Abstract
In an effort to increase use of preventive health care, The Patient Protection and Affordable Care Act (ACA) eliminated cost-sharing for preventive cancer screening services for the privately insured. The impact on patient spending and use of these screenings is still poorly understood.
We used an interrupted time series analysis with the Massachusetts All-Payer Claims Database (2009–2012) to assess changes in trends in costs and use of breast, cervical and colorectal cancer screenings after the ACA policy. We find that the ACA was associated with a 0.024 (95% CI: −0.031, −0.017, p < 0.001) and 0.424 (95% CI: −0.481, −0.368, p < 0.001) percentage point decrease in the likelihood of a copayment each week for preventive breast and cervical cancer screenings respectively. The likelihood of copayment for colon cancer screening declined throughout the study period, with the rate of decline slowing following the ACA (trend in percent of screenings with copayment −0.130 before vs −0.071 after ACA, p = 0.014). Overall, we find only weak evidence that the ACA policy increased screenings. We find no significant effect on utilization for cervical cancer or colon cancer screening. For breast cancer screening, we find a small immediate increase in the utilization rate in the month after the policy change, with no change in trend after the ACA policy. Policy makers may need to consider other complementary policy options to increase screening rates.
1. Introduction
Breast, cervical and colorectal cancer pose serious threats to population health in the United States. Breast cancer is the most common cancer among women in the United States, where 12% of women will develop breast cancer in their lifetime (Siegel et al., 2018). Colorectal cancer is the third most common cancer among both men and women and the second leading cause of cancer mortality when men and women are considered together (Siegel et al., 2018). Though less common than breast and colorectal cancer, cervical cancer is the second leading cause of cancer deaths in women aged 20 to 39 years (Siegel et al., 2018).
A reduction in the number of new cancer cases, as well as illness, disability, and death caused by cancer is included among the United States Department of Health and Human Services' Healthy People 2020 Goals. Among the objectives used to measure progress, Healthy People 2020 set target cancer screening rates. As of 2015 (the most recent year of data available), national screening rates for breast (71.5%), colorectal (62.4%) and cervical cancer (83.0%) were all still below Healthy People 2020 goals (White et al., 2017).
In an effort to increase screening rates for these cancers, the Patient Protection and Affordable Care Act (ACA) eliminated all patient cost-sharing for breast, cervical and colorectal cancer screenings for individuals with private insurance coverage or covered by Medicare as of September 23, 2010 (The Henry J. Kaiser Family Foundation, 2015). While there is some debate about the relative benefits of cancer screenings (Welch et al., 2016), the US Preventive Services Task Force (USPSTF) determined that all three of these screenings provide a moderate to substantial net benefit to population health when considered alongside the potential risks of over-diagnosis (The Henry J. Kaiser Family Foundation, 2015; Lin et al., 2016; Moyer, 2012; Nelson, 2016). The policy to eliminate cost-sharing is motivated in part by economic theory, which predicts that reducing a patient's cost of a screening test should lead greater numbers of patients to be tested. Evidence from experimental and observational studies demonstrates that preventive care is price sensitive, supporting this theory (Baicker et al., 2013; Lohr et al., 1986; Meeker et al., 2011; Solanki and Schauffler, 1999; Karter et al., 2003).
In evaluating the impact of the ACA on health behaviors, it is first important to understand how this policy reform actually impacted patient out-of-pocket costs for preventive care. There are several reasons why the actual out-of-pocket costs for cancer screenings may not have changed much after the ACA. First, screening services are exempt from cost-sharing only if they are provided by an in-network provider and if the patient is insured through a non-grandfathered plan (The Henry J. Kaiser Family Foundation, 2015). Second, if the cancer screening is not the main purpose of the office visit, then a copayment can be charged for the office visit. Further, the cancer screening has to be billed in a way that it is distinguished from diagnostic screening in billing claims (Department of the Treasury, and Department of Labor, Health and Human Services, 2010). Finally, cost-sharing for these cancer screenings may have been limited even before the start of the ACA so that the new policy would not actually affect the status quo and thus would not lead to changes in behavior.
Even if the ACA reduced out-of-pocket costs for preventive care, rates of use of these cancer screenings could have remained the same. If screening utilization is driven in large part by physician recommendation rather than patient demand, or patients are not aware of the cost of preventive screenings (Lafata et al., 2014; Ramdass et al., 2014), then reducing out-of-pocket costs may also not have affected screening rates.
Previous studies assessing whether the ACA increased cancer screenings have reported inconsistent results (Sabik and Adunlin, 2017). Several of these studies compare yearly estimates from population-based surveys, however, these studies could be confounded by temporal changes in screenings that may have occurred during the years of the ACA reforms (Fedewa et al., 2015; Hamman and Kapinos, 2015; Sabatino et al., 2016; Jensen et al., 2015; Richman et al., 2015; Han et al., 2015; Wan et al., 2015). A few other studies have addressed these concerns by using more rigorous quasi-experimental designs; however, one of these studies was limited to small business beneficiaries enrolled in Humana insurance plans (Mehta et al., 2015), and the remaining studies have focused on Medicare patients over age 65 (Jena et al., 2017; Lissenden and Yao, 2017). Additionally, most previous research has not examined changes in documented out-of-pocket costs. In this paper, we add to this evidence base through the use of an interrupted time series design in the full population of privately insured enrollees from Massachusetts to assess whether the ACA's no cost-sharing policy was associated with out-of-pocket costs and use of preventive cancer screenings.
2. Methods
2.1. Data and sampling
The data source for this study was the Massachusetts All-Payer Claims Database (APCD), which includes all medical, pharmacy, and dental claims in the state of Massachusetts. We obtained a 10% random sample of commercially-insured patient encounters for medical care from January 2009 to December 2012. A patient encounter included all of a patient's claims submitted for medical care with the same service date.
2.2. Preventive service codes and data exclusions used to create the dataset for analysis
Cancer screening services were identified using Current Procedural Terminology (CPT) codes and International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes retrieved from billing guidelines released from the major insurance companies operating in Massachusetts, including Tufts, Harvard Pilgrim, Blue Cross Blue Shield and Aetna, which together account for 85% of the private insurance market. Breast cancer screening included mammography which was considered preventive regardless of ICD9 code, cervical cancer screening included cytology (Papanicolaou smear) or human papillomavirus test with a preventive ICD9 code and colon cancer screening included colonoscopy with a preventive ICD9 code. Similar to previous studies using claims data (Mehta et al., 2015), we included only colonoscopy to measure colon cancer screening as it was the primary modality for colorectal cancer screening in Massachusetts during the study period (Joseph et al., 2012). However, we include results for fecal occult blood testing (FOBT) in the Appendix.
