Abstract
There are many examples of the interaction between prokaryotes and eukaryotes. One such example is the polymicrobial colonization/infection by the various opportunistic pathogenic yeasts belonging to the genus Candida and the ubiquitous bacterium, Pseudomonas aeruginosa. Although this interaction has simplistically been characterized as antagonistic to the yeast, this review highlights the complexity of the interaction with various factors influencing both microbes. The first section deals with the interactions in vitro, looking specifically at the role of cell wall components, quorum sensing molecules, phenazines, fatty acid metabolites and competition for iron in the interaction. The second part of this review places all these interactions in the context of various infection or colonization sites, i.e., lungs, wounds, and the gastrointestinal tract. Here we see that the role of the host, as well as the methodology used to establish co-infection, are important factors, influencing the outcome of the disease. Suggested future perspectives for the study of this interaction include determining the influence of newly identified participants of the QS network of P. aeruginosa, oxylipin production by both species, as well as the genetic and phenotypic plasticity of these microbes, on the interaction and outcome of co-infection.
Keywords: Candida albicans, interaction, Pseudomonas aeruginosa
1. Introduction
Nature provides many examples of the interaction between prokaryotes and eukaryotes [1,2]. These interactions can have various outcomes and may provide an advantage to one party (e.g., through antagonism of the other) or both parties (mutualism). Interactions between fungi and bacteria are abundant in many habitats, including the human body [3,4,5]. The identification of these interactions, especially in the human host, have been performed through culture-dependent techniques or culture-independent techniques, the latter being able to identify the presence of microbial intruders at low cell counts that could play a large role in infection. One of the most well-documented bodily sites where polymicrobial interaction takes place is the gastrointestinal tract, colonized by a plethora of microbial species [6]. Furthermore, polymicrobial infections occur frequently in the urinary tract, wounds, where it can negatively affect wound healing, as well as in the lungs, especially in patients predisposed to infection by altered immunity, as in the case with cystic fibrosis (CF). The competition posed by close proximity and nutrient limitation can result in altered metabolism and virulence factor expression as well as altered host immunity that may lead to a competitive advantage to species within a polymicrobial infection [6,7]. This interaction between fungi and bacteria can cause a considerable increase in morbidity and mortality compared to single species infection [8,9].
One of the principle models for studying the interaction between eukaryotic and prokaryotic interaction in the context of human health is the association between Candida albicans, a polymorphic fungus, and the ubiquitous bacterium Pseudomonas aeruginosa. The study of this interaction dates back to the 1970s, when several authors observed that P. aeruginosa had an inhibitory effect on the growth of C. albicans [10,11]. This phenomenon is not confined to these two species. Kerr (1994) found that Pseudomonas cepacia also has this inhibitory effect, while other potentially pathogenic Candida spp. (C. dubliniensis, C. glabrata, C. guillermondii, C. kefyr, C. krusei, C. lusitaniae, C. parapsilosis, C. pseudotropicalis and C. tropicalis) can also be susceptible to inhibition by Pseudomonas [12,13,14]. Today we know that many factors can mediate the interaction between these organisms, with the best-studied example being the interaction between P. aeruginosa and C. albicans.
2. In Vitro Interaction between Pseudomonas aeruginosa and Candida Species
2.1. Role of Cell Wall Components in the Interaction
Hogan and Kolter (2002) showed that P. aeruginosa preferentially attaches to C. albicans hyphae, effectively killing them, probably to gain nutrients for growth [1]. This was not the case for C. albicans yeast cells. In addition, they found that P. aeruginosa mutants lacking type IV pili, could attach to C. albicans hyphae, but were unable to kill them. They speculated that the role of pili might involve pilus retraction to bring the bacterium into close contact with the hyphae or that the pili act as sensors, signaling attachment to the fungal surface. Other cell wall-associated compounds of both P. aeruginosa and C. albicans are also important in this interaction. Bacterial lipopolysaccharide (LPS), including P. aeruginosa LPS, has adverse effects on C. albicans biofilms [14,15]. These include a decrease in filamentation, biofilm metabolic activity (including glycolysis) and growth. In contrast, peptidoglycan triggers filamentation in C. albicans [16]. Brand and co-workers (2008) investigated the role of C. albicans hyphal-specific cell wall proteins and mannoproteins in the selective attachment and killing of hyphae, and found that neither the major hyphal-specific proteins (Hyr1p, Hwp1p and Als3p), nor enzymes involved in N-glycosylation of cell wall proteins played a role, but that O-mannan probably protects against P. aeruginosa killing activity [17]. Whereas the bulk of research has been done on the interaction between C. albicans and P. aeruginosa, less is known regarding the interaction of P. aeruginosa with other Candida species. However, Bandara and co-workers (2010) did indicate a mutually suppressive interaction between P. aeruginosa and five non-albicans Candida species, i.e., C. glabrata, C. tropicalis, C. parapsilosis, C. dubliniensis and C. krusei in an in vitro dual species biofilm model [14]. They observed species-specific variations of inhibition of Candida biofilms, with almost complete inhibition of C. albicans, C. glabrata and C. tropicalis biofilms after 48 hours. Interestingly, although research from Brand and co-workers (2008) indicated selective attachment and killing of hyphal cells, Bandara and co-workers (2010) observed attachment of bacterial cells to blastospores, indicating that the hyphal phenotype may not be an absolute requirement for the physical association of P. aeruginosa to fungal cells [14,17].
2.2. Role of Quorum Sensing Molecules in the Interaction
P. aeruginosa secretes several types of molecules that may influence the growth of Candida spp. [18]. An important category of secreted molecules is quorum sensing molecules (QSM), produced by both organisms [19]. Gram-negative bacteria use N-acyl homoserine lactones (AHL) as QSM. At high population densities, when the concentration of AHL is above the threshold level, it can bind to and activate transcriptional activators to induce expression of target genes [20]. P. aeruginosa has two AHL-dependent QS systems, las and rhl. The LasI autoinducer synthase controls the production of the autoinducer, 3-oxododecanoyl-l-homoserine lactone (3-oxo-HSL), while production of butanoyl homoserine lactone is regulated by RhlI autoinducer synthase. These autoinducers act on their respective transcriptional activators, LasR and RhlR [21,22] and, depending on the culture conditions, may regulate up to 10% of the P. aeruginosa genome [23,24]. 3-oxododecanoyl-l-homoserine lactone (3-oxo-HSL), which is required for the production of surface adherence proteins on P. aeruginosa cells, is also important for adherence of P. aeruginosa to C. albicans hyphae [25]. In addition, 3-oxo-HSL could inhibit the yeast to hyphal switch of C. albicans in a dose-dependent manner [26,27]. Interestingly, Trejo-Hernández and co-workers (2014) found that hypoxia influences the ability of P. aeruginosa to inhibit C. albicans filamentation, due to a decrease in AHL production [28]. Another QS signal, 2-heptyl-3-hydroxyl-4-quinolone signal or Pseudomonas quinolone signal (PQS), which is released by P. aeruginosa during late exponential phase [29,30], is induced by the LasI/R system and inhibited by the RhlI/R system [31]. This signal not only modulates swarming motility of P. aeruginosa [32,33], but also induces the production of several virulence factors, including phenazines [34]. Pseudomonas quinolone signal and its precursor, 2-heptyl-4-quinolone, represses C. albicans biofilm formation [35]. These combined effects may lead to the dispersal of C. albicans cells in the presence of P. aeruginosa [25,36].
Lee and co-workers (2013) provided evidence of an additional QS network, integrated QS system or IQS, that is capable of integrating the stress cues from the environment with the QS network [37,38]. Although dependent on environmental factors, this QS system was shown to function below the las QS circuit in a hierarchical manner and was capable of regulating virulence factors through induction of the PQS and rhl QS systems. The impact of this addition player in the QS regulatory circuit in the interaction with C. albicans is unknown.
Recently, Martínez and co-workers (2019) discovered another cell-density dependent system that is reliant on the production of oxidized fatty acids (oxylipins), termed oxylipin-dependent quorum sensing (ODS) [39]. This system affects the motility of P. aeruginosa, but functions independently of the hierarchical circuit of P. aeruginosa. The activity of this system was induced with the addition of the polyunsaturated fatty acid, oleic acid, that may be available during infection by this pathogen. This raises the question if oxylipins may play a role in the interaction between P. aeruginosa and C. albicans, as C. albicans is also known to produce oxylipins from polyunsaturated fatty acids that can affect morphology in a QS manner [40]. The role of fatty acid metabolites in the interaction is discussed later.
Candida albicans also produces QSMs [41]. The QSM, farnesol, inhibits germ tube formation, similar to 3-oxo-HSL. This effect may be due to inhibition of the Ras1p-controlled pathway involved in hyphal growth [36]. Farnesol inhibits P. aeruginosa PQS and pyocyanin (PYO) production in a dose dependent manner [19]. It also influences expression of P. aeruginosa virulence-related proteins [42], including haemolysin production [43] and inhibits swarming motility [27]. Another C. albicans QSM, tyrosol, stimulates hyphal formation [44], especially during early and intermediate stages of biofilm formation [45]. Physiologically relevant concentrations of tyrosol could also inhibit haemolysin production and secretion of protease by clinical isolates of P. aeruginosa [43].
