Supplemental Digital Content is available in the text
Keywords: drug-susceptible tuberculosis, outpatient treatment, tuberculosis
Abstract
Kazakhstan has a high burden of multidrug-resistant tuberculosis (TB). The patient-centered National Program for the treatment and prevention of TB has been implemented in Kazakhstan. The program is aimed at meeting the needs of patients and expansion of the outpatient treatment of TB in the country.
The aim of the study was to compare the efficacy of the outpatient and inpatient treatment of drug-susceptible TB.
This study was a retrospective cohort study.
A total of 36.926 TB cases were included. The majority of patients were treated as inpatients. The socioeconomic factors, sex, age, HIV status, and other diagnostic factors (e.g., sputum smear results, extrapulmonary disease) may serve as risk factors to estimate the likely TB treatment outcome. The outpatient treatment of drug-susceptible TB seems to be a comparable option to the inpatient treatment in terms of efficacy.
The socioeconomic factors are the main modifiable risk factors for treatment failure. The outpatient treatment of drug-susceptible TB is safe and effective.
1. Introduction
Tuberculosis (TB) is the ninth main cause of death worldwide. In 2017, 10.0 million people (range 9.0–11.1 million) developed the TB disease. It was also estimated that most of the TB cases occurred in India, China, and Indonesia, together accounting for 45% of all TB cases. The WHO has developed a list of countries with a high TB burden, where Kazakhstan is listed as a country with a high burden of multidrug-resistant TB (MDR TB). In 2016, the general TB incidence in Kazakhstan was 67 cases per 100,000 population and that of MDR TB or rifampicin-resistant TB was 39 cases per 100,000 population.[1] The primary MDR TB accounts for 25% of cases and acquired MDR TB accounts for 43%.[2] Some countries with a high burden of MDR TB in the European region, including Kazakhstan (where 85% of drug-susceptible TB cases are treated as inpatients),[3] provide a significant amount of TB medical care in hospitals, although the outpatient treatment of TB, including MDR TB , has been regarded as an effective and cost-effective approach.[4–6] The need for the development of reliable outpatient TB treatment strategies is of great importance, especially in resource-limited settings, where intensive adherence support strategies, such as Direct Observation of Therapy (DOT), may be unfeasible due to a lack of medical personnel or patient access.[7,8]
The new WHO strategies “The END TB”[9] and “Tuberculosis action plan for the WHO European Region 2016–2020”[10] propose the development of integrated, patient-centered National Programs for the treatment and prophylaxis of TB, together with creation of incentives for the use of innovative approaches toward prevention, diagnostics, and treatment of TB. The aforementioned documents recommend the expansion of scope and coverage of treatment and prevention of TB, with the focus on highly effective, integrated patient-centered approaches.
Primary health care should play a central role in the patient-centered combat against TB.[7] Thus, a government-led initiative seeking to modernize the medical care and expand the outpatient treatment [including the psychological and social assistance] of TB patients by optimizing the anti-TB service in Kazakhstan (in accordance with the WHO recommendations[9]) was launched in 2013, in the Akmola region. This initiative was later expanded to include other regions (Aktobe, Zhambyl, Kyzylorda regions, and Astana). The National Scientific Center of Phthisiopulmonology was instituted to supervise and monitor the reformed and integrated system of TB treatment. In this article, we present the results of the aforementioned reform.
The aims of our study were
-
1.
to compare the treatment results of drug-susceptible TB between the outpatient and inpatient settings;
-
2.
to identify potential outpatient treatment failure factors.
2. Methodology
2.1. Study design
This study was a retrospective cohort study with continuous sampling. All patients with drug-susceptible TB registered during the period lasting from 2014 to 2016 throughout the Republic of Kazakhstan were included in the study.
