Abstract
The composition of glycan in immunoglobulin G (IgG) has shown to affect various diseases and can be regulated by drugs and preventive vaccination. A hepatitis B surface antigen (HBsAg)-hepatitis B immunoglobulin (HBIG) immune complex (YIC) therapeutic vaccine for chronic hepatitis B (CHB) patients has undergone clinical trials. To explore for markers of CHB, which could be associated with responsiveness to YIC therapeutic vaccine, serum IgG glycosylation in CHB patients was analyzed.
Kinetic changes of serum galactosylated IgG in 53 hepatitis Be antigen (HBeAg)-positive CHB patients treated with YIC were monitored by matrix-assisted laser desorption/ionization (MALDI) mass spectrometry (MS) analysis. Whole blood cytokines were assayed by cytokine binding assay kits. All samples were back assayed before treatment, during therapy and follow-up for 6 months from a previous completed clinical trial.
During YIC treatment, 26 patients with lower IgG galactosylation level at baseline [galactosylation level (Gal-ratio) = −0.29, 0.18 (mean, SD)] showed sustained increase of serum galactosylated IgG, and responded to YIC treatment by HBeAg seroconversion. While those who did not respond to YIC treatment [Gal-ratio = −0.40, 0.15 (mean, SD)] failed to show similar changes. Furthermore, this kinetic increase of galactosylated IgG correlated with marked up-regulated IL-2 level, confirming that effective cellular immune responses have participated in responsiveness.
For HBeAg-positive CHB patients lower serum IgG galactosylation level may serve as an indicator for selecting a suitable subpopulation of candidates for YIC therapeutic vaccination.
Keywords: chronic hepatitis B, galactosylation, hepatitis Be antigen seroconversion, immunoglobulin G, therapeutic vaccine
1. Introduction
The fragment crystallizable (Fc) domain of immunoglobulin G (IgG) plays an important immunoregulatory role via binding to its cellular receptors.[1] The Fc domain displays remarkable heterogeneity, determined both by the amino acid sequence of various IgG subclasses and by the complex Fc-associated N-linked glycan. These determinants regulate the conformational flexibility of the Fc domain and affect its capacity to interact with Fcγ receptors. The glycosylation patterns of IgG have been studied in immune disorders, inflammation, infections, and metabolic diseases.[2] Furthermore, recent data showed that antibody glycosylation not only is related to disease progression, but also can be manipulated to regulate host immune responses.[3] Many reports have showed the serum IgG galactosylation characteristics in autoimmune diseases, infectious diseases, and cancers.[4,5] Fc galactosylation of IgG1 and IgG3 can enhance the complement-dependent cytotoxicity by improving binding of C1q.[6] Antibody-dependent cell-mediated cytotoxicity (ADCC) was increased in some monoclonal antibodies by Fc galactose.[7] IgG from the malaria-endemic region adults could inhibit parasite growth by activating ADCC with natural killer cells.[8] IgG deficient of galactose has a close correlation with inflammation.[9] HIV-specific antibodies agalactosylation was associated with enhanced Fc-mediated reduction of viral replication, resulting robust antiviral activity.[10]These findings provide promising clues for improvement of the current therapeutics and for the development of new therapeutics and novel vaccines.
Viral hepatitis B is an infectious disease caused by hepatitis B virus (HBV). Belonging to the hepadnaviridae, with a small circular DNA genome, HBV replicates via an RNA intermediate and secretes huge amount of small envelope protein particles [hepatitis B surface antigen (HBsAg)].[11] Notwithstanding the tremendous success of preventive vaccine in control of viral hepatitis B, there are still around 300 million individuals infected with HBV, and the number of chronic hepatitis B (CHB) patients may attain 20 million worldwide. Aimed at restoring the immune responses in CHB patients, we have developed a HBsAg-hepatitis B immunoglobulin (HBIG) immune complex (YIC) therapeutic vaccine for CHB patients, and these clinical trial studies have been published elsewhere.[12–14] To explore individualized effective treatment by YIC, the kinetic changes of IgG galactosylation in responsive and non-responsive hepatitis Be antigen (HBeAg)-positive patients under YIC treatment were analyzed. Serum samples from the phase III clinical trial[12] were back assayed for glycosylation of their IgG in relation to responsiveness to therapeutic vaccine treatment.
