Abstract
Acute onset neurological symptoms evoked by traumatic, surgical, or emotional events in Wilson disease (WD) have never been reported and its clinical characteristics are unclear.
We aimed to summarize the clinical characteristics of a special WD whose neurological symptoms acutely developed after traumatic, surgical, or emotional events.
Retrospective pilot study.
Thirty-one patients who had acute onset neurological symptom as an initial presentation of WD or a new presentation of hepatic WD after mild trauma, surgery, or emotional events were retrospectively studied. All patients were followed for half to 1 year after regular anti-copper treatment.
The averaged latency for neurological symptom presentation was 2.79 ± 1.21 hours. The most frequent neurological symptoms were tremor (74%) and basal ganglia (BG) lesions were detected on magnetic resonance imaging in all patients. Lesions in other regions were much less frequently detected. Neurological symptom score and its recovery after treatment were correlated with lesion location: BG area and BG plus other brain areas. Neurological symptoms improved in 21 patients who received timely anti-copper treatment but continued to deteriorate in 6 patients who did not accept regular anti-copper treatment for delayed diagnosis.
A diagnosis of WD should be considered when adolescents or adults experience acute presentation of extrapyramidal systems after traumatic, surgical, or emotional stimulation. Timely anti-copper therapy usually gives rise to an excellent prognosis.
Keywords: acute onset, emotional stimulation, mild trauma, neurological symptoms, surgery, Wilson disease
1. Introduction
Wilson disease (WD) is an autosomal recessive disorder of copper metabolism resulting in pathological accumulation of copper predominantly in liver, brain, cornea, kidneys, and in other tissues and organs. Toxic copper accumulation in these tissues and organs contributes to a variety of clinical conditions with primarily neurological, psychiatric, and hepatic symptoms.[1] Commonly, the younger the age of symptom onset, the greater is the degree of liver involvement.[2] Infants with genetically confirmed WD all presented with hepatic symptoms.[3] Approximately 40% to 50% of patients with WD present with neurological symptoms and these patients typically have a later onset than those with hepatic disease, presenting in the second or third decade.[4,5] Acute manifestations of liver involvement have been commonly reported as an initial presentation of WD.[6–10] And it is not uncommon that acute development of hemolytic anemia associated with or without acute hepatic failure presents as an initial symptom of WD.[6,10–14] However, neurological symptoms usually follow a subacute or chronic course; thus, acute onset are rare.[4,15] In very rare circumstances, acute onset neurological symptoms including anarthria, extrapyramidal syndromes, and seizures may also present as initial manifestations of WD.[16–18] But, it has never been reported in literature that traumatic, surgical, or emotional events caused an acute onset of neurological symptoms in patients with no complaint of pre-existing WD symptoms or in patients with pre-existing hepatic WD. Herein, we describe for the first time the clinical characteristics of 31 patients who acutely developed neurological symptoms after traumatic, surgical, or emotional events as initial presentation of WD or as new presentation in hepatic WD.
2. Materials and methods
2.1. Study population
Among 4530 patients with WD hospitalized in our WD center, we encountered 31 patients who had no complaint of pre-existing neurological manifestation but complaint of acute development of neurological symptoms after trauma, surgery, or emotional stimulation. A complete history was obtained for each patient, including sex, age, events causing acute neurological symptoms, and latency of neurological symptom onset after trauma, surgery, or emotional stimulation. A complete physical and neurological examination was recorded in all cases. Serum copper and ceruloplasmin, 24-hour urinary copper excretion, and brain magnetic resonance imaging (MRI) data were obtained within 3 days after trauma or emotional stimulation in all patients except for those patients with delayed diagnosis.
Diagnosis of WD was based on our previous standard,[19] including characteristic clinical manifestations, a positive family history, low serum ceruloplasmin (<0.2 g/L), elevated 24-hour urinary copper excretion (>100 μg/24 h), presence of a Kayser-Fleischer (KF) ring, and elevated 24-hour urinary copper excretion following administration of 2 × 500 mg doses of penicillamine (>1600 μg/24 h) and MRI of brain,[20,21] and met the WD diagnostic criteria formed at the 8th International Meeting on WD[22] except liver copper >250 μg/g dry weight was not among our criteria.
2.2. Examination and classification
Neurological symptoms and signs were evaluated based on neurological examinations and detailed questionnaire including speech, salivation, dysphagia, writing and gait disturbances, limb weakness, epileptic seizures, and involuntary movements. Psychiatric symptoms were evaluated based on mood disorders, anxiety, depression, and cognitive impairment assessed with mini-mental state examination (MMSE) and montreal cognitive assessment (MoCA). Hepatic symptoms and signs were also evaluated based on physical examinations and detailed questionnaire including fatigue, weight loss, jaundice, leg edema, abdominal swelling, nausea or vomiting, hematemesis, hemorrhages, as well as on laboratory examinations of aminotransferases, bilirubin, prothrombin time and international normalized ratio, albumen and liver and spleen assessment of hepatomegaly, nodular liver, liver cirrhosis, portal hypertension, and splenomegaly confirmed by ultrasonography or computed tomography scanning.
