Abstract
Introduction
Alzheimer's disease (AD) has few available treatments, and there is a high rate of failure in AD drug development programs. Study of the AD drug development pipeline can provide insight into the evolution of drug development and how best to optimize development practices.
Methods
We reviewed clinicaltrials.gov and identified all pharmacologic AD trials of all agents currently being developed for treatment of AD.
Results
There are 132 agents in clinical trials for the treatment of AD. Twenty-eight agents are in 42 phase 3 trials; 74 agents are in 83 phase 2 trials; and 30 agents are in 31 phase 1 trials. There is an increase in the number of agents in each phase compared with that in the 2018 pipeline. Nineteen agents in trials target cognitive enhancement, and 14 are intended to treat neuropsychiatric and behavioral symptoms. There are 96 agents in disease modification trials; of these, 38 (40%) have amyloid as the primary target or as one of several effects. Eighteen of the antiamyloid agents are small molecules, and 20 are monoclonal antibodies or biological therapies. Seven small molecules and ten biologics have tau as a primary or combination target (18%). Amyloid is the most common specific target in phase 3 and phase 2 disease modification trials. Novel biomarkers (e.g., neurofilament light), new outcomes (e.g., AD Composite Score [ADCOMS]), enrollment of earlier populations, and innovative trial designs (e.g., Bayesian adaptive designs) are new features in recent clinical trials.
Discussion
Drug development continues robustly at all phases despite setbacks in several programs in the recent past. Continuing unmet needs require a commitment to growing and accelerating the pipeline.
Keywords: Alzheimer's disease, Drug development, Clinical trials, Biomarkers, Bayesian design, Adaptive design, Repurposed drugs
1. Introduction
Drug discovery and development for Alzheimer's disease (AD) is arduous. There have been no new drugs approved since 2003, and there are no approved disease-modifying treatments (DMTs) for AD. The challenges of drug development have become more complex as potential trial populations have expanded to include preclinical and prodromal AD, as well as AD dementia [1], [2], [3]. The US Food and Drug Administration (FDA) has provided guidance for clinical trials in AD dementia and predementia AD including use of a single primary outcome in trials of prodromal AD, the role of biomarkers in staging preclinical and prodromal AD, and the use of Bayesian statistics and adaptive clinical trial designs [4], [5], [6]. A new research framework for the diagnosis of AD based on amyloid, tau, and neurodegeneration (ATN) biomarkers was introduced by the National Institute on Aging (NIA) and the Alzheimer's Association [7]. This framework allows more precise classification of stages of AD, especially predementia stages, and may facilitate clinical trials of DMTs in AD [8]. Progress in biomarkers relevant to clinical trials of AD include increased understanding of the role of tau positron emission tomography (PET) in characterizing and staging AD and development of new fluid biomarkers such as neurofilament light and neurogranin that are increasingly integrated into clinical trials [9], [10]. These advances comprise the foundations for progress in drug development and demonstrate collaboration among key stakeholders including basic and translational neuroscientists, clinician-scientists, pharmacy benefit managers, regulators, the National Institutes of Health (NIH), advocacy groups, and participants and family members.
In our annual update on the state of the AD drug development pipeline, we build on prior contributions to discuss the current phase 1, phase 2, and phase 3 clinical trials in AD [11], [12], [13]. We describe clinical trials and experimental treatments for disease modification, cognitive enhancement, and neuropsychiatric symptoms of AD. We note changes from 2018 and discuss specific areas of interest including repurposed agents, immunotherapies, novel mechanisms, the use of biomarkers in drug development, and new trends in AD clinical trials. Our goal is to continuously learn from the drug development process, identify best practices, and provide an update and overview of the current state.
2. Methods
Clinicaltrials.gov provides the source of information for this review. There are other clinical trial registries, and our review does not represent an exhaustive listing of every clinical trial in AD. However, the “Common Rule” governing clinicaltrials.gov mandates registration of all trials from sponsors with an investigational new drug or investigational new device [14], [15] being assessed in the US. Compliance with the required trial registration is high [16], [17], [18]. The US has more clinical trials than any other nation, and thus clinicaltrials.gov includes most agents currently in clinical trials for AD.
We assayed clinicaltrials.gov as of February 12, 2019, and the tables and discussion provided apply to the information available at that time. We comment on terminated trials if the information has become publicly available but is not yet reflected on clinicaltrials.gov. We include all trials of all agents in phase 1, 2, and 3; if trials are presented as 1/2 or 2/3 in the clinicaltrials.gov database, we use that nomenclature in the review. Our trial database tracks trial title; trial number in clinicaltrials.gov; beginning date; projected end date; calculated trial duration; duration of treatment exposure; number of subjects planned for enrollment; number of arms of the study (usually a placebo arm and one or more treatment arms with different doses); whether a biomarker was described; subject characteristics; and sponsorship (a biopharmaceutical company, NIH, academic medical center, “other” entity such as a consortium or a philanthropic organization or a combination of these sponsors). We used the clinicaltrials.gov labeling and included trials that were recruiting, active but not recruiting (e.g., trials that have completed recruiting and are continuing with the exposure portion of the trial), enrolling by invitation, and not yet recruiting. We did not include trials listed as completed, terminated, suspended, unknown, or withdrawn. Information on these trials and reasons for their current status may not be publicly revealed. We do not include trials of nonpharmacologic therapeutic approaches such as cognitive therapies and caregiver interventions; we do not include studies of supplements and medical foods. We provide a table and brief discussion of new device trials (not included in Fig. 1). We do not include trials of biomarkers, although we note whether biomarkers were used in the trials reviewed. We include stem cell therapies among the interventions reviewed (not integrated into Fig. 1).
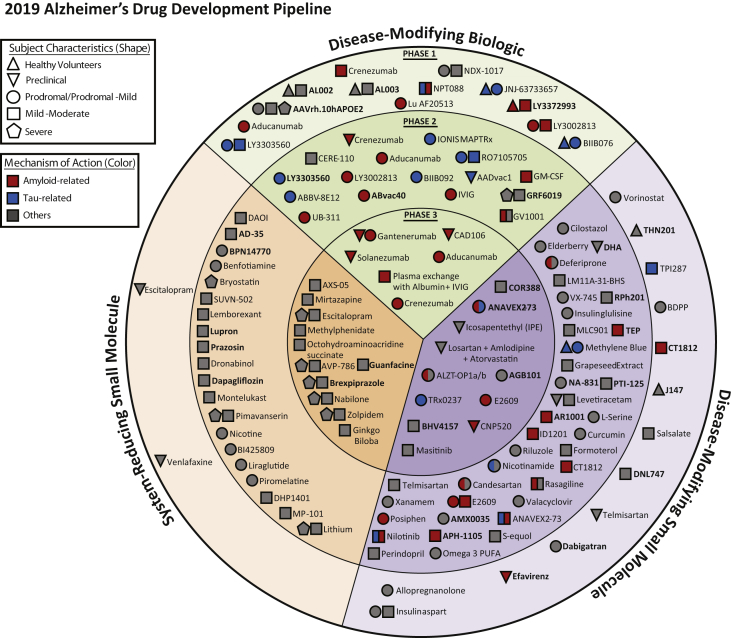
Fig. 1.
All compounds in AD clinical trials as of February 12, 2019 (the inner ring shows phase 3 agents; the middle ring is comprised of phase 2 agents; the outer ring presents phase 1 compounds; agents in green areas are biologics; agents in purple areas are disease-modifying small molecules; agents in orange areas are symptomatic agents addressing cognitive enhancement or behavioral and neuropsychiatric symptoms; the shape of the icon shows the population of the trial; the icon color shows the class of target for the agent.). Bolded names represent agents new to that phase since 2018.
Drug targets and mechanisms of action (MOA) are important aspects of this review. MOA was determined from the information on clinicaltrials.gov or from a comprehensive search of the literature. In a few cases, the mechanism is undisclosed and could not be identified in the literature; we note these agents as having an “unknown” or “undisclosed” MOA. We grouped the mechanisms into symptomatic agents or DMTs. We divided the symptomatic agents into those that are putative cognitive enhancing agents or those that address neuropsychiatric and behavioral symptoms. DMTs were divided into small molecules or biologics including immunotherapies. DMTs were further divided into those targeting amyloid-related mechanisms, those that have tau-related MOAs, and those with “other” mechanisms such as neuroprotection, anti-inflammatory effects, growth factor promotion, or metabolic effects. The distinction between symptomatic and disease-modifying agents can be arbitrary, and some agents may have both properties. For purposes of this review, we chose what appears to be the principal MOA.
3. Results
3.1. Overview
As of February 12, 2019, there were 132 agents in 156 trials of anti-AD therapies. Fig. 1 shows the universe of pharmacologic compounds currently in clinical trials for AD. Nineteen (14%) agents in trials target cognitive enhancement, and 14 (11%) are intended to treat neuropsychiatric and behavioral symptoms. There are 96 (73%) agents that intend to achieve disease modification; 38 (40%) of these have amyloid; and 17 (18%) have tau as the primary target or as one of several effects seen in nonclinical studies. Eighteen of the antiamyloid agents are small molecules, and 20 are monoclonal antibodies or biological therapies. Anti-tau agents include seven small molecules and ten biologics.
All compounds in AD clinical trials as of February 12, 2019 (the inner ring shows phase 3 agents; the middle ring is comprised of phase 2 agents; the outer ring presents phase 1 compounds; agents in green areas are biologics; agents in purple areas are disease-modifying small molecules; agents in orange areas are symptomatic agents addressing cognitive enhancement or behavioral and neuropsychiatric symptoms; the shape of the icon shows the population of the trial; the icon color shows the class of target for the agent.). Bolded names represent agents new to that phase since 2018.
3.2. Phase 3
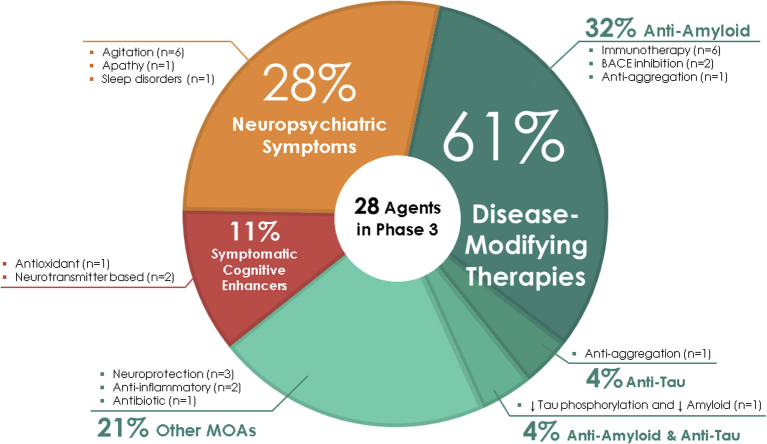
In phase 3, there are 28 agents in 42 trials (Figs. 1 and 2, Table 1). There are 11 symptomatic agents in phase 3; three cognitive enhancers and eight targeting behavioral symptoms. There are six biological therapies and 11 oral agents/small molecules in phase 3 that target disease modification. All the biological therapies and four of the small molecules have amyloid as the primary or one of several targets. There is one anti-tau agent in phase 3: LMTX (TRx0237). A phase 3 trial of this agent failed to show a drug-placebo difference [19], and based on the results, a new phase 2/3 trial (LUCIDITY) was started in 2018 with a lower dose of LMTX as monotherapy. Other mechanisms represented among phase 3 DMT molecules include neuroprotection, anti-inflammatory approaches, and metabolic interventions. Of the DMTs, two are repurposed agents approved for use in another indication (losartan plus amlodipine plus atorvastatin; and levetiracetam). Of the drugs with amyloid targets, there were six biologics, two beta-site amyloid precursor protein cleavage enzyme (BACE) inhibitors, and one anti-aggregation agent. Fig. 2 shows the MOAs of agents in phase 3.