We excluded patient encounters where patients had any interaction with a provider outside of the patient's insurance network, because the ACA's no cost-sharing policy applies only to health services rendered by in-network providers. We conducted analyses for each screening type on separate samples, with each sample restricted to the age group and sex for which the service was recommended according to the US Preventive Services Task Force (USPSTF) (Appendix). In the 10% sample, there were a total of 177,368 breast cancer screenings, 205,960 cervical cancer screenings, and 27,078 colon cancer screening.
2.3. Cost calculations
Coinsurance was charged in only a very small proportion of encounters for the three screenings (2%), and deductible payments for these screenings were eliminated in Massachusetts before the ACA; therefore, copayments were the main source of out-of-pocket spending for cancer screenings before the ACA. Our primary measure of patient payment for preventive cancer screenings was whether a copayment was charged either on a claim line for a screening service or on a claim line for a preventive office visit during which a preventive cancer screening was conducted. We report the percentage of weekly encounters for breast cancer, cervical and colon cancer screenings for which any copayment was charged. In sensitivity analyses, we tested two alternative measures of patient payment. The first included copayments listed on any claim line in claims that included the preventive screening or office visit, and the second included any copayment listed in the health encounter (i.e. including copayments in claims from the encounter without a preventive cancer screening). A detailed description of these methods and the results of these analyses appear in the Appendix.
2.4. Utilization calculations
We measured utilization as the weekly screening rate per 1000 health care encounters. For each screening type, the total number of encounters included in the denominator was restricted to the population eligible for the preventive screening without cost sharing per ACA regulations (The Henry J. Kaiser Family Foundation, 2015). For instance, the denominator for the breast cancer calculation was all health care encounters among women over 40 years of age. The numerator was a count of health care encounters among this population that included a breast cancer screening. As a sensitivity analysis, we examined changes in the total number of encounters involving a screening (rather than a rate per total encounters) (Appendix). This sensitivity analysis was implemented to ensure that any changes in the screening rate per 1000 encounters, our main utilization outcome, were not driven by a change in total encounters over time.
2.5. Descriptive analysis
Trends in the cost of preventive cancer screenings are displayed using graphs of the weekly percentage of screening encounters for which any copayment was charged over the study period. To display these trends, we plotted the percent of weekly screening encounters with a copayment between January 2009 and December 2012. In practice most insurance plans would not implement the policy until the plan renewal. However, given that plans renew at different times throughout the next calendar year, we chose to use the policy start date as the point from which to assess a change in trend in the analyses as this should yield the most conservative estimates of impact. We also graphed the weekly screening rate per 1000 health care encounters during the study period. A vertical line at September 20, 2010 was included to represent the start of the ACA's no-cost sharing policy.
2.6. Regression analyses
We estimated two interrupted time series models to test whether the ACA's no cost-sharing policy changed the probability that a copayment was charged for preventive cancer screening encounters, and whether the utilization of cancer screenings changed.
In the copayment regression, the dependent variable measured the percentage of weekly screening encounters for a given cancer screening in which a copayment was paid. In the utilization regression the dependent variable was the screening rate per 1000 encounters among individuals eligible for each cancer screening. In both models, the dependent variable was regressed on a binary variable indicating whether the service billed in the claim occurred after the ACA policy started (e.g., after September 19, 2010), a linear weekly time trend starting from January 2009 through December 2012, and an interaction term between the post policy indicator and the time trend. All regression models included 11 calendar month dummies to control for seasonality. In addition, the breast cancer screening rate analysis controlled for a change in USPSTF breast cancer screening recommendations in November 2009. The cervical cancer screening rate analysis controlled for the November 2009 recommendation change by the American Congress of Obstetricians and Gynecologists and the March 2012 USPSTF recommendation change. All recommendation changes were controlled for using a binary variable that was equal to zero before the recommendation change, and one after the change. We used the actest command in Stata to conduct the Cumby-Huizinga general test for autocorrelation in time series data examining weekly lags of one to ten. We then used ordinary least squares regression with Newey-West standard errors to adjust for autocorrelation. The smallest p-value identified among the ten lags tested was used as the lag value in the Newey-West standard error adjustment. A p-value below 0.05 was considered statistically significant.
3. Results
In 2009, 4.1% of encounters for preventive breast cancer screenings were charged a copayment and the average copayment was $19.04. Among encounters with a preventive cervical cancer screening in 2009, 65.8% of patients were charged a copayment, and the average payment among those encounters was $18.07. A copayment was charged in 21.6% encounters that included a colon cancer screening in 2009, and the average copayment amount was $142.19 (Table 1).
Table 1.
Copayment amounts (USD) for cancer screenings among privately insured individuals, Massachusetts All Payers Claims Database in 2009.
| Percent paying a copayment |
Average copayment amount among those who paid |
Screening rate per 1000 encounters |
Average age |
|
|---|---|---|---|---|
| Percent | Mean dollar amount | Percent | Years | |
| Breast cancer screening | 4.13 | 19.04 | 38.38 | 55.84 |
| Cervical cancer screening | 65.81 | 18.07 | 38.71 | 42.53 |
| Colon cancer screening | 21.60 | 142.19 | 5.37 | 56.94 |
Source: Massachusetts All-Payer Claims Database.
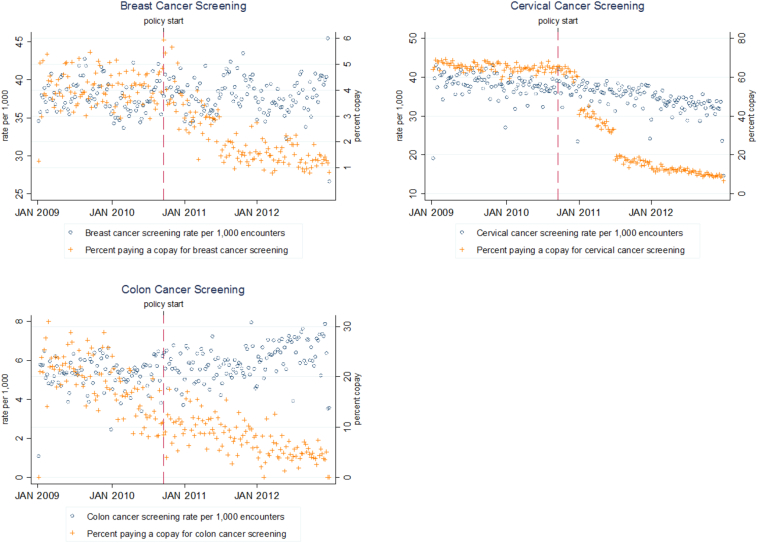
Fig. 1 displays unadjusted trends in copayments and utilization for the three cancer screening tests, with the right axis (and “+” markers) corresponding to copayments and the left axis (and “o” markers) corresponding to screenings. There were declines in the percentage of encounters that included a patient copayment for all cancer types, with notable abrupt changes in trend after the ACA for breast and cervical cancer screening encounters. Distinct trend breaks in the percent of cervical cancer screenings charged a copayment are apparent in January and June 2011, corresponding to the most common plan renewal dates when some plans lost their grandfathered status. Screening rates for breast cancer appear unchanged after the ACA, while colon cancer screenings appear to have increased and cervical cancer screenings appear to have decreased.
