Abstract
Simple Summary
The vascular endothelium plays an essential role in regulating cardiovascular homeostasis by controlling the vascular tone. However, it is known that variations of sex hormones in the female during the reproductive cycle have a significant impact on the homeostasis of the female cardiovascular system. Therefore, this review shows an overview of how the vascular smooth muscle response is altered in the presence of some endogenous and exogenous substances with vasoactive actions during the estrous cycle and during pregnancy in rats. We performed a systematic review of the literature using the Web of Science, PubMed, and Scielo database through multiple combinations of terms. We selected only those terms that coincided with the objectives of this study. The result of the review shows that several studies have observed both vasoconstrictive and vasodilatory responses induced by vasoactive substances during the estrous cycle and in ovariectomized and pregnant rats. The understanding of these effects is essential to optimize and develop new treatments for some vascular pathologies.
Abstract
Vascular endothelium plays a key role in regulating cardiovascular homeostasis by controlling the vascular tone. Variations in sex hormones during the reproductive cycle of females affect the homeostasis of the cardiovascular system. Also, the evidence shows that estrogens show a cardioprotective effect. On this basis, this study describes some vascular responses induced by vasoactive substances during the estrous cycle in rats. We obtained the information available on this topic from the online databases that included scientific articles published in the Web of Science, PubMed, and Scielo. Many investigations have evaluated the vasoactive response of substances such as acetylcholine and norepinephrine during the estrous cycle. In this review, we specifically described the vascular response to vasoactive substances in rats during the estrous cycle, pregnancy, and in ovariectomized rats. In addition, we discussed the existence of different signaling pathways that modulate vascular function. The knowledge of these effects is relevant for the optimization and development of new treatments for some vascular pathologies.
Keywords: vascular reactivity, estrous cycle, vasoactive substances, cardiovascular diseases, endothelium, endothelial cells, pregnancy
1. Introduction
The vascular endothelium is a tissue of mesodermal origin made up by a monolayer of polygonal cells of 10–50 μm thickness called endothelial cells (ECs). ECs are oriented longitudinally toward blood flow, covering the interior of all vessels [1]. The endothelium is a metabolically active tissue with great functional versatility playing a key role in the regulation, maintenance, and control of the vascular tone, through the production and release of various substances [2,3,4]. The regulation of the vascular tone and reactivity involves several factors, so that the balance resulting from the action of endothelial factors that induce vasoconstriction and cell proliferation (e.g., Ang-II, endothelin (ET), thromboxane A2 (TXA2), superoxide anion) and the factors inducing vasodilation and antiproliferation (e.g., nitric oxide (NO), prostacyclin (PGI2) and hyperpolarizing factor (EDHF)) is essential for the maintenance of the vascular tone [5,6,7]. Also, the vascular endothelium is responsible for the modulation of vascular cell growth, regulation of leukocyte and platelet adhesion, selective capillary transport, regulation of plasma lipids, and maintenance of a non-thrombogenic capillary surface [2,3,8,9,10,11].
ECs, in their normal state, act by regulating the balance between procoagulant and anticoagulant factors, for example, elaborating thrombomodulin and inhibiting the formation of the tissue factor (TF) that avoid the formation of thrombus. They also inhibit inflammation by releasing PGI2, preventing the adhesion of leukocytes, and thus maintaining vascular homeostasis [12,13,14]. The imbalance in the bioavailability of some of these modulators or loss in the integrity of the vascular endothelium can lead to endothelial dysfunction associated with inflammation, vasoconstriction and increased vascular permeability and the appearance of associated cardiovascular pathologies [15,16,17].
Sex hormones are specific in men and women and influence many functions. The relationship between sex hormone levels and cardiovascular diseases has been demonstrated [18]. The incidence of these pathologies is lower in premenopausal women than in men of the same age, later equaling during menopause [19]. In this sense, the role of estrogens in the vascular system has received considerable interest, since there is experimental and clinical evidence that they have beneficial or protective effects on the cardiovascular system in females [20,21,22,23,24,25,26]. Evidence confirms that the hormonal status of women fluctuates with each reproductive cycle and during pregnancy [27,28,29]. However, little information is available on the mechanisms of control of reactivity during the ovarian cycle and pregnancy, especially regarding how reproductive hormones regulate their functions. This information is an important milestone for a thorough understanding of the homeostasis of blood pressure.
The discovery of the endothelial function in the acetylcholine (ACh)-induced vasodilation represented a milestone in the biological sciences and also had an important consequence in preparing the isolated rat aorta as an experimental model [30]. Since then, the evaluation of changes in the vascular reactivity in the isolated rat aorta model has been a widely used method [30]. Also, the estrous cycle of the rodent has turned out to be an ideal model for cognitive and reproductive research since most of the cytological data are well characterized [31,32].
Because of these advances, some aspects of the vascular function have been studied in depth, while other issues remain unexplored. An important issue to consider is the decrease of sex hormones in aging, which is often associated with an increased risk of cardiovascular disease, the leading cause of death worldwide. The knowledge of how variations in sex hormones are involved in the development of vascular disorders is essential to find better strategies to prevent and treat cardiovascular diseases [33].
Various endogenous and exogenous substances affect the vascular reactivity in different ways; most times, the specific effect of such agents during the ovarian cycle or human pregnancy is unknown [31,32]. Therefore, this review aims to show a general overview of how the vascular response is altered in the presence of endogenous and exogenous vasoactive substances under the action of the estrous cycle and pregnancy using rats as an experimental model.
2. Materials and Methods
We performed a systematic review of the scientific literature using the Web of Science, PubMed, and Scielo databases through multiple combinations of MeSH terms: “Vascular Reactivity”, “Endothelial Factors”, “Rat Estral Cycle”, “Pregnancy”, “Vasoactive Substances” and “Vasoactive Natural Extracts”. We limited the search to studies in rats. We excluded articles written in a language other than English or Spanish. We obtained over 6000 articles that were analyzed and subsequently those that correspond to the objectives of this study were selected. Following this criterion, we chose and used 182 articles as a reference for this review.
2.1. Endothelial Factors Involved in the Modulation of Vascular Tone
Currently, it is known that the maintenance of the vascular tone and its regulation is due to the production and release of various endothelium-dependent factors. These factors, whether released by autocrine or paracrine mechanisms, are the determinants of the active participation of the endothelium in the vascular tone. According to their function, they are grouped into endothelial factors that induce vasoconstriction and proliferation, and factors that produce vasodilation and antiproliferation (see Table 1) and are described below:
Table 1.
Factors released by the vascular endothelium (Adapted from: De la Serna (2010) [14]).
| Vasoactive Factors | |
| Vasodilators | -Nitric oxide (NO) |
| -Endothelium-derived hyperpolarizing factor (EDHF) | |
| -Prostacyclin (PGI2) | |
| Vasoconstrictors | -Endothelin 1 (ET-1) |
| -Angiotensin II (Ang II) | |
| -Thromboxane A2 (TXA2) | |
| Growth modulators | |
| Growth promoters | -Platelet-derived growth factor (PDGF) |
| -Basic fibroblast growth factor (bFGF) | |
| -Somatomedin-1 (IGF-1) | |
| -Endothelin 1 (ET-1) | |
| -Angiotensin II (Ang II) | |
| -Heparan sulfate (HPS) | |
| Growth inhibitors | -Transforming growth factor (TGF) |
| -Nitric oxide (NO) | |
| -Prostacyclin (PGI2) | |
| Modulators of inflammation | |
| Adhesion molecules | -Endothelial leukocyte adhesion molecule (ELAM) -Intercellular adhesion molecule (ICAM) -Vascular cell adhesion molecule (VCAM) |
| Hemostatic and thrombolytic factors | |
| -Tissue plasminogen activator (t-PA) -Plasminogen activator inhibitor 1 (PAI-1) -Thrombomodulin |
|
2.1.1. Angiotensin II (Ang-II)
Ang-II is a vasoactive peptide hormone of relevance for maintaining the structural and functional integrity of the arterial wall, and for its role in regulating the renal, vascular, and cardiac function. This vasoactive peptide comes from its precursor angiotensin I (Ang-I) which is transformed into Ang II through the action of the dipeptidyl carboxypeptidase enzyme located in the membrane of ECs, also called angiotensin conversion enzyme (ECA). The effects of Ang II are related to the modulation of synaptic transmission, stimulation of vasopressin secretion, vasoconstriction, and stimulation of aldosterone secretion by the adrenal cortex. Besides, it can act in an endocrine manner to maintain blood pressure and electrolyte balance; it can also regulate highly specific cellular functions, such as cytokine secretion and cell proliferation [34,35,36]. These actions are mediated by complex signaling systems activated by the binding of the hormone to specific receptors, namely AT1 and AT2 [37,38,39,40]. In the vasculature, the AT1 and AT2 receptors are present in large numbers, concentrating mainly on smooth muscle cells; these receptors are in a low number in the adventitia, and practically they are not expressed in ECs [34]. AT1 and AT2 differ in the G protein they preferentially activate and in the variety of signals they initiate. Ang II is involved in various cardiovascular diseases, maintaining arterial hypertension by vasoconstrictor mechanisms, stimulating the sympathetic nervous system, increasing the release of aldosterone and increasing the production of superoxide anions, mainly in the endothelium and the adventitia through NADPH oxidase-linked membrane [41,42,43]. Recently, it was demonstrated that the ECs of cava and portal veins show important properties to be activated by Ang II, probably influencing the whole circulatory system [44].
2.1.2. Endothelin (ET)
Endothelins are a family of vasoconstrictor peptides that contribute to regulating the vascular tone and cell proliferation constituted by three isoforms of 21 amino acids with four cysteine residues, establishing two disulfide intramolecular bridges, forming an unconventional semi-conical structure [45,46]: Endothelin-1 (ET-1), endothelin-2 (ET-2) and endothelin-3 (ET-3) differing in their potency of vasoconstrictive action [47]. The biological effects of ET-1 are relevant in the maintenance of the basal vasomotor tone and blood pressure in humans [48,49,50,51,52]. They have direct mitogenic effects on smooth muscle cells, stimulating the production of cytokines and growth factors [26,53,54]. They also induce the formation of extracellular matrix proteins and fibronectin and potentiate the effect of transforming the growth factor-beta (TGF-b) and platelet-derived growth factor (PDGF) [26,53]. On the other hand, vasodilators such as NO and PGI2 act by inhibiting the production of ET-1 through common mechanisms involving the production of cyclic guanosine monophosphate (cGMP). The same function fulfills the auricular natriuretic hormone, inhibiting the basal ET production. These same hormones inhibit the mitogenic and vasoconstrictor effect stimulated by ET [55,56]. It has been proven that the synthesis of ET-1 is stimulated and associated with various cardiovascular risk factors, the main ones are high cholesterol levels, low-density lipoproteins, and glucose, estrogen deficiency, obesity, pro-coagulant mediators similar to thrombin. Also, vasoconstrictors, growth factors, cytokines, and adhesion molecules also stimulate endothelin production [52,57,58,59,60,61].
2.1.3. Thromboxane A2 (TXA2)
TXA2 is an eicosanoid resulting from the transformation of the arachidonic acid (AA) derivative by the enzyme TXA2 synthase. It has been shown that TXA2 is a potent platelet aggregator and vasoconstrictor produced by the vascular wall and its biological actions involve platelet aggregation, contraction of muscle cells [62], regulation of cell migration processes endothelial, angiogenesis and tumor metastasis [26,63]. Such functions explain their participation in the process of hemostasis, while in the respiratory system it is a powerful bronchoconstrictor. The specific receptor for TXA2 is called TP and has two isoforms called α and β, through which TXA2 exerts its actions. They are distinguished by their carboxy-terminal end located inside the cell. This fact explains the multiple transduction pathways of signals activated by this receptor through different G proteins [64,65,66,67]. The release of TXA2 and other PGs vasoconstrictors can be stimulated by different vasoconstrictive agents such as ET, 5-HT, NA, and A-II, as well as by vasodilator agents such as ACh or by mechanical actions on endothelial cells [68,69,70]. It has been described that both endothelial cells [61,69] and the smooth muscle [71,72] synthesize TXA2. The synthesis of TXA2 is increased in a variety of cardiovascular disorders, in which TXA2 is implicated as a key cellular mediator [73]. TXA2 participates in the endothelial dysfunction associated with different cardiovascular risk factors such as diabetes and hypertension [74,75]. The inhibition of the synthesis of TXA2 by aspirin has been associated with a decrease in mortality caused by cardiovascular diseases [73]. Recently, it has been proposed that the interaction between LPA/LPA1 and TXA2/TP pathway plays a significant role in vasoregulation, hemostasis, thrombosis, and vascular remodeling (LAP = Lysophosphatidic acid; TP = Thromboxane prostanoid) [76].