2. Materials and methods
Formal approvals from the ethics committees in 21 evaluation centers were completed, and enrollment of patients started at the end of October 2007. A signed written inform consent for participation in this trial was obtained from each patient prior to enrollment.
2.1. Patients
As reported previously, a double-blind, randomized, and placebo-controlled trial was conducted to determine the efficacy of therapeutic vaccine (YIC) for chronic hepatitis B patients who were aged 18 to 65 years, HBsAg and HBeAg positive for at least 6 months. Study design was published elsewhere.[12] Briefly, all participants allocated in the YIC group were administrated with 12 intramuscular injections at 4-week interval, with an 8-week break between the 6th and 7th injections, the patients were followed-up for 24 weeks after the termination of immunization. Serum samples were collected at week 0, 12, 24, 40, 52, and 76 after initial injection.[12]
In this study, 53 patients were enrolled from the YIC treatment group. At the end of follow-up, 26 patients reached the primary endpoint (HBeAg seroconversion). Another 27 patients (non-responders) from the same group whose HBeAg did not convert were selected randomly as controls by matching the age, gender, and genotype. Serum samples were back assayed at 3 time points, namely, week 0 (baseline), week 52 (completion of treatment with YIC for 12 injections), and week 72 (followed for 6 months), with informed consent of utilizing the serum samples for further analysis. Responder (HBeAg seroconversion) was defined as loss of HBeAg, and appearance of anti-HBe at the end of follow-up.
2.2. Isolation of IgG
IgG from blood serum sample was isolated by Protein A Spin Plate for IgG Screening (Thermo Fisher Scientific, Rockford). The isolation was manipulated according to the manufacturer instructions.
2.3. Release and purification of IgG N-glycan
As reported previously,[15] IgG N-glycan was released by mixing denatured portion of IgG-containing fractions of Protein A column elution and PNGase F (New England Biolabs, Inc., USA) with incubation for 12 hours at 37°C. The released oligosaccharides were subsequently purified using a porous graphitic carbon-containing 96-well plate for mass spectrometry (MS) analysis.
2.4. Matrix-assisted laser desorption/ionization (MALDI) MS analysis
The collected N-glycan samples (0.5 μl) were deposited onto a standard MALDI plate. Then, 0.5 μl of matrix solution, 10 mg/ml 2,5-dihydroxybenzoicacid (Sigma-Aldrich, Germany) in 0.1% (v/v) trifluoroacetic acid in 50% acetonitrile/H2O (v/v), was added onto the sample layer, followed by re-crystallization to form homogeneity of the spot surface with ethanol. Each sample was spotted in triplicate. The samples were interrogated automatically in a “batch mode” by AXIMA Resonance MALDI-quadrupole ion trap (QIT)-time of flight (TOF) MS (Shimadzu Corp. JP) equipped with a 337 nm nitrogen laser in reflector positive ionization mode. The m/z range was monitored to span from 500 to 5000. Tandem mass spectrometry was utilized to validate the component of the detected glycans. The GlycoWorkbench software was used for the annotation of MS spectra. The MALDI MS spectra data were pre-processed, normalized and extracted using the software of Progenesis MALDI before further analysis.
2.5. Whole blood cytokines assay
Whole blood samples from 53 patients at baseline, 52 and 76 weeks were assayed for IL-2, IL-4, IL-6, TNF-α, interferon-γ, and IL-17A by FACS Calibur (BD Biosciences, Sunnyvale, CA, USA), using cytokine binding assay kits (BD PharMingen, BD Biosciences, USA), which has been published recently.[16]
2.6. Data management and statistical analysis
SAS statistical software (SAS Institute Inc., Cary, North Carolina, USA) and GraphPad Prism 5 were used for analysis in this study. HBV DNA, HBsAg, HBeAg, IgG galactosylation and alanine aminotransferase (ALT) levels of responders and non-responders were compared at baseline, week 52 and week 76. For binary data, the Chi-Square test, or Fisher exact test were employed as appropriate. For dichotomous outcomes, such as HBV DNA, HBsAg, HBeAg, and IgG galactosylation level, ANOVA was used after logarithmically transform. Wilcoxon rank-sun test was applied for the comparison of ALT level.