Fig. 1.
Percent of weekly preventive breast, cervical and colon cancer screenings for which a copayment was charged and screening rate per 1000 encounters that included a preventive cancer screening, Massachusetts, 2009–2012.
Source: Massachusetts All-Payer Claims Database.
Results from the copayment interrupted time series regression models are presented in Table 2. The probability of copayment for breast cancer screening was essentially unchanging before the ACA. The ACA was associated with a small but significant 0.024 percentage point decrease per week (95% CI: −0.031, −0.017, p-value<0.001) in the probability of a copayment for breast cancer screening. For cervical cancer screening, the ACA was associated with a 0.424 weekly percentage point (95% CI: −0.481, −0.368, p-value <0.001) decrease in the probability of copayment. In addition to statistically significant changes in trends, we find a statistically significant negative coefficient on the post policy indicator for breast and cervical cancer. The ACA policy was associated with an immediate 0.583 (95% CI: −1.027, −0.140, p-value = 0.010) and 12.385 (95% CI: −17.161, −7.610, p-value<0.001) percentage point decrease in the percent of breast and cervical cancer screenings respectively charged a copay in the month following the policy change.
Table 2.
Change in the percentage of weekly preventive cancer screenings with a copayment, Massachusetts 2009–2012.
| Breast cancer screeninga (n = 177,368b) |
Cervical cancer screeninga (n = 205,960b) |
Colon cancer screeninga (n = 27,078b) |
||||
|---|---|---|---|---|---|---|
| β (95% CI) |
p-Value | β (95% CI) |
p-Value | β (95% CI) |
p-Value | |
| Weekly time trend | −0.000 (−0.005, 0.005) |
0.986 | −0.036⁎⁎ (−0.064, −0.009) |
0.009 | −0.130⁎⁎⁎ (−0.174, −0.087) |
0.000 |
| Post ACA policy indicator | −0.583⁎ (−1.027, −0.140) |
0.010 | −12.385⁎⁎⁎ (−17.161, −7.610) |
0.000 | −0.861 (−3.062, 1.339) |
0.441 |
| Post X weekly time interaction | −0.024⁎⁎⁎ (−0.031, −0.017) |
0.000 | −0.424⁎⁎⁎ (−0.481, −0.368) |
0.000 | 0.060⁎ (0.012, 0.107) |
0.014 |
| Baseline meanc | 5.05 | 65.12 | 20.80 | |||
| N | 210 | 210 | 210 |
p < 0.05.
p < 0.01.
p < 0.001.
Trends in likelihood of copayment for cancer screenings, calculated from interrupted time series regression models assessing the change in trend before (January 2009–September 19, 2010) and after (September 20, 2010–December 2012) the ACA policy controlling for seasonality using month indicator variables. Newey-West standard errors were used to adjust for autocorrelation. 95% confidence intervals in parentheses.
Total number of screenings among the population eligible during the study period from 10% sample.
Baseline mean represents the mean from January 5th to 11th, 2009 or the second week of 2009 because the first week from January 1st to January 4th had few observations/screenings.
Source: Massachusetts All-Payer Claims Database.
Finally, while the probability of copayment for colon cancer screening was significantly decreasing before the ACA, the weekly decline became smaller after the ACA. Therefore, the ACA was associated with an increase in the trend in the probability of copayment for colon cancer screening by 0.060 percentage points per week (95% CI: 0.012, 0.107, p-value = 0.014). The ACA policy was not associated with a change in the probability of copayment for colon cancer screening in the month after the policy change. Full regression output for these models is provided in Appendix Table 2. The sensitivity analysis in Appendix Table 3 presents these interrupted time series results with the two alternative constructions of the copayment variable. Results for breast and cervical cancer screening using these alternative measures are similar to our main results, but the finding that the ACA slowed the decreasing trend in probability of copayment for colon cancer screening is no longer present in these alternative specifications.
Results from the interrupted time series regressions for the utilization of screenings are presented in Table 3. The ACA was not associated with a trend change in the rate of breast cancer or cervical cancer screenings. However, for breast cancer screening, the ACA policy was associated with a 1.145 immediate increase in screenings per 1000 encounters (95% CI: 0.059, 2.230, p-value = 0.039) in the month after the policy change. The ACA was not associated with an immediate increase in the rate of colon cancer screening after the policy change. The increase in trend of 0.009 colon cancer screenings per 1000 encounters each week (95% CI: −0.001, 0.018, p-value = 0.065) was not statistically significant. Full regression output for these models is provided in Appendix Table 4. As a sensitivity analysis, we tested screening counts as our measure of utilization (Appendix Table 5) and results are similar; however, the breast cancer screening result is no longer statistically significant. The ACA was associated with a decrease in copayment for FOBT but was not significantly associated with the rate of FOBT screening per 1000 encounters.
Table 3.
Change in the screening rate per 1000 encounters that included a preventive cancer screening, Massachusetts 2009–2012.
| Breast cancer screeninga (n = 4,768,206b) |
Cervical cancer screeninga (n = 5,680,269b) |
Colon cancer screeninga (n = 5,053,333b) |
||||
|---|---|---|---|---|---|---|
| β (95% CI) |
p-Value | β (95% CI) |
p-Value | β (95% CI) |
p-Value | |
| Weekly time trend | −0.015 (−0.050, 0.021) |
0.420 | 0.016 (−0.054, 0.085) |
0.656 | 0.001 (−0.007, 0.008) |
0.844 |
| Post ACA policy indicator | 1.145⁎ (0.059, 2.230) |
0.039 | 0.237 (−1.846, 2.321) |
0.822 | 0.130 (−0.343, 0.602) |
0.588 |
| Post X weekly time interaction | 0.007 (−0.029, 0.042) |
0.718 | −0.057 (−0.126, 0.012) |
0.104 | 0.009 (−0.001, 0.018) |
0.065 |
| Baseline meanc | 35.78 | 39.72 | 5.79 | |||
| N | 210 | 210 | 210 |
p < 0.05.
Trends in the screening rate per 1000 health encounters, calculated from interrupted time series regression models assessing the change in trend before (January 2009–September 19, 2010) and after (September 20, 2010–December 2012) the ACA policy controlling for seasonality using month indicator variables. Newey-West standard errors were used to adjust for autocorrelation. 95% confidence intervals in parentheses. Population restricted to privately insured adults that meet eligibility criteria for each cancer screening.
Total number of encounters during the study period among the population eligible for each screening type from 10% sample.