2.3. Role of Phenazines in the Interaction
Pyocyanin or methyl-1-hydroxyphenazine, is produced by P. aeruginosa during the early stationary phase [46,47] and is the best studied bacterial phenazine. It plays a major role in maintaining NADH/NAD+ ratio stability in P. aeruginosa cells under oxygen limitation, since P. aeruginosa has a limited fermentation capability [48]. It acts as an alternative terminal electron acceptor and decreases the NADH/NAD+ ratio. When oxygen becomes available again, PYO is re-oxidized, possibly leading to the production of reactive oxygen species (ROS). This production of ROS may contribute to the toxic effect of phenazines on eukaryotes, including fungi [49,50]. Pyocyanin also decreases cyclic adenosine monophosphate (cAMP) [51], which explains the observed inhibition of the C. albicans yeast to hyphae transition, which requires cAMP. Gibson and co-workers (2009) co-incubated P. aeruginosa and C. albicans and observed a red pigment inside C. albicans cells [52]. The presence of the red pigment was linked to repression of C. albicans viability. The precursor of this red pigment was identified as 5-methyl phenazine-1-carboxylic acid (5-MPCA). The related phenazine, phenazine-1-carboximide (PCN), also showed broad-spectrum antifungal activity, including against C. albicans and C. glabrata and inhibited the yeast to hyphal switch in C. albicans [53]. The mechanism of action of PCN was increased ROS production, mitochondrial membrane hyperpolarization, and eventually, apoptosis. Interestingly, Morales and co-workers (2013) found that low concentrations of PYO, phenazine methosulfate, and PCN specifically inhibited respiration, leading to increased fermentation and lower extracellular pH, which inhibited C. albicans yeast-to-hyphal switch [54]. Furthermore, Briard and co-workers (2015) indicated that sub-inhibitory concentrations of phenazines were capable of reducing ferric iron, making it more bio-available for co-inhabitants, such as Aspergillus fumigatus [55]. This is a phenomenon that may apply to C. albicans as well.
Chen and co-workers (2014) studied the effect of the C. albicans fermentation product, ethanol, on P. aeruginosa [56]. They found that ethanol inhibited swarming motility, but stimulated adhesion, subsequent biofilm formation and production of 5-MPCA and PCN. This implies the existence of a positive feedback loop where C. albicans-produced ethanol enhances P. aeruginosa biofilm formation and production of antifungal phenazines, which in turn stimulates ethanol production in C. albicans. Several other phenazines (phenazine-1-ol, phenazine-1-carboxylic acid, and PCN) are also inhibitory towards C. albicans and C. tropicalis, with phenazine-1-carboxylic acid having the lowest minimum inhibitory concentration [57]. These phenazines also have a synergistic effect with azoles against Candida species. All of this suggests that the presence of phenazine-producing organisms, such as P. aeruginosa, may influence co-infection by Candida spp. as well as the treatment of such fungal co-infection.
2.4. Role of Fatty Acid Metabolites in the Interaction
As stated above (Section 2.2), Candida spp. can convert exogenous (or host-derived) fatty acids into various lipid metabolites, including hydroxy fatty acids and eicosanoids, such as prostaglandins [40,58,59,60,61,62,63,64,65,66,67], that may influence co-infecting microbes [68] or the host [69,70]. One of the best-studied Candida lipid metabolites is the eicosanoid, prostaglandin E2 (PGE2), which has been reported in C. albicans, C. dubliniensis, C. glabrata, C. parapsilosis and C. tropicalis. P. aeruginosa also secretes an array of fatty acid metabolites, including hydroxy fatty acids [71,72,73] as well as PGE2 and prostaglandin F2α [74]. Martínez and coworkers (2019) studied the effect of hydroxy fatty acids produced by the action of diol synthase in P. aeruginosa and found that the products, 10-hydroxy-octadecenoic acid (10-HOME) and 7,10-dihydroxy-octadecenoic acid (7,10-DiHOME), caused transcriptomic and proteomic alterations in P. aeruginosa [39,71]. This reflected in reduced swarming as well as swimming motility in a dose-dependent manner, as well as strongly promoting type IV pilus-driven twitching movement. These metabolites also promoted micro-colony and subsequent biofilm formation in vitro. Interestingly, 7,10-DiHOME has an inhibitory effect on the growth of C. albicans [75], while another hydroxy fatty acid, 3-hydroxy tetradecadienoic acid (3-OH 14:2), which is an endogenous linoleic acid metabolite of C. albicans, stimulates germ tube formation, even in the presence of farnesol [40]. Fourie and co-workers (2017) investigated the influence of co-incubation of C. albicans and P. aeruginosa on eicosanoid production from exogenous arachidonic acid by mixed species biofilms [74]. They found that, although co-incubation decreased the ability of individual colony forming units (CFUs) in the biofilm to produce eicosanoids, the final concentrations of all the studied eicosanoids (i.e., PGE2, PGF2α, and 15-hydroxy eicosatetraenoic acid) increased compared to single species biofilms. This was due to the increase in P. aeruginosa CFUs in the mixed species biofilms. Although it is known that PGE2 enhances serum-induced germ tube formation in both C. albicans and C. dubliniensis [60,66,76], the influence of these eicosanoids on P. aeruginosa is unknown.
2.5. Role of Competition for Iron in the Interaction
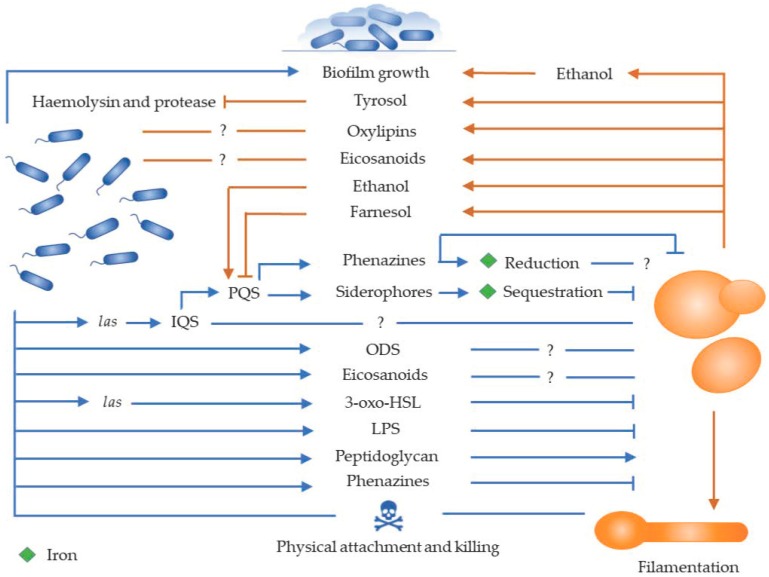
Purscke and co-workers (2012) compared the secretome of single and mixed species biofilms of C. albicans and P. aeruginosa [77]. They found an overall increase in secreted proteins of mixed species biofilms relative to single species biofilms. This increase was due to increased P. aeruginosa secreted proteins, many of them related to production of the P. aeruginosa siderophore, pyoverdine. The authors speculated that this was caused by competition for iron in multispecies biofilms. Chelation of iron by pyoverdine decreases its availability to C. albicans, leading to the observed inhibition of yeast growth. Because pyoverdine production is increased in mixed biofilms, the virulence of P. aeruginosa might also be upregulated in mixed biofilms [28]. An interesting observation was made by Nazik and co-workers (2017) who found that P. aeruginosa bacteriophages can bind to and inhibit C. albicans (and possibly C. kefyr) [78]. This inhibition is likely due to the ability of the bacteriophages to bind iron into tight complexes, precipitating it out of the medium. This denies iron to the yeast, inhibiting both biofilm and planktonic cells. A summary of the interaction between C. albicans and P. aeruginosa is given in Figure 1.
Figure 1.
Schematic of the interaction between Candida albicans and Pseudomonas aeruginosa in vitro. P. aeruginosa kills C. albicans hyphae through physical attachment. In addition, cell wall components such as lipopolysaccharide (LPS) and peptidoglycan affect filamentation. Furthermore, secreted factors such as phenazines and siderophores, as well as quorum sensing systems, such as las (controls the production of autoinducer 3-oxododecanoyl-l-homoserine lactone or 3-oxo-HSL), Pseudomomas quinolone signal (PQS), integrated quorum sensing (IQS), and oxylipin dependent sensing (ODS) may affect C. albicans. Lastly, C. albicans secreted factors such as farnesol, ethanol, oxylipins, and eicosanoids may affect P. aeruginosa growth characteristics and biofilm formation. Question mark (?) indicate unknown roles in the interaction.
3. In Vivo Interactions between Candida spp. and Pseudomonas aeruginosa
Although studying the interaction between organisms in vitro has the advantage of being able to limit the number of variables, this does not always provide a true picture of the potential interactions of co-infecting organisms in a living host with an active immune system. In addition, different body sites provide unique environments in terms of nutrient availability, presence of microbiota and immunity. Therefore, pathogens may exhibit unique patterns of growth and virulence in separate niches of the human body [79]. Due to this, a brief review of the documented interaction between C. albicans and P. aeruginosa in different body sites will be given.
3.1. Interaction in the Lungs
Kerr (1994) was the first to report inhibition of C. albicans in the lungs of postoperative patients after subsequent colonization by P. aeruginosa [80]. This inhibition was reversed by antibiotic treatment of the P. aeruginosa infection. This observation was followed by studies that indicated that prior colonization by Candida spp. increases the susceptibility of the host to P. aeruginosa infection [81,82,83,84] and that the risk can be reduced by antifungal treatment [85]. This could be explained by Roux and co-workers (2013) who found that the Candida-induced Th1-Th17 immune response caused an increase in interferon-gamma (IFN-gamma) [86]. This led to the inhibition of the expression of scavenger receptors on rat alveolar macrophages, and thus to a decrease in P. aeruginosa phagocytosis [87]. Interestingly, Chen and co-workers (2014) found that C. albicans produced ethanol promotes P. aeruginosa colonization of lung epithelial cells, and Greenberg and co-workers (1999) demonstrated that ethanol could inhibit the clearance of P. aeruginosa from the lungs in a rat model of infection by inhibiting macrophage recruitment [56,88]. Ader and co-workers (2011) established a murine infection model with short term colonization by C. albicans prior to P. aeruginosa infection and found that this leads to a reduction in P. aeruginosa load compared to infection in the absence of C. albicans [89]. Mear and co-workers (2014) found that C. albicans induces secretion of interleukin-17 (IL-17) and IL- 22, leading to the production of antimicrobial peptides, as well as the mobilization of phagocytic cells [90]. Bergeron and co-workers (2017) developed a zebra fish swimbladder infection model to study the effect of C. albicans-P. aeruginosa co-infection on virulence [91]. This model clearly showed a synergistic virulence associated with increased C. albicans pathogenesis and inflammation. It is thus clear that the model and the specific methods used for infection influence the outcome of infection and should be taken into account when comparing results regarding the interactions of Candida spp. and P. aeruginosa in animal models.