Repeated measures analysis was performed using a generalized estimating equations (GEEs) method to adjust the dependence among repeated observations made on the same patient while testing the group (responder and non-responder) and time effects as well as the potential interaction between genotype, gender, group and time.[17] The unstructured working covariance matrix which provided robust estimation of covariance to the structure was applied. Adjustments were made for group, gender, genotype, age, level of HBV DNA, HBsAg, and HBeAg.
To explore the potential prediction value of IgG galactosylation level at baseline with regard to the outcome after YIC treatment, which was HBeAg seroconversion, the receiver operator characteristic (ROC) curve as well as Youden index method was applied to define the optimal cutoff of Gal-ratio at baseline. Hereafter, the sensitivity, specificity, positive predictive value, negative predictive value, likelihood ratio, as well as 95% confidence interval for sensitivity and specificity[18] were calculated. A P-value < .05 (2-tailed) was considered as statistically of significance.
3. Results
3.1. Profiling serum IgG N-glycan by MALDI MS analysis
Using a simple and accurate method for the relative quantification of serum IgG galactosylation in our previous study,[19] serum IgG N-glycan profile from each CHB patient was identified. A representative MALDI-QIT-TOF MS spectrum model displaying the N-glycoforms of serum IgG from CHB patients is shown in Figure 1. The most abundant components are fucosylated biantennary glycans, including G0 (carrying no galactose), G1 (carrying 1 galactose), and G2 (carrying 2 galactose) as shown in the MS spectrum. The IgG galactosylation level (referred to as Gal-ratio) was measured by calculating the relative intensities of agalactosylated (G0) vs monogalactosyl (G1) and digalactosyl (G2) N-glycans according to the formula of G0/(G1 + G2 × 2) as described previously.[19]
Figure 1.
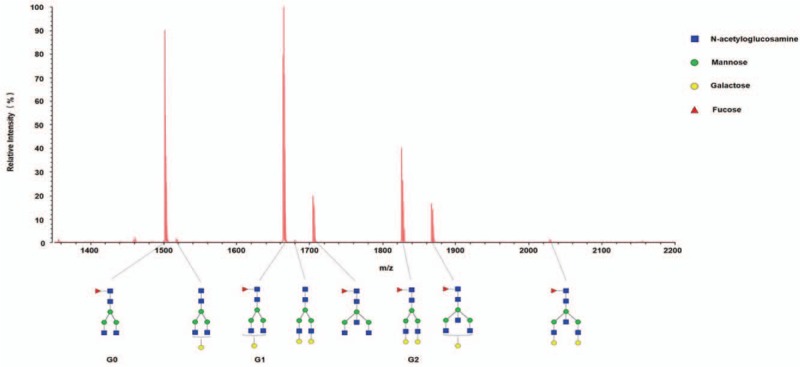
Representative MALDI-QIT-TOF MS spectrum of serum IgG N-glycan profiles from CHB patients. In the mass spectrum, the x-axis (m/z) and the y-axis (relative intensity) represent mass-to-charge ratio and relative signal intensity of the IgG N-glycans, respectively. The annotation of peaks on mass spectrum was based on the relative molecular weight of IgG N-glycans. IgG N-glycans are comprised of N-acetylglucosamines, mannose, galactose, and fucose residues. Structure abbreviations: G0, G1, and G2 indicate fucosylated biantennary glycans with no galactose, 1 galactose, and 2 galactose, respectively.
