Abstract
The present study aims to measure the retinal thickness of the macular region of AIDS patients with normal ocular fundus, HIV-related microvascular retinopathy patients and cytomegalovirus retinitis (CMVR) patients by optical coherence tomography, and generalize the characteristics of retinal thickness of these 3 groups of patients.
In this retrospective case series, the study object comprised of 111 AIDS patients who received diagnosis and treatment in the Ophthalmology Department of Beijing Youan Hospital. There are 33 patients in the AIDS normal ocular fundus group, 47 patients in the HIV-related microvascular retinopathy group, and 31 patients in the CMVR group. The retinal thickness of the macular region of these above patients was measured. The main indicators were retinal thickness of 9 macular partitions, best corrected visual acuity, CD4+ T lymphocyte count, and the start of highly active antiretroviral therapy.
In the CMVR group, except for the nasal-outer and temporal-outer sectors, the thickness of the affected eye of the rest of the regions was greater than that of healthy eye (P < .05). Furthermore, there was a difference in thickness of the superior-outer and inferior-outer sectors between the AIDS normal ocular fundus group and HIV-related microvascular retinopathy group. The difference in thickness of the superior-inner sector between patients in the AIDS normal ocular fundus group and CMVR group was not statistically significant, while the difference in thickness of the rest of the regions was statistically significant. The difference in thickness of various regions between patients in the HIV-related microvascular retinopathy group and CMVR group was statistically significant.
The retinal thickness of patients in the CMVR group generally increased, the retinal thickness of superior-outer and inferior-outer sections of patients in the HIV-related microvascular retinopathy group increased, when compared to the AIDS normal ocular fundus group. These optical coherence tomography (OCT) examination results present its own characteristics in different eye diseases in AIDS patients, and different stages of eye disease.
Keywords: AIDS, CD4+ T-lymphocyte, CMV-DNA, cytomegalovirus retina, HIV, microvascular retinopathy, optical coherence tomography
1. Introduction
Various retinal anomalies can be found in AIDS patients, and the most commonly used diseases include microvascular retinopathy (MVR) and cytomegalovirus retinitis (CMVR).
The MVR[1,2] can be found in approximately 50% to 70% of AIDS patients. It often manifests as a cotton wool spot and retinal hemorrhage, and affects the patient's visual function. CMVR is often found in the patients with serious and advanced HIV-1 affection. It may lead to the complete loss of sight and increased mortality,[3] even in the highly active antiretroviral therapy (HAART) period. Furthermore, it is the main reason for the visual acuity impairment of AIDS patients, and the majority of visual acuity impairment cannot be recovered.
OCT is a noncontact, noninvasive, high-resolution, and quantifiable biological tissue imaging technology. With the advantages of fast image capture, clearer imaging, increased axial resolution, and support for 3D image reconstruction, spectral-domain OCT (SD-OCT) can perform thickness measurement, analyze various layers of the retina, and provide accurate data.[4] At present, OCT has been widely applied to the quantitative evaluation[5] of the thickness of the fibrous layer of the retinal nerve. However, there is a lack of corresponding reports on the law of retinal thickness of the macular region in AIDS patients. The present study performed an intra-group and inter-group comparison of patients in the AIDS normal ocular fundus group, HIV-related MVR group, and CMVR group, and analyzed the respective characteristics, providing a certain basis for clinical diagnosis and treatment.
2. Data and method
2.1. General data
2.1.1. Inclusion criteria
AIDS patients, who received treatment in the Ophthalmology Department of Beijing YouAn Hospital, Capital Medical University and confirmed by the specialist in the Infection Department from January 2012 to April 2015, patients with normal ocular fundus and patients diagnosed with CMVR or HIV-related MVR upon examination of the eyes without anticytomegalovirus treatment. The time period between the initial diagnosis of HIV-positive and the beginning of the study was 1 day to 3 months.