The inclusion criteria were the following: individuals with drug-susceptible TB, treated with either category 1 (2 months of rifampicin (R), isoniazid (H), ethambutol (E), pyrazinamide (Z), and 4 moths of HR) or category 2 (retreatment regimen composed of 2 months of streptomycin (S), rifampicin (R), isoniazid (H), ethambutol (E), and pyrazinamide (Z); 1 month of R, H, E, and Z; and 5 months of R, H, and E)[11] regimens during the period lasting from December 26, 2013 to December 25, 2016, ≥18 years of age at the time of inclusion in the study, and the outcome of treatment had to be registered at the time of data collection (May 1, 2017). Duration of the study: 3 years—from 2014 to 2016.
2.2. Inpatient and outpatient treatment definition.
All patients, regardless of the category and treatment regimen, underwent the continuation phase in the outpatient settings, provided mainly by the primary healthcare organizations. If the entire course of treatment (including both the intensive and continuation phases) was administered on an outpatient basis or if a short-term hospitalization occurred before sputum conversion, but the intensive and continuation phases were still administered on an outpatient basis, such treatment was designated as the complete outpatient treatment of TB; otherwise, the patient was classified as an individual treated as an inpatient.
3. Materials and methods
The national database, the National Tuberculosis Register of the Ministry of Health of the Republic of Kazakhstan, was used to collect the data. The following patient data were collected: the patient's registration number, surname and name, the medical institution where the patient was treated, diagnosis, category, patient type, region, place of residence, social status, risk factors, survey results, microscopy data (direct microscopic examination by Ziehl-Neelsen method), chest X-ray examination, diagnosis, including accelerated method GeneXpert MTB/RIF, Bacteс, polymerase chain reaction line-probe assay (Hain test), and disease outcomes. The socially disadaptive group included migrant workers, homeless people, persons serving jail or prison sentences, persons under arrest pending investigation or trial, and unemployed persons; other individuals were identified as socially adaptive individuals.
Outpatient treatment of TB was provided primarily by primary health care organizations. Outpatient treatment of TB was defined as the treatment that was carried out fully on an outpatient basis (intensive and continuation phases), including cases with short-term hospitalization before sputum conversion and subsequent transfer to outpatient treatment (intensive and continuation phases) with patient-oriented support. Detailed definition of variables is provided in the Supplementary material.
3.1. Diagnostic methods
GeneXpert MTB/RIF (GXpert) is a single-use sample-processing cartridge system with integrated multicolor real-time polymerase chain reaction capacity that detects the presence of drug-resistant Mycobacterium tuberculosis complex DNA and its susceptibility to rifampicin in a single reaction.[12] Bactec MGIT 960 (Bacteс) system is used to cultivate M. tuberculosis isolates that are later confirmed by colony morphology.[13] Drug-susceptible TB was defined as a case were isolated M. tuberculosis were susceptible to isoniazid (INH) and rifampicin (RFP), as identified with Bactec MGIT 960 culture media and GeneXpert MTB/RIF.[14]
3.2. Patient outcomes assessment
In accordance with the WHO framework,[15] patient outcomes were defined in 6 categories: cured (an individual with pulmonary TB and bacteriological confirmation at the treatment initiation, who later became smear- or culture-negative in the last month of treatment), treatment completed (the patient completed the prescribed treatment with no evidence of treatment failure, without the results of last month of treatment sputum smear of culture), died (for any reason), lost to follow-up, and not evaluated (patients with no treatment outcome assigned); other definitions are included in the Supplementary material. These outcome categories were then collapsed to form the variable “treatment success,” with “successful” including cases that were cured or individuals who completed the treatment. The “unsuccessful” category included all other outcomes.
All information was recorded with the dates of the results. To assess the effectiveness of TB treatment and identify risk factors, the following data were collected: sociodemographic data, social status, risk factors; medical information (data on TB status of the patient, diagnosis, localization of the TB process, type of patient, category, date of treatment, diagnostic results); type of medical care (outpatient, inpatient); treatment regimen; and outcome (of patients with drug-sensitive TB). The data in the final database were depersonalized.
Ethical statement. The permission to perform this study was issued by the Local Ethical Committee of Asfendiyarov Kazakh National Medical University as of March 29, 2017, Decision number #430.