3.2. Virological and clinical characteristics between responders and non-responders
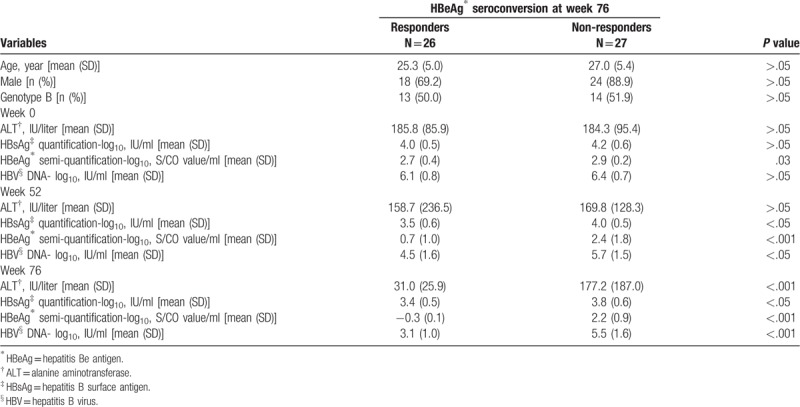
These characteristics were extracted from our previous publication.[12] Of the 53 patients, 27 were infected with HBV genotype B, while other 26 were infected with genotype C. There was no significant difference in age, gender, and distribution of genotype B and C between responders and non-responders (P > .05) (Table 1). At week 0, levels of serum HBsAg, HBV DNA, and ALT were comparable between responders and non-responders. At the end of YIC treatment, decrease in HBV DNA and conversion from HBeAg positive to negative were observed in responders, compared to non-responders. At the end of follow-up, all responders approached HBeAg seroconversion to anti-HBe positive, ALT normalization and an average of 0.6 log10 decrease of HBsAg were only observed in the responders (Table 1).
Table 1.
Clinical and virological characteristics between responders and non-responders.
3.3. Increase of IgG galactosylation in responders during YIC treatment
During YIC treatment, a sustained and dramatic increase of IgG galactosylation was found in responders versus non-responders (Fig. 2). The increase continued after cessation of treatment even in the follow-up 6 months (P = .02). Interestingly, significant statistical higher IgG Gal-ratio was found in responders [−0.29, 0.18 (mean, SD)] than that in non-responders [−0.40, 0.15 (mean, SD)] even at baseline (P = .02). IgG Gal-ratio difference was not statistically significant between responders and non-responders at 72 weeks. After adjusting the age, gender, HBV genotype, as well as HBV DNA, HBsAg, and HBeAg levels at baseline, the pattern of differences in IgG galactosylation between responders and non-responders was statistically significant (P < .001) (Table 2). In addition, increase of IgG galactosylation was more marked in responsive HBV genotype B patients compared to those in genotype C patients (Table 3).
Figure 2.
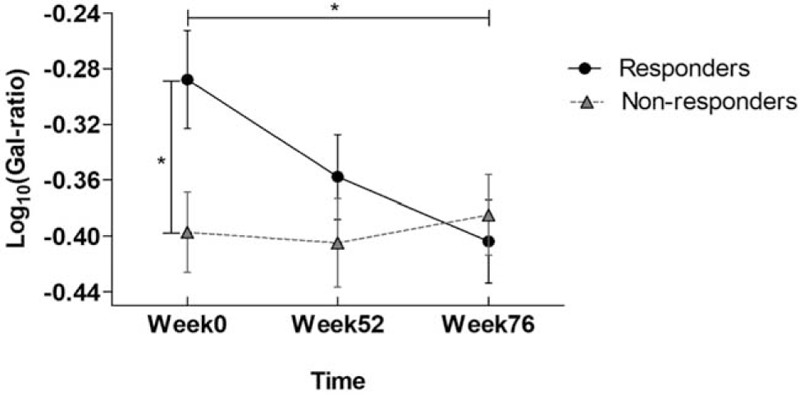
The kinetic trend of IgG galactosylation level in responders and non-responders. Comparison of Gal-ratio (log10 transformed) values at week 0, week 52, and week 76 in the responders and non-responders. P value for comparison between week 0 and week 76 in responders was.02 from paired t test. P value for comparison between responders and non-responders at week 0 was.02 from 2-tailed independent t test.
Table 2.
The coefficient of generalized estimating equation in estimating the trend of IgG∗ galactosylation.
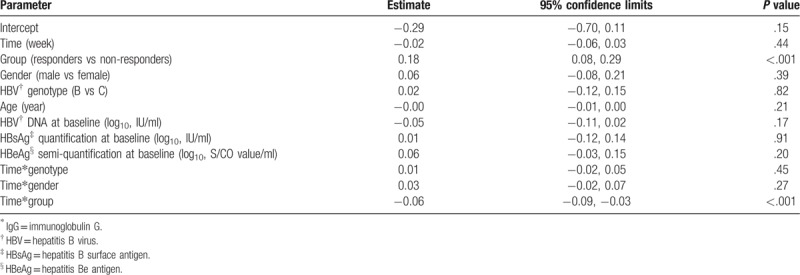
Table 3.
