Abstract
Accumulating evidence suggests altered function of the mesolimbic reward system resulting from exposure to early adversity. The present study investigated the combined long-term impact of adversity until young adulthood on neuronal reward processing and its interaction with individual resilience processes. In this functional magnetic resonance imaging study, 97 healthy young adults performed a reward-based decision-making task. Adversity as well as resilience were assessed retrospectively using the validated childhood trauma questionnaire, trauma history questionnaire and a resilience scale. Subjects with high adversity load showed reduced reward-related bottom-up activation in the ventral striatum (VS), ventral tegmental area (VTA) and hippocampus (HP) as compared to the low adversity group. However, high resilience traits in individuals with high adversity load were associated with an increased activation in the VTA and HP, indicating a possible resilience-related protective mechanism. Moreover, when comparing groups with high to low adversity, psychophysiological interaction analyses highlighted an increased negative functional coupling between VS and VTA as well as between VS and anteroventral prefrontal cortex (avPFC) during reward acceptance, and an impaired top-down control of the VS by the avPFC during reward rejection. In turn, combination of high adversity and high resilience traits was associated with an improved functional coupling between VTA, VS and HP. Thereby, the present findings identify neural mechanisms mediating interacting effects of adversity and resilience, which could be targeted by early intervention and prevention.
Keywords: Trauma, Mesolimbic dopamine system, Ventral striatum, Functional activity, fMRI, Resilience traits
Highlights
-
•
fMRI activity in the reward system decresead with adversity load.
-
•
Stress exposure associated with impaired connectivity in the reward system.
-
•
fMRI activity in VTA and hippocampus incresead with resilience to adversity.
-
•
Functional coupling within reward circuitry improved with resilience to adversity.
-
•
Evidence for protective resilience-related mechanisms mediated by VTA & hippocampus.
1. Introduction
Growing evidence suggests that childhood maltreatment may impair human brain development and mental health later in life (Lupien et al., 2009; Heim and Binder, 2012). Exposure to stress early during development has detrimental effects on the brain's dopaminergic system (Abercrombie et al., 1989; Pani et al., 2000), and is a well-known risk factor for the development of a variety of psychiatric disorders, such as schizophrenia (Wicks et al., 2005; Matheson et al., 2013), mood and anxiety disorders (Dube et al., 2001; Chapman et al., 2004), in which aberrant dopamine neuromodulation is implicated in the pathogenesis.
However, there is striking variability in the outcomes of early and later life stress among humans. Although exposed to childhood maltreatment, some individuals remain resilient and high functioning even when exposed to additional stressors in adulthood. It can be assumed that protective factors such as resilience traits or processes play an important role (Masten and Coatsworth, 1998). Fundamentally, resilience is the capacity to cope effectively within the context of significant adversity and to maintain or regain mental health. It is a dynamic, multidimensional process encompassing positive adaptation despite experiencing stress (Wald et al., 2006; Herrman et al., 2011; Masten, 2011), and, from a more psychobiological view, a short-term and long-term response that reduces allostatic load (Curtis and Cicchetti, 2003; Charney, 2004).
Alterations of the mesocorticolimbic system have been hypothesized to play a significant role mediating the devastating effect of childhood maltreatment on psychopathology and brain development (Dillon et al., 2009). Within the mesocorticolimbic pathway dopaminergic neurons in the ventral tegmental area (VTA) project to the ventral striatum (VS), hippocampus (HP) and prefrontal cortex (PFC), collectively mediating reward and learning-related processing as well as motivated behavior (Schultz et al., 1997; Haber and Knutson, 2010). Even more importantly, the mesocorticolimbic dopamine system plays a critical role in adapting to ever-changing environments and in regulating the entry of novel information into long-term memory by signaling to the HP (Wise, 2004; Lisman and Grace, 2005).
There is evidence for decreased VS reactivity to rewarding events and for reduced HP activity and poorer declarative memory retrieval in subjects exposed to early stress (Carrión et al., 2010; Goff et al., 2013). The impact of early life stress on reward processing is possibly mediated by alterations of the hypothalamus-pituitary-adrenal (HPA) axis (Ulrich-Lai and Herman, 2009). Yet, not much is known about the neural mechanisms of resilience to early life adversity. The limited number of studies designed to investigate resilience have mainly focused on examining neural correlates of emotion processing (Van der Werff et al., 2013). But it has become clear that a broader view of the neural underpinnings is required.
In the present study, the impact of the variation of childhood and young adulthood adversity and resilience as well as their interaction on reward-related functional activity and connectivity has been investigated in a large sample of young adults. It is known that cumulative trauma is a significant predictor of clinical outcomes. This approach has previously been applied in a study demonstrating a combined impact of childhood trauma and adult trauma on post-traumatic stress and depressive symptoms (Gillespie et al., 2009). By combining childhood and young adulthood adversity in our group of 97 subjects, we aimed to investigate whether childhood trauma and young adulthood trauma operate on the same neural circuitry and have synergistic or buffering effects. Using functional magnetic resonance imaging (fMRI), the desire-reason-dilemma (DRD) paradigm (Diekhof and Gruber, 2010) was applied in order to measure blood-oxygen-level-dependent (BOLD) responses in key regions of the dopaminergic reward system, namely the VS, VTA and HP during acceptance or rejection of immediate rewards. This paradigm consists of two different task contexts, one in which subjects were allowed to select previously conditioned reward stimuli, and another in which these conditioned reward stimuli had to be rejected in favor of a superordinate long-term goal. Therewith, it allows to directly investigate reward-related brain activations depending on bottom-up mechanisms when accepting the reward stimuli in the first context, and top-down mechanisms, when these stimuli had to be rejected in the second context. We hypothesized that activation of the mesocorticolimbic system during reward acceptance would decrease with the level of early life adversity. Furthermore, we expected the functional interaction within and beyond the mesocorticolimbic dopaminergic system to be affected by adversity load. Resilience traits, however, were hypothesized to lead to a change in activation of these brain regions despite the experience of early life adversity.
2. Materials and methods
2.1. Sample
The initial sample consisted of 306 healthy Caucasian subjects who were recruited from the university environment and underwent fMRI while performing the DRD paradigm. 78 participants had to be excluded due to missing imaging data, excessive head movements, low behavioral task performance rates (overall rate < 75%) and voluntary abortion of the task. Three years after completion of the fMRI study, information about adversity during childhood and lifetime as well as resilience traits were assessed retrospectively as part of a comprehensive online survey. 135 participants took part in this online survey. From these, however, 38 individuals were discarded due to insufficient information in the online survey (not fully completed questionnaires), leaving a final sample of 97 participants (65 females, mean age at time of fMRI measurement (±SD): 23.78 years (±2.50 years). All subjects had normal or corrected-to-normal vision and no history of neurological or psychiatric disorders. Additionally, they were screened for MRI contraindications, current or past psychopathology, neurological disorders and actual or previous drug as well as psychotropic medication use. The study was carried out in accordance with the latest version of the Declaration of Helsinki and all procedures in this study were approved by the Ethics Committee of the University Medical Center Göttingen. After complete description of all study procedures, participants gave written informed consent and were paid for both participation in the fMRI study (30–60 Euro depending on task performance) and online survey (30 Euro).
2.2. Psychological assessment and group classification
Childhood adversity was assessed retrospectively using the psychometrically validated 25-item childhood trauma questionnaire (CTQ; Klinitzke et al., 2012). In addition, information about traumatic events during lifetime were collected using the validated trauma history questionnaire consisting of 24 yes/no questions (THQ; Hooper et al., 2011). For each category of the instrument, having had the exposure was scored ‘1′ and no exposure ‘0′. Overall, a score was formed by counting the number of items of the CTQ and THQ experienced until their mid-20s (mean: 34.42 ± 6.88; range: 25–57). The cumulative risk hypothesis has been largely confirmed by empirical evidence that the likelihood of unfavorable child outcomes increases with the number of adversity factors (Evans et al., 2013). It has been shown that cumulative adversity is an important predictor of trauma and health-related outcomes. Recently, studies investigated the combined effect of childhood and adult trauma on post-traumatic stress and depressive symptoms (Gillespie et al., 2009), and linked cumulative trauma exposure to trauma symptoms (Martin et al., 2013). Finally, resilience was measured with the validated 25-item, self-rated resilience scale (RS; Wagnild and Young, 1993). Scores varied between 1 and 175 with higher scores reflecting higher resilience (mean: 132.94 ± 22.92; range: 33–169). All participants were assigned to either a high adversity (highA, n = 48) or low adversity group (lowA, n = 49) as well as high resilience (highR) or low resilience (lowR) group based on the frequently used method for dichotomizing scores using a median split (Roy et al., 2007; Von Eisenhart Rothe et al., 2013; Aas et al., 2017).