4. Data and statistical analysis
The data were analyzed using the SPSS Statistics package (version 17) and SAS University Edition and summarized with descriptive statistics. The data cross-tabulation was performed; the presence of association between categorical variables was assessed with χ2 test; and odds ratio was presented for the 2 × 2 cross-tabulations. The independent samples t test was used to compare the means of continuous variables (that were symmetrically distributed) between the groups, where appropriate. Data unification principles are presented in Supplementary material.
5. Results
The sample included all 36,926 patients with drug-sensitive forms of TB registered in the Registry (the Kazakhstan national tuberculosis database for drug susceptible TB patients) during the 3 years (2014–2016) before May 1, 2017, who met the criteria of being ≥18 years of age and having had a registered outcome of the disease. In the vast majority of cases, TB infection was pulmonary localized. Nearly two-thirds of the patients were new cases, and a little over one-third had been previously treated for TB. Full results of data analysis and summary statistics are provided in Tables 1–5. The majority (88.5%; n = 32,687) of TB patients were treated on an inpatient basis, 10.8% were treated as outpatients, and in 0.7% of the cases; the treatment setting data was missing. The failure rates were comparable across the inpatient and outpatient treatment groups (Table 4 ); however, the majority of the cases were treated as inpatients and, to some extent, the inpatients seemed to have a lower successful treatment rate. In addition, a number of risk factors had a significant relationship with treatment success, but with a negligible effect size. For patients with 2 risk factors, the success rate for treatment was lower than for those with one risk factor. The treatment duration seemed to be similar between the outpatient and inpatient groups: the treatment duration in days (mean [SD]) in the outpatient group was 197.4 (85.1), and in the inpatient group, it was 194.7 (98.5), t (5411.5) = 1.90, P = .06.
Table 1.
Frequency distributions for variables.

Table 4 (Continued).
Comparison of patient related factors between outpatient and inpatient treatment groups.

Table 4.
Comparison of patient related factors between outpatient and inpatient treatment groups.

Table 2.
Treatment outcome comparison.

Table 4 (Continued).
Comparison of patient related factors between outpatient and inpatient treatment groups.

Table 4 (Continued).
Comparison of patient related factors between outpatient and inpatient treatment groups.
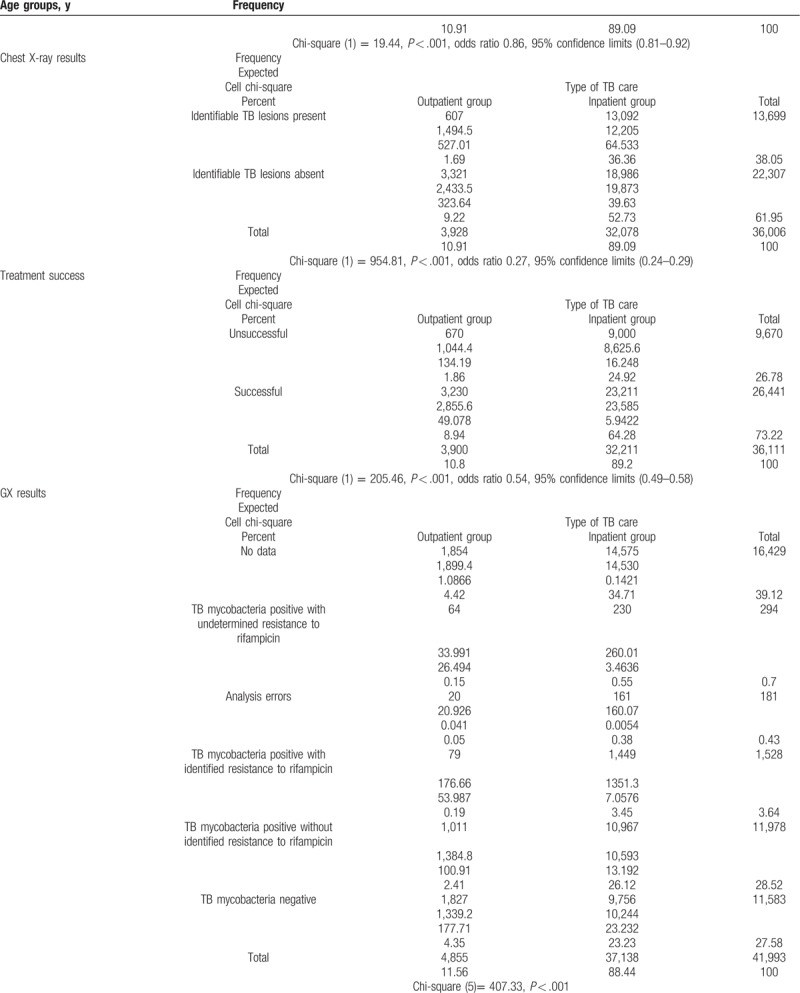
Table 5.
