Abstract
Urinary catheterization can cause catheter-related bladder discomfort (CRBD). Ketorolac is widely used for pain control. Therefore, we evaluated the effect of ketorolac on the prevention of CRBD in patients undergoing robot-assisted laparoscopic radical prostatectomy (RALP). All patients were randomly allocated to the ketorolac group or the control group. The primary outcome was CRBD above a moderate grade at 0 h postoperatively. CRBD above a moderate grade at 1, 2, and 6 h was also assessed. Postoperative pain, opioid requirement, ketorolac-related complications, patient satisfaction, and hospitalization duration were also assessed. The incidence of CRBD above a moderate grade at 0 h postoperatively was significantly lower in the ketorolac group (21.5% vs. 50.8%, p = 0.001) as were those at 1, 2, and 6 h. Pain scores at 0 and 1 h and opioid requirement over 24 h were significantly lower in the ketorolac group, while patient satisfaction scores were significantly higher in the ketorolac group. Ketorolac-related complications and hospitalization duration were not significantly different between the two groups. This study shows ketorolac can reduce postoperative CRBD above a moderate grade and increase patient satisfaction in patients undergoing RALP, suggesting it is a useful option to prevent postoperative CRBD.
Keywords: catheter-related bladder discomfort, ketorolac, robot-assisted laparoscopic prostatectomy
1. Introduction
Urinary catheterization is generally used during surgery, although it can cause postoperative catheter-related bladder discomfort (CRBD) [1]. CRBD is characterized by discomfort in the suprapubic region, manifesting as urinary urgency and frequency with or without urge incontinence [1,2]. The incidence of CRBD is 47–90% in patients who have undergone elective surgery [3]. CRBD that is above a moderate grade, which is often intolerable and requires treatment [4,5], occurs in 38–57% of patients with a urinary bladder catheter in a post-anesthesia care unit (PACU) [6,7]. Indwelling urinary catheter size and male gender are known independent predictors of CRBD above a moderate grade in the PACU [8]. CRBD can be so disturbing to patients that it is accompanied by postoperative agitation, poor satisfaction of a hospital day, extension of hospital stay, and an increased workload for medical staff [3].
For postoperative pain management, multimodal techniques that include administration of two or more drugs of different mechanisms have been used [9]. Nonsteroidal anti-inflammatory drugs (NSAIDs), cyclooxygenase-2 selective NSAIDs, and opioids should be individualized for the multimodal technique [9]. Opioid and non-opioid analgesics have been administered to 58.9% and 96.5% of patients to manage postoperative pain in general surgery, respectively [10]. However, in contrast to postoperative pain, CRBD has been under-treated and may be resistant to conventional pain management such as opioids because different mechanisms are involved in developing postoperative pain and CRBD [1]. CRBD is induced by involuntary contractions of the urinary bladder mediated by type 3 muscarinic receptor activation [4]. Agents associated with anti-muscarinic effects, such as tolterodine [11], oxybutynin [12], butylscopolamine [2,13], ketamine [4,14], robinul [15], and dexmedetomidine [6], have been used to prevent and manage CRBD, with varying degrees of success. However, these agents are limited because of various adverse effects, including dry mouth, postoperative nausea and vomiting, facial flushing, and blurred vision [1].
Ketorolac tromethamine, a NSAID, induces a decrease in prostaglandin levels driven by the inhibition of cyclooxygenase [16]. Ketorolac is widely used as an analgesic in various clinical practices [17,18]. In particular, multimodal analgesia including ketorolac is recommended for effective postoperative pain management [19]. However, the preventive effect of ketorolac on postoperative CRBD has not been studied. Based on these considerations, we hypothesized that ketorolac could prevent CRBD after surgery in male patients requiring urinary catheterization. We evaluated the effect of ketorolac on the prevention of postoperative CRBD above a moderate grade in patients undergoing robot-assisted laparoscopic radical prostatectomy (RALP).
2. Materials and Methods
This prospective, randomized, double-blinded, placebo-controlled study was approved by the Institutional Review Board of Asan Medical Center, Seoul, Korea (2018-0749). This study was registered at the Clinical Research Information Service (KCT0003064).
2.1. Patients
Patients aged 20–79 years of age with an American Society of Anesthesiologists physical status ≤2, who were scheduled to undergo elective RALP and voluntarily agreed to this clinical study, were enrolled as participants. Patients with chronic kidney disease, uncontrolled hypertension, morbid obesity, psychiatric disorders, a history of preexisting bladder disease, gastrointestinal ulcers or perforation, hemorrhage, coagulopathy, or asthma were excluded. Those with a history of ketorolac allergy were also excluded.
2.2. Randomization, Concealment, and Blinding
Patients enrolled in the present study were randomized. For the randomization, a web-based randomization software (Random Allocation Software version 1.0, Isfahan University of Medical Sciences, Isfahan, Iran) was used. Randomization was determined with block sizes of four and an allocation ratio of 1:1. Eligible participants were allocated to be given either intravenous ketorolac (ketorolac group) or intravenous normal saline as a placebo (control group) by a computer-generated randomization schedule.
The randomization codes were enclosed in sequentially numbered, identical, opaque, and sealed envelopes. The envelope was kept in a closed box during the study period. The codes were concealed by the first investigator and were given to the second investigator, who prepared the medications of either ketorolac or normal saline. These medications were prepared in identical syringes with a volume of 2 mL, and labelled with the patients’ names and hospital registration numbers. After the completion of urethrovesical anastomosis during surgery, the medications were administered by the third investigator, who was blinded to the allocation groups. The fourth investigator, who was also blinded to the allocation groups, assessed the outcomes of the study. All other investigators and participants, except the first and second investigators, did not know the group allocation until the data analyses were completed.
2.3. Anesthetic and Surgical Techniques
Before surgery, patients were instructed on symptoms of CRBD (i.e., a burning sensation with an urge to void, or discomfort in the suprapubic area). No premedication was administered before the induction of general anesthesia.