For classification of WD types, symptoms scoring system was adopted and mildly modified from Litwin et al's articles.[23,24] Briefly, assessment of neurological symptoms were ranged from 0 to 3 representing neurological impairment from completely normal (0) to severely impaired (3). Assessment of hepatic symptoms were ranged from 0 to 3 representing completely normal (0), increased serum level of liver enzymes with no liver cirrhosis (1), compensated liver cirrhosis (2), decompensated liver cirrhosis, or acute liver failure (3). Patients with neurological score ≥1 and hepatic score ≤1 were classified as neurological WD, and patients with neurological score ≥1 and hepatic score ≥2 classified as neurological and hepatic WD.
2.3. Follow-up
Outcomes of therapeutic results were also determined based on the symptoms scoring system half to 1 year after the traumatic, surgical, or emotional events for those patients accepting regular anti-copper treatment in hospital, and we followed up the patients who did not accept regular treatment through telephone.
2.4. Statistical analysis
Statistical analyses were performed using a commercial statistical software package (SPSS for windows, version 13.0). Data were expressed as mean ± standard deviation. Paired Student t test was used for comparison between groups of pre- and post-treatment, and chi square and Fisher tests were used to compare percentage. Differences were considered significant at P < .05.
The First Affiliated Hospital of Anhui University of Traditional Chinese Medicine review board approved this study.
3. Results
All 31 patients complained of acute onset of neurological symptoms after trauma, surgery, or emotional stimulation. The demographic and clinical features including the types of trauma, emotional stimulations, and surgeries of 31 patients are listed in Table 1. For all the patients with trauma, no consciousness loss was reported and no hemorrhage, infarction, edema, or other structural alterations noticed on MRI. Among the 31 patients, 20 were men and 11 were women. Acute neurological onset ages ranged from 9 to 46 years (mean 21.26 ± 5.54 years). Averaged diagnosing age was 19.29 ± 6.70, ranging from 5 to 48 years. Averaged latency for acute neurological symptom onset was 2.79 ± 1.21 hours, ranging from 0.5 to 7 hours. The number of 0 (19 patients) in column “A-B” means the diagnosis of WD was made due to the acute neurological symptom onset. A positive number (6 patients) in column “A-B” means a hepatic or asymptomatic WD had been diagnosed before the acute neurological symptom onset. A negative number (6 patients) in column “A-B” means a diagnosis of WD was delayed. These indicate that 19 patients who had no pre-existing clinical WD presentation developed acute neurological symptoms and the diagnosis of WD was made shortly after traumatic, surgical, or emotional events, 6 patients with hepatic or asymptomatic presentations developed acute neurological symptoms, and 6 patients had delayed diagnosis of WD though acute neurological presentations were noticed shortly after traumatic, surgical, or emotional events. There were 6 patients who had previously hepatic or symptomatic WD and developed acute neurological presentations after traumatic, surgical, or emotional events. Patient 7 and 24 had familial histories of WD with their elder sister and elder brother affected with symptomatic WD, and they were detected with asymptomatic WD for 10 (age at diagnosis, 11 years old) and 9 years (age at diagnosis, 17 years old) respectively until they were slapped on face (patient 7) and frightened (patient 24). Patient 2 was diagnosed as hepatic WD for finding of abnormal hepatic function while he was 5 years old and penicillamine treatment gave rise to an asymptomatic status for 23 years. Patient 8 and 28 were found to have albuminuria and were then diagnosed as WD at age of 7 years for 10 and 8 years, respectively. Lastly, patient 12 was diagnosed as hepatic WD due to findings of splenomegaly, hypersplenism and liver cirrhosis and the patient accepted splenectomy at the age of 16 years. All these 6 patients who had previously hepatic or symptomatic WD accepted regular penicillamine treatment and came to our center for intensive treatment with intravenous sodium 2,3-dimercapto-1-propane sulfonate (DMPS) once a year and the therapeutic course would last for 2 weeks. Thus, neurological examination was conducted once a year with no obvious neurological sign recorded. The latest neurological examination before events was performed 3 to 10 days before traumatic, surgical, or emotional events during hospitalization for DMPS treatment and no positive neurological sign was detected in these 6 patients.
Table 1.
Demographic and clinical features of 31 patients.