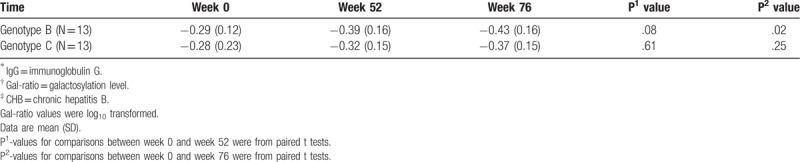
Distribution of IgG∗ Gal-ratio† in two genotype responders of CHB‡ patients.
3.4. Increase of IgG galactosylation with upregulation of IL-2 in responders
To explore the association between levels of IgG galactosylation and cytokine levels in patients, correlation analysis was carried out using IgG galactosylation levels and cytokine levels at baseline and at the end of follow-up (76 weeks). Only at the end of follow-up (week 76), among the 6 cytokines monitored, IL-2 significantly negatively correlated with levels of IgG Gal-ratio, while IL-17A also showed substantial negative correlation (IL-2: r = −0.54, P < .002; IL-17A: r = −0.41, P = .04; respectively).
3.5. Association between IgG galactosylation at baseline and HBeAg seroconversion
The optimal cutoff Gal-ratio value to predict HBeAg seroconversion at week 76 in HBeAg-positive CHB patients was defined by receiver operator characteristic (ROC) curve (Fig. 3). The area under the curve (AUC) of ROC reached 0.691 (P = .02). A cutoff Gal-ratio value of 0.60 was calculated by Youden index method. By this cutoff, a satisfied performance of prediction for HBeAg seroconversion after YIC treatment was concluded, with a sensitivity of 42% (95% CI: 23–61%) and a specificity of 93% (95% CI: 83–100%). In particular, the positive predictive value was quite promising, which reached 85%. The accuracy was 0.68, and negative predictive value, positive likelihood ratio, and negative likelihood ratio were 63%, 5.7 and 0.62, respectively.
Figure 3.
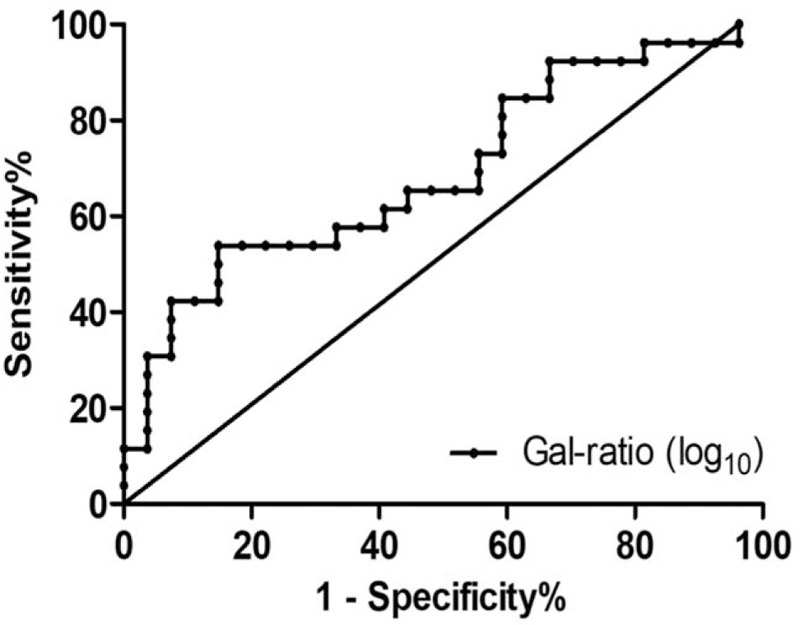
Performance of baseline IgG galactosylation level to differentiate responders from non-responders. ROC curve for baseline IgG Gal-ratio (log10 transformed) values to differentiate responders from non-responders. AUC of ROC = 0.691. SE = 0.073, P = .02. Sensitivity = 42% (95% CI: 23–61%). Specificity = 93% (95% CI: 83–100%). Cutoff value of Gal-ratio (log10) = −0.219.