Fig. 2.
Mechanisms of action of agents in phase 3.
Table 1.
Agents currently in phase 3 of Alzheimer's disease drug development (as of February 12, 2019)
| Agent | Agent mechanism class | Mechanism of action | Therapeutic purpose | ClinicalTrials.gov ID | Status | Sponsor | Start date | Estimated end date |
|---|---|---|---|---|---|---|---|---|
| Aducanumab∗ | Antiamyloid | Monoclonal antibody directed at plaque and oligomers | Remove amyloid (DMT) | NCT02484547 | Active, not recruiting | Biogen | Sep 2015 | Apr 2022 |
| NCT02477800 | Active, not recruiting | Biogen | Aug 2015 | Apr 2022 | ||||
| AGB101 (low-dose levetiracetam) | Neuroprotective | SV2A modulator | Decrease amyloid-induced neuronal hyperactivity (DMT) | NCT03486938 | Recruiting | AgeneBio, NIA | Jan 2019 | Nov 2022 |
| Plasma exchange with albumin + immunoglobulin∗ | Antiamyloid | Plasma exchange | Remove amyloid (DMT) | NCT01561053† | Active, not recruiting | Grifols | Mar 2012 | Dec 2017 |
| ALZT-OP1a + ALZT-OP1b (cromolyn + ibuprofen) | Antiamyloid, anti-inflammatory | Mast cell stabilizer (cromolyn), anti-inflammatory (ibuprofen) | Reduce neuronal damage; mast cells may also play a role in amyloid pathology (DMT) | NCT02547818 | Recruiting | AZTherapies, Pharma Consulting Group, KCAS Bio, APCER Life Sciences | Sep 2015 | Nov 2019 |
| ANAVEX2-73 | Anti-tau, antiamyloid, anti-inflammatory | Sigma-1 receptor agonist (high affinity), muscarinic agonist (low affinity), GSK-3β inhibitor | Improve cell signaling (cognitive enhancer) and reduce tau phosphorylation and amyloid (DMT) | NCT03790709† | Recruiting | Anavex Life Sciences | Jul 2018 | Mar 2021 |
| AVP-786 | Neurotransmitter based | Sigma-1 receptor agonist; NMDA receptor antagonist | Improve neuropsychiatric symptoms (agitation) | NCT02442765 | Active, not recruiting | Avanir | Sep 2015 | Apr 2019 |
| NCT02442778 | Recruiting | Avanir | Sep 2015 | Dec 2019 | ||||
| NCT02446132 | Recruiting, extension | Avanir | Dec 2015 | Jun 2022 | ||||
| NCT03393520 | Recruiting | Avanir | Oct 2017 | Jun 2021 | ||||
| AXS-05 | Neurotransmitter based | Sigma-1 receptor agonist; NMDA receptor antagonist (dextromethorphan); dopamine-norepinephrine reuptake inhibitor (bupropion) | Improve neuropsychiatric symptoms (agitation) | NCT03226522† | Recruiting | Axsome Therapeutics | Jul 2017 | Sep 2019 |
| BHV4157 (troriluzole) | Neuroprotective | Glutamate modulator | Reduce synaptic levels of glutamate (DMT) | NCT03605667† | Recruiting | Biohaven Pharma, ADCS | Jul 2018 | Feb 2020 |
| Brexpiprazole | Neurotransmitter based | Atypical antipsychotic; D2 receptor partial agonist and serotonin-dopamine modulator | Improve neuropsychiatric symptoms (agitation) | NCT03620981† | Recruiting | Otsuka | Aug 2018 | Nov 2021 |
| NCT03594123 | Recruiting, extension | Otsuka | Oct 2018 | Aug 2021 | ||||
| NCT03548584 | Recruiting | Otsuka | May 2018 | Dec 2020 | ||||
| NCT03724942 | Recruiting, extension | Otsuka | Nov 2018 | May 2021 | ||||
| CAD106 & CNP520 | Antiamyloid | Amyloid vaccine (CAD106), BACE inhibitor (CNP520) | Remove amyloid (vaccine); prevent amyloid production (BACE inhibitor) (DMT) | NCT02565511† | Recruiting | Novartis, Amgen, NIA, Alzheimer's Association, Banner Alzheimer's Institute |
Feb 2016 | Jan 2025 |
| CNP520 | Antiamyloid | BACE inhibitor | Prevent amyloid production (DMT) | NCT03131453† | Recruiting | Novartis, Amgen, Banner Alzheimer's Institute | Aug 2017 | Mar 2025 |
| COR388 | Anti-inflammatory | Bacterial protease inhibitor targeting a periodontal pathogen | Reduce neuroinflammation and hippocampal degeneration (DMT) | NCT03823404† | Not yet recruiting | Cortexyme | Apr 2019 | Dec 2022 |
| Crenezumab∗ | Antiamyloid | Monoclonal antibody directed at oligomers | Remove amyloid (DMT) | NCT02670083 | Active, not recruiting | Roche | Mar 2016 | Jul 2021 |
| NCT03114657 | Recruiting | Roche | Mar 2017 | Oct 2022 | ||||
| NCT03491150 | Recruiting, extension | Roche | Apr 2018 | Nov 2022 | ||||
| E2609 (elenbecestat) | Antiamyloid | BACE inhibitor | Reduce amyloid production (DMT) | NCT02956486 | Recruiting | Eisai, Biogen | Oct 2016 | Jun 2021 |
| NCT03036280 | Recruiting | Eisai, Biogen | Dec 2016 | Jun 2021 | ||||
| Escitalopram | Neurotransmitter based | Serotonin reuptake inhibition | Improve neuropsychiatric symptoms (agitation) | NCT03108846 | Recruiting | NIA, JHSPH Center for Clinical Trials | Jan 2018 | Aug 2022 |
| Gantenerumab | Antiamyloid | Monoclonal antibody | Remove amyloid (DMT) | NCT02051608 | Active, not recruiting | Roche | Mar 2014 | Nov 2020 |
| NCT01224106 | Active, not recruiting | Roche | Nov 2010 | Aug 2020 | ||||
| NCT03444870 | Recruiting | Roche | Jun 2018 | May 2023 | ||||
| NCT03443973 | Recruiting | Roche | Jun 2018 | May 2023 | ||||
| Gantenerumab & Solanezumab | Antiamyloid | Monoclonal antibody directed at plaque and oligomers (gantenerumab); Monoclonal antibody directed at monomers (solanezumab) | Remove amyloid/reduce amyloid production (DMT) | NCT01760005† | Active, not recruiting | Washington University, Eli Lilly, Roche, NIA, Alzheimer's Association | Dec 2012 | Dec 2023 |
| Ginkgo Biloba | Metabolic | Plant extract with antioxidant properties | Improve brain blood flow and mitochondrial function (cognitive enhancer) | NCT03090516† | Recruiting | Nanjing Medical University | Aug 2016 | Mar 2018 |
| Guanfacine | Neurotransmitter based | Alpha-2 adrenergic agonist | Modulation of noradrenergic deficit (cognitive enhancer) | NCT03116126 | Not yet recruiting | Imperial College London, UK National Institute of Health Research | Sep 2018 | Sep 2019 |
| Icosapent ethyl (IPE) | Neuroprotective | Purified form of the omega-3 fatty acid EPA | Protect neurons from disease pathology (DMT) | NCT02719327† | Recruiting | VA Office of Research and Development, University of Wisconsin, Madison | Jun 2017 | Nov 2021 |
| Losartan & Amlodipine & Atorvastatin + exercise | Anti-inflammatory, metabolic | Angiotensin II receptor blocker (losartan), calcium channel blocker (amlodipine), cholesterol agent (atorvastatin) | Intensive vascular risk reduction can preserve cognitive function (DMT) | NCT02913664† | Recruiting | University of Texas Southwestern | Sep 2016 | Sep 2022 |
| Masitinib | Anti-inflammatory | Selective tyrosine kinase inhibitor | Activity on mast cells, modulation of inflammatory processes (DMT) | NCT01872598 | Active, not recruiting | AB Science | Jan 2012 | Oct 2019 |
| Methylphenidate | Neurotransmitter based | Dopamine reuptake inhibitor | Improve neuropsychiatric symptoms (apathy) | NCT02346201 | Recruiting | Johns Hopkins, NIA | Jan 2016 | Aug 2020 |
| Mirtazapine | Neurotransmitter based | Alpha-1 antagonist | Improve neuropsychiatric symptoms (agitation) | NCT03031184 | Recruiting | University of Sussex | Jan 2017 | Jul 2020 |
| Nabilone∗ | Neurotransmitter based | Cannabinoid (receptor agent) | Improve neuropsychiatric symptoms (agitation) | NCT02351882† | Active, not recruiting | Sunnybrook Health Sciences Center | Jan 2015 | Mar 2019 |
| Octohydroaminoacridine Succinate | Neurotransmitter based | Acetylcholinesterase inhibitor | Improve acetylcholine signaling (cognitive enhancer) | NCT03283059 | Recruiting | Shanghai Mental Health Center, Changchun-Huayang High-tech Co., Jiangsu Sheneryang High-tech Co. | Aug 2017 | Feb 2020 |
| Solanezumab | Antiamyloid | Monoclonal antibody directed at monomers | Remove amyloid and prevent aggregation (DMT) | NCT02008357 | Active, not recruiting | Eli Lilly, ATRI | Feb 2014 | Jul 2022 |
| TRx0237 (LMTX) | Anti-tau | Tau protein aggregation inhibitor | Reduce tau-mediated neuronal damage (DMT) | NCT03446001† | Recruiting | TauRx Therapeutics | Jan 2018 | Jun 2020 |
| Zolpidem | Neurotransmitter based | Positive allosteric modulator of GABA-A receptors | Improve neuropsychiatric symptoms (sleep disorders) | NCT03075241 | Recruiting | Brasilia University Hospital | Oct 2016 | Dec 2018 |
Abbreviations: ATRI, Alzheimer's Therapeutic Research Institute; BACE, beta-site amyloid precursor protein cleaving enzyme; DMT, disease-modifying therapy; EPA, eicosapentaenoic acid; GABA, gamma-aminobutyric acid; GSK, glycogen synthase kinase; NIA, National Institute on Aging; SV2A, synaptic vesicle protein 2A.
NOTE. Twenty-eight agents in 42 phase 3 clinical trials currently ongoing as of February 12, 2019 according to clinicaltrials.gov.
Bolded terms represent new agents into the 2019 phase 3 pipeline.
Reported as terminated or completed after the data collection date of February 12, 2019.
Phase 2/3 trials.
There were six prevention trials enrolling cognitively normal participants; 14 trials in patients with prodromal AD/mild cognitive impairment (MCI) or prodromal-to-mild AD; 12 trials of patients with mild-to-moderate AD; and 10 trials of patients with mild-to-severe AD.
