Supplemental Digital Content is available in the text
Keywords: Gensini score, major adverse cardiac event (MACE), monocyte/lymphocyte ratio (MLR), non-ST-elevation myocardial infarction (NSTEMI)
Abstract
Monocyte/lymphocyte ratio (MLR), a widely used inflammation maker for prognosis of cancer, tuberculosis, and autoimmune diseases, has attracted more and more attention for its application to cardiovascular disease. The aim of the present study was to investigate the relationship of MLR with the severity of coronary lesion and clinical outcomes in non-ST-elevation myocardial infarction (NSTEMI) patients.
963 consecutive NSTEMI patients (mean age, 60.77 ± 11.34; 758 male) undergoing coronary angiography were analyzed and followed in 3 groups according to the average MLR tertile (low MLR <0.23, n = 321; intermediate MLR 0.23–0.35, n = 322; high MLR >0.35, n = 320) in this study. The severity of coronary lesion was determined by Gensini score. Multiple linear regression analysis was used to examine the correlation between MLR and the severity of coronary lesion. Kaplan–Meier curve was performed to compare the long-term major adverse cardiac event (MACE)-free survival. Logistic regression analysis and Cox proportional hazard regression model were used to assess the independent predictors for in-hospital and long-term MACE.
MLR (B: 0.281, 95% confidence interval [CI]: 0.130–0.432, P < .001) and high-sensitivity C-reactive protein (B: 0.017, 95% CI: 0.010–0.024, P < .001) were both independently correlated with the severity of coronary lesion, while neutrophil/lymphocyte ratio was not. The frequencies of in-hospital MACE (1.6%, 2.2%, 4.7%, P = .016) and long-term MACE (13.3%, 16.2%, 27.2%, P < .001) both increased among the 3 groups. Kaplan–Meier curve analysis indicated that patients in high MLR group had worse long-term MACE-free survival than the patients in low MLR group (P2 < .001) and intermediate MLR group (P3 = .004) during a median follow-up of 22 (12–35) months. MLR was an independent predictor for in-hospital MACE (adjusted odds ratio: 2.891, 95% CI: 1.265–8.354, P = .026) and long-term MACE (adjusted hazard ratio: 1.793, 95% CI: 1.169–2.515, P = .012) in NSTEMI patients.
MLR is independently correlated with the severity of coronary lesion and has better performance to reflect the severity of coronary lesion than NLR. MLR is an independent predictor for the MACE in NSTEMI patients.
1. Introduction
Coronary artery disease (CAD) is a leading cause of morbidity and mortality worldwide,[1,2] and non-ST-elevation myocardial infarction (NSTEMI) is one of the leading causes of death in patients with CAD.[3] Patients with NSTEMI tend to have multivessel coronary artery lesions and more unfavorable long-term outcome than the patients with ST-elevation myocardial infarction (STEMI).[4,5] Therefore, the early risk stratification and management of patients in NSTEMI are crucial for improving the prognosis of these patients. The severity of coronary artery lesions is an important index for risk stratification of CAD and directly determines the optimal treatment strategy.
The role of inflammation in the initiation and progression of coronary atherosclerosis is well described, high levels of inflammatory markers have been found in association with the severity of CAD and the prognosis of CAD patients.[6,7] Therefore, an appropriate coronary inflammatory marker is of great significance for early risk stratification and prognostic evaluation of CAD patients. High-sensitivity C-reactive protein (hs-CRP), a nonspecific inflammatory marker, has been reported to be related to the risk of coronary heart disease and cardiovascular events in patients with acute coronary syndrome (ACS),[8,9] but it is susceptible to tissue injury, infection, abnormal liver and kidney function, and other noncardiac factors. White blood cell subtypes have been considered to play a crucial role in the pathogenesis of atherogenesis and atherothrombosis, which are closely related to the cardiovascular adverse events.[10,11] Currently, numerous studies have shown that neutrophil/lymphocyte ratio (NLR) is related to the severity of CAD, the degree of myocardial damage and the clinical outcome of CAD.[12–14]
Compared with neutrophils, monocytes, and monocyte-derived macrophages play more important roles in the initiation and progression of atherosclerotic disease.[15,16] There have been several studies showing that high monocyte count or low lymphocyte are closely related to the adverse cardiovascular events of CAD patient.[11,17] Monocyte/lymphocyte ratio (MLR), a combined inflammatory marker with monocyte count divided by lymphocyte count, has been widely used to the studies of cancer, tuberculosis, and autoimmune diseases.[18–21] In recent years, more and more attention has been attracted to MLR's applications for cardiovascular diseases.[22–24] However, the relationship of MLR with the severity of coronary artery lesions and clinical outcomes in NSTEMI patients has not been fully elucidated.
The aim of this study was to investigate the relationship of MLR with the severity of coronary lesion (through Gensini scoring system) and the major adverse cardiac event (MACE) in NSTEMI patients Table S1, Fig. S1 and Fig. S2.