2.3. Assessment of personality characteristics
All subjects completed the German version of the Temperament and Character Inventory (TCI) (Cloninger et al., 1993) and the Barratt Impulsivity Scale (BIS) (Patton et al., 1995) to assess individual personality characteristics. BIS is a measure of attentional and motor impulsivity and reflects the inability to plan ahead. Temperament, as measured by the TCI, has been defined as a heritable and stable personality component throughout life, and may further be linked to the functional integrity of the brain's dopamine system (Cloninger et al., 1993).
2.4. Experimental paradigm
Prior to the fMRI measurement, participants performed an operant conditioning task and a training session. In the conditioning task, eight differently colored squares were presented in a shuffled mode on a monitor. By free button press subjects had to figure out which of these colors were associated with an immediate reward (bonus points) or a neutral outcome. After successful completion of this task, subjects have learned that the red and green stimuli were always rewarded.
Afterwards, subjects underwent fMRI while performing the DRD paradigm (event-related design). The superordinate task goal of a sequence of four to eight trials was indicated by a cue showing the two target colors at the beginning of each task block. Subjects were encouraged to collect all target stimuli to reach 50 points at the end of a block. Additionally, in the ‘desire context’ (DC), subjects were allowed to select the previously conditioned reward stimuli for immediate bonus points which were added to the total amount. In the ‘reason context’ (RC), however, subjects had to reject the reward to achieve the superordinate task goal. Otherwise they lost the collected points. The context changed after every second block and was indicated by a cue. For more information regarding task timing see Supplementary Information (SI) and Fig. S1.
2.5. Analyses of behavioral data, personality measures, adversity and resilience scores
All data were analyzed using the software package SPSS (IBM SPSS statistics 25.0). Normal distribution was tested using the Kolmogorov-Smirnov test. Chi-squared tests were used to determine differences between groups regarding gender and handedness. Independent sample t-tests were used to analyze differences between groups according to age and education level. To account for possible interaction effects, ANOVAs were performed for personality measures, performance and reaction time data using adversity load and resilience as between-subject factor. Differences between groups were assessed with independent t-tests or Mann-Whitney U tests, Bonferroni corrected for multiple comparisons.
2.6. Imaging acquisition and analyses
The experiment was performed on a 3 T MRI scanner (Magnetom TRIO, Siemens Healthcare, Erlangen, Germany). Functional images were acquired using a T2*-sensitive echo planar imaging (EPI) sequence (31 slices; voxel size 3 × 3 × 3 mm3; gap 20%; TR 1900 ms; TE 30 ms; flip angle 70°, FOV 192 mm) parallel to the anterior-posterior commissure plane in ascending direction. A total of 370 image volumes were acquired over the course of two functional sessions.
The fMRI data were preprocessed and analyzed with SPM12 (Wellcome Department of Cognitive Neurology, University College London, London, UK). To avoid motion artifacts a cutoff threshold of 3 mm was set over the entire paradigm for the three planes of translation and 3° for the three planes of rotation. All subjects displaying movements above the cutoff threshold were excluded from the final analysis. Preprocessing included realignment and unwarping, corrections for slice-time acquisition differences and low-frequency fluctuations, normalization into standard stereotactic space [skull-stripped EPI template by the Montreal Neurological Institute (MNI)], resampling to 2 × 2 × 2 mm3 and spatial smoothing with an isotropic Gaussian kernel filter of 6 mm full-width at half-maximum isotropic Gaussian kernel. Statistical analyses used a general linear model (GLM). The following 11 onset regressors went into the GLM: three cues, block feedbacks for goal completion or failure, and goal-relevant targets, neutral non-targets, conditioned reward non-targets both for the DC and RC. A vector representing the temporal onset of stimulus presentation was convolved with a canonical hemodynamic response function to produce a predicted hemodynamic response to each experimental condition. All statistical analyses of the single-subject data included a high-pass filter with a 128-s cut off and an autoregressive AR(1) model to account for serial correlations in fMRI time series. Linear t contrasts were defined for assessing the specific effects elicited by the conditioned reward stimuli presented in the DC, where it was allowed to collect the stimuli, and in the RC, where subjects had to refrain from collecting them. Single-subject contrast images were taken to the second level to assess group effects with random-effects analyses. A three-way ANOVA was used with the factors ‘adversity’ (low, high), ‘resilience’ (low, high) and ‘experimental context’ (DC, RC) to determine both the associated main effects and significant interactions between factors. Age and gender were embedded as covariates of no interest. Post-hoc t-tests were used to test for specific differences between subjects with different adversity load and resilience traits in brain activation modulated by the ‘desire-reason-dilemma’. Statistical effects were determined at a search criterion of p < .005, uncorrected, with a minimum cluster size of 10 voxels. Corrections for multiple comparisons were performed using family-wise error (FWE) at p < .05 for the whole brain. For brain regions with a priori hypotheses i.e., for the VS, VTA and HP small volume corrections (pSVC) were used (VS: ±12 12–4; VTA: ±8–16 –16, ±12–18 –20; 6 mm sphere, coordinates taken from Diekhof and Gruber, 2010; Bunzeck and Düzel, 2006; HP: ±34–17 –15, 8 mm sphere, taken from Krebs et al., 2011). Furthermore, we calculated FWE-adjusted p-values (p < .017) to account for multiple parallel tests between different regions of interest.
2.7. PPI analyses
The functional interaction between key regions of the reward circuitry was assessed to reveal the impact of adversity and resilience on the mesocorticolimbic system by performing psychophysiological interaction (PPI) analyses (Friston et al., 1997). As seed regions, individual BOLD signal time courses were extracted from first eigenvariate time series (VOI; sphere of 6 mm) of the local activation maxima within the VS (12 4–4; −14 10–2) which were the second-level local activation maxima in response to the reward stimuli (see Table 2). We conducted PPI analyses during reward acceptance in the desire context (DC) and during the rejection of conditioned rewarding stimuli in the desire-reason-dilemma. Furthermore, to examine functional interactions between VTA, HP and other brain regions, VOIs of the second-level local activation maxima within the VTA (8–26 −14; −10 −20 −8) and HP (36–18 −14; −36 −22 −12) in response to the reward stimuli were extracted (Table 2). Using Matlab and SPM12, the hemodynamic signals were first deconvolved using a parametric empirical Bayesian formulation and mean-corrected. Subsequently, the PPI term was built separately for the seed regions by multiplying the deconvolved and mean-corrected BOLD signal with the psychological vector. After convolution with the hemodynamic response function, mean correction, and orthogonalization, the three regressors (PPI term, physiological vector, and psychological vector) went into the statistical analysis to determine context-dependent changes of functional connectivity over and above any main effect of task or any main effect of activity in the corresponding brain areas. In the PPI contrasts, the PPI term was computed against implicit baseline. Random-effect analyses were performed on single-subject PPI contrast images with a statistical search criterion of p < .005, uncorrected with a minimum cluster size of 10 voxels. To correct for multiple comparisons family-wise error (FWE) at p < .05 for whole brain was applied. We also used small-volume correction (pSVC) for brain regions with a priori hypotheses i.e., for the VS and VTA (VS: ±12 12–4; VTA: ±8–16 –16, ±12–18 −20; 6 mm sphere, coordinates taken from Diekhof and Gruber 2010; Bunzeck and Düzel, 2006) as well as hippocampus (HP: ±34–17 –15, 8 mm sphere, taken from Krebs et al., 2011). Again, FWE-adjusted p-values (p < .017) were calculated to account for multiple testing between different regions of interest.