On arrival to the operating room, patient monitoring was performed according to our institutional standards. Intraoperative monitoring included electrocardiography, intra-arterial blood pressure, end-tidal carbon dioxide concentration, and peripheral oxygen saturation. Anesthesia was induced with 5 mg/kg thiopental sodium and target-controlled infusion of remifentanil (Orchestra Base Primea; Fresenius Kabi, Bad Homburg, Germany) with an effect site concentration of 2 ng/mL. Subsequently, 0.6 mg/kg rocuronium bromide was given to facilitate tracheal intubation. Anesthesia was maintained with 2 to 3 vol% sevoflurane and 50% oxygen in medical air. Also, the effect site concentration of remifentanil target-controlled infusion was adjusted to between 2 and 5 ng/mL. The depth of anesthesia was monitored using the bispectral index (A-1050 Monitor; Aspect Medical Systems, Newton, MA, USA), which was maintained between 40 and 60. Systolic blood pressure was maintained at 80–130 mmHg and heart rate was maintained at 60–100 beat/min. Sevoflurane administration and remifentanil target-controlled infusion were intermittently adjusted during surgery according to the bispectral index and hemodynamic parameters. Train-of-four monitoring was used for neuromuscular blockade monitoring. Rocuronium bromide was administered intermittently to maintain train-of-four ≤2 throughout surgery. Mechanical ventilation using an anesthetic machine (Primus; Dräger, Lübeck, Germany) was performed using a fixed tidal volume of 8 mL/kg (ideal body weight) and a respiratory rate of 10–16 frequency/min to maintain an end-tidal carbon dioxide concentration of 35–40 mmHg. A positive end-expiratory pressure of 5 cm H2O was applied. The maintenance infusion rate of Plasma Solution A (CJ Pharmaceutical, Seoul, Korea) as a crystalloid fluid was 2–4 mL/kg/h. A colloid fluid was not used during RALP.
The RALP was performed according to our standard protocols using the da Vinci robot system (Intuitive Surgical, Inc., Sunnyvale, CA, USA). During surgery, all patients were placed in the Trendelenburg position and pneumoperitoneum, which was achieved by continuous carbon dioxide insufflation maintaining an intra-abdominal pressure of 12 cm H2O. After skin sterilization for surgery, urinary bladder catheterization was performed with a 16 to 20-Fr Foley catheter after lubrication with lidocaine jelly, and its balloon was inflated with 10 mL of normal saline. To access the space of Retzius, bladder mobilization was performed. A transperitoneal antegrade approach was used to dissect the prostate, and nerve sparing was carried out on all patients on sides that were not suspected for extension of cancer. Pelvis lymph node dissection was performed in intermediate- to high-risk groups as designated by the D’Amico criteria [20]. Urethrovesical anastomosis was performed with a continuous suture.
Patients were given either ketorolac (30 mg) or an equivalent volume of placebo (0.9% normal saline) intravenously just after urethrovesical anastomosis. At the end of the procedure, sugammadex 2 mg/kg was administered to antagonize residual neuromuscular block after confirming that train-of-four count was ≥2. In the PACU, when the patient reported CRBD above a moderate grade, 50 mg tramadol was administered intravenously as rescue therapy to decrease CRBD as tramadol has a potent antimuscarinic effect [21]. Postoperative pain, defined as sharp pain at the surgical site, was assessed using a numerical rating scale (0 = no pain to 10 = worst imaginable pain). In case of postoperative pain without urgency or suprapubic discomfort like CRBD, 50 μg fentanyl was administered as rescue therapy when pain scores were ≥4 on a numeric rating scale. If the patient complained of both CRBD and postoperative pain, we treated the main chief complaint by either tramadol or fentanyl, and then reassessed the patient.
2.4. Assessments
Patient characteristics assessed were age, sex, body mass index, American Society of Anesthesiologists physical status, underlying disease (diabetes mellitus and hypertension), Gleason score, and tumor category. Gleason score was assessed by adding the numeric value of the two most prevalent differentiation patterns on histology obtained during preoperative transrectal ultrasound-guided needle biopsy [22]. Gleason scores were categorized into three groups: under 7, equal to 7, and above 7 [23,24,25]. Tumor category was assessed by digital rectal exam, transrectal ultrasound-guided biopsy, and imaging studies performed preoperatively [26]. Tumor category was classified by the American Joint Committee on Cancer (AJCC) Cancer Staging Manual [27]. Intraoperative variables included operation time, intraoperative fluid administered, and urinary catheter size used.
CRBD above a moderate grade was assessed at 0, 1, 2, and 6 h postoperatively. CRBD above a moderate grade at 0 h postoperatively was assessed just after the patients were transferred to the PACU. The severity of CRBD was considered “mild” when reported by patients only on questioning, “moderate” when reported by patients on their own without questioning and not accompanied by any behavioral response, and “severe” when reported by patients on their own with accompanying behavioral responses, such as flailing limbs, a strong vocal response, or an attempt to remove the catheter [4].
Postoperative pain was assessed using a numerical rating scale at 0, 1, 2, and 6 h postoperatively. Doses of all opioids and tramadol administered to patients during the 24 h following surgery were converted to intravenous fentanyl equianalgesic doses according to published conversion factors (intravenous fentanyl 100 μg = intravenous tramadol 100 mg) [28,29]. Patient satisfaction was assessed with a seven-point Likert scale (1 = strongly dissatisfied, 2 = moderately dissatisfied, 3 = slightly dissatisfied, 4 = neutral, 5 = slightly satisfied, 6 = moderately satisfied, 7 = extremely satisfied) [30] at 6 h postoperatively.
Ketorolac-related complications included acute kidney injury, hemoglobin changes, gastrointestinal bleeding, and desaturation events. Acute kidney injury was assessed by Kidney Disease: Improving Global Outcomes (KDIGO) criteria. According to KDIGO criteria, acute kidney injury is defined as an increase in serum creatinine by 0.3 mg/dL or more within 48 h, or an increase in serum creatinine of 1.5 times or more within the prior 7 days. However, the urine output criterion was not included because of the inconsistency in urine output measurements [5]. Hemoglobin changes, calculated by subtracting preoperative hemoglobin levels from hemoglobin levels at postoperative day 1, were evaluated. Gastrointestinal bleeding during hospitalization was evaluated according to criteria fulfilling one or more of the following conditions: physician-documented frank hematemesis, physician-documented frank melena, heme-positive stool associated with a documented upper gastrointestinal lesion judged to be the source of the bleeding, and active upper gastrointestinal bleeding documented by endoscopy or angiography [31]. Desaturation events, defined as events of saturation below 90%, were assessed in the PACU or general ward until postoperative day 1 [32].
