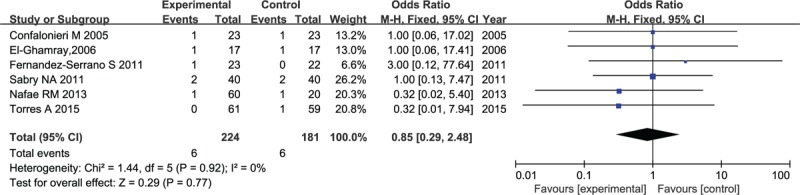
Forest plot for incidence of upper gastrointestinal bleeding in patients with corticosteroid versus placebo.
3.4.5.2. Incidence of super infection
Super infection outcome was reported in 3 published RCTs, comprising a total of 259 patients. There was a significant evidence of high heterogeneity among these studies (I2 = 59%). Conducting by random-effects model, the corticosteroid group had no significant difference from the placebo group in the rate of super infection (OR: 1.11, 95% CI, 0.14–9.13, P = .92), as illustrated by the forest plot shown in Figure 10.
Figure 10.
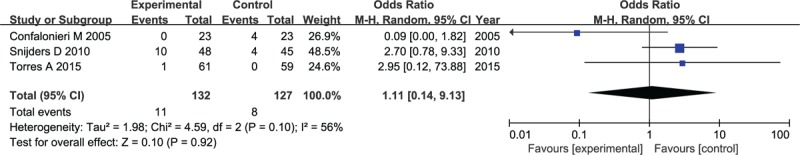
Forest plot for incidence of super infection in patients with corticosteroid versus placebo.
3.4.5.3. Incidence of hyperglycemia
The data about incidence of hyperglycemia were available for 4 studies comprising 338 patients. No heterogeneity was found among trials (I2 = 0%). Although corticosteroid was associated with an increased risk of hyperglycemia, as determined by the forest plot presented in Figure 11, there was no statistically significant difference observed in the occurrence of hyperglycemia reported in 192 patients receiving corticosteroid compared with 146 patients receiving antimicrobial therapy alone (OR: 1.3, 95% CI, 0.68–2.49, P = .42).
Figure 11.
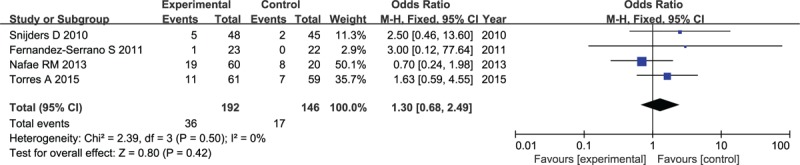
Forest plot for incidence of hyperglycemia in patients with corticosteroid versus placebo.
3.5. Sensitive analysis
Most of the consistency test carried out in each comparison indicated no or low degree of heterogeneity with I2 ranged from 0% to 25%, except for comparison on the level of CRP and the risk of super infection where heterogeneity was significantly higher up to 59% and 56%, respectively. Hence, sensitive analysis was required for outcomes of CRP level and the incidence of super infection to search for the source of heterogeneity. When comparing CRP level associated with corticosteroid versus antimicrobial therapy alone in patients with severe CAP, the study by Sarbry 2011 was found probably to affect the stability of the pooled results following the removal of included studies one by one. Exclusion of this study did not significantly alter the treatment estimates with regard to the overall CRP level, whereas statistically significance was lost on the contrary (SMD: −1.1, 95% CI, −1.51 to −0.69, P < .001, I2 = 0%), revealing the source of heterogeneity.
In addition, we obtained similar results where no association between super infection with corticosteroid was detected in the meta-analysis conducted by sensitive analysis through dislodging each individual trail at a time. In the process of analysis, the study by Conflonieri 2005 contributed to the inner-study heterogeneity. Similarly, no heterogeneity was obtained after the elimination of this study. Notably, the P value calculated again without Conflonieri's study in treatment estimate decreased significantly with regard to the overall analysis but the difference was not significant enough for the incidence of super infection statistically (OR: 2.73, 95% CI, 0.86–8.69, P = .09, I2 = 0%). However, the reason of the high heterogeneity triggered by Conflonieri M might be ascribe to the higher baseline of complications in patients with severe CAP comprising hypertension, diabetes, ischemic heart disease, alcoholic hepatitis, and so on, which lead to the decrease in the immune function.