Phase 3 trials included an average of 640 participants and had a mean duration of 246 weeks (including the recruitment and the treatment period). Mean treatment exposure period was 73 weeks. DMT trials were longer and larger than trials of symptomatic agents with a mean duration of 297 weeks including 112 treatment weeks, and included an average of 862 participants. The mean duration of cognitive enhancer trials was 88 weeks (17 treatment weeks), and they included an average of 333 participants. Trials of agents for behavioral symptoms had a mean duration of 187 weeks (15 treatment weeks) and included a mean of 311 subjects.
The average duration of treatment exposure for phase 3 DMTs is 112 weeks, and the mean period from trial initiation to primary completion date (final data collection date for primary outcome measures) is 269 weeks. This indicates that 157 weeks, more than the treatment period, is the average anticipated recruitment time. When examined by trial population, DMT prevention trials are 405 weeks in duration (192 treatment weeks); trials for patients with MCI/prodromal/prodromal-to-mild AD are 263 weeks in duration (98 treatment weeks); and trials for patients with mild-to-moderate AD are 264 weeks in duration (57 treatment weeks). Planned recruitment periods for these three types of trials are 192, 130, and 191 weeks, respectively.
3.3. Phase 2
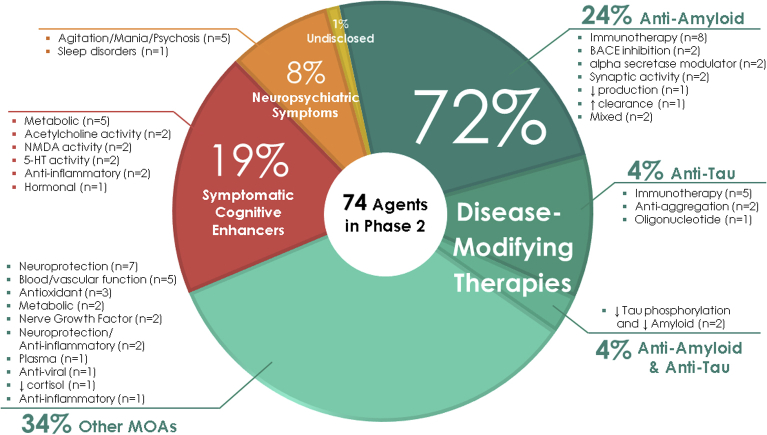
Phase 2 has a larger array of therapies and mechanisms that are being assessed than are represented in phase 3. There are 74 agents in 83 trials (Figs. 1 and 3, Table 2). Of these, there are 20 symptomatic agents; 14 cognitive enhancers; and six agents targeting behavioral symptoms. There are 53 potential disease-modifying agents in phase 2 trials; 16 biologics and 37 small molecules. One agent had an undisclosed mechanism. Twelve of the small molecules and eight of the biologics have amyloid reduction as one of the mechanisms observed in nonclinical studies (38% of DMTs). Four small molecules and six biologics in phase 2 target tau as one of their mechanisms (19% of DMTs). There are 24 small molecules and two biologics with neuroprotection as one of the mechanisms (49% of DMTs). Other mechanisms represented in phase 2 include anti-inflammatory and metabolic interventions as the primary or one of a combination of effects documented in animal models. There are six trials involving stem cell therapies. Sixteen of the DMT agents are repurposed agents approved for use in another indication.
Fig. 3.
Mechanisms of action of agents in phase 2.
Table 2.
Agents currently in phase 2 of Alzheimer's disease drug development (as of February 12, 2019)
| Agent | Agent mechanism class | Mechanism of action | Therapeutic purpose | ClinicalTrials.gov ID | Status | Sponsor | Start date | Estimated end date |
|---|---|---|---|---|---|---|---|---|
| AADvac1 | Anti-tau | Active immunotherapy | Remove tau and prevent tau propagation (DMT) | NCT02579252 | Active, not recruiting | Axon Neuroscience | Mar 2016 | Jun 2019 |
| ABBV-8E12 | Anti-tau | Monoclonal antibody | Remove tau and prevent tau propagation (DMT) | NCT02880956 | Recruiting | AbbVie | Oct 2016 | Sep 2022 |
| NCT03712787 | Not yet recruiting, extension | AbbVie | Nov 2018 | Aug 2027 | ||||
| ABvac40 | Antiamyloid | Active immunotherapy | Remove amyloid (DMT) | NCT03461276 | Recruiting | Araclon Biotech | Feb 2018 | Feb 2021 |
| AD-35 | Neurotransmitter based | Acetylcholinesterase inhibitor | Improve acetylcholine signaling (cognitive enhancer) | NCT03625401 | Recruiting | Zhejiang Hisun Pharmaceutical, Medpace, Inc. | Oct 2018 | Jul 2020 |
| NCT03790982 | Active, not recruiting | Zhejiang Hisun Pharmaceutical | Dec 2018 | Jul 2021 | ||||
| Aducanumab∗ | Antiamyloid | Monoclonal antibody directed at plaque and oligomers | Remove amyloid (DMT) | NCT03639987 | Recruiting | Biogen | Dec 2018 | Nov 2023 |
| AMX0035 | Neuroprotective | Blocks mitochondrial and endoplasmic reticulum stress | Blocks nerve cell death and neuroinflammation (DMT) | NCT03533257 | Recruiting | Amylyx Pharmaceuticals, ADDF, Alzheimer's Association | Aug 2018 | Sep 2020 |
| ANAVEX 2-73 | Anti-tau, antiamyloid, anti-inflammatory | Sigma-1 receptor agonist (high affinity); muscarinic agonist (low affinity); GSK-3β inhibitor | Improve cell signaling (cognitive enhancer) and reduce tau phosphorylation and amyloid (DMT) | NCT02756858 | Active, not recruiting, extension | Anavex Life Sciences | Mar 2016 | Nov 2020 |
| APH-1105 | Antiamyloid | Alpha-secretase modulator | Reduce amyloid (DMT) | NCT03806478 | Not yet recruiting | Aphios | Jun 2021 | Dec 2022 |
| AR1001 | Antiamyloid | PDE 5 inhibitor | Improve synaptic plasticity and reduce amyloid (DMT) | NCT03625622 | Recruiting | AriBio Co. | Jan 2019 | Aug 2020 |
| AstroStem | Regenerative | Stem cell therapy; autologous adipose tissue derived mesenchymal stem cells | Regenerate neurons (DMT) | NCT03117738† | Recruiting | Nature Cell Co. | Apr 2017 | Jul 2019 |
| BAC | Undisclosed | Undisclosed | Undisclosed | NCT02886494 | Recruiting | Charsire Biotechnology | Dec 2016 | Nov 2019 |
| NCT02467413 | Not yet recruiting | Charsire Biotechnology, A2 Healthcare Taiwan Corporation |
Dec 2019 | Dec 2021 | ||||
| Benfotiamine | Metabolic | Synthetic thiamine (B1) | Improve multiple cellular processes (cognitive enhancer) | NCT02292238 | Recruiting | Burke Medical Research Institute, Columbia University, NIA, ADDF | Nov 2014 | Nov 2019 |
| BI425809 | Neurotransmitter based | Glycine transporter 1 inhibitor | Facilitate NMDA receptor activity (cognitive enhancer) | NCT02788513 | Recruiting | Boehringer Ingelheim | Aug 2016 | Mar 2020 |
| BIIB092 | Anti-tau | Monoclonal antibody | Remove tau and reduce tau propagation (DMT) | NCT03352557 | Recruiting | Biogen | May 2018 | Jul 2021 |
| BPN14770 | Anti-inflammatory | PDE4D inhibitor | Prolongs cAMP activity (cognitive enhancer) | NCT03817684 | Not yet recruiting | Tetra Discovery Partners | Apr 2019 | Jun 2020 |
| Byrostatin | Metabolic | Protein kinase C modulator | Improve multiple cellular processes (cognitive enhancer) | NCT03560245 | Recruiting | Neurotrope Bioscience | Jun 2018 | Jul 2019 |
| Candesartan | Neuroprotective, metabolic, antiamyloid | Angiotensin receptor blocker | Improve vascular functioning and reduce amyloid (DMT) | NCT02646982 | Recruiting | Emory University | Jun 2016 | Sep 2021 |
| CERE-110∗ | Neuroprotective | Adeno-associated virus-based gene delivery vector of nerve growth factor | Cholinergic neuronal hypertrophy; slows age-related neurodegeneration (DMT) | NCT00876863 | Active, not recruiting | Sangamo Therapeutics, ADCS | Sep 2009 | Mar 2020 |
| Cilostazol | Neuroprotective | PDE-3 inhibitor | Reduce accumulation of amyloid and reduce tau phosphorylation; improve cerebral circulation (DMT) | NCT02491268 | Recruiting | National Cerebral and Cardiovascular Center, Japan | Jul 2015 | Dec 2020 |
| Crenezumab∗ | Antiamyloid | Monoclonal antibody targeting soluble oligomers | Remove amyloid (DMT) | NCT01998841 | Active, not recruiting | Genentech, NIA Banner Alzheimer's Institute |
Dec 2013 | Feb 2022 |
| CT1812 | Antiamyloid | Sigma-2 receptor antagonist | Reduce amyloid-beta protein-induced synaptic toxicity (DMT) | NCT03507790 | Recruiting | Cognition Therapeutics | Oct 2018 | Dec 2019 |
| NCT03493282† | Recruiting | Cognition Therapeutics | Apr 2018 | Jan 2020 | ||||
| Curcumin + aerobic yoga | Neuroprotective | Herb with antioxidant and anti-inflammatory properties | Reduce amyloid production, decrease neuroglial cell proliferation (DMT) | NCT01811381 | Recruiting | VA Office of Research and Development | Jan 2014 | Dec 2019 |
| DAOI | Neurotransmitter based | NMDA receptor modulation | Enhance NMDA activity (cognitive enhancer) | NCT03752463 | Recruiting | Chang Gung Memorial Hospital, Taiwan | May 2015 | Dec 2019 |
| Dapagliflozin | Metabolic | SGLT2 inhibitor | Improve insulin sensitivity (cognitive enhancer) | NCT03801642† | Recruiting | University of Kansas | Feb 2019 | Oct 2020 |
| Deferiprone | Antiamyloid, neuroprotective | Iron chelating agent | Reduce reactive oxygen species that damage neurons; effect on amyloid and BACE pathology (DMT) | NCT03234686 | Recruiting | Neuroscience Trials Australia | Jan 2018 | Dec 2021 |
| DHA | Neuroprotective | Omega-3 fatty acid in high concentration in the brain | Reduce amyloid production, improve synaptic function (DMT) | NCT03613844 | Recruiting | University of Southern California | Jul 2018 | Sep 2024 |
| DHP1401 | Metabolic | Affects cAMP activity | Improve synaptic function (cognitive enhancer) | NCT03055741 | Active, not recruiting | Daehwa Pharmaceutical Co. | Dec 2016 | Jun 2019 |
| Dronabinol | Neurotransmitter based | CB1 and CB2 endocannabinoid receptor partial agonist | Improve neuropsychiatric symptoms (agitation) | NCT02792257 | Recruiting | Mclean Hospital, Johns Hopkins University | Mar 2017 | Dec 2020 |
| E2609 (elenbecestat) | Antiamyloid | BACE inhibitor | Reduce amyloid production (DMT) | NCT02322021 | Active, not recruiting | Eisai, Biogen | Nov 2014 | Jun 2020 |
| Elderberry Juice | Anti-inflammatory, neuroprotective | Antioxidant rich in anthocyanins | Improve mitochondrial function (DMT) | NCT02414607† | Recruiting | University of Missouri | Sep 2016 | Apr 2019 |
| Formoterol | Metabolic | Beta-2 adrenergic receptor agonist | Effects on multiple cellular pathways (DMT) | NCT02500784 | Recruiting | Palo Alto Veterans Institute for Research, Mylan, Alzheimer's Association |
Jan 2015 | Jul 2018 |
| Grapeseed Extract | Neuroprotective | Polyphenolic compounds; antioxidant | Anti-oligomerization agent; prevents aggregation of amyloid and tau (DMT) | NCT02033941 | Recruiting | Mount Sinai School of Medicine, NCCIH | Nov 2014 | Sep 2018 |
| GRF6019 | Anti-inflammatory | Human plasma protein fraction infusions | Young blood parabiosis can counteract inflammatory and age-related processes in the brain (DMT) | NCT03520998 | Recruiting | Alkahest | Apr 2018 | Nov 2019 |
| NCT03765762 | Recruiting | Alkahest | Dec 2018 | Nov 2019 | ||||
| GV1001 | Antiamyloid, metabolic | Telomerase reverse transcriptase peptide vaccine | Effects on multiple cellular pathways including amyloid pathology (DMT) | NCT03184467 | Recruiting | GemVax & Kael | Jun 2017 | Jun 2019 |