2. Methods
2.1. Study population and protocol
One thousand three hundred sixty-five participants who were diagnosed with NSTEMI were consecutively screened at the First Affiliated Hospital of Xi’an Jiao Tong University from December 2013 to December 2017. The diagnosis of NSTEMI was based on 2014 AHA/ACC guideline.[25] Exclusion criteria included age <18 years, congenital heart disease, cardiomyopathy, valvular disease, hematologic disease, severe liver or renal dysfunction, stroke, tumor, thyroid disease, autoimmune disease, acute and chronic infectious disease, chronic obstructive pulmonary disease, the patients without coronary angiography, and the patients without informed consent. Of the 1365 patients, only 963 were eligible for study inclusion. According to the average MLR tertile (low MLR <0.23, n = 321; intermediate MLR 0.23–0.35, n = 322; high MLR >0.35, n = 320), all the included patients were divided into 3 groups for data analysis, and then followed up until October 2018 or until the occurrence of a MACE (Fig. 1). Global registry of acute coronary events (GRACE) risk score and thrombolysis in myocardial infarction (TIMI) risk score were calculated for each eligible index case on admission according to 2014 AHA/ACC guideline.[25] The study protocol was approved by the Ethical Committee of the First Affiliated Hospital of Xi’an Jiao Tong University, and the study conforms to the Declaration of Helsinki.
Figure 1.

The research flow chart. In-hospital MACE = in-hospital major adverse cardiac event, long-term MACE = long-term major adverse cardiac event, MLR = monocyte/lymphocyte ratio, NSTEMI = non-ST-elevation myocardial infarction.
2.2. Definition of coronary risk factors
Hypertension was defined as >140/90 mm Hg or taking antihypertensive medication. Diabetes mellitus was defined by the patient having been informed of this diagnosis by a physician before admission or was receiving hypoglycemic treatments (dietary, oral anti-diabetic agents, or insulin) or the patients who presented to the serum glycosylated hemoglobin (HbA1c%) levels ≥6.5%. Dyslipidemia was defined by fasting serum total cholesterol (TC) >220 mg/dL, low density lipoprotein cholesterol (LDL-C) >140 mg/dL, high-density lipoprotein cholesterol (HDL-C) <35 mg/dL, triglyceride (TG) >150 mg/dL or the use of lipid-lowering medications. Smoking index was calculated from the average number of smoking root per day multiplied by the number of smoking years.
2.3. Measurement of blood parameters
Venous blood samples of all patients were drawn from upper limb. The blood parameters were measured by clinical laboratory of the First Affiliated Hospital of Xi’an Jiao Tong University. The leukocyte count, monocyte count, neutrophil count, lymphocyte count, and serum hs-CRP were measured on admission. The MLR and NLR were respectively computed using the absolute monocyte or neutrophil count divided by the absolute lymphocyte count. Patients were advised to fast at least for 12 hours before biochemical indicators investigations. The biochemical indicators included HbA1c%, TC, LDL-C, HDL-C, TG, serum creatinine (SCr), and uric acid. Plasma hs-troponin T, creatine kinase isoenzyme (CKMB) and N-terminal pro-brain natriuretic peptide (NT-proBNP) were measured on admission and serially every 6 hours until the detection of 2 consecutive declining measurements and for at least 36 hours to determine the Peak hs-troponin T, Peak CKMB, and peak NT-proBNP in NSTEMI patients.
2.4. Assessment of the severity of coronary lesion
Coronary angiogram was assessed by 2 interventional physicians blindly. The diseased coronary vessel was defined as a lesion with ≥50% stenosis. The results of coronary angiograms were scored based on the Gensini scoring system. Gensini score is a widely used evaluation system to reflect the severity of coronary lesion, which is determined according to the severity of stenosis as follows: 1 point for <25% stenosis, 2 points for 26% to 50% stenosis, 4 points for 51% to 75% stenosis, 8 points for 76% to 90% stenosis, 16 points for 91% to 99% stenosis, and 32 points for total occlusion. The score is then multiplied by a factor representing the importance of the lesion's position in the coronary artery system. For example, 0.5 for small branch of coronary artery, 1 for the distal left anterior descending, overall region of right coronary artery or mid-distal region of the left circumflex artery, 1.5 for the mid-region, 2.5 for the proximal left anterior descending or proximal left anterior descending or proximal left circumflex artery, and 5 for the left main coronary artery.[26]
2.5. Definition of in-hospital MACE and long-term MACE
The in-hospital MACE was defined as cardiac death, cardiac arrest, cardiac rupture, cardiogenic shock, cardiogenic syncope, acute congestive heart failure, malignant arrhythmic events during hospitalization. The long-term MACE, including cardiac death, myocardial infarction, unstable angina pectoris, malignant arrhythmia, cardiac arrest, cardiogenic shock, cardiogenic syncope, and rehospitalization for heart failure decompensation or coronary revascularization, were ascertained from a review of medical records and confirmed by direct dialogue with the patients, their families, and physicians following discharge.