Table 2.
Main and interaction effects of experimental context, adversity and resilience in the ventral striatum, ventral tegmental area and hippocampus in the three-way ANOVA.
| Brain region | MNI coordinates (F-value) |
|---|---|
| Main effect of experimental context | |
| R VS | 10 12–2 (35.66)⁎ |
| L VS | –8 8–2 (22.62)⁎⁎ |
| R VTA | 4–20 –18 (11.85) |
| L VTA | –4–20–14 (26.43)⁎ |
| Main effect of adversity | |
| L VS | −10 6 0 (10.24) |
| L VTA | −6 –20 –16 (11.08) |
| R hippocampus | 40–16–12 (13.99)⁎⁎ |
| Main effect of resilience | |
| R VS | 8 8–6 (15.83)⁎⁎ |
| R VTA | 12–22 –14 (10.05) |
| R hippocampus | 30–20 –14 (10.49) |
| Interaction between adversity and resilience | |
| R VS | 14 8–8 (11.68) |
| R VTA | 14–20 –18 (16.63)⁎⁎ |
| L VTA | −12 –14 –14 (11.09) |
| R hippocampus | 36–18 –20 (17.81)⁎⁎ |
| L hippocampus | −34 –12 − 14 (15.85)⁎⁎ |
Abbreviations: L, left; n.s., not significant; R, right; VS, ventral striatum; VTA, ventral tegmental area.
Activations are reported at p < .005, uncorrected with a minimum cluster size of 10 voxels; ⁎p < .05, FWE-corrected (whole brain); ⁎⁎p < .017 FWE-corrected for small volume and adjusted for multiple parallel testing.
3. Results
3.1. Effect of adversity and resilience on behavioral data and personality measures
Groups did not differ on gender, age, handedness and years of education (see Table 1 for statistical details). Analyses of performance and reaction time data as well as temperament and character traits (TCI) revealed no significant main effect of adversity load and resilience. Attentional impulsivity measures (BIS), however, showed a main effect of resilience (F = 10.33, p = .002). Post hoc t-tests demonstrated significantly higher attentional impulsivity traits in the highA-lowR group when compared to the lowA-highR group (U = 177.50, p < .0005) and when compared to the highA-highR group (U = 126.00, p = .002).
Table 1.
Demographic, personality and behavioral data of the sample.
| Low adversity |
High adversity |
lowA-lowR vs. lowA-highR |
lowA-lowR vs. highA-lowR |
lowA-lowR vs. highA-highR |
lowA-highR vs. highA-lowR |
lowA-highR vs. highA-highR |
highA-lowR vs. highA-highR |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Low resilience (n = 20) |
High resilience (n = 29) |
Low resilience (n = 29) |
High resilience (n = 19) |
|||||||
| Mean values ± standard deviation | p-value (2-tailed) | |||||||||
| Demographic data | ||||||||||
| Gender | 13 F, 7 M | 19 F, 10 M | 18 F, 11 M | 15 F, 4 M | .97a | .83a | .33a | .79a | .32a | .22a |
| Age at time of fMRI scan (in years) | 22.75 ± 2.24 | 24.24 ± 2.01 | 24.31 ± 2.82 | 23.37 ± 2.65 | 0.02 | 0.05 | 0.44 | 0.82 | 0.24 | 0.29 |
| Handedness | 20 R, 0 L | 29 R, 1 L | 29 R, 1 L | 19 R, 0 L | .40a | .40a | 1.0a | 1.0a | .41a | .41a |
| Education (in years) | 15.85 ± 1.68 | 16.90 ± 1.52 | 16.62 ± 1.45 | 15.95 ± 1.47 | 0.03 | 0.10 | 0.85 | 0.59 | 0.06 | 0.16 |
| CTQ | 27.45 ± 1.90 | 27.66 ± 2.00 | 35.83 ± 4.77 | 37.42 ± 8.66 | .65b | <.0005b | <.0005b | <.0005b | <.0005b | .94b |
| THQ | 1.70 ± 1.22 | 2.10 ± 1.45 | 2.72 ± 2.30 | 3.37 ± 2.45 | 0.31 | 0.05 | 0.01 | 0.22 | 0.05 | 0.36 |
| Summarized score | 29.15 ± 1.76 | 29.76 ± 1.92 | 38.55 ± 5.01 | 40.79 ± 8.40 | .23b | <.0005b | <.0005b | <.0005b | <.0005b | .74b |
| RS | 115.20 ± 26.77 | 148.28 ± 9.43 | 120.41 ± 20.71 | 147.32 ± 8.32 | <.0005b | 0.18 | <.0005b | <.0005b | 0.74 | <.0005b |
| TCI | ||||||||||
| Novelty Seeking | 22.10 ± 5.64 | 21.25 ± 5.62 | 21.43 ± 6.90 | 21.00 ± 6.90 | 0.61 | 0.72 | 0.59 | 0.92 | 0.89 | 0.84 |
| Harm Avoidance | 13.15 ± 4.27 | 11.96 ± 6.53 | 16.07 ± 6.08 | 12.53 ± 5.93 | 0.45 | 0.07 | 0.71 | 0.02 | 0.77 | 0.05 |
| Reward dependence | 16.85 ± 2.98 | 17.11 ± 3.00 | 16.32 ± 3.96 | 16.74 ± 2.62 | 0.77 | 0.62 | 0.90 | 0.41 | 0.66 | 0.69 |
| Persistence | 4.05 ± 2.06 | 4.39 ± 2.38 | 4.75 ± 1.97 | 5.68 ± 2.16 | 0.61 | 0.24 | 0.02 | 0.54 | 0.07 | 0.13 |
| BIS | ||||||||||
| Attentional impulsivity | 16.15 ± 3.47 | 15.38 ± 2.29 | 18.61 ± 3.18 | 15.37 ± 3.17 | .48b | .01b | 0.38 | <.0005b | .87b | .002b |
| Motor-impulsivity | 21.80 ± 4.15 | 21.76 ± 3.25 | 22.57 ± 3.78 | 21.74 ± 3.51 | 0.97 | 0.51 | 0.96 | 0.39 | 0.95 | 0.45 |
| Non-planning impulsivity | 24.40 ± 4.27 | 22.83 ± 3.82 | 25.04 ± 5.51 | 22.63 ± 5.31 | 0.18 | 0.67 | 0.26 | 0.08 | 0.99 | 0.14 |
| Total | 62.35 ± 9.16 | 59.97 ± 6.69 | 66.21 ± 10.78 | 59.74 ± 10.12 | 0.30 | 0.20 | 0.40 | 0.01 | 0.93 | 0.04 |
| Performance and RT | ||||||||||
| Goal failure (absolute value) | 5.20 ± 3.30 | 7.48 ± 4.11 | 6.55 ± 3.36 | 7.00 ± 3.18 | 0.05 | 0.17 | 0.09 | 0.35 | 0.67 | 0.18 |
| RT (in ms) | 488.90 ± 55.62 | 507.42 ± 41.38 | 489.87 ± 34.59 | 479.54 ± 41.84 | 0.19 | 0.94 | 0.56 | 0.09 | 0.03 | 0.36 |
| Desire context | ||||||||||
| Correct acceptance of reward stimuli (in %) | 90.62 ± 12.07 | 92.51 ± 7.07 | 92.15 ± 6.59 | 90.41 ± 8.20 | 0.50 | 0.55 | 0.96 | 0.88 | 0.36 | 0.41 |
| Reason context | ||||||||||
| Correct rejection of reward stimuli (in %) | 97.50 ± 4.41 | 96.34 ± 4.21 | 97.08 ± 3.53 | 96.70 ± 2.78 | 0.36 | 0.64 | 0.52 | 0.55 | 0.72 | 0.82 |
Abbreviations: BIS, Barratt Impulsiveness Scale; CTQ, Childhood Trauma Questionnaire; F, Female; highA, high adversity; highR, high resilience; L, left; lowA, low adversity; lowR, low resilience; M, Male; R, right; RS, Resilience Scale; RT, reaction times; TCI, Temperament and Character Inventory; THQ, Trauma History Questionnaire.
p-values derived from t-tests between independent groups; Figures in bold indicate p < .008 Bonferroni-corrected to account for multiple testing.
