Abstract
Objective
To demonstrate DNA sequencing analysis (DNAsa) of sinus cultures in patients with CRS is a reliable method of detecting pathogens in polymicrobial CRS infections.
Methods
After obtaining Institutional Review Board approval for this prospective cohort study, we selected a random sample of 50 patients with CRS at Medstar Georgetown University Hospital between September 2016 and March 2017. We defined CRS as a history of rhinosinusitis refractory to maximal medical therapy and prior endoscopic sinus surgery. Patients demonstrating active purulence in a sinus cavity were prospectively selected to undergo standard hospital cultures (SHC) and DNAsa cultures. Organisms identified in both methods were compared for each patient.
Results
Specimens were obtained from 29 female and 16 male patients with a mean age of 50 years. A total of 45 cultures were included in our final analysis; five cultures were excluded after inappropriate laboratory processing. Results from these patients were compared and analyzed. Cohen's weighted kappa analysis showed agreement between the two testing methods in identifying predominant microorganisms. DNAsa detected 31.9% more microorganisms compared to SHC (P < 0.05). When multiple microorganisms were detected, DNAsa yielded more positive results compared to SHC (P < 0.05).
Conclusions
DNAsa detects all microorganisms identified by SHC as well as predominant microorganisms not detected by SHC. Thus molecular pathogen identification may be more reliable for identifying multiple microorganisms as compared to standard culture techniques that identify only one or two microorganisms. In recalcitrant cases of CRS, DNAsa may provide better guidance in selection of appropriate antimicrobial treatment.
Keywords: Chronic rhinosinusitis (CRS), DNA sequencing analysis (DNAsa), Microbiome, Molecular sequencing, Microbiology culture
Introduction
Chronic rhinosinusitis (CRS) is an inflammatory condition of the mucosal lining of the nasal cavities and paranasal sinuses that affects both children and adults. When the inflammation persists beyond twelve weeks, a diagnosis of CRS is established. CRS is a multifactorial inflammatory process and when superimposed with infection, the inflammation becomes more complex and difficult to treat.1 Multiple classes of bacteria and fungi have been implicated in these chronic infections, believed to play a key role in the pathophysiology of CRS. Identifying the causative organisms is paramount for tailoring treatment and eradicating the underlying infection. Culture-directed treatment is critical for successful patient outcomes and prevention of antibiotic resistance,2 however, given the chronic nature of these infections, identifying the causative pathogens can be challenging.3 In contrast with acute bacterial sinusitis – where the most commonly isolated bacteria are Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis – anaerobes and Staphylococcus aureus are more commonly isolates in CRS.2, 3
Much like the gut, the sinonasal cavities have a microbiome that maintains an optimal environment for respiratory health. It is suspected that disparities in this environment lead to states of chronic infection, so numerous attempts have been made to delineate the sinonasal microbiome. And yet no single, consistent pattern in healthy and diseased subjects has been noted. Interestingly, identical microbes in varying proportions have been seen in both healthy and diseased patients, adding to the challenge of how to treat infections when the underlying bacteria may ordinarily be contributing to a healthy microbial flora.4
Previous studies seeking to detect the microbiomes of chronically infected wounds have demonstrated that molecular culture analysis identifies pathogens more accurately than traditional culture methods.3 More specifically, molecular culture analyses are capable of detecting resistance genes of bacteria such as those in methicillin and vancomycin resistance as well as identifying the presence of pathogens even in cases when a patient is taking or has taken antibiotic therapy.5 Molecular culture analysis is also less cumbersome than traditional culture detection methods because it does not entail growing patient cultures on selective media to ensure the isolation of infectious pathogens. Furthermore, unlike SHCs, detection of bacterial pathogens with molecular analysis does not require the employment of meticulous methods of specimens' transportation and cultivation.