4. Discussion
N-linked glycosylation of IgG is an important posttranslational modification of host proteins. Because of the high abundance of IgG in serum, and the Fc-Fc receptor binding roles in initiating immune responses, IgG glycosylation has been studied in a number of diseases.[20] In rheumatoid arthritis patients, nongalactosylated IgG is associated with disease status and may act as an indicator for prognosis, and decrease of galactose in IgG is a common feature in various cancers.[15] Increase of serum IgG level commonly occurs in patients with chronic liver disease and is highly related to disease progression.[3,21] In addition, CHB patients presented higher galactose deficiency than healthy controls.[3,21] Recently, studies on glycosylation have been reported in other areas, including nephropathy, breast adenocarcinoma,[22] colonc caner,[23] septecimia,[24] HIV,[10] metabolic diseases,[9] and vaccinations.[20]
As YIC therapeutic vaccination is an active immuno-therapy, and its therapeutic efficacy is based on modulating host immune responses via Fc-Fc recepoter interactions, the IgG glycosylation of each patient could affect his/her therapeutic responsiveness. By analysis of dynamic changes of IgG glycosylation in YIC treated patients in association with cytokines, the increase in serum IgG galactosylation was highly correlated with up-regulation of IL-2 expression in responsive HBeAg-positive CHB patients. This observation indicates that modulation in IgG glycosylation is in association with regulation of immune responses in CHB patients. IL-2 as the T cell growth factor has pleiotropic natures and multiple functions. The up-regulation of IL-2 supports that YIC therapy was functioned through accelerated T cell-mediated immune response. This observation is in accordance with our recent immune cellular study, showing that by administration of YIC, significant upregulated IL-2 expression was observed in CD4+ and CD8+ cells, with increase in interferon-γ expression and decrease of inhibitory factors (IL-10 and TGF-ß [16]). Another recent article reported that changes in glycosylation of IgG in CHB patients treated with antiviral nucleotides were related to changes in TGF-ß expression,[21] which also showed that modification in IgG glycosylation was associated with modified expression of cytokines.
To explore for individualized treatment with YIC, based on this back assay from a previous clinical trial, lower baseline levels of IgG galactosylation in CHB patients may serve as a potential predictor for selecting a subpopulation of CHB patients for YIC therapy. Besides, viral genotype B infected CHB patients may also be considered as another selective parameter for using YIC treatment.
Unlike drug therapy, efficacy of immunotherapy highly depends on individual functional immune status of patients. To practice precision medicine in immunotherapy, evaluation of individual immune status is critical. Two approaches may be employed. One is to do a complete survey of the immune elements in patients before clinical trial and identify the positive and negative factors after completion of trial. The other is to use results of completed clinical trials to reveal certain immune elements as potential predictors by back assay. Using the latter approach, we have detected a candidate predictor for favorable responses to YIC treatment.
Although the number of samples stated here was limited, the information provided by these findings is valuable for further investigations. By studying the underlying mechanisms between IgG galactosylation and cytokines will deepen our knowledge on interactions between humoral and cellular immune responses. The increased natural killer cell expression of FcγRIIIa and ADCC activity to antibodies is related to the FcγRIIIa-158 V/V and V/F polymorphism.[25] Studying the FcγRIIIa polymorphism might help to understand the association between IgG galactosylation and response to YIC therapy in CHB patients in future. Further studies on Fc receptors in CHB patients are necessary, to improve the strategy of using immune complexes or other therapies via Fc for treatment of diseases.
Acknowledgments
We would like to thank the dedicated people of the YIC Efficacy Trial Study Team (Drs. Dao-Zhen Xu, Guo-Zhong Gong, Hong Ren, Li-Min Guo, Ai-Min Sun, Min Xu, Lan-Juan Li, Xin-Hui Guo, Zhen Zhen, Hui-Fen Wang, Huan-Yu Gong, Cheng Xu, Nan Jiang, Chen Pan, Zuo-Jiong Gong, Ji-Ming Zhang, Jia Shang, Jie Xu, Qing Xie, Tie-Feng Wu, Wen-Xiang Huang, Yong-Guo Li) who made this study possible.