Chi-squared tests were performed for gender and handedness between groups.
Mann-Whitney-U tests were used for data violating normal distribution.
3.2. Functional neuroimaging data
3.2.1. Main and interaction effects of experimental context, adversity and resilience
First, we demonstrated that activation in a priori brain regions of the mesolimbic system, namely the VS and VTA was significantly modulated by experimental context (Table 2), replicating previous findings (Diekhof and Gruber, 2010; Diekhof et al., 2012; Richter et al., 2015).
Second, we examined whether reward-related activation in these brain regions may be modulated by adversity load (low adversity versus high adversity). Indeed, we found that activation in the VS, VTA and HP was significantly modulated by adversity.
Third, we tested whether activation in brain regions of the mesolimbic dopamine system may vary with the factor resilience. Again, activation in the VS, VTA and HP was significantly modulated by resilience traits in the present sample of 97 subjects.
Moreover, we found a significant interaction between adversity and resilience in the activation of the VS, VTA and HP in the three-way ANOVA (Table 2). The direction of these main and interaction effects was subsequently determined by post-hoc t-contrasts and are reported in the following subsections.
3.2.2. Effect of adversity on reward processing in the desire and reason context
A significant effect of adversity exposure was revealed when it was allowed to collect the previously conditioned reward stimuli in the DC, and when it was not allowed in the RC. Subjects with low adversity load showed significantly increased activation in the VS and VTA during reward acceptance (DC, Table 3), which is well in line with previous findings of healthy subjects (Diekhof and Gruber, 2010; Diekhof et al., 2012).
Table 3.
Brain activation in a priori regions of interest during reward acceptance in the desire context (DC).
| Brain region | Low adversity |
High adversity |
||
|---|---|---|---|---|
| Low resilience | High resilience | Low resilience | High resilience | |
| MNI coordinates (t-value) | ||||
| R VS | 12 4–4 (5.55)⁎ | 12 8 0 (4.42)⁎⁎ | n.s. | n.s. |
| L VS | –14 10–2 (5.12)⁎ | −12 8 0 (4.83)⁎⁎ | n.s. | n.s. |
| R VTA | 6–24 –12 (5.31)⁎ | 8–26 –14 (7.75)⁎ | 6–24 –12 (3.70)⁎⁎ | 8–26 –14 (5.14)⁎ |
| L VTA | −6 –20 –14 (7.05)⁎ | −4 –22 –14 (8.18)⁎ | −8 –22–18 (3.60)⁎⁎ | −10 –20 –8 (5.64)⁎ |
| R HP | –– | 36–18 –18 (3.40)⁎⁎ | n.s. | –– |
| L HP | −34 –16 –14 (3.45)⁎⁎ | −32 –16 –10 (2.77) | –– | −36 –22 –12 (2.62) |
| a) Group comparison – effect of adversity | ||||
| highA-lowR < lowA-lowR | highA-lowR > lowA-lowR | highA-highR < lowA-highR | highA-highR > lowA-highR | |
| R VS | 12 14–2 (3.49)⁎⁎ | n.s. | 12 14–4 (2.98)⁎⁎ | n.s. |
| L VS | −10 12–2 (2.71) | n.s. | −10 12 0 (2.91) | n.s. |
| L VTA | −6 –18 –14 (3.31)⁎⁎ | n.s. | −4 –20 –14 (3.20)⁎⁎ | n.s. |
| R HP | n.s. | n.s. | 24–38 2 (2.54) | n.s. |
| b) Group comparison – effect of resilience | ||||
| lowA-highR < lowA-lowR | lowA-highR > lowA-lowR | highA-highR < highA-lowR | highA-highR > highA-lowR | |
| R VS | 10 6–6 (4.00)⁎⁎ | n.s. | 12 10–8 (2.81) | n.s. |
| L VS | n.s. | n.s. | n.s. | n.s. |
| R VTA | n.s. | 14–20 –16 (3.31)⁎⁎ | n.s. | 14–22–18 (3.45)⁎⁎ |
| L VTA | n.s. | n.s. | n.s. | n.s. |
| R HP | n.s. | 36–18 –20 (3.84)⁎⁎ | n.s. | 38–10 –24 (2.86) |
Abbreviations: highA, high adversity; highR, high resilience; HP, hippocampus; L, left; lowA, low adversity; lowR, low resilience; n.s., not significant; R, right; VS, ventral striatum; VTA, ventral tegmental area.
Activations are reported at p < .005, uncorrected, with a minimum cluster size of 10 voxels; ⁎p < .05, FWE-corrected (whole brain); ⁎⁎p < .017 FWE-corrected for small volume and adjusted for multiple parallel testing.
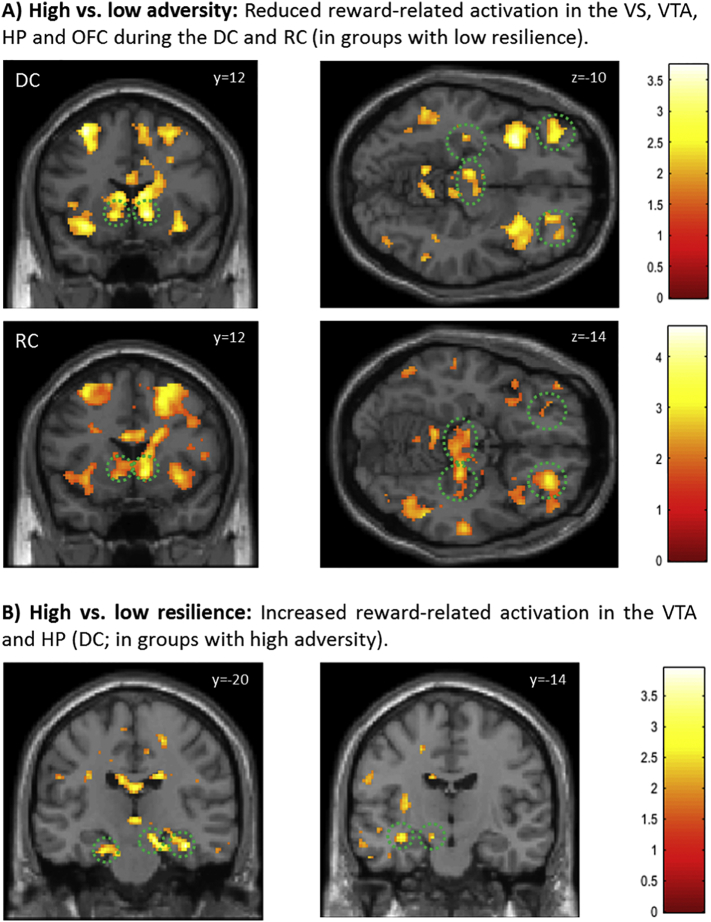
However, comparison of subjects with high adversity level to those with low adversity revealed significantly reduced activation in the VS, VTA, HP and orbitofrontal cortex (OFC), among other brain regions, in response to the reward stimuli when it was allowed to collect them (DC) (Fig. 1A; Table 3a, Supplementary Table S1 and S2), and when subjects had to reject them (RC) (Table S3 and S4), suggesting an impaired bottom-up mechanism in subjects with high adversity experiences. The decrease in activation with increasing adversity load has been observed when both groups showed the same resilience level (low and high).
Fig. 1.
Reward-related activations modulated by adversity load and resilience traits.
