Abstract
Introduction
Chronic suppurative otitis media (CSOM) is a common problem in worldwide and untreated CSOM leads to fatal complications like facial nerve paralysis, lateral sinus thrombosis, labyrinthitis, meningitis and brain abscess in developing country like India.
Objective
To isolate causative bacteria and antibiotic sensitivity pattern for CSOM and to know the prevalence of extended spectrum beta lactamases (ESBL) and Metallobetalactamases (MBL) in CSOM patients.
Methods
A total of 500 ear swabs of clinical suspected CSOM patients were cultured on specific cultured medium and identified the bacteria with conventional methods. Then all the identified bacteria were subjected with specific antibiotics by the Kirby–Bauer's method to know the resistance pattern of antibiotics. ESBL and MBL strains were detected by double disc diffusion test.
Results
A total of 384 bacteria were isolated from 500 CSOM patients, among them 86 P. aeruginosa (22.40%), 112 Staphylococcus aureus (29.17%), 53 A. baumannii (13.80%), 32 E. aerogenes (18%), 26 C. freundii (6.77%), 24 K. oxytoca (6.25%), 23 P. vulgaris (5.99%), 18 K. pneumoniae (4.69%) and 10 P. mirabilis (2.60%) identified with conventional methods. From antibiotic disc diffusion methods 74.22% ESBL strains and 9.90% MBL strains were documented. Multidrug resistant strains of P. aeruginosa (86/384,22.40%) were more prevalent than those of S. aureus (112/384,29.17%) and other bacteria in ear discharges. Imipenem and vancomicin could control to gram negative bacteria and gram positive bacteria respectively.
Conclusion
Continuous and periodic evaluation of microbiological profile and antimicrobial sensitivity pattern of bacterial is essential for optimum management of CSOM patients.
Keywords: Antibiotics, Chronic suppurative otitis media, Gram negative bacteria, ESBL, MBL
Introduction
Chronic suppurative otitis media (CSOM) is chronic inflammatory condition of the middle ear cleft with permanent perforation, ear discharge and hearing loss. Clinicians dispute the duration of otorrhea for more than three months.1, 2 There are two types of CSOM and these are safe or unsafe depending on the presence of cholesteatoma3: Safe CSOM is CSOM without cholesteatoma and can be subdivided into active or inactive depending on whether or not infection is present. Unsafe CSOM is often associated with cholesteatoma which may lead to fatal complications.
The most common bacteria for CSOM are Pseudomonas aeruginosa, Klebsiella sp., Proteus sp., and Staphylococcus aureus.4 Whereas, the bacteria commonly isolated from patients with acute otitis media (AOM) are Moraxella catarrhalis and Streptococcus pneumonia, Haemophilus influenzae.5 Additionally, the P. aeruginosa had been seen as a notorious pathogen in the hospital too.6, 7 Certainly, the ability to form biofilm by these organisms contributes to their frequency in CSOM.8 It is often found in immune compromised patient as compared to the normal patients.9 Mostly, with several clonal variants of the bacteria were resistant to the penicillin group of antibiotics, after which different antibiotics were introduced for the control.10 The most horrible situation is that Methicillin resistant Staphylococcus aureus (MRSA) strains have emerged with subsequent resistance to most commonly used antibiotics of groups, macrolides, aminoglycosides, fluoroquinolones, chloramphenicol and tetracycline and many more such as, to cephalosporin, cefepime and other betalactams, ampicillin-sulbactam, amoxicillin-clavulanic acid, ticarcillin-clavulanic acid, piperacillin-tazobactam and the carbapenem, imipenem. So the isolated MRSA are MDR too.11 In a study extended spectrum beta lactamases (ESBL) and ampicillin were detected in 11 (18.3%) and 12 (20.0%) Gram negative bacteria, respectively. Whereas the MBL producer was not detected.12
This work describes the surveillance of bacterial flora from ear infections of patients attending the Outpatients Department (OPD) of Otorhinolaryngology department of the hospital, in a year. Antibiotic sensitivity pattern of isolated bacterial were determined to assess the variety of CSOM that would help in empirical therapy with the antimicrobial stewardship program of the teaching hospital at the eastern India. Also, the incident of ESBL and MBL strains from the CSOM were documented.
Materials and methods
Selection of CSOM patients
CSOM is a long-standing suppurative middle ear infection with permanent perforation, otorrhea and hearing loss with duration of more than three months. CSOM patients were diagnosed clinically by the consultant otolaryngologist. On the basis of clinical presentations and otoscopic examinations, patients were selected for this study. The patients of acute infections of the middle ear cleft of less than three months and traumatic perforations of the tympanic membrane were excluded from this study. The pus is collected from the ear canal in CSOM patients with the help of the sterile ear swab (Himedia mumbai PW003) by consultant otolaryngologist with all aseptic measures.