2.1.2. Exclusion criteria
Patients who have other definite infectious eye lesions, such as herpes virus-related eye diseases (pure herpes simplex viral keratitis, acute retinal necrosis, herpes viral palpebral dermatitis, etc), pneumocystis carinii choroidopathy, ocular toxoplasmosis, fungoid eye disease, bacterial and mycobacterium retinitis, cryptococcus optic nerve retinitis, syphilis-related optic nerve retinitis, glaucoma, maculopathy, and so on. Patients who had hypertension, diabetes, or other comorbid diseases that might damage the vascular structures of the eye. Previous CMVR patients who received anticytomegalovirus treatment, or had complications.
2.1.3. Ethics approval
This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Beijing You An Hospital. A written informed consent was obtained from all participants.
2.2. Methods of examination
2.2.1. Ophthalmologic examination
Best corrected visual acuity (BCVA), intraocular pressure, and anterior segment examination with a slit lamp: the anterior segment, including the eye conjunctiva, cornea, anterior chamber, iris, and lens, was observed. If the anterior chamber is flashing, the suspended cell, keratic precipitates, and pupil synechia can be identified in the anterior chamber. This will be recorded as an anterior segment abnormity. Indirect ophthalmoscopy and colored ocular fundus photography were also performed.
2.2.2. OCT examination
Using a NIDEK RS-3000 light interference tomoscanner, the eyes of all subjects were examined through the OCT instrument after mydriasis. Through Macula Map mode scanning, the mode of 9 partitions was dividing the macular region into the fovea, inner and outer region, according to a distance of ≤1 mm, >1 to 3 mm and >3 to 6 mm from the macula central fovea, and dividing the macular region into 4 quadrants (superior, inferior, temporal, and nasal quadrant) according to the 2 straight lines that passed the macula central fovea, with angles of 45° and 135°, thereby obtaining the retinal thickness of the 9 partitions: fovea, superior-inner, nasal-inner, inferior-inner, temporal-inner, superior-outer, nasal-outer, inferior-outer, and temporal-outer. The value was automatically obtained through the software system, according to the scanning results. No treatment was given to CMVR patients before the OCT examination.
2.2.3. General check-up
AIDS-related immunological detection was carried out for all patients: The blood auxiliary CD4+ T-lymphocyte count detection was carried out, and the HAART state of all patients was recorded.
2.3. Diagnostic criteria
2.3.1. Diagnostic criteria of CMVR
According to the ACTGA standard, the diagnosis of CMVR can be confirmed by an experienced ophthalmologist according to the characteristic retinal change observed in the ocular fundus.[6] The characteristic manifestation of CMVR is as follows: the lesion retina manifests with a yellowish-white retinal focus and regional infiltration, accompanied by a dropsical white boundary or granular white boundary, with or without retinal hemorrhage.[7] The progress of the CMVR is recorded by ocular fundus photography. The exclusion criteria include other necrotic retinitis[8] caused by VZV, HSV, syphilis, toxoplasmosis, or lymphoma.
2.3.2. Diagnosis of HIV-related MVR
Most of these patients have no symptoms. The clinical manifestations are cotton wool spot, and sometimes retinal hemorrhage and hemangioma capillanisum, and these have little impact on the patient's visual function. Furthermore, there is no the manifestation of iritis or hyaloiditis.[7,8]
2.4. Data processing and statistical analysis
The SPSS 22 software is used for the statistical analysis, and the difference was statistically significant in the case of P < .05. The statistical description was determined for the measurement of normally distributed data according to the mean ± standard deviation, and the comparison among groups was performed using t test or variance analysis. For non-normally distributed data, the statistical description was determined according to the median (minimum and maximum), and the comparison was performed using the rank-sum test. The description for count data was determined according to the number of cases (percentage). The comparison among groups was performed for data that conformed to the chi-square test conditions, and the Fisher exact test was performed for data that did not conform to the chi-square test conditions. A matched pair design was used for the comparison of quantitative data. The internal subtraction of pairs was performed to determine the difference of the samples, namely, the quantitative data. A normality test was carried out for the difference sample. If the difference was normally distribution, a paired t test was carried out. Otherwise, a signed rank-sum test of the paired data was carried out. The normality test and homogeneity test of variance were carried out for the mutually independent random samples of the 3 groups. If these 3 groups of samples were normally distributed and have equal variance, 1-way analysis of variance was performed. Otherwise, Kruskal–Wallis test was carried out. Multiple linear regression analysis was performed to analyze the relationship between the measured parameters and the thickness of the macular region.