Baseline rate represents the rate from January 5th to 11th, 2009 or the second week of 2009 because the first week from January 1st to January 4th had few observations/screenings.
Source: Massachusetts All-Payer Claims Database.
4. Discussion
This study examined the effect of the ACA's policy eliminating out-of-pocket payments for preventive breast, cervical and colon cancer screenings on patient spending and use of these cancer screenings among the privately insured in Massachusetts. The policy was associated with a decrease in the likelihood that a copayment was charged for breast and cervical cancer screenings. Despite these declines in copayments, the elimination of cost-sharing was only associated with a modest immediate increase in breast cancer screenings and not associated with an increase in the trend of breast cancer screenings. We find no significant association between the ACA policy change itself and the likelihood of being screened for cervical cancer; declines in cervical cancer screenings observed in the period after the ACA were likely due to changes in cervical cancer screening guidelines.
The percent of preventive colon cancer screenings charged a copayment was decreasing strongly throughout the study period. We find evidence that this rate of decline was lessened following the ACA, though this result was not robust in sensitivity analysis. The ACA was not associated change in utilization of colon cancer screening.
Previous studies of the impact of the ACA no cost policy on colorectal cancer screening and mammography have found positive effects among the Medicare population (Hamman and Kapinos, 2015; Sabatino et al., 2016; Wan et al., 2015; Jena et al., 2017; Trivedi et al., 2018), but mixed or null results among the commercially insured (Fedewa et al., 2015; Richman et al., 2015; Han et al., 2015; Mehta et al., 2015). The single previous study that included cervical cancer screenings is consistent with our finding of no result (Han et al., 2015). Further, our finding that out-of-pocket costs for breast cancer screening decreased from an already low proportion before the ACA is consistent with the single previous study that examined trends in out-of-pocket costs, indicating that low cost sharing before the ACA among the commercially insured may explain the null effect found in most studies (Mehta, 2015).
Our results suggest that price sensitivity is unlikely to be driving the decision to be screened for these cancers among the privately insured. One potential reason for this is that patients lack information either about the policy change itself or about their specific cost-sharing amount before and after the policy change. A 2014 Kaiser tracking poll found that only 43% of the population was aware that the ACA eliminated out-of-pocket expenses for preventive services (Hamel et al., 2014), lending some support to this explanation. Other evidence has shown that consumers lack information about important features of their health plan (Reed et al., 2012). Beyond information gaps, other important behavioral barriers to preventive care are often present (e.g. procrastination, information aversion, etc.) (Mehta et al., 2018; Kessler et al., 2018; Volpp and Asch, 2017), and the reductions in cost may not have been enough to overcome these barriers for some patients.
Another reason that we find minimal response to the ACA's no cost-sharing policy with respect to cancer screening could be the relatively high rate of screening in Massachusetts before the ACA (83.6%, 84.5% and 75.1% for breast, cervical and colorectal cancer, respectively) (Massachusetts Department of Public Health, 2011). Copayment amounts for breast and cervical cancer screenings in our sample prior to the ACA were roughly $20–30. It is possible that patients in Massachusetts who were not accessing screening before the ACA may have required a larger incentive to induce a change in care-seeking behavior. However, previous research has shown that even modest copayments of $20, the median amount for breast and cervical cancer in our data, can affect preventive care use (Trivedi et al., 2008).
In the years before the ACA's no cost-sharing policy came into effect, Massachusetts implemented a state-wide health reform which reduced the number of uninsured and mandated exemption of these preventive cancer screenings from deductible payments (McDonough et al., 2008; Kolstad and Kowalski, 2012). The states' largest commercial payer implemented an alternative payment model that included quality bonuses for provision of preventive care (Chernew et al., 2011; Song et al., 2012). These changes should not introduce confounding as the reforms all occurred at least one year before the ACA policy change. While these reforms may limit the generalizability of our findings, these findings are still broadly relevant as the majority of states have undertaken some similar efforts. By 2010, all 50 states had passed mandates requiring coverage of breast cancer screenings, and 30 states had passed mandates requiring coverage of colorectal cancer screening (The National Colorectal Cancer Research Alliance, 2010). Further, prior to the ACA, over 87% of employer sponsored plans nationally covered preventive care before the enrollee met the deductible (Henry J. Kaiser Family Foundation and Health Research and Educational Trust (HRET), 2010).
There are several limitations to this study. First, we are unable to use this data to determine why some patients continued to be charged copayments after the policy change and if charging these copayments was appropriate. For example, we are unable to identify patients enrolled in grandfathered health care plans where copayments could be permitted, or instances where the preventive service was received during an office visit for a non-preventive service and for which a copayment was owed. Second, our utilization measure reflects screening trends among people who use health care but does not capture trends in the uninsured or among those who do not use health care. Third, Massachusetts, had higher screening rates than most other states before the ACA, and had enacted some preventive care policy reforms before the ACA. Further, this study does not include a comparison group, such as grandfathered plans, and therefore could be confounded by other policy changes affecting screening utilization, if they occurred at the same time as the ACA policy change. Finally, the use of interrupted times series requires the selection of a discrete time of the policy change, but the ACA likely has had a more gradual impact on payments and screenings due to the gradual decline in grandfathered plans that are not subject to the policy. As late as 2014, 26% of employer sponsored health insurance plans were still grandfathered. Further, as colonoscopy screening is recommended less frequently than other screening types, any effect of the ACA no cost policy would be more gradual, and potentially more difficult to detect using interrupted time series methodology.
5. Conclusion
Using a rigorous interrupted time series design that reduces the possibility of confounding factors, this study finds evidence that the ACA had a modest or null effect on preventive cancer screenings among the general commercially insured population, despite the fact that the policy was associated with decreased copayments for breast and cervical cancer screening. More evidence is needed to understand why eliminating cost-sharing was not an effective way to increase screening in this population, and whether alternative approaches could help raise screening rates.
Declaration of Competing Interest
None.
Acknowledgements
We obtained funding for this study from the Office on Women’s Health, United States Department of Health and Human Services and the American Cancer Society Research, Scholar Grant – Insurance (RSGI-16-251-01-CPHPS).
Appendix A.
Appendix Table 1.
Age and medical eligibility conditions for cost sharing under the ACA's preventive services policy.
| Screening | Recommendation | Age and gender exclusions |
|---|---|---|
| Breast cancer screening |
|
Restricted to women ≥40 years |
| Cervical cancer screening |
|
Restricted to women ≥21 years & ≤65 years |
| Colorectal cancer screening |
|
Restrict to ≥50 and ≤75 |
Though the ACA generally considered the most recent USPSTF recommendation, the law specified that the 2009 recommendation for mammography would not be considered. Therefore, during the study period the USPSTF 2002 recommendation was used to determine eligibility for mammography without cost sharing.