The most frequent neurological symptoms observed were tremor (23/31, 74%), slurred speech (21/31, 67%), and drooling (6/31, 19.5%), whereas less frequent were abnormal gait (2/31, 6.5%) and increased muscle tension (2/31, 6.5%). No obvious psychiatric and behavioral disturbances, such as swings of mood, outbursts of anger, changes of personality, depression, anxiety, and memory impairment,[25,26] were reported or observed. And cognitive function examinations with MMSE and MoCA were normal in all patients. Except for the 4 patients with pre-existing diagnosis of hepatic WD, all 27 patients had neither severe hepatic function impairment nor severe hepatic presentations such as jaundice, nausea or vomiting, and bloating[27] but obvious fatigue in 7 patients. Liver functions of alanine aminotransferase (ALT) (normal ranges, 5–35 U/L) and aspartate aminotransferase (AST) (normal ranges, 8–40 U/L) were normal in 27 out of the 31 patients. Only 4 patients had mildly elevated ALT (36, 36, and 49 U/L in patient 5, 25, and 28, respectively) and AST (41, 43, and 45 U/L in patient 5, 7, and 28, respectively). Decreased white blood cell number was found in 12 patients and decreased platelet number in 17 patients. Concerning neurological examinations, KF ring was detected in all 31 patients, pyramidal sign in 5 patients, and cerebellar ataxia in 1 patient, but muscle weakness or sensation disturbance in no patient. Except for 6 patients (patient 5, 11, 15, 16, 23, and 25) who did not accept brain MRI examination shortly after the traumatic, surgical, or emotional events and had delayed diagnosis of WD, the rest 25 patients all had brain MRI examination showing lesions in the brain. Finally, basal ganglia (BG) lesion was detected in all 31 patients, but lesions in other areas outside of BG, such as thalamus (8/31, 25.81%), brainstem (14/31, 45.16%), cerebellum (3.23%), and cortical white matter (12.90%) were less frequently detected on MRI. MRI data before traumatic, surgical, or emotional events was available only in 1 patient (patient 8) who was accepting regular treatment in our center. MRI of this patient showed that the mild trauma caused an expansion of BG lesion from bilateral thalamus to bilateral lenticular nucleus (Fig. 1). In order to determine what might be associated with brain lesions, 31 patients were divided into group of patients with brain lesions only in BG and group of patients with brain lesions not only in BG but also in other brain areas and the neurological symptoms, sex, age, and WD type were compared between groups (Table 2). The results showed that the percentage of patients with single symptom was significantly higher in group of patients with lesions only in BG than that in group of patients with lesions not only in BG but also in other brain areas, indicating that lesion restricted in BG caused less severe neurological symptoms. And the sex, age, and WD type were not significantly different between 2 groups, indicating the brain lesions were not associated with sex, age, and WD type (Table 2).
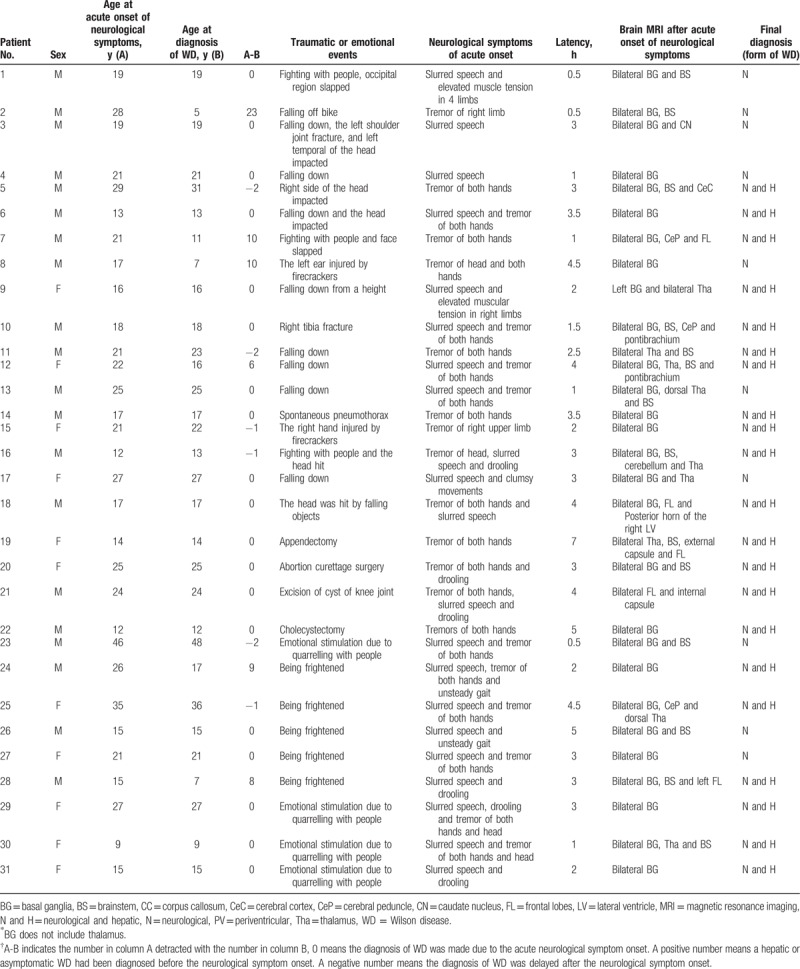
Figure 1.