2.6. Statistical analysis
Continuous variables were defined as mean ± standard deviation or median (interquartile range); categorical variables were expressed as percentages. For continuous variables, the Kolmogorov–Smirnov test was applied to test the normality of distribution, 1-way analysis of variance or Kruskal–Wallis test was used to compare depending on the probability distribution of the variables. For categorical variables, the chi-square test was used. Because of a positively skewed distribution, Gensini score was lg-transformed for linear regression analysis. Linear regression analysis was used to test univariate correlations in the whole study population, and the significant correlation variables were further taken into multiple linear regression analysis (stepwise). Kaplan–Meier curve analysis was performed and comparisons of the long-term MACE-free survival was performed using the log-rank test among the 3 groups. Logistic regression analysis was used to assess the independent predictors for in-hospital MACE of all NSTEMI patients. The effects of different variables on in-hospital MACE were calculated using univariate analyses for related variables. The variables for which the unadjusted P value was less than .10 in logistic regression analysis were identified as potential risk markers and included in the full model. Cox proportional hazard regression model was used to estimate the long-term MACE hazard ratio (HR) and its 95% confidence interval (CI) in all NSTEMI patients by univariate and multivariate analysis, including various clinical and inflammatory indicators. Receiver operating characteristic (ROC) curve analysis was performed to assess the predictive value of MLR, NLR, and hs-CRP for in-hospital and long-term MACE of NSTEMI patients, and c-statistics was used to compare the difference of predictive value among MLR, NLR, and hs-CRP for in-hospital or long-term MACE. Statistical analyses were performed using IBM SPSS 20.0. All probabilities were 2-sided and P values <.05 were considered statistically significant.
3. Results
3.1. Baseline characteristics of NSTEMI patients based on the tertile of MLR
A total of 963 NSTEMI patients referred for coronary angiography at the First Affiliated Hospital of Xi’an Jiao Tong University are included in the final analysis. The mean age of study population is 60.77 ± 11.34, and 78.7% are males. The baseline characteristics of the study population based on the tertile of MLR (low MLR <0.23, n = 321; intermediate MLR 0.23–0.35, n = 322; high MLR >0.35, n = 320) are outlined in Table 1. The results show that there are significant differences in age, gender, heart rate (HR) and the frequencies of hypertension, history of CAD and history of MI among the different groups. Interestingly, low MLR group tends to have higher value of body mass index (BMI), HbA1c%, TC, and TG than that of the other 2 groups (P < .05). Leukocytes, neutrophils, monocytes, MLR, NLR, hs-CRP, and SCr levels in high MLR group are statistically higher than that of other 2 groups (P < .01), while there is a decrease of the absolute number of lymphocytes in the 3 groups (1.96 ± 0.72, 1.56 ± 0.53, 1.22 ± 0.52, P < .001). High MLR group has significantly higher levels of Killip grades, peak hs-troponin T, peak CKMB, and peak NT-pro BNP than the other 2 groups (P < .001), indicating that high MLR NSTEMI patients have worse clinical condition. Besides, there are significant increases of TIMI risk score and GRACE risk score among the 3 groups (3.2 ± 1.3, 3.4 ± 1.3, 3.7 ± 1.3, P < .001; 104.9 ± 28.9, 115.6 ± 30.4, 124.7 ± 36.1, P < .001). There is no significant difference in prior medications (aspirin, beta-blocker, stain and angiotensin-converting enzyme inhibitor/ angiotensin receptor blocker) among the 3 groups (P > .05).
Table 1.
Baseline characteristics of the NSTEMI patients based on the tertile of MLR.

3.2. Angiographic characteristics of NSTEMI patients based on the tertile of MLR
The angiographic characteristics of the NSTEMI patients based on the tertile of MLR are shown in Table 1. The results demonstrate that the frequency of coronary arteriography TIMI flow = 0/1 in high MLR group is statistically higher than that of other 2 groups. With the increase of MLR level, the frequencies of diseased vessels in each coronary artery branch increase (P < .05). The high MLR group has higher frequency of triple diseased vessels (P < .001) and lower frequency of single diseased vessels (P = .007) than that of other 2 groups. The number of stents in high MLR group is also higher than that of other 2 groups (P = .007). There is an increase in the Gensini score in the 3 groups (60 [36–85], 68 [40–101], 85 [52–116]; P < .001).
3.3. Correlation between MLR and lgGensini-score
In the univariate correlation analysis, age, male, DM, history of CAD, HR, HbA1c%, Scr, leucocyte, neutrophil, lymphocyte, monocyte, MLR, NLR, hs-CRP all have significant correlation with the lgGensini-score, and MLR has a positive correlation with the lgGensini-score (r = 0.260, P < .001) (Table 2). All above significant correlation variables are taken into multiple linear regression analysis (stepwise) for further analysis. The results show that age, gender, history of CAD, MLR (B: 0.281, 95% CI: 0.130–0.432, P < .001) and hs-CRP (B: 0.017, 95% CI: 0.010–0.024, P < .001) still have strong significant correlations with lgGensini-score (Table 3), while NLR does not.
Table 2.
Univariate correlation of lgGensini-score.

Table 3.