Study population
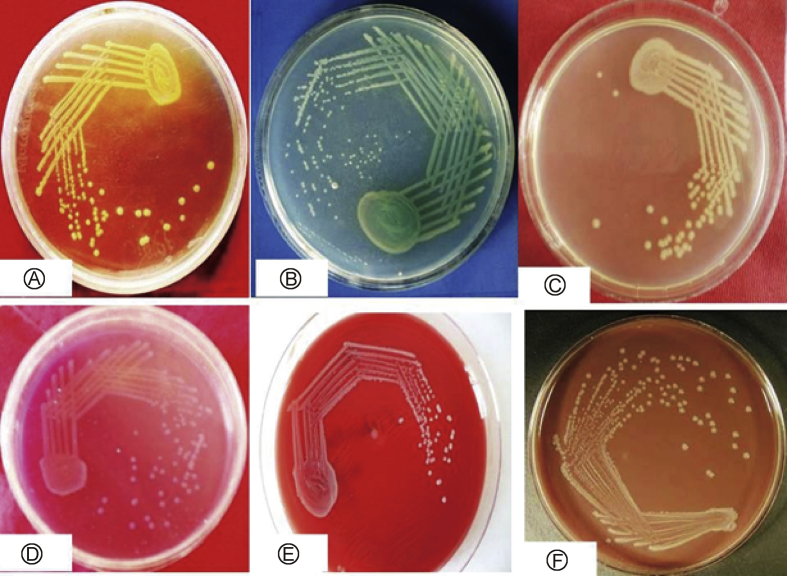
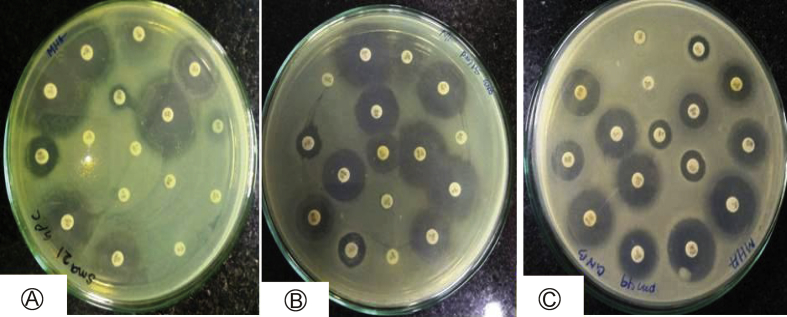
A total of 500 ear swabs from clinically diagnosed CSOM cases were collected, during July 2016 to June 2017 with sterile cotton swab sticks. Pus swabs were cultured on blood and MacConkey agar plates that were incubated at 37 °C overnight for pathogenic bacteria, which were identified according to the standard method used for bacteria (Fig. 1A–F).13 Antibiotic susceptibility tests of isolated bacteria were done according to Clinical Laboratory Standard Institute guidelines (Fig. 2A–C), as described.14 Standard antimicrobial disks (HiMedia, Mumbai) used for isolated bacteria were amikacin 30, gentamicin 30, ciprofloxacin, amoxyclav 30, aztreonam 30, piperacillin 100, piperacillin/tazobactam 100/10, cefepime 30, cefoperazone 75, cefoperazone/sulbactam 75/30, ceftazidime 10, ceftriaxone 10, imipenem 10, gatifloxacin 30, Oxacilin and Vancomicin. The standard MTCC strains and all the clinical isolated bacteria were subjected to antibiotic sensitivity tests with antibiotics, by the Kirby–Bauer's method (disk diffusion) detailed previously.11
Fig. 1.
Macroscopic photograph of isolated bacteria on specific medium from human chronic suppurative otitis media A: Stphylococcous aureus; B: Pseudomonas aeruginosa; C: Acenetobacter baumanii; D: Citrobacter freundii; E: Enterobacter sp.; F: Proteus sp.
Fig. 2.
Antibiotic sensitivity pattern of isolated bacteria from human chronic suppurative otitis media on MHA (Mueller Hinton Agar) A: Psueudomonas aeruginosa; B: Acenetobacter baumanii; C: Citrobacter fruindii.