4. Discussion
The crucial element that determines the prognosis of severe CAP is the inflammatory response of the host, which aims to destroy the microorganism and control infection, whereas excessive inflammatory response will cause damage to the host on contrary, leading to early death of patients with severe CAP when it goes worse. Corticosteroid as the most effective anti-inflammatory medicine can not only control the excessive inflammatory response, but also supply a sufficient evidence of benefit to adrenal insufficiency triggered by severe CAP, Mikami investigation indicated, which the insufficiency of adrenal hormone secretion was ameliorated significantly, decreasing the occurrence of CIRCI. Despite glucocorticoids had certain curative effect on patients with severe CAP, the application of GCS may lead to serious side effects, such as super infection, upper gastrointestinal bleeding, hyperglycemia, and so on, in patients with severe CAP. Therefore, whether systemic glucocorticoids can improve the prognosis and safety of patients with severe CAP has become the most controversial topic at present.
This paper systematically reviewed the literature and meta-analysis on the clinical efficacy of systemic glucocorticoids therapy in patients with severe community-acquired pneumonia, involving 665 patients included in a total of 10 randomized controlled trials where 357 was assigned to corticosteroid group and 308 to placebo group. To the best of our knowledge, this is the most updated and comprehensive meta-analysis to now. Our principle findings were as follows: systematically adjunctive corticosteroid treatment was superior compared with antimicrobial therapy merely in reducing all-cause mortality in patients with severe CAP. Compared with conventional therapy, corticosteroid was associated with a significant reduction of C-reactive protein on day 8. Corticosteroid was associated with a reduced risk of severe complications (the incidence of septic shock and the need for mechanical ventilation) in patients with severe CAP. Corticosteroid was associated with an increased risk of super infection, but there were no statistically significant differences in terms of the risks of upper gastrointestinal bleeding, hyperglycemia or super infection.
Whether patients with severe CAP benefited from adjunctive corticosteroid has been a long-debated issue, with conflicting evidence reported in different literatures. The findings of this systematic review and meta-analysis comparing adjunctive corticosteroid treatment with antimicrobial therapy suggested that adjunctive corticosteroid was significantly superior to conventional treatment in decreasing all-cause mortality in patients with severe CAP. Although given the adverse effect of corticosteroid on patients with severe CAP, how to maximize therapeutic effect while minimizing adverse effect is a key point that should be carefully considered by clinicians so that they can make optimal corticosteroid therapy decision. To date, the optimal corticosteroid therapeutic regimen for patients with severe CAP has not been fully explored currently. Thus, subgroup analysis of all-cause mortality was performed in terms of dose, duration, and dosage modus to estimate the approximate range of above index, respectively.
The dosage modus of corticosteroid is the most crucial tissue that has an impact on efficacy and safety. In contrast with previous meta-analysis, it is the first time that we have been able to look at the effects of corticosteroid administration on all-cause mortality where the mode delivering a maintenance dose after a bolus was associated with a more significant reduction in death in patients with severe CAP compared with the mode delivering single maintenance dose. However, as only 4 RCTs were reported to be undergoing the administration of maintenance dose after a bolus, it is regrettable that there is not enough data included in the RCTs to be used for statistically analysis to derive the optimal dosage of maintenance and loading dose. As for the duration of corticosteroid, despite the trials, such as Horita et al, that showed no difference in patients with long-term use of corticosteroid versus those with a short-term course, our meta analysis demonstrate an apparent benefit in all-cause mortality where the death was significantly lower in patients receiving prolonged use of corticosteroid (>5 days [median of duration ranging from 1 to 9 days in included studies]) compared with those receiving a short course (<5 days). Still, it is worth noting that long-period use of corticosteroid tends to increase the risk of complications, such as upper gastrointestinal bleeding, whereas no related detailed data on the upper limit of the suitable duration of corticosteroid is available in the published randomized controlled trails at present. As a result, we can draw a conclusion that duration of at least 5 days is the most effective, acceptable safe and well tolerated. In addition, dose is also a critical factor affecting the magnitude of corticosteroid action. Dose in the included studies ranging from 40 to 176 mg prednisone per day was put into subgroup analysis with a median of 86 mg/d, which indicated that a dose of <86 mg/d prednisone was the optimal dose of corticosteroid that maximized therapeutic effect, as well as reducing the risk of complications at the same time.