Independent correlation of variables with lgGensini-score in multiple linear regression analysis.

3.4. MLR and Gensini score are independent predictors for in-hospital MACE and long-term MACE
A total of 27 NSTEMI patients have in-hospital MACE, including 10 patients with acute left heart failure, 2 patients with cardiac arrest, 3 patients with malignant arrhythmia, 10 patients with cardiogenic shock, and 2 patients with cardiac rupture. The frequency of in-hospital MACE increases among 3 groups (1.6%, 2.2%, 4.7%, P = .016) (Fig. 2). During a median follow-up of 22 (12–35) months, 176 long-term MACE are recorded in the total cohort of NSTEMI patients, and there is a significant increase of long-term MACE in 3 groups (13.3%, 16.2%, 27.2%, P < .001) (Fig. 2). The long-term MACE-free survival result of Kaplan–Meier curve analysis based on the tertile of MLR is shown in Figure 3. Patients in high MLR group have significantly worse long-term MACE-free survival than patients in low MLR group (P2 < .001) and intermediate MLR group (P3 = .004). While the long-term MACE-free survival of the low MLR group and Intermediate MLR group does not significantly differ (P1 = .203). Logistic regression analysis reveals history of CAD (adjusted odds ratio [OR]: 1.461, 95% CI: 1.118–4.301, P = .006), MLR (adjusted OR: 2.891, 95% CI: 1.265–8.354, P = .026), NLR (adjusted OR: 1.102, 95% CI: 1.012–1.201, P = .022), hs-CRP (adjusted OR: 1.207, 95% CI: 1.033–1.410, P = .018), and Gensini score (adjusted OR: 1.018, 95% CI: 1.008–1.028, P = .001) are all independent predictors for in-hospital MACE in patients with NSTEMI (Table 4). Cox proportional hazard regression analysis indicates that age (adjusted HR: 1.016, 95% CI: 1.007–1.028, P = .011), MLR (adjusted HR: 1.793, 95% CI: 1.169–2.515, P = .012), NLR (adjusted HR: 1.029, 95% CI: 1.004–1.064, P = .022), hs-CRP (adjusted HR: 1.055, 95% CI: 1.019–1.095, P = .007), and Gensini score (adjusted HR: 1.006, 95% CI: 1.003–1.010, P = .001) are all independent risk factors for long-term MACE in NSTEMI patients (Table 5).
Figure 2.

The frequency of in-hospital MACE in patients with NSTEMI based on the tertile of MLR; low MLR <0.23, n = 321; intermediate MLR 0.23–0.35, n = 322; high MLR >0.35, n = 320; P1, the trend for in-hospital MACE frequency in 3 groups; P2, the trend for long-term MACE frequency in 3 groups. In-hospital MACE = in-hospital major adverse cardiac event, long-term MACE = long-term major adverse cardiac event, MLR = monocyte/lymphocyte ratio, NSTEMI = non-ST-elevation myocardial infarction.
Figure 3.

Kaplan–Meier curve analysis for long-term MACE-free survival in patients with NSTEMI based on the tertile of MLR; low MLR <0.23, n = 321; intermediate MLR 0.23–0.35, n = 322; high MLR >0.35, n = 320; the difference between low MLR group and intermediate MLR group, P1 = .203; the difference between low MLR group and high MLR group, P2 < .001; the difference between intermediate MLR group and high MLR group, P3 = .004. MACE = major adverse cardiac event, MLR = monocyte/lymphocyte ratio, NSTEMI = non-ST-elevation myocardial infarction.
Table 4.
Effects of related variables on in-hospital MACE in univariate and multivariate logistic regression analysis.

Table 5.
The risk of related variables for long-term MACE in univariate and multivariate Cox proportional hazard regression analysis.

3.5. The predictive value of MLR for in-hospital MACE and long-term MACE
ROC curve analysis is applied to test the predictive value of MLR, NLR, and hs-CRP for in-hospital MACE and long-term MACE in patients with NSTEMI (Figs. 4 and 5). Results show that MLR, NLR, and hs-CRP all have significant predictive value for in-hospital MACE and long-term MACE (P < .05) (Table 6), and there is no significant difference among the 3 biomarkers of their predictive value for in-hospital MACE (P1 = .245) and long-term MACE (P2 = .486) (Table 6). MLR has the largest AUC at 0.728 (95% CI: 0.604–0.852, P = .001) among all the makers, and a MLR of 0.43 is identified to be an effective cut-off point for predicting in-hospital MACE with a sensitivity of 63.2% and a specificity of 80.8%. Similarly, MLR also has a significant predictive value for long-term MACE in NSTEMI patients with the largest AUC at 0.609 (95% CI: 0.556–0.661, P < .001), and the cut-off point is 0.31 with a sensitivity of 57.4% and a specificity of 61.5%.
Figure 4.