Brain magnetic resonance imaging (MRI) in a patient with hepatic Wilson disease (WD) before and after a traumatic event. Normal signal on T1 (A1) and T2 (A2) weighted imaging, and high signals involving small areas of bilateral thalamus on flair (A3) weighted imaging 1 week before traumatic events. Low intensive signals involving small areas of left caudate nucleus and bilateral thalamus on T1-weighted imaging (B1), and high signals involving bilateral caudate nucleus and thalamus on T2 (B2) and flair (B3) weighted imaging 4.5 hours after traumatic event.
Table 2.
Comparison of different parameters between patients with lesion only in basal ganglia lesion and patients with lesion in basal ganglia and other brain areas.

Final diagnosis of WD type (neurological or hepatic) was made at the time the patients came to our department hours to days after traumatic, surgery, or emotional events for most patients except for those (patient 5, 11, 15, 16, 23, and 25) who had delayed WD diagnosis. Diagnosis of neurological WD was made in 10 patients (patient 1, 2, 3, 4, 8, 13, 17, 23, 26, and 27), neurological and hepatic WD in the rest 21 patients. Among these 10 patients, patient 2 was diagnosed as hepatic WD for the finding of abnormal liver function while he was 5 years old and penicillamine treatment gave rise to an asymptomatic state for 23 years, and was diagnosed as neurological WD after traumatic event. Twenty-one out of 25 (84%) patients accepting regular treatment with penicillamine or in combination with sodium DMPS, which had been used in our center for decades,[19] had well improvement in neurological and hepatic symptoms. Three (patient 9, 19, and 28) out of 24 (12.5%) patients accepting regular treatment had neither improvement nor obvious deterioration in neurological or hepatic symptoms. Among the 25 patients accepting regular anti-copper treatment, 9 were finally diagnosed as neurological WD and the neurological symptoms improved to a normal level in 8 patients after regular treatment for half to 1 year. Six patients (patient 5, 11, 15, 16, 23, and 25) including 1 patient (patient 23) with final diagnosis of neurological WD did not accept regular treatment due to the delayed diagnosis and the symptoms deteriorated thereafter. In order to determine whether brain lesions were associated with treatment responses, 25 patients accepting regular treatment were divided into group of patients with brain lesions only in BG and group of patients with brain lesions not only in BG but also in other brain areas and the neurological symptom scores were compared between groups (Table 3). The results showed that the neurological symptom scores were significantly improved in patients with brain lesions restricted in BG as well as in patients with brain lesions not only in BG but also in other brain areas. But the percentage of patients with neurological symptoms recovered to normal level after treatment was significantly higher in group of patients with brain lesions only in BG than that in group of patients with brain lesions not only in BG but also in other brain areas (Table 3). This indicated that patients with lesions restricted in BG area had better prognosis.
Table 3.
Comparison of treatment responses between patients with lesion only in basal ganglia lesion and patients with lesion in basal ganglia and other brain areas.

4. Discussion
Here we report 31 patients with WD who developed neurological symptoms shortly (0.5–7 hours) after traumatic, surgical, or emotional events. The short latency (2.79 ± 1.21 hours) for the neurological symptom onset indicated a causal relationship between the neurological symptoms and the traumatic, surgical, or emotional events. The negative results of neurological examination short time (3–10 days) before the traumatic, surgical, and emotional events in 6 patients further confirmed that the newly developed neurological symptoms were initiated by these events. All the acute symptoms were exclusively those of neurological but not hepatic or psychiatric involvement though 21 patients were finally diagnosed as hepatic and neurological WD. Here, the head trauma triggering the onset of neurological symptoms was all mild as no hemorrhage, infarction, edema, or other structural alterations was noticed on brain MRI and no consciousness alteration reported.