3. Results
3.1. General clinical data
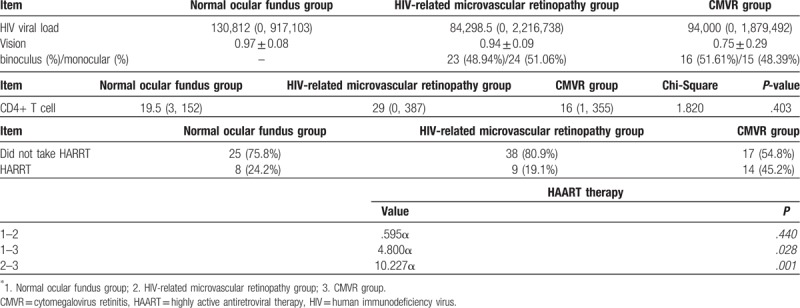
Among the 111 AIDS patients, 33 patients had normal ocular fundus upon examination of the eyes, 31 patients were diagnosed with CMVR, and 47 patients were diagnosed with HIV-related MVR. The viral loads were 130,812 (0, 917,103) in normal ocular fundus group, 84,298.5 (0, 2,216,738) in HIV-related microvascular retinopathy group, and 94,000 (0, 1,879,492) in CMVR group. Furthermore, 105 patients were male and 6 patients were female, and the age of these patients ranged within 20 to 65 years old. In the retinal microangiopathy group, 23 (48.94%) patients had binocular disease, while 24 (51.06%) patients had monocular disease. In the CMVR group, 16 (51.61%) patients had binocular disease, while 15 (48.39%) patients had monocular disease.
3.2. Basic examination of the 3 groups of patients
The BCVA of patients in the AIDS normal ocular fundus group, HIV-related MVR group, and CMVR group was 0.97 ± 0.08, 0.94 ± 0.09, and 0.75 ± 0.29, respectively. Furthermore, the blood CD4+ T-lymphocyte count of patients in the AIDS normal ocular fundus group, HIV-related MVR group, and CMVR group was 19.5 (3, 152), 29 (0, 387), and 16 (1, 355), respectively, and no significant difference was found among groups. Moreover, 25 of 33 patients in the AIDS normal ocular fundus group, 38 of 47 patients in the HIV-related MVR group, and 17 of 31 patients in the CMVR group did not take HAART, occupying 75.8%, 80.9%, and 54.8%, respectively. In the statistical analysis of the number of patients who took HAART, there was significant difference (P < .017) in HIV-related microvascular retinopathy group and CMVR group. No significant difference was found in normal versus HIV-related microvascular retinopathy group and normal versus CMVR group (Table 1).
Table 1.
Basic examination of the 3 groups of patients.
3.3. Difference in variable values of the left and right eyes of patients in the AIDS normal ocular fundus group
For the 33 AIDS patients with normal ocular fundus, their average age was 33 ± 11 years old, the mean visual acuity was 0.97 ± 0.08, and the difference in retinal thickness of the 9 macular partitions between the left eye and right eye of AIDS patients with normal ocular fundus was not statistically significant (P > .05) (Tables 2 and 3).
Table 2.
Paired rank-sum test of variables of the left and right eyes of patients in the AIDS normal ocular fundus group.
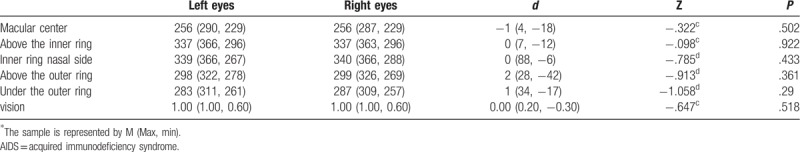
Table 3.
Paired t test of variables for the left and right eyes of patients in the AIDS normal ocular fundus group.

3.4. Retinal thickness of the macular region and visual acuity analysis of the affected eye and healthy eye of patients with monocular MVR
For the 47 patients in the retinal microangiopathy group, their average age was 34 ± 11 years old, and the number of patients with monocular disease was 24 (51.06%). After the normality test, the P-value was >.05 through the rank-sum test or paired t test of the paired data, and there is no difference in retinal thickness of the macular region and visual acuity between the affected eye and healthy eye of patients with monocular MVR (Tables 4 and 5).