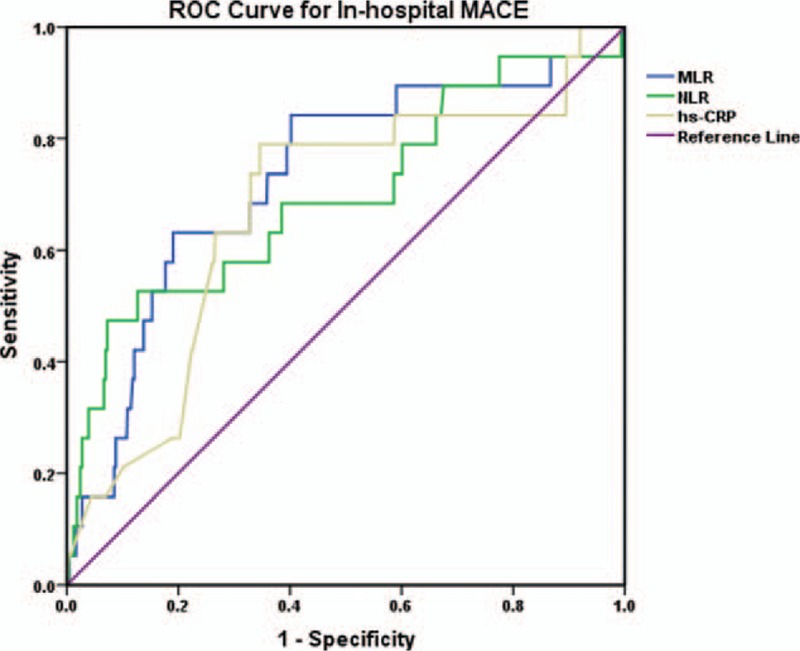
ROC curve analysis of the predictive value of MLR, NLR, and hs-CRP for in-hospital MACE. Hs-CRP = serum high-sensitivity C-reactive protein, In-hospital MACE = in-hospital major adverse cardiac event, MLR = monocyte/lymphocyte ratio, NLR = neutrophil/lymphocyte ratio, ROC curve = receiver operating characteristic curve.
Figure 5.
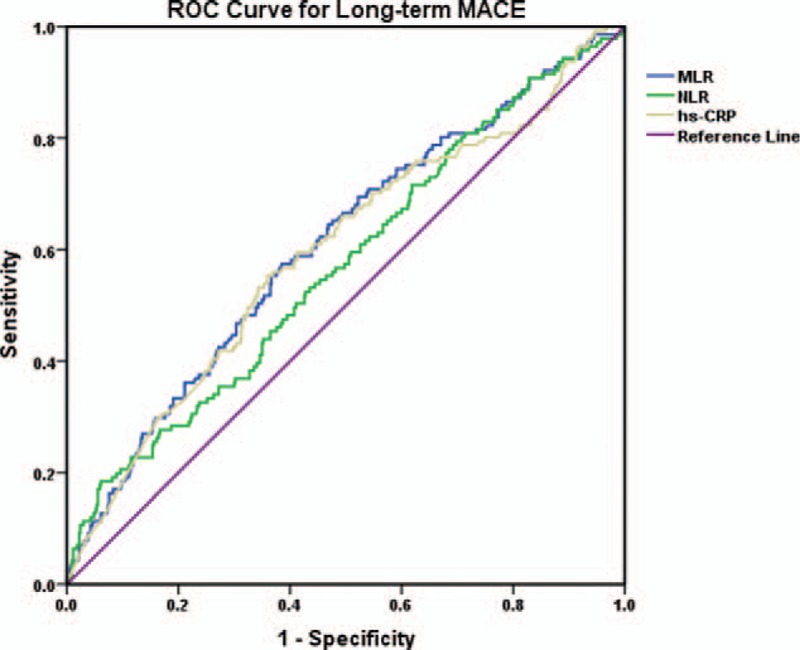
ROC curve analysis of the predictive value of MLR, NLR, and hs-CRP for long-term MACE. Hs-CRP = serum high-sensitivity C-reactive protein, long-term MACE = long-term major adverse cardiac event, MLR = monocyte/lymphocyte ratio, NLR = neutrophil/lymphocyte ratio, ROC curve = receiver operating characteristic curve.
Table 6.
ROC curve analysis of the predictive value of MLR, NLR, and hs-CRP for in-hospital MACE and long-term MACE.

4. Discussion
MLR, a widely used inflammation maker for the prognosis of cancer, tuberculosis, and autoimmune diseases,[18–21] has also been confirmed to be closely related to the cardiovascular events in patients with CAD recently.[22–24] In the present study, we demonstrated that
-
(1)
The NSTEMI patients in high MLR group had worse clinical condition, higher GRACE and TIMI risk scores on admission, more diseased vessels in each coronary artery branch, more severe degree of coronary stenosis, and higher incidence of MACE compared with the patients in low MLR or intermediate MLR groups;
-
(2)
MLR and hs-CRP were both independently correlated with the severity of coronary lesion in patients with NSTEMI, while NLR was not;
-
(3)
MLR was an independent predictor for in-hospital MACE and long-term MACE in NSTEMI patients and had the same good predictive value as the well-known coronary artery inflammatory marker -hs-CRP and NLR.