2.5. Primary and Secondary Outcomes
The primary outcome was CRBD above a moderate grade at 0 h postoperatively. The secondary outcomes were CRBD levels above a moderate grade at 1, 2, and 6 h postoperatively. Postoperative pain, postoperative opioid requirement, ketorolac-related complications, patient satisfaction, and hospitalization duration were also assessed.
2.6. Statistical Analysis
From the pilot study and our experience, 48% of patients complained of CRBD above a moderate grade at 0 h after RALP. We assumed that ketorolac might decrease the incidence of CRBD above a moderate grade by 50% (i.e., 48% vs. 24%). We calculated that 59 patients would be necessary for each group to acquire statistical significance, with α = 0.05 and β = 0.20. Considering a 10% dropout rate, 66 patients were included in each group.
The analyses were performed on a modified intention-to-treat basis, which included all randomly assigned participants with all eligible criteria, who performed the study intervention and did not withdraw consent to participate in this study. Data are expressed as mean ± standard deviation, median (interquartile range), or number (%) as appropriate. Normality was assessed using the Kolmogorov–Smirnov test. Categorical variables were compared using the chi-square test or Fisher’s exact test as appropriate. Continuous variables were compared using the independent t-test or Mann–Whitney U test as appropriate. The comparisons of postoperative pain scores between the two groups at each time point were analyzed by independent t-test and a p value < 0.0125 (0.05/4) was considered significant after using Bonferroni correction. Otherwise, a p value < 0.05 was considered significant. Statistical analysis was conducted using MedCalc (version 11.3.3.0; MedCalc Software bvba, Mariakerke, Belgium) and SPSS 21 for Windows (version 21.0.0; IBM Corporation, Chicago, IL, USA).
3. Results
The CONSORT flowchart of this study is presented in Figure 1. During the enrollment process, 143 patients were assessed for eligibility, and 11 patients were excluded. Therefore, a total of 132 patients were included in the present study. Two patients did not receive an intervention, as their surgeries were converted to open prostatectomies. Finally, 130 patients were included in the analysis. All patients were Asian. There were no significant differences in age, gender, body mass index, American Society of Anesthesiologists physical status, underlying disease, Gleason score, tumor category, operation time, intraoperative fluid, and urinary catheter size between the two groups (Table 1).
Figure 1.
A CONSORT flow chart.
Table 1.
Characteristics of study participants.
| Variables | Control Group (n = 65) | Ketorolac Group (n = 65) | p-Value |
|---|---|---|---|
| Age (years) | 66.7 ± 6.5 | 65.4 ± 7.0 | 0.282 |
| Sex (male/female) | 65/0 (100/0) | 65/0 (100/0) | 1.000 |
| BMI (kg/m2) | 24.5 (23.0–26.6) | 24.2 (22.4–25.8) | 0.329 |
| ASA PS | 0.440 | ||
| 1/2 | 2/63 (3.1/96.9) | 5/60 (7.7/92.3) | |
| DM | 9 (13.8) | 9 (13.8) | 1.000 |
| Hypertension | 27 (41.5) | 30 (46.2) | 0.596 |
| Gleason score (points) | 0.940 | ||
| ≤6 | 15 (23.1) | 16 (24.6) | |
| 7 | 33 (50.8) | 31 (47.7) | |
| >7 | 17 (26.2) | 18 (27.7) | |
| T category | 0.720 | ||
| T2 | 38 (58.5) | 40 (61.5) | |
| T3a | 24 (36.9) | 18 (27.7) | |
| T3b | 2 (3.1) | 6 (4.0) | |
| T4 | 1 (1.5) | 1 (1.5) | |
| Operation time (min) | 168.0 (156.5–186.5) | 167.0 (150.0–184.0) | 0.373 |
| Intraoperative fluid (mL) | 1100.0 (900.0–1325.0) | 1150.0 (800.0–1325.0) | 0.393 |
| Urinary catheter size (F) | 0.387 | ||
| 16/18/20 | 32 (49.2)/5 (7.7)/28 (43.1) | 30 (46.2)/10 (15.4)/25 (38.5) |
Data are presented as mean ± standard deviation, median (interquartile range), or number (%) as appropriate. BMI = body mass index; ASA PS = American Society of Anesthesiologists physical status; DM = diabetes mellitus; T category = tumor category.
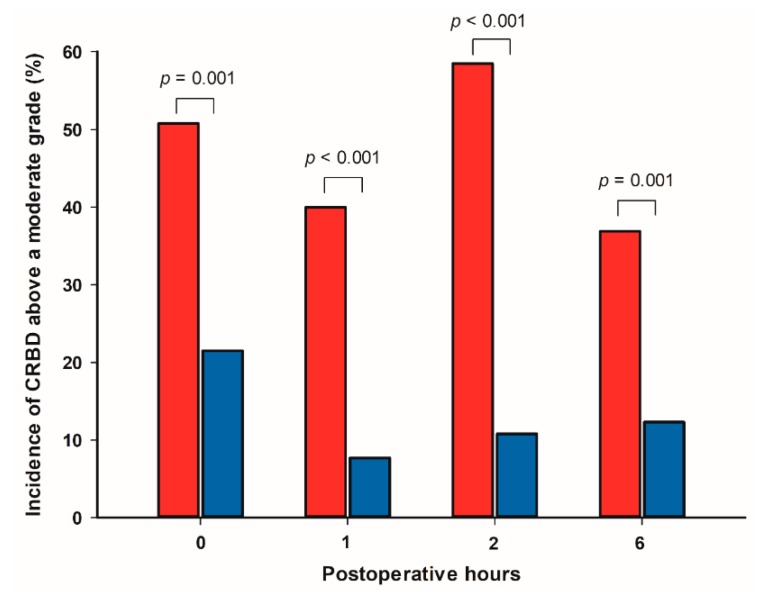
Incidences of CRBD at 0, 1, 2, and 6 h postoperatively are shown in Table 2. The incidence of CRBD above a moderate grade at 0 h postoperatively was significantly lower in the ketorolac group compared with the control group (14 (21.5%) vs. 33 (50.8%), p = 0.001) (Figure 2). In addition, incidences of CRBD above a moderate grade were significantly lower in the ketorolac group compared with the control group at 1, 2, and 6 h postoperatively (5 (7.7%) vs. 26 (40.0%), p < 0.001; 7 (10.8%) vs. 38 (58.5%), p < 0.001; 8 (12.3%) vs. 24 (36.9%), p = 0.001; respectively) (Figure 2).