Our study aims to compare the sensitivity and specificity of SHCs to DNA sequencing analysis (DNAsa) with polymerase chain reaction (PCR)-based identification in CRS. We saw a need to improve our method of diagnosing the underlying potential pathogens in our patients with CRS, since our SHCs often yield negative or non-diagnostic culture results despite having been obtained from frank pus in the setting of clinical sinus infection. Since infections in patients with CRS are often polymicrobial, we hypothesize that SHCs may be inaccurate in diagnosing all the potential pathogens.
Methods
Between September 2016 and March 2017, patients presenting to Medstar Georgetown University Hospital with clinically diagnosed CRS defined as history of disease refractory to medical therapy were identified for possible enrollment in the study. Sinus cultures were then collected from 50 patients diagnosed with CRS who demonstrated active purulence in a sinus cavity and mucosal erythema. Patients with CRS who had not undergone previous functional endoscopic sinus surgery (FESS) were not included in this study to enable easy access of sinus-directed cultures in a clinic setting. The sinus cultures were obtained under endoscopic visualization in the Otolaryngology clinic by inserting the culture swab into the sinus cavity. If more than one sinus in a patient showed evidence of infection then the sinus that clinically appeared to be the more infected sinus, defined by the presence of increased erythema and purulent exudate, was cultured.
After informed consent was obtained, nasal endoscopy was performed and the appropriate sinus was swabbed three times; once for an aerobic culture, once for an anaerobic culture, and once for a DNAsa culture. Collected samples were transferred in a BD Culturette CultureSwab EZ (BD, Franklin Lakes, NJ) for standard culture at the respective laboratories, either Labcorp, Quest Diagnostics through Medstar Georgetown University Hospital, or PathoGenius.
In accordance with standard culturing guidelines at our institution, each sample was first plated on blood agar and chocolate agar. The cultures were then incubated at 35 degrees Celsius in 5% CO2 for a minimum of 48 h for aerobic culturing. For anaerobic culturing, specimens were plated onto pre-reduced Brucella agar with 5% sheep blood, phenethyl alcohol blood agar with vitamin K, and Bacteriodes fragilis isolation agar, and then incubated at 35 degrees Celsius with 9.5% H2, 10% CO2, and 80.5% N2 for 7 d. Standard aerobic cultures were grown for 48 h, and anaerobic cultures for 72 h. Fungal cultures were grown for 4 weeks before declaring no growth. The DNAsa culture from each patient was sent to PathoGenius (Lubbock, TX), which uses comparative DNAsa to detect bacterial, atypical mycobacterial and fungal DNA, identifying what pathogens are present and their relative abundance. These samples, however, were not analyzed for antimicrobial sensitivities or susceptibilities. The results of bacterial and fungal cultures sent for DNAsa were available within 5–7 d whereas hospital bacterial cultures could take up-to 2 weeks to speciate with sensitivities and fungal cultures could take up to 4 weeks for results.
The pathogens identified in each sample were recorded along with the date the specimen was collected, the sinus from which the specimen was collected, and patient demographics such as age and gender. For each patient the isolated microorganism from each method of identification were compared to determine whether there was a difference in microorganism detection. Statistical analysis was then performed to identify if there was any significant difference between the SHC and DNAsa culture results.
The study was approved by the Institutional Review Board at Medstar Georgetown University Hospital.