| hUCB-MSCs | Regenerative | Stem cell therapy | Regenerate neurons; reduce amyloid plaque deposition and soluble amyloid; decrease microglial systemic inflammation (DMT) | NCT02054208† | Recruiting | Medipost Co. | Feb 2014 | Jul 2019 |
| NCT03172117† | Recruiting, extension | Medipost Co. | May 2017 | Dec 2021 | ||||
| NCT02513706 | Ongoing | South China Research Center | Oct 2017 | Oct 2019 | ||||
| NCT02672306† | Ongoing | South China Research Center | Oct 2017 | Oct 2019 | ||||
| NCT02833792 | Active, not recruiting | Stemedica Cell Technologies | Jun 2016 | Jun 2020 | ||||
| ID1201 | Antiamyloid | Alpha-secretase enhancer | Reduce amyloid (DMT) | NCT03363269 | Ongoing | IlDong Pharmaceutical | Apr 2016 | Dec 2018 |
| Insulin glulisine (intranasal) | Metabolic | Increase insulin signaling in the brain | Enhance cell signaling and growth; promote neuronal metabolism (DMT) | NCT02503501 | Ongoing | HealthPartners Institute | Aug 2015 | May 2019 |
| IONIS MAPTRx (BIIB080) | RNA-based anti-tau | MAPT RNA inhibitor; antisense oligonucleotide | Reduce tau production (DMT) | NCT03186989† | Recruiting | Ionis Pharmaceuticals, Biogen | Jun 2017 | Feb 2020 |
| Lemborexant | Neurotransmitter based | Dual antagonist of orexin OX1 and OX2 receptors | Improve neuropsychiatric symptoms (sleep disorders) | NCT03001557 | Active, not recruiting | Eisai, Purdue | Dec 2016 | Apr 2020 |
| Levetiracetam | Neuroprotective | SV2A modulator | Decrease amyloid-induced neuronal hyperactivity (DMT) | NCT02002819 | Recruiting | University of California, San Francisco | Jun 2014 | Dec 2019 |
| NCT03489044 | Recruiting | University of Oxford, NHS Foundation Trust, UCB Pharma | Nov 2018 | Jan 2020 | ||||
| NCT03461861 | Recruiting | Medical College of Wisconsin, NIA | Nov 2018 | Mar 2019 | ||||
| Liraglutide | Metabolic | Glucagon-like peptide 1 receptor agonist | Enhance cell signaling (cognitive enhancer) | NCT01843075 | Recruiting | Imperial College London | Jan 2014 | Mar 2019 |
| Lithium | Neurotransmitter based | Ion channel modulator | Improve neuropsychiatric symptoms (agitation, mania, psychosis) | NCT02129348 | Recruiting | New York State Psychiatric Institute, NIA | Jun 2014 | Apr 2019 |
| LM11A-31-BHS | Neuroprotective | p75 neurotrophin receptor ligand | Inhibits tau phosphorylation and synaptic dysfunction; prevents amyloid-induced toxicity (DMT) | NCT03069014† | Recruiting | PharmatrophiX Inc., NIA |
Feb 2017 | Oct 2019 |
| Lupron (leuprolide acetate depot) | Metabolic | Gonadotropin-releasing hormone receptor agonist | Suppresses brain-produced gonadotropin-releasing hormone (cognitive enhancer) | NCT03649724 | Not yet recruiting | New York University | Dec 2018 | Dec 2020 |
| L-Serine | Neuroprotective | Amino acid | Stabilizes protein misfolding (DMT) | NCT03062449 | Recruiting | Dartmouth-Hitchcock Medical Center, Brain Chemistry Laboratories | Mar 2017 | Aug 2019 |
| LY3002813 | Antiamyloid | Monoclonal antibody | Remove amyloid (DMT) | NCT03367403 | Recruiting | Eli Lilly | Dec 2017 | Sep 2021 |
| LY3303560 | Anti-tau | Monoclonal antibody | Remove tau and reduce tau propagation (DMT) | NCT03518073 | Recruiting | Eli Lilly | Apr 2018 | Oct 2021 |
| Methylene blue | Anti-tau | Tau protein aggregation inhibitor | Reduce neurofibrillary tangle formation (DMT) | NCT02380573 | Active, not recruiting | Texas Alzheimer's Research and Care Consortium | Jul 2015 | Jul 2019 |
| MLC901 (NeuroAiD) | Neuroprotective, anti-inflammatory | Traditional Chinese medicine consisting of several herbs | Multiple cellular pathways (DMT) | NCT03038035 | Recruiting | National University Hospital, Singapore | Dec 2016 | Jun 2019 |
| Montelukast | Anti-inflammatory | Leukotriene receptor antagonist | Reduce inflammatory pathways (cognitive enhancer) | NCT03402503 | Recruiting | IntelGenx Corp. | Nov 2018 | Oct 2020 |
| MP-101 | Neurotransmitter based | Enhance mitochondrial functioning | Improve neuropsychiatric symptoms (psychosis) | NCT03044249 | Recruiting | Mediti Pharma | May 2017 | Jan 2021 |
| NA-831 (traneurocin) | Neuroprotective | Undisclosed | Neurogenesis and neuroprotection (DMT) | NCT03538522 | Not yet recruiting | NeuroActiva | Sep 2018 | Apr 2019 |
| Neflamapimod (VX-745) | Anti-inflammatory | Selective p38 MAPK inhibitor | Affects multiple cellular processes including inflammation and cellular plasticity; reduces amyloid plaque burden (DMT) | NCT03402659 | Recruiting | EIP Pharma, VU University | Dec 2017 | Jul 2019 |
| NCT03435861 | Recruiting | EIP Pharma, Toulouse University, Foundation Plan Alzheimer | Oct 2018 | Jan 2021 | ||||
| Nicotinamide | Anti-tau, neuroprotective | Histone deacetylase inhibitor | Reduce tau-induced microtubule depolymerization (DMT) | NCT03061474 | Recruiting | University of California, Irvine | Jul 2017 | Feb 2019 |
| Nicotine | Neurotransmitter based | Nicotinic acetylcholine receptor agonist | Enhance acetylcholine signaling (cognitive enhancer) | NCT02720445 | Recruiting | Univ. of Southern California, NIA, ATRI, Vanderbilt University | Jan 2017 | Dec 2019 |
| Nilotinib | Antiamyloid, anti-tau | Tyrosine kinase inhibitor | Reduce amyloid and tau phosphorylation (DMT) | NCT02947893 | Active, not recruiting | Georgetown University | Jan 2017 | Dec 2019 |
| Octagam 10% | Antiamyloid | 10% human normal immunoglobulin | Remove amyloid (DMT) | NCT03319810 | Recruiting | Sutter Health | Jan 2018 | May 2019 |
| Omega-3 PUFA | Neuroprotective | Fish oil concentrate standardized to long chain in n-3 PUFA content | Support small blood vessels in the brain (DMT) | NCT01953705 | Active, not recruiting | Oregon Health and Science University, NIA | May 2014 | Sep 2019 |
| Pimavanserin | Neurotransmitter based | 5-HT2A inverse agonist | Improve neuropsychiatric symptoms (psychosis) | NCT03118947 | Active, not recruiting, extension | Acadia | Feb 2017 | Aug 2019 |
| Piromelatine | Neurotransmitter based | Melatonin receptor agonist; 5-HT 1A and 1D serotonin receptor agonist | Enhance cellular signaling (cognitive enhancer) | NCT02615002 | Recruiting | Neurim Pharmaceuticals | Nov 2015 | Apr 2019 |
| Posiphen | Antiamyloid | Selective inhibitor of APP production | Reduce amyloid production (DMT) | NCT02925650† | Recruiting | QR Pharma, ADCS | Mar 2017 | Dec 2019 |
| Prazosin | Neurotransmitter based | Alpha-1 adrenoreceptor antagonist | Improve neuropsychiatric symptoms (agitation) | NCT03710642 | Recruiting | ADCS, NIA | Jan 2019 | Dec 2022 |
| PTI-125 | Neuroprotective, anti-inflammatory | FLNA inhibitor | Reduce amyloid, prevent tau hyperphosphorylation and inflammatory toxicity (DMT) | NCT03748706 | Recruiting | Pain Therapeutics, NIH | Nov 2018 | Mar 2019 |
| Rasagiline∗ | Antiamyloid, neuroprotective, metabolic | Monoamine oxidase B inhibitor | Enhance mitochondria activity and inactivate reactive oxygen species (cognitive enhancer), also effect on amyloid pathology (DMT) | NCT02359552 | Active, not recruiting | The Cleveland Clinic | May 2015 | Feb 2019 |
| Riluzole | Neuroprotective | Glutamate receptor antagonist | Inhibit glutamate neurotransmission (DMT) | NCT01703117 | Recruiting | Rockefeller University | Nov 2013 | Nov 2019 |
| RO7105705 (MTAU9937 A) | Anti-tau | Monoclonal antibody | Remove tau (DMT) | NCT03289143 | Recruiting | Genentech | Oct 2017 | Sep 2022 |
| NCT03828747 | Recruiting | Genentech | Feb 2019 | Sep 2021 | ||||
| RPh201 | Neuroprotective | Undisclosed | Promote neurogenesis (DMT) | NCT03462121 | Recruiting | Regenera Pharma | Mar 2018 | Apr 2019 |
| Sargramostim∗ (GM-CSF) | Antiamyloid, neuroprotective | Synthetic granulocyte colony stimulator | Stimulate innate immune system to remove amyloid pathology; increase neuronal connectivity (DMT) | NCT01409915 | Active, not recruiting | University of Colorado, Denver, The Dana Foundation |
Mar 2011 | Nov 2019 |
| S-equol (AUS-131) | Neuroprotective | Nonhormonal estrogen receptor B agonist | Mitochondrial function potentiation; improve synaptic functioning, protects neurons (DMT) | NCT03101085† | Recruiting | Ausio Pharmaceuticals, University of Kansas | May 2017 | Oct 2019 |
| SUVN-502 | Neurotransmitter based | 5-HT 6 antagonist | Improve neuronal signaling (cognitive enhancer) | NCT02580305 | Active, not recruiting | Suven Life Sciences | Sep 2015 | May 2019 |
| Telmisartan & Perindopril | Neuroprotective, anti-inflammatory | Angiotensin II receptor blocker, PPAR-gamma agonist (telmisartan); angiotensin converting enzyme inhibitor (perindopril) | Improve vascular functioning (DMT) | NCT02085265 | Recruiting | Sunnybrook Health Sciences Center, ADDF |
Mar 2014 | Mar 2021 |
| TEP | Antiamyloid | Antiemetic; activates transport protein ABCC1 | Remove amyloid (DMT) | NCT03417986 | Recruiting | Immungenetics AG | Nov 2017 | Jul 2021 |
| UB-311 | Antiamyloid | Active immunotherapy | Reduce amyloid (DMT) | NCT03531710 | Recruiting, extension | United Neuroscience | Aug 2018 | Mar 2021 |
| Valacyclovir | Neuroprotective, anti-inflammatory | Antiviral agent | Protects against HSV-1/2 infection and inflammation (DMT) | NCT02997982 | Recruiting | Umea University | Dec 2016 | Apr 2019 |
| NCT03282916 | Recruiting | New York State Psychiatric Institue, NIH, NIA | Feb 2018 | Aug 2022 | ||||
| Xanamem (UE2343) | Neuroprotective | Blocks 11 beta-HSD1 enzyme activity | Decrease cortisol production and neurodegeneration (DMT) | NCT02727699 | Active, not recruiting | Actinogen | Mar 2017 | Jul 2019 |
Abbreviations: ABCC1, ATP binding cassette subfamily C member 1; ADCS, Alzheimer's Disease Cooperative Study; ADDF, Alzheimer's Drug Discovery Foundation; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; APOE, apolipoprotein E; APP, amyloid precursor protein; ATRI, Alzheimer's Therapeutic Research Institute; BACE, beta-site amyloid precursor protein cleaving enzyme; cAMP, cycling adenosine monophosphate; CB, cannabinoid; DHA, docosahexaenoic acid; DMT, disease-modifying therapy; FLNA, Filamin A; GM-CSF, granulocyte-macrophage colony-stimulating factor; GSK, glycogen synthase kinase; HSD, hydroxysteroid dehydrogenase; HT, hydroxytriptamine; hUCB-MSCs, human umbilical cord blood derived mesenchymal stem cells; MAPK, mitogen-activated protein kinase; MAPT, microtubule-associated tau; NCCIH, National Center for Complementary and Integrative Health; NIA, National Institute on Aging; NMDA, N-methyl-D-aspartate; PDE, phosphodiesterase; PPAR, peroxisome proliferator-activated receptor; PUFA, polyunsaturated fatty acids; SGLT2, sodium-glucose transporter 2; SV2A, synaptic vesicle protein 2A; TEP, thiethylperazine.