Table 2.
Postoperative CRBD in patients undergoing robot-assisted laparoscopic radical prostatectomy.
| CRBD | Control Group (n = 65) | Ketorolac Group (n = 65) | p-Value |
|---|---|---|---|
| Postoperative hour 0 | <0.001 | ||
| None | 12 (18.5) | 20 (30.8) | |
| Mild | 20 (30.8) | 31 (47.7) | |
| Moderate | 17 (26.2) | 14 (21.5) | |
| Severe | 16 (24.6) | 0 (0.0) | |
| Postoperative hour 1 | <0.001 | ||
| None | 5 (7.7) | 9 (13.8) | |
| Mild | 34 (52.3) | 51 (78.5) | |
| Moderate | 25 (38.5) | 5 (7.7) | |
| Severe | 1 (1.5) | 0 (0.0) | |
| Postoperative hour 2 | <0.001 | ||
| None | 0 (0.0) | 1 (1.5) | |
| Mild | 27 (41.5) | 57 (87.7) | |
| Moderate | 38 (58.5) | 7 (10.8) | |
| Severe | 0 (0.0) | 0 (0.0) | |
| Postoperative hour 6 | <0.001 | ||
| None | 0 (0.0) | 16 (24.6) | |
| Mild | 41 (63.1) | 41 (63.1) | |
| Moderate | 24 (36.9) | 8 (12.3) | |
| Severe | 0 (0.0) | 0 (0.0) |
Data are presented as number (%). CRBD = catheter-related bladder discomfort; Postoperative hour 0 = upon admission to the postanesthetic care unit.
Figure 2.
Comparisons of incidences of CRBD above a moderate grade between the control group (red bar) and ketorolac group (blue bar) at 0, 1, 2, and 6 h postoperatively. Each column indicates the incidence of CRBD above a moderate grade. CRBD = catheter-related bladder discomfort; Postoperative hour 0 = upon admission to the postanesthetic care unit.
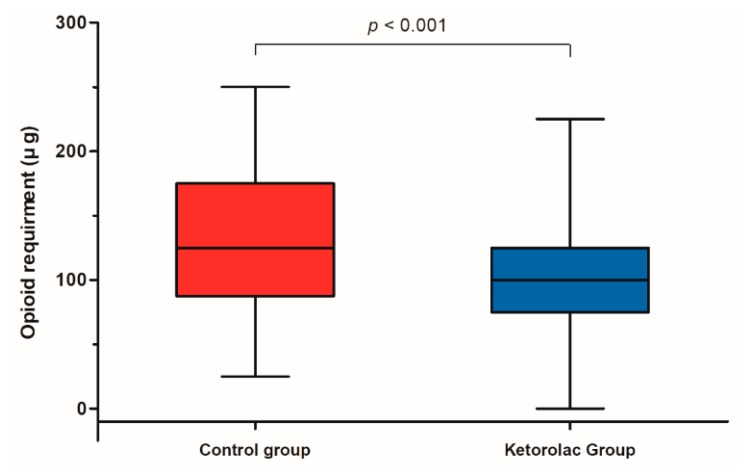
Pain scores at 0 and 1 h postoperatively were significantly lower in the ketorolac group than in the control group (p = 0.012, p = 0.007, respectively) (Table 3). However, pain scores at 2 and 6 h postoperatively did not significantly differ between the two groups (p = 0.766, p = 0.132, respectively). Opioid requirement during the 24 h following surgery was significantly lower in the ketorolac group than in the control group (100.0 μg (75.0–125.0) vs. 125.0 μg (87.5–175.0), p < 0.001) (Figure 3). There were no significant differences in acute kidney injury, hemoglobin changes, gastrointestinal bleeding, and desaturation events between the two groups (Table 3). Patient satisfaction scores were significantly higher in the ketorolac group than in the control group (5.0 (4.0–6.0) vs. 4.0 (4.0–4.0), p < 0.001) (Table 3). There was no significant difference in hospitalization duration between the two groups (7.0 days (5.0–7.0) vs. 7.0 days (5.0–7.0), p = 0.722) (Table 3).
Table 3.
Postoperative pain score, opioid requirement, ketorolac-related complications, patient satisfaction score, and hospitalization duration in patients undergoing robot-assisted laparoscopic radical prostatectomy.
| Variables | Control Group (n = 65) | Ketorolac Group (n = 65) | p-Value |
|---|---|---|---|
| Postoperative pain score | |||
| Postoperative hour 0 | 5.4 ± 1.1 | 4.8 ± 1.5 | 0.012 * |
| Postoperative hour 1 | 3.0 ± 1.3 | 2.5 ± 0.7 | 0.007 * |
| Postoperative hour 2 | 4.5 ± 1.5 | 4.4 ± 1.5 | 0.766 |
| Postoperative hour 6 | 2.9 ± 1.0 | 2.7 ± 0.9 | 0.132 |
| Opioid requirement during 24 h after surgery (μg) | 125.0 (87.5–175.0) | 100 (75.0–125.0) | <0.001 |
| Ketorolac-related complications | |||
| Acute kidney injury | 2 (3.7) | 1 (2.2) | >0.999 |
| Hemoglobin changes (mg/dL) | −1.7 ± 1.2 | −2.0 ± 1.1 | 0.148 |
| Gastrointestinal bleeding | 0 (0.0) | 0 (0.0) | 1.000 |
| Desaturation events | 1 (1.5) | 0 (0.0) | >0.999 |
| Patient satisfaction score | 4.0 (4.0–4.0) | 5.0 (4.0–6.0) | <0.001 |
| Hospitalization duration (days) | 7.0 (5.0–7.0) | 7.0 (5.0–7.0) | 0.722 |
Data are presented as mean ± standard deviation, median (interquartile range), or number (%) as appropriate. Hemoglobin change is calculated by subtracting preoperative hemoglobin levels from hemoglobin levels at postoperative day 1. Desaturation events are defined as events of saturation below 90% confirmed in the post-anesthesia care unit or in the general ward until postoperative day 1. Postoperative hour 0 = upon admission to the postanesthetic care unit. * p < 0.0125 (Bonferroni-corrected significance level).