Several studies of CF patients have indicated pulmonary co-infection with C. albicans and bacteria [92,93,94,95,96]. In this context, the co-infection of C. albicans and P. aeruginosa has been well documented [97]. Haiko and co-workers (2019) indicated a higher incidence of co-existence of P. aeruginosa with Candida spp. compared to other respiratory disorders [98]. Although the clinical impact of fungi in lungs is not yet fully understood [99], it is known that the presence of C. albicans in CF sputum is a very strong predictor of co-colonization with Pseudomonas spp., as well as of hospital-treated exacerbations [100]. However, Haiko and co-workers (2019) provided evidence that Candida spp. colonization does not exclusively lead to higher existence of pathogenic bacteria, indicating that this aspect still needs further validation [98]. Co-colonization with C. albicans and P. aeruginosa also led to a significant decline in lung function compared to patients without C. albicans. Kim and co-workers (2015) provided evidence of adaptation of C. albicans in the CF lung [101]. They isolated C. albicans strains that independently accumulated loss-of-function mutations in the NRG1 gene, the product of which is a transcription factor that is known to repress filamentation in C. albicans [102]. Strikingly, these strains were impervious to the filamentation-suppressing effects of co-culture with P. aeruginosa and an analog of PYO, phenazine methosulfate. This study indicated that this adaptation may be common in the CF lung environment [101].
Patients on mechanical ventilation are also particularly susceptible to colonization by Candida spp. and subsequent P. aeruginosa ventilator-associated pneumonia (VAP) [81]. In 214 patients with Pseudomonas spp. VAP included in this study, who also had Candida spp. in their respiratory tracts, the most common Candida spp. were C. albicans, C. glabrata and C. tropicalis. Hamet and co-workers [83] found that mortality of intensive care patients with VAP was significantly increased by Candida spp. co-colonization and that Candida colonization was an independent predictor of death. According to Delisle and co-workers (2011), it is unclear whether colonization of the respiratory tract by Candida spp. is merely an indication of disease severity or contributes to the observed worse clinical outcomes in these patients [103]. Since respiratory colonization with Candida spp. alone can increase levels of tumor necrosis factor alpha and IFN-gamma in the lung [82] and, as stated above, IFN-gamma can impair the function of alveolar macrophages, it may be possible that colonization by C. albicans could inhibit an antibacterial immune response. In a recent review by Pendleton and co-workers [104] dealing with the significance of Candida in the human respiratory tract, the question regarding the clinical relevance of treating such occurrences with antifungal drugs is raised. Although treatments with antifungals in experimental models have yielded positive results [86,105], this was not always the case in human patients [85,106,107]. Due to these discrepancies in the results, no firm treatment guidelines exist, and clinical practice varies.
3.2. Interaction in Wounds
Chronic wounds, such as diabetic foot ulcers, pressure ulcers, and venous leg ulcers are prone to infection by polymicrobial biofilms [108,109]. Many of these are co-infections by Candida spp. (C. parapsilosis, C. tropicalis and C. albicans) and bacteria, including P. aeruginosa [110]. Despite this, infections in chronic wounds are typically treated with antibiotics, ignoring the fungal component, often leading to unresolved infections. Townsend and co-workers (2017) used a biofilm model, consisting of a complex cellulose matrix, to mimic the wound environment and polymicrobial biofilms consisting of C. albicans, P. aeruginosa and Staphylococcus aureus [109]. By treating the polymicrobial biofilms with antibiotics alone or in combination with fluconazole, they could change the compositions of the biofilms without affecting the overall cell numbers. They also found that the C. albicans population is an important driving force within the biofilm and is correlated to the total number of cells in the biofilm. This is due to the physical support and protection provided by the hyphae and extracellular matrix of C. albicans.
Another type of wound prone to infection by both Candida spp. and P. aeruginosa is thermal burn wounds. Although burn wounds are initially sterile, within the first 48 hours they typically become infected by Gram-positive bacteria. During the first week the population shifts to Gram-negative bacteria [111]. Lethal Candida infections often follow or occur concomitantly with bacterial infections [112]. The Candida spp. identified from burn wounds include C. albicans, C. tropicalis, and C. parapsilosis [113]. Using a burned mouse model to study the effect of a recent P. aeruginosa infection on the development of systemic C. albicans infection, Neely and co-workers (1986) found that burned mice pre-infected with a sublethal dose of P. aeruginosa, followed by a sublethal challenge with C. albicans, had a significantly higher mortality rate than either unburned mice, challenged in the same way, or burned mice infected with only one of the microbes [112]. They found that burned mice challenged with both organisms died due to Candida infection and indicated the importance of proteolytic enzymes secreted by P. aeruginosa in allowing the establishment of lethal C. albicans infections. This is in contrast to a study by Gupta and co-workers (2005), who found significant in vivo inhibition of C. albicans growth in the presence of P. aeruginosa in burn wounds [114].
3.3. Interaction in the Gastrointestinal Tract
C. albicans is a commensal member of the gastrointestinal microbiota of many mammals, including humans [115,116]. Certain immunocompromised patients, such as cancer patients, are at risk of developing invasive Candida infections that may originate from the gastrointestinal system. Similarly, these patients are also at higher risk of P. aeruginosa bloodstream infections originating from the gastrointestinal tract [117]. In order to study the interaction between C. albicans and P. aeruginosa, a neutropenic murine model for gastrointestinal co-colonization was developed by Lopez-Medina and co-workers (2015) [118]. The reported antagonism between C. albicans and P. aeruginosa, often seen in vitro, was not seen in this model, with levels of both P. aeruginosa and C. albicans unaffected by co-colonization. In addition, mice co-colonized with P. aeruginosa and C. albicans had significantly lower mortality compared to mice colonised with only P. aeruginosa. All dead mice exhibited evidence of P. aeruginosa dissemination. The authors showed that C. albicans supressed expression of P. aeruginosa pyochelin and pyoverdine genes. Deletion of these genes did not affect the ability of P. aeruginosa to colonize the gastrointestinal tract, but did decrease the dissemination and virulence of P. aeruginosa. This virulence was restored after oral iron supplementation. This points to the importance of iron in the in vivo interaction between C. albicans and P. aeruginosa. In addition, using cultured colonocytes, they also found that C. albicans secreted proteins inhibited the production of cytotoxic exotoxin A. Lamont and co-workers (2002) indicated that pyoverdine regulates the production of exotoxin A, so the decrease in exotoxin A levels may also be an additional effect of C. albicans mediated decrease in pyoverdine production [119]. Regardless of the mechanism behind the reduction in exotoxin A, this may prevent the damage caused by P. aeruginosa in the gastrointestinal tract and possibly prevent dissemination. Table 1 summarizes the interaction between C. albicans and P. aeruginosa in vivo.
Table 1.
Summary of the interaction between Candida spp. and Pseudomonas aeruginosa in vivo in various infection sites.
| Infection Site | Type of Study/Model | Observations | Effect of P. aeruginosa on Candida | Effect of Candida on P. aeruginosa | Number(s) in Reference List |
|---|---|---|---|---|---|
| Lungs | Postoperative monitoring of surgery patients | Inhibition of C. albicans after subsequent colonization with P. aeruginosa. Reversed with antibiotic treatment | Inhibited | - | [80] |
| Lungs | Monitoring of patients with mechanical ventilation | Colonization with Candida spp. associated with increased risk of P. aeruginosa VAP1 | - | Promoted | [81] |
| Lungs | Monitoring of patients with VAP 1 | Increase in isolation of multidrug resistant bacteria such as P. aeruginosa when Candida spp. are present. Reduced risk for P. aeruginosa VAP 1 with antifungal treatment |
- | Promoted | [83,85] |
| Lungs | Analysis of sputum samples from CF 2 patients | Higher incidence of co-existence between P. aeruginosa and Candida spp. in CF2 patients compared to other respiratory disorders | - | - | [98,100] |
| Lungs | Analysis of sputum samples from CF 2 patients | Presence of hyperfilamentous C. albicans with loss of function mutation in NGR1 in presence of P. aeruginosa | No inhibition of filamentation | _ | [101] |
| Lungs | Wistar rat model | Fungal colonization promoted pneumonia by P. aeruginosa Reversed with antifungal treatment |
- | Promoted | [82,86] |
| Lungs | Mouse model | Prior C. albicans colonization promoted P. aeruginosa clearance | - | Clearance enhanced | [89,90] |
| Mucosa | Zebrafish swimbladder model | Synergistic increase in virulence in co-infection compared to single species infection | Increased virulence | Increased virulence | [91] |
| Wounds | Microbial populations of deep tissue wounds of patients with type 2 diabetes cultured | C. albicans not found in combination with P. aeruginosa | Inhibited | - | [106] |
| Wounds | Mouse model | Pre-infection with P. aeruginosa predisposed mice to lethal C. albicans infection | P. aeruginosa proteolytic enzymes promote lethal C. albicans infection | - | [108] |
| Wounds | Microbial analysis of wounds of burn patients | Inhibition of Candida spp. when Pseudomonas spp. were present | Inhibited | - | [110] |
| Wound model | In vitro biofilm model to mimic wounds | Biofilms of C. albicans, Staphylococcus aureus and P. aeruginosa created. Monotreatment with antifungal/antibiotic only shifted population dynamics without affecting overall biofilm bioburden |
Provides physical support and protection to bacteria | [105] | |
| Gastro-intestinal tract | Neutropenic mouse model | Levels of both P. aeruginosa and C. albicans unaffected by co-incubation | Decrease in siderophore production and virulence | [114] |