NSTEMI is one kind of ACS, and increased levels of inflammatory markers have been found in association with the severity of coronary atherosclerosis and prognosis in ACS.[27] Monocytes are the main component of the innate immune system, and are also involved in endogenous inflammatory processes.[7] Monocytes and monocyte-derived macrophages play pivotal roles in the initiation and progression of atherosclerotic disease. Monocytes can migrate from blood to tissues in response to signals and differentiate to inflammatory dendritic cells, macrophages, and foam cells, and then, activate the production of pro-inflammatory cytokines secretion, matrix metalloproteinases, and reactive oxidative species which play key roles in the initiation, formation, and rupture of atherosclerotic plaque.[15,16] Therefore, the increase in the number of peripheral blood monocytes indicates that the monocytes and macrophages raised around the coronary plaque in response to signals can also increased, thus enhancing the inflammatory reaction process, the degree of coronary stenosis and the risk of atherosclerotic plaque rupture. Lymphocytes consisted with T lymphocytes, B lymphocytes and natural killer cells, represent the regulatory pathway of the immune system in contrast. Lymphocytopenia is related to major cardiac events for acute chest pain patients, poor outcomes for patients of heart failure with reduced ejection fraction, impaired coronary microcirculation, and cardiac remodeling,[17,28–30] which can be interpreted as the excessive apoptosis of lymphocytes caused by increased catecholamine and cortisol level under stress.[31,32] Thus, the combination of elevated monocytes and low levels of lymphocytes into a single composite marker of inflammation could provide more additive information than either parameter alone, which could reflect the severity of CAD better.
Our study showed that MLR and hs-CRP were both independent correlation variables with the severity of coronary lesion in NSTEMI patients, while NLR was not, which is different from Kurtul's study results.[33] We speculate that the main reason is that Kurtul's study did not include MLR value in their linear regression analysis model, which indirectly indicates that MLR has stronger correlation with the severity of coronary lesion than NLR. Besides, monocytes and monocyte-derived macrophages play more important roles in the initiation and progression of atherosclerotic plaque than neutrophils.[16] Ji et al have also demonstrated that compared to NLR, MLR has better performance to reflect the severity of coronary lesion in CAD patients on the basis of Syntax score in a research including 543 Chinese population, which is consistent with our research findings.[22]
With the improvement of medical level, more and more attention has been paid to the prognosis of NSTEMI patients. The FAST-MI Program has shown that the 6-month mortality of acute myocardial infarction patients has decreased considerably over the past 20 years, and the mortality of STEMI patients continued to decline until 2015, whereas the mortality in patients with NSTEMI appears stable since 2010.[34] Thus, the risk stratification and management of patients with NSTEMI in early phase are very crucial for improving the prognosis of these patients. Recently, Wang et al have demonstrated that lymphocyte/monocyte is an independent predictor for major adverse cardiac and cerebrovascular events in STEMI patients in a research including 306 patients,[24] so we speculated that whether MLR is also a predictor for MACE in NSTEMI patients. In the present study, we found that MLR was an independent predictor for in-hospital and long-term MACE in patients with NSTEMI, and the MLR's predictive value for the MACE was as good as that of the well-known coronary artery inflammatory marker -hs-CRP and NLR. Similarly, a newly published research also reported that MLR was an independent predictor for long-term MACE in a retrospective cohort population of 678 NSTEMI patients.[35]
MLR is more specific and efficient than NLR in evaluating the severity of coronary lesion, and more routine, stable, immediately obtainable, and inexpensive than hs-CRP in predicting in-hospital and long-term MACE in patients with NSTEMI. Therefore, MLR has a great clinical practical value, and could be considered as a new indicator for early risk stratification in patients with NSTEMI.
In addition, we have observed that low MLR group tended to have higher value of BMI, HbA1c%, TC, and TG than that of the other 2 groups (P < .05) in the present study. Obesity may produce systemic inflammatory condition, and the macrophage infiltration in adipose tissue is a central event leading to the metabolic complications of obesity. Accordingly, we consider that the relatively obese patients with low MLR are the result of increased migration of monocytes from the intravascular compartment to adipose tissues due to an increase chemotactic and adhesion molecule activities in the adipose tissues of the obese patients.[36,37]
There are several limitations in this study. First, this was a single-center study with a relatively small patient population. Further large multicenter studies involving larger numbers of patients will be required to determine the relationship of MLR with NSTEMI. Second, this study was a observational study without interventions. The benefits of anti-inflammatory therapy for NSTEMI patients are still unclear. Therefore, prospective interventional studies of NSTEMI patients in a large-scale population are necessary. Third, other inflammatory markers such as tumor necrosis factor-alpha, monocyte chemotactic protein-1, and interleukin-6, were not measured in the study, so we could not evaluate their addictive effects on the severity of CAD and the MACE of NSTEMI patients in analysis models.