Comparison of patient related factors between successful and unsuccessful treatment outcomes.

Table 5 (Continued).
Comparison of patient related factors between successful and unsuccessful treatment outcomes.

Table 5 (Continued).
Comparison of patient related factors between successful and unsuccessful treatment outcomes.

Table 5 (Continued).
Comparison of patient related factors between successful and unsuccessful treatment outcomes.
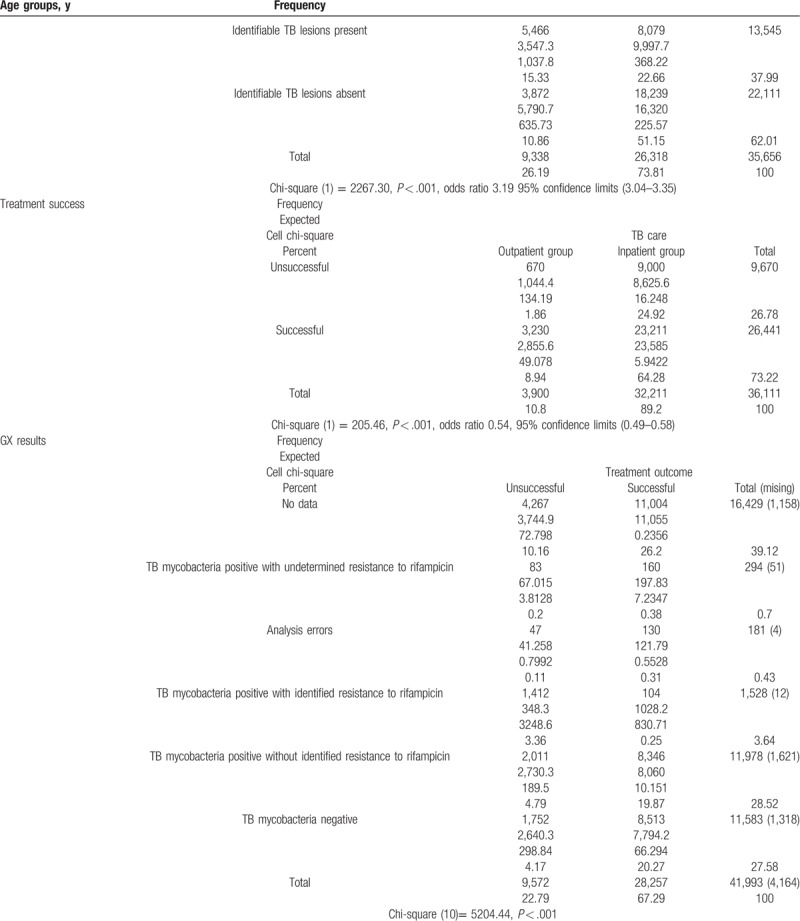
5.1. Comparison of patient-related factors between outpatient and inpatient treatment groups
The comparison of patient-related factors between the groups revealed that the inpatient and outpatient treatment groups were quite heterogeneous, as it seems that older, male, HIV positive, relapsed, socially disadaptive patients with sputum smear positive, identifiable TB lesions in chest X-ray, and MDR TB (with GX identified rifampicin resistance) in rural areas were more likely to be treated as inpatients (Table 4 ).