1 VAP – Ventilator-associated pneumonia; 2 CF – Cystic fibrosis.
4. Phenotypic Plasticity in Polymicrobial Interactions
An impressive characteristic of C. albicans is its phenotypic plasticity, with this fungus able to exhibit up to nine distinct cell types that are dependent on environmental cues [120]. These include the classical cell types, yeast, hyphae, pseudohyphae, and chlamydospores. Furthermore, in addition to the white phenotype with heterozygosity at the mating type locus (a/α; mostly observed in laboratory conditions and infection), a white phenotype exists with either the a or α genotype, as well as an opaque phenotype with heterozygosity at the mating type locus, a/α, with opaque (homozygous, a or α), grey, and gastrointestinally-induced transition (GUT) phenotypes [120,121,122,123]. These non-classical phenotypes are highly dependent on the expression of the white-opaque regulator, Wor1p, that causes heritable changes, eliciting transcriptional changes, which flow over into virulence. The expression of this regulator is dependent on environmental cues, such as N-acetylglucosamine, a component of the bacterial cell wall, CO2, and hypoxia—an environmental circumstance frequently found in the gastrointestinal tract [122,124,125]. These phenotypes exhibit altered metabolic specialization and mating potential. For example, expression of GUT cell genes involved in iron uptake and glucose utilization is down-regulated, with an upregulation in genes involved in utilization of N-acetylglucosamine and short-chain fatty acids, making these cells well suited for commensal growth with resident bacteria in the gastrointestinal tract [122]. Furthermore, whereas white cells have an increased expression of genes involved in pathways associated with fermentative growth, opaque cells are more primed for oxidative respiration [121,123]. Furthermore, Malavia and co-workers (2017) described a hyper-adherent goliath phenotype induced by zinc limitation [126].
The influence of some of the classical phenotypes, such as yeast and hyphae, on the interaction with P. aeruginosa has been evaluated. Less information regarding the influence of non-classical phenotypic alterations on the interaction between C. albicans and pathobionts such as P. aeruginosa is available. This is especially interesting as certain niches, where co-occurrence of C. albicans and P. aeruginosa has been observed, may have the presence of environmental cues that could potentially elicit a partial or full switch to the mentioned opaque or GUT phenotypes in C. albicans, such as in the CF lung where bacterial cell wall components such as N-acetylglucosamine, are abundant due to the presence of bacteria [102], and hypoxic conditions are present [127]. However, further research is required to determine if P. aeruginosa could encounter other phenotypes of C. albicans besides the classical cell types, and whether this would cause any alterations in the interaction with this bacterium.
Similar to the functional specialization of C. albicans, is the remarkable phenotypic variation exhibited by P. aeruginosa, driven by hypermutable strains and richness in oxidative and nitrosative stresses, when in the environment of the CF lung [128,129,130]. Genetic and functional phenotypic variation lead to P. aeruginosa isolates exhibiting differences in colony morphology, extracellular polysaccharide production, motility, quorum sensing, protease activity, auxotrophy, siderophore production, and growth profiles [131]. These different phenotypes are found together, even in a single patient, and comprise a highly dynamic population of P. aeruginosa in chronic CF lung infections due to spatial heterogeneity and niche partitioning [131,132]. In addition, large variation in susceptibility to antimicrobials is seen with little association to morphotype. How these different phenotypes may influence the interaction of P. aeruginosa with pathobionts in the CF lung such as C. albicans has received little attention. Of special interest is the ethanol production by C. albicans that may be able to promote a mucoid phenotype of P. aeruginosa in the CF lung [56,133]. This phenotype, associated with an increase in alginate production, a component of the extracellular matrix of P. aeruginosa, is associated with long term chronic infection of the CF lung by P. aeruginosa. In addition to overproduction of alginate, the adaptation of P. aeruginosa to long term chronic CF infection also includes decreased expression and loss of flagellin, the type III secretion system, as well as changes in LPS structure that aid in immune evasion [128,134,135,136,137]. The possible influence of this adaptation of P. aeruginosa on C. albicans deserves further attention.
5. Conclusions and Future Perspectives
The interaction between C. albicans and P. aeruginosa is frequently used as a model to study the complex interplay between cross-kingdom co-infectious agents. This interaction is often characterized as antagonistic in vitro, with suppression of fungal growth by both physical association as well as secreted factors. Notably, QSMs produced by both species alter the phenotype of the other during association. The discovery of an additional P. aeruginosa QS participant in the las, rhl, and PQS systems, namely IQS, still warrants investigation into the influence during polymicrobial growth with C. albicans. Additionally, the identification of the QS activity of oxylipins and eicosanoid production in P. aeruginosa, as well as the known production of oxylipins and eicosanoids by C. albicans, further motivates research into their effects on fungal-bacterial interactions.
The in vitro antagonism of the interaction between C. albicans and P. aeruginosa may be a simplistic view of an interaction in which both participants influence the outcome of infection. In addition, this interaction is much more complex in vivo, where either species can alter colonization by the other, promoting or inhibiting disease. This seems to be highly dependent on the site of infection, immune functionality, and prior antimicrobial treatment. Both pathogens exhibit complex regulatory systems that facilitate adaptability to multiple niches within the human body. Due to this, they are rarely isolated in only one niche, as described above in the various infection sites, and exhibit alterations in their phenotypes. The occurrence of the functional specialization of both C. albicans and P. aeruginosa leading to altered phenotypes also prompts the investigation into the occurrence of these phenotypes in the presence of other microbial species, i.e., C. albicans in the presence of P. aeruginosa, and vice versa. Additionally, the influence of these phenotypes on the interaction of these two species still requires investigation.
Author Contributions
R.E. and C.P. co-wrote the article. Both authors collected literature and placed it into context for the review. R.E. designed the figures and C.P. edited the final manuscript and supervised the first author.
Funding
This research was funded by the National Research foundation of South Africa, who provided a bursary to R.E, grant number 102594.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of the literature; in the writing of the manuscript, or in the decision to publish the review.
References
- 1.Hogan D.A., Kolter R. Pseudomonas-Candida interactions: An ecological role for virulence factors. Science. 2002;296:2229–2232. doi: 10.1126/science.1070784. [DOI] [PubMed] [Google Scholar]
- 2.Pohl C.H., Kock J.L.F. Oxidized fatty acids as inter-kingdom signaling molecules. Molecules. 2014;19:1273–1285. doi: 10.3390/molecules19011273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frey-Klett P., Burlinson P., Deveau A., Barret M., Tarkka M., Sarniguet A. Bacterial-fungal interactions: Hyphens between agricultural, clinical, environmental, and food microbiologists. Microbiol. Mol. Biol. Rev. 2011;75:583–609. doi: 10.1128/MMBR.00020-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanley C.E., Stöckli M., van Swaay D., Sabotič J., Kallio P.T., Künzler M., deMello A.J., Aebi M. Probing bacterial-fungal interactions at the single cell level. Integr. Biol. (Camb.) 2014;6:935–945. doi: 10.1039/C4IB00154K. [DOI] [PubMed] [Google Scholar]
- 5.Rashid M.I., Mujawar L.H., Shahzad T., Almeelbi T., Ismail I.M.I., Oves M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 2016;183:26–41. doi: 10.1016/j.micres.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Tay W.H., Chong K.K.L., Kline K.A. Polymicrobial–host interactions during infection. J. Mol. Biol. 2016;428:3355–3371. doi: 10.1016/j.jmb.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 7.He J., Kim D., Zhou X., Ahn S.-J., Burne R.A., Richards V.P., Koo H. RNA-seq reveals enhanced sugar metabolism in Streptococcus mutans co-cultured with Candida albicans within mixed-species biofilms. Front. Microbiol. 2017;8:1036. doi: 10.3389/fmicb.2017.01036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlson E. Synergistic effect of Candida albicans and Staphylococcus aureus on mouse mortality. Infect. Immun. 1982;38:921–924. doi: 10.1128/iai.38.3.921-924.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diaz P.I., Xie Z., Sobue T., Thompson A., Biyikoglu B., Ricker A., Ikonomou L., Dongari-Bagtzoglou A. Synergistic interaction between Candida albicans and commensal oral streptococci in a novel in vitro mucosal model. Infect. Immun. 2012;80:620–632. doi: 10.1128/IAI.05896-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Auger P., Joly J. Factors influencing germ tube production in Candida albicans. Mycopathologia. 1977;61:183–186. doi: 10.1007/BF00468014. [DOI] [PubMed] [Google Scholar]