In a report of 119 WD cases, the mean age at neurological symptom onset was 19.6 years old and the age ranged from 7 to 37 years old.[28] In another large cohort report, only 31 out of 1223 (2.5%) WD cases became to present with neurological symptoms beyond 40 years of age.[29] This is in consistence with our current report of mean age at acute neurological symptom onset (20.87 ± 7.50 years old), age ranges (9–46 years old) and percentage (3.23%) of cases presenting with acute neurological symptoms, indicating the age at acute neurological presentations caused by traumatic, surgical, or emotional events is similar to that following regular clinical course. Among these 31 patients, 21 were diagnosed as hepatic and neurological WD for detection out hepatic disease and 10 diagnosed as neurological WD. This suggested that neurological symptom presented as an initial manifestation of WD in 10 patients. And the finding of hepatomegaly, nodular liver, liver cirrhosis, or splenomegaly shortly after traumatic, surgical, or emotional events indicated that hepatic WD already existed in these 21 patients and the acute neurological symptoms presented as new manifestations. The pathological changes on brain MRI and the KF findings shortly after mild trauma, surgery, or emotional stimulation indicated that the 31 patients might be either those with asymptomatic or subclinical WD (10 neurological WD patients) or those with pathological involvement of brain but neurologically asymptomatic before traumatic, surgical, or emotional events.
The symptoms of neurological WD are very variable, and the frequency of distinct neurological symptoms varies widely in different case series.[3] But it is suggested that the most frequent 3 distinct neurological symptoms of WD are dystonic, ataxic, and parkinsonian syndromes.[3] In Machado et al's[28] report of 119 untreated WD cases, the most frequent neurological manifestations were dysarthria (91%), abnormal gait (75%), risus sardonicus (72%), dystonia (69%), rigidity (66%), tremor (60%), and dysphagia (50%), less frequent manifestations were chorea (16%) and athetosis (14%), and rare neurological presentations were seizures (4.2%) and pyramidal signs (3%). In current report, the most frequent neurological symptoms observed were tremor (74%), slurred speech (67%), and drooling (19.5%), whereas less frequent were abnormal gait (6.5%) and increased muscle tension (6.5%). Compared to Machado et al's case series, significant more frequent manifestations of tremor and drooling but less frequent of slurred speech, abnormal gait, and dystonia were observed in current case series. And there were no manifestations of risus sardonicus, rigidity, dysphagia, chorea, athetosis, and seizures in our case series but common in literature case series.[28] In addition, none of the 31 cases had acute psychiatric presentations which are common in WD following regular clinical course.[25,26] Especially, there were much fewer cases presenting with a combination of >2 types of neurological symptoms in our case series than that in literature case series.[28] This indicates that the neurological symptoms in WD of acute presentation caused by traumatic, surgical, or emotional events are less severe than that following regular clinical course.
All patients had been detected with lesions in BG on brain MRI indicating that BG is the area most vulnerable to trauma, surgery, and emotional stimulation. Earlier literature report also showed that BG is the most frequent (86%) lesion area on MRI in untreated patients with WD[30] but less frequent than that (100%) in current case series. But lesions in other areas outside of BG, such as brainstem (45.16% vs 82%), cerebellum (3.23% vs 50%), and cortical white matter (12.90% vs 59%) were less frequently detected on MRI in current case series than those in corresponding areas in untreated patients with WD from literature report.[30] This high frequency of BG involvement in pathology is consistent with their most frequent (74%) clinical presentation of tremor in current case series, as WD tremor is associated with BG lesions on MRI.[31] On the contrary, the less combination with lesions in other brain areas on MRI is in line with the less severe neurological presentations. This is further supported by current findings that the percentage of patients with single symptom was significantly higher than that of patients with brain lesions not only in BG but also in other brain areas. In 1 patient (patient 8), the MRI showed that the mild trauma caused an expansion of BG lesion from bilateral thalamus to bilateral lenticular nucleus. In another patient (not listed in Table 1) who had been diagnosed as WD for 2 years, a mild trauma caused a severe worsening of presentations from mild tremor and slurred speech to severe tremor, dysarthria, gait disturbance and body rigidity, and a correspondent expansion of brain MRI lesions from brainstem and bilateral lenticular nucleus to bilateral caudate nucleus, thalamus, and frontal lobes (Fig. 2). These may indicate that a mild trauma can cause BG lesion on MRI. This is consistent with literature reports showing that BG is most vulnerable to different insults.[32–35] Concerning treatment, most (84%) of the patients who accepted regular anti-copper treatment had well improvement in neurological symptoms and some became asymptomatic. This perfect prognosis might be associated with fewer lesions in other areas outside of BG.[36] This is further supported by current findings that patients with lesions restricted in BG area had better prognosis than patients with lesions in wider areas.