2.1.4. Reactive Oxygen Species (ROS)
The generation of reactive oxygen species (ROS), such as superoxide anion (O2-), is generally produced in all tissues [77,78]. However, the presence of ROS in the vasculature is a consequence of the abnormal generation and/or an inability of the endogenous reducer and antioxidant systems to eliminate these reactive species [79]. These species can exert their action directly on the vascular system or serve as a substrate for the formation of other ROS, such as hydrogen peroxide (H2O2), peroxynitrite (OONO-), hypochlorous acid (HOCl) and the hydroxyl radical (OH.), among others.
In vascular cells, potential sources of O2- include the mitochondrial electron transport chain and the participation of nicotinamide adenine dinucleotide/nicotinamide adenine dinucleotide phosphate (NADH/NADPH) oxidase, xanthine oxidase and NO endothelial synthase (eNOS) [80,81,82,83]. However, the NADH/NADPH oxidase system is the primary source of O2-vascular and comprises membrane-bound oxidases that use NADH and NADPH as substrates [84,85,86]. In non-pathological conditions, the generation of ROS by eNOS or mitochondria is limited, and in the case of mitochondria, it is probably more related to signaling pathways [87,88]. However, there is significant evidence pointing to the generation of ROS by decoupling eNOS; this situation arises when NADPH consumption is not correlated with a stoichiometric production of NO, what is called decoupling of the eNOS [89,90,91]. This phenomenon is caused by several factors that can alter the transfer of electrons in NOS, so that the amount of NADPH required to generate NO is relatively higher, primarily explained by the reduction of other substrates mainly molecular oxygen to produce superoxide [79].
These species have an unpaired electron, so they are highly reactive. Like these species, NO has an unpaired electron so that it can react with them through a chemically spontaneous and irreversible reaction [92,93]. When O2- reacts with NO, it inactivates, hindering vasorelaxation and inducing apoptosis in endothelial cells, favoring the appearance of thrombotic phenomena and promoting the adhesion of different cells to the endothelium. Additionally, the product of the reaction, OONO-, is a powerful oxidant that produces critical biological effects such as a protein nitrosylation [26,93,94].
The participation of ROS in the vasorelaxation has been demonstrated in at least two ways: First, in the interaction with NO and second, through the direct effects of H2O2. It is well established that the NO derived from the endothelium undergoes a rapid reaction with O2- reducing its bioavailability [95]. A growing number of experimental evidences in animals and humans suggest that the oxidative inactivation of NO plays an essential role in several vascular pathologies such as hypertension, diabetes, hypercholesterolemia, and arteriosclerosis [96,97,98,99].
2.1.5. Nitric Oxide (NO)
NO is a free radical with an unpaired electron that can participate in several reactions, acting as a weak oxidant or reducer [100,101]. Its neutral charge allows it to diffuse freely through the biological membranes, explaining its potential to act in different biological targets [102,103]. NO can react with O2- forming peroxynitrite (ONOO-), a potent oxidant involved in the oxidation of proteins under physiological conditions [94].
The synthesis of NO is carried out by a family of enzymes called nitric oxide synthases (NOS), of which three isoforms exist, exhibiting different characteristics reflecting their specific functions in vivo [100,104,105,106]. There are two constitutive isoforms called eNOS or endothelial, present mainly in endothelial cells [107] and nNOS or neuronal present mainly in neurons. Both isoforms are dependent on Ca2+ and calmodulin [108]. The third isoform, called iNOS, is inducible and is independent of Ca2+ and calmodulin and is present in different cell types. iNOS is expressed in abnormal cellular processes such as heart failure [109]. Also, iNOS is induced by cytokines and inflammatory agents determining high NO levels [26,110,111,112]. Furchgott et al. (1980) [113], elegantly demonstrated that the endothelium was instrumental in the vascular smooth muscle relaxation. In rats, Rees et al. (1989) [114], found that blocking the formation of NO with the inhibitor L-NMMA resulted in an immediate rise in blood pressure and that the subsequent administration of the L-arginine substrate reversed this effect. NO also plays a role in the endothelium-dependent venous relaxation [115].
2.1.6. Prostaglandins
Prostaglandins, like thromboxanes, are cellular mediator eicosanoids synthesized from AA by the activation of cyclooxygenases (COX) [116,117]. The (COX) are bifunctional enzymes that have two catalytic activities and a cyclooxygenase which incorporates two molecules of O2 to AA forming PGG2, and another peroxidase, which catalyzes the reduction of PGG2 to PGH2 [118] generating prostanoids, lipid mediators of inflammation with autocrine and paracrine action, including both prostaglandins (PGs) and thromboxanes (TXs). There are two isoforms of the COX enzymes: COX1, a constitutive enzyme present in most of the body’s cells and that regulates the basal homeostasis of prostanoid synthesis [119,120], and COX-2, an inducible enzyme against inflammatory stimuli such as the action of pro-inflammatory cytokines, bacterial lipopolysaccharides (LPS), growth factors and tumor-promoting agents [117,121,122].
Prostaglandin I2 (PGI2) is one of the primary metabolites of the COX pathway. It was first discovered by Needleman and Vane in 1976 [123,124]. PGI2 is produced in ECs and the lung parenchyma by the action of the enzyme prostacyclin synthase on prostaglandin H2 [116,125,126,127]. The main action of PGI2 is to avoid the aggregation of platelets; however, it is also an effective vasodilator [128]. PGI2 has the opposite effect of TXA2, suggesting a homeostatic mechanism between these two hormones. It has been shown that the release of PGI2 depends mainly on the exit of Ca2+ from intracellular deposits [129]: At low concentrations, PGI2 activates its specific IP receptor producing vasodilation by reducing the intracellular concentration of Ca2+ in smooth muscle cells by the action of cAMP after the activation of adenylate cyclase (AC). On the contrary, at high concentrations, PGI2 can activate IP receptors and produce vasoconstriction [130,131,132]. It has been observed that the stimulation of PGI2 synthesis by factors such as Ang-II, acetylcholine (ACh) or bradykinin (Bk), and products released from platelets such as 5-HT, interleukin-1 and platelet-derived growth factor (PDGF) [133], NO [134], ROS [135] and the prostanoids themselves [136,137], indicates a rich interaction between different cellular mediators [26,138]. Additionally, the cardioprotective effect of PGI2 has been related to a protective action against atherogenesis and heart disease, because PGI2 decreases cholesterol synthesis and reduces the activity of LDL receptors in leukocytes [139]. Likewise, it has been described that some of its analogs inhibit the progression of atherosclerosis in the aorta of hamsters and rabbits [26,140,141].
On the other hand, other eicosanoids that give rise to AA are leukotrienes (LTs) and lipoxins (LXs) through a pathway mediated by lipoxygenases (LOX); besides, hydroxyeicosatetraenoic acids (HETEs) and epoxyeicosatrienoic acids (EETs) are generated by a cytochrome P 450-mediated pathway (CYP450). Concerning the route mediated by LOXs, the three main enzymes involved are the 5-, 12- and 15-lipoxygenases (5-LOX, 12-LOX, 15-LOX) present in leukocytes, platelets, and endothelial cells, respectively [142]. LTs act as lipid mediators with the paracrine action involved in inflammatory and immune processes mediated by specific rhodopsin-receptors coupled to G-protein [117,143].
The cytochrome P450 family (CYP450) are enzymes anchored to the cell membrane with a heme group in their structure. These enzymes catalyze the conversion of fatty acids, including AA, to various derivatives, including EETs obtained from AA by the action of CYP2C and CYP2J enzymes or hydroxylated derivatives such as HETEs mediate by the CYP4A enzyme [142]. It has been suggested that the EETs could act as EDHF with the vasodilator action. One example is the conversion of AA to EETs in the vascular endothelium, with anti-inflammatory activity and anti-platelet aggregation effect, while, in the vascular smooth muscle it is hydroxylated and converted to 20-HETE with vasoconstrictive effect. The action of both compounds contributes to maintaining vascular homeostasis [143].
2.1.7. Endothelium-dependent Hyperpolarizing Factor (EDHF)
The EDHF group includes epoxyeicosatrienoic acids (EETs), which respond to stimuli such as ACh, Bk, and shear stress, causing the opening of K+ channels [144,145]. EETs correspond to metabolites of AA in which the cytochrome P-450 is involved [146]. They are produced by the endothelium and contribute to the regulation of the vascular tone by inducing relaxation through a hyperpolarization of the smooth muscle membrane [147]. Hyperpolarization and relaxation produced by EDHF seem to be due to an increase in the conductance of the K+ in the vascular smooth muscle membrane, through the Ca2+ or ATP-dependent K+ channels [148], depending on the vascular bed [26]. Several studies suggest that EDHF is particularly important in response to endothelium-dependent vasodilator agents in coronary and mesenteric circulation [149]. Their participation is modified in pathophysiological situations in which there is a decrease in other endothelial factors such as NO [150]. Endothelial cells induce smooth muscle hyperpolarization in a contact-dependent manner with H2O2 [151], K ions [152], CNP [153], H2S [154]. Putative diffusible candidates for the EDHF activity have increased over the past 15 years [155]. Besides, EDHF appears to be the predominant endothelial factor in the vascular resistance in women, a response influenced by sex [19].
Physiologically, a lower bioavailability of NO, alterations in the production of prostanoids (including PGI2, TXA2 and/or isoprostanes), deterioration of endothelium-dependent hyperpolarization, and a higher release of ET-1, can contribute individually or associated with endothelial dysfunction. Endothelial dysfunction can be understood as an endothelium situation where ECs do not adequately fulfill the functions mentioned above, either in basal or stimulated conditions.
According to the above, and based on the evidence, it can be indicated that the ECs produce various bioactive compounds, including products generated by the enzymes eNOS and COX, capable of regulating the vascular tone. It is interesting to note that, through the activation of COX, vasoconstrictor prostanoids and vasodilators are produced, and both can be modulated in their synthesis by NO. This phenomenon and the diversity of interrelationships that occur between the signals that determine the vascular tone shows the importance of considering the influence of sex hormones on the cardiovascular system during the estrous cycle and pregnancy.
3. Estrous Cycle and Vascular Reactivity
An estrous cycle is a periodic event, with regular but restricted phases of sexual receptivity associated with the release of fit ovules to be fertilized [156]. This period typically covers between 17 and 24 days, considering 21 days as the average time, depending on the species [157,158]. In the rat, the estrous cycle has a relatively short duration of four days approximately and is divided into four phases, perfectly distinguishable by vaginal smear [159,160]: (1) Proestrous, follicular phase or regression of the corpus luteum with a duration of 12 h; (2) estrous, a period of sexual receptivity, at the end of which ovulation occurs with a duration of 12 h; (3) metestrous, where the initial development of the corpus luteum occurs with a duration of 21 h; and (4) diestrous, beginning of the activity of the mature corpus luteum with a duration of approximately 65 h [156,161]. Its short duration allows the rat to be an ideal species for the investigation of the changes that occur during the reproductive cycle [162].