- 11.Hughes W.T., Kim H.K. Mycoflora in cystic fibrosis: Some ecologic aspects of Pseudomonas aeruginosa and Candida albicans. Mycopathol. Mycol. Appl. 1973;50:261–269. doi: 10.1007/BF02053377. [DOI] [PubMed] [Google Scholar]
- 12.Kerr J. Inhibition of fungal growth by Pseudomonas aeruginosa and Pseudomonas cepacia isolated from patients with cystic fibrosis. J. Infect. 1994;28:305–310. doi: 10.1016/S0163-4453(94)91943-7. [DOI] [PubMed] [Google Scholar]
- 13.Kaleli I., Cevahir N., Demir M., Yildirim U., Sahin R. Anticandidal activity of Pseudomonas aeruginosa strains isolated from clinical specimens. Mycoses. 2006;50:74–78. doi: 10.1111/j.1439-0507.2006.01322.x. [DOI] [PubMed] [Google Scholar]
- 14.Bandara H.M.H.N., Yau J.Y.Y., Watt R.M., Jin L.J., Samaranayake L.P. Pseudomonas aeruginosa inhibits in-vitro Candida biofilm development. BMC Microbiol. 2010;10:125. doi: 10.1111/j.1439-0507.2006.01322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bandara H.M.H.N., Cheung B.P.K., Watt R.M., Jin L.J., Samaranayake L.P. Pseudomonas aeruginosa lipopolysaccharide inhibits Candida albicans hyphae formation and alters gene expression during biofilm development. Mol. Oral Microbiol. 2013;28:54–69. doi: 10.1111/omi.12006. [DOI] [PubMed] [Google Scholar]
- 16.Xu X.L., Lee R.T.H., Fang H.M., Wang Y.M., Li R., Zou H., Zhu Y., Wang Y. Bacterial peptidoglycan triggers Candida albicans hyphal growth by directly activating the adenylyl cyclase Cyr1p. Cell Host Microbe. 2008;4:28–39. doi: 10.1016/j.chom.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 17.Brand A., Barnes J.D., Mackenzie K.S., Odds F.C., Gow N.A.R. Cell wall glycans and soluble factors determine the interactions between the hyphae of Candida albicans and Pseudomonas aeruginosa. FEMS Microbiol. Lett. 2008;287:48–55. doi: 10.1111/j.1574-6968.2008.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holcombe L.J., McAlester G., Munro C.A., Enjalbert B., Brown A.J.P., Gow N.A.R., Ding C., Butler G., O’Gara F., Morrissey J.P. Pseudomonas aeruginosa secreted factors impair biofilm development in Candida albicans. Microbiology. 2010;156:1476–1485. doi: 10.1099/mic.0.037549-0. [DOI] [PubMed] [Google Scholar]
- 19.Cugini C., Calfee M.W., Farrow J.M., Morales D.K., Pesci E.C., Hogan D.A. Farnesol, a common sesquiterpene, inhibits PQS production in Pseudomonas aeruginosa. Mol. Microbiol. 2007;65:896–906. doi: 10.1111/j.1365-2958.2007.05840.x. [DOI] [PubMed] [Google Scholar]
- 20.de Kievit T.R., Iglewski B.H. Bacterial quorum sensing in pathogenic relationships. Infect. Immun. 2000;68:4839–4849. doi: 10.1128/IAI.68.9.4839-4849.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Passador L., Cook J.M., Gambello M.J., Rust L., Iglewski B.H. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science. 1993;260:1127–1130. doi: 10.1126/science.8493556. [DOI] [PubMed] [Google Scholar]
- 22.Pearson J.P., Passador L., Iglewski B.H., Greenberg E.P. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. 1995;92:1490–1494. doi: 10.1073/pnas.92.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hentzer M., Wu H., Andersen J.B., Riedel K., Rasmussen T.B., Bagge N., Kumar N., Schembri M.A., Song Z., Kristoffersen P., et al. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 2003;22:3803–3815. doi: 10.1093/emboj/cdg366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner V.E., Bushnell D., Passador L., Brooks A.I., Iglewski B.H. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: Effects of growth phase and environment. J. Bacteriol. 2003;185:2080–2095. doi: 10.1128/JB.185.7.2080-2095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ovchinnikova E.S., Krom B.P., van der Mei H.C., Busscher H.J. Force microscopic and thermodynamic analysis of the adhesion between Pseudomonas aeruginosa and Candida albicans. Soft Matter. 2012;8:6454. doi: 10.1039/c2sm25100k. [DOI] [Google Scholar]
- 26.Hogan D.A., Vik Å., Kolter R. A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol. Microbiol. 2004;54:1212–1223. doi: 10.1111/j.1365-2958.2004.04349.x. [DOI] [PubMed] [Google Scholar]
- 27.McAlester G., O’Gara F., Morrissey J.P. Signal-mediated interactions between Pseudomonas aeruginosa and Candida albicans. J. Med. Microbiol. 2008;57:563–569. doi: 10.1099/jmm.0.47705-0. [DOI] [PubMed] [Google Scholar]
- 28.Trejo-Hernández A., Andrade-Domínguez A., Hernández M., Encarnación S. Interspecies competition triggers virulence and mutability in Candida albicans-Pseudomonas aeruginosa mixed biofilms. ISME J. 2014;8:1974–1988. doi: 10.1038/ismej.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pesci E.C., Milbank J.B.J., Pearson J.P., McKnight S., Kende A.S., Greenberg E.P., Iglewski B.H. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. 1999;96:11229–11234. doi: 10.1073/pnas.96.20.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lépine F., Déziel E., Milot S., Rahme L.G. A stable isotope dilution assay for the quantification of the Pseudomonas quinolone signal in Pseudomonas aeruginosa cultures. Biochim. Biophys. Acta - Gen. Subj. 2003;1622:36–41. doi: 10.1016/S0304-4165(03)00103-X. [DOI] [PubMed] [Google Scholar]
- 31.De Sordi L., Mühlschlegel F.A. Quorum sensing and fungal-bacterial interactions in Candida albicans: A communicative network regulating microbial coexistence and virulence. FEMS Yeast Res. 2009;9:990–999. doi: 10.1111/j.1567-1364.2009.00573.x. [DOI] [PubMed] [Google Scholar]
- 32.Déziel E., Lépine F., Milot S., He J., Mindrinos M.N., Tompkins R.G., Rahme L.G. Analysis of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines (HAQs) reveals a role for 4-hydroxy-2-heptylquinoline in cell-to-cell communication. Proc. Natl. Acad. Sci. USA. 2004;101:1339–1344. doi: 10.1073/pnas.0307694100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ha D.G., Merritt J.H., Hampton T.H., Hodgkinson J.T., Janecek M., Spring D.R., Welch M., O’Toole G.A. 2-Heptyl-4-quinolone, a precursor of the Pseudomonas quinolone signal molecule, modulates swarming motility in Pseudomonas aeruginosa. J. Bacteriol. 2011;193:6770–6780. doi: 10.1128/JB.05929-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phelan V.V., Moree W.J., Aguilar J., Cornett D.S., Koumoutsi A., Noble S.M., Pogliano K., Guerrero C.A., Dorrestein P.C. Impact of a transposon insertion in phzF2 on the specialized metabolite production and interkingdom interactions of Pseudomonas aeruginosa. J. Bacteriol. 2014;196:1683–1693. doi: 10.1128/JB.01258-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reen F.J., Mooij M.J., Holcombe L.J., Mcsweeney C.M., Mcglacken G.P., Morrissey J.P., O’Gara F. The Pseudomonas quinolone signal (PQS), and its precursor HHQ, modulate interspecies and interkingdom behaviour. FEMS Microbiol. Ecol. 2011;77:413–428. doi: 10.1111/j.1574-6941.2011.01121.x. [DOI] [PubMed] [Google Scholar]
- 36.Morales D.K., Hogan D.A. Candida albicans interactions with bacteria in the context of human health and disease. PLoS Pathog. 2010;6:e1000886. doi: 10.1371/journal.ppat.1000886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J., Zhang L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell. 2015;6:26–41. doi: 10.1007/s13238-014-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee J., Wu J., Deng Y., Wang J., Wang C., Wang J., Chang C., Dong Y., Williams P., Zhang L.-H. A cell-cell communication signal integrates quorum sensing and stress response. Nat. Chem. Biol. 2013;9:339–343. doi: 10.1038/nchembio.1225. [DOI] [PubMed] [Google Scholar]
- 39.Martínez E., Cosnahan R.K., Wu M., Gadila S.K., Quick E.B., Mobley J.A., Campos-Gómez J. Oxylipins mediate cell-to-cell communication in Pseudomonas aeruginosa. Commun. Biol. 2019;2:66. doi: 10.1038/s42003-019-0310-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nigam S., Ciccoli R., Ivanov I., Sczepanski M., Deva R. On mechanism of quorum sensing in Candida albicans by 3(R)-hydroxy-tetradecaenoic acid. Curr. Microbiol. 2011;62:55–63. doi: 10.1007/s00284-010-9666-6. [DOI] [PubMed] [Google Scholar]
- 41.Hornby J.M., Jensen E.C., Lisec A.D., Tasto J.J., Jahnke B., Shoemaker R., Dussault P., Nickerson K.W. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 2001;67:2982–2992. doi: 10.1128/AEM.67.7.2982-2992.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones-Dozier S.L. Ph.D. Thesis. Georgia State University; Atlanta, GA, USA: 2008. Proteomic analysis of the response of Pseudomonas aeruginosa PAO1 to the cell to cell signaling molecule trans,trans-farnesol of Candida albicans. [Google Scholar]