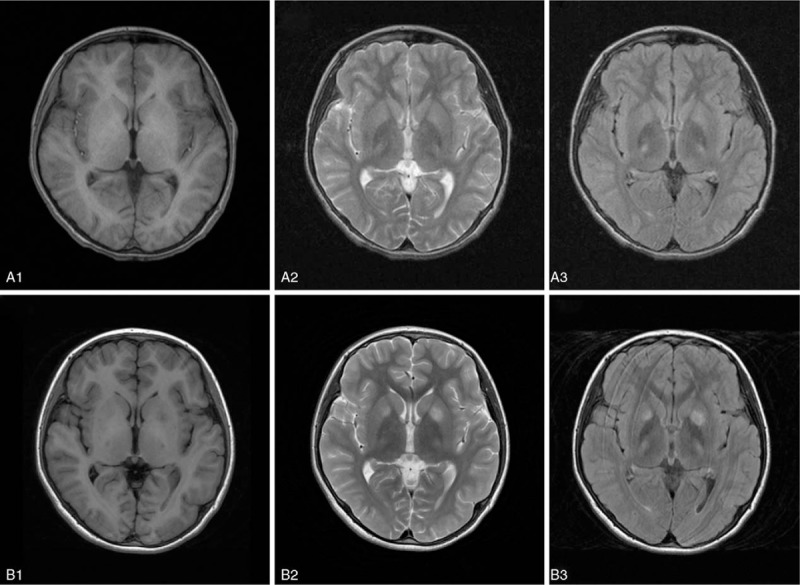
Figure 2.
Brain magnetic resonance imaging (MRI) in a patient with Wilson disease (WD) before and after a traumatic event. These images were from a 25 years old female patient who had been affected with WD with mixed hepatic and neurological presentations for 2 years. Before trauma (A1-A5), the neurological symptoms were slurred speech and drooling, and these symptoms became more severe to be both hand and head tremors and clumsy movement apart from the initial symptoms of slurred speech and drooling 1 hour after falling down with head impacted on ground while walking (B1-B5). Three days before trauma (A1-A5), high signals in bilateral Pons (A1), midbrain (A2), putamen (A3, A4), and left frontal lobe (A5) on flair-weighted MRI. One hour after trauma (B1-B5), apart from the high signal in Pons (B1), the areas of high signals in midbrain(B2), putamen (B3, B4), and left frontal lobe (B5) on flair weighted MRI became wider than those before trauma, and high signals in bilateral thalamus (B3 and B4) and caudate nucleus (B3) were also noticed after traumatic event.
The essential pathological mechanism of WD is an excessive copper deposition and the consequent copper intoxication. It is quite unclear how mild trauma caused an increased copper deposition in brain areas especially in BG area as reflected on MRI in our patients. Because of the vulnerability of BG to different insults, a copper redistribution to BG might be expected as a potential mechanism as different stress including mild trauma and emotional stimuli can cause activation of BG structure accompanied with inflammatory responses[37–40] and copper is required to alleviate BG inflammation.[41,42]
4.1. Limitations
Our study is a cross-sectional study generating a proof of concept that patients with WD with acute onset neurological symptoms due to mild trauma and emotional stimulation would have excellent prognosis if they accept regular anti-copper treatment since there is no matched control group, that is, the patients with regularly developed WD and the follow-up is relatively short. In addition, it is not clear whether the patients already had asymptomatic WD with brain lesion before traumatic and emotional events and these events facilitated the brain lesion and provoked the presentation of neurological symptoms, as we have no MRI data before the events.
5. Conclusions
A diagnosis of WD should be considered when adolescents or young adults experience acute neurological symptoms, especially the symptoms of tremor and slurred speech, after mild trauma or emotional events. Patients with WD should avoid trauma and severe mood swings. Patients with WD with acute neurological symptoms after mild trauma, surgery, or emotional events should accept timely anti-copper therapy to have excellent prognosis.
Acknowledgments
The authors thank all doctors who have contributed by providing imaging data and other clinical information of the patients.
Author contributions
Conceptualization: Liang-Yong Li, Yu Wang.
Data curation: Liang-Yong Li, Xiao-Qun Zhu, Wei-Wei Tao, Yu Wang.
Formal analysis: Xiao-Qun Zhu, Wei-Wei Tao.
Funding acquisition: Yu Wang.
Investigation: Liang-Yong Li, Wen-Ming Yang, Huai-Zhen Chen, Yu Wang.
Methodology: Yu Wang.
Project administration: Yu Wang.
Resources: Liang-Yong Li, Xiao-Qun Zhu, Wen-Ming Yang, Huai-Zhen Chen.
Supervision: Liang-Yong Li., Yu Wang.
Validation: Liang-Yong Li, Xiao-Qun Zhu, Wen-Ming Yang, Huai-Zhen Chen.
Writing – original draft: Liang-Yong Li, Xiao-Qun Zhu, Wei-Wei Tao.
Writing – review and editing: Wei-Wei Tao, Wen-Ming Yang, Yu Wang, Xiao-Qun Zhu.