Detection of MBL strain
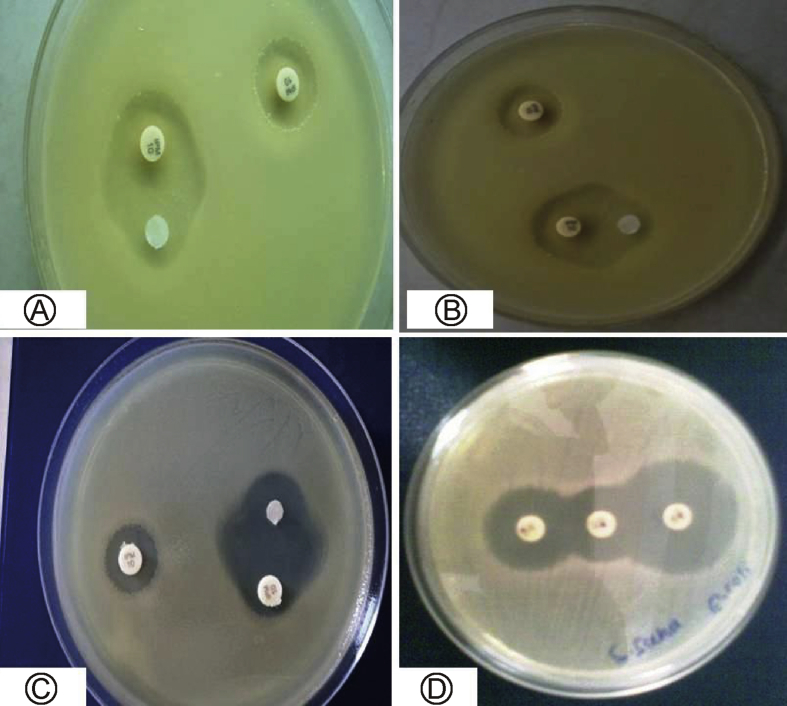
The metallo β-lactamases (MBL) production was detected by the imipenem – EDTA-DDST. The organisms were considered to be MBL producers, if the increase in the inhibition zone of the β-lactam + EDTA disc was ≥5 mm (Fig. 3A–C).15 The MBL production in this isolate, for the synergy test imipenem-EDTA disk was used developed, as followed. Earlier reports have recommended that the imipenem-EDTA disk synergy test is a reliable method for initial screening of MBL production in clinical isolates.16
Fig. 3.
Detection of Metallobetalactamases (MBL) and extended spectrum beta lactamases (ESBL) with disc diffusion methods A: MBL strain of Pseudomonas aeruginosa; B: MBL strain of Klebsiela pneumaneae; C: ESBL strain of Citrobacter freundii; D: ESBL strain of Acenetobacter baumanii.
Detection of ESBL strains
The double disc synergy test (DDST) was used to detect the ESBL producing activity of selected bacterial strains on a lawn culture on a MH agar plate. The augmentin 30 μg disc (20 μg amoxicillin and 10 μg clavulanic acid) in the middle was flanked by a disc of ceftazidime 30 μg and a disc of cefotaxime 30 μg (both third generation cephalosporin), at 30 mm apart on a lawn culture. The set up was done in triplicates and plates were incubated at 37 °C for overnight for observation of inhibition zones. With the augmentin disc inhibition the action of the ESBL enzyme give an inhibition zone, which was formed from a peripheral disc towards the middle, due to the synergistic action of the augmentin and the corresponding cephalosporin disc (Fig. 3D). In general, if the organism is resistant to both cephalosporins, because of the production of the ESBL enzyme, the action of augmentin deactivated the enzyme with a consequent reactivation of a cephalosporin resulting in the extension of the inhibition zone.17
Results
Growth of bacteria from ear swabs of CSOM patients
A total of 500 ear swabs were subjected to culture on specific culture media for bacterial growth. In this study the prevalence of bacteria among CSOM patients was 82.6%. It was observed that varieties of colonies were grown on the specific cultured medium. In certain plate single type of colony and in other cases two, three or more than 3 types of colonies were grown. The percentage of frequency of the colony was documented (Table 1). The single colony frequency was found more as compare to other types of colonies from the ear swab. Three or more colonies were treated as contaminated samples. With specific culture media different type of colonies were grown and it was identified with conventional method by both macroscopically and biochemically (Table 2, Table 3). All the grown positive bacteria were processed with catalase and coagulase and Staphylococcus aureus were identified basing upon the results of catalase and coagulase. Similarly all the gram negative bacterial were processed with different biochemical tests and basing up on their results the bacteria were identified (Table 3). For the species identification the carbohydrate fermentation tests were carried out and results were documented (Table 4).