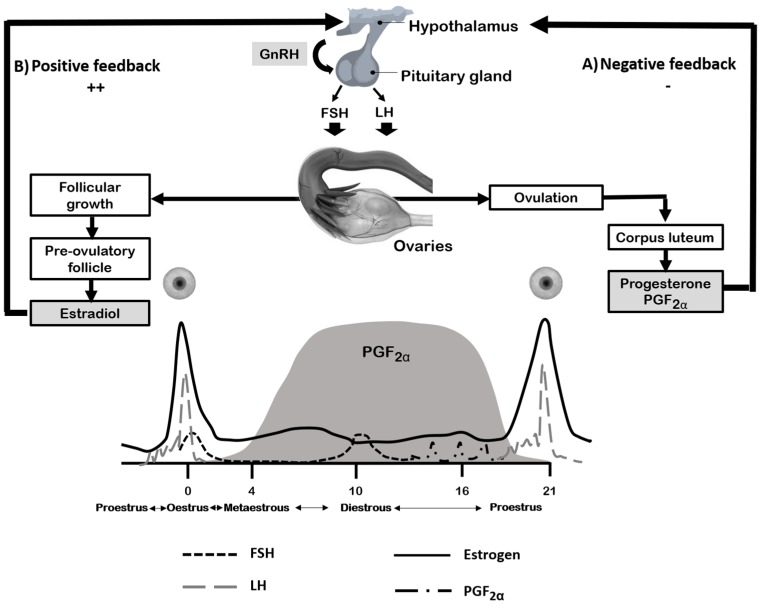
The hormonal regulation of the estrous cycle is under the neurological control of the hypothalamus-pituitary, ovary, and uterus axis (Figure 1). The activity begins with the pulsatile secretion of gonadotropins (GnRH) from the neurosecretory parvicellular neurons located in the arcuate nucleus, the periventricular nucleus and the preoptic area [163]. The hormone acts as a messenger, regulating each of the phases of the estrous cycle [164]. During estrous, metestrous and diestrous, the secretion of GnRH, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), are at basal levels by the inhibitory or feedback-negative action of the ovarian hormones, mainly estradiol, but also, progesterone and inhibins [161,165]. The amplitude and frequency of the GnRH pulses and feedback from progesterone and estrogens control the processes of synthesis and secretion of LH and FSH from adenohypophyseal gonadotrophs. GnRH stimulates the growth of antral follicles and increases the production of ovarian steroids, which affects the secretion of LH/FSH for the benefit of negative feedback. A low frequency and high pulse amplitude of GnRH leads to the release of FSH, while a high frequency and low amplitude pulses stimulate the release of LH. However, in the female the frequency of the GnRH pulses varies during the estrous cycle, being higher during the pre-ovulatory surge of LH and lower during the luteal phase of the estrous cycle [166,167,168]. Progesterone is also produced in the corpus luteum by the action of LH and prepares the uterus to allow implantation of the embryo and to maintain pregnancy. Progesterone exerts a negative feedback effect on the hypothalamus [161].
Figure 1.
Simplified scheme of the hormonal interactions of the hypothalamus-pituitary-ovarian axis during the estrous cycle. (A) Negative feedback: Decreased LH concentration characteristic of the late follicular phase. The initial effects of ovarian steroids eliminate the responsiveness of the pituitary to the action of GnRH, since the secretion of these by the hypothalamus is continuous at this stage; (B) positive feedback: Continuation of the previous period, here is a significant increase (about 90%) in the concentration of LH. The effect produced result from the combined action of high levels of estrogen and progesterone (of ovarian origin) on the hypothalamus and of the latter (through hormonal release factors) on the pituitary. This phenomenon constitutes the endocrine event that characterizes the preovulatory period.
On the other hand, during proestrous, estrogens exert a positive feedback at the level of secretion of GnRH, LH, and FSH. Among the positive actions are: (1) Cooperation with GnRH in the synthesis of its receptors at the pituitary level; (2) pituitary sensitization to GnRH (probably through a post-receptor mechanism), (3) increase of the prostaglandin receptor at the pituitary and hypothalamic level, (4) regulation of the GnRH synthesis, (5) cooperation in the appearance of the preovulatory secretion of hypothalamic decapeptide, and (6) decrease in the metabolism of GnRH in the pituitary gland, all them increasing effectiveness [168]. In the pituitary gland, estrogens carry out inhibitory and stimulatory actions on the secretion of gonadotropins by activating their receptors in the gonadotrope, among which are different types of localization and isoforms (intracellular or membrane, α or β) [161,169].
Lower amounts of estrogen are synthesized in the adipose tissue and liver from testosterone by aromatase [170], enzyme P-450 responsible for the aromatization of cholesterol for conversion to estrogen in the endoplasmic reticulum in the ovary and placenta where the synthesis of estrogens occurs, and in the adrenal gland and testes. This enzyme is also found in the vasculature and can synthesize estrogen locally for paracrine/autocrine effects in vessels [171]. Although human endothelial cells do not contain aromatase, the vascular smooth muscle cells of the aorta and the pulmonary arteries contain them [172].
These hormones play an essential role in the vascular reactivity [173], and their most prominent effects are mediated by direct actions on the endothelial function [174]. Several propositions try to explain the mechanism of action by which estrogenic hormones exert cardiovascular protection in women. One of the most accepted explanations is related to the effects of estrogen on lipid metabolism [175]. Estrogen decreases the levels of low-density lipoprotein and lipoprotein (A) in the blood [170] while it increases the cholesterol bound to high-density lipids [176,177,178]. These actions may explain the protective effect of estrogen in women against the development of atherosclerosis, a disease closely associated with menopause [175]. Atherosclerosis begins with the deposit of lipid material and blood cells, together with the formation of lesions that give rise to the fibrous plaque, which provides rigidity to the blood vessels affecting the hemodynamic properties of the circulatory system [177].
Conversely, in ECs, estrogens trigger the synthesis of factors derived from the endothelium, such as NO, ET-1, and prostacyclins [179,180]. Estrogen can bind to the α-estrogen receptor in the ECs membrane of the caveolae [181] activating the NOS [177,182]. These findings were confirmed in a clinical study where the alteration of the endothelium-dependent vasodilation induced by ACh was normalized after an estrogen replacement therapy [183]. The vascular reactivity in the estral cycle was studied in rat aortic rings [184]. Recently our group has reported that the indomethacin affects the norepinephrine-induced contraction in the vascular smooth muscle in different stages of the estrous cycle suggesting that cyclooxygenase produces an increase in contraction especially during proestrous [185].
4. Pregnancy and Vascular Reactivity
Pregnancy begins with the union of the egg and sperm in the tube; the egg is transferred to the uterus to complete the development of the fetus. After pregnancy, the corpus luteum, responsible for the secretion of estrogen and progesterone, increases its size and its function is maintained until the third month of gestation [29], secreting slightly higher amounts of hormones than those that occur after ovulation and second half of the menstrual cycle. From the fourth month of gestation, the placenta secretes progressively elevated amounts of these hormones, reaching a maximum at the end of pregnancy [27]. Steroids produced by the placenta come from steroidal precursors that enter from the maternal bloodstream or the fetus. Therefore, maternal estrogen levels throughout pregnancy reach concentrations thirty times higher than those found in the luteal phase.
On the other hand, progesterone originates from the maternal cholesterol, and 90% of the progesterone produced in the placenta passes into the maternal circulation and the remaining 10% into the fetal circulation. Progesterone levels throughout pregnancy increase progressively, reaching concentrations ten times higher than those found during the luteal phase of the ovarian cycle [29]. In this sense, rats show similar changes during pregnancy, thus providing an adequate initial experimental model for pregnancy and vascular reactivity studies [186,187,188,189].
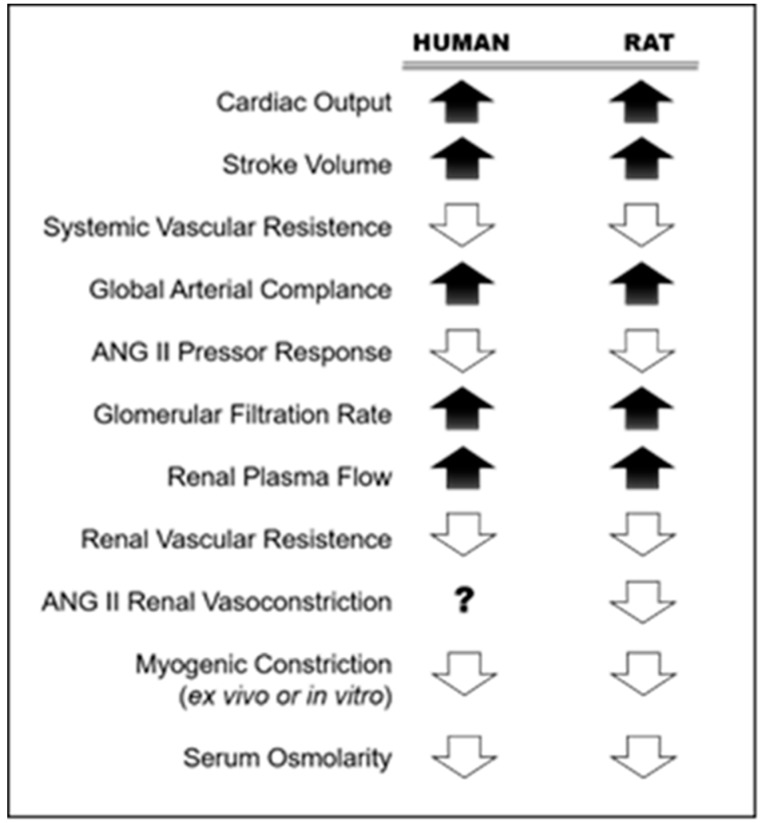
During pregnancy, there are alterations in the maternal cardiovascular system that leads to the appearance of systemic vascular manifestations of the same. These changes occur rapidly to provide an efficient uteroplacental circulation that facilitates the development of the fetus [190]. It has been proposed that estrogens act as mediators of vascular changes during pregnancy due to their effects on the vasculature and its high concentration during this period. There are a number of well-described changes in the cardiovascular system (Figure 2) that include significant increases in cardiac output and blood volume with a reduction in peripheral vascular resistance [190,191,192,193,194], a significant decrease in the pressor response to vasoconstrictors administered exogenously [195], and vascular remodeling conducting to higher compliance [196]. However, the mechanisms that could explain such vascular modifications during normal pregnancy remain little known and of high relevance considering the inadequate adaptation of the cardiovascular system to pregnancy associated with hypertensive disorders during pregnancy, such as preeclampsia and fetal growth impairment [197,198].
Figure 2.
Renal and systemic vasodilation during pregnancy in human and rat. Adapted from: Conrad, 2011 [198].
5. Actions of Exogenous Vasoactive Substances on The Endothelial Function during the Estrous Cycle and Pregnancy
The difficulties associated with carrying out in vivo studies in humans have led researchers to examine the vascular changes that occur during pregnancy and the ovarian cycle through indirect methods. These have included animal models, mainly rats, and have focused on understanding the effect of vasoactive substances during the estrous cycle. Regarding pregnancy, to date, most investigations have focused on the exogenous administration of estrogens to non-pregnant animals where estrogen levels are close to those of pregnancy [199]. Likewise, the results have been contradictory with some reports of attenuation of the pressor response capacity [200,201,202,203]. However, other studies have shown no effect [204]. The differing data could be due to methodological differences including dose used, type of administration (acute versus chronic), duration of oophorectomy before administration of steroids and heterogeneity of the vascular bed [199]. In this sense, the actions of substances of non-estrogenic origin on the vascular endothelium have also been studied, and the effects of pregnancy and the influence of the estrous cycle on its effects have been determined. Table 2 summarizes some results.
Table 2.