Footnotes
Abbreviations: BG = basal ganglia, DMPS = 2,3-dimercapto-1-propane sulfonate, MRI = magnetic resonance imaging, WD = Wilson disease.
L-YL, X-QZ, and W-WT contributed equally to this work.
This work was supported by Natural Science grants to YW (grant number: 81671290) from the National Natural Science Foundation of China.
The authors report no conflicts of interest.
References
- [1].Harada M. Pathogenesis and management of Wilson disease. Hepatol Res 2014;44:395–402. [DOI] [PubMed] [Google Scholar]
- [2].Aggarwal A, Bhatt M. Update on Wilson disease. Int Rev Neurobiol 2013;110:313–48. [DOI] [PubMed] [Google Scholar]
- [3].Bandmann O, Weiss KH, Kaler SG. Wilson's disease and other neurological copper disorders. Lancet Neurol 2015;14:103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Das SK, Ray K. Wilson's disease: an update. Nat Clin Pract Neurol 2006;2:482–93. [DOI] [PubMed] [Google Scholar]
- [5].Gouider-Khouja N. Wilson's disease. Parkinsonism Relat Disord 2009;15suppl 3:S126–9. [DOI] [PubMed] [Google Scholar]
- [6].Roche-Sicot J, Benhamou JP. Acute intravascular hemolysis and acute liver failure associated as a first manifestation of Wilson's disease. Ann Intern Med 1977;86:301–3. [DOI] [PubMed] [Google Scholar]
- [7].Shiraishi A, Hoshina T, Ihara K, et al. Acute liver failure as the initial manifestation of Wilson disease triggered by human parvovirus b19 infection. Pediatr Infect Dis J 2012;31:103–4. [DOI] [PubMed] [Google Scholar]
- [8].Kegley KM, Sellers MA, Ferber MJ, et al. Fulminant Wilson's disease requiring liver transplantation in one monozygotic twin despite identical genetic mutation. Am J Transplant 2010;10:1325–9. [DOI] [PubMed] [Google Scholar]
- [9].Korman JD, Volenberg I, Balko J, et al. Screening for Wilson disease in acute liver failure: a comparison of currently available diagnostic tests. Hepatology 2008;48:1167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dabrowska E, Jablonska-Kaszewska I, Ozieblowski A, et al. Acute haemolytic syndrome and liver failure as the first manifestations of Wilson's disease. Med Sci Monit 2001;7 suppl 1:246–51. [PubMed] [Google Scholar]
- [11].Agrawal AK, Haddad FG, Matsunaga A. Acute nonimmune hemolytic anemia without fulminant hepatitis in Wilson disease. J Pediatr Hematol Oncol 2011;33:e163–5. [DOI] [PubMed] [Google Scholar]
- [12].Thapa R, Mukherjee K. Acute Wilson disease associated with E beta-thalassemia. J Pediatr Hematol Oncol 2008;30:925–7. [DOI] [PubMed] [Google Scholar]
- [13].Buchanan GR. Acute hemolytic anemia as a presenting manifestation of Wilson disease. J Pediatr 1975;86:245–7. [DOI] [PubMed] [Google Scholar]
- [14].El Raziky MS, Ali A, El Shahawy A, et al. Acute hemolytic anemia as an initial presentation of Wilson disease in children. J Pediatr Hematol Oncol 2014;36:173–8. [DOI] [PubMed] [Google Scholar]
- [15].Huster D, Hermann W, Bartels M. Acute Wilson disease [in German]. Internist (Berl) 2011;52:815–22. [DOI] [PubMed] [Google Scholar]
- [16].Verma R, Bhandari A, Tiwari N, et al. Acute onset anarthria without hepatic manifestation: a rare presentation of Wilson disease. BMJ Case Rep 2013;2013:pii: bcr2013010415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Verma R, Patil TB, Lalla RS. Acute extrapyramidal syndrome and seizures as heralding manifestation of Wilson disease. Neurol India 2012;60:363–4. [DOI] [PubMed] [Google Scholar]
- [18].Kawamura N, Ohyagi Y, Kawajiri M, et al. Serial diffusion-weighted MRI in a case of Wilson's disease with acute onset hemichorea. J Neurol 2004;251:1413–4. [DOI] [PubMed] [Google Scholar]
- [19].Li LY, Yang WM, Chen HZ, et al. Successful splenectomy for hypersplenism in Wilson's disease: a single center experience from China. PLoS One 2015;10:e0124569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rosencrantz R, Schilsky M. Wilson disease: pathogenesis and clinical considerations in diagnosis and treatment. Semin Liver Dis 2011;31:245–59. [DOI] [PubMed] [Google Scholar]