NOTE. Seventy-four agents in 83 phase 2 clinical trials currently ongoing as of February 12, 2019 according to clinicaltrials.gov.
Bolded terms represent new agents into the 2019 phase 2 pipeline.
Reported as terminated or completed after the data collection date of February 12, 2019.
Phase 1/2 trials.
Of the drugs with amyloid targets, there were seven immunotherapies, one colony-stimulating factor, two BACE inhibitors, and two alpha-secretase modulators. Two agents targeted synaptic activity, two were anti-aggregation agents, and two agents involved neuroprotection or a metabolic MOA. There were two agents targeting both amyloid and tau reduction. Fig. 3 shows the MOAs of agents in phase 2.
Three of the phase 2 trials were prevention trials; 36 trials involved patients with prodromal or prodromal and mild AD; 38 were trials for mild-to-moderate AD; two trials were for patients with severe AD; two included patients with mild, moderate, or severe AD; one included patients with MCI or healthy volunteers; and one trial was for prodromal or mild-to-moderate AD.
Phase 2 trials are shorter in duration and smaller in terms of participant number than phase 3 trials; phase 2 trials had a mean duration of 178 weeks, average treatment period of 45 weeks, and included an average of 143 subjects in each trial.
3.4. Phase 1
Phase 1 has 30 agents in 31 trials (Fig. 1, Table 3). There are two cognitive enhancers being assessed in phase 1. There are currently no agents addressing neuropsychiatric symptoms in phase 1. In addition, there are 13 small molecules and 13 biologics being assessed in phase 1. The MOA was not identified for two agents. Two of the small molecules and six of the biologics have amyloid as a primary target or one among several targets. Tau is targeted by one small molecule and four biologics in phase 1 studies. Other mechanisms represented in phase 1 include neuroprotection, metabolic, anti-inflammatory, and regenerative interventions.
Table 3.
Agents currently in phase 1 of Alzheimer's disease drug development (as of February 12, 2019)
| Agent | Agent mechanism class | Mechanism of action | Therapeutic purpose | ClinicalTrials.gov ID | Status | Sponsor | Start date | Estimated end date |
|---|---|---|---|---|---|---|---|---|
| AAVrh.10hAPOE2 | Neuroprotective | Serotype rh. 10 adeno-associated virus gene transfer vector expressing the cDNA coding for human ApoE2 | Conversion of the ApoE protein isoforms in the CSF of ApoE4 homozygotes from ApoE4 to ApoE2-ApoE4 (DMT) | NCT03634007 | Not yet recruiting | Cornell University | Jan 2019 | Dec 2021 |
| Aducanumab∗ | Antiamyloid | Monoclonal antibody | Remove amyloid (DMT) | NCT01677572 | Active, not recruiting | Biogen | Oct 2012 | Oct 2021 |
| AL002 | Anti-inflammatory | Monoclonal antibody targeting TREM2 receptors | Prevents inflammatory activity (DMT) | NCT03635047 | Recruiting | Alector | Nov 2018 | Mar 2020 |
| AL003 | Anti-inflammatory | Monoclonal antibody targeting SIGLEC-3 | Reactivates microglia and immune cells in the brain (DMT) | NCT03822208 | Not yet recruiting | Alector | Mar 2019 | Jul 2020 |
| Allopregnanolone (Allo-IM) | Neuroprotective, metabolic | GABA receptor modulator | Improve neurogenesis (DMT) | NCT03748303 | Not yet recruiting | University of Southern California, University of Arizona, Alzheimer's Association | Dec 2018 | Dec 2020 |
| BDPP (bioactive dietary polyphenol preparation) | Neuroprotective | Combination of grape seed polyphenolic extract and resveratrol | Prevents amyloid and tau aggregation (DMT) | NCT02502253 | Recruiting | Johns Hopkins University, Mount Sinai School of Medicine | Jun 2015 | Oct 2019 |
| BIIB076 | Anti-tau | Monoclonal antibody | Remove tau and reduce tau propagation (DMT) | NCT03056729 | Recruiting | Biogen | Feb 2017 | Jul 2019 |
| CKD-355 | Undisclosed | Undisclosed | Undisclosed | NCT03802162 | Not yet recruiting | Chong Kun Dang Pharmaceutical | Feb 2019 | Jul 2019 |
| Crenezumab∗ | Antiamyloid | Monoclonal antibody targeting oligomers | Remove amyloid (DMT) | NCT02353598 | Active, not recruiting | Genentech | Feb 2015 | Sep 2023 |
| CT1812 | Antiamyloid | Sigma-2 receptor antagonist | Reduce amyloid-beta protein-induced synaptic toxicity (DMT) | NCT03522129 | Recruiting | Cognition Therapeutics | May 2018 | Dec 2019 |
| Dabigatran | Neuroprotective | Direct thrombin inhibitor; anticoagulant | Reduce neurovascular damage (DMT) | NCT03752294 | Not yet recruiting | University of Rhode Island, ADDF, Boehringer Ingelheim | Nov 2018 | Dec 2021 |
| DNL747 | Neuroprotective, anti-inflammatory | RIPK1 inhibitor | Reduce cytokines and other inflammatory factors (DMT) | NCT03757325 | Recruiting | Denali Therapeutics | Feb 2019 | Aug 2019 |
| Efavirenz | Antiamyloid | Antiretroviral; nonnucleoside reverse transcriptase inhibitor | Increase cholesterol removal and enhance amyloid reduction (DMT) | NCT03706885 | Recruiting | Case Western Reserve University, Cleveland Medical Center, Massachusetts General Hospital | Dec 2018 | May 2020 |
| Escitalopram & Venlafaxine | Neurotransmitter based | SSRI, SNRI | Improve neurotransmission (cognitive enhancer) | NCT03274817 | Recruiting | New York University | Jul 2017 | Jan 2019 |
| hMSCs (human mesenchymal stem cells) | Regenerative | Stem cell therapy | Regenerate neurons | NCT02600130 | Recruiting | Longeveron | Aug 2016 | Mar 2020 |
| Insulin aspart (intranasal) | Metabolic | Increase insulin signaling in the brain | Neuroprotection and enhanced neuronal function; protects against amyloid toxicity (DMT) | NCT02462161 | Recruiting | Wake Forest School of Medicine, NIA, General Electric | May 2015 | Sep 2019 |
| J147 | Neuroprotective | Mitochondrial ATP synthase inhibitor | Protects neurons from multiple toxicities associated with aging (DMT) | NCT03838185 | Recruiting | Abrexa | Jan 2019 | Jan 2020 |
| JNJ-63733657 | Anti-tau | Monoclonal antibody | Remove tau and reduce tau propagation (DMT) | NCT03375697 | Recruiting | Janssen | Dec 2017 | Oct 2019 |
| Lu AF20513 | Antiamyloid | Active immunotherapy | Remove amyloid (DMT) | NCT02388152 | Active, not recruiting | Lundbeck | Mar 2015 | Dec 2019 |
| NCT03668405 | Recruiting, extension | Lundbeck | Jun 2018 | Nov 2020 | ||||
| NCT03819699 | Recruiting | Lundbeck | Dec 2018 | Jun 2019 | ||||
| LY3002813 | Antiamyloid | Monoclonal antibody | Remove amyloid (DMT) | NCT02624778 | Active, not recruiting | Eli Lilly | Dec 2015 | May 2020 |
| LY3303560 | Anti-tau | Monoclonal antibody | Remove tau and reduce tau propagation (DMT) | NCT03019536 | Active, not recruiting | Eli Lilly | Jan 2017 | Jun 2020 |
| LY3372993 | Antiamyloid | Monoclonal antibody | Remove amyloid (DMT) | NCT03720548 | Recruiting | Eli Lilly | Nov 2018 | Sep 2021 |
| MK-4334 | Undisclosed | Undisclosed | Undisclosed | NCT03740178 | Not yet recruiting | Merck | Jan 2019 | Jun 2019 |
| NDX-1017 | Regenerative | Hepatocyte growth factor | Regenerate neurons (DMT) | NCT03298672 | Recruiting | M3 Biotechnology, ADDF, Biotrial Inc. | Oct 2017 | Apr 2019 |
| NPT088 | Antiamyloid, anti-tau | IgG1-Fc-GAIM fusion protein | Clear amyloid and tau (DMT) | NCT03008161 | Active, not recruiting | Proclara Biosciences, Alzheimer's Association | Dec 2016 | Apr 2019 |
| Salsalate | Anti-inflammatory | Nonsteroidal anti-inflammatory | Reduce neuronal injury (DMT) | NCT03277573 | Recruiting | University of California, San Francisco | Jul 2017 | Oct 2019 |
| Telmisartan | Neuroprotective, anti-inflammatory | Angiotensin II receptor blocker, PPAR-gamma agonist | Improve vascular functioning and effects on amyloid pathology (DMT) | NCT02471833 | Recruiting | Emory University | Apr 2015 | Apr 2019 |
| THN201 | Neurotransmitter based | Cholinesterase inhibitor + antimalarial glial cell modulator | Improve acetylcholine signaling and modulate astrocyte function (DMT) | NCT03698695 | Recruiting | Theranexus | Sep 2018 | Jul 2019 |
| TPI-287 | Anti-tau | Microtubule protein modulator | Reduce tau-mediated cellular damage (DMT) | NCT01966666 | Active, not recruiting | University of California, San Francisco | Nov 2013 | Mar 2019 |
| Vorinostat | Neuroprotective | Histone deacetylase inhibitor | Enhance multiple cellular processes including tau aggregation and amyloid deposition (DMT) | NCT03056495 | Recruiting | German Center for Neurodegenerative Diseases, University Hospital, Bonn, University of Gottingen | Sep 2017 | Oct 2019 |
Abbreviations: ADDF, Alzheimer's Drug Discovery Foundation; ApoE, apolipoprotein E; BACE, beta-site amyloid precursor protein cleaving enzyme; CSF, cerebrospinal fluid; DMT, disease-modifying therapy; GABA, gamma-aminobutyric acid; GAIM, general amyloid interaction motif; NIA, National Institute on Aging; PPAR, peroxisome proliferator-activated receptor; RIPK1, receptor-interacting serine/threonine-protein kinase 1; SIGLEC-3, sialic acid-binding Ig-like lectin 3; SSRI, selective serotonin reuptake inhibitor; SNRI, serotonin-norepinephrine reuptake inhibitor; TREM2, triggering receptor expressed on myeloid cells 2.