- 43.Abdel-Rhman S.H., El-Mahdy A.M., El-Mowafy M. Effect of tyrosol and farnesol on virulence and antibiotic resistance of clinical isolates of Pseudomonas aeruginosa. Biomed Res. Int. 2015;2015:456463. doi: 10.1155/2015/456463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fink G.R., Clardy J., Feng Q., Fujita M., Chen H. Tyrosol is a quorum-sensing molecule in Candida albicans. Proc. Natl. Acad. Sci. 2004;101:5048–5052. doi: 10.1073/pnas.0401416101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alem M.A.S., Oteef M.D.Y., Flowers T.H., Douglas L.J. Production of tyrosol by Candida albicans biofilms and its role in quorum sensing and biofilm development. Eukaryot. Cell. 2006;5:1770–1779. doi: 10.1128/EC.00219-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hernandez M.E., Kappler A., Newman D.K. Phenazines and other redox-active antibiotics promote microbial mineral reduction. Appl. Environ. Microbiol. 2004;70:921–928. doi: 10.1128/AEM.70.2.921-928.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Price-Whelan A., Dietrich L.E.P., Newman D.K. Pyocyanin alters redox homeostasis and carbon flux through central metabolic pathways in Pseudomonas aeruginosa PA14. J. Bacteriol. 2007;189:6372–6381. doi: 10.1128/JB.00505-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dietrich L.E.P., Price-Whelan A., Petersen A., Whiteley M., Newman D.K. The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol. Microbiol. 2006;61:1308–1321. doi: 10.1111/j.1365-2958.2006.05306.x. [DOI] [PubMed] [Google Scholar]
- 49.O’Malley Y.Q., Abdalla M.Y., McCormick M.L., Reszka K.J., Denning G.M., Britigan B.E. Subcellular localization of Pseudomonas pyocyanin cytotoxicity in human lung epithelial cells. Am. J. Physiol. Cell. Mol. Physiol. 2003;284:L420–L430. doi: 10.1152/ajplung.00316.2002. [DOI] [PubMed] [Google Scholar]
- 50.Gloyne L.S., Grant G.D., Perkins A.V., Powell K.L., McDermott C.M., Johnson P.V., Anderson G.J., Kiefel M., Anoopkumar-Dukie S. Pyocyanin-induced toxicity in A549 respiratory cells is causally linked to oxidative stress. Toxicol. Vitr. 2011;25:1353–1358. doi: 10.1016/j.tiv.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 51.Kerr J.R., Taylor G.W., Rutman A., Høiby N., Cole P.J., Wilson R. Pseudomonas aeruginosa pyocyanin and 1-hydroxyphenazine inhibit fungal growth. J. Clin. Pathol. 1999;52:385–387. doi: 10.1136/jcp.52.5.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gibson J., Sood A., Hogan D.A. Pseudomonas aeruginosa-Candida albicans interactions: Localization and fungal toxicity of a phenazine derivative. Appl. Environ. Microbiol. 2009;75:504–513. doi: 10.1128/AEM.01037-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tupe S.G., Kulkarni R.R., Shirazi F., Sant D.G., Joshi S.P., Deshpande M.V. Possible mechanism of antifungal phenazine-1-carboxamide from Pseudomonas sp. against dimorphic fungi Benjaminiella poitrasii and human pathogen Candida albicans. J. Appl. Microbiol. 2015;118:39–48. doi: 10.1111/jam.12675. [DOI] [PubMed] [Google Scholar]
- 54.Morales D.K., Grahl N., Okegbe C., Dietrich L.E.P., Jacobs N.J., Hogan D.A. Control of Candida albicans metabolism and biofilm formation by Pseudomonas aeruginosa phenazines. MBio. 2013;4:1–9. doi: 10.1128/mBio.00526-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Briard B., Bomme P., Lechner B.E., Mislin G.L.A., Lair V., Prévost M.-C., Latgé J.-P., Haas H., Beauvais A. Pseudomonas aeruginosa manipulates redox and iron homeostasis of its microbiota partner Aspergillus fumigatus via phenazines. Sci. Rep. 2015;5:8220. doi: 10.1038/srep08220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen A.I., Dolben E.F., Okegbe C., Harty C.E., Golub Y., Thao S., Ha D.G., Willger S.D., O’Toole G.A., Harwood C.S., et al. Candida albicans ethanol stimulates Pseudomonas aeruginosa WspR-controlled biofilm formation as part of a cyclic relationship involving phenazines. PLoS Pathog. 2014;10:e1004480. doi: 10.1371/journal.ppat.1004480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishanth Kumar S., Nisha G.V., Sudaresan A., Venugopal V.V., Sree Kumar M.M., Lankalapalli R.S., Dileep Kumar B.S. Synergistic activity of phenazines isolated from Pseudomonas aeruginosa in combination with azoles against Candida species. Med. Mycol. 2014;52:482–490. doi: 10.1093/mmy/myu012. [DOI] [PubMed] [Google Scholar]
- 58.Deva R., Ciccoli R., Kock L., Nigam S. Involvement of aspirin-sensitive oxylipins in vulvovaginal candidiasis. FEMS Microbiol. Lett. 2001;198:37–43. doi: 10.1111/j.1574-6968.2001.tb10616.x. [DOI] [PubMed] [Google Scholar]
- 59.Grózer Z., Tóth A., Tóth R., Kecskeméti A., Vágvölgyi C., Nosanchuk J.D., Szekeres A., Gácser A. Candida parapsilosis produces prostaglandins from exogenous arachidonic acid and OLE2 is not required for their synthesis. Virulence. 2015;6:85–92. doi: 10.4161/21505594.2014.988097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Noverr M.C., Phare S.M., Toews G.B., Coffey M.J., Huffnagle G.B. Pathogenic yeasts Cryptococcus neoformans and Candida albicans produce immunomodulatory prostaglandins. Infect. Immun. 2001;69:2957–2963. doi: 10.1128/IAI.69.5.2957-2963.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Noverr M.C., Toews G.B., Huffnagle G.B. Production of prostaglandins and leukotrienes by pathogenic fungi. Infect. Immun. 2002;70:400–402. doi: 10.1128/IAI.70.1.400-402.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alem M.A., Douglas L.J. Prostaglandin production during growth of Candida albicans biofilms. J. Med. Microbiol. 2005;54:1001–1005. doi: 10.1099/jmm.0.46172-0. [DOI] [PubMed] [Google Scholar]
- 63.Erb-Downward J.R., Noverr M.C. Characterization of prostaglandin E2 production by Candida albicans. Infect. Immun. 2007;75:3498–3505. doi: 10.1128/IAI.00232-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haas-Stapleton E.J., Lu Y., Hong S., Arita M., Favoreto S., Nigam S., Serhan C.N., Agabian N. Candida albicans modulates host defense by biosynthesizing the pro-resolving mediator resolvin E1. PLoS ONE. 2007;2:e1316. doi: 10.1371/journal.pone.0001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shiraki Y., Ishibashi Y., Hiruma M., Nishikawa A., Ikeda S. Candida albicans abrogates the expression of interferon-γ-inducible protein-10 in human keratinocytes. FEMS Immunol. Med. Microbiol. 2008;54:122–128. doi: 10.1111/j.1574-695X.2008.00457.x. [DOI] [PubMed] [Google Scholar]
- 66.Ells R., Kock J.L.F., Albertyn J., Kemp G., Pohl C.H. Effect of inhibitors of arachidonic acid metabolism on prostaglandin E2 production by Candida albicans and Candida dubliniensis biofilms. Med. Microbiol. Immunol. 2011;200:23–28. doi: 10.1007/s00430-010-0169-7. [DOI] [PubMed] [Google Scholar]
- 67.Mishra N.N., Ali S., Shukla P.K. Arachidonic acid affects biofilm formation and PGE2 level in Candida albicans and non-albicans species in presence of subinhibitory concentration of fluconazole and terbinafine. Braz. J. Infect. Dis. 2014;18:287–293. doi: 10.1016/j.bjid.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krause J., Geginat G., Tammer I. Prostaglandin E2 from Candida albicans stimulates the growth of Staphylococcus aureus in mixed biofilms. PLoS ONE. 2015;10:e0135404. doi: 10.1371/journal.pone.0135404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Romani L. Innate and adaptive immunity in Candida albicans infections and saprophytism. J. Leukoc. Biol. 2000;68:175–179. [PubMed] [Google Scholar]
- 70.Smeekens S.P., van de Veerdonk F.L., van der Meer J.W.M., Kullberg B.J., Joosten L.A.B., Netea M.G. The Candida Th17 response is dependent on mannanand B-glucan-induced prostaglandin E2. Int. Immunol. 2010;22:889–895. doi: 10.1093/intimm/dxq442. [DOI] [PubMed] [Google Scholar]
- 71.Martínez E., Campos-Gómez J. Oxylipins produced by Pseudomonas aeruginosa promote biofilm formation and virulence. Nat. Commun. 2016;7:13823. doi: 10.1038/ncomms13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Serhan C.N. Lipoxins and aspirin-triggered 15-epi-lipoxin biosynthesis: An update and role in anti-inflammation and pro-resolution. Prostaglandins Other Lipid Mediat. 2002;68–69:433–455. doi: 10.1016/S0090-6980(02)00047-3. [DOI] [PubMed] [Google Scholar]
- 73.Vance R.E., Hong S., Gronert K., Serhan C.N., Mekalanos J.J. The opportunistic pathogen Pseudomonas aeruginosa carries a secretable arachidonate 15-lipoxygenase. Proc. Natl. Acad. Sci. USA. 2004;101:2135–2139. doi: 10.1073/pnas.0307308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fourie R., Ells R., Kemp G., Sebolai O.M., Albertyn J., Pohl C.H. Pseudomonas aeruginosa produces aspirin insensitive eicosanoids and contributes to the eicosanoid profile of polymicrobial biofilms with Candida albicans. Prostaglandins Leukot. Essent. Fat. Acids. 2017;117:36–46. doi: 10.1016/j.plefa.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 75.Hou C.T. New bioactive fatty acids. Asia Pac. J. Clin. Nutr. 2008;17(Suppl. 1):192–195. [PubMed] [Google Scholar]