Author contributions
Shi-Fang Ren, Guo-Zhong Gong, Yu-Mei Wen, Xuan-Yi Wang and Jian-Xin Gu contributed to the study's conception and design. Guo-Zhong Gong, and Jian-Hua Lei collected the serum samples. Shi-Fang Ren, Jing Han, Jing Xu, Wen-Jun Qin and Rui-Huan Qin carried out the study. Jian-Hua Lei and Xuan-Yi Wang analyzed the data. Jing Han, Shi-Fang Ren, Yu-Mei Wen and Jian-Xin Gu wrote the paper.
Conceptualization: Guozhong Gong, Xuanyi Wang, Jianxin Gu, Shifang Ren, Yumei Wen.
Data curation: Xuanyi Wang, Shifang Ren, Yumei Wen.
Formal analysis: Jing Han, Guozhong Gong, Jianhua Lei, Wenjun Qin, Ruihuan Qin, Shifang Ren.
Methodology: Jing Han, Guozhong Gong, Jianhua Lei, Wenjun Qin, Ruihuan Qin, Xuanyi Wang, Shifang Ren.
Project administration: Xuanyi Wang, Jianxin Gu, Shifang Ren, Yumei Wen.
Supervision: Jianxin Gu, Shifang Ren, Yumei Wen.
Writing - original draft: Jing Han, Jianxin Gu, Shifang Ren, Yumei Wen.
Writing - review & editing: Jing Han, Jianxin Gu, Shifang Ren, Yumei Wen.
Footnotes
Abbreviations: ADCC = antibody-dependent cell-mediated cytotoxicity, ALT = alanine aminotransferase, AUC = area under the curve, CHB = chronic hepatitis B, Fc = fragment crystallizable, G0 = fucosylated biantennary glycan carrying no galactose, G1 = fucosylated biantennary glycan carrying one galactose, G2 = fucosylated biantennary glycan carrying two galactose, Gal-ratio = galactosylation level, GEEs = generalized estimating equations, HBeAg = hepatitis Be antigen, HBIG = hepatitis B immunoglobulin, HBsAg = hepatitis B surface antigen, HBV = hepatitis B virus, IgG = immunoglobulin G, MALDI = matrix-assisted laser desorption/ionization, MS = mass spectrometry, QIT = quadrupole ion trap, ROC = receiver operator characteristic, TOF = time of flight, YIC = hepatitis B surface antigen-hepatitis B immunoglobulin immune complex.
Jing Han and Guo-Zhong Gong contributed equally.
We gratefully acknowledge financial support from the National Key Research and Development Program of China (2018YFC0910300 & 2016YFA0501303) and National Natural Science Foundation of China (31770858), and National Science and Technology Project of Major Infectious Diseases (2008ZX10002-003 & 2012ZX10002002004, 2017ZX10202201002) and Zhixian Biotech.
The authors declare no conflict of interests.
References
- [1].Bournazos S, Ravetch JV. Fcgamma receptor pathways during active and passive immunization. Immunol Rev 2015;268:88–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Russell A, Adua E, Ugrina I, et al. Unravelling immunoglobulin G Fc N-glycosylation: a dynamic marker potentiating predictive, preventive and personalised medicine. Int J Mol Sci 2018;19: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ho CH, Chien RN, Cheng PN, et al. Aberrant serum immunoglobulin g glycosylation in chronic hepatitis b is associated with histological liver damage and reversible by antiviral therapy. J Infect Dis 2015;211:115–24. [DOI] [PubMed] [Google Scholar]
- [4].Parekh RB, Dwek RA, Sutton BJ, et al. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature 1985;316:452–7. [DOI] [PubMed] [Google Scholar]
- [5].Huhn C, Selman MH, Ruhaak LR, et al. IgG glycosylation analysis. Proteomics 2009;9:882–913. [DOI] [PubMed] [Google Scholar]
- [6].Peschke B, Keller CW, Weber P, et al. Fc-Galactosylation of human immunoglobulin gamma isotypes improves C1q binding and enhances complement-dependent cytotoxicity. Front Immunol 2017. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Thomann M, Reckermann K, Reusch D, et al. Fc-galactosylation modulates antibody-dependent cellular cytotoxicity of therapeutic antibodies. Mol Immunol 2016;73:69–75. [DOI] [PubMed] [Google Scholar]