- [21].Ferenci P. Review article: diagnosis and current therapy of Wilson's disease. Aliment Pharmacol Ther 2004;19:157–65. [DOI] [PubMed] [Google Scholar]
- [22].Ferenci P, Caca K, Loudianos G, et al. Diagnosis and phenotypic classification of Wilson disease. Liver Int 2003;23:139–42. [DOI] [PubMed] [Google Scholar]
- [23].Litwin T, Gromadzka G, Czlonkowska A. Gender differences in Wilson's disease. J Neurol Sci 2012;312:31–5. [DOI] [PubMed] [Google Scholar]
- [24].Litwin T, Gromadzka G, Czlonkowska A, et al. The effect of gender on brain MRI pathology in Wilson's disease. Metab Brain Dis 2013;28:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Akil M, Brewer GJ. Psychiatric and behavioral abnormalities in Wilson's disease. Adv Neurol 1995;65:171–8. [PubMed] [Google Scholar]
- [26].Akil M, Schwartz JA, Dutchak D, et al. The psychiatric presentations of Wilson's disease. J Neuropsychiatry Clin Neurosci 1991;3:377–82. [DOI] [PubMed] [Google Scholar]
- [27].Lin L, Wang D, Ding N, et al. Hepatic manifestations in Wilson's disease: report of 110 cases. Hepatogastroenterology 2015;62:657–60. [PubMed] [Google Scholar]
- [28].Machado A, Chien HF, Deguti MM, et al. Neurological manifestations in Wilson's disease: report of 119 cases. Mov Disord 2006;21:2192–6. [DOI] [PubMed] [Google Scholar]
- [29].Ferenci P, Czlonkowska A, Merle U, et al. Late-onset Wilson's disease. Gastroenterology 2007;132:1294–8. [DOI] [PubMed] [Google Scholar]
- [30].King AD, Walshe JM, Kendall BE, et al. Cranial MR imaging in Wilson's disease. AJR Am J Roentgenol 1996;167:1579–84. [DOI] [PubMed] [Google Scholar]
- [31].Sudmeyer M, Saleh A, Wojtecki L, et al. Wilson's disease tremor is associated with magnetic resonance imaging lesions in basal ganglia structures. Mov Disord 2006;21:2134–9. [DOI] [PubMed] [Google Scholar]
- [32].Jensen N, Oliveira JR. Basal ganglia vulnerability to oxidative stress. Front Neurosci 2014;8:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nishino H, Hida H, Kumazaki M, et al. The striatum is the most vulnerable region in the brain to mitochondrial energy compromise: a hypothesis to explain its specific vulnerability. J Neurotrauma 2000;17:251–60. [DOI] [PubMed] [Google Scholar]
- [34].Newsome MR, Durgerian S, Mourany L, et al. Disruption of caudate working memory activation in chronic blast-related traumatic brain injury. Neuroimage Clin 2015;8:543–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yang FH, Wang H, Zhang JM, et al. Cerebral infarction after mild head trauma in children. Indian Pediatr 2013;50:875–8. [DOI] [PubMed] [Google Scholar]
- [36].Litwin T, Dziezyc K, Karlinski M, et al. Early neurological worsening in patients with Wilson's disease. J Neurol Sci 2015;355:162–7. [DOI] [PubMed] [Google Scholar]
- [37].Almeida I, Van Asselen M, Castelo-Branco M. The role of the amygdala and the basal ganglia in visual processing of central vs. peripheral emotional content. Neuropsychologia 2013;51:2120–9. [DOI] [PubMed] [Google Scholar]
- [38].Pohlack ST, Nees F, Ruttorf M, et al. Activation of the ventral striatum during aversive contextual conditioning in humans. Biol Psychol 2012;91:74–80. [DOI] [PubMed] [Google Scholar]
- [39].Lobo MK, Zaman S, Damez-Werno DM, et al. DeltaFosB induction in striatal medium spiny neuron subtypes in response to chronic pharmacological, emotional, and optogenetic stimuli. J Neurosci 2013;33:18381–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kishioka A, Uemura T, Fukushima F, et al. Consolidation of auditory fear memories formed by weak unconditioned stimuli requires NMDA receptor activation and de novo protein synthesis in the striatum. Mol Brain 2013;6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zucconi GG, Cipriani S, Scattoni R, et al. Copper deficiency elicits glial and neuronal response typical of neurodegenerative disorders. Neuropathol Appl Neurobiol 2007;33:212–25. [DOI] [PubMed] [Google Scholar]
- [42].Choo XY, Alukaidey L, White AR, et al. Neuroinflammation and copper in Alzheimer's disease. Int J Alzheimers Dis 2013;2013:145345. [DOI] [PMC free article] [PubMed] [Google Scholar]