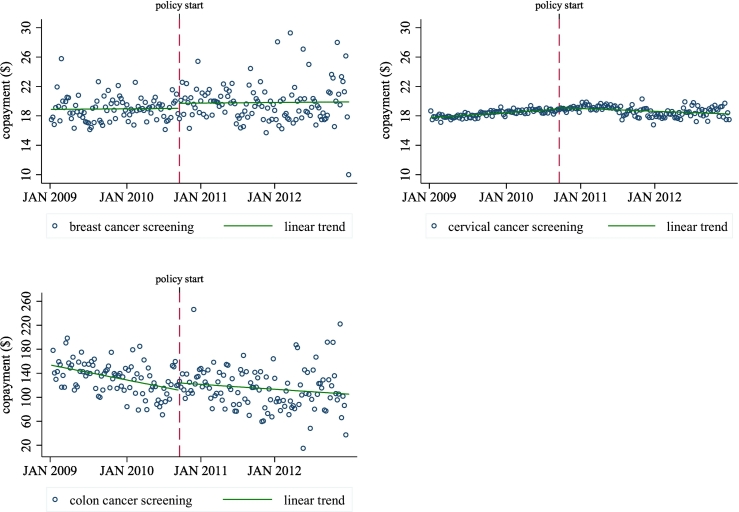
Appendix Fig. 1.
Average weekly copayment amount charged for preventive breast, cervical and colon cancer screenings among patients who paid a copayment in Massachusetts, 2009–2012.
Source: Massachusetts All-Payer Claims Database.
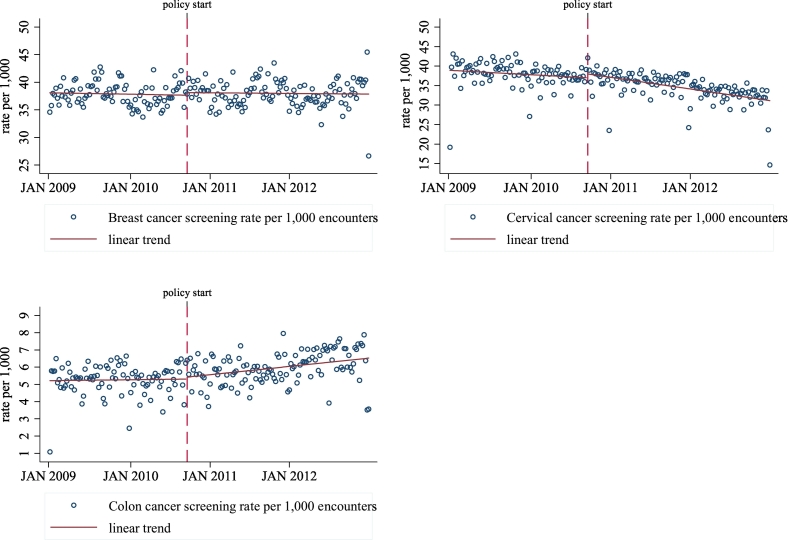
Appendix Fig. 2.
Screening rate per 1000 encounters that included a preventive cancer screening, Massachusetts 2009–2012.
Appendix Table 2.
Results from interrupted time series models assessing the effect of the ACA on the percentage of weekly preventive cancer screenings for which a copayment was billed in Massachusetts, 2009–2012.
| Breast cancer screening |
Cervical cancer screening |
Colon cancer screening |
|
|---|---|---|---|
| β (95% CI) |
β (95% CI) |
β (95% CI) |
|
| Weekly time trend | −0.000 (−0.005, 0.005) |
−0.036⁎⁎ (−0.064, −0.009) |
−0.130⁎⁎⁎ (−0.174, −0.087) |
| Post ACA policy indicator | −0.583⁎ (−1.027, −0.140) |
−12.385⁎⁎⁎ (−17.161, −7.610) |
−0.861 (−3.062, 1.339) |
| Post X weekly time interaction | −0.024⁎⁎⁎ (−0.031, −0.017) |
−0.424⁎⁎⁎ (−0.481, −0.368) |
0.060⁎ (0.012, 0.107) |
| February indicator | 0.206 (−0.198, 0.610) |
1.157 (−1.331, 3.644) |
0.694 (−2.989, 4.377) |
| March indicator | 0.002 (−0.359, 0.363) |
1.760 (−0.516, 4.036) |
1.423 (−1.820, 4.666) |
| April indicator | 0.128 (−0.246, 0.503) |
1.343 (−0.875, 3.560) |
1.046 (−2.176, 4.269) |
| May indicator | 0.455⁎ (0.062, 0.848) |
2.366⁎ (0.227, 4.506) |
1.032 (−1.942, 4.006) |
| June indicator | 0.359 (−0.013, 0.731) |
2.182 (−0.289, 4.652) |
1.275 (−1.950, 4.499) |
| July indicator | −0.012 (−0.563, 0.538) |
0.605 (−4.360, 5.570) |
1.056 (−1.951, 4.063) |
| August indicator | −0.087 (−0.468, 0.294) |
0.449 (−4.233, 5.130) |
0.354 (−2.886, 3.594) |
| September indicator | 0.383 (−0.329, 1.096) |
3.140 (−2.960, 9.240) |
1.386 (−1.672, 4.443) |
| October indicator | 0.235 (−0.223, 0.693) |
5.610 (−0.290, 11.511) |
0.454 (−2.719, 3.628) |
| November indicator | 0.465 (−0.110, 1.039) |
7.212⁎ (1.342, 13.081) |
2.104 (−1.183, 5.391) |
| December indicator | 0.390 (−0.049, 0.830) |
7.215⁎ (0.926, 13.504) |
1.171 (−1.758, 4.100) |
| Constant | 3.893⁎⁎⁎ (3.425, 4.362) |
64.644⁎⁎⁎ (62.442, 66.847) |
23.474⁎⁎⁎ (19.155, 27.794) |
| N | 210 | 210 | 210 |
Note. Trends in likelihood of copayment for cancer screenings, calculated from interrupted time series regression models assessing the change in trend before (January 2009–September 19, 2010) and after (September 20, 2010–December 2012) the ACA policy controlling for seasonality using month indicator variables. Newey-West standard errors were used to adjust for autocorrelation 95% confidence intervals in parentheses. Population restricted to privately insured adults that meet eligibility criteria for each cancer screening.
p < 0.05.
p < 0.01.
p < 0.001.
Source: Massachusetts All-Payer Claims Database.
A.1. Cost sensitivity analyses
To test the sensitivity of our copayment variable to our choice of variable construction, we tested two alterative copayment variable possibilities. In the first of these analyses, we considered costs from the claims used to bill for the preventive screening or the preventive office visit. This method would include copayments for all health services billed in the same claim as the preventive screening or the preventive office visit. In the second sensitivity analysis we considered all of the claim lines from claims submitted for the entire health service encounter in which a preventive service was billed. This method included any costs, including costs for services that occurred during the same patient day but may not have been related to the screening, and was the most conservative measure. The table below shows that our finding that the trend in copayments declined significantly after the ACA was not sensitive to the construction of our copayment variable.