NOTE. Thirty agents in 31 phase 1 clinical trials currently ongoing as of February 12, 2019 according to clinicaltrials.gov.
Bolded terms represent new agents into the 2019 phase 1 pipeline.
Reported as terminated or completed after the data collection date of February 12, 2019.
Phase 1 trials had an average duration of 141 weeks (recruitment and treatment period) and included a mean number of 58 participants in each trial.
3.5. Trial sponsors
Across all trials, 54% are sponsored by the biopharma industry, 35% by Academic Medical Centers (with funding from NIH, industry, or other entities), and 10% by others. Table 4 shows the sponsor of agents in each phase of development.
Table 4.
Trial sponsor for each phase of development (clinicaltrials.gov as of February 12, 2019)
| Sponsor | N of trials (%) |
||
|---|---|---|---|
| Phase 1 | Phase 2 | Phase 3 | |
| Biopharma | 18 (58) | 39 (47) | 28 (67) |
| Academic Medical Centers | 8 (26) | 20 (24) | 7 (17) |
| NIH | 0 | 0 | 0 |
| NIH and Academic Medical Centers | 0 | 5 (6) | 2 (5) |
| NIH and Industry | 0 | 2 (2) | 1 (2) |
| Consortium/foundation | 0 | 2 (2) | 0 |
| Industry and consortium/foundation | 2 (6) | 5 (6) | 2 (5) |
| Academic Medical Centers and consortium/foundation | 1 (3) | 2 (2) | 0 |
| Industry, Academic Medical Centers, and consortium/foundation | 2 (6) | 2 (2) | 0 |
| Other combinations | 0 | 6 (7) | 2 (5) |
Abbreviation: NIH, National Institutes of Health.
3.6. Biomarkers
Table 5 shows the biomarkers used as outcome measures in current phase 2 and phase 3 AD clinical trials as described in the federal website; not all trial descriptions in clinicaltrials.gov note if biomarkers are included in the trial.
Table 5.
Biomarkers as outcome measures in phase 2 and phase 3 trials for agents in the Alzheimer's disease drug development pipeline (clinicaltrials.gov as of February 12, 2019)
| Biomarker | N of trials (%) |
|
|---|---|---|
| Phase 3 | Phase 2 | |
| CSF amyloid | 14 (33) | 15 (18) |
| CSF tau | 13 (31) | 17 (20) |
| FDG-PET | 1 (2) | 10 (12) |
| vMRI | 10 (24) | 8 (10) |
| Plasma amyloid | 5 (12) | 5 (6) |
| Plasma tau | 2 (5) | 1 (1) |
| Amyloid PET | 11 (26) | 6 (7) |
| Tau PET | 8 (19) | 2 (2) |
Abbreviations: CSF, cerebrospinal fluid; FDG, fluorodeoxyglucose; PET, positron emission tomography; vMRI, volumetric magnetic resonance imaging.
AD biomarkers served as secondary outcome measures in 16 phase 3 DMT trials and 29 phase 2 DMT trials. The most common biomarkers used were cerebrospinal fluid (CSF) amyloid, CSF tau, volumetric magnetic resonance imaging, and amyloid PET. Of the 25 phase 3 DMT trials, five trials (20%) used amyloid PET as an entry criterion, two (8%) used CSF amyloid, and eight (32%) used either amyloid PET or CSF amyloid. Ten (17%) out of 60 phase 2 DMT trials used amyloid PET as an entry criterion, seven (12%) used CSF amyloid, and six (10%) used either amyloid PET or CSF amyloid. Ten DMT trials in phase 3 and 37 in phase 2 did not require biomarker confirmation of AD for trial entry.
Table 5. Biomarkers as outcome measures in phase 2 and phase 3 trials for agents in the Alzheimer's disease drug development pipeline (clinicaltrials.gov as of February 12, 2019)
3.7. Devices
A variety of approaches to brain stimulation are under study in clinical trials for AD (Table 6). These range from deep brain stimulation with implanted electrodes to surface application of light, electric current, and laser therapy. Most of the trials target cognitive enhancement; a few trials posit effects on amyloid, tau, inflammation, oxidative stress, or mitochondrial function [20], [21]. Targets have varied from deep brain stimulation of fornix and memory-related structures to surface stimulation of parieto-frontal regions. The few completed studies have shown no consistent cognitive benefit; the techniques have been safe with acceptable adverse event profiles [22]. There are no FDA-defined phases for device trials, and most trials did not list the phase on clinicaltrials.gov. The stages of development for device studies can be divided into pilot, pivotal, and postapproval phases.
Table 6.
Devices in clinical trials for treatment of Alzheimer's disease (as of February 12, 2019)
| Device | Mechanism of action | Clinicaltrials.gov ID | Sponsor |
|---|---|---|---|
| tDCS | Low intensity electric current to modulate cortical excitability and brain plasticity | NCT02772185 | Federal University of Paraiba, Brazil |
| NCT03638284 | Center for Addiction and Mental Health | ||
| NCT03288363 | Centre Hospitalier Esquirol | ||
| NCT02873546 | Centre Hospitalier Universitaire de Besancon | ||
| NCT02155946 | VA Office of Research and Development | ||
| Transcranial alternating current stimulation (tACS) | Gamma frequency stimulation to the region of maximum amyloid burden; microglia activation and decrease amyloid and tau depositions |
NCT03290326, NCT03412604 |
Beth Israel Deaconess Medical Center |
| SonoCloud | Low intensity contact ultrasound implant to open the blood-brain barrier; allows increased intracerebral bioavailability of anti-AD drugs; may also allow endogenous antibodies to penetrate the brain parenchyma and target amyloid plaques even without any adjunct antiamyloid treatment | NCT03119961 | CarThera |
| MemorEM 1000 | Transcranial electromagnetic treatment; disaggregation of toxic oligomers; mitochondrial enhancement | NCT02958930 | NeuroEm Therapeutics |
| Neuro Gamma | Photobiomodulation–administers low energy, near-infrared LED light to the brain transcranially and intranasally; reduces oxidative stress and neuroinflammation |
NCT03484143, NCT03328195 |
Vielight |
|
NCT03160027, NCT03405662 |
University of California, San Francisco | ||
| RGn530 | Photobiomodulation device; reduces oxidative stress and neuroinflammation | NCT03672474 | University Hospital, Montpellier (device by REGEnLIFE) |
| Electroconvulsive therapy | Improve cognition by increasing brain-derived neurotrophic factor levels | NCT02438202 | Central Institute of Mental Health, Mannheim |
| ExAblate Model 4000 | Blood-brain barrier disruption by focal ultrasound |
NCT03671889, NCT03739905 |
InSightec |
| GammaSense stimulation system | Visual sensory stimulation device flickering lights at gamma frequency to drive gamma oscillations in brain areas; increase cerebral blood flow and reduce amyloid | NCT03556280 | Cognito Therapeutics |
| NCT03543878 | Emory University, Georgia Institute of Technology | ||
| Low level laser therapy | Modulate cellular metabolism and regeneration | NCT02537626 | Erchonia Corporation |
| DBS | Directly target and modulate the activity of brain structures implicated in memory functioning; improve cognition | NCT03347084 | University of California, Los Angeles |
| NCT03352739 | Xuanwu Hospital, China, Beijing Pins Medical Co. | ||
| NCT03622905 | Functional Neuromodulation | ||
| NCT03290274 | Hospital San Carlos, Madrid | ||
| rTMS | Stimulate different areas of the brain to induce changes in brain activity and modify impaired neural networks | NCT03121066 | Universitat Oberta de Catalunya |
| NCT02908815 | University of Manitoba | ||
| NCT03270137 | Instituto Nacional de Psipquiatria Dr. Ramon de la Fuente | ||
| NCT02190084 | Central Arkansas Veterans Healthcare System | ||
| NCT03778151 | Fondazione Santa Lucia | ||
| NEUROLITH | TPS consisting of short acoustic pulses with an ultrasound frequency to stimulate the brain; maintains and improves cognitive abilities | NCT03770182 | Storz Medical |
| tVNS | Stimulation of the auricular branch with electrodes on the external ear to improve cognition | NCT03359902 | University of Florida, NIA |
| NeuroAD | Combination of TMS and cognitive training; stimulates areas of the brain responsible for cognitive functions that have been impaired by AD and makes them more receptive to cognitive training | NCT01825330 | Neuronix |
Abbreviations: AD, Alzheimer's disease; DBS, deep brain stimulation; rTMS, repetitive transcranial magnetic stimulation; tDCS, Transcranial direct current stimulation; tACS, Transcranial alternating current stimulation; TMS, transcranial magnetic stimulation; TPS, Transcranial pulse stimulation; tVNS, Transcutaneous vagal nerve stimulation
NOTE: Thirty-three device trials currently ongoing (“recruiting,” “active, not recruiting,” and “not yet recruiting”) as of February 12, 2019 according to clinicaltrials.gov.