(A) Subjects with high adversity load as compared to subjects with low adversity load showed a reduced bottom-up responsiveness of the VS, VTA, HP and OFC (green circles) to the conditioned reward stimuli during the DC and RC. (B) Subjects with high resilience traits as compared to those with low resilience displayed an increased functional activation in the VTA and HP when exposed to adversity. Activation was thresholded at p < .05, uncorrected for illustration purposes. T-values are indicated by color bars. Regions listed in Table 3 and Supplementary Information.
3.2.3. Effect of resilience on reward processing in individuals with adversity exposure
In subjects with high adversity level and high resilience traits compared to those with low resilience we found decreased activation in the right VS. Otherwise, they showed a significantly increased activation within the right VTA and HP in the DC (Fig. 1B; Table 3b). In addition, the insula was also significantly activated in resilient subjects (Table S1, S2). For more information, see SI.
3.3. PPI data
3.3.1. Effect of adversity on functional connectivity within the mesocorticolimbic system
During reward acceptance in the DC, PPI analyses revealed a positive functional coupling between VS and VTA in the low adversity-low resilience group (R VS: −2 −18 −12, t = 2.30; L VS: −4 −10 −8, t = 2.27) and a negative functional interaction in the high adversity-low resilience-group (R VS: −14 −14 −16, t = 2.56; L VS: 4 -14 -16, t=3.10, pSVC; −6 −12 −10, t = 3.02, pSVC). Furthermore, comparison of groups with high adversity level to those with low adversity while low resilience highlighted an increased negative functional coupling between VS-VTA and VS-avPFC, confirming an impaired bottom-up mechanism with increasing adversity load (Table 4a).
Table 4.
Psychophysiological interactions of the bilateral VS in the desire context (DC) and during the desire-reason dilemma (DRD) (a, b), and of the bilateral VTA and hippocampus in the DC (c, d).
|
a) Influence of adversity during reward acceptance in the desire context (DC): Increased negative functional coupling between VS-VTA and VS-avPFC in the high adversity group | ||||
|---|---|---|---|---|
| Brain region | Seed area: R VS (12 4–4) |
Seed area: L VS (−14 10–2) |
||
| highA-lowR < lowA-lowR | highA-lowR > lowA-lowR | highA-lowR < lowA-lowR | highA-lowR > lowA-lowR | |
| MNI coordinates (t-value) | ||||
| L VTA | −12 −14 −16 (2.41)+ | n.s. | −4 −10 −10 (3.25) | n.s. |
| R avPFC | 14 52 16 (2.82) | n.s. | 8 66 18 (2.41)+ | n.s. |
| L avPFC | −18 50 10 (3.32) | n.s. | n.s. | n.s. |
| b) Influence of adversity during reward rejection in the ‘desire-reason dilemma’ (DRD): Impaired top-down control expressed by reduced negative VS-avPFC-coupling in the high adversity group | ||||
| Seed area: R VS (12 4–4) | Seed area: L VS (−14 10–2) | |||
| highA-lowR < lowA-lowR | highA-lowR > lowA-lowR | highA-lowR < lowA-lowR | highA-lowR > lowA-lowR | |
| R OFC | n.s. | 38 40–10 (3.45)⁎ | n.s. | 42 44–8 (3.28)⁎ |
| R avPFC | n.s. | 16 52 16 (3.13) | n.s. | 18 54 12 (2.93) |
| L avPFC | n.s. | −18 54 10 (3.09) | n.s. | −22 64 14 (4.23) |
| c) Influence of adversity and resilience during reward acceptance in the desire context (DC): Less negative VTA- VS -coupling in the high adversity and high resilient group | ||||
| Seed area: R VTA (8–26 –14) | Seed area: L VTA (−10–20 −8) | |||
| highA-highR < highA-lowR | highA-highR > highA-lowR | highA-highR < highA-lowR | highA-highR > highA-lowR | |
| L VS | n.s. | −12 16 2 (2.39)+ | n.s. | −16 4–6 (2.31)+ |
| d) Influence of adversity and resilience during reward acceptance in the desire context (DC): Less negative hippocampus-VS-coupling and hippocampus-VTA-coupling in the high adversity and high resilient group | ||||
| Seed area: R HP (36–18 −14) | Seed area: L HP (−36–22 –12) | |||
| highA-highR < highA-lowR | highA-highR > highA-lowR | highA-highR < highA-lowR | highA-highR > highA-lowR | |
| L VS | n.s. | −14 6–4 (2.26)+ | n.s. | n.s. |
| R VTA | n.s. | 4–16 –16 (3.00)⁎⁎ | n.s. | n.s. |
| L VTA | n.s. | n.s. | n.s. | −8 −16 −14 (2.73) |
Abbreviations: avPFC, anteroventral prefrontal cortex; highA, high adversity; highR, high resilience; L, left; HP, hippocampus; lowA, low adversity; lowR, low resilience; n.s., not significant; OFC, orbitofrontal cortex; R, right; VS, ventral striatum; VTA, ventral tegmental area.
Activations are reported at p < .005, uncorrected, with a minimum cluster size of 10 voxels; +p < .05, uncorrected, with a minimum cluster size of 10 voxels; ⁎p < .05, FWE-corrected (whole brain); ⁎⁎p < .017 FWE-corrected for small volume and adjusted for multiple parallel testing.
During the desire-reason-dilemma, we found a negative functional interaction between VS and avPFC in the low adversity group (R VS: 16 52 16, t = 3.93; −26 52 0 (2.65); L VS: 18 54 14, t = 2.35; −26 52 6, t = 2.56), replicating previous findings of healthy subjects (Diekhof and Gruber, 2010). The high adversity group, however, showed a positive functional interaction between the VS and avPFC/OFC (R VS: 2 56–2, t = 3.42; 38 40–10, t = 3.85, pSVC; −18 54 10, t = 3.18; 42 40–2, t = 3.19, pSVC; L VS: 18 62 16, t = 3.15; −22 64 14, t = 5.13, pSVC). Indeed, group comparisons demonstrated an impaired top-down control expressed by an impaired VS-avPFC-coupling in the high adversity group (Table 4b).
3.3.2. Effect of resilience on functional connectivity within the mesocorticolimbic system in individuals with adversity exposure
During reward acceptance in the DC, using the VTA as seed region analyses revealed a negative functional interaction between VTA and VS in both the highA-lowR group (R VTA: 16 14 0, t = 3.25, pSVC; −14 16 0, t = 3.51, pSVC; L VTA: 16 10–2, t = 2.12; −16 14–6, t = 3.17, pSVC) and the highA-highR group (R VTA: 16 8–4, t = 2.64; −16 10–6, t = 3.16, pSVC; L VTA: 12 12 2, t = 3.05, pSVC; −10 22–2, t = 2.43).