Table 1.
Number of varieties of colony observed macroscopically on the cultured plate.
| No. of different Colony | Frequency | Number of bacteria | Percentage of bacteria isolated (%) |
|---|---|---|---|
| No growth | 87 | 0 | 17.4 |
| Single colony | 216 | 216 | 43.2 |
| Two colony | 84 | 168 | 16.8 |
| Three or more colony | 29 | Treated as contaminated | |
| Total | 500 | 384 | 76.8 |
Table 2.
dentification of bacteria with culture morphology.
| Bacterium | MTCC no. | Agar media | Colony morphology |
|---|---|---|---|
| Staphylococcus aureus | 7443 | Nutrient agar | Golden yellow, opaque, circular colonies white butyrous consistency |
| Mannitol salt agar | Yellow colonies, | ||
| Acinetobacter baumannii | 1425 | Nutrient agar (NA) | Colourless smooth, opaque, raised and pinpoint colonies. |
| MacConkey agar | Colourless smooth, opaque, raised, NLF colonies | ||
| Enterobacter aerogenes | 2990 | Blood agar | Small, round and pin -point colony. |
| MacConkey agar | LF and mucoid colonies | ||
| Citrobacter freundii | 1658 | MacConkey agar | Late LF colonies light pink after 48 h |
| Klebsiella sp. | 4031 | MacConkey agar | LF, pink, mucoid colonies. |
| CLED agar | Yellow and mucoid colonies | ||
| Proteus sp. | 1771 | Blood agar | Swarming colonies |
| CLED | translucent blue colonies | ||
| Pseudomonas aeruginosa | 1688 | Nutrient agar, | Large, irregular opaque colonies, with bluish green pigment. |
MTCC: Microbial type culture collection; Na: not available; EMB: Eosin methylene blue agar; XLD: Xylose lysine deoxycholate; CLED: cystine lactose electrolyte deficient medium; LF, lactose fermenting; NLF: Non-lactose fermenting.
Table 3.
Summary of results of biochemical tests of isolated Gram-negative bacteria.
| Bacteria | Catalase | Oxidase | Indole | MR | VP | Citrate | Urease | TSI | Nitrate | Motility |
|---|---|---|---|---|---|---|---|---|---|---|
| A. baumannii | + | – | – | – | + | + | V | ND | – | M |
| Citrobacter sp. | + | – | – | + | – | + | – | K/A + H2S | + | M |
| Enterobacter sp | + | – | – | – | + | + | V | A/A | + | M |
| K. oxytoca | + | – | + | – | + | + | + | A/AG | + | NM |
| K. pneumoniae | + | – | – | – | + | + | + | A/AG | + | NM |
| P. vulgaris | + | – | – | + | – | V | + | K/A H2S | + | M |
| P. mirabilis | + | – | + | + | – | V | + | K/A H2S | + | M |
| P. aeruginosa | + | + | – | – | – | + | + | ND | + | M |
+: positive; –: negative; V: variable; MR: methyl red; VP: Voges-Proskauer; TSI: triple sugar iron; A: acid; K: alkali; G: gas; H2S: H2S production; M: motile; NM: non-motile; ND: not done.
Table 4.
Summary of results of carbohydrate fermentation tests of isolated Gram-negative bacteria.
| Bacteria | Glucose | Lactose | Sucrose | Maltose | Mannitol |
|---|---|---|---|---|---|
| A. baumannii | – | – | – | – | – |
| Citrobacter sp. | A + G | LLF | + | + | + |
| Enterobacter sp. | A + G | A | + | + | + |
| P. vulgaris | G | – | – | – | + |
| P. mirabilis | G | – | – | – | + |
| K. oxytoca | A + G | A | + | – | + |
| K. pneumoniae | A + G | A | + | – | + |
| P. aeruginosa | A | – | + | + | V |
A: acid; A + G: acid + gas; V: variable; LLF: late lactose fermentation; LSF: late sucrose fermentation; +: positive; –: negative.
Antibiotic sensitivity test of isolated bacteria from CSOM patients
From the antibiotic tests, it was revealed that ceftazidime (CAZ) was highest resistant (78%) to A. baumannii and Amoxyclav is the highest sensitive (86%) to A. baumannii. Similarly the antibiotic resistance percentage for other bacteria was documented (Table 5). But in CSOM patients were prescribed the antibiotic amoxyclav and ciprofloxacin mainly. The ciprofloxacin is resistant to 20% to A. baumannii, 12% to C. freundii, 51% to E. aerogenes, 32% to K. oxytoca, 62% to K. pneumonia, 81% to P. vulgaris, 31% to P. mirabilis, 28% to P. aeruginosa and 43% to S. aureus. Similarly, Amoxyclav is resistant to 14% to A. baumannii, 31% to C. freundii, 32% to E. aerogenes,16% to K. oxytoca, 21% to K. pneumonia, 25% to P. vulgaris, 23% to P. mirabilis,17% to P. aeruginosa and 38% to S. aureus (Table 5).