Table 4.
Paired rank-sum test of the affected eye and healthy eye of patients in the MVR group.
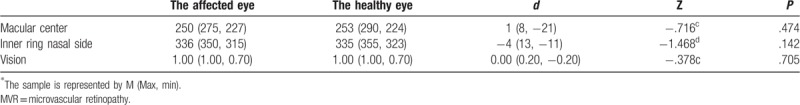
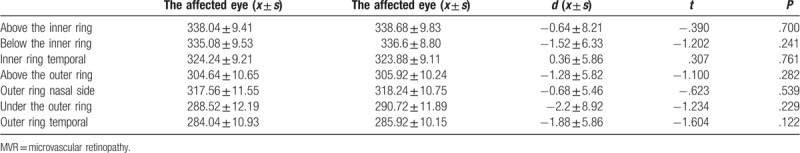
Table 5.
Paired t test of the affected eye and healthy eye of patients in the MVR group.
3.5. Retinal thickness of the macular region and visual acuity analysis of the affected eye and healthy eye of patients with CMVR
For the 31 patients in the CMVR group, the number of patients with monocular disease was 15, and their average age was 39 ± 9.68 years old. Based on the statistical analysis, the BCVA of the affected eye of patients with CMVR was lower than that of the healthy eye, while the retinal thickness of the affected eye was larger than that of the healthy eye in the fovea, inferior-inner, temporal-inner superior-outer, and inferior-outer (P < .05). The difference was statistically significant (Tables 2–6 and 2–7).
Table 6.
Paired rank-sum test of the affected eye and healthy eye of patients in the CMVR group.
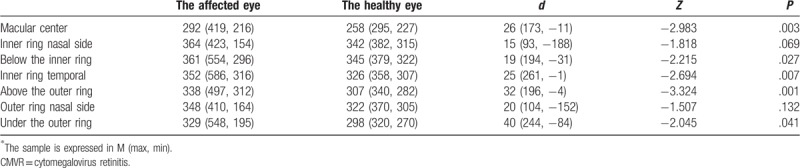
Table 7.
Paired t test of the affected eye and healthy eye of patients in the CMVR group.
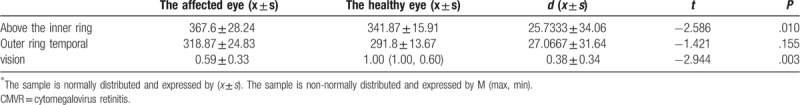
3.6. Retinal thickness of the macular region, visual acuity, start of HAART, and CD4+ T-lymphocyte count among patients in the 3 groups
The start of HAART was significantly different in the HIV-related MVR group and CMVR group (P < .017). The proportion of patients in the CMVR group that started to take the HAART was high, but there was no difference in such indicator among patients in the AIDS normal ocular fundus group, HIV-related MVR group, and CMVR group. (Tables 2–8).
Table 8.
Summary of indicator pairwise comparison results among the 3 groups.