In terms of efficacy outcomes, the decreased risk of septic shock, along with the dramatic reduction in CRP level on day 8 and the requirement of mechanical ventilation, might provide an explanation to the possible risk reduction in death. First, consistent with other previous systematic reviews and meta-analysis, a large pooled analysis of 3 randomized trials found that corticosteroid was associated with more significant and faster reduction in CRP level during the 8 days after hospitalization. Inflammatory response in patients with severe CAP is a process of balancing inflammatory mediator. When the balance is broken, the various inflammatory cell excessively releases inflammatory mediators that stimulate the release of mononuclear cells and inflammatory transmitters to form inflammatory waterfalls, which, in turn, produce local and systemic inflammatory responses, destroy organization, and allow bacteria enter blood, resulting in death from SIRS, sepsis, septic shock, or multiple organ dysfunction (MODS). Therefore, it is of potential value for the measurement of various inflammatory biomarkers, consisting of CRP, PCT, IL-6/8/10, various cytokines, TNF, and so on, to predict the severity and prognosis of the disease. Although the included data of RCTs were not sufficient for a complete meta-analysis except for the C-reactive protein level, so the prediction of the inflammatory status was performed with C-reactive protein merely. Our meta-analysis was consistent with the demonstration that corticosteroid showed additional positive benefits on inhibition of excessive inflammatory response compared with antimicrobial therapy.[22] Second, severe CAP could be associated with CIRCI which might lead to insufficient GCS activity to lower the inflammatory response. A prospective study performed by Cornelia Mueller[23] confirmed above theory. As a marker of stress, serum cortisol showed the activation degree of HPA axis, reflecting the severity of disease. Meanwhile, researchers also found that the adrenal function measured by DHEA, which could be used as a prognostic indicator sepsis, was associated with the severity of CAP. However, both cortisol and DHEA were predictive indicators of mortality. Thus, the application of adjunctive corticosteroid supplemented the shortage of GCS, thereby inhibiting progression of inflammatory and cutting down patients in death. In our meta-analysis, the finding was not confirmed in sequential analysis, which disclosed an insufficient evidence for this association with only one study reported vary in adrenocortical hormone level before and after treatment.[24] Third, in contrast to the trials led by Torres et al where the proportion of patients suffering septic shock and in need of mechanical ventilation did not differ between groups, our meta-analysis suggested that adjunctive corticosteroid regimens significantly lowered the risk of septic shock and the incidence of mechanical ventilation, with consistent homogeneity between the trials.
As regard to safety outcomes, limited by insufficient data in adverse events of corticosteroid, involving super infection, upper gastrointestinal bleeding, acute kidney injury, acute liver failure, muscle weakness or muscle atrophy, lower extremity thrombosis, fracture, and so on, this paper evaluated the safety of corticosteroid therapy for severe CAP in terms of the risk of secondary infection, along with upper gastrointestinal bleeding and hyperglycemia. In this meta-analysis, there was no difference in terms of the incidence of upper gastrointestinal bleeding and hyperglycemia. In addition, corticosteroid-based regimens did not statistically reduce the risk of super infection, despite it was associated with an increased risk of super infection. Consistent with the data in our study, previous studies of community-acquired pneumonia found no evidence of increased risk for nosocomial infections or high rates of other potentially adverse events in corticosteroid-treated patients,[10,13,16] except for study by Confalonieri[15] (high super infection induced by corticosteroid) and study by Nafae[20] (hyperglycemia induced by corticosteroid). However, due to small sample size for each indication, the current analysis still would not confirm that glucocorticoids treatment would not lead to serious side effects such as super infection, gastrointestinal bleeding and hyperglycemia in patients with severe CAP. Thus, a prospective study is warranted for such conclusion.