Table 5.
Percentage of antibiotic resistance with corresponding bacteria (μg/disc).
| Bacteria | AK | GEN | CIP | AMC | AT | PI | PIT | CPM | CPZ | CFS | CAZ | CTR | IPM | GAT | OX | V |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. baumannii | 23 | 30 | 20 | 14 | 71 | 30 | 21 | 71 | 62 | 34 | 78 | 72 | 21 | 20 | – | – |
| C. freundii | 7 | 8 | 12 | 31 | 63 | 40 | 39 | 69 | 52 | 15 | 82 | 74 | 31 | 22 | – | – |
| E. aerogenes | 78 | 56 | 51 | 32 | 60 | 78 | 34 | 61 | 56 | 24 | 74 | 68 | 42 | 16 | – | – |
| K. oxytoca | 24 | 36 | 32 | 16 | 74 | 82 | 25 | 54 | 62 | 21 | 82 | 74 | 38 | 19 | – | – |
| K. pneumoniae | 63 | 83 | 62 | 21 | 61 | 87 | 28 | 57 | 71 | 21 | 89 | 82 | 56 | 71 | – | – |
| P. vulgaris | 69 | 48 | 81 | 25 | 58 | 91 | 34 | 64 | 52 | 16 | 91 | 92 | 58 | 69 | – | – |
| P. mirabilis | 49 | 54 | 31 | 23 | 46 | 69 | 21 | 78 | 61 | 14 | 78 | 79 | 37 | 78 | – | – |
| P. aeruginosa | 68 | 59 | 28 | 17 | 51 | 67 | 23 | 83 | 73 | 31 | 73 | 74 | 39 | 31 | – | – |
| S.aureus | 75 | 46 | 43 | 38 | 87 | 59 | – | 68 | 53 | 48 | 27 | 43 | – | 39 | 54 | 36 |
Ak: amikacin 30; GEN: gentamicin 30; CIP: ciprofloxacin; AMC: amoxyclav 30; AT: aztreonam 30; PI: piperacillin 100; PIT: piperacillin/tazobactam 100/10; CPM: cefepime 30; CPZ: cefoperazone 75; CFS: cefoperazone/sulbactam 75/30; CAZ: ceftazidime 10; CTR: ceftriaxone 10; IPM: imipenem 10 (Carbap., carbapenem); GAT: gatifloxacin 30; OX: Oxacilin; V:Vancomicin.
Incidence of ESBL and MBL strains from CSOM patients
All the strains were screened for ESBL and MBL and it was revealed that 74.22% ESBL and 9.90% MBL strains were found. Among the ESBL strains 89 S. aureus and 79 P. aeruginosa strains were found. Similarly, 2.08% S. aureus and 4.43% P. aeruginosa MBL strains were found during this study period (Table 6).
Table 6.
Number of isolated ESBL and MBL strains from Gram negative (GN) bacteria.
| Bacteria | Number of isolated strains | % | ESBL strain | % with respective bacteria | % with total bacteria | MBL strain | % with respective bacteria | % with total bacteria |
|---|---|---|---|---|---|---|---|---|
| A. baumannii | 53 | 13.80 | 34 | 64.15 | 8.85 | 6 | 11.32 | 1.56 |
| C. freundii | 26 | 6.77 | 11 | 42.31 | 2.86 | 1 | 3.85 | 0.26 |
| E. aerogenes | 32 | 8.33 | 18 | 56.25 | 4.69 | 0 | 0 | 0.00 |
| K. oxytoca | 24 | 6.25 | 20 | 83.33 | 5.21 | 0 | 0 | 0.00 |
| K. pneumoniae | 18 | 4.69 | 9 | 50 | 2.34 | 1 | 5.56 | 0.26 |
| P. vulgaris | 23 | 5.99 | 21 | 91.3 | 5.47 | 2 | 8.70 | 0.52 |
| P. mirabilis | 10 | 2.60 | 4 | 40 | 1.04 | 3 | 30.00 | 0.78 |
| P. aeruginosa | 112 | 29.17 | 89 | 79.46 | 23.18 | 8 | 7.14 | 2.08 |
| S. aureus | 86 | 22.40 | 79 | 91.86 | 20.57 | 17 | 19.77 | 4.43 |
| Total | 384 | 100.00 | 285 | 74.22 | 74.22 | 38 | 9.90 | 9.90 |
ESBL: Extended spectrum beta lactamse; MBL: mettalo beta lactamase.