Some vasoactive substances capable of modulating vascular tone in different endothelial tissues.
| Vasoactive Substance | Model | Tissue | Actions in Endothelium | References |
|---|---|---|---|---|
| Norepinephrine (NE) | Control group: Cyclic rats Experimental group: Ovariectomized rats (OVX) |
Aorta (vascular smooth muscle) Treated in isometric myography system |
-The vascular synthesis of both PGE2 and PGF2a was significantly higher in the group of OVX rats compared to the proestrous. -The vascular response to NE was significantly higher in OVX rats, compared to normal cycling rats during proestrous. |
[205] |
| Cirazoline (α1-Adrenergic agonist) |
Pregnant, proestrous and diestrous rats | Mesenteric vascular bed Treated by perfusion |
-The tone induced by cirazoline was lower in the proestrous and pregnant groups, but the increase in the tone of L-NA is higher in pregnant compared to proestrous and diestrous group. The participation of EDRF in this effect is suggested. | [206] |
| Methacholine (Muscarinic agonist, endothelium-dependent vasodilator) |
Control group: Experimental group: Pregnant mice |
Uterine and mesenteric arteries Treated in isometric myography system |
-The relaxation induced by methacholine was higher in pregnant mice, in both uterine artery and mesenteric vessels, with a more pronounced effect on the uterine vasculature. -Modulation of relaxation is endothelium-dependent PGHS or NOS pathways is reinforced in the uterine arteries. |
[207] |
|
Aspilia africana (Ethanolic extract) |
Cyclic rats | Uterine endothelium Treatment by using an oropharyngeal cannula and calibrated hypodermic syringe |
-Dose-dependent decrease in duration of estrous cycle and histoarchitecture of the uterus. | [208] |
|
Buddleja globosa (Ethanolic extract) |
Cyclic rats Ovariectomized rats (OVX) |
Uterine endothelium Treatment administered subcutaneously with hypodermic syringe |
Anti-estrogenic effect of extract of Buddleja globosa at the highest dose evaluated. | [209] |
6. Conclusions
After a thorough review of the literature, it is possible to conclude that sex hormones, mainly estrogens, play an important role as modulators of the vascular tone through the vascular endothelium. Evidence shows that these effects are associated with the cardio protection observed in young (premenopausal) women and in pregnancy.
Likewise, the vascular effects of some natural and synthetic products have been discussed as agents capable of modulating the vascular tone in rats. However, more research is still needed on this issue to better understand the effective impact of these vasoactive substances during the estrous cycle. Herbs have a potential therapeutic value in the prevention and/or complementary treatment of vascular disorders that may occur during human pregnancy.
Acknowledgments
We thanks to Grant R17A10001 from CREAS for facilities support.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “conceptualization, L.J. and J.L.M.; methodology, L.J., C.L. and B.M.; investigation, L.J., M.K. and J.B.; resources, J.L.M.; writing-original draft preparation, L.J.; writing-review and editing, R.V.; supervision, J.L.M.; funding acquisition, J.L.M.”, please turn to the CRediT taxonomy for the term explanation. Authorship must be limited to those who have contributed substantially to the work reported.
Funding
Funded by the DICYT-Universidad de Santiago de Chile. Project No. 021643MS of José L. Martínez.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Aird W.C. Phenotypic Heterogeneity of the Endothelium: I. Structure, Function and Mechanisms. Circ. Res. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 2.Tran Q.K., Ohashi K., Watanabe H. Calcium signaling in endothelial cells. Cardiovasc. Res. 2000;48:13–22. doi: 10.1016/S0008-6363(00)00172-3. [DOI] [PubMed] [Google Scholar]
- 3.Bonetti P.O., Lerman L.O., Lerman A. Endothelial dysfunction. A marker of atherosclerotic risk. Art. Thromb. Vasc. Biol. 2003;23:168–175. doi: 10.1161/01.ATV.0000051384.43104.FC. [DOI] [PubMed] [Google Scholar]
- 4.Vogel R.A. Heads and hearts. The endotelial connection. Circulation. 2003;107:2766–2768. doi: 10.1161/01.CIR.0000080660.63404.68. [DOI] [PubMed] [Google Scholar]
- 5.Furchgott R.F., Vanhoutte P.M. Endothelium-derived relaxing and contracting factors. FASEB J. 1989;3:2007–2018. doi: 10.1096/fasebj.3.9.2545495. [DOI] [PubMed] [Google Scholar]
- 6.Lüscher T.F., Vanhoutte P.M. The Endothelium: Modulator of Cardiovascular Function. 1st ed. CRC Press; Boca Ratón, FL, USA: 1990. pp. 1–23. [Google Scholar]
- 7.Lévesque H., Cailleux N., Courtois H. Vasoactive peptides of endothelial origin. Therapeutic perspectives. Presse Med. 1994;23:1172–1177. [PubMed] [Google Scholar]
- 8.Anderson T.J. Assessment and treatment of endothelial dysfunction in humans. J. Am. Coll. Cardiol. 1999;34:631–638. doi: 10.1016/S0735-1097(99)00259-4. [DOI] [PubMed] [Google Scholar]
- 9.Behrendt D., Ganz P. Endothelial function: From vascular biology to clinical applications. Am. J. Cardiol. 2002;90:40L–48L. doi: 10.1016/S0002-9149(02)02963-6. [DOI] [PubMed] [Google Scholar]
- 10.Celermajer D.S. Endothelial dysfunction. Does it matter? Is it reversible? J. Am. Coll. Cardiol. 1997;30:325–333. doi: 10.1016/S0735-1097(97)00189-7. [DOI] [PubMed] [Google Scholar]
- 11.Vogel R.A. Coronary risk factors, endothelial function, and atherosclerosis: A review. Clin. Cardiol. 1997;20:426–432. doi: 10.1002/clc.4960200505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saadi S., Holzknecht R.A., Patte C.P., Stern D.M., Platt J.L. Endothelial cell activation by pore-form structures. Pivotal role for interleukin-1 alpha. Circulation. 2000;101:1867–1873. doi: 10.1161/01.CIR.101.15.1867. [DOI] [PubMed] [Google Scholar]
- 13.Luther T., Mackman N. Tissue factor in the heart. Multiple roles in hemostasis, thrombosis, and inflammation. Trends Cardiovasc. Med. 2001;11:307–312. doi: 10.1016/S1050-1738(01)00129-3. [DOI] [PubMed] [Google Scholar]
- 14.De la Serna F. Insuficiencia Cardíaca Crónica. 3rd ed. Editorial Federación Argentina de Cardiología; Buenos Aires, Argentina: 2010. pp. 114–171. [Google Scholar]
- 15.Badimon L., Badimon J.J., Penny W., Webster M.W., Chesebro J.H., Fuster V. Endothelium and atherosclerosis. J. Hypertens. 1992;10:S43–S50. [PubMed] [Google Scholar]
- 16.Badimon L., Martínez-González J. Endotelio en la protección vascular: Nuevos conocimientos. Rev. Esp. Cardiol. 2002;55:S17–S26. [PubMed] [Google Scholar]
- 17.Badimon L. Estatinas y función endotelial. Rev. Esp. Cardiol. 2003;3:C25–C40. [Google Scholar]
- 18.Teede H.J. Sex hormones and the cardiovascular system: Effects on arterial function in women. Clin. Exp. Pharmacol. Physiol. 2007;34:672–676. doi: 10.1111/j.1440-1681.2007.04658.x. [DOI] [PubMed] [Google Scholar]
- 19.Villar I., Hobbs A.J., Ahluwalia A. Sex differences in vascular function: Implication of endothelium-derived hyperpolarizing factor. J. Endocrinol. 2008;197:447–462. doi: 10.1677/JOE-08-0070. [DOI] [PubMed] [Google Scholar]
- 20.Farhat M.Y., Lavigne M.C., Ramwell P.W. The vascular protective effects of estrogen. FASEB J. 1996;10:615–624. doi: 10.1096/fasebj.10.5.8621060. [DOI] [PubMed] [Google Scholar]
- 21.Joswig M., Hach-Wunderle V., Ziegler R., Nawroth P.P. Postmenopausal hormone replacement therapy and the vascular wall: Mechanisms of 17 beta-estradiol’s effects on vascular biology. Exp. Clin. Endocrinol. Diabetes. 1999;107:477–487. doi: 10.1055/s-0029-1232556. [DOI] [PubMed] [Google Scholar]
- 22.Jones R.D., Hugh Jones T., Channer K.S. The influence of testosterone upon vascular reactivity. Eur. J. Endocrinol. 2004;151:29–37. doi: 10.1530/eje.0.1510029. [DOI] [PubMed] [Google Scholar]
- 23.Liu P.Y., Death A.K., Handelsman D.J. Androgens and cardiovascular disease. Endocrinol. Rev. 2003;24:313–340. doi: 10.1210/er.2003-0005. [DOI] [PubMed] [Google Scholar]
- 24.Perusquia M. Androgen-induced vasorelaxation: A potential vascular protective effect. Exp. Clin. Endocrinol. Diabetes. 2003;111:55–59. doi: 10.1055/s-2003-39229. [DOI] [PubMed] [Google Scholar]
- 25.Littleton-Kearney M., Hurn P.D. Testosterone as a modulator of vascular behavior. Biol. Res. Nurs. 2004;5:276–285. doi: 10.1177/1099800403262927. [DOI] [PubMed] [Google Scholar]
- 26.Martorell A. Ph.D. Thesis. Universidad Autónoma de Madrid; Madrid, Spain: 2008. Influencia de las Hormonas Sexuales Endógenas en la Respuesta Vasodilatadora Inducida por Acetilcolina. Participación de Protanoides e Interacción con el Óxido Nítrico. [Google Scholar]
- 27.Netter F.H. Sistema Reproductor. 2nd ed. Editorial Salvat; Barcelona, Spain: 1990. pp. 85–105. [Google Scholar]
- 28.Kalin M.F., Zumoff B. Sex hormones and coronary disease: A review of the clinical studies. Steroids. 1990;55:330–351. doi: 10.1016/0039-128X(90)90058-J. [DOI] [PubMed] [Google Scholar]
- 29.Usandizaga J.A., de la Fuente P. Tratado de Obstetricia y Ginecología. 1st ed. Editorial McGraw-Hill Interamericana; Madrid, Spain: 1997. pp. 532–768. [Google Scholar]
- 30.Vinet R., Knox M., Mascher D., Paredes-Carbajal C., Martinez J.L. Isolated aorta model and its contribution to phytopharmacology. Bol. Latinoam. Caribe. Plant. Med. Aromat. 2012;11:35–45. [Google Scholar]
- 31.Fujime M., Tomimatsu T., Okaue Y., Koyama S., Kanagawa T., Taniguchi T., Kimura T. Central aortic blood pressure and augmentation index during normal pregnancy. Hypertens. Res. 2012;35:633–638. doi: 10.1038/hr.2012.1. [DOI] [PubMed] [Google Scholar]
- 32.Khalil A., Jauniaux E., Cooper D., Harrington K. Pulse wave analysis in normal pregnancy: A prospective longitudinal study. PLoS ONE. 2009;4:e6134. doi: 10.1371/journal.pone.0006134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams B., Mancia G., Spiering W., Rosei E.A., Azizi M., Burnier M., Clement D., Coca A., De Simone G., Dominiczak A., et al. Practice guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology. J. Hypertens. 2018;36:2284–2309. doi: 10.1097/HJH.0000000000001961. [DOI] [PubMed] [Google Scholar]