Figure 3.
Comparison of opioid requirements within 24 h after surgery between the control group (red box) and ketorolac group (blue box). The line inside the rectangle shows the median. The upper and lower ends of the box indicate the third quartile and first quartile, respectively. Whiskers above and below the box designate 90% and 10%, respectively.
4. Discussion
In the present study, we found that ketorolac administration significantly decreased the incidences of CRBD above a moderate grade, not only at 0 h postoperatively, but also at 1, 2, and 6 h in male patients undergoing RALP. In addition, pain scores were significantly lower in the ketorolac group than in the control group at 0 and 1 h, but not at 2 and 6 h. The opioid requirement during the 24 h following surgery was significantly lower in the ketorolac group compared with the control group. There were no significant differences in ketorolac-related complications between the two groups. Patient satisfaction scores were significantly higher in the ketorolac group compared with the control group.
Bladder urinary catheterization may induce CRBD after awakening from general anesthesia in the postoperative period [1]. CRBD is a known risk factor for emergence agitation occurring during anesthetic recovery [33]. The incidence of CRBD was higher in the early postoperative period compared with the late postoperative period. Therefore, CRBD is an important issue in the PACU, as patients who receive urinary catheterization usually stay in the PACU for about 1 h postoperatively [15,34,35]. In particular, urgent treatment may be needed for patients experiencing CRBD above a moderate grade, which occurs in 38–57% of patients in the PACU [5,6,7]. Unlike routine perioperative pain management, opioid administration may be regarded as ineffective for CRBD because of the difference between mechanisms of postoperative pain and CRBD [6,34]. For the mechanism of CRBD, the antimuscarinic actions, particularly type 3 muscarinic receptor blockade, are considered to be responsible for the effective management of postoperative CRBD. Therefore, antimuscarinic agents, such as oxybutynin, ketamine, tramadol, and gabapentin, have been evaluated for either treatment or prevention of CRBD [4,21,36]. However, even if such medications are used, the incidence of postoperative CRBD is reported to be high [4,21].
In our study, ketorolac effectively reduced the incidence of CRBD above a moderate grade after RALP. Ketorolac, an NSAID and cyclooxygenase inhibitor, inhibits prostaglandin synthesis [37]. Prostaglandins in the bladder, which increase with the occurrence of obstruction, inflammation, and mucosal injury in the urinary bladder [38], induce detrusor muscle contraction. In addition, capsaicin-sensitive C fibers activated by prostaglandins contract the bladder detrusor muscle [39,40,41]. Therefore, ketorolac is thought to reduce CRBD induced by the contraction of the detrusor muscle from the secretion of inflammatory substances and the synthesis of prostaglandins resulting from urinary catheterization. Similarly, paracetamol, which is not an NSAID but is another cyclooxygenase inhibitor, is reported to reduce CRBD scores 0.5, 1, 2, and 6 h after percutaneous nephrolithotomy [42]. However, paracetamol has various adverse effects [42]. Most importantly, paracetamol overdose is a common cause of acute liver failure [43]. Severe liver impairment or hepatic necrosis may occur when the maximum amount of the recommended dose is administered [44,45,46]. In patients with weakened hepatic function, paracetamol can more easily lead to hepatic impairment [44]. Renal impairment not associated with hepatic failure has also been reported [47].
We found that ketorolac decreased pain scores at 0 and 1 h postoperatively, but not at 2 and 6 h. This result may be, at least in part, because of the analgesic characteristics of ketorolac. It is known that 30 mg ketorolac is equivalent to 10 mg morphine sulfate or 100 μg fentanyl [28,29]. The peak effect of intravenous ketorolac tromethamine occurs at 75–150 min [18]. In the present study, ketorolac was administered just after the completion of urethrovesical anastomosis, with the surgery ending 30–60 min after ketorolac administration. Therefore, ketorolac administration may influence the postoperative pain score, especially at 0 and 1 h postoperatively.
NSAIDs such as ketorolac may uncommonly induce renal impairment, peptic ulceration, bleeding diathesis, gastrointestinal bleeding, respiratory distress, and asthma exacerbation [18]. Incidences of these side effects are reported to be 0.17% for renal failure, 1.04% for surgical bleeding, and 0.04% for gastrointestinal bleeding in patients intravenously administered 90 mg ketorolac tromethamine daily for 7 days after surgery [48,49]. Ketorolac is related with an increased risk of gastrointestinal bleeding and renal insufficiency in elderly patients with use exceeding 5 days and higher than 105 mg/day [18]. In the present study, there were no significant differences in acute kidney injury, hemoglobin changes, gastrointestinal bleeding, or desaturation events between the ketorolac group and control group. Therefore, we found that a single administration of 30 mg ketorolac tromethamine does not induce adverse effects associated with NSAID regimens postoperatively.
Unlike postoperative pain, CRBD might not be managed properly by clinicians. This seems to be partly because clinicians pay less attention to CRBD than postoperative pain. Moreover, conventional analgesics such as opioids may not manage CRBD effectively [1]. In our study, ketorolac reduced postoperative CRBD above a moderate grade and increased patient satisfaction in patients who underwent RALP. In addition, postoperative complications related to ketorolac were not significantly different between the two groups. Therefore, we assume that all patients requiring urinary catheterization can be administered ketorolac for preventing postoperative CRBD when there are no risk factors of side effects associated with NSAIDs, such as renal disease, gastrointestinal ulcers, coagulopathy, and asthma.