Results
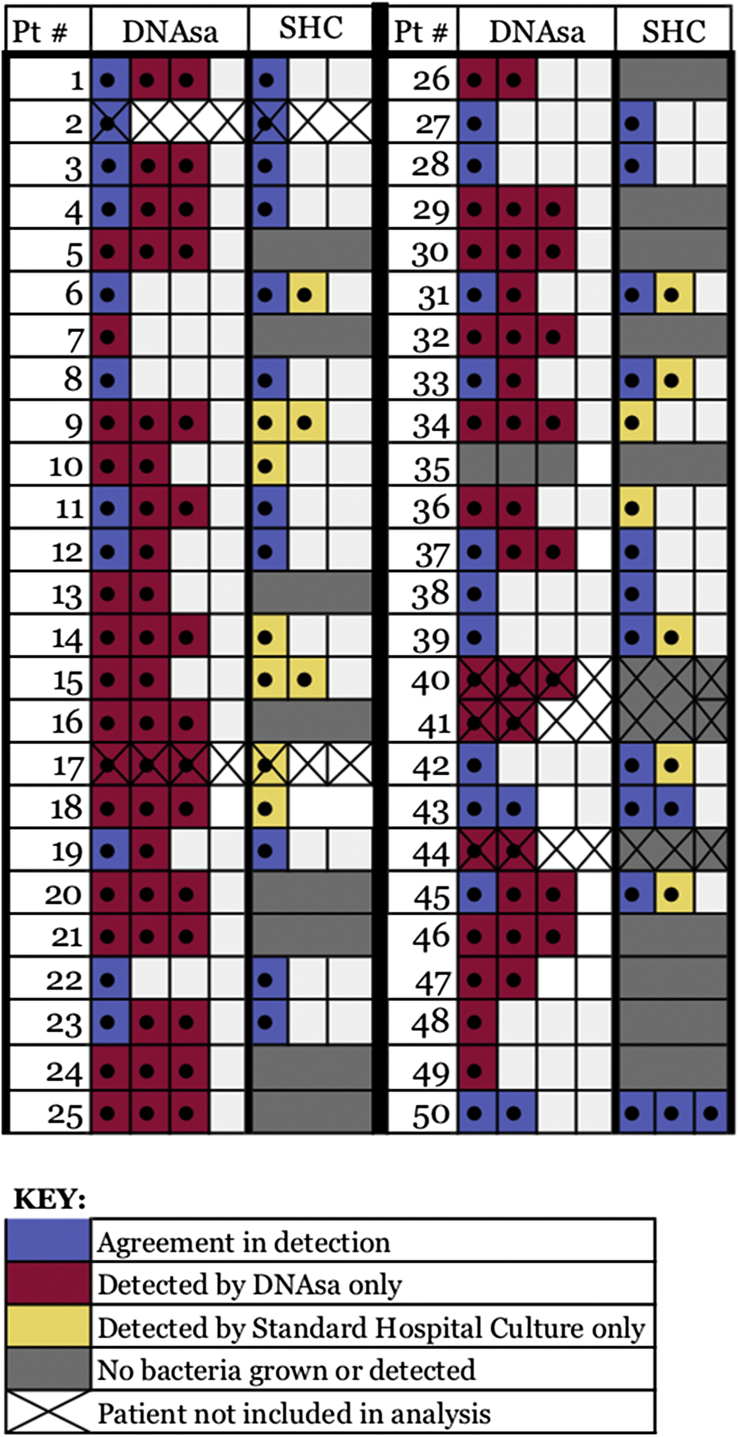
Sinus-directed cultures were obtained from 50 patients who had previously undergone FESS and demonstrated purulence on clinical exam. Of the 50 patients originally cultured, 45 patients were included in the final analysis and five were excluded. All five excluded patients were due to inappropriate processing of specimens by the standard hospital laboratory issues such as inappropriate processing. No complications, delays in processing or handling errors occurred during the DNAsa of specimens. The DNAsa of the 5 excluded patients were not included in the results analysis for this paper.
Specimens were obtained from 29 female and 16 male patients. These included 32 maxillary sinuses (71.1%), 8 ethmoid sinuses (17.8%), 3 sphenoid sinuses (6.7%) and 2 frontal sinuses (4.4%).