- 34.Dostal D.E., Baker K.M. The cardiac rennin-angiotensin system. Conceptual, or a regulator of cardiac function? Circ. Res. 1999;85:643–650. doi: 10.1161/01.RES.85.7.643. [DOI] [PubMed] [Google Scholar]
- 35.Touyz R.M., Schiffrin E.L. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol. Rev. 2000;52:639–672. [PubMed] [Google Scholar]
- 36.Dinh D.T., Fauman A.G., Johnston C.I., Fabiani M.E. Angiotensin receptors: Distribution, signalling and function. Clin. Sci. 2001;100:482–492. doi: 10.1042/cs1000481. [DOI] [PubMed] [Google Scholar]
- 37.Heeneman S., Sluimer I.J., Daemen M.J. Angiotensin converting enzyme and vascular remodeling. Circ. Res. 2007;101:441–454. doi: 10.1161/CIRCRESAHA.107.148338. [DOI] [PubMed] [Google Scholar]
- 38.De Gasparo M., Whitebread S., Mele M., Motani A.S., Whitcombe P.J., Ramjoué H.P., Kamber B. Biochemical characterization of two angiotensin II receptor subtypes in the rat. J. Cardiovasc. Pharmacol. 1990;16:S31–S35. doi: 10.1097/00005344-199016004-00008. [DOI] [PubMed] [Google Scholar]
- 39.Horiuchi M., Akishita M., Dzau V.J. Recent progress in angiotensin II type 2 receptor research in the cardiovascular system. Hypertension. 1999;33:613–621. doi: 10.1161/01.HYP.33.2.613. [DOI] [PubMed] [Google Scholar]
- 40.Escobar E., Rodríguez-Reyna T.S., Arrieta O., Sotelo J. Angiotensin II, cell proliferation and angiogénesis regulator: Biologic and therapeutic implications in cancer. Curr. Vasc. Pharmacol. 2004;2:1–15. doi: 10.2174/1570161043385556. [DOI] [PubMed] [Google Scholar]
- 41.Ferrario C.M., Brosnihan K.B., Diz D.I., Jaiswal N., Khosla M.C., Milsted A., Tallant E.A. Angiotensin-(1-7): A new hormone of the angiotensin system. Hypertension. 1991;18:126–133. doi: 10.1161/01.HYP.18.5_Suppl.III126. [DOI] [PubMed] [Google Scholar]
- 42.Laursen J.B., Rajagopalan S., Galis Z., Tarpey M., Freeman B.A., Harrison D.G. Role of superoxide in angiotensin II iinduced but not catecholamine-induced hypertension. Circulation. 1997;95:588–593. doi: 10.1161/01.CIR.95.3.588. [DOI] [PubMed] [Google Scholar]
- 43.Risler N., Miatello R., Montserrat C., Castro C., Bardi V. Bases moleculares, genéticas y fisiopatológicas de la hipertensión arterial. Procardio. 1998;2:19–63. [Google Scholar]
- 44.Trindade M.R., Assuncao H.C.R., Torres T.C., Beertolina J.S., Fernandes L. Venous endothelium reactivity to Angiotensine II: A study in primary endothelial cultures of rat vena cava and portal vein. Exp. Cell Res. 2018;362:188–194. doi: 10.1016/j.yexcr.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 45.Inoue A., Yanagisawa M., Kimura S., Miyauchi T., Goto K., Masaki T. The human endothelin family: Three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc. Natl. Acad. Sci. USA. 1989;86:2864–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davenport A.P., Hyndman K.A., Dhaun N., Southan C., Kohan D.E., Pollok J.S., Pollok D.M., Webb D.J., Maguire J.J. Endothelin. Pharmacol. Rev. 2016;68:257–418. doi: 10.1124/pr.115.011833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yanagisawa M., Masaki T. Endothelin, a novel endothelium-derived peptide. Pharmacological activities, regulation and possible roles in cardiovascular control. Biochem. Pharmacol. 1989;38:1877–1883. doi: 10.1016/0006-2952(89)90484-X. [DOI] [PubMed] [Google Scholar]
- 48.Leppaluoto J., Ruskoaho H. Endothelin peptides: Biological activities, celular signaling and clinical significance. Ann. Med. 1992;24:153–161. doi: 10.3109/07853899209147813. [DOI] [PubMed] [Google Scholar]
- 49.Haynes W.G., Ferro C.E., Webb D.J. Physiologic role of endothelin in maintenance of vascular tone in humans. J. Cardiovasc. Pharmacol. 1995;26:183–185. doi: 10.1097/00005344-199506263-00055. [DOI] [PubMed] [Google Scholar]
- 50.Balakrishnan S., Pandhi P. Endothelins: A brief review. Ind. J. Pharmacol. 1997;29:281–288. [Google Scholar]
- 51.Baltazares M., Rodríguez H., Ortega J., Sotres-Vega A., Baltazares M.E. Sistema endotelina. Rev. Inst. Nac. Enf. Resp. Mex. 2005;18:308–320. [Google Scholar]
- 52.Schorlemmer A., Matter M.L., Shohet R.V. Cardioprotective signaling by endothelin. Trends Cardiovasc. Med. 2008;18:233–239. doi: 10.1016/j.tcm.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luscher T.F., Barton M. Endothelins and endothelin receptor antagonists: Therapeutic considerations for a novel class of cardiovascular drugs. Circulation. 2000;102:2434–2440. doi: 10.1161/01.CIR.102.19.2434. [DOI] [PubMed] [Google Scholar]
- 54.Galie N., Manes A., Branzi A. The endothelin systemnin pulmonary arterial hypertension. Cardiovasc. Res. 2004;61:227–237. doi: 10.1016/j.cardiores.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 55.Balakrishnan S.M., Wang H.D., Gopalakrishnan V., Wilson T.W., McNeil J.R. Effect of an endothelin antagonist on hemodynamic responses to angiotensin II. Hypertension. 1996;28:806–809. doi: 10.1161/01.HYP.28.5.806. [DOI] [PubMed] [Google Scholar]
- 56.Savoia C., Schiffrin E.L. Significance of recently identified peptides in hypertension: Endothelin, natriuretic peptides, adrenomedullin, leptin. Med. Clin. N. Am. 2004;88:39–62. doi: 10.1016/S0025-7125(03)00122-6. [DOI] [PubMed] [Google Scholar]
- 57.Malek A., Izumo S. Physiological fluid shear stress causes down regulation of endothelin-1 mRNA in bovine aortic endothelium. Am. J. Physiol. 1992;263:389–396. doi: 10.1152/ajpcell.1992.263.2.C389. [DOI] [PubMed] [Google Scholar]
- 58.Gray G.A., Battistini B., Webb D.J. Endothelins are potent vasoconstrictor, and much more besides. Trend Pharmacol. Sci. 2000;21:38–40. doi: 10.1016/S0165-6147(99)01431-5. [DOI] [PubMed] [Google Scholar]
- 59.Quehenberger P., Exner M., Sunder-Plassmann R., Ruzicka K., Bieglmayer C., Endler G., Muellner C., Speiser W., Wagner O. Leptin induces endothelin-1 in endothelial cells in vitro. Circ. Res. 2002;90:711–718. doi: 10.1161/01.RES.0000014226.74709.90. [DOI] [PubMed] [Google Scholar]
- 60.Clozel M., Flores S. Endothelin receptor as drug targets in chronic cardiovascular disease: The rationale for dual antagonism. Drug Develop. Res. 2006;67:825–834. doi: 10.1002/ddr.20156. [DOI] [Google Scholar]
- 61.Barton M., Yanagisawa M. Endothelin: 20 years from discovery to therapy. Can. J. Physiol. Pharmacol. 2008;86:485–498. doi: 10.1139/Y08-059. [DOI] [PubMed] [Google Scholar]
- 62.Buzzard C.J., Pfister S.L., Campbell W.B. Endothelium-dependent contractions in rabbit pulmonary artery are mediated by thromboxane A2. Circ. Res. 1993;72:1023–1034. doi: 10.1161/01.RES.72.5.1023. [DOI] [PubMed] [Google Scholar]
- 63.Nie D., Lamberti M., Zacharek A., Li L., Szekeres K., Tang K., Chen Y., Honn K.V. Thromboxane A(2) regulation of endothelial cell migration, angiogenesis, and tumor metastasis. Biochem. Biophys. Res. Commun. 2000;267:245–251. doi: 10.1006/bbrc.1999.1840. [DOI] [PubMed] [Google Scholar]
- 64.Mayeux P.R., Mais D.E., Carr C., Halushka P.V. Human erythroleukemia cells express functional thromboxane A2/prostaglandin H2 receptors. J. Pharmacol. Exp. Ther. 1989;250:923–927. [PubMed] [Google Scholar]
- 65.Hagemann C., Rapp U.R. Isotype-specific functions of raf kinases. Exp. Cell Res. 1999;253:34–46. doi: 10.1006/excr.1999.4689. [DOI] [PubMed] [Google Scholar]
- 66.Narumiya S., Sugimoto Y., Ushikubi F. Prostanoid receptors: Structures, properties, and functions. Physiol. Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 67.Miggin S.M., Kinsella B.T. Regulation of extracellular signal-regulated kinase cascades by alpha- and beta-isoforms of the human thromboxane A2 receptor. Mol. Pharmacol. 2002;61:817–831. doi: 10.1124/mol.61.4.817. [DOI] [PubMed] [Google Scholar]
- 68.Manabe K., Shirahase H., Usui H., Kurahashi K., Fujiwara M. Endothelium-dependent contractions induced by angiotensin I and angiotensin II in canine cerebral artery. J. Pharmacol. Exp. Ther. 1989;251:317–320. [PubMed] [Google Scholar]
- 69.Taddei S., Vanhoutte P.M. Endothelium-dependent contractions to endothelin in the rat aorta are mediated by thromboxane A2. J. Cardiovasc. Pharmacol. 1993;22:S328–S331. doi: 10.1097/00005344-199322008-00086. [DOI] [PubMed] [Google Scholar]
- 70.Srisawat S., Phivthong-Ngam L., Unchern S., Chantharaksri U., Govitrapong P., Sanvarinda Y. Improvement of vascular function by chronic administration of a cyclo-oxygenase inhibitor in cholesterol-fed rabbits. Clin. Exp. Pharmacol. Physiol. 2003;30:405–412. doi: 10.1046/j.1440-1681.2003.03850.x. [DOI] [PubMed] [Google Scholar]
- 71.Gonzales R.J., Ghaffari A.A., Duckles S.P., Krause D.N. Testosterone treatment increases thromboxane function in rat cerebral arteries. Am. J. Physiol. Heart Circ. Physiol. 2005;289:578–585. doi: 10.1152/ajpheart.00958.2004. [DOI] [PubMed] [Google Scholar]
- 72.Blanco-Rivero J., Balfagón G., Ferrer M. Orchidectomy modulates alpha2-adrenoceptor reactivity in rat mesenteric artery through increased thromboxane A2 formation. J. Vasc. Res. 2006;43:101–108. doi: 10.1159/000089791. [DOI] [PubMed] [Google Scholar]
- 73.Fitzgerald G.A. Mechanisms of platelet activation: Thromboxane A2 as an amplifying signal for other agonists. Am. J. Cardiol. 1991;68:11B–15B. doi: 10.1016/0002-9149(91)90379-Y. [DOI] [PubMed] [Google Scholar]
- 74.Shimokawa H. Endothelial dysfunction in hypertension. J. Atheroscler. Thromb. 1998;4:118–127. doi: 10.5551/jat1994.4.118. [DOI] [PubMed] [Google Scholar]
- 75.Matz R.L., de Sotomayor M.A., Schott C., Stoclet J.C., Andriantsitohaina R. Vascular bed heterogeneity in age-related endothelial dysfunction with respect to NO and eicosanoids. Br. J. Pharmacol. 2000;131:303–311. doi: 10.1038/sj.bjp.0703568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dancs P.T., Ruisanchez E., Balogh A., Panta C.R., Miklos Z., Nüsing R.M., Aoki J., Chun J., Offermanns S., Tigyi G., et al. LPA1 receptor-mediated Thromboxane A2 release is responsible for lysophosphatidic acid-induced vascular smooth muscle contraction. FASEB J. 2017;31:1547–1555. doi: 10.1096/fj.201600735R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheeseman K.H., Slater T.F. An introduction of free radical radical biochemistry. Br. Med. Bull. 1993;49:481–493. doi: 10.1093/oxfordjournals.bmb.a072625. [DOI] [PubMed] [Google Scholar]