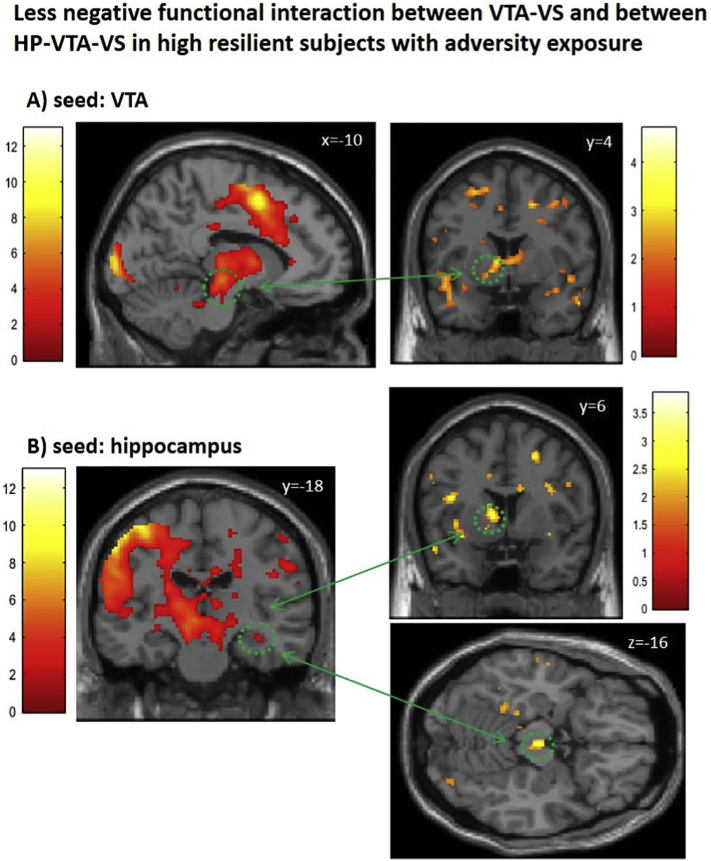
Using the HP as seed region, PPI analyses demonstrated a negative functional coupling between HP and VS as well as HP and VTA in the highA-lowR group (R HP: VS –12 12 2, t = 3.40, pSVC / VTA 2–16 –16, t = 3.48, pSVC; L HP: −6 –18 –10, t = 3.50, pSVC) and a negative functional coupling between HP and VS but a positive coupling between HP and VTA in the highA-highR group (R HP: VS –10 14–2, t = 2.34 / VTA 4–20 –14, t = 2.49; L HP: −2 –24–22, t = 5.11). Furthermore, in groups with high adversity, the high resilient group when compared to the low resilient group showed a less negative VTA-VS-coupling (Fig. 2A; Table 4c) and a less negative HP-VTA and HP-VS-coupling (Fig. 2B; Table 4d), confirming a protective mechanism mediated by the VTA and HP.
Fig. 2.
Functional connectivity modulated by adversity load and resilience traits.
Subjects with high adversity and high resilience traits showed a less negative functional interaction between (A) the VTA and VS, and (B) HP, VTA and VS, respectively, during reward acceptance. Activation was thresholded at p < .05, uncorrected for illustration purposes. T-values are indicated by color bars. Regions listed in Table 4.
4. Discussion
The present fMRI study examined the long-term impact of childhood and young adulthood adversity as well as its interaction with individual resilience traits on neural alterations of the mesolimbic system into adulthood. The findings provided evidence that childhood and young adulthood adversity had shared consequences on the mesocorticolimbic dopamine system by demonstrating that activity in the VS, VTA, HP and PFC decreased with the level of adversity load during reward acceptance. Stress exposure was further associated with impaired functional connectivity between VS, VTA and avPFC. Remarkably, high resilience in subjects with high adversity experiences was accompanied by an increased activation in the VTA and HP, and improved functional interaction within the mesocorticolimbic system.
Here we demonstrate that experienced childhood and young adulthood adversity before the age of 30 had synergistic effects on neural activity and connectivity in the mesocorticolimbic system. This extends previous findings showing a combined effect of childhood and adult trauma on clinical outcome in impoverished women (Gillespie et al., 2009). The observed hyporesponsivity of VS and VTA in subjects with high adversity in childhood and young adulthood is in accordance with previous studies investigating the impact of childhood adversity on reward anticipation (Dillon et al., 2009; Boecker et al., 2014; Boecker-Schlier et al., 2016). We extend these findings by showing this neural pattern of blunted activity in response to conditioned reward stimuli when it was allowed (DC) and when it was not allowed to collect them (RC), indicating an impaired bottom-up mechanism in subjects with high adversity load. Recent findings demonstrated that dopamine neurons in the VTA respond to chronic stress by attenuating activity in this brain region (Chang and Grace, 2014). The VTA has a multitude of afferent connections to brain regions involved in reward processing and in regulating the stress response, such as the VS, HP, and PFC, among others (Ulrich-Lai and Herman, 2009; Haber and Knutson, 2010). All these brain regions in this corticosubcortical network exhibited a reduced activation in response to stress and adversity. Moreover, PPI analyses highlighted a more negative functional coupling between VS and VTA, as well as between VS and avPFC during reward acceptance, confirming an impaired bottom-up mechanism with increasing maltreatment. Previous studies applying the DRD paradigm provided evidence for a negative functional coupling between VS and avPFC in healthy subjects during reward rejection (Diekhof and Gruber, 2010; Diekhof et al., 2012). The findings presented above, however, revealed a reduced negative VS-avPFC-coupling in subjects with high adversity load when compared to those with low during reward rejection, indicating not only a disturbed bottom-up mechanism but also an impaired top-down control of the mesolimbic system by prefrontal brain regions. Using the DRD paradigm, pathophysiological changes in the functional activity and connectivity within the dopaminergic reward circuitry have previously been proven in schizophrenia (Richter et al., 2015), bipolar disorder (Trost et al., 2014) and depression (Goya-Maldonado et al., 2015). Here we demonstrate that even in healthy subjects without psychopathology exposure to stress in childhood and young adulthood is associated with altered neural mechanisms underlying reward processing.
Furthermore, we showed that subjects with high adversity load but also high resilience traits displayed reduced reward-related activation in the VS, and increased activation in the VTA and HP. The association between resilience and increased VTA/HP responsivity under stress could be interpreted as a mechanism of adaptive neuroplasticity in the midbrain and HP (Xin et al., 2016), characterizing resilience. Furthermore, high resilience in subjects with high adversity experiences was associated with less negative functional interactions between VTA, VS and HP, suggesting a compensatory or protective mechanism counteracting the effects of adversity. In line with this, reduced resting-state connectivity between VTA and HP in adolescents exposed to early threat has been shown in a previous fMRI study (Marusak et al., 2017).
Specifically, the stress and reward system show considerable overlap on both the structural and functional level (Ulrich-Lai and Herman, 2009). Exposure to stress stimulates the HPA axis leading to cortisol release. In turn, HPA axis activity is influenced by limbic regions such as the HP (Ulrich-Lai and Herman, 2009). HP stimulation decreases HPA axis activity (Rubin et al., 1966; Dunn and Orr, 1984), whereas HP damage increases cortisol release (Herman et al., 2003). The ventral subiculum (vSub) of the HP controls burst firing of dopamine neurons in the VTA (Grace, 2010). By carrying context-related information, the vSub adjust sresponsivity of the dopaminergic system based on the needs of the individual and the environment. In turn, dopaminergic projections from the VTA to the HP enhance long-term potentiation and long-term memory (Lisman and Grace, 2005). To successfully cope with trauma, increased VTA and hippocampal responsivity in resilient subjects may help effective behavioral and emotional regulation of stress later in life. In addition, the VTA-hippocampal loop is thought to be critical for the modulation of memory by behavioral significance and novel information. Increased activation and connectivity of the VTA and HP may reflect entry of new and possibly positive information into long-term memory, leading to experience-dependent modifications of previous stress-related memories. As adversity caused a reduced drive, presumably dopaminergic, within the mesocorticolimbic system in this study, the HP and VTA may represent effective targets for therapeutic interventions aiming to increase hippocampal-dependent contextual learning in individuals at psychiatric risk, such as certain forms of psychotherapy or pharmacological regulation of dopamine. It has been shown previously that HP activation in individuals with childhood trauma was dependent on a catechol-O-methyltransferase (COMT) gene variation, in which Val/Val subjects may develop better mechanisms to cope with high levels of early maltreatment (Van Rooij et al., 2016). Furthermore, activation within the salience network was highlighted as important neural correlate underlying resilience in healthy young adults (Kong et al., 2015). By examining neural mechanisms of emotional characteristics of trait resilience, one study found insula activation only in response to aversive pictures in high resilient individuals, reflecting flexible adaptation of emotional resources to meet the demands of a situation (Waugh et al., 2008). Indeed, we also detected increased activation in the anterior insular cortex in subjects with high resilience traits despite maltreatment, confirming the critical role of the insula in psychological resilience.
In addition, we found elevated attentional impulsivity scores in subjects with low resilience traits and high adversity load when compared to those with high resilience and different adversity level. This finding supports the fMRI results of impaired top-down control mechanisms expressed by enhanced attentional impulsivity in low resilient subjects exposed to stress. In turn, high resilient individuals irrespective of trauma load may have developed flexible cognitive strategies in regulating the subject's affective state depending on recruitment of prefrontal brain regions.