- 76.Noverr M.C., Huffnagle G.B. Regulation of Candida albicans morphogenesis by fatty acid metabolites. Infect. Immun. 2004;72:6206–6210. doi: 10.1128/IAI.72.11.6206-6210.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Purschke F.G., Hiller E., Trick I., Rupp S. Flexible survival strategies of Pseudomonas aeruginosa in biofilms result in increased fitness compared with Candida albicans. Mol. Cell. Proteomics. 2012;11:1652–1669. doi: 10.1074/mcp.M112.017673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nazik H., Joubert L.-M., Secor P.R., Sweere J.M., Bollyky P.L., Sass G., Cegelski L., Stevens D.A. Pseudomonas phage inhibition of Candida albicans. Microbiology. 2017;163:1568–1577. doi: 10.1099/mic.0.000539. [DOI] [PubMed] [Google Scholar]
- 79.Jabra-Rizk M.A., Kong E.F., Tsui C., Nguyen M.H., Clancy C.J., Fidel P.L., Noverr M. Candida albicans pathogenesis: Fitting within the host-microbe damage response framework. Infect. Immun. 2016;84:2724–2739. doi: 10.1128/IAI.00469-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kerr J.R. Suppression of fungal growth exhibited by Pseudomonas aeruginosa. J. Clin. Microbiol. 1994;32:525–527. doi: 10.1128/jcm.32.2.525-527.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Azoulay E., Timsit J.-F., Tafflet M., de Lassence A., Darmon M., Zahar J.-R., Adrie C., Garrouste-Orgeas M., Cohen Y., Mourvillier B., et al. Candida colonization of the respiratory tract and subsequent Pseudomonas ventilator-associated pneumonia. Chest. 2006;129:110–117. doi: 10.1378/chest.129.1.110. [DOI] [PubMed] [Google Scholar]
- 82.Roux D., Gaudry S., Dreyfuss D., El-Benna J., De Prost N., Denamur E., Saumon G., Ricard J.D. Candida albicans impairs macrophage function and facilitates Pseudomonas aeruginosa pneumonia in rat. Crit. Care Med. 2009;37:1062–1067. doi: 10.1097/CCM.0b013e31819629d2. [DOI] [PubMed] [Google Scholar]
- 83.Hamet M., Pavon A., Dalle F., Pechinot A., Prin S., Quenot J.P., Charles P.E. Candida spp. airway colonization could promote antibiotic-resistant bacteria selection in patients with suspected ventilator-associated pneumonia. Intensive Care Med. 2012;38:1272–1279. doi: 10.1007/s00134-012-2584-2. [DOI] [PubMed] [Google Scholar]
- 84.Xu L., Wang F., Shen Y., Hou H., Liu W., Liu C., Jian C., Wang Y., Sun M., Sun Z. Pseudomonas aeruginosa inhibits the growth of pathogenic fungi: In vitro and in vivo studies. Exp. Ther. Med. 2014;7:1516–1520. doi: 10.3892/etm.2014.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nseir S., Jozefowicz E., Cavestri B., Sendid B., Di Pompeo C., Dewavrin F., Favory R., Roussel-Delvallez M., Durocher A. Impact of antifungal treatment on Candida-Pseudomonas interaction: A preliminary retrospective case-control study. Intensive Care Med. 2007;33:137–142. doi: 10.1007/s00134-006-0422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roux D., Gaudry S., Khoy-Ear L., Aloulou M., Phillips-Houlbracq M., Bex J., Skurnik D., Denamur E., Monteiro R.C., Dreyfuss D., et al. Airway fungal colonization compromises the immune system allowing bacterial pneumonia to prevail. Crit. Care Med. 2013;41:e191–e199. doi: 10.1097/CCM.0b013e31828a25d6. [DOI] [PubMed] [Google Scholar]
- 87.Sun K., Metzger D.W. Inhibition of pulmonary antibacterial defense by interferon-γ during recovery from influenza infection. Nat. Med. 2008;14:558–564. doi: 10.1038/nm1765. [DOI] [PubMed] [Google Scholar]
- 88.Greenberg S.S., Zhao X., Hua L., Wang J.F., Nelson S., Ouyang J. Ethanol inhibits lung clearance of Pseudomonas aeruginosa by a neutrophil and nitric oxide-dependent mechanism, in vivo. Alcohol. Clin. Exp. Res. 1999;23:735–744. doi: 10.1111/j.1530-0277.1999.tb04177.x. [DOI] [PubMed] [Google Scholar]
- 89.Ader F., Jawhara S., Nseir S., Kipnis E., Faure K., Vuotto F., Chemani C., Sendid B., Poulain D., Guery B. Short term Candida albicans colonization reduces Pseudomonas aeruginosa-related lung injury and bacterial burden in a murine model. Crit. Care. 2011;15:R150. doi: 10.1186/cc10276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mear J.B., Gosset P., Kipnis E., Faure E., Dessein R., Jawhara S., Fradin C., Faure K., Poulain D., Sendid B., et al. Candida albicans airway exposure primes the lung innate immune response against Pseudomonas aeruginosa infection through innate lymphoid cell recruitment and interleukin-22-associated mucosal response. Infect. Immun. 2014;82:306–315. doi: 10.1128/IAI.01085-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bergeron A.C., Seman B.G., Hammond J.H., Archambault L.S., Hogan D.A., Wheeler R.T. Candida albicans and Pseudomonas aeruginosa interact to enhance virulence of mucosal infection in transparent zebrafish. Infect. Immun. 2017;85 doi: 10.1128/IAI.00475-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Conrad D.J., Bailey B.A. Multidimensional clinical phenotyping of an adult cystic fibrosis patient population. PLoS ONE. 2015;10:e0122705. doi: 10.1371/journal.pone.0122705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grahl N., Dolben E.L., Filkins L.M., Crocker A.W., Willger S.D., Morrison H.G., Sogin M.L., Ashare A., Gifford A.H., Jacobs N.J., et al. Profiling of bacterial and fungal microbial communities in cystic fibrosis sputum using RNA. mSphere. 2018;3:e00292-18. doi: 10.1128/mSphere.00292-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Güngör Ö., Tamay Z., Güler N., Erturan Z. Frequency of fungi in respiratory samples from Turkish cystic fibrosis patients. Mycoses. 2013;56:123–129. doi: 10.1111/j.1439-0507.2012.02221.x. [DOI] [PubMed] [Google Scholar]
- 95.Valenza G., Tappe D., Turnwald D., Frosch M., König C., Hebestreit H., Abele-Horn M. Prevalence and antimicrobial susceptibility of microorganisms isolated from sputa of patients with cystic fibrosis. J. Cyst. Fibros. 2008;7:123–127. doi: 10.1016/j.jcf.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 96.Williamson D.R., Albert M., Perreault M.M., Delisle M.-S., Muscedere J., Rotstein C., Jiang X., Heyland D.K. The relationship between Candida species cultured from the respiratory tract and systemic inflammation in critically ill patients with ventilator-associated pneumonia. Can. J. Anesth. Can. d’anesthésie. 2011;58:275–284. doi: 10.1007/s12630-010-9439-5. [DOI] [PubMed] [Google Scholar]
- 97.Leclair L.W., Hogan D.A. Mixed bacterial-fungal infections in the CF respiratory tract. Med. Mycol. 2010;48:S125–S132. doi: 10.3109/13693786.2010.521522. [DOI] [PubMed] [Google Scholar]
- 98.Haiko J., Saeedi B., Bagger G., Karpati F., Özenci V. Coexistence of Candida species and bacteria in patients with cystic fibrosis. Eur. J. Clin. Microbiol. Infect. Dis. 2019 doi: 10.1007/s10096-019-03493-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pendleton K.M., Erb-Downward J.R., Bao Y., Branton W.R., Falkowski N.R., Newton D.W., Huffnagle G.B., Dickson R.P. Rapid pathogen identification in bacterial pneumonia using real-time metagenomics. Am. J. Respir. Crit. Care Med. 2017;196:1610–1612. doi: 10.1164/rccm.201703-0537LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chotirmall S.H., O’Donoghue E., Bennett K., Gunaratnam C., O’Neill S.J., McElvaney N.G. Sputum Candida albicans presages FEV 1 decline and hospital-treated exacerbations in cystic fibrosis. Chest. 2010;138:1186–1195. doi: 10.1378/chest.09-2996. [DOI] [PubMed] [Google Scholar]
- 101.Kim S.H., Clark S.T., Surendra A., Copeland J.K., Wang P.W., Ammar R., Collins C., Tullis D.E., Nislow C., Hwang D.M., et al. Global analysis of the fungal microbiome in cystic fibrosis patients reveals loss of function of the transcriptional repressor Nrg1 as a mechanism of pathogen adaptation. PLOS Pathog. 2015;11:e1005308. doi: 10.1371/journal.ppat.1005308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Murad A.M.A., Leng P., Straffon M., Wishart J., Macaskill S., MacCallum D., Schnell N., Talibi D., Marechal D., Tekaia F., et al. NRG1 represses yeast-hypha morphogenesis and hypha-specific gene expression in Candida albicans. EMBO J. 2001;20:4742–4752. doi: 10.1093/emboj/20.17.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Delisle M.-S., Williamson D.R., Albert M., Perreault M.M., Jiang X., Day A.G., Heyland D.K. Impact of Candida species on clinical outcomes in patients with suspected ventilator-associated pneumonia. Can. Respir. J. 2011;18:131–136. doi: 10.1155/2011/827692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pendleton K.M., Huffnagle G.B., Dickson R.P. The significance of Candida in the human respiratory tract: Our evolving understanding. Pathogens and Disease. 2017;75:fxt029. doi: 10.1093/femspd/ftx029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Venkatesh M.P., Pham D., Fein M., Kong L., Weisman L.E. Neonatal coinfection model of coagulase-negative Staphylococcus (Staphylococcus epidermidis) and Candida albicans: Fluconazole prophylaxis enhances survival and growth. Antimicrob. Agents. Chemother. 