- 78.Thomas C. Oxygen Radicals and Disease Process. 1st ed. Harwood Academic; Amsterdam, The Netherland: 1997. pp. 327–377. [Google Scholar]
- 79.Tejero J., Shiva S., Gladwin M. Sources of vascular nitric oxide and reactive oxygen species and their regulation. Physiol. Rev. 2019;99:311–379. doi: 10.1152/physrev.00036.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pagano P.J., Ito Y., Tornheim K., Gallop P.M., Tauber A.I., Cohen R.A. An NADPH oxidase superoxide generating system in the rabbit aorta. Am. J. Physiol. 1995;268:H2274–H2280. doi: 10.1152/ajpheart.1995.268.6.H2274. [DOI] [PubMed] [Google Scholar]
- 81.Halliwell B. Mechanisms involved in the generation of free radicals. Pathol. Biol. 1996;44:6–13. [PubMed] [Google Scholar]
- 82.Poli G., Leonarduzzi G., Biasi F., Chiarpotto E. Oxidative stress and cell signalling. Curr. Med. Chem. 2004;11:1163–1182. doi: 10.2174/0929867043365323. [DOI] [PubMed] [Google Scholar]
- 83.Zalba G., Beaumont F.J., San José G., Fortuño A., Fortuño M.A., Díez J. Vascular oxidant stress: Molecular mechanisms and pathophysiological implications. J. Physiol. Biochem. 2000;56:57–64. doi: 10.1007/BF03179777. [DOI] [PubMed] [Google Scholar]
- 84.DeKeulenaer G.W., Alexander R.W., Ushio-Fukai M., Ishizaka N., Griendling K.K. Tumor necrosis factor-α activates a p22phox-based NADH oxidase in vascular smooth muscle cells. Biochem. J. 1998;329:653–657. doi: 10.1042/bj3290653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Griendling K.K., Minieri C.A., Ollerenshaw J.D., Alexander R.W. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ. Res. 1994;74:1141–1148. doi: 10.1161/01.RES.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 86.Wang H.D., Pagano P.J., Du Y., Cayatte A.J., Quinn M.T., Brecher P., Cohen R.A. Superoxide anion from the adventitia of the rat thoracic aorta inactivates nitric oxide. Circ. Res. 1998;82:810–818. doi: 10.1161/01.RES.82.7.810. [DOI] [PubMed] [Google Scholar]
- 87.Finkel T. Oxygen radicals and signaling. Curr. Opin. Cell Biol. 1998;10:248–253. doi: 10.1016/S0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- 88.Finkel T. Signal transduction by mitochondrial oxidants. J. Biol. Chem. 2012;287:4434–4440. doi: 10.1074/jbc.R111.271999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Karbach S., Wenzel P., Waisman A., Munzel T., Daiber A. eNOS uncoupling in cardiovascular diseases–the role of oxidative stress and inflammation. Curr. Pharm. Des. 2014;20:3579–3594. doi: 10.2174/13816128113196660748. [DOI] [PubMed] [Google Scholar]
- 90.Li H., Förstermann U. Uncoupling of endothelial NO synthase in atherosclerosis and vascular disease. Curr. Opin. Pharmacol. 2013;13:161–167. doi: 10.1016/j.coph.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 91.Siragusa M., Fleming I. The eNOS signalosome and its link to endothelial dysfunction. Pflugers Arch. 2016;468:1125–1137. doi: 10.1007/s00424-016-1839-0. [DOI] [PubMed] [Google Scholar]
- 92.Squadrito G.L., Pryor W.A. The formation of peroxynitrite in vivo from nitric oxide and superoxide. Chem. Biol. Interact. 1995;96:203–206. doi: 10.1016/0009-2797(94)03591-U. [DOI] [PubMed] [Google Scholar]
- 93.Munzel T., Heitzer T., Harrison D.G. The physiology and pathophysiology of the nitric oxide/superoxide system. Herz. 1997;22:158–172. doi: 10.1007/BF03044353. [DOI] [PubMed] [Google Scholar]
- 94.Halliwell B., Zhao K., Whiteman M. Nitric oxide and peroxynitrite. The ugly, the uglier and the not so good: A personal view of recent controversies. Free Radic. Res. 1999;31:651–669. doi: 10.1080/10715769900301221. [DOI] [PubMed] [Google Scholar]
- 95.Muller G., Morawietz H. NAD(P)H oxidase and endothelial dysfunction. Horm. Metab. Res. 2008;41:152–158. doi: 10.1055/s-0028-1086023. [DOI] [PubMed] [Google Scholar]
- 96.Ohara Y., Peterson T.E., Harrison D.G. Hypercholesterolemia increases endothelial superoxide anion production. J. Clin. Investig. 1993;91:2546–2551. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cai H., Harrison D.G. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ. Res. 2000;87:840–844. doi: 10.1161/01.RES.87.10.840. [DOI] [PubMed] [Google Scholar]
- 98.Hogg N. Free radicals in disease. Semin. Reprod Endocrinol. 1998;16:241–248. doi: 10.1055/s-2007-1016284. [DOI] [PubMed] [Google Scholar]
- 99.Wolin M.S. Interaction of oxidants with vascular signaling system. Arterioscler. Thromb. Vasc. Biol. 2000;20:1430–1442. doi: 10.1161/01.ATV.20.6.1430. [DOI] [PubMed] [Google Scholar]
- 100.Moncada S., Palmer R.M.J., Higgs E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 101.Kojda G., Harrison D. Interactions between NO and reactive oxygen species: Pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc. Res. 1999;43:562–571. doi: 10.1016/S0008-6363(99)00169-8. [DOI] [PubMed] [Google Scholar]
- 102.Palmer R.M.J., Ferrige A.G., Moncada S. Nitric oxide reléase accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 103.Birks E.J., Yacoub M.H. The role of nitric oxide and cytokines in heart failure. Coron. Artery Dis. 1997;8:389–402. doi: 10.1097/00019501-199706000-00007. [DOI] [PubMed] [Google Scholar]
- 104.Marletta M.A. Nitric oxide synthase: Aspects concerning structure and catalysis. Cell. 1994;78:927–930. doi: 10.1016/0092-8674(94)90268-2. [DOI] [PubMed] [Google Scholar]
- 105.Stuehr D.J. Structure-function aspects in the nitric oxide synthases. Ann. Rev. Pharmacol. Toxicol. 1997;37:339–359. doi: 10.1146/annurev.pharmtox.37.1.339. [DOI] [PubMed] [Google Scholar]
- 106.Govers R., Rabelink T.J. Cellular regulation of endothelial nitric oxide synthase. Am. J. Physiol. Renal. Physiol. 2001;280:193–206. doi: 10.1152/ajprenal.2001.280.2.F193. [DOI] [PubMed] [Google Scholar]
- 107.Pollock J.S., Klinghofer V., Forstermann U., Murad F. Endothelial nitric oxide synthase is myristylated. FEBS Lett. 1993;309:402–404. doi: 10.1016/0014-5793(92)80816-Y. [DOI] [PubMed] [Google Scholar]
- 108.Bredt D.S., Hwang P.M., Snyder S.H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990;347:768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- 109.Ferreiro C.R., Chagas A.C., Carvalho M.H., Dantas A.P., Scavone C., Souza L.C., Buffolo E., da Luz P.L. Expression of inducible nitric oxide synthase is increased in patients with heart failure due to ischemic disease. Braz. J. Med. Biol. Res. 2004;37:1313–1320. doi: 10.1590/S0100-879X2004000900005. [DOI] [PubMed] [Google Scholar]
- 110.Pollock J.S., Forstermann U., Tracey W.R., Nakane M. Nitric oxide synthase isozymes antibodies. Histochem. J. 1995;27:738–744. doi: 10.1007/BF02388299. [DOI] [PubMed] [Google Scholar]
- 111.Andrew P.J., Mayer B. Enzymatic function of nitric oxide synthases. Cardiovasc. Res. 1999;43:521–531. doi: 10.1016/S0008-6363(99)00115-7. [DOI] [PubMed] [Google Scholar]
- 112.Alderton W.K., Cooper C.E., Knowles R.G. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001;357:593–615. doi: 10.1042/bj3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Furchgott R.F., Zawadski J.V. The obligatory role of endothelial cells in the relaxation of smooth muscle by acethilcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 114.Rees D.D., Palmer R.M., Moncada S. Role of endothelium-derived nitric oxide in the regulation of bllod pressure. Proc. Natl. Acad. Sci. USA. 1989;86:3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Raffetto J.D., Yu P., Reslan O.M., Xia Y., Khalil R.A. Endothelium-dependent nitric oxide and hyperpolarization-mediated venous relaxation pathways in rat inferior vena cava. J. Vasc. Surg. 2012;55:1716–1725. doi: 10.1016/j.jvs.2011.10.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vane J.R., Bakhle Y.S., Botting R.M. Cyclooxygenases 1 and 2. Ann. Rev. Pharmacol. Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 117.Meirer K., Steinhilber D., Proschak E. Inhibitors of the arachidonic acid cascade: Interfering with multiple pathways. Basic Clin. Pharmacol. Toxicol. 2014;114:83–91. doi: 10.1111/bcpt.12134. [DOI] [PubMed] [Google Scholar]
- 118.Davidge S.T. Prostaglandin H synthase and vascular function. Circ Res. 2001;89:650–660. doi: 10.1161/hh2001.098351. [DOI] [PubMed] [Google Scholar]
- 119.Tanabe T., Tohnai N. Cyclooxygenase isozymes and their gene structures and expression. Prostaglandins Other Lipid Mediat. 2002;68:95–114. doi: 10.1016/S0090-6980(02)00024-2. [DOI] [PubMed] [Google Scholar]
- 120.Ramsay R.G., Ciznadija D., Vanevski M., Mantamadiotis T. Transcriptional regulation of cyclo-oxygenase expression: Three pillars of control. Int. J. Immunopathol. Pharmacol. 2003;16:59–67. [PubMed] [Google Scholar]
- 121.Briones A.M., Salaices M., Vila E. Ageing alters the production of nitric oxide and prostanoids after IL-1β exposure in mesenteric resistance arteries. Mech. Ageing Dev. 2005;126:710–721. doi: 10.1016/j.mad.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 122.Ricciotti E., FitzGerald G.A. Prostaglandins and Inflammation. Arterioscler. Thromb. Vasc. Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dorris S.L., Peebles R.S. PGI2 as a regulator of inflammatory diseases. Med. Inflamm. 2012;2012:926968–926969. doi: 10.1155/2012/926968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rahman M.S. Prostacyclin: A major prostaglandin in the regulation of adipose tissue development. J. Cell Physiol. 2019;234:3254–3262. doi: 10.1002/jcp.26932. [DOI] [PubMed] [Google Scholar]
- 125.Bergstrom S., Danielsson H., Samuelsson B. The enzymatic formation of prostaglandin E2 from arachidonic acid. Prostaglandins and related factors. Biochim. Biophys. Acta. 1974;90:207–210. doi: 10.1016/0304-4165(64)90145-X. [DOI] [PubMed] [Google Scholar]
- 126.Yamamoto S., Ogino N., Ohki S., Yoshimoto T., Bhat S.G., Oka J., Hayaishi O. Enzymological studies on prostaglandin biosynthesis. In: Kharasch N., Freid J., editors. Biochemical Aspects of Prostaglandins and Thromboxanes. 1st ed. Academic; New York, NY, USA: 1977. p. 278. [Google Scholar]