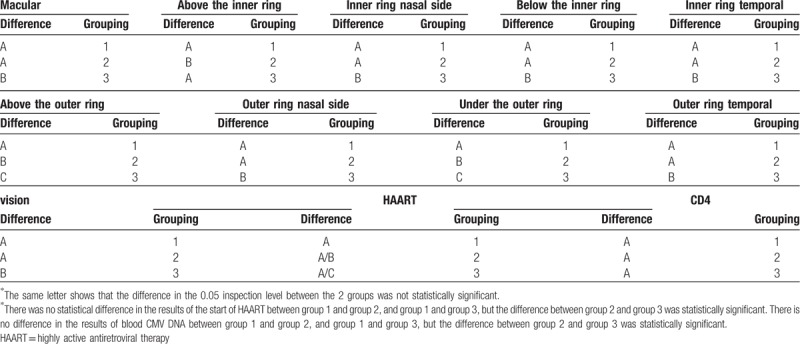
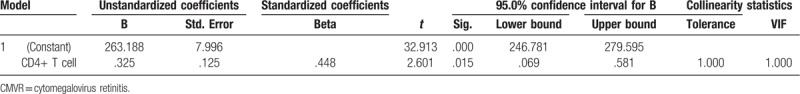
The difference in retinal thickness of the macular region in the superior-outer and inferior-outer of patients between the AIDS normal ocular fundus group and HIV-related MVR group was statistically significant. Furthermore, the thickness in these regions in patients in the HIV-related MVR group was larger than that in patients in the AIDS normal ocular fundus group. However, the remaining test indicators were not statistically significant between these 2 groups. Although the difference in retinal thickness of the superior-inner region in patients between the AIDS normal ocular fundus group and CMVR group was not statistically significant, this was statistically different in the other regions. The thickness of various regions obviously increased in patients in the CMVR group, but visual acuity obviously decreased. The difference in thickness of various regions in patients between the HIV-related MVR group and CMVR group was statistically significant. The thickness in patients in the CMVR group obviously increased, but visual acuity obviously decreased (Table 8). No independent variables were correlated to the thickening in normal ocular fundus group and HIV-related microvascular retinopathy group. In CMVR group, the multiple linear regression analysis showed that the blood CD4+ T-lymphocyte count was correlated with macular thickness (Table 9).
Table 9.
Multiple linear regression analysis in CMVR group.
4. Discussion
The present study conducted an intra-group and inter-group comparison of the visual acuity, CD4+ T-lymphocyte count, start of HAART and retinal thickness of the macular region in patients in the AIDS normal ocular fundus group, HIV-related MVR group and CMVR group.
-
(1)
OCT characteristics of patients in the AIDS normal ocular fundus group: The present study performed a statistical analysis of various data of the left eye and right eye of patients in this group. The binocular difference in retinal thickness of various partitions of the macular region was not statistically significant, and the median of the retinal thickness of the fovea of the left eye and right eye was 256 (290, 229) μm and 256 (287, 229) μm, respectively. The fovea retina was the thinnest, the temporal retina was thinner, the nasal and superior retinas were thicker, and the inner retina was thicker than the outer retina. Consistently, the superior and inferior retinal nerve fiber layers of the macular region were thicker, the nasal retina of the macular region was closer to the optic nerve head, and the maculary fasciculi of optic nerve head were richer than the temporal. This is similar to the study conducted by Kakinoki et al[9] with the same mode for normal Japanese subjects with an average age of 49.9 years old and an average fovea retinal thickness of 257.6 ± 19.6 μm.
-
(2)
OCT characteristics of patients in the HIV-related microvascular retinopathy group: Among the 47 patients in the HIV-related MVR group, 24 patients had monocular disease. The statistical analysis of the retinal thickness of the healthy eye and affected eye of patients with monocular disease was performed, and the difference in retinal thickness between the healthy eye and affected eye was not statistically significant. In this group, the fovea retina was the thinnest, the temporal retina was thinner, the nasal and superior retinas were thicker, and the inner retina was thicker than the outer retina.
-
(3)
OCT characteristics of patients in the CMVR group: In the present study, the thickness of various macular regions in the CMVR group generally increased, and the reflection of the entorretina increased.[10] This is consistent with the study results reported by Japanese scholar Yashiro et al,[11] but is contradictory to the study results reported by Pathai[12] and Plummer.[13] Plummer et al used a Heidelberg retina tomograph to measure the thickness of the nerve fiber layer in 38 HIV positive patients with or without CMVR and 24 HIV negative controls, and found that the thickness of the nerve fiber layer of HIV positive patients was smaller than that of the controls, regardless of CMVR. Pathai et al verified the deficiency of the retinal nerve fiber layer of patients with HIV infection through OCT examination. The reason for the analysis was that the CMVR patients were at the early stage of CMVR when they took the OCT examination, and the ocular fundus mainly manifested in the exudation of yellowish-white particles, accompanied by retinal hemorrhage. This clinical manifestation was deemed to be retinal necrosis and different degrees of edema histologically, and significant damage[14] to the retinal structure was the most serious[15] in the active retinitis area, thereby leading to increased retinal thickness of the affected eye in the CMVR examination results. The study conducted by Invernizzi et al revealed that patients with active CMVR have 100% hemorrhage and retinal swelling.[16] With the implementation of treatment or progress of the course of disease, the early lesion would gradually become static. With the clearing of necrotic tissue, this would gradually become the gray or transparent neuroglia scar tissue, allowing the retinal and RPE atrophy to be obvious seen in the scar tissue area. This study shows that the retinal atrophy caused by the CMVR would gradually develop[17] at 2 years after the attack. If the OCT examination is carried out at this time, the retina and retinal nerve fiber layer will become thin.