2007;51:1240–1245. doi: 10.1128/AAC.01298-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lindau S., Nadermann M., Ackermann H., Bingold T.M., Stephan C., Kempf V.A.J., Herzberger P., Beiras-Fernandez A., Zacharowski K., Meybohm P. Antifungal therapy in patients with pulmonary Candida spp. colonization may have no beneficial effects. J. Intensive Care. 2015;3:31. doi: 10.1186/s40560-015-0097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Terraneo S., Ferrer M., Martin-Loeches I., Esperatti M., Di Pasquale M., Giunta V., Rinaudo M., de Rosa F., Li Bassi G., Centanni S., Torres A. Impact of Candida spp. isolation in the respiratory tract in patients with intensive care unit-acquired pneumonia. Clin. Microbiol. Infect. 2016;22:94.e1–94.e8. doi: 10.1016/j.cmi.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 108.James G.A., Swogger E., Wolcott R., Pulcini E.D., Secor P., Sestrich J., Costerton J.W., Stewart P.S. Biofilms in chronic wounds. Wound Repair Regen. 2008;16:37–44. doi: 10.1111/j.1524-475X.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 109.Townsend E.M., Sherry L., Kean R., Hansom D., Mackay W.G., Williams C., Butcher J., Ramage G. Implications of antimicrobial combinations in complex wound biofilms containing fungi. Antimicrob. Agents Chemother. 2017;61:e00672-17. doi: 10.1128/AAC.00672-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chellan G., Shivaprakash S., Karimassery Ramaiyar S., Varma A.K., Varma N., Thekkeparambil Sukumaran M., Rohinivilasam Vasukutty J., Bal A., Kumar H. Spectrum and prevalence of fungi infecting deep tissues of lower-limb wounds in patients with type 2 diabetes. J. Clin. Microbiol. 2010;48:2097–2102. doi: 10.1128/JCM.02035-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Church D., Elsayed S., Reid O., Winston B., Lindsay R. Burn wound infections. Clin. Microbiol. Rev. 2006;19:403–434. doi: 10.1128/CMR.19.2.403-434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Neely A.N., Law E.J., Holder I.A. Increased susceptibility to lethal Candida infections in burned mice preinfected with Pseudomonas aeruginosa or pretreated with proteolytic enzymes. Infect. Immun. 1986;52:200–204. doi: 10.1128/iai.52.1.200-204.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.de Macedo J.L.S., Santos J.B. Bacterial and fungal colonization of burn wounds. Mem. Inst. Oswaldo Cruz. 2005;100:535–539. doi: 10.1590/S0074-02762005000500014. [DOI] [PubMed] [Google Scholar]
- 114.Gupta N., Haque A., Mukhopadhyay G., Narayan R.P., Prasad R. Interactions between bacteria and Candida in the burn wound. Burns. 2005;31:375–378. doi: 10.1016/j.burns.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 115.Neville B.A., d’Enfert C., Bougnoux M.-E. Candida albicans commensalism in the gastrointestinal tract. FEMS Yeast Res. 2015;15:fov081. doi: 10.1093/femsyr/fov081. [DOI] [PubMed] [Google Scholar]
- 116.Lopez-Medina E., Koh A.Y. The complexities of bacterial-fungal interactions in the mammalian gastrointestinal tract. Microb. Cell. 2016;3:191–195. doi: 10.15698/mic2016.05.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tancrède C.H., Andremont A.O. Bacterial translocation and gram-negative bacteremia in patients with hematological malignancies. J. Infect. Dis. 1985;152:99–103. doi: 10.1093/infdis/152.1.99. [DOI] [PubMed] [Google Scholar]
- 118.Lopez-Medina E., Fan D., Coughlin L.A., Ho E.X., Lamont I.L., Reimmann C., Hooper L.V., Koh A.Y. Candida albicans inhibits Pseudomonas aeruginosa virulence through suppression of pyochelin and pyoverdine biosynthesis. PLoS Pathog. 2015;11:e1005129. doi: 10.1371/journal.ppat.1005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lamont I.L., Beare P.A., Ochsner U., Vasil A.I., Vasil M.L. Siderophore-mediated signaling regulates virulence factor production in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA. 2002;99:7072–7077. doi: 10.1073/pnas.092016999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Noble S.M., Gianetti B.A., Witchley J.N. Candida albicans cell-type switching and functional plasticity in the mammalian host. Nat. Rev. Microbiol. 2017;15:96–108. doi: 10.1038/nrmicro.2016.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lan C.-Y., Newport G., Murillo L.A., Jones T., Scherer S., Davis R.W., Agabian N. Metabolic specialization associated with phenotypic switching in Candida albicans. Proc. Natl. Acad. Sci. USA. 2002;99:14907–14912. doi: 10.1073/pnas.232566499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pande K., Chen C., Noble S.M. Passage through the mammalian gut triggers a phenotypic switch that promotes Candida albicans commensalism. Nat. Genet. 2013;45:1088–1091. doi: 10.1038/ng.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tong Y., Cao C., Xie J., Ni J., Guan G., Tao L., Zhang L., Huang G. N-acetylglucosamine-induced white-to-opaque switching in Candida albicans is independent of the Wor2 transcription factor. Fungal Genet. Biol. 2014;62:71–77. doi: 10.1016/j.fgb.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 124.Fox E.P., Cowley E.S., Nobile C.J., Hartooni N., Newman D.K., Johnson A.D. Anaerobic bacteria grow within Candida albicans biofilms and induce biofilm formation in suspension cultures. Curr. Biol. 2014;24:2411–2416. doi: 10.1016/j.cub.2014.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ramírez-Zavala B., Reuß O., Park Y.-N., Ohlsen K., Morschhäuser J. Environmental induction of white–opaque switching in Candida albicans. PLoS Pathog. 2008;4:e1000089. doi: 10.1371/journal.ppat.1000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Malavia D., Lehtovirta-Morley L.E., Alamir O., Weiß E., Gow N.A.R., Hube B., Wilson D. Zinc limitation induces a hyper-adherent goliath phenotype in Candida albicans. Front. Microbiol. 2017;8:2238. doi: 10.3389/fmicb.2017.02238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Montgomery S.T., Mall M.A., Kicic A., Stick S.M. AREST CF Hypoxia and sterile inflammation in cystic fibrosis airways: Mechanisms and potential therapies. Eur. Respir. J. 2017;49:1600903. doi: 10.1183/13993003.00903-2016. [DOI] [PubMed] [Google Scholar]
- 128.Faure E., Kwong K., Nguyen D. Pseudomonas aeruginosa in chronic lung infections: How to adapt within the host? Front. Immunol. 2018;9:2416. doi: 10.3389/fimmu.2018.02416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Oliver A., Cantón R., Campo P., Baquero F., Blázquez J. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science. 2000;288:1251–1254. doi: 10.1126/science.288.5469.1251. [DOI] [PubMed] [Google Scholar]
- 130.Smith E.E., Buckley D.G., Wu Z., Saenphimmachak C., Hoffman L.R., D’Argenio D.A., Miller S.I., Ramsey B.W., Speert D.P., Moskowitz S.M., et al. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc. Natl. Acad. Sci. 2006;103:8487–8492. doi: 10.1073/pnas.0602138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Workentine M.L., Sibley C.D., Glezerson B., Purighalla S., Norgaard-Gron J.C., Parkins M.D., Rabin H.R., Surette M.G. Phenotypic heterogeneity of Pseudomonas aeruginosa populations in a cystic fibrosis patient. PLoS ONE. 2013;8:e60225. doi: 10.1371/journal.pone.0060225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Clark S.T., Diaz Caballero J., Cheang M., Coburn B., Wang P.W., Donaldson S.L., Zhang Y., Liu M., Keshavjee S., Yau Y.C.W., et al. Phenotypic diversity within a Pseudomonas aeruginosa population infecting an adult with cystic fibrosis. Sci. Rep. 2015;5:10932. doi: 10.1038/srep10932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.DeVault J.D., Kimbara K., Chakrabarty A.M. Pulmonary dehydration and infection in cystic fibrosis: Evidence that ethanol activates alginate gene expression and induction of mucoidy in Pseudomonas aeruginosa. Mol. Microbiol. 1990;4:737–745. doi: 10.1111/j.1365-2958.1990.tb00644.x. [DOI] [PubMed] [Google Scholar]
- 134.Casilag F., Lorenz A., Krueger J., Klawonn F., Weiss S., Häussler S. The LasB elastase of Pseudomonas aeruginosa acts in concert with alkaline protease AprA to prevent flagellin-mediated immune recognition. Infect. Immun. 2015 doi: 10.1128/IAI.00939-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dettman J.R., Rodrigue N., Aaron S.D., Kassen R. Evolutionary genomics of epidemic and nonepidemic strains of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. 2013;110:21065–21070. doi: 10.1073/pnas.1307862110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jain M., Ramirez D., Seshadri R., Cullina J.F., Powers C.A., Schulert G.S., Bar-Meir M., Sullivan C.L., McColley S.A., Hauser A.R. Type III secretion phenotypes of Pseudomonas aeruginosa strains change during infection of individuals with cystic fibrosis. J. Clin. Microbiol. 2004;42:5229–5237. doi: 10.1128/JCM.42.11.5229-5237.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tart A.H., Blanks M.J., Wozniak D.J. The AlgT-dependent transcriptional regulator AmrZ (AlgZ) inhibits flagellum biosynthesis in mucoid, nonmotile Pseudomonas aeruginosa cystic fibrosis isolates. J. Bacteriol. 2006;188:6483–6489. doi: 10.1128/JB.00636-06. [DOI] [PMC free article] [PubMed] [Google Scholar]