Several limitations have to be considered in the interpretation of the present findings. First, by following a more naturalistic approach where trauma is multifaceted and complex, we demonstrate that childhood and young adulthood trauma have synergistic effects on the reward circuitry and interact with resilience processes in the same neural pathway. However, it is important to note that we cannot make any assumptions about distinct effects on neural systems. Even though exposure to multiple traumatic events across the lifespan is relatively common and trauma rarely occurs in isolation (Thomason and Marusak, 2017). In addition, it is assumable that interaction between resilience and adversity might differ depending on whether adversity occurred in childhood or in young adulthood. One solution may be to narrow subject selection criteria to reduce variation in these characteristics, requiring larger sample sizes within a longitudinal context. Thus, the present findings warrant confirmation in larger longitudinal neuroimaging studies systematically investigating and comparing the neural underpinnings of both individual and combined effects of these measures. Second, retrospective self-reports of childhood and lifetime experiences are not completely accurate and influenced by factors such as forgetting, repression or reporting biases. However, the lack of validity of retrospective self-reports may lead to underestimation of the actual occurrence rather than overestimation (Hardt and Rutter, 2004). Additionally, self-reports of resilience might also be biased by these factors, though the measure chosen has been shown to have good validity and reliability (Wagnild, 1993). Third, current research has emphasized striking variability in the effects of early and later life stress among humans. The interplay between environmental and genetic factors affecting reward processing may further contribute to a better understanding of the underlying mechanisms. Fourth, all participants involved in this study were healthy and without DSM-IV-classified psychiatric disorder. Advantage of this approach is that effects do not reflect consequences of psychopathology. Nevertheless, it must be noted that our conclusions are limited to a mild to moderate adversity level as evidenced by the moderate average scores in both adversity groups.
5. Conclusions
Finally, we found adversity-related differences in brain function in regions involved in reward processing and stress regulation. Neurofunctional changes in these brain networks play an important role in the development and pathophysiology of mental disorders. Yet potential factors that might explain outcome variability or resilience to the effects of childhood and young adulthood stress in humans are poorly understood. Here we found protective resilience-related neural mechanisms mediated by the VTA and HP. The findings suggest that interventions targeting motivation and contextual learning may be useful for reducing the negative consequences of childhood and young adulthood adversity. The reported findings may assist in identification of individuals at greatest risk of adverse functioning after trauma exposure who hold a higher risk of psychiatric disturbance and help to deliver resilience-enhancing interventions in time.
Disclosure
The authors report no biomedical relationships with financial or commercial interests and identify no potential conflicts of interest.
Acknowledgements
We acknowledge Maria Keil, Peter Dechent and the staff of the unit “MR-Research in Neurology and Psychiatry” at the University Medical Center Göttingen (Germany) for their valuable work with MRI data acquisition. We thank Julia Hasenkamp for help with contacting all participants again, and Till Düsberg for assistance in data handling of the online survey. We are very grateful to our participants.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.101920.
Appendix A. Supplementary data
Supplementary material
References
- Aas M., Kauppi K., Brandt C.L., Tesli M., Kaufmann T., Steen N.E. Childhood trauma is associated with increased brain responses to emotionally negative as compared with positive faces in patients with psychotic disorders. Psychol. Med. 2017;47(4):669–679. doi: 10.1017/S0033291716002762. [DOI] [PubMed] [Google Scholar]
- Abercrombie E.D., Keefe K.A., DiFrischia D.S., Zigmond M.J. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial prefrontal cortex. J. Neurochem. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Boecker R., Holz N.E., Buchmann A.F., Blomeyer D., Plichta M.M., Wolf I., Baumeister S., Meyer-Lindenberg A., Banaschewski T., Brandeis D., Laucht M. Impact of early life adversity on reward processing in young adults: EEG-fMRI results from a prospective study over 25 years. PLoS One. 2014;9(8) doi: 10.1371/journal.pone.0104185. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boecker-Schlier R., Holz N.E., Buchmann A.F., Blomeyer D., Plichta M.M., Jennen-Steinmetz C., Wolf I., Baumeister S., Treutlein J., Rietschel M., Meyer-Lindenberg A., Banaschewski T., Brandeis D., Laucht M. Interaction between COMT Val158Met polymorphism and childhood adversity affects reward processing in adulthood. NeuroImage. 2016;132:556–570. doi: 10.1016/j.neuroimage.2016.02.006. [DOI] [PubMed] [Google Scholar]
- Bunzeck N., Düzel E. Absolute coding of stimulus novelty in the human substantia nigra/VTA. Neuron. 2006;51:369–379. doi: 10.1016/j.neuron.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Carrión V.G., Haas B.W., Garrett A., Song S., Reiss A.L. Reduced hippocampal activity in youth with posttraumatic stress symptoms: an fMRI study. J. Pediatr. Psychol. 2010;35:559–569. doi: 10.1093/jpepsy/jsp112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Grace A.A. Amygdala-ventral pallidum pathway decreases dopamine activity after chronic mild stress in rats. Biol. Psychiatry. 2014;76(3):223–230. doi: 10.1016/j.biopsych.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman D.P., Whitfield C.L., Felitti V.J., Dube S.R., Edwards V.J., Anda R.F. Adverse childhood experiences and the risk of depressive disorders in adulthood. J. Affect. Disord. 2004;82:217–225. doi: 10.1016/j.jad.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Charney D.S. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am. J. Psychiatr. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- Cloninger C.R., Svrakic D.M., Pryzbeck T.R. A psychobiological model of temperament and character. Arch. Gen. Psychiatry. 1993;50:975–990. doi: 10.1001/archpsyc.1993.01820240059008. [DOI] [PubMed] [Google Scholar]
- Curtis W.J., Cicchetti D. Moving research on resilience into the 21st century: theoretical and methodological considerations in examining the biological contributors to resilience. Dev. Psychopathol. 2003;15:773–810. doi: 10.1017/s0954579403000373. [DOI] [PubMed] [Google Scholar]
- Diekhof E.K., Gruber O. When desire collides with reason: functional interactions between anteroventral prefrontal cortex and nucleus accumbens underlie the human ability to resist impulsive desires. J. Neurosci. 2010;30(4):1488–1493. doi: 10.1523/JNEUROSCI.4690-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof E.K., Kaps L., Falkai P., Gruber O. The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude - an activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia. 2012;50(7):1252–1266. doi: 10.1016/j.neuropsychologia.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Dillon D.G., Holmes A.J., Birk J.L., Brooks N., Lyons-Ruth K., Pizzagalli D.A. Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biol. Psychiatry. 2009;66(3):206–213. doi: 10.1016/j.biopsych.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube S.R., Anda R.F., Felitti V.J., Chapman D.P., Whilliamson D.F., Giles W.H. Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: findings from the adverse childhood experiences study. J. Am. Med. Assoc. 2001;286:3089–3096. doi: 10.1001/jama.286.24.3089. [DOI] [PubMed] [Google Scholar]
- Dunn J.D., Orr S.E. Differential plasma corticosterone responses to hippocampal stimulation. Exp. Brain Res. 1984;54:1–6. doi: 10.1007/BF00235813. [DOI] [PubMed] [Google Scholar]