5. Limitation
The findings of this study should be interpreted cautiously as there are several potential limitations that should be carefully addressed. First, the potential role of adrenal function as an important marker for assessing the effect of corticosteroid has not been directly appraised restrained by insufficient data. Second, as there is no general agreement on standard definition of scoring system in severe CAP, the clinical heterogeneity inherent among the included studies could not be resolved. In addition, the severity, along with complexity and laboratory examination of the enrolled patients, is difficult to reach a unified standard. Third, it is underpowered to assess the risk of death as demonstrated by analysis because of a lack of long-term follow-up data. Fourth, the sample size of the included literatures and studies is small, which has a significant impact on the reliability of the results.
6. Conclusion
Adjunctive corticosteroid yielded favorable outcomes in the treatment of SCAP as evidenced by decreased all-cause mortality, incidence of septic shock and requirement for mechanical ventilation without increasing risk of adverse events. Low-dose (≤86 mg/d), prolonged use (>5 days) of corticosteroid in dosage modus of a maintenance dose after a bolus can be recommended as preferred regimen to guard against SCAP.
Acknowledgment
We thank the patients taking part in the original studies.
Author contributions
SJ and KW are the primary authors who are responsible for the entire project. TL, YH, and RL contributed to the systematic literature review and database search. XD and XJ performed the data collection and reference search. SJ and YW analyzed the data and drafted the writing of the manuscript. KW drafted the first revision of the manuscript. All authors approved the interpretation of the results and took part in the final revision of the manuscript.
Conceptualization: Yuxin Hu, Ranwei Li, Xin Di, Xin Jin.
Data curation: Xin Di, Xin Jin, Yanqiao Wang.
Formal analysis: Shan Jiang, Yanqiao Wang.
Funding acquisition: Xin Di, Xin Jin, Ke Wang.
Investigation: Tiecheng Liu, Yuxin Hu.
Methodology: Ranwei Li, Xin Di, Xin Jin.
Project administration: Ke Wang.
Resources: Tiecheng Liu, Yuxin Hu, Xin Di, Xin Jin.
Software: Yanqiao Wang.
Supervision: Ranwei Li, Ke Wang.
Writing – original draft: Shan Jiang.
Writing – review and editing: Ke Wang.
Footnotes
Abbreviations: ATS = American Thoracic Society, BTS = England Thoracic Society, CAP = community acquired pneumonia, CIRCI = critical illness-related corticosteroid insufficiency, CRP = C-reactive protein, DB = double blind, OR = odds ratio, RCT = randomized controlled trial, RR = risk ratio, SCAP = severe community acquired pneumonia, SIRS = systemic inflammatory response syndrome, SMD = standard mean difference.
Availability of data and materials: All relevant data supporting the conclusion of this study are included within the paper.
This work was supported by Special Preferential Support Project for Medical Health (No. 20191102012YY) for Ranwei Li and Technological Breakthrough and Social Development Project (No.20190303162SF) for Ke Wang.
Ethics Approval and consent to participate: Ethics approval was not applicable for this meta-analysis.
The authors have no conflicts of interest to disclose.