Appendix Table 3.
Change in the percentage of weekly preventive cancer screenings charged a copayment, using alternative methods to measure whether copayment was billed in Massachusetts 2009–2012.
| Breast cancer screening |
Cervical cancer screening |
Colon cancer screening |
||||
|---|---|---|---|---|---|---|
| β (95% CI) |
p-Value | β (95% CI) |
p-Value | β (95% CI) |
p-Value | |
| Including costs from claims with the preventive screening or a preventive office visit | ||||||
| Weekly time trend | 0.001 (−0.005, 0.006) |
0.798 | −0.023 (−0.050, 0.004) |
0.094 | −0.100⁎⁎⁎ (−0.145, −0.054) |
0.000 |
| Post ACA policy indicator | −0.641⁎⁎ (−1.066, −0.216) |
0.003 | −11.361⁎⁎⁎ (−15.771, −6.951) |
0.000 | −1.607 (−3.864, 0.651) |
0.162 |
| Post X weekly time interaction | −0.021⁎⁎⁎ (−0.029, −0.014) |
0.000 | −0.413⁎⁎⁎ (−0.467, −0.360) |
0.000 | 0.039 (−0.010, 0.089) |
0.119 |
| Baseline meana | 5.57 | 67.83 | 21.43 | |||
| Including costs from all claim lines on a patient-day | ||||||
| Weekly time trend | 0.008 (−0.001, 0.016) |
0.091 | −0.021 (−0.047, 0.006) |
0.126 | −0.107⁎⁎⁎ (−0.156, −0.057) |
0.000 |
| Post ACA policy indicator | −0.781⁎⁎ (−1.334, −0.227) |
0.006 | −11.291⁎⁎⁎ (−15.729, −6.852) |
0.000 | −1.466 (−3.859, 0.927) |
0.162 |
| Post X weekly time interaction | −0.031⁎⁎⁎ (−0.042, −0.019) |
0.000 | −0.409⁎⁎⁎ (−0.462, −0.356) |
0.000 | 0.043 (−0.012, 0.098) |
0.119 |
| Baseline meana | 10.49 | 74.59 | 27.78 | |||
| N | 210 | 210 | 210 | |||
Note. Trends in likelihood of copayment for cancer screenings, calculated from interrupted time series regression models assessing the change in trend before (January 2009–September 19, 2010) and after (September 20, 2010–December 2012) the ACA policy controlling for seasonality using month indicator variables. Newey-West standard errors were used to adjust for autocorrelation 95% confidence intervals in parentheses. Population restricted to privately insured adults that meet eligibility criteria for each cancer screening.
p < 0.01.
p < 0.001.
Baseline mean represents the mean from January 5th to 11th, 2009 or the second week of 2009 because the first week from January 1st to January 4th had too few observations/screenings.
Source: Massachusetts All-Payer Claims Database.
Appendix Table 4.
Results from interrupted time series models assessing the effect of the ACA on the number of screenings per 1000 encounters billed to private insurers in Massachusetts, 2009–2012.
| Breast cancer screening |
Cervical cancer screening |
Colon cancer screening |
|
|---|---|---|---|
| β (95% CI) |
β (95% CI) |
β (95% CI) |
|
| Weekly time trend | −0.015 (−0.050, 0.021) |
0.016 (−0.054, 0.085) |
0.001 (−0.007, 0.008) |
| Post ACA policy indicator | 1.145⁎ (0.059, 2.230) |
0.237 (−1.846, 2.321) |
0.130 (−0.343, 0.602) |
| Post X weekly time interaction | 0.007 (−0.029, 0.042) |
−0.057 (−0.126, 0.012) |
0.009 (−0.001, 0.018) |
| February indicator | 0.580 (−0.472, 1.631) |
0.154 (−2.001, 2.309) |
−0.167 (−0.759, 0.425) |
| March indicator | 0.956 (−0.075, 1.987) |
0.755 (−1.410, 2.919) |
0.069 (−0.517, 0.655) |
| April indicator | 1.990⁎⁎⁎ (0.876, 3.104) |
0.164 (−1.904, 2.232) |
−0.049 (−0.600, 0.502) |
| May indicator | −0.135 (−1.136, 0.866) |
0.902 (−1.100, 2.904) |
−0.138 (−0.735, 0.458) |
| June indicator | 0.930 (−0.064, 1.924) |
0.560 (−1.375, 2.495) |
0.294 (−0.285, 0.874) |
| July indicator | 2.935⁎⁎⁎ (1.877, 3.994) |
0.337 (−1.872, 2.547) |
−0.105 (−0.771, 0.560) |
| August indicator | 4.042⁎⁎⁎ (2.813, 5.271) |
0.966 (−0.848, 2.779) |
0.127 (−0.473, 0.727) |
| September indicator | 1.797⁎⁎ (0.475, 3.119) |
1.067 (−0.966, 3.100) |
−0.012 (−0.584, 0.559) |
| October indicator | 2.259⁎⁎ (0.909, 3.609) |
0.255 (−1.795, 2.305) |
0.034 (−0.544, 0.611) |
| November indicator | 3.201⁎⁎⁎ (2.046, 4.356) |
0.604 (−1.338, 2.546) |
0.246 (−0.352, 0.845) |
| December indicator | 2.553⁎⁎⁎ (1.098, 4.007) |
−2.127 (−5.641, 1.387) |
−0.362 (−1.294, 0.571) |
| Constant | 37.016⁎⁎⁎ (36.024, 38.008) |
37.962⁎⁎⁎ (34.490, 41.433) |
5.223⁎⁎⁎ (4.467, 5.979) |
| N | 210 | 210 | 210 |
Note. Trends in the screening rate per 1000 health encounters, calculated from interrupted time series regression models assessing the change in trend before (January 2009–September 19, 2010) and after (September 20, 2010–December 2012) the ACA policy controlling for seasonality using month indicator variables. Newey-West standard errors were used to adjust for autocorrelation 95% confidence intervals in parentheses. Population restricted to privately insured adults that meet eligibility criteria for each cancer screening.
p < 0.05.
p < 0.01.
p < 0.001.
Source: Massachusetts All-Payer Claims Database.
Appendix Table 5.