5. Conclusion
In conclusion, MLR is independently correlated with the severity of coronary lesion in NSTEMI patients and has better performance to reflect the severity of coronary lesion than NLR. MLR is also an independent predictor for in-hospital and long-term MACE in patients with NSTEMI. Therefore, MLR, an efficient, routine, immediately obtainable and inexpensive inflammatory biomarker, may be considered as a useful indicator for early risk stratification of NSTEMI patients, so that we can provide more aggressive therapeutic approach, intensive care and close follow-up for high-risk patients.
Author contributions
Conceptualization: Hui Chen, Danjun Zhu, Gang Tian.
Data curation: Hui Chen, Min Li, Xiawei Dang.
Formal analysis: Lei Liu.
Funding acquisition: Gang Tian.
Investigation: Min Li.
Project administration: Hui Chen, Gang Tian.
Resources: Xiawei Dang.
Software: Lei Liu.
Supervision: Danjun Zhu.
Validation: Danjun Zhu.
Writing – original draft: Hui Chen.
Writing – review and editing: Danjun Zhu, Gang Tian.
Supplementary Material
Footnotes
Abbreviations: ACS = acute coronary syndrome, BMI = body mass index, CAD = coronary artery disease, CKMB = creatine kinase isoenzyme, DBP = diastolic pressure, DM = diabetes mellitus, HbA1c = glycosylated hemoglobin, HDL-C = high density lipoprotein cholesterol, HR = heart rate, hs-CRP = high-sensitivity C-reactive protein, LDL-C = low density lipoprotein cholesterol, MACE = major adverse cardiac event, MLR = monocyte/lymphocyte ratio, NLR = neutrophil/lymphocyte ratio, NSTEMI = non-ST-elevation myocardial infarction, NT-proBNP = N-terminal pro-brain natriuretic peptide, SBP = systolic blood pressure, SCr = serum creatinine, STEMI = ST-elevation myocardial infarction, TC = total cholesterol, TG = triglyceride.
This study was supported by the Nature Science Foundation of China (grant no. 81873513, 81600574, and 30871042), Key Projects of Shaanxi Science and Technology Research and Development Plan (No. 2018ZDXM-SF-049) and Shaanxi Science and Technology Research and Development Plan of International Science and Technology (No. 2012 kw-40-01 and 2014 JM2-8145).
The authors declare that they have no conflict of interest.
Supplemental Digital Content is available for this article.
References
- [1].Roth GA, Forouzanfar MH, Moran AE, et al. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med 2015;372:1333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;385:117–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yeh RW, Sidney S, Chandra M, et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med 2010;362:2155–65. [DOI] [PubMed] [Google Scholar]
- [4].Terkelsen CJ, Lassen JF, Nørgaard BL, et al. Mortality rates in patients with ST-elevation vs. non-ST-elevation acute myocardial infarction: observations from an unselected cohort. Eur Heart J 2005;26:18–26. [DOI] [PubMed] [Google Scholar]
- [5].Plakht Y, Gilutz H, Shiyovich A. Excess long-term mortality among hospital survivors of acute myocardial infarction. Soroka Acute Myocardial Infarction (SAMI) project. Public Health 2017;143:25–36. [DOI] [PubMed] [Google Scholar]
- [6].Kriszbacher I, Koppan M, Bodis J. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;353:429–30. [PubMed] [Google Scholar]
- [7].Santos-Gallego CG, Picatoste B, Badimon JJ. Pathophysiology of acute coronary syndrome. Curr Atheroscler Rep 2014;16:401. [DOI] [PubMed] [Google Scholar]
- [8].Kaptoge S, Di Angelantonio E, Lowe G, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 2010;375:132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cheng JM, Oemrawsingh RM, Garcia-Garcia HM, et al. Relation of C-reactive protein to coronary plaque characteristics on grayscale, radiofrequency intravascular ultrasound, and cardiovascular outcome in patients with acute coronary syndrome or stable angina pectoris (from the ATHEROREMO-IVUS study). Am J Cardiol 2014;114:1497–503. [DOI] [PubMed] [Google Scholar]
- [10].Kounis NG, Soufras GD, Tsigkas G, et al. White blood cell counts, leukocyte ratios, and eosinophils as inflammatory markers in patients with coronary artery disease. Clin Appl Thromb Hemost 2015;21:139–43. [DOI] [PubMed] [Google Scholar]
- [11].Yamamoto E, Sugiyama S, Hirata Y, et al. Prognostic significance of circulating leukocyte subtype counts in patients with coronary artery disease. Atherosclerosis 2016;255:210–6. [DOI] [PubMed] [Google Scholar]
- [12].Chen J, Chen MH, Li S, et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting the severity of coronary artery disease: a Gensini score assessment. J Atheroscler Thromb 2014;21:1271–82. [DOI] [PubMed] [Google Scholar]
- [13].Azab B, Zaher M, Weiserbs KF, et al. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am J Cardiol 2010;106:470–6. [DOI] [PubMed] [Google Scholar]
- [14].Arbel Y, Finkelstein A, Halkin A, et al. Neutrophil/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients undergoing angiography. Atherosclerosis 2012;225:456–60. [DOI] [PubMed] [Google Scholar]