- Evans G.W., Li D., Whipple S.S. Cumulative risk and child development. Psychol. Bull. 2013;139:1342–1396. doi: 10.1037/a0031808. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Buechel C., Fink G.R., Morris J., Rolls E., Dolan R.J. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Gillespie G.F., Bradley B., Mercer K., Smith A.K., Conneely K., Gapen M. Trauma exposure and stress-related disorders in inner city primary care patients. Gen. Hosp. Psychiatry. 2009;31(6):505–514. doi: 10.1016/j.genhosppsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff B., Gee D.G., Telzer E.H., Humphreys K.L., Gabard-Durnam L., Flannery J. Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience. 2013;249:129–138. doi: 10.1016/j.neuroscience.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goya-Maldonado R., Weber K., Trost S., Diekhof E.K., Keil M., Dechent P., Gruber O. Dissociating pathomechanisms of depression with fMRI: bottom-up or top-down dysfunctions of the reward system. Eur. Arch. Psychiatry Clin. Neurosci. 2015;265(1):57–66. doi: 10.1007/s00406-014-0552-2. [DOI] [PubMed] [Google Scholar]
- Grace A.A. Dopamine system dysregulation by the ventral subiculum as the common pathophysiological basis for schizophrenia psychosis, psychostimulant abuse, and stress. Neurotox. Res. 2010;18(3–4):367–376. doi: 10.1007/s12640-010-9154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S.N., Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacol. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt J., Rutter M. Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. J. Child Psychol. Psychiatry. 2004;45:260–273. doi: 10.1111/j.1469-7610.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- Heim C., Binder E.B. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp. Neurol. 2012;233:102–111. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Herman J.P., Figueiredo H., Mueller N.K., Ulrich-Lai Y., Ostrander M.M., Choi D.C., Cullinan W.E. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front. Neuroendocrinol. 2003;24(3):151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Herrman H., Stewart D.E., Diaz-Granados N., Berger E.L., Jackson B., Yuen T. What is resilience. Can. J. Psychiatr. 2011;56(5):258–265. doi: 10.1177/070674371105600504. [DOI] [PubMed] [Google Scholar]
- Hooper L.M., Stockton P., Krupnick J.L., Green B.L. Development, use, and psychometric properties of the trauma history questionnaire. J. Loss Trauma. 2011;16:258–283. [Google Scholar]
- Klinitzke G., Rompel M., Häuser W., Brähler E., Glaesmer H. The German version of the childhood trauma questionnaire (CTQ): psychometric characteristics in a representative sample of the general population. Psychother. Psychosom. Med. Psychol. 2012;62(2):47–51. doi: 10.1055/s-0031-1295495. [DOI] [PubMed] [Google Scholar]
- Kong F., Wang X., Liu J. Neural correlates of psychological resilience and their relation to life satisfaction in a sample of healthy young adults. Neuroimage. 2015;123:165–172. doi: 10.1016/j.neuroimage.2015.08.020. [DOI] [PubMed] [Google Scholar]
- Krebs R.M., Heipertz D., Schuetze H., Duzel E. Novelty increased the mesolimbic functional connectivity of the substantia nigra/ventral tegmental area (SN/VTA) during reward anticipation: evidence from high-resolution fMRI. NeuroImage. 2011;58:647–655. doi: 10.1016/j.neuroimage.2011.06.038. [DOI] [PubMed] [Google Scholar]
- Lisman J.E., Grace A.A. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Lupien S.J., McEwen B.S., Gunnar M.R., Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Martin C.G., DeMarni Cromer L., DePrince A.P., FreyD J.J. The role of cumulative trauma, betrayal, and appraisals in understanding trauma symptomatology. Psychol. Trauma. 2013;52(2):110–118. doi: 10.1037/a0025686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak H.A., Hatfield J.R.B., Thomason M.E., Rabinak C.A. Reduced ventral tegmental area-hippocampal connectivity in children and adolescents exposed to early threat. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2017;2(2):130–137. doi: 10.1016/j.bpsc.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten A.S. Resilience in children threated by extreme adversity: frameworks for research, practice, and translational synergy. Dev. Psychopathol. 2011;23(2):493–506. doi: 10.1017/S0954579411000198. [DOI] [PubMed] [Google Scholar]
- Masten A.S., Coatsworth J.D. The development of competence in favorable and unfavorable environments: lessons from research on successful children. Am. Psychol. 1998;53(2):205–220. doi: 10.1037//0003-066x.53.2.205. [DOI] [PubMed] [Google Scholar]
- Matheson S.L., Shepherd A.M., Pinchbeck R.M., Laurens K.R., Carr V.J. Childhood adversity in schizophrenia: a systematic meta-analysis. Psychol. Med. 2013;43(2):225–238. doi: 10.1017/S0033291712000785. [DOI] [PubMed] [Google Scholar]
- Pani L., Porcella A., Gessa G.L. The role of stress in the pathophysiology of the dopaminergic system. Mol. Psychiatry. 2000;5:14–21. doi: 10.1038/sj.mp.4000589. [DOI] [PubMed] [Google Scholar]
- Patton J.H., Stanford M.S., Barratt E.S. Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Richter A., Petrovic A., Diekhof E.K., Trost S., Wolter S., Gruber O. Hyperresponsivity and impaired prefrontal control of the mesolimbic reward system in schizophrenia. J. Psychiatr. Res. 2015;71:8–15. doi: 10.1016/j.jpsychires.2015.09.005. [DOI] [PubMed] [Google Scholar]
- Roy A., Hu X.Z., Janal M.N., Goldman D. Interaction between childhood trauma and serotonin transporter gene variation in suicide. Neuropsychopharmacology. 2007;32:2046–2052. doi: 10.1038/sj.npp.1301331. [DOI] [PubMed] [Google Scholar]
- Rubin R.T., Mandell A.J., Crandall P.H. Corticosteroid responses to limbic stimulation in man: localization of stimulation sites. Science. 1966;153:1212–1215. doi: 10.1126/science.153.3737.767. [DOI] [PubMed] [Google Scholar]
- Schultz W., Dayan P., Montague P.R. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Thomason M.E., Marusak H.A. Toward understanding the impact of trauma on the early developing human brain. Neuroscience. 2017;342:55–67. doi: 10.1016/j.neuroscience.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost S., Diekhof E.K., Zvonik K., Lewandowski M., Usher J., Keil M., Zilles D., Falkai P., Dechent P., Gruber O. Disturbed anterior prefrontal control of the mesolimbic reward system and increased impulsivity in bipolar disorder. Neuropsychopharmacology. 2014;39(8):1914–1923. doi: 10.1038/npp.2014.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai Y.M., Herman J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009;10(6):397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Werff S.J.A., Pannekoek J.N., Stein D.J., van der Wee N.J.A. Neuroimaging of resilience to stress: current state of affairs. Hum. Psychopharmacol. Clin. Exp. 2013;28:529–532. doi: 10.1002/hup.2336. [DOI] [PubMed] [Google Scholar]
- Van Rooij S.J.H., Stevens J.S., Ely T.D., Fani N., Smith A.K., Kerley K.A., Lori A., Ressler K.J., Jovanovic T. Childhood trauma and COMT genotype interact to increase hippocampal activation in resilient individuals. Front. Psychiatr. 2016;7(156):1–12. doi: 10.3389/fpsyt.2016.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Eisenhart Rothe A., Zenger M., Lacruz M.E., Emeny R., Baumert J., Haefner S., Ladwig K.H. Validation and development of a shorter version of the resilience scale RS-11: results from the population-based KORA-age study. BMC Psychol. 2013;1:25. doi: 10.1186/2050-7283-1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagnild G.M., Young H.M. Development and psychometric evaluation of the resilience scale. J. Nurs. Meas. 1993;1:165–178. [PubMed] [Google Scholar]
- Wald J., Taylor S., Asmundson G.J.G. Defence R&D Canada; Toronto (ON): 2006. Literature Review of Concepts: Psychological Resiliency. [Google Scholar]
- Waugh C.E., Wager T.D., Fredrickson B.L., Noll D.C., Taylor S.F. The neural correlates of trait resilience when anticipating and recovering from threat. Soc. Cogn. Affect. Neurosci. 2008;3:322–332. doi: 10.1093/scan/nsn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicks S., Hjern A., Gunnell D., Lewis G., Dalman C. Social adversity in childhood and the risk of developing psychosis: a national cohort study. Am. J. Psychiatr. 2005;162(9):1652–1657. doi: 10.1176/appi.ajp.162.9.1652. [DOI] [PubMed] [Google Scholar]
- Wise R.A. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Xin W., Edwards N., Bonci A. VTA dopamine neuron plasticity – the unusual suspects. Eur. J. Neurosci. 2016;44:2975–2983. doi: 10.1111/ejn.13425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material