- 127.Schulman E.S., Newball H.H., Demers L.M., Fitzpatrick F.A., Adkinson N.F. Anaphylactic release of Thromboxane A2, Prostaglandin D2 and Prostacyclin from human lung parenchyma. Am. Rev. Respir. Dis. 1981;124:402–406. doi: 10.1164/arrd.1981.124.4.402. [DOI] [PubMed] [Google Scholar]
- 128.Higgs E.A., Moncada S. Prostacyclin-physiology and clinical uses. Gen. Pharmacol. 1983;14:7–11. doi: 10.1016/0306-3623(83)90054-X. [DOI] [PubMed] [Google Scholar]
- 129.Lüsher T.F., Oemar B.S., Boulanger C.M., Hahn A.W.A. Molecular and cellular biology of endothelin and its receptor. Part I. Hypertens. 1993;11:121–126. doi: 10.1097/00004872-199302000-00002. [DOI] [PubMed] [Google Scholar]
- 130.Parsaee H., Mcewan J.R., Joseph S., MacDermot J. Differential sensitivities of the prostacyclin and nitric oxide biosynthetic pathways to cytosolic calcium in bovine aortic endothelial cells. Br. J. Pharmacol. 1992;107:1013–1019. doi: 10.1111/j.1476-5381.1992.tb13400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Williams S.P., Dorn G.W., Rapoport R.M. Prostaglandin I2 mediates contraction and relaxation of vascular smooth muscle. Am. J. Physiol. 1994;267:H796–H803. doi: 10.1152/ajpheart.1994.267.2.H796. [DOI] [PubMed] [Google Scholar]
- 132.Blanco-Rivero J., Cachofeiro V., Lahera V., Aras-Lopez R., Márquez-Rodas I., Salaices M., Xavier F.E., Ferrer M., Balfagón G. Participation of prostacyclin in endothelial dysfunction induced by aldosterone in normotensive and hypertensive rats. Hipertension. 2005;46:107–112. doi: 10.1161/01.HYP.0000171479.36880.17. [DOI] [PubMed] [Google Scholar]
- 133.Mitchell J.A., De Nucci G., Warner T.D., Vane J.R. Different patterns of release of endothelium-derived relaxing factor and prostacyclin. Br. J. Pharmacol. 1992;105:485–489. doi: 10.1111/j.1476-5381.1992.tb14280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mollace V., Muscoli C., Masini E., Cuzzocrea S., Salvemini D. Modulation of prostaglandin biosynthesis by nitric oxide and nitric oxide donors. Pharmacol. Rev. 2005;57:217–252. doi: 10.1124/pr.57.2.1. [DOI] [PubMed] [Google Scholar]
- 135.Bachschmid M., Schildknecht S., Ullrich V. Redox regulation of vascular prostanoid synthesis by the nitric oxide-superoxide system. Biochem. Biophys. Res. Commun. 2005;338:536–542. doi: 10.1016/j.bbrc.2005.08.157. [DOI] [PubMed] [Google Scholar]
- 136.Catella-Lawson F., Crofford L.J. Cyclooxygenase inhibition and thrombogenicity. Am. J. Med. 2001;110:28S–32S. doi: 10.1016/S0002-9343(00)00683-5. [DOI] [PubMed] [Google Scholar]
- 137.Mukherjee D., Nissen S.E., Topol E.J. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001;286:954–959. doi: 10.1001/jama.286.8.954. [DOI] [PubMed] [Google Scholar]
- 138.Busse R., Förstermann U., Matsuda H., Pohl U. The role of prostaglandins in the endothelium-mediated vasodilatory response to hypoxia. Pflügers Arch. 1984;401:77–83. doi: 10.1007/BF00581536. [DOI] [PubMed] [Google Scholar]
- 139.Krone W., Klass A., Nagele H., Behnke B., Greten H. Effects of prostaglandins on LDL receptor activity and cholesterol synthesis in freshly isolated human mononuclear leukocytes. J. Lipid Res. 1988;29:1663–1669. [PubMed] [Google Scholar]
- 140.Braun M., Hohlfeld T., Kienbaum P., Weber A.A., Sarbia M., Schror K. Antiatherosclerotic effects of oral cicaprost in experimental hypercholesterolemia in rabbits. Atherosclerosis. 1993;103:93–105. doi: 10.1016/0021-9150(93)90043-T. [DOI] [PubMed] [Google Scholar]
- 141.Kowala M.C., Mazzucco C.E., Hartl K.S., Seiler S.M., Warr G.A., Abid S., Grove R.I. Prostacyclin agonists reduce early atherosclerosis in hyperlipidemic hamsters. Octimibate and BMY 42393 suppress monocyte chemotaxis, macrophage cholesteryl ester accumulation, scavenger receptor activity, and tumor necrosis factor production. Arterioscler. Thromb. 1993;13:435–444. doi: 10.1161/01.ATV.13.3.435. [DOI] [PubMed] [Google Scholar]
- 142.Stables M.J., Gilroy D.W. Old and new generation lipid mediators in acute inflammation and resolution. Progress Lipid Res. 2011;50:35–51. doi: 10.1016/j.plipres.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 143.Hwang S.H., Wecksler A.T., Wagner K., Hammock B.D. Rationally designed multitarget. agents against inflammation and pain. Curr. Med. Chem. 2013;20:1783–1799. doi: 10.2174/0929867311320130013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vanhoutte P.M. Other endothelium-derived vasoactive factors. Circulation. 1993;87:V9–V17. [Google Scholar]
- 145.Vanhoutte P.M., Boulanger C.M., Mombouli J.V. Endothelium-derived relaxing factors and converting enzyme inhibition. Am. J. Cardiol. 1995;76:3E–12E. doi: 10.1016/S0002-9149(99)80496-2. [DOI] [PubMed] [Google Scholar]
- 146.Campbell W.B., Gebremedhin D., Pratt P.F., Harder D.R. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ. Res. 1996;78:415–423. doi: 10.1161/01.RES.78.3.415. [DOI] [PubMed] [Google Scholar]
- 147.Komori K., Suzuki H. Heterogenous distribution of muscarinic receptors in the rabbit saphenous artery. Br. J. Pharmacol. 1987;92:657–664. doi: 10.1111/j.1476-5381.1987.tb11369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gluais P., Edwards G., Weston A.H., Falck J.R., Vanhoutte P.M., Félétou M. Role of SK (Ca) and IK (Ca) in endothelium-dependent hyperpolarization of the guinea-pig isolated carotid artery. Br. J. Pharmacol. 2005;144:477–485. doi: 10.1038/sj.bjp.0706003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vázquez-Pérez S., Navarro-Cid J., De Las Heras N., Cediel E., Sanz-Rosa D., Ruilope L.M., Cachofeiro V., Lahera V. Relevance of endothelium-derived hyperpolarizing factor in the effects of hypertension on rat coronary relaxations. J. Hypertens. 2001;19:539–545. doi: 10.1097/00004872-200103001-00004. [DOI] [PubMed] [Google Scholar]
- 150.Luksha L., Agewall S., Kublickiene K. Endothelium-derived hyperpolarizing factor in vascular physiology and cardiovascular disease. Atherosclerosis. 2009;202:330–344. doi: 10.1016/j.atherosclerosis.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 151.Ellinsworth D.F.C., Sandow S.L., Shukla A., Liu Y., Jeremy J.Y., Gutterman D.D. Endothelium-derived hyperpolarization and coronary vasodilation: Diverse and integrated roles of epoxyeicosatrienoic acids, hydrogen peroxide, and gap junctions. Microcirculation. 2016;23:15–32. doi: 10.1111/micc.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shimokawa H., Morikawa K. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in animals and humans. J. Mol. Cell. Cardiol. 2005;39:725–732. doi: 10.1016/j.yjmcc.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 153.Sandow S.L., Tare M. C-type natriuretic peptide: A new endothelium-derived hyperpolarizing factor? Trends Pharmacol. Sci. 2007;28:61–67. doi: 10.1016/j.tips.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 154.Majed B.H., Khalil R.A. Molecular mechanisms regulating the vascular prostacyclin pathways and their adaptation during pregnancy and in the newborn. Pharmacol. Rev. 2012;64:540–582. doi: 10.1124/pr.111.004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Köhler R., Hoyer J. The endothelium-derived hyperpolarizing factor: Insights from genetic animal models. Kidney Int. 2007;72:145–150. doi: 10.1038/sj.ki.5002303. [DOI] [PubMed] [Google Scholar]
- 156.Levine J.E. Neuroendocrine control of the ovarian cycle of the rat. In: Plant T.M., Zeleznik A.J., editors. Knobil and Neill’s Physiology of Reproduction. 4th ed. Academic Press; Oxford, UK: 2015. pp. 1199–1257. Chapter 26. [Google Scholar]
- 157.Leavitt W.W., Toft D.O., Strott C.A., O’Malley B.W. A specific progesterone receptor in the Hamster uterus: Physiologic properties and regulation during the estrous cycle. Endocrinology. 1974;94:1041–1053. doi: 10.1210/endo-94-4-1041. [DOI] [PubMed] [Google Scholar]
- 158.Goldman J.M., Murr A.S., Coper R.L. The rodent estrous cycle: Characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- 159.Rothchild I. The regulation of the mammalian corpus luteum. Recent Prog. Horm. Res. 1981;37:183–298. doi: 10.1016/b978-0-12-571137-1.50009-8. [DOI] [PubMed] [Google Scholar]
- 160.Linares N., Millán Y., Garrido-Gracia J.C., Aguilar R., Gordon A., Sánchez-Criado J.E., Martín de las Mulas J. Utilidad de los agonistas, moduladores selectivos y antagonistas puros del receptor de estrógenos en estudios morfofuncionales del útero de la rata. Anal. Real Acad. Cienc. Vet. Andalucía Orient. 2010;23:97–109. [Google Scholar]
- 161.Belozertseva I.V., Merkulovs D.D., Vilitis O.J., Skryabin B.V. Instrumental methods for determining the estrous cycle stages in small laboratory rodents. Lab. Anim. Sci. 2018;4:125–135. doi: 10.29926/2618723X-2018-04-10. [DOI] [Google Scholar]
- 162.Marcondes F.K., Miguel K., Melo L.L., Spadari-Bratfisch R.C. Estrous cycle influences the response of female rats in the elevated plus-maze. Physiol. Behav. 2001;74:435–440. doi: 10.1016/S0031-9384(01)00593-5. [DOI] [PubMed] [Google Scholar]
- 163.Tanriverdi F., Silveira L., MacColl G., Bouloux P.M. The hypothalamic-pituitary-gonadal axis: Immune function and autoimmunity. J. Endocrinol. 2003;176:293–304. doi: 10.1677/joe.0.1760293. [DOI] [PubMed] [Google Scholar]
- 164.Lamb G.C., Smith M.F., Perry G.A., Atkins J.A., Risley M.E., Busch D.C., Patterson D.J. Reproductive endocrinology and hormonal control of the estrous cycle. Bovine Practitioner. 2010;44:18–26. [Google Scholar]
- 165.Freeman M.E. The ovarian cycle of the rat. In: Plant T.M., Zeleznik A.J., editors. Knobil and Neill’s Physiology of Reproduction. 4th ed. Academic Press; Oxford, UK: 2015. [Google Scholar]
- 166.Herbison A.E. Noradrenergic regulation of cyclic GnRH secretion. Rev. Reprod. 1997;2:1–6. doi: 10.1530/ror.0.0020001. [DOI] [PubMed] [Google Scholar]
- 167.Turkstra J., Meloen R. Active immunization against gonadotropin-releasing hormone, an active tool to block the fertility axis in mammals. Vet. Sci. Tomorrow. 2006 [Google Scholar]
- 168.Fink G. Gonadotropin secretion and its control. In: Plant T.M., Zeleznik A.J., editors. Knobil and Neill’s Physiology of Reproduction. 4th ed. Academic Press; Oxford, UK: 2015. [Google Scholar]