5.2. General treatment success factors
The women had a higher treatment success rate than the men. The younger adults (aged under 30) also seemed to have greater success rates than the middle age and older groups. The rural residents were more likely to complete the treatment successfully. New cases and other previously treated cases were also noticeably more successful than relapse cases and patients who were re-treated after previous failure (by roughly 12%–18%). Incident case patients were more likely to be treated successfully than previously treated patients. Social status was also significantly related to the treatment success. Socially adaptive patients were more likely to successfully complete the TB treatment than disadaptive patients. The distribution of general risk factors of patients with susceptible forms of TB is provided in Table 3.
Table 3.
Risk factors.

5.3. Diagnostics related treatment success factors
Smear negative pulmonary TB patients and extrapulmonary TB patients had noticeably higher rates of successful treatment (24%–30%) than those with smear-positive pulmonary TB and generalized TB. The type of TB care had a significant relationship with treatment success. X-ray test results also were significantly related to treatment success. The patients who had chest X-rays showing no pulmonary damage were more likely to successfully complete treatment than the patients who had X-rays showing the presence of pulmonary damage. The smear-negative patients were more likely to have successful treatment outcomes than smear-positive patients. Most noticeably, the patients with drug-resistant TB had drastically lower success rates, although 93% of these patients who were designated as having been treated unsuccessfully were in fact referred to a different treatment scheme, with their eventual treatment outcomes not included in the source registry. The HIV-negative patients had a 25% greater rate of successful treatment than HIV-positive patients. The one patient who refused the test was successfully treated. Comparison of diagnostics related success factors is presented in detail in Table 5 .
6. Discussion
In our study, we present a successful attempt to improve the outpatient TB treatment system in Kazakhstan. The outpatient TB treatment seems to be a safe and effective alternative to the inpatient treatment for patients with a less complicated course of TB (as indicated by the heterogeneity of inpatient and outpatient population comparison). At the same time, it should be acknowledged, that the development of outpatient treatment for drug-susceptible and drug-resistant TB is challenging in countries that have a hospital-based healthcare system. A similar attempt has recently been made in Uzbekistan.[16]
Previous research has shown that the treatment outcomes do not differ among inpatient/outpatient with tuberculosis or outpatient only groups.[17] It has been brought to attention that inpatients may suffer from more severe TB, and this may result in inferior treatment outcomes.[18] Our results show that the outpatient treatment may be an effective alternative to the inpatient treatment for patients that have a less complicated therapeutic status. In addition, TB patients with HIV infection may have less favorable outcomes,[19] as is also seen in our study. However, the retrospective nature of our study can only be used to draw limited conclusions.
Previous research describes that female sex, illiterate status, and presence of comorbidities may be risk factors for the TB treatment failure.[20] Patient age, TB form, baseline smear,[21] TB/HIV coinfection, age over 64 years, intravenous drugs abuse, other diseases (excluding HIV and diabetes), and need for retreatment[22] have previously been implicated as factors predisposing to the unsuccessful treatment outcome.