- [8].Arora G, Hart GT, Manzella-Lapeira J, et al. NK cells inhibit Plasmodium falciparum growth in red blood cells via antibody-dependent cellular cytotoxicity. Elife 2018. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Plomp R, Ruhaak LR, Uh HW, et al. Subclass-specific IgG glycosylation is associated with markers of inflammation and metabolic health. Sci Rep 2017. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ackerman ME, Crispin M, Yu X, et al. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J Clin Invest 2013;123:2183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ma ZM, Lin X, Wang YX, et al. A double-spliced defective hepatitis b virus genome derived from hepatocellular carcinoma tissue enhanced replication of full-length virus. J Med Virol 2009;81:230–7. [DOI] [PubMed] [Google Scholar]
- [12].Xu DZ, Wang XY, Shen XL, et al. Results of a phase III clinical trial with an HBsAg-HBIG immunogenic complex therapeutic vaccine for chronic hepatitis B patients: experiences and findings. J Hepatol 2013;59:450–6. [DOI] [PubMed] [Google Scholar]
- [13].Xu DZ, Huang KL, Zhao K, et al. Vaccination with recombinant HBsAg-HBIG complex in healthy adults. Vaccine 2005;23:2658–64. [DOI] [PubMed] [Google Scholar]
- [14].Xu DZ, Zhao K, Guo LM, et al. A randomized controlled phase IIb trial of antigen-antibody immunogenic complex therapeutic vaccine in chronic hepatitis B patients. PLoS One 2008;3: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ren SF, Zhang ZJ, Xu CJ, et al. Distribution of IgG galactosylation as a promising biomarker for cancer screening in multiple cancer types. Cell Res 2016;26:963–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhou CL, Li CF, Gong GZ, et al. Analysis of immunological mechanisms exerted by HBsAg-HBIG therapeutic vaccine combined with Adefovir in chronic hepatitis B patients. Hum Vaccin Immunother 2017;13:1989–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zeger SL, Liang KY. Longitudinal data-analysis for discrete and continuous outcomes. Biometrics 1986;42:121–30. [PubMed] [Google Scholar]
- [18].Habbema JDFERKP. Knottnerus JA. Analysis of data on the accuracy of diagnostic tests. The evidence base of clinical diagnosis.. London: BMJ Publishing Group; 2002. 117–44. [Google Scholar]
- [19].Qian YF, Wang YS, Zhang XW, et al. Quantitative analysis of serum IgG galactosylation assists differential diagnosis of ovarian cancer. J Proteome Res 2013;12:4046–55. [DOI] [PubMed] [Google Scholar]
- [20].Vestrheim AC, Moen A, Egge-Jacobsen W, et al. A pilot study showing differences in glycosylation patterns of IgG subclasses induced by pneumococcal, meningococcal, and two types of influenza vaccines. Immun Inflamm Dis 2014;2:76–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ho CH, Chien RN, Cheng PN, et al. Association of serum IgG N-glycome and transforming growth factor-beta1 with hepatitis B virus e antigen seroconversion during entecavir therapy. Antiviral Res 2014;111:121–8. [DOI] [PubMed] [Google Scholar]
- [22].Stuchlova Horynova M, Raska M, Clausen H, et al. Aberrant O-glycosylation and anti-glycan antibodies in an autoimmune disease IgA nephropathy and breast adenocarcinoma. Cell Mol Life Sci 2013;70:829–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Theodoratou E, Thaci K, Agakov F, et al. Glycosylation of plasma IgG in colorectal cancer prognosis. Sci Rep 2016;6:28098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].De Haan N, Boeddha NP, Ekinci E, et al. Differences in IgG Fc glycosylation are associated with outcome of pediatric meningococcal sepsis. MBio 2018;9: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hatjiharissi E, Xu L, Santos DD, et al. Increased natural killer cell expression of CD16, augmented binding and ADCC activity to rituximab among individuals expressing the Fc{gamma}RIIIa-158 V/V and V/F polymorphism. Blood 2007;110:2561–4. [DOI] [PMC free article] [PubMed] [Google Scholar]