In the present study, patient satisfaction scores were significantly higher in the ketorolac group than in the control group. This may be explained by the reduction of CRBD above a moderate grade within 6 h after RALP in the ketorolac group. In addition, higher patient satisfaction scores are thought to be related to the reduction of pain scores at 0 and 1 h after surgery in the ketorolac group. Therefore, it seems feasible to administer ketorolac to prevent CRBD after RALP.
Our study has several limitations. First, we administered 30 mg ketorolac only once in the present study. Although this single dose of ketorolac had a beneficial effect on the prevention of CRBD above a moderate grade, we did not confirm whether this dose of ketorolac was the optimal dose to prevent CRBD. Therefore, further studies are needed to evaluate optimal doses of ketorolac to prevent CRBD in patients requiring urinary catheterization during surgery. Second, we administered ketorolac just after the completion of urethrovesical anastomosis, and we did not confirm whether this timing of ketorolac administration was optimal to prevent postoperative CRBD. Accordingly, further studies are needed to determine the optimal timing of ketorolac to prevent postoperative CRBD. Third, urinary urgency and urge incontinence are unique characteristics of CRBD, unlike nociceptive postoperative pain. However, it may be difficult to distinguish CRBD and postoperative pain by asking a patient about suprapubic pain after RALP. Therefore, further study will be needed to evaluate the effect of ketorolac on CRBD in patients undergoing surgery in another surgical site.
5. Conclusions
Ketorolac reduced the incidence of CRBD above a moderate grade in male patients undergoing RALP. In addition, 30 mg ketorolac administered intravenously during RALP was not associated with any adverse effects. These results suggest that ketorolac administration is an effective and safe option to prevent CRBD above a moderate grade in male patients undergoing RALP who require urinary catheterization.
Abbreviations
| CRBD | catheter-related bladder discomfort |
| PACU | post-anesthesia care unit |
| NSAIDs | nonsteroidal anti-inflammatory drugs |
| RALP | robot-assisted laparoscopic radical prostatectomy |
| KDIGO | Kidney Disease: IMPROVING Global Outcomes |
| SD | standard deviation |
| IQR | interquartile range |
| BMI | body mass index |
| ASA PS | American Society of Anesthesiologists physical status |
| DM | diabetes mellitus |
| T category | tumor category |
Author Contributions
Conceptualization, Y.-G.K. and Y.-K.K.; Methodology, J.-Y.P. and J.-H.H.; Validation, J.Y. and D.-H.K.; Formal analysis, J.-Y.P. and Y.-K.K.; Investigation, J.-Y.P., J.Y., D.-H.K. and G.-H.K.; Resources, J.-Y.P., J.Y., D.-H.K. and G.-H.K.; Data curation, S.-A.L.; Writing—Original Draft preparation, J.-Y.P.; Writing—Review and Editing, J.H.H., Y.-G.K. and Y.-K.K.; Visualization, J.-Y.P. and J.H.H.; Supervision, J.-H.H., Y.-G.K. and Y.-K.K.; Project administration, J.-Y.P., Y.-G.K. and Y.-K.K.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hu B., Li C., Pan M., Zhong M., Cao Y., Zhang N., Yuan H., Duan H. Strategies for the prevention of catheter-related bladder discomfort: A PRISMA-compliant systematic review and meta-analysis of randomized controlled trials. Medicine. 2016;95:e4859. doi: 10.1097/MD.0000000000004859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryu J., Hwang J., Lee J., Seo J., Park H., Oh A., Jeon Y., Do S. Efficacy of butylscopolamine for the treatment of catheter-related bladder discomfort: A prospective, randomized, placebo-controlled, double-blind study. Br. J. Anaesth. 2013;111:932–937. doi: 10.1093/bja/aet249. [DOI] [PubMed] [Google Scholar]
- 3.Bai Y., Wang X., Li X., Pu C., Yuan H., Tang Y., Li J., Wei Q., Han P. Management of catheter-related bladder discomfort in patients who underwent elective surgery. J. Endourol. 2015;29:640–649. doi: 10.1089/end.2014.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal A., Dhiraaj S., Singhal V., Kapoor R., Tandon M. Comparison of efficacy of oxybutynin and tolterodine for prevention of catheter related bladder discomfort: A prospective, randomized, placebo-controlled, double-blind study. Br. J. Anaesth. 2006;96:377–380. doi: 10.1093/bja/ael003. [DOI] [PubMed] [Google Scholar]
- 5.Ketteler M., Block G.A., Evenepoel P., Fukagawa M., Herzog C.A., McCann L., Moe S.M., Shroff R., Tonelli M.A., Toussaint N.D. Diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder: Synopsis of the kidney disease: Improving global outcomes 2017 clinical practice guideline update. Ann. Intern. Med. 2018;168:422–430. doi: 10.7326/M17-2640. [DOI] [PubMed] [Google Scholar]
- 6.Kim H.-C., Lee Y.-H., Jeon Y.-T., Hwang J.-W., Lim Y.-J., Park J.-E., Park H.-P. The effect of intraoperative dexmedetomidine on postoperative catheter-related bladder discomfort in patients undergoing transurethral bladder tumour resection: A double-blind randomised study. Eur. J. Anaesthesiol. 2015;32:596–601. doi: 10.1097/EJA.0000000000000196. [DOI] [PubMed] [Google Scholar]