4. Discussion
In 2018, the FDA approved 59 novel pharmacotherapies across all therapeutic areas, breaking the 1996 record of 53 drug approvals [23], [24]. There were 42 small molecule therapies and 17 biological therapies approved [24]. Eight new neurological drugs were included among the new therapies: 3 migraine treatments (all were calcitonin gene-related peptide receptor antibodies), 2 for seizures in Dravet syndrome (1 included Dravet syndrome and Lennox-Gastaut syndrome), two for hereditary transthyretin-mediated amyloidosis, and 1 for Fabry disease. The latter three agents can be regarded as DMTs, the others provide relief of symptoms albeit life-threatening symptoms in the case of the epilepsies. The therapies for transthyretin-mediated amyloidosis are RNA-based interventions (antisense oligonucleotide or interference RNA) representing a new approach to neurological disorders. Oligonucleotide-based therapies have shown initial promise in Huntington's disease and may have applications in other neurodegenerative disorders including AD [25]. There is one RNA-based treatment in the AD pipeline (IONIS MAPTRx).
Several agents have completed clinical trials since the time of last year's pipeline analysis and shown no drug-placebo difference. LTMX targeted tau pathology in AD and did not establish efficacy [19]. Azeliragon is a receptor for advanced glycation end products inhibitor and was found to produce no drug-placebo difference in a trial of mild-to-moderate AD. Crenezumab is a monoclonal antibody that targeted oligomeric forms of amyloid-beta protein (Aβ) [26]. It failed to show a drug-placebo difference at the time of a futility analysis in two large clinical trials, and development of the agent was halted. Similarly, aducanumab trials were recently stopped after a futility analysis. Verubecestat is a BACE inhibitor whose development was halted for futility in a mild-to-moderate AD clinical trial [27]. A trial of verubecestat in patients with prodromal AD defined by clinical and amyloid PET measures was halted after a futility analysis suggested that the agent could not succeed. Similarly, lanabecestat did not meet the criteria to continue after a futility analysis. Atabecestat is a BACE inhibitor being assessed in preclinical AD; the trial was discontinued when elevated liver enzymes were observed among some trial participants. Intranasal insulin was assessed in mild-to-moderate AD and showed no drug-placebo difference [28]. Pioglitazone, an insulin sensitizing agent was stopped for futility in a preclinical AD trial. A trial of a FYN inhibitor (AZD0530) used fluorodeoxyglucose PET as the primary outcome and showed no drug-placebo difference on the biomarker or any clinical measure [29]. Fluorodeoxyglucose PET performed well in this multisite trial suggesting it can be used in multicenter trials to show drug-placebo differences with agents that affect brain metabolism. ITI-007 is a multitransmitter agent being developed for the treatment of schizophrenia and was tested in a clinical trial to determine its effect on agitation in AD. No drug-placebo difference was observed in the trial. Of the 17 phase 3 DMTs listed in our 2018 review, eight have been terminated.
GV-971 is a multitargeted molecule that completed a phase 3 clinical trial in China in 2018 [30]. GV-971 has nonclinical evidence of effects on neuroinflammation, amyloid plaques, neurofibrillary tangles, mitochondrial function, and cholinergic function [30]. In a phase 3 trial conducted in China by an international contract research organization, GV-971 showed a statistically significant benefit over placebo on the Alzheimer's Disease Assessment Scale–cognitive subscale [31]; a trend toward improvement was noted on the clinical interview-based impression of change [32]. There was no impact on functional and behavioral measures. The outcomes appear to have met the criteria required for approval by the Chinese FDA, and the agent is under review.
Biomarkers play an increasingly important role in AD drug development. Participant selection, target engagement, disease course prediction, evidence of disease modification, and side effect monitoring all involve biomarkers [33]. The NIA–Alzheimer's Association established the biomarker-based ATN framework for the diagnosis and characterization of AD [7]. This framework will assist in trials with both accurate diagnosis of AD and biological staging of AD relevant to matching the trial population to the MOA of the agent being assessed [8]. Of the disease-modifying trials currently in the AD pipeline, 52 use amyloid imaging and/or CSF to support the diagnosis, 20 have amyloid imaging as an outcome, ten have tau imaging as an outcome of the intervention. In addition to the specific biomarkers included in the ATN framework, evidence is accruing that the plasma amyloid 40/42 ratio corresponds to the presence of cerebral amyloidosis [34] and that plasma neurofilament light is indicative of neurodegeneration [35]. These increasingly available biomarkers will facilitate screening for clinical trials and may have a role in course prediction and assessing treatment outcome.
Design innovations are evident in recent trials of AD therapeutics. Futility analyses were used to terminate development programs for pioglitazone, verubecestat [27], crenezumab, ITI-007, LY3314814 (lanabecestat), and aducanumab. Futility analyses are conducted when the trial is incomplete but when sufficient data are available to predict if continuing the trial could meet prespecified criteria [36].
The Alzheimer's Disease Composite Score (ADCOMS) [37] has been introduced as a cognitive outcome in several development programs including BAN2401, elenbecestat, and xanamem. The ADCOMS is an analytic approach whose score is based on combining scores on items derived from the Alzheimer's Disease Assessment Scale–cognitive subscale, clinical dementia rating, and Mini-Mental State Examination [38] after these tools are administered in the standard way. ADCOMS constituents were derived from trials of patients with MCI that showed the most change over a one-year period to develop a score that is most likely to show a drug-placebo difference in trials of patients with early-stage disease and very limited cognitive deficits.
Bayesian adaptive designs are being implemented in AD trials. These have been used broadly in non-AD trials including development of cancer and diabetes therapies [39], [40]. Adaptive trials are being used in the BAN2401 trial, Dominantly Inherited Alzheimer Network–Treatment Unit [41], European Prevention of Alzheimer Disease initiative [42], and the Intranasal Oxytocin for Fronto-temporal Dementia (FOXY) trial of intranasal oxytocin for frontotemporal dementia [43]. The trial of ABT-089 pioneered the use of an adaptive design in AD [44]. Bayesian designs use data derived from the ongoing trial to inform dose allocation, trial duration, sample size, or response to adverse events; decisions are prespecified before trial initiation. Dose-adaptive designs are participant-centric in that they allow study subjects to be assigned to the doses most likely to succeed or least likely to produce adverse events.
It has been argued that there is no “pipeline” of drug development because agents often do not proceed systematically from phase 1 to phase 3 and irregularities are common [45]. We use the word “pipeline” to categorize agents in early, middle, and late-stage trials. In AD drug development, agents tend to proceed from phase 1 single and multiple ascending dose studies to phase 2 proof-of-concept (POC) studies, and then to phase 3 registration-type trials. Testing for POC in phase 2 depends on dose ranges and safety established in phase 1 and provides the foundation for phase 3. Repurposed agents may have irregular pathways going from approved status for one indication to phase 1 or phase 2 to define dose and POC before testing in phase 3 for the AD indication [46], [47]. The concept of “pipeline” applies as an imprecise but generally accurate overview of drug development for AD.
There are more agents in the AD pipeline in 2019 than was observed in the 2018 pipeline. There are 28 agents in phase 3 (compared with 26 in 2018), 74 agents in phase 2 (compared with 63 in 2018), and 30 in phase 1 (compared with 23 in 2018).
The lack of success in AD drug development has given rise to nihilism with regard to the ability of the field to develop agents that meaningfully modify the progression of AD. Suggestions to abandon the amyloid hypothesis, focus exclusively to combination therapies, place more emphasis on lifestyle interventions to prevent AD or reassess our assumptions and build new models to drive drug development are all voiced, and each of these perspectives have merit. Reviews of the pipeline show that lessons are learned from all trials; even negative and futile outcomes are highly informative and provide guidance for future trials. The overview of trials document a shift toward more diversification of targets between phase 3 and phase 2, the entry of combination therapies into the pipeline, and the use of biomarkers to allow early assessments of the impact of candidate interventions on disease biology.
Several agents have shown no drug-placebo difference, and the development programs have been discontinued. A few programs successfully demonstrated drug-placebo differences in phase 2 and are advancing. Progress depends on innovation and learning from exploration of new targets, assessment of new candidates, and implementation of new trial features. As in other chronic disease such as cancer, human immunodeficiency virus (HIV), and cardiovascular disease, a learning phase preceded periods whose sequential incremental successes led to meaningful treatments.
Research in context.
-
1.
Systematic review: There is a high rate of failure of drug development for Alzheimer's disease. New treatments are urgently needed, and review of the drug development pipeline can improve our understanding of how best to advance new therapies. We reviewed all drugs currently in clinical trials for Alzheimer's disease listed in the federal government database clinicaltrials.gov.
-
2.
Interpretation: We showed that there are 132 agents in clinical trials for the treatment for Alzheimer's disease. Ninety-six of these drugs are disease-modifying agents intended to change the underlying biology of Alzheimer's disease. Nineteen of the drugs are intended to be cognitive enhancing agents, and 14 are being developed for the treatment of neuropsychiatric and behavioral symptoms. We provide an overview of drugs currently in clinical trials for Alzheimer's disease.
-
3.
Future directions: Progress is being made in terms of defining new targets for the treatment of Alzheimer's disease, developing new agents, introducing innovative clinical trial designs, incorporating a broader range of populations in clinical trials, and developing new biomarkers that provide insight into the impact of emerging therapies. Improvements in drug development success rates are anticipated.
Acknowledgments
JC acknowledges funding from the National Institute of General Medical Sciences (Grant: P20GM109025) and support from Keep Memory Alive.
Footnotes
Disclosures: Dr. Cummings has provided consultation to Acadia, Accera, Actinogen, Alkahest, Allergan, Alzheon, Avanir, Axsome, BiOasis Technologies, Biogen, Cognicity, Diadem, EIP Pharma, Eisai, Genentech, Green Valley, Grifols, Hisun, Idorsia, Karuna, Kyowa Kirin, Lilly, Lundbeck, Merck, Otsuka, Proclara, QR, Resverlogix, Roche, Samus, Samumed, Sunovion, Suven, Takeda, Teva, Toyama, and United Neuroscience pharmaceutical and assessment companies. JC acknowledges funding from the National Institute of General Medical Sciences (Grant: P20GM109025) and support from Keep Memory Alive. Dr Sabbagh reported Royalties from Harper Collins, Stock/Equity from uMethodHealth, Brain Health Inc, Optimal Cognitive Health Company, M3 Biosciences, Versanum; Speakers Bureau from Peerview and Rockpointe, Consultant/Advisor for Allergan, Biogen, Bracket, Neurotrope, Cortexyme, Roche, Grifols, Regeneron, VTV therapeutics, Alzheon. Dr. Zhong is the CEO of CNS Innovations and has provided consultation to Green Valley. GL and AR have no disclosures.