Discussion
In this study the prevalence of bacteria among CSOM patients was 82.6%. This was in tandem with reports from other parts of 91.7%,18 89.4%,19 89.5%,20 100%21 and Nigeria, 81.9%.22 Gram-negative bacteria, 59.6% were the dominant isolates of the discharging ears compared to gram-positive bacteria. Similar reports were seen from Gonder 56.4%,20 Dessie 74.2%,18 Addis Ababa 60.5%23 and Nigeria 75%24 though the proportion varies.
The CSOM is a major health problem both in children and adults world-wide, but more so in developing countries. It can cause chronic hearing loss which has a negative impact on the development of speech, language and social interaction as well as school and workplace performance and is responsible for significant morbidity and mortality due to complications. According to a report by WHO, India belongs to the highest (>4%) CSOM prevalent countries.4 Topical antibiotics are the mainstay of therapy while systemic antibiotics are given in acute exacerbations and in complications due to CSOM.25 The poor living conditions, less access to medical care, poor medical treatment, recurrent upper respiratory tract infections and the nasal diseases have been recognized as risk factors for CSOM.25 Atticoantral disease is the most commonly involved with the posterior superior part of the middle ear cleft and it is characterized by the formation of a retraction pocket with cholesteatoma; eventually it is considered to be a dangerous form of the disease because of the development of several intracranial and extra-cranial complications.26 Moreover, staphylococci are a part of the normal flora, but those remain invasive causing a variety of body infections. S. aureus is the most notorious nosocomial pathogen and in community too.27 Although the clinical relevance of Coagulase negative Staphylococcus Sp. (CONS) is still controversial; patients at risk of CONS infections include neonates, those with intravascular catheters, prosthetic devices and surgical wounds in the immune-compromised individuals. The remarkable ability of S. aureus and CONS to acquire antibiotic resistance limits therapeutic options, attended with high rates of morbidity and mortality, including costs of hospitalization.28, 29 Particularly, several clonal variants of S. aureus and MRSA were reported resistant to the penicillin group of antibiotics, methicillin/oxacillin. Moreover, in a German study, it was reported that a majority of MRSA strains were from wound infections (56.9%), with pneumonia cases being the second most common (21.0%), followed by BSI (15.1%).30
In our study, from 500 samples 384 bacteria were isolated. In other studies, Nazir et al31 and Sanjana et al32 have reported less number of bacteria as compare to our study results. Gram negative bacteria predominance (60.6%) matches other studies in India.33 S. aureus was the predominant bacteria followed by P. aeruginosa which are in opposite with other studies.4, 34 Whereas similar results reported in other studies i.e. S. aureus as predominant isolate followed by P. aeruginosa.35, 36, 37 In this study higher resistance demonstrated in Gram positive bacteria 54% S. aureus exhibiting methicillin resistance. On the other hand resistance among Gram negative bacteria was much lower with 9.90% MBL producer detected but high rate of 74.22% ESBL strains documented.
Conclusion
CSOM is a common clinical entity where topical and systemic antibiotic are the main treatment. However the emergence of antibiotic resistant strains is leading to increasing treatment failure. MDR strains of P. aeruginosa and MRSA were most prevalent in ear discharges of patients with CSOM. Continuous and periodic evaluation of microbiological profile and antimicrobial sensitivity pattern of bacterial is essential for optimum management of CSOM patients.
Conflicts of interest
The authors declare no conflicts of interest.
Funding
This work was supported by the NPDF research project file NO. PDF/2016/000773 on CSOM, from SERB, DST, Govt. of India, New Delhi.