- [15].Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol 2010;7:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ley K, Miller YI, Hedrick CC. Monocyte and macrophage dynamics during atherogenesis. Arterioscler Thromb Vasc Biol 2011;31:1506–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nunez J, Sanchis J, Bodi V, et al. Relationship between low lymphocyte count and major cardiac events in patients with acute chest pain, a non-diagnostic electrocardiogram and normal troponin levels. Atherosclerosis 2009;206:251–7. [DOI] [PubMed] [Google Scholar]
- [18].Yuan C, Li N, Mao X, et al. Elevated pretreatment neutrophil/white blood cell ratio and monocyte/lymphocyte ratio predict poor survival in patients with curatively resected cell lung cancer: results from a large cohort. Thorac Cancer 2017;8:350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chen L, Zeng H, Yang J, et al. Survival and prognostic analysis of preoperative inflammatory markers in patients undergoing surgical resection for laryngeal squamous cell carcinoma. BMC Cancer 2018;18:816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Naranbhai V, Kim S, Fletcher H, et al. The association between the ratio of monocytes:lymphocytes at age 3 months and risk of tuberculosis (TB) in the first two years of life. BMC Med 2014;12:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Du J, Chen S, Shi J, et al. The association between the lymphocyte-monocyte ratio and disease activity in rheumatoid arthritis. Clin Rheumatol 2017;36:2689–95. [DOI] [PubMed] [Google Scholar]
- [22].Ji H, Li Y, Fan Z, et al. Monocyte/lymphocyte ratio predicts the severity of coronary artery disease: a syntax score assessment. BMC Cardiovasc Disord 2017;17:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gijsberts CM, Ellenbroek GHJM, Ten Berg MJ, et al. Effect of monocyte-to-lymphocyte ratio on heart failure characteristics and hospitalizations in a coronary angiography cohort. Am J Cardiol 2017;120:911–6. [DOI] [PubMed] [Google Scholar]
- [24].Wang Q, Ma J, Jiang Z, et al. Association of lymphocyte-to-monocyte ratio with in-hospital and long-term major adverse cardiac and cerebrovascular events in patients with ST-elevated myocardial infarction. Medicine (Baltimore) 2017;96:e7897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;64:e139–228. [DOI] [PubMed] [Google Scholar]
- [26].Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol 1983;51:606. [DOI] [PubMed] [Google Scholar]
- [27].Zamani P, Schwartz GG, Olsson AG, et al. Inflammatory biomarkers, death, and recurrent nonfatal coronary events after an acute coronary syndrome in the MIRACL study. J Am Heart Assoc 2013;2:e003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Vaduganathan M, Ambrosy AP, Greene SJ, et al. Predictive value of low relative lymphocyte count in patients hospitalized for heart failure with reduced ejection fraction: insights from the EVEREST trial. Circ Heart Fail 2012;5:750–8. [DOI] [PubMed] [Google Scholar]
- [29].Camici PG, D’Amati G, Rimoldi O. Coronary microvascular dysfunction: mechanisms and functional assessment. Nat Rev Cardiol 2015;12:48–62. [DOI] [PubMed] [Google Scholar]
- [30].Tang TT, Yuan J, Zhu ZF, et al. Regulatory T cells ameliorate cardiac remodeling after myocardial infarction. Basic Res Cardiol 2012;107:232. [DOI] [PubMed] [Google Scholar]
- [31].Cioca DP, Watanabe N, Isobe M. Apoptosis of peripheral blood lymphocytes is induced by catecholamines. Jpn Heart J 2000;41:385–98. [DOI] [PubMed] [Google Scholar]
- [32].Kruger K, Agnischock S, Lechtermann A, et al. Intensive resistance exercise induces lymphocyte apoptosis via cortisol and glucocorticoid receptor-dependent pathways. J Appl Physiol (1985) 2011;110:1226–32. [DOI] [PubMed] [Google Scholar]
- [33].Kurtul S, Sarli B, Baktir AO, et al. Neutrophil to lymphocyte ratio predicts SYNTAX score in patients with non-ST segment elevation myocardial infarction. Int Heart J 2015;56:18–21. [DOI] [PubMed] [Google Scholar]
- [34].Puymirat E, Simon T, Cayla G, et al. Acute myocardial infarction: changes in patient characteristics, management, and 6-month outcomes over a period of 20 years in the FAST-MI program (French Registry of Acute ST-Elevation or Non-ST-Elevation Myocardial Infarction) 1995 to 2015. Circulation 2017;136:1908–19. [DOI] [PubMed] [Google Scholar]
- [35].Fan Z, Li Y, Ji H, et al. Prognostic utility of the combination of monocyte-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in patients with NSTEMI after primary percutaneous coronary intervention: a retrospective cohort study. BMJ Open 2018;8:e023459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Oh DY, Morinaga H, Talukdar S, et al. Increased macrophage migration into adipose tissue in obese mice. Diabetes 2012;61:346–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lolmede K, Duffaut C, Zakaroff-Girard A, et al. Immune cells in adipose tissue: key players in metabolic disorders. Diabetes Metab 2011;37:283–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