The risk factors associated with the drug-susceptible TB treatment failure are age, retreatment, nonadherence to medications, failure to monitor treatment, and positive culture at the end of treatment months 1 or 2.[23] Older age, unemployment, HIV infection, and alcohol use have also been identified as independent risk factors of unsuccessful treatment (e.g., death, lost to follow-up, failure, transfer out, and other).[24] Diabetes mellitus seems to be a contributing factor to culture-positive rates at the end of the second month, treatment failure, and death.[25] The cavitation on chest radiographs bilateral involvement and combined pleural effusion are seemingly related with smear positivity after ≥ 5 months of treatment and may potentially be treatment failure risk factors.[26] Furthermore, the financial burden of treatment, medication side effects, and beliefs may lead patients to treatment discontinuation and may predispose them to treatment failure.[27]
Successful MDR-TB treatment is associated with non-HIV patients, sputum-negativity at baseline, unilateral disease and no prior drug-resistant TB diagnosis.[28] HIV-positive, younger patients are less likely to be treated successfully.[29] Sputum smear conversion [that is acid fast bacilli are no longer detectable] results obtained 2 months after the treatment initiation,[30] and identification of resistance to fluoroquinolones[31] have also been described as factors determining the treatment outcome. Interestingly, outpatient care for MDR TB has also been successfully implemented[32] and does not seem to carry additional risks in comparison with inpatient care, thus supporting the World Health Organization's (WHO) recommendation that patients with TB should be treated using mainly ambulatory care.[4,32]
Our study has several limitations. The main limitation of our study is its retrospective nature, leading to imbalance between the outpatient (e.g., with regard to sex balance as a result of more women being included in this study) and inpatient treatment groups, aggravating the straightforward comparison between the treatment outcomes. This imbalance seems to reflect the clinical reasoning of physicians, as it is reasonable to treat more complicated cases in hospital (e.g., older patients with positive sputum smear and MDR TB).
The incomplete documentation of medical data in our retrospective study might be a source of bias. In many cases, the medical personnel failed to enter the results of TB diagnostics or risk factors. An extreme amount of missing data was observed in the provision of TB diagnostics data, where data were missing in >40% of the cases.
7. Conclusions
Kazakhstan has successfully started an advanced outpatient TB treatment program by reforming both the infrastructure and legislative environment. The development of patient-centered outpatient TB treatment is an ongoing process that can also be successfully implemented, without apparent issues regarding the quality and efficacy of TB treatment. It would be reasonable to conclude that to generate more data and to draw more reliable conclusions, regarding the efficacy of TB treatment and treatment failure risk factors, more studies of prospective nature should be performed.
Socioeconomic factors, HIV positivity, and TB diagnostics related factors (like smear negativity and extrapulmonary TB infection, chest X-ray results) may be risk factors for treatment failure.
Author contributions
Participated in research design: ŽP, AN, TM, ES.
Conducted the study: SA, LS, AN, TM, EB, KK.
Performed data analysis: LS, SA, ES, EB, TC, KK.
Wrote or contributed to the writing of the manuscript: LS, SA, ŽP, AN, TC, ES.
Conceptualization: Laura Sadykova, Talgat Maimakov, Elmira Berikova, Kural Kurakbayev, Žilvinas Padaiga, Edgaras Stankevičius.
Data curation: Laura Sadykova, Silvijus Abramavičius, Talgat Maimakov, Albinas Naudžiūnas.
Formal analysis: Laura Sadykova, Silvijus Abramavičius, Albinas Naudžiūnas, Edgaras Stankevičius.
Funding acquisition: Laura Sadykova, Talgat Maimakov.
Investigation: Talgat Maimakov, Kural Kurakbayev, Edgaras Stankevičius.
Methodology: Laura Sadykova, Talgat Maimakov, Elmira Berikova, Kural Kurakbayev, Albinas Naudžiūnas, Edgaras Stankevičius, Silvijus Abramavičius.
Project administration: Albinas Naudžiūnas, Edgaras Stankevičius.
Resources: Talgat Maimakov, Elmira Berikova, Edgaras Stankevičius.
Software: Kural Kurakbayev, Edgaras Stankevičius, Silvijus Abramavičius.
Supervision: Laura Sadykova, Talgat Maimakov, Nathan T. Carr, Albinas Naudžiūnas, Edgaras Stankevičius.
Validation: Laura Sadykova, Silvijus Abramavičius, Albinas Naudžiūnas, Edgaras Stankevičius.
Visualization: Žilvinas Padaiga.
Writing – original draft: Silvijus Abramavičius, Talgat Maimakov, Nathan T. Carr, Albinas Naudžiūnas.
Writing – review and editing: Silvijus Abramavičius, Nathan T. Carr, Albinas Naudžiūnas.