- 7.Li X., Zhang X., Liu J., Bechu S.M., Yin X., Wan Q., Luo L., Zhu Y., Luo J. Efficacy of pudendal nerve block for alleviation of catheter-related bladder discomfort in male patients undergoing lower urinary tract surgeries: A randomized, controlled, double-blind trial. Medicine. 2017;96:8932. doi: 10.1097/MD.0000000000008932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li C., Liu Z., Yang F. Predictors of catheter-related bladder discomfort after urological surgery. J. Huazhong Univ. Sci. Technol. Med. Sci. 2014;34:559–562. doi: 10.1007/s11596-014-1315-z. [DOI] [PubMed] [Google Scholar]
- 9.American Society of Anesthesiologists Task Force on Acute Pain Management Practice guidelines for acute pain management in the perioperative setting: An updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116:248–273. doi: 10.1097/ALN.0b013e31823c1030. [DOI] [PubMed] [Google Scholar]
- 10.Polanco-Garcia M., Garcia-Lopez J., Fabregas N., Meissner W., Puig M.M. Postoperative Pain Management in Spanish Hospitals: A Cohort Study Using the PAIN-OUT Registry. J. Pain Off. J. Am. Pain Soc. 2017;18:1237–1252. doi: 10.1016/j.jpain.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Tijani K., Akanmu N., Olatosi J., Ojewola R. Role of tolterodine in the management of postoperative catheter-related bladder discomfort: Findings in a Nigerian teaching hospital. Niger. J. Clin. Pract. 2017;20:484–488. doi: 10.4103/1119-3077.196036. [DOI] [PubMed] [Google Scholar]
- 12.Tauzin-Fin P., Sesay M., Svartz L., Krol-Houdek M.-C., Maurette P. Sublingual oxybutynin reduces postoperative pain related to indwelling bladder catheter after radical retropubic prostatectomy. Br. J. Anaesth. 2007;99:572–575. doi: 10.1093/bja/aem232. [DOI] [PubMed] [Google Scholar]
- 13.Nam K., Seo J.-H., Ryu J.-H., Oh A.-Y., Lee T., Park H.-P., Jeon Y.-T., Hwang J.-W. Randomized, clinical trial on the preventive effects of butylscopolamine on early postoperative catheter-related bladder discomfort. Surgery. 2015;157:396–401. doi: 10.1016/j.surg.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 14.Shariat Moharari R., Lajevardi M., Khajavi M., Najafi A., Shariat Moharari G., Etezadi F. Effects of Intra-Operative Ketamine Administration on Postoperative Catheter-Related Bladder Discomfort: A Double-Blind Clinical Trial. Pain Pract. 2014;14:146–150. doi: 10.1111/papr.12055. [DOI] [PubMed] [Google Scholar]
- 15.Kim H.-C., Lim S.-M., Seo H., Park H.-P. Effect of glycopyrrolate versus atropine coadministered with neostigmine for reversal of rocuronium on postoperative catheter-related bladder discomfort in patients undergoing transurethral resection of bladder tumor: A prospective randomized study. J. Anesth. 2015;29:831–835. doi: 10.1007/s00540-015-2064-2. [DOI] [PubMed] [Google Scholar]
- 16.Ricciotti E., FitzGerald G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forrest J.B., Camu F., Greer I.A., Kehlet H., Abdalla M., Bonnet F., Ebrahim S., Escolar G., Jage J., Pocock S., et al. Ketorolac, diclofenac, and ketoprofen are equally safe for pain relief after major surgery. Br. J. Anaesth. 2002;88:227–233. doi: 10.1093/bja/88.2.227. [DOI] [PubMed] [Google Scholar]
- 18.Vadivelu N., Gowda A.M., Urman R.D., Jolly S., Kodumudi V., Maria M., Taylor R., Pergolizzi J.V. Ketorolac Tromethamine—Routes and Clinical Implications. Pain Pract. 2015;15:175–193. doi: 10.1111/papr.12198. [DOI] [PubMed] [Google Scholar]
- 19.Wick E.C., Grant M.C., Wu C.L. Postoperative Multimodal Analgesia Pain Management with Nonopioid Analgesics and Techniques: A Review. JAMA Surg. 2017;152:691–697. doi: 10.1001/jamasurg.2017.0898. [DOI] [PubMed] [Google Scholar]
- 20.D’Amico A.V., Whittington R., Malkowicz S.B., Schultz D., Blank K., Broderick G.A., Tomaszewski J.E., Renshaw A.A., Kaplan I., Beard C.J., et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal A., Yadav G., Gupta D., Singh P., Singh U. Evaluation of intra-operative tramadol for prevention of catheter-related bladder discomfort: A prospective, randomized, double-blind study. Br. J. Anaesth. 2008;101:506–510. doi: 10.1093/bja/aen217. [DOI] [PubMed] [Google Scholar]
- 22.King C.R. Patterns of prostate cancer biopsy grading: Trends and clinical implications. Int. J. Cancer. 2000;90:305–311. doi: 10.1002/1097-0215(20001220)90:6<305::AID-IJC1>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 23.Partin A.W., Kattan M.W., Subong E.N., Walsh P.C., Wojno K.J., Oesterling J.E., Scardino P.T., Pearson J. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer: A multi-institutional update. JAMA. 1997;277:1445–1451. doi: 10.1001/jama.1997.03540420041027. [DOI] [PubMed] [Google Scholar]
- 24.Epstein J.I., Zelefsky M.J., Sjoberg D.D., Nelson J.B., Egevad L., Magi-Galluzzi C., Vickers A.J., Parwani A.V., Reuter V.E., Fine S.W. A contemporary prostate cancer grading system: A validated alternative to the Gleason score. Eur. Urol. 2016;69:428–435. doi: 10.1016/j.eururo.2015.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Epstein J.I., Allsbrook W.C., Jr., Amin M.B., Egevad L.L., Committee I.G. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am. J. Surg. Pathol. 2005;29:1228–1242. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 26.Paner G.P., Stadler W.M., Hansel D.E., Montironi R., Lin D.W., Amin M.B. Updates in the Eighth Edition of the Tumor-Node-Metastasis Staging Classification for Urologic Cancers. Eur. Urol. 2018;73:560–569. doi: 10.1016/j.eururo.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 27.Edge S.B., Byrd D.R., Compton C.C., Fritz A.G., Greene F.L., Trotti A. JCC Cancer Staging Manual. 7th ed. Springer; New York, NY, USA: 2010. [Google Scholar]