Compared with the normal ocular fundus group, the thickness of the remaining regions, except for the superior-inner retinal thickness, obviously increased and visual acuity obviously decreased in the CMVR group. The reason may be that the CMVR lesion location of patients in this group was not in the superior-inner area. Different results may be attained when the sample size of patients in this group is increased.
-
(1)
The difference in nasal-outer and temporal-outer retinal thickness of patients in the CMVR group between the affected eye and healthy eye was not statistically significant. The thickness of the healthy eye increased, which may have be caused by the retinal pigment epithelium decompensation[18] induced by the subclinical dysfunction of the macular photoreceptor and inner retina, as well as damage to the blood-retina barrier.
-
(2)
The cotton wool spot in the SD-OCT image can be shown as an increase in nerve fiber layer,[19] and the superior-outer and inferior-outer retinal thickness of patients in the HIV-related MVR group increased, when compared with that in patients in the AIDS normal ocular fundus group. This may be due to the appearance of the cotton wool spot in this region.
Healthy subjects were not included in the present study. Hence, there may be bias in comparing the thickness of various OCT regions of AIDS patients in various groups with the study results of domestic and foreign literatures. Healthy Chinese subjects should be included in a comparative study in the future. In addition, a study revealed that the macular region of newly diagnosed patients with higher viral load obviously thickened,[20] when compared with HIV infection patients that received treatment. The OCT characteristics can be further summarized by refining patients in the CMVR group according to the course of disease and ocular fundus lesion area.
5. Conclusion
The retinal thickness of macular region of patients in the CMVR group generally increased, and the superior-outer and inferior-outer retinal thickness of patients in the HIV-related MVR group increased, when compared with patients in the AIDS normal ocular fundus group. Furthermore, the OCT examination results of AIDS patients with different eye diseases have their own characteristics.
Author contributions
Conceptualization: Lian-Yong Xie, Wen-Bin Wei.
Data curation: Lian-Yong Xie, Chao Chen.
Formal analysis: Chao Chen, Wen-Jun Kong, Chun-Gang Guo.
Investigation: Wen-Jun Kong, Kui-Fang Du.
Methodology: Wen-Bin Wei.
Resources: Kui-Fang Du, Chun-Gang Guo.
Software: Wen-Jun Kong, Kui-Fang Du, Chun-Gang Guo.
Visualization: Wen-Bin Wei.
Writing – original draft: Lian-Yong Xie, Chao Chen.
Writing – review and editing: Wen-Jun Kong, Kui-Fang Du, Chun-Gang Guo, Wen-Bin Wei.
Footnotes
Abbreviations: BCVA = best corrected visual acuity, CMVR = cytomegalovirus retinitis, HAART = highly active antiretroviral therapy, MVR = microvascular retinopathy, OCT = optical coherence tomography, SD-OCT = spectral-domain OCT.
Funding was provided by Open Research Project of Key Laboratory of Capital Medical University (2017YKSJ04) and Capital Medical University Fundamental Clinical Research Cooperation Fund (No.16JL73).
The authors have no conflicts of interest to disclose.