Results from interrupted time series models assessing the effect of the ACA on the number of preventive cancer screenings billed to private insurers in Massachusetts, 2009–2012.
| Breast cancer screeninga |
Cervical cancer screeninga |
Colon cancer screeninga |
||||
|---|---|---|---|---|---|---|
| β (95% CI) |
p-Value | β (95% CI) |
p-Value | β (95% CI) |
p-Value | |
| Weekly time trend | 0.237 (−2.195, 2.668) |
0.848 | 1.687 (−2.054, 5.429) |
0.375 | 0.215⁎ (0.015, 0.416) |
0.035 |
| Post ACA policy indicator | 21.482 (−55.645, 98.609) |
0.583 | −19.223 (−128.832, 90.386) |
0.73 | 0.964 (−14.539, 16.467) |
0.903 |
| Post X weekly time interaction | −0.280 (−2.577, 2.017) |
0.810 | −2.658 (−6.381, 1.064) |
0.161 | 0.093 (−0.196, 0.383) |
0.525 |
| Baseline meanc | 779 | 1035 | 135 | |||
| N | 210 | 210 | 210 |
Note: Trends in the number of preventive cancer screenings, calculated from interrupted time series regression models assessing the change in trend before (January 2009–September 19, 2010) and after (September 20, 2010–December 2012) the ACA policy controlling for seasonality using month indicator variables. Newey-West standard errors were used to adjust for autocorrelation. Population restricted to privately insured adults that meet eligibility criteria for each cancer screening.
p < 0.05.
Baseline mean represents the total from January 5th to 11th, 2009 or the second week of 2009 because the first week from January 1st to January 4th had too few observations/screenings.
Source: Massachusetts All-Payer Claims Database.
Appendix Table 6.
Results from interrupted time series models assessing the effect of the ACA on fecal occult blood test screening in Massachusetts, 2009–2012.
| Fecal occult blood test screening | Percent copayment |
Screening rate per 1000 encounters |
||
|---|---|---|---|---|
| β (95% CI) |
p-Value | β (95% CI) |
p-Value | |
| Weekly time trend | −0.058⁎ (−0.102, −0.013) |
0.011 | −0.013⁎⁎ (−0.022, −0.003) |
0.007 |
| Post ACA policy indicator | −12.181⁎⁎⁎ (−16.079, −8.283) |
0.000 | 0.391 (−0.102, 0.883) |
0.119 |
| Post X weekly time interaction | −0.335⁎⁎⁎ (−0.397, −0.273) |
0.000 | 0.004 (−0.007, 0.015) |
0.482 |
| Baseline meana | 82.39% | 9.22 | ||
| N | 210 | 210 |
Note: Trends in copayment and screening rate per 1000 health encounters for fecal occult blood testing calculated from interrupted time series regression models assessing the change in trend before (January 2009–September 19, 2010) and after (September 20, 2010–December 2012) the ACA policy controlling for seasonality using month indicator variables. Newey-West standard errors were used to adjust for autocorrelation. Population restricted to privately insured adults that meet eligibility criteria for each cancer screening.
p < 0.05.
p < 0.01.
p < 0.001.
Baseline mean represents the total from January 5th to 11th, 2009 or the second week of 2009 because the first week from January 1st to January 4th had too few observations/screenings.
Source: Massachusetts All-Payer Claims Database.
References
- Baicker K., Taubman S.L., Allen H.L., Bernstein M., Gruber J.H., Newhouse J.P. The Oregon experiment—effects of Medicaid on clinical outcomes. N. Engl. J. Med. 2013;368(18):1713–1722. doi: 10.1056/NEJMsa1212321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernew M.E., Mechanic R.E., Landon B.E., Safran D.G. Private-payer innovation in Massachusetts: the ‘alternative quality contract’. Health Aff (Millwood) 2011;30(1):51–61. doi: 10.1377/hlthaff.2010.0980. [DOI] [PubMed] [Google Scholar]
- Department of the Treasury, Department of Labor, Health and Human Services . 2010. Interim Final Rules for Group Health Plans and Health Insurance Issuers Relating to Coverage of Preventive Services Under the Patient Protection and Affordable Care Act. [Google Scholar]
- Fedewa S.A., Goodman M., Flanders W.D., Han X., Smith R.A., M Ward E. Elimination of cost-sharing and receipt of screening for colorectal and breast cancer. Cancer. 2015;121(18):3272–3280. doi: 10.1002/cncr.29494. [DOI] [PubMed] [Google Scholar]
- . Hamel L, Firth J, Brodie M. Kaiser Health Tracking Poll: March 2014. Menlo Park, CA: Henry J. Kaiser Family Foundation 2014.
- Hamman M.K., Kapinos K.A. Affordable care act provision lowered out-of-pocket cost and increased colonoscopy rates among men in Medicare. Health Aff (Millwood) 2015;34(12):2069–2076. doi: 10.1377/hlthaff.2015.0571. [DOI] [PubMed] [Google Scholar]
- Han X., Yabroff K.R., Guy G.P., Zheng Z., Jemal A. Has recommended preventive service use increased after elimination of cost-sharing as part of the Affordable Care Act in the United States? Prev. Med. 2015;78:85–91. doi: 10.1016/j.ypmed.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry J. Kaiser Family Foundation, Health Research and Educational Trust (HRET) Henry J. Kaiser Family Foundation and HRET; Menlo Park, CA: 2010. Employer Health Benefits: 2010 Annual Survey. [Google Scholar]
- Jena A.B., Huang J., Fireman B., Fung V., Gazelle S., Landrum M.B. Screening mammography for free: impact of eliminating cost sharing on cancer screening rates. Health Serv. Res. 2017;52(1):191–206. doi: 10.1111/1475-6773.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen G.A., Salloum R.G., Hu J., Ferdows N.B., Tarraf W. A slow start: use of preventive services among seniors following the Affordable Care Act's enhancement of Medicare benefits in the US. Prev. Med. 2015;76:37–42. doi: 10.1016/j.ypmed.2015.03.023. [DOI] [PubMed] [Google Scholar]
- Joseph D.A., King J.B., Miller J.W., Richardson L.C. Prevalence of colorectal cancer screening among adults—behavioral risk factor surveillance system, United States, 2010. MMWR Morb. Mortal. Wkly Rep. 2012;61(Suppl):51–56. [PubMed] [Google Scholar]
- Karter A.J., Stevens M.R., Herman W.H., Ettner S., Marrero D.G., Safford M.M. Out-of-pocket costs and diabetes preventive services: the Translating Research Into Action for Diabetes (TRIAD) study. Diabetes Care. 2003;26(8):2294–2299. doi: 10.2337/diacare.26.8.2294. [DOI] [PubMed] [Google Scholar]