References
- [1].Qu JM, Cao B. Guidelines for the diagnosis and treatment of adult community acquired pneumonia in China (2016 Edition). Zhonghua Jie He He Hu Xi Za Zhi 2016;39:241–2. [DOI] [PubMed] [Google Scholar]
- [2].Sibila O, Ferrer M, Agustí C, et al. Corticosteroids as adjunctive treatment in community-acquired pneumonia. Minerva Anestesiol 2014;80:1336–44. [PubMed] [Google Scholar]
- [3].Tian XL, Jiang M, Sun XF, et al. The indications for glucocorticoids in treating community-acquired pneumonia in adults: a meta-analysis. Zhonghua Jie He He Hu Xi Za Zhi 2016;39:280–5. [DOI] [PubMed] [Google Scholar]
- [4].Welte T, Torres A, Nathwani D. Clinical and economic burden of community- acquired pneumonia among adults in Europe. Thorax 2012;67:71–9. [DOI] [PubMed] [Google Scholar]
- [5].De Pascale G, Bello G, Antonelli M. Steroids in severe pneumonia: a literature review. Minerva Anestesiol 2011;77:902–10. [PubMed] [Google Scholar]
- [6].Moran JL, Graham PL, Rockliff S, et al. Updating the evidence for the role of corticosteroids in severe sepsis and septic shock: a Bayesian meta-analytic perspective. Crit Care 2010;14:R134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tagami T, Matsui H, Horiguchi H, et al. Low-dose corticosteroid use and mortality in severe community-acquired pneumonia patients. Eur Respir J 2015;45:463–72. [DOI] [PubMed] [Google Scholar]
- [8].Sibila O, Mortensen EM, Anzueto A, et al. Prior cardiovascular disease increases long-term mortality in COPD patients with pneumonia. Eur Respir J 2014;43:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Marik PE, Pastores SM, Annane D, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med 2008;36:1937–49. [DOI] [PubMed] [Google Scholar]
- [10].Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA 2015;313:677–86. [DOI] [PubMed] [Google Scholar]
- [11].Gu H, Wang Y, Li W. The application of cochrane risk of bias tools in meta-analysis of RCT. Chinese Circ J 2014;29:147–8. [Google Scholar]
- [12].Sabry NA, Omar EE. Corticosteroids and ICU course of community acquired pneumonia in Egyptian settings. Pharmacol Pharm 2011;2:73–81. [Google Scholar]
- [13].Fernandez-Serrano S, Dorca J, Garcia-Vidal C, et al. Effect of corticosteroids on the clinical course of community-acquired pneumonia: a randomized controlled trial. Crit Care 2011;15:R96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mikami K, Suzuki M, Kitagawa H, et al. Efficacy of corticosteroids in the treatment of community-acquired pneumonia requiring hospitalization. Lung 2007;185:249–55. [DOI] [PubMed] [Google Scholar]
- [15].Confalonieri M, Urbino R, Potena A, et al. Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med 2005;171:242–8. [DOI] [PubMed] [Google Scholar]
- [16].Snijders D, Daniels JM, de Graaff CS, et al. Efficacy of corticosteroids in community-acquired pneumonia: a randomized double-blinded clinical trial. Am J Respir Crit Care Med 2010;181:975–82. [DOI] [PubMed] [Google Scholar]
- [17].Ceccato A, Cilloniz C, Ranzani OT, et al. Treatment with macrolides and glucocorticosteroids in severe community-acquired pneumonia: A post-hoc exploratory analysis of a randomized controlled trial. PLoS One 2017;12:e0178022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Marik P, Kraus P, Sribante J, et al. Hydrocortisone and tumor necrosis factor in severe community-acquired pneumonia. A randomized controlled study. Chest 1993;104:389–92. [DOI] [PubMed] [Google Scholar]
- [19].El-Ghamrawy AH, Shokeir MH, Esmat AA. Effects of low-dose hydrocortisone in ICU patients with severe community-acquired pneumonia. Egypt J Chest 2006;55:91–9. [Google Scholar]
- [20].Nafae RM, Ragab MI, Amany FM, et al. Adjuvant role of corticosteroids in the treatment of community-acquired pneumonia. Egypt J Chest Dis Tuberc 2013;62:439–45. [Google Scholar]
- [21].Fernandez-Botran R, Uriarte SM, Arnold FW, et al. Contrasting inflammatory responses in severe and non-severe community-acquired pneumonia. Inflammation 2014;37:1158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lorenzo M-J, Moret I, Sarria B, et al. Lung inflammatory pattern and antibiotic treatment in pneumonia. Respir Res 2015;16:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mueller C, Blum CA, Trummler M, et al. Christ-Crain M. Association of adrenal function and disease severity in community-acquired pneumonia. PLoS One 2014;9:e99518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ariani F, Liu K, Jing Z, et al. Glucocorticosteroid in treatment of severe pneumonia. Mediators Inflamm 2013;2013:865635. [DOI] [PMC free article] [PubMed] [Google Scholar]