- 28.Power I., Noble D.W., Douglas E., Spence A.A. Comparison of i.m. ketorolac trometamol and morphine sulphate for pain relief after cholecystectomy. Br. J. Anaesth. 1990;65:448–455. doi: 10.1093/bja/65.4.448. [DOI] [PubMed] [Google Scholar]
- 29.McPherson M.L. Demystifying Opioid Conversion Calculations: A Guide for Effective Dosing. ASHP; Bethesda, MD, USA: 2009. [Google Scholar]
- 30.Bhardwaj P., Yadav R.K. Measuring pain in clinical trials: Pain scales, endpoints, and challenges. Int. J. Clin. Exp. Physiol. 2015;2:151. doi: 10.4103/2348-8093.169965. [DOI] [Google Scholar]
- 31.Langman M.J., Jensen D.M., Watson D.J., Harper S.E., Zhao P.-L., Quan H., Bolognese J.A., Simon T.J. Adverse upper gastrointestinal effects of rofecoxib compared with NSAIDs. JAMA. 1999;282:1929–1933. doi: 10.1001/jama.282.20.1929. [DOI] [PubMed] [Google Scholar]
- 32.Siddiqui N., Arzola C., Teresi J., Fox G., Guerina L., Friedman Z. Predictors of desaturation in the postoperative anesthesia care unit: An observational study. J. Clin. Anesth. 2013;25:612–617. doi: 10.1016/j.jclinane.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 33.Lepouse C., Lautner C., Liu L., Gomis P., Leon A. Emergence delirium in adults in the post-anaesthesia care unit. Br. J. Anaesth. 2006;96:747–753. doi: 10.1093/bja/ael094. [DOI] [PubMed] [Google Scholar]
- 34.Kim H.C., Park H.P., Lee J., Jeong M.H., Lee K.H. Sevoflurane vs. propofol in post-operative catheter-related bladder discomfort: A prospective randomized study. Acta Anaesthesiol. Scand. 2017;61:773–780. doi: 10.1111/aas.12922. [DOI] [PubMed] [Google Scholar]
- 35.Kim H.-C., Hong W.-P., Lim Y.-J., Park H.-P. The effect of sevoflurane versus desflurane on postoperative catheter-related bladder discomfort in patients undergoing transurethral excision of a bladder tumour: A randomized controlled trial. Can. J. Anaesth. 2016;63:596–602. doi: 10.1007/s12630-016-0600-7. [DOI] [PubMed] [Google Scholar]
- 36.Agarwal A., Dhiraaj S., Pawar S., Kapoor R., Gupta D., Singh P.K. An evaluation of the efficacy of gabapentin for prevention of catheter-related bladder discomfort: A prospective, randomized, placebo-controlled, double-blind study. Anesth. Analg. 2007;105:1454–1457. doi: 10.1213/01.ane.0000281154.03887.2b. [DOI] [PubMed] [Google Scholar]
- 37.Green G.A. Understanding NSAIDs: From aspirin to COX-2. Clin. Cornerstone. 2001;3:50–60. doi: 10.1016/S1098-3597(01)90069-9. [DOI] [PubMed] [Google Scholar]
- 38.Mikhailidis D., Jeremy J., Dandona P. Urinary bladder prostanoids—Their synthesis, function and possible role in the pathogenesis and treatment of disease. J. Urol. 1987;137:577–582. doi: 10.1016/S0022-5347(17)44109-7. [DOI] [PubMed] [Google Scholar]
- 39.De Groat W. Anatomy and physiology of the lower urinary tract. Urol. Clin. N. Am. 1993;20:383. [PubMed] [Google Scholar]
- 40.Maggi C., Santicioli F., Meli A. Evidence for the involvement of a capsaicin-sensitive innervation in the afferent branch of micturition reflex in rats. Acta Physiol. Hung. 1987;69:425–435. [PubMed] [Google Scholar]
- 41.Maggi C.A., Giuliani S., Conte B., Furio M., Santicioli P., Meli P., Gragnani L., Meli A. Prostanoids modulate reflex micturition by acting through capsaicin-sensitive afferents. Eur. J. Pharmacol. 1988;145:105–112. doi: 10.1016/0014-2999(88)90221-X. [DOI] [PubMed] [Google Scholar]
- 42.Ergenoglu P., Akin S., Cok O.Y., Eker E., Kuzgunbay B., Turunc T., Aribogan A. Effect of intraoperative paracetamol on catheter-related bladder discomfort: A prospective, randomized, double-blind study. Curr. Ther. Res. Clin. Exp. 2012;73:186–194. doi: 10.1016/j.curtheres.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee W.M. Acetaminophen and the US Acute Liver Failure Study Group: Lowering the risks of hepatic failure. Hepatology. 2004;40:6–9. doi: 10.1002/hep.20293. [DOI] [PubMed] [Google Scholar]
- 44.Jozwiak-Bebenista M., Nowak J.Z. Paracetamol: Mechanism of action, applications and safety concern. Acta Pol. Pharm. 2014;71:11–23. [PubMed] [Google Scholar]
- 45.Claridge L.C., Eksteen B., Smith A., Shah T., Holt A.P. Acute liver failure after administration of paracetamol at the maximum recommended daily dose in adults. BMJ. 2010;341:c6764. doi: 10.1136/bmj.c6764. [DOI] [PubMed] [Google Scholar]
- 46.Bray G.P. Liver failure induced by paracetamol. BMJ. 1993;306:157. doi: 10.1136/bmj.306.6871.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilkinson S., Moodie H., Arroyo V., Williams R. Frequency of renal impairment in paracetamol overdose compared with other causes of acute liver damage. J. Clin. Pathol. 1977;30:141–143. doi: 10.1136/jcp.30.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jaquenod M., Rönnhedh C., Cousins M.J., Eckstein R.P., Jordan V., Mather L.E., Power I. Factors influencing ketorolac-associated perioperative renal dysfunction. Anesth. Analg. 1998;86:1090–1097. doi: 10.1213/00000539-199805000-00036. [DOI] [PubMed] [Google Scholar]
- 49.Bjarnason I. Gastrointestinal safety of NSAIDs and over-the-counter analgesics. Int. J. Clin. Pract. Suppl. 2013:37–42. doi: 10.1111/ijcp.12048. [DOI] [PubMed] [Google Scholar]