References
- 1.Dubois B., Feldman H.H., Jacova C., Cummings J.L., Dekosky S.T., Barberger-Gateau P. Revising the definition of Alzheimer's disease: a new lexicon. Lancet Neurol. 2010;9:1118–1127. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- 2.Dubois B., Hampel H., Feldman H.H., Scheltens P., Aisen P., Andrieu S. Preclinical Alzheimer's disease: definition, natural history, and diagnostic criteria. Alzheimer's & dementia. J Alzheimer's Assoc. 2016;12:292–323. doi: 10.1016/j.jalz.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aisen P.S., Andrieu S., Sampaio C., Carrillo M., Khachaturian Z.S., Dubois B. Report of the task force on designing clinical trials in early (predementia) AD. Neurology. 2011;76:280–286. doi: 10.1212/WNL.0b013e318207b1b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. Food and Drug Administration . 2013. Guidance for Industry Alzheimer's Disease: Developing Drugs for the Treatment of Early Stage Disease. Food and Drug Administration, Washington, D.C. [Google Scholar]
- 5.U.S. Food and Drug Administration. U.S. Department of Health and Human Services . Guidance for Industry; Washington, DC: 2018. Early Alzheimer's Disease: Developing Drugs for Treatment. [Google Scholar]
- 6.U.S. Food and Drug Administration. U.S. Department of Health and Human Services . Draft Guidance for Industry; Washington, DC: 2018. Adaptive Designs for Clinical Trials of Drugs and Biologics. [Google Scholar]
- 7.Jack C.R., Jr., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B. NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings J. The National Institute on Aging-Alzheimer's Association framework on Alzheimer's disease: application to clinical trials. Alzheimers Dement. 2018;15:172–178. doi: 10.1016/j.jalz.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pontecorvo M.J., Devous M.D., Sr., Navitsky M., Lu M., Salloway S., Schaerf F.W. Relationships between flortaucipir PET tau binding and amyloid burden, clinical diagnosis, age and cognition. Brain. 2017;140:748–763. doi: 10.1093/brain/aww334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molinuevo J.L., Ayton S., Batrla R., Bednar M.M., Bittner T., Cummings J. Current state of Alzheimer's fluid biomarkers. Acta Neuropathol. 2018;136:821–853. doi: 10.1007/s00401-018-1932-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cummings J., Morstorf T., Lee G. Alzheimer's disease drug development pipeline: 2016. Alzheimer's Demen. 2016;2:222–232. doi: 10.1016/j.trci.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cummings J., Lee G., Mortsdorf T., Ritter A., Zhong K. Alzheimer's disease drug development pipeline: 2017. Alzheimer's & Dementia. Translational Res Clin Interventions. 2017;3:367–384. doi: 10.1016/j.trci.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cummings J., Lee G., Ritter A., Zhong K. Alzheimer's disease drug development pipeline: 2018. Alzheimers Dement. 2018;4:195–214. doi: 10.1016/j.trci.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curry S., DeCory H.H., J.G . Phase I: The first opportunity for extrapolation from animal data to human exposure. In: Edwards L.D., Fox A.W., Stonier, editors. Principles and Practice of Pharmaceutical Medicine. Wiley-Blackwell; Oxford, UK: 2011. pp. 84–106. [Google Scholar]
- 15.Kelley J. Wiley-blackwell; Oxford, UK: 2009. Principles of CNS Drug Development: From Test Tube to Patient. [Google Scholar]
- 16.Lassman S.M., Shopshear O.M., Jazic I., Ulrich J., Francer J. Clinical trial transparency: a reassessment of industry compliance with clinical trial registration and reporting requirements in the United States. BMJ Open. 2017;7:e015110. doi: 10.1136/bmjopen-2016-015110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller J.E., Wilenzick M., Ritcey N., Ross J.S., Mello M.M. Measuring clinical trial transparency: an empirical analysis of newly approved drugs and large pharmaceutical companies. BMJ Open. 2017;7:e017917. doi: 10.1136/bmjopen-2017-017917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson M.L., Chiswell K., Peterson E.D., Tasneem A., Topping J., Califf R.M. Compliance with results reporting at ClinicalTrials.gov. N Engl J Med. 2015;372:1031–1039. doi: 10.1056/NEJMsa1409364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gauthier S., Feldman H.H., Schneider L.S., Wilcock G.K., Frisoni G.B., Hardlund J.H. Efficacy and safety of tau-aggregation inhibitor therapy in patients with mild or moderate Alzheimer's disease: a randomised, controlled, double-blind, parallel-arm, phase 3 trial. Lancet. 2016;388:2873–2884. doi: 10.1016/S0140-6736(16)31275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonsalvez I., Baror R., Fried P., Santarnecchi E., Pascual-Leone A. Therapeutic Noninvasive Brain Stimulation in Alzheimer's Disease. Curr Alzheimer Res. 2017;14:362–376. doi: 10.2174/1567205013666160930113907. [DOI] [PubMed] [Google Scholar]
- 21.Hsu W.Y., Ku Y., Zanto T.P., Gazzaley A. Effects of noninvasive brain stimulation on cognitive function in healthy aging and Alzheimer's disease: a systematic review and meta-analysis. Neurobiol Aging. 2015;36:2348–2359. doi: 10.1016/j.neurobiolaging.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lozano A.M., Fosdick L., Chakravarty M.M., Leoutsakos J.M., Munro C., Oh E. A Phase II study of Fornix deep brain stimulation in mild Alzheimer's disease. J Alzheimers Dis. 2016;54:777–787. doi: 10.3233/JAD-160017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrison C. Fresh from the biotech pipeline-2017. Nat Biotechnol. 2018 doi: 10.1038/nbt.4068. [DOI] [PubMed] [Google Scholar]
- 24.Mullard A. 2018 FDA drug approvals. Nat Rev Drug Discov. 2019;18:85–89. doi: 10.1038/d41573-019-00014-x. [DOI] [PubMed] [Google Scholar]
- 25.van Roon-Mom W.M.C., Roos R.A.C., de Bot S.T. Dose-dependent lowering of mutant Huntingtin using antisense oligonucleotides in Huntington disease patients. Nucleic Acid Ther. 2018;28:59–62. doi: 10.1089/nat.2018.0720. [DOI] [PubMed] [Google Scholar]
- 26.Cummings J.L., Cohen S., van Dyck C.H., Brody M., Curtis C., Cho W. ABBY: A phase 2 randomized trial of crenezumab in mild to moderate Alzheimer disease. Neurology. 2018;90:e1889–e1897. doi: 10.1212/WNL.0000000000005550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egan M.F., Kost J., Tariot P.N., Aisen P.S., Cummings J.L., Vellas B. Randomized trial of verubecestat for mild-to-moderate Alzheimer's disease. N Engl J Med. 2018;378:1691–1703. doi: 10.1056/NEJMoa1706441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Craft S., Raman R., Chow T., Rafii M.S., Rissman R.A., Brewer J.B. 2018. Primary results from a phase II/III trial of intranasal insulin: a novel multi-target molecule and delivery mode for AD therapeutics. Clinical Trials on Alzheimer's disease. Barcelona, Spain, October 25. [Google Scholar]
- 29.van Dyck C.H., Nygaard H.B., Chen K., Donohue M.C., Raman R., Rissman R.A. 2018. Pahse 2a Trial of AZD0530 Evaluating 18F-FDG PET, Safety, Tolerability in Mild Alzheimer's Dementia. Clinical Trials on Alzheimer's Disease. Barcelona, Spain, October 25. [Google Scholar]
- 30.Xiao S., Zhang Z., Geng M., GV-971 Study Group . 2018. Phase 3 Clinical Trial of a Novel and Multi-targeted Oligosaccharide in Patients with Mild-moderate AD in China. Clinical Trials on Alzheimer's Disease. Barcelona, Spain, October 25. [Google Scholar]
- 31.Rosen W.G., Mohs R.C., Davis K.L. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 32.Boothby H., Mann A.H., Barker A. Factors determining inter rater agreement with rating global change in dementia: the cibic-plus. Int J Geriatr Psychiatry. 1995;10:1037–1045. [Google Scholar]
- 33.Cummings J. The role of biomarkers in Alzheimer's disease drug development. In: Guest P.C., editor. Reviews on Biomarker Studies in Neurological Disorders. Springer; United Kingdom: 2019. In Press. [Google Scholar]
- 34.Nakamura A., Kaneko N., Villemagne V.L., Kato T., Doecke J., Dore V. High performance plasma amyloid-beta biomarkers for Alzheimer's disease. Nature. 2018;554:249–254. doi: 10.1038/nature25456. [DOI] [PubMed] [Google Scholar]
- 35.Ashton N.J., Leuzy A., Lim Y.M., Troakes C., Hortobagyi T., Hoglund K. Increased plasma neurofilament light chain concentration correlates with severity of post-mortem neurofibrillary tangle pathology and neurodegeneration. Acta Neuropathol Commun. 2019;7:5. doi: 10.1186/s40478-018-0649-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shan G., Banks S., Miller J.B., Ritter A., Bernick C., Lombardo J. Statistical advances in clinical trials and clinical research. Alzheimers Dement (N Y) 2018;4:366–371. doi: 10.1016/j.trci.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J., Logovinsky V., Hendrix S.B., Stanworth S.H., Perdomo C., Xu L. ADCOMS: a composite clinical outcome for prodromal Alzheimer's disease trials. J Neurol Neurosurg Psychiatry. 2016;87:993–999. doi: 10.1136/jnnp-2015-312383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 39.Berry S.M., Broglio K.R., Groshen S., Berry D.A. Bayesian hierarchical modeling of patient subpopulations: efficient designs of Phase II oncology clinical trials. Clin Trials. 2013;10:720–734. doi: 10.1177/1740774513497539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skrivanek Z., Gaydos B.L., Chien J.Y., Geiger M.J., Heathman M.A., Berry S. Dose-finding results in an adaptive, seamless, randomized trial of once-weekly dulaglutide combined with metformin in type 2 diabetes patients (AWARD-5) Diabetes Obes Metab. 2014;16:748–756. doi: 10.1111/dom.12305. [DOI] [PubMed] [Google Scholar]
- 41.Bateman R.J., Benzinger T.L., Berry S., Clifford D.B., Duggan C., Fagan A.M. The DIAN-TU Next Generation Alzheimer's prevention trial: adaptive design and disease progression model. Alzheimers Dement. 2017;13:8–19. doi: 10.1016/j.jalz.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ritchie C.W., Molinuevo J.L., Truyen L., Satlin A., Van der Geyten S., Lovestone S. Development of interventions for the secondary prevention of Alzheimer's dementia: the European Prevention of Alzheimer's Dementia (EPAD) project. Lancet Psychiatry. 2016;3:179–186. doi: 10.1016/S2215-0366(15)00454-X. [DOI] [PubMed] [Google Scholar]
- 43.Finger E., Berry S., Cummings J., Coleman K., Hsiung R., Feldman H.H. Adaptive crossover designs for assessment of symptomatic treatments targeting behaviour in neurodegenerative disease: a phase 2 clinical trial of intranasal oxytocin for frontotemporal dementia (FOXY) Alzheimer's Res Ther. 2018;10:102. doi: 10.1186/s13195-018-0427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lenz R.A., Pritchett Y.L., Berry S.M., Llano D.A., Han S., Berry D.A. Adaptive, dose-finding phase 2 trial evaluating the safety and efficacy of ABT-089 in mild to moderate Alzheimer disease. Alzheimer Dis Assoc Disord. 2015;29:192–199. doi: 10.1097/WAD.0000000000000093. [DOI] [PubMed] [Google Scholar]
- 45.Baxter K., Horn E., Gal-Edd N., Zonno K., O'Leary J., Terry P.F. An end to the myth: there is no drug development pipeline. Sci Transl Med. 2013;5:171cm1. doi: 10.1126/scitranslmed.3003505. [DOI] [PubMed] [Google Scholar]
- 46.Appleby B.S., Nacopoulos D., Milano N., Zhong K., Cummings J.L. A review: treatment of Alzheimer's disease discovered in repurposed agents. Dement Geriatr Cogn Disord. 2013;35:1–22. doi: 10.1159/000345791. [DOI] [PubMed] [Google Scholar]
- 47.Appleby B.S., Cummings J.L. Discovering new treatments for Alzheimer's disease by repurposing approved medications. Curr Top Med Chem. 2013;13:2306–2327. doi: 10.2174/15680266113136660162. [DOI] [PubMed] [Google Scholar]