- 169.Alonso R., Marín F., González M., Guelmes P., Bellido C., Hernández G., Marín R., Díaz M., Sánchez-Criado J. The hypothalamus-pituitary-ovarian axis as a model system for the study of SERM effects: An overview of experimental and clinical studies. In: Cano A., Calaf i Alsina J., Dueñas-Diez J.L., editors. Selective Estrogen Receptor Modulators. A New Brand of Multitarget Drugs. 1st ed. Springer; Berlin, Germany: 2006. pp. 103–139. Chapter 5. [Google Scholar]
- 170.Berne R.M., Levy M.N. Physiology. 7th ed. Elsevier Science; St. Louis, MO, USA: 1998. pp. 1–880. [Google Scholar]
- 171.Simpson E.R., Clyne C., Rubin G., Boon W.C., Robertson K., Britt K., Speed C., Jones M. Aromatase—A brief overview. Ann. Rev. Physiol. 2002;64:93–127. doi: 10.1146/annurev.physiol.64.081601.142703. [DOI] [PubMed] [Google Scholar]
- 172.Harada N., Sasano H., Murakami H., Ohkuma T., Nagura H., Takagi Y. Localized expression of aromatase in human vascular tissues. Circ. Res. 1999;84:1285–1291. doi: 10.1161/01.RES.84.11.1285. [DOI] [PubMed] [Google Scholar]
- 173.Miller V.M., Duckles S.P. Vascular actions of estrogens: Functional implications. Pharmacol Rev. 2008;60:210–241. doi: 10.1124/pr.107.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Miller V.M., Mulvagh S.L. Sex steroids and endothelial function: Translating basic science to clinical practice. Trends Pharmacol. Sci. 2007;28:263–270. doi: 10.1016/j.tips.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 175.Nathan L., Chaudhuri G. Estrogens and atherosclerosis. Ann. Rev. Pharmacol. Toxicol. 1997;37:477–515. doi: 10.1146/annurev.pharmtox.37.1.477. [DOI] [PubMed] [Google Scholar]
- 176.Herrington D.M., Reboussin D.M., Brosnihan K.B., Sharp P.C., Shumaker S.A., Snyder T.E., Furberg C.D., Kowalchuk G.J., Stuckey T.D., Rogers W.J., et al. Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. N. Engl. J. Med. 2000;343:522–529. doi: 10.1056/NEJM200008243430801. [DOI] [PubMed] [Google Scholar]
- 177.Mendelsohn M.E., Karas R.H. The protective effects of estrogen on the cardiovascular system. N. Engl. J. Med. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 178.Nasr A., Breckwoldt M. Estrogen replacement therapy and cardiovascular protection: Lipid mechanisms are the tip of an iceburg. Gynecol. Endocrinol. 1998;12:43–59. doi: 10.3109/09513599809024970. [DOI] [PubMed] [Google Scholar]
- 179.Raddino R., Pela G., Uberti D., Portera C., Ferrari R., Scarabelli T.M., Cooper T.J., Manca C. Estrogen derivative relaxes rabbit aorta via the endotelial receptor system. Ital. Heart J. 2001;2:49–54. [PubMed] [Google Scholar]
- 180.Wild R.A., Reis S.E. Estrogens, progestins, selective estrogen receptor modulators, and the arterial tree. Am. J. Obstet. Gynecol. 2001;184:1031–1039. doi: 10.1067/mob.2001.112902. [DOI] [PubMed] [Google Scholar]
- 181.Mendelsohn M.E. Mechanisms of estrogen action in the cardiovascular system. J. Steroid Biochem. Mol. Biol. 2000;74:337–343. doi: 10.1016/S0960-0760(00)00110-2. [DOI] [PubMed] [Google Scholar]
- 182.Hayashi T., Yamada K., Esaki T., Kuzuya M., Satake S., Ishikawa T., Hidaka H., Iguchi A. Estrogen increases endothelial nitric oxide by a receptor-mediated system. Biochem. Biophys. Res. Commun. 1995;214:847–855. doi: 10.1006/bbrc.1995.2364. [DOI] [PubMed] [Google Scholar]
- 183.Virdis A., Ghiadoni L., Pinto S., Lombardo M., Petraglia F., Gennazzani A., Buralli S., Taddei S., Salvetti A. Mechanisms responsible for endothelial dysfunction associated with acute estrogen deprivation in normotensive women. Circulation. 2000;101:2258–2263. doi: 10.1161/01.CIR.101.19.2258. [DOI] [PubMed] [Google Scholar]
- 184.Zamorano B., Bruzzone M.E., Martínez J.L. Influence of estrous cycle on norepinephrine-induced contraction in rat aorta: Relationship to vascular prostanoids biosynthesis. Biol. Res. 1994;27:209–215. [PubMed] [Google Scholar]
- 185.Martinez J.L., Bruzzone M.E., Morales B., Palacios J., Knox M., Vinet R., Laurido C. Effects of indomethacin on the norepinephrine-induced contraction in vascular smooth muscle in different stages of the estrous cycle. Pak. Vet. J. 2019 in press. [Google Scholar]
- 186.Conrad K.P., Colpoys M.C. Evidence against the hypothesis that prostaglandins are the vasodepressor agents of pregnancy. Serial studies in chronically instrumented, conscious rats. J. Clin. Investig. 1986;77:236–245. doi: 10.1172/JCI112282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Debrah D.O., Novak J., Matthews J.E., Ramirez R.J., Shroff S.G., Conrad K.P. Relaxin is essential for systemic vasodilation and increased global arterial compliance during early pregnancy in conscious rats. Endocrinology. 2006;147:5126–5131. doi: 10.1210/en.2006-0567. [DOI] [PubMed] [Google Scholar]
- 188.Gilson G.J., Mosher M.D., Conrad K.P. Systemic hemodynamics and oxygen transport during pregnancy in chronically instrumented, conscious rats. Am. J. Physiol. Heart Circ. Physiol. 1992;263:H1911–H1918. doi: 10.1152/ajpheart.1992.263.6.H1911. [DOI] [PubMed] [Google Scholar]
- 189.Slangen B.F., Out I.C., Verkeste C.M., Peeters L.L. Hemodynamic changes in early pregnancy in chronically instrumented, conscious rats. Am. J. Physiol. Heart Circ. Physiol. 1996;270:H1779–H1784. doi: 10.1152/ajpheart.1996.270.5.H1779. [DOI] [PubMed] [Google Scholar]
- 190.Iacobaeus C., Andolf E., Thorsell M., Bremme K., Jörneskog G., Östlund E., Kahan T. Longitudinal study of vascular structure and function during normal pregnancy. Ultrasound Obstet. Gynecol. 2017;49:46–53. doi: 10.1002/uog.17326. [DOI] [PubMed] [Google Scholar]
- 191.Clapp J.F., Capeless E. Cardiovascular function before, during, and after the first and subsequent pregnancies. Am. J. Cardiol. 1997;80:1469–1473. doi: 10.1016/S0002-9149(97)00738-8. [DOI] [PubMed] [Google Scholar]
- 192.Sladek S.M., Magness R.R., Conrad K.P. Nitric oxide and pregnancy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1997;272:R441–R463. doi: 10.1152/ajpregu.1997.272.2.R441. [DOI] [PubMed] [Google Scholar]
- 193.Lenton E.A., Neal L.M., Sulaiman R. Plasma concentrations of human chorionic gonadotropin from the time of implantation until the second week of pregnancy. Fertil Steril. 1982;37:773–778. doi: 10.1016/S0015-0282(16)46337-5. [DOI] [PubMed] [Google Scholar]
- 194.Mahendru A.A., Everett T.R., Wilkinson I.B., Lees C.C., McEniery C.M. Maternal cardiovascular changes from pre-pregnancy to very early pregnancy. J. Hypertens. 2012;30:2168–2172. doi: 10.1097/HJH.0b013e3283588189. [DOI] [PubMed] [Google Scholar]
- 195.Weiner C.P., Thompson L.P. Nitric oxide and pregnancy. Semin. Perinatol. 1997;21:367–380. doi: 10.1016/S0146-0005(97)80003-1. [DOI] [PubMed] [Google Scholar]
- 196.Osol G., Cipolla M. Pregnancy-induced changes in the three-dimensional mechanical properties of pressurized rat uteroplacental (radial) arteries. Am. J. Obstet. Gynecol. 1993;168:268–274. doi: 10.1016/S0002-9378(12)90924-2. [DOI] [PubMed] [Google Scholar]
- 197.Goulopoulou S. Maternal vascular physiology in preeclampsia. Hypertension. 2017;70:1076–1083. doi: 10.1161/HYPERTENSIONAHA.117.08821. [DOI] [PubMed] [Google Scholar]
- 198.Conrad K.P. Maternal vasodilation in pregnancy: The emerging role of relaxin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;301:R267–R275. doi: 10.1152/ajpregu.00156.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhang Y., Stewart K., Davidge S. Endogenous estrogen mediates vascular reactivity and distensibility in pregnant rat mesenteric arteries. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H956–H961. doi: 10.1152/ajpheart.2001.280.3.H956. [DOI] [PubMed] [Google Scholar]
- 200.Geary G.G., Krause D.N., Duckles S.P. Estrogen reduces myogenic tone through a nitric oxide-dependent mechanism in rat cerebral arteries. Am. J. Physiol. Heart Circ. Physiol. 1998;275:H292–H300. doi: 10.1152/ajpheart.1998.275.1.H292. [DOI] [PubMed] [Google Scholar]
- 201.Magness R.R., Parker C.R., Rosenfeld C.R. Systemic and uterine responses to chronic infusion of estradiol-17beta. Am. J. Physiol. Endocrinol. Metab. 1993;265:E690–E698. doi: 10.1152/ajpendo.1993.265.5.E690. [DOI] [PubMed] [Google Scholar]
- 202.Magness R.R., Phernetton T.M., Zheng J. Systemic and uterine blood flow distribution during prolonged infusion of 17beta estradiol. Am. J. Physiol. Heart Circ. Physiol. 1998;275:H731–H743. doi: 10.1152/ajpheart.1998.275.3.H731. [DOI] [PubMed] [Google Scholar]
- 203.Zhang Y., Davidge S.T. Effect of estrogen replacement on vasoconstrictor responses in rat mesenteric arteries. Hypertension. 1999;34:1117–1122. doi: 10.1161/01.HYP.34.5.1117. [DOI] [PubMed] [Google Scholar]
- 204.Conrad K.P., Mosher M.D., Brinck-Johnsen T., Colpoys M.C. Effects of 17b-estradiol and progesterone on pressor responses in conscious ovariectomized rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1994;266:R1267–R1272. doi: 10.1152/ajpregu.1994.266.4.R1267. [DOI] [PubMed] [Google Scholar]
- 205.Zamorano B., Bruzzone M.E., Martínez J.L. Vascular smooth muscle reactivity to norepinephrine in ovariectomized rats: Relationship to vascular PGE2/PGF2α ratio. Gen. Pharmac. 1995;26:1613–1618. doi: 10.1016/0306-3623(95)00060-7. [DOI] [PubMed] [Google Scholar]
- 206.Dalle Lucca J.J., Adeagbo A.S.O., Alsip N.L. Influence of estrous cycle and pregnacy on the reactivity of yhe rat mesenteric vascular bed. Hum. Rep. 2000;15:961–968. doi: 10.1093/humrep/15.4.961. [DOI] [PubMed] [Google Scholar]
- 207.Cooke C.L.M., Davidge S.T. Pregnancy-induced alterations of vascular function in mouse mesenteric and uterine arteries. Biol. Reprod. 2003;68:1072–1077. doi: 10.1095/biolreprod.102.009886. [DOI] [PubMed] [Google Scholar]
- 208.Oluyemi A., Okwuonu C., Baxter D., Oyesola T.O. Toxic effects of methanolic extract of Aspilia africana leaf on the estrous cycle and uterine tissues of Wistar rats. Int. J. Morphol. 2007;25:609–614. doi: 10.4067/S0717-95022007000300023. [DOI] [Google Scholar]
- 209.Parada M., Valenzuela-Barra G., Delporte C., Lara H. Estrous cycle disruptor effect of an ethanolic extract from Buddleja globosa leaves and its main component (verbascoside) Bol. Latinoam. Caribe Plant. Med. Aromat. 2014;13:189–197. [Google Scholar]