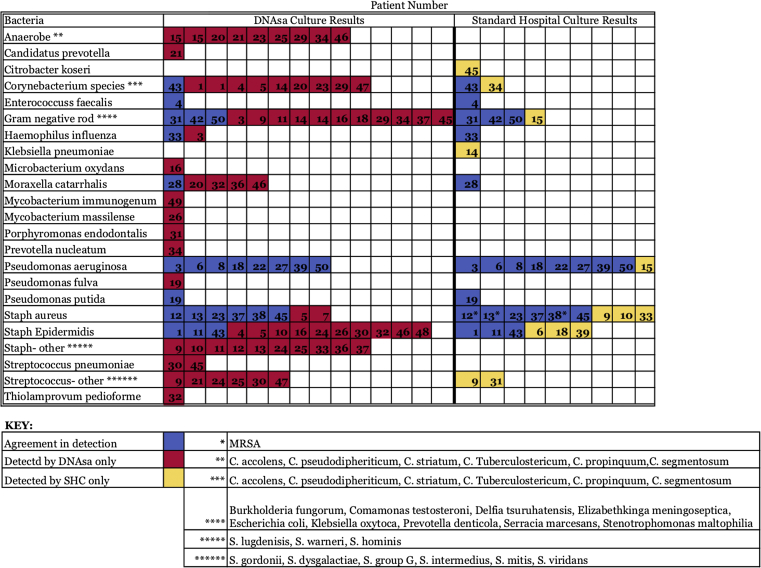
The most common organism identified using SHC were Pseudomonas aeruginosa and Staphylococcus aureus, each identified in 10 cultures. In total, 14 different bacterial taxa were identified by SHC. These organisms, their frequency of isolation, and the corresponding frequency of detection by SHC are reported in Fig. 1, Fig. 2.
Fig. 1.
Bacterial taxa between DNA sequencing analysis (DNAsa) and standard hospital cultures (SHC).
Fig. 2.
Frequency of bacterial detection and agreement in pathogens between DNA sequencing analysis (DNAsa) and standard hospital cultures (SHC).
Comparatively, when considering the 45 patients, Gram-negative rods were the most common class of bacteria detected by DNAsa in 14 specimens (31.1%). The most frequently detected bacteria among the Gram-negative rods were Serratia marcescens, Stenotrophomona maltophilia and Escherichia coli. The next most common classes of bacteria detected by DNAsa were Corneybacterium and Staphylococcus species each detected in 10 cultures (22.2%) where Corneybacterium pseudodiptheriticum and Staphylococcus lugdenisis were the most commonly detected bacterial species, respectively. The single most commonly detected organism identified using DNAsa was Staphylococcus epidermidis, detected in 13 cultures and perhaps a common contaminant (28.9%). In total, 32 different bacterial taxa were identified by the DNAsa culture. These organisms, the frequency of culture of each organism, and the corresponding frequency of detection by DNAsa are also reported in Fig. 1, Fig. 2. Of note, there was a statistically significant difference in detection of methicillin-resistant Staphylococcus aureus by DNAsa (P = 0.016), Pseudomonas aeruginosa (P = 0.013) and anaerobes (P = 0.035) when compared to detection of these species by SHC.
When comparing the ability of each culture type to detect a dominant and multiple bacterial pathogens, we found that the SHC was able to grow bacteria and identify a dominant infectious pathogen in 28/45 patients (62.2%) while DNAsa detected and identified bacteria in 44/45 patients (97.8%). In the 28 cases in which the SHC and DNAsa both identified bacteria, the pathogen detected by SHC concurred with the dominant pathogen detected by the DNAsa in 21/28 cultures (75.0%). In the cases where the DNAsa culture detected a Gram-negative rod or Anaerobe as the primary organism, the SHC did not detect a dominant organism and the culture results returned as “no growth”. When comparing the 23 specimens where the SHC detected a dominant organism with these patients' results detected by the DNAsa probe, the two detection methods agreed on the primary pathogen in 21/23 cultures (91.3%).
Discussion
The primary goal of our study was to compare the utility of our SHC methods with newer DNAsa technology to determine if we could obtain more accurate results with the DNAsa culture probe. Identifying a more accurate culture method could potentially lead to optimal infection resolution in both surgically and medically treated patients.6 Our results demonstrate that the SHC was unable to reliably detect the presence of multiple organisms in the sinus cavity. The SHC detected two or more pathogens in 13/43 (30.2%, P < 0.001) of the patients' cultures and three or more pathogens in 1/43 (2.3%, P < 0.001), while the DNAsa detected two or more organisms in 32/43 (74.4%, P < 0.001) of our patients and three or more pathogens in 21/43 (48.8%, P < 0.001). These results underscore the superiority of DNAsa over SCHs and how chronic infections are often polymicrobial in nature and therefore unlikely to respond to narrow spectrum antimicrobials or monotherapy.7 Relying on bacterial culture that often yield only the most dominant organism or only a single one is unlikely to lead to appropriate antimicrobial therapy and the resolution of infection. Therefore, when the SHC results produced a dominant isolate, they often failed to detect other potential pathogens that may contribute to the infectious microbiome.