Silvijus Abramavičius orcid: 0000-0002-9183-7783.
Supplementary Material
Footnotes
Abbreviations: Bacteс = Bactec MGIT 960 system, E = ethambutol, GXpert = GeneXpert MTB/RIF assay, H = isoniazid, HIV = human immunodeficiency virus, MDR = multidrug-resistant, R = rifampicin, S = streptomycin, TB = tuberculosis, WHO = World Health Organization, XDR TB = extensively drug-resistant tuberculosis, Z = pyrazinamide.
SL and SA equally contributed to this work.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].WHO | Global tuberculosis report 2017. WHO. 2017. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed February 1, 2018. [Google Scholar]
- [2].WHO | Tuberculosis country profiles. WHO. 2017. Available at: http://www.who.int/tb/country/data/profiles/en/. Accessed February 1, 2018. [Google Scholar]
- [3].Global Tuberculosis Report. 2016. Available at: http://apps.who.int/medicinedocs/en/d/Js23098en/. Accessed February 1, 2018. [Google Scholar]
- [4].Bassili A, Fitzpatrick C, Qadeer E, et al. A systematic review of the effectiveness of hospital- and ambulatory-based management of multidrug-resistant tuberculosis. Am J Trop Med Hyg 2013;89:271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fitzpatrick C, Floyd K. A systematic review of the cost and cost effectiveness of treatment for multidrug-resistant tuberculosis. Pharmacoeconomics 2012;30:63–80. [DOI] [PubMed] [Google Scholar]
- [6].Bada FO, Okpokoro E, Blok N, et al. Cost of three models of care for drug-resistant tuberculosis patients in Nigeria. BMC Infect Dis 2019;19:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Uplekar M, Weil D, Lonnroth K, et al. WHO's new end TB strategy. Lancet 2015;385:1799–801. [DOI] [PubMed] [Google Scholar]
- [8].Nguyen TA, Pham MT, Nguyen TL, et al. Video Directly Observed Therapy to support adherence with treatment for tuberculosis in Vietnam: a prospective cohort study. Int J Infect Dis 2017;65:85–9. [DOI] [PubMed] [Google Scholar]
- [9].WHO | WHO End TB Strategy. WHO. 2015. Available at: http://www.who.int/tb/post2015_strategy/en/. Accessed February 1, 2018. [Google Scholar]
- [10].EUR/RC65/17 Rev.1 Tuberculosis action plan for the WHO European Region 2016-2020. 2017. Available at: http://www.euro.who.int/en/about-us/governance/regional-committee-for-europe/past-sessions/65th-session/documentation/working-documents/eurrc6517-rev.1-tuberculosis-action-plan-for-the-who-european-region-20162020. Accessed February 1, 2018. [Google Scholar]
- [11].Jones-López EC, Ayakaka I, Levin J, et al. Effectiveness of the standard WHO recommended retreatment regimen (category II) for tuberculosis in Kampala, Uganda: a prospective cohort study. PLoS Med 2011;8:e1000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ioannidis P, Papaventsis D, Karabela S, et al. Cepheid GeneXpert MTB/RIF assay for Mycobacterium tuberculosis detection and rifampin resistance identification in patients with substantial clinical indications of tuberculosis and smear-negative microscopy results. J Clin Microbiol 2011;49:3068–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ardito F, Posteraro B, Sanguinetti M, et al. Evaluation of BACTEC Mycobacteria Growth Indicator Tube (MGIT 960) automated system for drug susceptibility testing of Mycobacterium tuberculosis. J Clin Microbiol 2001;39:4440–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kim SJ. Drug-susceptibility testing in tuberculosis: methods and reliability of results. Eur Respir J 2005;25:564–9. [DOI] [PubMed] [Google Scholar]
- [15].Eurosurveillance Editorial Team. WHO revised definitions and reporting framework for tuberculosis. Euro Surveill 2013;18:20455. [PubMed] [Google Scholar]