Edited by Yu-Xin Fang
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Arslan I.B., Genc S., Kayhan B.C., Gumussoy M., Ozel G., Cukurova I. Bacterial change in external auditory canal upon antisepsis with povidone-iodine during tympanoplasty. Eur Arch Otorhinolaryngol. 2015;272:551–555. doi: 10.1007/s00405-013-2864-7. [DOI] [PubMed] [Google Scholar]
- 2.Thompson P.L. University of London, School of Pharmacy; London: 2009. Effect of Antibiotics for Otitis Media on Mastoiditis in Children. [Google Scholar]
- 3.Chirwa M., Mulwafu W., Aswani J.M., Masinde P.W., Mkakosya R., Soko D. Microbiology of chronic suppurative otitis media at Queen Elizabeth Central Hospital, Blantyre, Malawi: a cross-sectional descriptive study. Malawi Med J. 2015;27:120–124. [PMC free article] [PubMed] [Google Scholar]
- 4.Shyamala R., Reddy P.S. The study of bacteriological agents of chronic suppurative otitis media-Aerobic culture and evaluation. J Microbiol Biotechnol Res. 2017;2:152–162. [Google Scholar]
- 5.Berman S. Otitis media in children. N Engl J Med. 1995;332:1560–1565. doi: 10.1056/NEJM199506083322307. [DOI] [PubMed] [Google Scholar]
- 6.Sahu M.C., Dubey D., Rath S., Debata N.K., Padhy R.N. Multidrug resistance of Pseudomonas aeruginosa as known from surveillance of nosocomial and community infections in an Indian teaching hospital. J Publ Health. 2012;20:413–423. [Google Scholar]
- 7.Afolabi O.A., Fadare J.O., Omokanye H.K. Socioeconomic challenges of chronic suppurative otitis media management in state tertiary health facility in Nigeria. Egypt J Ear Nose Throat Allied Sci. 2014;15:17–22. [Google Scholar]
- 8.Couzos S., Lea T., Mueller R., Murray R., Culbong M. Effectiveness of ototopical antibiotics for chronic suppurative otitis media in Aboriginal children: a community-based, multicentre, double-blind randomised controlled trial. Med J Aust. 2003;179:185–190. doi: 10.5694/j.1326-5377.2003.tb05496.x. [DOI] [PubMed] [Google Scholar]
- 9.Bluestone C.D., Klein J.O. Otitis Media in Infants and Children. 3rd ed. WB Saunders; Philadelphia, PA: 2001. pp. 79–101. [Google Scholar]
- 10.Sahu M.C., Padhy R.N. Bayesian evaluation of two conventional diagnostic methods for pathogenic fungal infections. J Acute Med. 2014;4:109–119. [Google Scholar]
- 11.Askarian M., Hosseini R.S., Kheirandish P., Assadian O. Incidence and outcome of nosocomial infections in female burn patients in Shiraz, Iran. Am J Infect Control. 2004;32:23–26. doi: 10.1016/j.ajic.2003.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Khatoon A., Rizvi M., Sultan A. Chronic suppurative otitis media: a clinico-microbiological menace. Int J Res Med Sci. 2015;3:1932–1936. [Google Scholar]
- 13.Ahmed B., Hydri A.S., Ejaz A., Farooq S., Zaidi S.K., Afridi A.A. Microbiology of ear discharge in Quetta. J Coll Phys Surg Pak. 2005;15:583–584. [PubMed] [Google Scholar]
- 14.Wisplinghoff H., Bischoff T., Tallent S.M., Seifert H., Wenzel R.P., Edmond M.B. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 15.Pitout J.D., Gregson D.B., Poirel L., McClure J.A., Le P., Church D.L. Detection of pseudomonas aeruginosa producing metallo-beta-lactamases in a large centralized laboratory. J Clin Microbiol. 2005;43:3129–3135. doi: 10.1128/JCM.43.7.3129-3135.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irfan S., Zafar A., Guhar D., Ahsan T., Hasan R. Metallo-beta-lactamase-producing clinical isolates of Acinetobacter species and Pseudomonas aeruginosa from intensive care unit patients of a tertiary care hospital. Indian J Med Microbiol. 2008;26:243–245. doi: 10.4103/0255-0857.42035. [DOI] [PubMed] [Google Scholar]
- 17.Peña C., Pujol M., Ricart A. Risk factors for faecal carriage of Klebsiella pneumoniae producing extended spectrum beta-lactamase (ESBL-KP) in the intensive care unit. J Hosp Infect. 1997;35:9–16. doi: 10.1016/s0195-6701(97)90163-8. [DOI] [PubMed] [Google Scholar]
- 18.Abera B., Kibret M. Bacteriology and antimicrobial susceptibility of otitis media at dessie regional health research laboratory, Ethiopia. Ethiopian J Health Develop. 2011;25:161–167. [Google Scholar]