Consequently, antimicrobial therapy based on SHC results would have been adequate for only 19/43 (44%) of the patients in this study. However, basing treatment on the DNAsa results, proper antimicrobial therapy directed at the polymicrobial infection would have been administered to 74.4% of the cases. Lastly, the SHC did not speciate any anaerobic bacteria while the DNAsa cultures detected anaerobic bacteria in 9 patients further underscoring the advantage of the molecular pathogen detection probes in detecting these organisms.
DNAsa results allow for the treatment of bacteria that would not have otherwise been isolated or detected by SHC. Moreover, empiric antimicrobial therapy selection can be used for DNAsa results even when susceptibility tests are unavailable. Most commonly, empiric treatment regimens for common CRS bacterial pathogens include a beta lactam antibiotic combined with a beta lactamase inhibitor such as clavulanic acid for anaerobics, quinolones for aerobic and facultative Gram negatives, and clindamycin or linezolid for Staphylococcus aureus. However we suspect that empiric treatment of sinus infections will not always be necessary as tailored antibiotic therapy can be chosen based on known patterns of resistance in local hospitals and communities.
Additionally, although we did not have a case of fungal sinusitis during our study, we did note that it took on average 4–8 weeks for standard hospital fungal cultures to yield results while the DNAsa probe was able to yield fungal culture results as quickly as it determined bacterial results. Such timely results would help in the selection of the proper antifungal agents. A more timely detection of potential pathogens can facilitate the selection and initiation of the most appropriate antimicrobial therapy. Our findings support the efficacy of DNAsa molecular probe in the earlier detection of fungal infection.
Our findings are in concordance with prior DNAsa-based culture studies, such as Rhoads et al,8 who demonstrate how molecular aerobic and anaerobic bacterial and fungal detection methods reveal a greater order of magnitude of bacterial species in chronic infections than standard hospital culture results demonstrate. When the DNAsa and SHC results are directly compared, there is a marked distinction between the results, impacting our perception of the patients' infectious microbiomes. In SHC chronic recalcitrant infections refractory to medical management are based on single-pathogen results. For effective treatment and eradication of the infectious microbiome in a chronic wound, multiple bacteria must be targeted simultaneously, which requires multiple antibiotics targeting pathogens co-existing in the patient's chronic infection.6
Unfortunately, since DNAsa culture results do not determine culture sensitivities and specificities, they are unable to recommend which antibiotics to avoid in cases of bacterial resistance. Especially in cases of chronically infected patients who have a history of prior antibiotic treatment, ensuring that bacterial resistance is not propagated using new antimicrobial regimens is critical. While such information would optimize treatment recommendations, the DNAsa has not yet developed the capacity to determine a pathogen's sensitivity or resistance to specific antibiotics.9 However, detection of resistance genes using DNAsa is possible and may help accurately identify resistant organisms, so antibiotic therapy can be selectively targeted.
While SHC are currently the first-line diagnostic tests used to detect the underlying pathogen in a CRS patient, when a patient has recalcitrant infections or a culture result that returns inconclusive despite being taken under visibly purulent, active infection, DNAsa provides a clear advantage and should be considered the next test to determine the causative organism. Though the DNAsa technology is new and may be more expensive than a SHC, the ability to accurately identify the infectious pathogens sooner can improve patient recovery time, minimize recurrent disease and requirement for prolonged antibiotic courses with various antiobiotics, and ultimately provide improved patient quality of life faster and more consistently.