- Kessler J.B., Troxel A.B., Asch D.A., Mehta S.J., Marcus N., Lim R. Partners and alerts in medication adherence: a randomized clinical trial. J. Gen. Intern. Med. 2018;33(9):1536–1542. doi: 10.1007/s11606-018-4389-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolstad J.T., Kowalski A.E. The impact of health care reform on hospital and preventive care: evidence from Massachusetts. J. Public Econ. 2012;96(11):909–929. doi: 10.1016/j.jpubeco.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafata J.E., Cooper G., Divine G., Oja-Tebbe N., Flocke S.A. Patient-physician colorectal cancer screening discussion content and patients' use of colorectal cancer screening. Patient Educ. Couns. 2014;94(1):76–82. doi: 10.1016/j.pec.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.S., Piper M.A., Perdue L.A., Rutter C., Webber E.M., O'Connor E. Screening for Colorectal Cancer: A Systematic Review for the U.S. Preventive Services Task Force. Agency for Healthcare Research and Quality (US); Rockville (MD): 2016. U.S. preventive services task force evidence syntheses, formerly systematic evidence reviews. [PubMed] [Google Scholar]
- Lissenden B., Yao N.A. Affordable care act changes to Medicare led to increased diagnoses of early-stage colorectal cancer among seniors. Health Aff (Millwood) 2017;36(1):101–107. doi: 10.1377/hlthaff.2016.0607. [DOI] [PubMed] [Google Scholar]
- Lohr K.N., Brook R.H., Kamberg C.J., Goldberg G.A., Leibowitz A., Keesey J. Use of medical care in the RAND Health Insurance Experiment: diagnosis-and service-specific analyses in a randomized controlled trial. Med. Care. 1986;24(9):S1–S87. [PubMed] [Google Scholar]
- Massachusetts Department of Public Health . Massachusetts Department of Public Health Health Survey Program; 2011. A Profile of Health Among Massachusetts Adults, 2010: Results From the Behavioral Risk Factor Surveillance System Massachusetts. [Google Scholar]
- McDonough J.E., Rosman B., Butt M., Tucker L., Howe L.K. Massachusetts health reform implementation: major progress and future challenges. Health Aff (Millwood) 2008;27(4):w285–w297. doi: 10.1377/hlthaff.27.4.w285. [DOI] [PubMed] [Google Scholar]
- Meeker D., Joyce G.F., Malkin J., Teutsch S.M., Haddix A.C., Goldman D.P. Coverage and preventive screening. Health Serv. Res. 2011;46(1 Pt 1):173–184. doi: 10.1111/j.1475-6773.2010.01188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta S.J., Polsky D., Zhu J., Lewis J.D., Kolstad J.T., Loewenstein G. ACA-mandated elimination of cost sharing for preventive screening has had limited early impact. Am. J. Manag. Care. 2015;21(7):511–517. [PMC free article] [PubMed] [Google Scholar]
- Mehta S.J., Khan T., Guerra C., Reitz C., McAuliffe T., Volpp K.G. A randomized controlled trial of opt-in versus opt-out colorectal cancer screening outreach. Am. J. Gastroenterol. 2018;113(12):1848–1854. doi: 10.1038/s41395-018-0151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer V.A. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2012;156(12):880–891. doi: 10.7326/0003-4819-156-12-201206190-00424. [DOI] [PubMed] [Google Scholar]
- Nelson H.D. 2016. Screening for Breast Cancer: A Systematic Review to Update the 2009 U.S. Preventive Services Task Force Recommendation. Rockville (MD) [PubMed] [Google Scholar]
- Ramdass P., Petraro P., Via C., Shahrokni A., Nawaz H. Providers role in colonoscopy screening for colorectal cancer. Am. J. Health Behav. 2014;38(2):234–244. doi: 10.5993/AJHB.38.2.9. [DOI] [PubMed] [Google Scholar]
- Reed M.E., Graetz I., Fung V., Newhouse J.P., Hsu J. In consumer-directed health plans, a majority of patients were unaware of free or low-cost preventive care. Health Aff (Millwood) 2012;31(12):2641–2648. doi: 10.1377/hlthaff.2012.0059. [DOI] [PubMed] [Google Scholar]
- Richman I., Asch S.M., Bhattacharya J., Owens D.K. Colorectal cancer screening in the era of the affordable care act. J. Gen. Intern. Med. 2015:1–6. doi: 10.1007/s11606-015-3504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatino S.A., Thompson T.D., Guy G.P., Jr., de Moor J.S., Tangka F.K. Mammography use among Medicare beneficiaries after elimination of cost sharing. Med. Care. 2016;54(4):394–399. doi: 10.1097/MLR.0000000000000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabik L.M., Adunlin G. The ACA and cancer screening and diagnosis. Cancer J. 2017;23(3):151–162. doi: 10.1097/PPO.0000000000000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J. Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- Solanki G., Schauffler H.H. Cost-sharing and the utilization of clinical preventive services. Am. J. Prev. Med. 1999;17(2):127–133. doi: 10.1016/s0749-3797(99)00057-4. [DOI] [PubMed] [Google Scholar]
- Song Z., Safran D.G., Landon B.E., Landrum M.B., He Y., Mechanic R.E. The ‘Alternative Quality Contract,’ based on a global budget, lowered medical spending and improved quality. Health Aff (Millwood) 2012;31(8):1885–1894. doi: 10.1377/hlthaff.2012.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Henry J. Kaiser Family Foundation . The Henry J. Kaiser Family Foundation. 2015. Preventive Services Covered by Private Health Plans Under the Affordable Care Act. [Google Scholar]
- The National Colorectal Cancer Research Alliance . In: 2010 Colorectal Cancer Legislation Report Card. Alliance T.N.C.C.R., editor. The National Colorectal Cancer Research Alliance; Los Angeles, CA: 2010. [Google Scholar]
- Trivedi A.N., Rakowski W., Ayanian J.Z. Effect of cost sharing on screening mammography in Medicare health plans. N. Engl. J. Med. 2008;358(4):375–383. doi: 10.1056/NEJMsa070929. [DOI] [PubMed] [Google Scholar]
- Trivedi A.N., Leyva B., Lee Y., Panagiotou O.A., Dahabreh I.J. Elimination of cost sharing for screening mammography in Medicare advantage plans. N. Engl. J. Med. 2018;378(3):262–269. doi: 10.1056/NEJMsa1706808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpp K.G., Asch D.A. Make the healthy choice the easy choice: using behavioral economics to advance a culture of health. QJM. 2017;110(5):271–275. doi: 10.1093/qjmed/hcw190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan T.T., Ortiz J., Berzon R., Lin Y.L. Variations in colorectal cancer screening of Medicare beneficiaries served by rural health clinics. Health Serv Res Manag Epidemiol. 2015;2 doi: 10.1177/2333392815597221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch H.G., Prorok P.C., O'Malley A.J., Kramer B.S. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N. Engl. J. Med. 2016;375(15):1438–1447. doi: 10.1056/NEJMoa1600249. [DOI] [PubMed] [Google Scholar]
- White A., Thompson T.D., White M.C., Sabatino S.A., de Moor J., Doria-Rose P.V. Cancer screening test use - United States, 2015. MMWR Morb. Mortal. Wkly Rep. 2017;66(8):201–206. doi: 10.15585/mmwr.mm6608a1. [DOI] [PMC free article] [PubMed] [Google Scholar]