- 19.Seid A., Deribe F., Ali K., Kibru G. Bacterial otitis media in all age group of patients seen at Dessie referral hospital, North East Ethiopia. Egypt J Ear Nose Throat Allied Sci. 2013;14:73–78. [Google Scholar]
- 20.Muluye D., Wondimeneh Y., Ferede G., Moges F., Nega T. Bacterial isolates and drug susceptibility patterns of ear discharge from patients with ear infection at Gondar University Hospital, Northwest Ethiopia. BMC Ear Nose Throat Disord. 2013;13:10. doi: 10.1186/1472-6815-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diriba M., Solomon G., Hailu N. Isolation and antimicrobial susceptibility pattern of bacterial pathogens causing otitis media in children in Jimma hospital, South Western Ethiopia. Ethiop J Health Sci. 2004;14:89–100. [Google Scholar]
- 22.Osazuwa F., Osazuwa E., Osime C. Etiologic agents of otitis media in Benin city, Nigeria. N Am J Med Sci. 2011;3:95–98. doi: 10.4297/najms.2011.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferede D., Geyid A., Lulseged S. Drug susceptibility pattern of bacterial isolates from children with chronic suppurative otitis media. Ethiop J Health Dev. 2001;15:89–96. [Google Scholar]
- 24.Iseh K.R., Adegbite T. Pattern and bacteriology of acute suppurative otitis media in Sokoto, Nigeria. Ann Afri Med. 2004;3:164–166. [Google Scholar]
- 25.Prajna L., Vijayakumar A. Jaypee Broth Med Publishers; New Delhi: 2008. pp. 24–28. (Atlas of Fungal Corneal Ulcers Clini-Cal Features and Laboratory Identification Methods). [Google Scholar]
- 26.Clinical and Laboratory Standards Institute Performance stan-dards for antimicrobial susceptibility testing. PA Wayne. 2011;1:100–181. [PubMed] [Google Scholar]
- 27.Dash A., Sahu K., Senapati J.N. Surveillance of antibiotic sensitivity and resistance pattern of bacteria isolated from orthopaedic wound discharge. Int J Pharm Sci Rev Res. 2016;36:208–211. [Google Scholar]
- 28.Vikram B.K., Khaja N., Udayashankar S.G., Venkatesha B.K., Manjunath D. Clinico-epidemiological study of complicated and uncomplicated chronic suppurative otitis media. J Laryngol Otol. 2008;122:442–446. doi: 10.1017/S0022215107000278. [DOI] [PubMed] [Google Scholar]
- 29.Chowdhury M.A., Alauddin M. Comparative study between tubotympanic and atticoantral types of chronic suppurative otitis media. Bangladesh Med Res Counc Bull. 2002;28:36–44. [PubMed] [Google Scholar]
- 30.Zong Z., Peng C., Lü X. Diversity of SCCmec elements in methicillin-resistant coagulase-negative staphylococci clinical isolates. PLoS One. 2011;6:e20191. doi: 10.1371/journal.pone.0020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanjana R.K., Singh Y.I., Reddy N.S. Aerobic bacteriology of chronic suppurative otitis media (CSOM) in a tertiary care hospital: a retrospective study. J Coll Med Sci Nepal. 2012;7:1–8. [Google Scholar]
- 32.Kumar H., Seth S. Bacterial and fungal study of chronic suppurative otitis media. J Clin Diagn Res. 2011;5:1224–1227. [Google Scholar]
- 33.Kumar K.R., Navya S., Basavarajappa K.G. A study of bacterial profile and antibiotic susceptibility pattern of chronic suppurative otitis media among patients attending a tertiary care centre, davangere. Sch J App Med Sci. 2014;2:1606–1612. [Google Scholar]
- 34.Sharma V., Kaur G. Microbiology and antimicrobial susceptibility pattern of cases of chronic suppurative otitis media in a tertiary care teaching hospital. Int J Bioassays. 2014;3:3033–3035. [Google Scholar]
- 35.Prakash M., Lakshmi K., Anuradha S., Swathi G.N. Bacteriological profile and their antibiotic susceptibility pattern of cases of chronic suppurative otitis media. Asian J Pharm Clin Res. 2013;6:210–212. [Google Scholar]
- 36.Mozafari N.K., Sepehri G., Khatmi H., Shakibaie M.R. Isolation and antimicrobial susceptibility of bacteria from chronic suppurative otitis media patients in kerman, Iran. Iran Red Crescent Med J. 2011;13:891–894. [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmed K., Mir A., Jan M., Imran R., Shah G., Latif A. Prevalence of bacteria in chronic suppurative otitis media patients and their sensitivity patterns against various antibiotics in human population of gilgit. Pakistan J Zool. 2013;45:1647–1653. [Google Scholar]