Conclusions
Optimizing the accuracy of identifying organisms involved in CRS greatly relies on the methods used. In cases of chronic polymicrobial infections, traditional SHC are limited in their ability to accurately isolate multiple bacterial isolates. As a result, treatment options can be inadequate. Transitioning means of pathogen detection in patients with CRS to methods of molecular detection such as DNAsa is warranted, especially in cases that have proven refractory to narrow spectrum or antimicrobial mono-therapy. In patients with recalcitrant sinus disease and intractable infections superimposed on CRS, conventional hospital cultures to determine infectious pathogens may need to be augmented with or replaced by more robust and dependable molecular-based probes. In addition, although our study did not focus on DNA molecular detection of fungal sinusitis, the molecular DNA probes are more timely allowing patients at risk of developing invasive fungal sinusitis to be promptly diagnosed and treated. Further investigation using a larger study population is warranted to confirm our findings. Results from a more powerful study may eventually shift the standard of care for pathogen and microbiome detection methods using molecular analysis.
Material support
PathoGenius Laboratories, a subsidiary of MicroGen DX, donated the 50 DNA Sequencing Analysis culture probes used for this study as well as the lab reports of the culture results for the samples obtained using the swabs.
Financial disclosures
Authors have no financial disclosures.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Edited by Yi Fang
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Rosenfeld R.M., Piccirillo J.F., Chandrasekhar S.S. Clinical practice guideline (update): adult sinusitis executive summary. Otolaryngol Head Neck Surg. 2015;152:598–609. doi: 10.1177/0194599815574247. http://www.ncbi.nlm.nih.gov/pubmed/25833927 [DOI] [PubMed] [Google Scholar]
- 2.Brook I. Microbiology of sinusitis. Proc Am Thorac Soc. 2011;8:90–100. doi: 10.1513/pats.201006-038RN. [DOI] [PubMed] [Google Scholar]
- 3.Ramakrishnan V.R., Gitomer S., Kofonow J.M., Robertson C.E., Frank D.N. Investigation of sinonasal microbiome spatial organization in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2017;7:16–23. doi: 10.1002/alr.21854. http://www.ncbi.nlm.nih.gov/pubmed/27627048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens W.W., Lee R.J., Schleimer R.P., Cohen N.A. Chronic rhinosinusitis pathogenesis. J Allergy Clin Immunol. 2015;136:1442–1453. doi: 10.1016/j.jaci.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kingdom T.T., Swain R.E. The microbiology and antimicrobial resistance patterns in chronic rhinosinusitis. Am J Otolaryngol. 2004;25:323–328. doi: 10.1016/j.amjoto.2004.03.003. http://www.ncbi.nlm.nih.gov/pubmed/15334396 [DOI] [PubMed] [Google Scholar]
- 6.Joss T.V., Burke C.M., Hudson B.J. Bacterial communities vary between sinuses in chronic rhinosinusitis patients. Front Microbiol. 2015;6:1532. doi: 10.3389/fmicb.2015.01532. http://www.ncbi.nlm.nih.gov/pubmed/26834708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boase S., Foreman A., Cleland E. The microbiome of chronic rhinosinusitis: culture, molecular diagnostics and biofilm detection. BMC Infect Dis. 2013;13:210. doi: 10.1186/1471-2334-13-210. http://www.ncbi.nlm.nih.gov/pubmed/23656607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhoads D.D., Wolcott R.D., Sun Y., Dowd S.E. Comparison of culture and molecular identification of bacteria in chronic wounds. Int J Mol Sci. 2012;13:2535–2550. doi: 10.3390/ijms13032535. http://www.ncbi.nlm.nih.gov/pubmed/22489109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagarajan K., Loh K.C. Molecular biology-based methods for quantification of bacteria in mixed culture: perspectives and limitations. Appl Microbiol Biotechnol. 2014;98:6907–6919. doi: 10.1007/s00253-014-5870-9. http://www.ncbi.nlm.nih.gov/pubmed/24928659 [DOI] [PubMed] [Google Scholar]