- [16].Kohler S, Asadov DA, Bründer A, et al. Health system support and health system strengthening: two key facilitators to the implementation of ambulatory tuberculosis treatment in Uzbekistan. Health Econ Rev 2016;6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Simonovska L, Ilievska-Popovska B. Comparison of results from inpatient and outpatient treatment of tuberculosis in Republic of Macedonia. Open access Maced J Med Sci 2015;3:337–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Perrechi MCT, Ribeiro SA. Outcomes of tuberculosis treatment among inpatients and outpatients in the city of São Paulo, Brazil. J Bras Pneumol 2011;37:783–90. [DOI] [PubMed] [Google Scholar]
- [19].Tornheim JA, Dooley KE. Tuberculosis associated with HIV infection. Microbiol Spectr 2017;5: doi: 10.1128/microbiolspec.TNMI7-0028-2016. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jaber AAS, Khan AH, Sulaiman SAS. Evaluating treatment outcomes and durations among cases of smear-positive pulmonary tuberculosis in Yemen: a prospective follow-up study. J Pharm Policy Pract 2017;10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ahmad T, Haroon, Khan M, et al. Treatment outcome of tuberculosis patients under directly observed treatment short course and its determinants in Shangla, Khyber-Pakhtunkhwa, Pakistan: a retrospective study. Int J Mycobacteriol 2017;6:360. [DOI] [PubMed] [Google Scholar]
- [22].Costa-Veiga A, Briz T, Nunes C. Unsuccessful treatment in pulmonary tuberculosis: factors and a consequent predictive model. Eur J Public Health 2017;28:352–8. [DOI] [PubMed] [Google Scholar]
- [23].Wang N, Ma Y, Liu YH, et al. Risk of treatment failure in patients with drug-susceptible pulmonary tuberculosis in China. Biomed Environ Sci 2016;29:612–7. [DOI] [PubMed] [Google Scholar]
- [24].Lucenko I, Riekstina V, Perevoscikovs J, et al. Treatment outcomes among drug-susceptible tuberculosis patients in Latvia, 2006-2010. Public Heal Action 2014;4:54–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ma Y, Huang ML, Li T, et al. Role of diabetes mellitus on treatment effects in drug-susceptible initial pulmonary tuberculosis patients in China. Biomed Environ Sci 2017;30:671–5. [DOI] [PubMed] [Google Scholar]
- [26].Kang HK, Jeong B-H, Lee H, et al. Clinical significance of smear positivity for acid-fast bacilli after ≥5 months of treatment in patients with drug-susceptible pulmonary tuberculosis. Medicine (Baltimore) 2016;95:e4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chida N, Ansari Z, Hussain H, et al. Determinants of default from tuberculosis treatment among patients with drug-susceptible tuberculosis in Karachi, Pakistan: a mixed methods study. PLoS One 2015;10:e0142384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bastos ML, Cosme LB, Fregona G, et al. Treatment outcomes of MDR-tuberculosis patients in Brazil: a retrospective cohort analysis. BMC Infect Dis 2017;17:718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Thu MK, Kumar AMV, Soe KT, et al. High treatment success rate among multidrug-resistant tuberculosis patients in Myanmar, 2012-2014: a retrospective cohort study. Trans R Soc Trop Med Hyg 2017;111:410–7. [DOI] [PubMed] [Google Scholar]
- [30].Lv L, Li T, Xu K, et al. Sputum bacteriology conversion and treatment outcome of patients with multidrug-resistant tuberculosis. Infect Drug Resist 2018;11:147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yu M-C, Chiang C-Y, Lee J-J, et al. OUP accepted manuscript. Clin Infect Dis 2018;Available from: http://www.ncbi.nlm.nih.gov/pubmed/29394358. Accessed February 18, 2018. [Google Scholar]
- [32].Fiseha D, Kumssa H, Tefera M, et al. Ambulatory care for multidrug-resistant tuberculosis: lessons learned in Addis Ababa, Ethiopia. Public Heal action 2014;4Suppl. 3:S37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