References
- [1].Wei WB. Wills Ophthalmological Manual. 2014;Beijing: Science Press, 544. [Google Scholar]
- [2].Luo J, Jing D, Kozak I, et al. Prevalence of ocular manifestations of HIV/AIDS in the highly active antiretroviral therapy (HAART) era: a different spectrum in Central South China. Ophthalm Epidemiol 2013;20:170–5. [DOI] [PubMed] [Google Scholar]
- [3].Nishijima T, Yashiro S, Teruya K, et al. Routine eye screening by an ophthalmologist is clinically useful for HIV-1-infected patients with CD4 count less than 200 /μL. PLoS One 2015;10: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Invernizzi A, Acquistapace A, Bochicchio S, et al. Correlation between inner retinal layer thickness and cognitive function in HIV. AIDS 2018;32:1485–90. [DOI] [PubMed] [Google Scholar]
- [5].Lim HB, Sung JY, Ahn SI, et al. Retinal nerve fiber layer thickness in various retinal diseases. Optom Vis Sci 2018;95:247–55. [DOI] [PubMed] [Google Scholar]
- [6].Benson CA, Kaplan JE, Masur H, et al. CDC; National Institutes of Health; Infectious Diseases Society of America. Treating opportunistic infections among HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association/Infectious Diseases Society of America. MMWR Recomm Rep 2004;53:35. [PubMed] [Google Scholar]
- [7].CE, Margolis TP. Ocular manifestations of HIV infection. Trans Am Ophthalmol Soc 1995;93:236–44. [PMC free article] [PubMed] [Google Scholar]
- [8].Becker KN, Becker NM. Ocular manifestations seen in HIV. Dis Mon 2014;60:268–75. [DOI] [PubMed] [Google Scholar]
- [9].Kakinoki M, Sawada O, Sawada T, et al. Comparison of macular thickness between cirrus HD-OCT and stratus OCT. Ophthalmic Surg Lasers Imaging 2009;40:135–40. [DOI] [PubMed] [Google Scholar]
- [10].Arevalo JF, Garcia RA, Arevalo FA, et al. Unilateral ischemic maculopathy associated with cytomegalovirus retinitis in patients with AIDS: optical coherence tomography findings. J Ophthalmic Vis Res 2015;10:487–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yashiro S, Nishijima T, Yamamoto Y, et al. Spectral domain optical coherence tomography and fundus autofluorescence findings in cytomegalovirus retinitis in HIV-infected patients. Japn J Ophthalmol 2018;62:373–89. [DOI] [PubMed] [Google Scholar]
- [12].Plummer DJ, Bartsch DU, Azen SP, et al. Retinal nerve fiber layer evaluation in human immunodeficiency virus positive patients. Am J Ophthalmol 2001;131:216–22. [DOI] [PubMed] [Google Scholar]
- [13].Pathai S, Lawn SD, Weiss HA, et al. Retinal nerve fibre layer thickness and contrast sensitivity in HIV infected individuals in South Africa: a case control study. PLoS One 2013;8:73694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sun LL. Optical coherence tomography changes in macular CMV retinitis. Digit J Ophthalmol 2012;18: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gupta MP, Patel S, Orlin A, et al. Spectral domain optical coherence tomography findings in Macula-involving cytomegalovirus retinitis. Retina 2018;38: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Invernizzi A, Agarwal A, Ravera V, et al. Optical coherence tomography findings in cytomegalovirus retinitis: a longitudinal study. Retina 2017;38: [DOI] [PubMed] [Google Scholar]
- [17].Arichika S, Uji A, Yoshimura N. Retinal structural features of cytomegalovirus retinitis with acquired immunodeficiency syndrome: an adaptive optics imaging and optical coherence tomography study. Clin Exp Ophthalmol 2016;44:62. [DOI] [PubMed] [Google Scholar]
- [18].Moschos MM, Margetis I, Markopoulos I, et al. Optical coherence tomography and multifocal electroretinogram study in human immunodeficiency virus positive children without infectious retinitis. Clin Exp Optom 2011;94:291–5. [DOI] [PubMed] [Google Scholar]
- [19].Jabs DA. Ocular manifestations of HIV infection. Trans Am Ophthalmol Soc 1998;339:236–44. [PMC free article] [PubMed] [Google Scholar]
- [20].Cetin EN, Kutlu SS, Parca O, et al. The thicknesses of choroid, macular segments, peripapillary retinal nerve fiber layer, and retinal vascular caliber in HIV-1-infected patients without infectious retinitis. Retina 2018;doi: 10.1097. [DOI] [PubMed] [Google Scholar]


