Abstract
Biomarkers that predict tumor response before surgical treatment are necessary to help select patients for preoperative chemoradiotherapy for rectal cancer. However, no definite predictive biomarker has been established. This study explored programmed death-ligand 1 (PD-L1), epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF), p-signal transducer and activator of transcription 3 (p-STAT3), and death-domain associated protein as predictive biomarkers with regard to preoperative chemoradiotherapy in rectal cancer.
Formalin-fixed paraffin-embedded cancer tissues from pretreatment biopsies from 31 patients who underwent preoperative chemoradiotherapy were studied. The biomarkers were evaluated by immunohistochemistry.
PD-L1 positivity was found in 22.6% of 31 patients and complete response (CR) showed 33.3% and non-CR showed 18.2%. EGFR positivity was found in 71.0% of 31 patients and CR showed 88.9% and non-CR showed 73.6%. VEGF positivity was found in 83.9% of 31 patients and CR showed 88.9% and non-CR showed 81.8%. p-STAT3 positivity was found in 80.6% of 31 patients and CR showed 88.9% and non-CR showed 77.3%. On multiple logistic regression analysis, only VEGF expression was found to be a significant predictive factor for CR (P = .001). VEGF expression in pretreatment biopsies might be a predictive marker for CR after preoperative chemoradiation in rectal cancer.
Although there is a restriction of small sample size, our finding suggested that this study can be foundation for a larger further study for biomarkers which can predict neoadjuvant therapy response of specimens obtained for diagnosis before surgery.
Keywords: chemoradiotherapy, programmed death-ligand 1, rectal neoplasm, signal transducer and activator of transcription 3, vascular endothelial growth factor
1. Introduction
Colorectal cancer is the third most common cancer in the world, with nearly 1.4 million new cases diagnosed in 2012.[1] The Republic of Korea has the highest rate for increasing incidence of colorectal cancer, followed by Slovakia and Hungary.[1] People with localized rectal cancers without metastasis to distant sites are usually treated with surgery.[2–4] Additional treatment with radiation and chemotherapy may also be used before or after surgery. Approximately 15% to 30% of patients experience a pathologic complete response; however, the association between responses and biomarkers is unclear.
At present, a limited number of studies reported that several biomarkers can predict response of neoadjuvant chemoradiotherapy in rectal cancer. These studies have sometimes conflicting results.[5–9]
If we were able to predict the response to neoadjuvant chemoradiation therapy (CRT) at diagnosis, it would be helpful to predict the prognosis of each patient and to implement the adaptive therapy.
The standard of care for patients with locally advanced rectal cancer is preoperative CRT. The feasibility of predicting tumor responses may significantly implicate the choice of patients for preoperative CRT as well as potentially modifying postoperative treatment plans.[2–4] However, the accuracy of currently available imaging modalities such as computed tomography, magnetic resonance imaging, or positron emission tomography on restaging patients with preoperative CRT is less favored than was originally expected.[10–12] Therefore, the search for molecular predictors for response to CRT in rectal cancer is necessary, and the identification of such biomarkers has been of great interest in the field of oncology in recent years.[7,12–16]
Promising candidate molecules have been reported for preoperative CRT in rectal cancer with potential roles in the prediction of radiation therapy, including factors of angiogenesis, apoptosis, and tumor cell proliferation.[7,12–14,16–26] Although these markers have been identified as potential surrogates of response, none have been simultaneously validated using the same group of patients. In addition, no specific tumor biology-based predictive markers have made their way into clinical practice.
Epidermal growth factor receptor (EGFR) expression has been reported to be overexpressed in 25% to 82% of colorectal cancers and therefore has become a molecular chemotherapeutic target.[27] However, the chemoradiotherapeutic significance of EGFR overexpression in colorectal cancer remains uncertain.[27,28] Death-domain associated protein (DAXX) might play an important role in colon carcinogenesis and apoptosis.[29] Programmed death-ligand 1 (PD-L1) positivity was reported to be associated with colorectal carcinomas with the right or transverse colon with poorly differentiated and mismatch repair deficiency.[30] Also, p-signal transducer and activator of transcription 3 (p-STAT3)has been reported as constitutively activated in colon cancer-initiating cells and necessary for proliferation and survival for colon cancer cells.[31] Lastly, vascular endothelial growth factor (VEGF) has an important role for the tumor microenvironment for radiotherapy.[32] It was therefore hypothesized that these 5 molecules might predict the response of CRT in colon cancer in the light of their function in colon cancer.
The aim of this study was to evaluate the potential of EGFR, VEGF, PD-L1, p-STAT3, and DAXX as molecular markers in pretreatment biopsies to predict complete response in rectal cancer treated with preoperative CRT followed by surgery.
2. Materials and methods
2.1. Patients
Thirty-one patients with locally advanced mid-to-low rectal cancer who received preoperative CRT and surgery between January 2000 and December 2006 were retrospectively enrolled in this study. The tumors were located 1 to 15 cm from the anal verge (AV) and the median distance from AV was 5.00 ± 2.701 cm. Radiotherapy was delivered with a median total dose of 54 Gy (range, 22–54 Gy) in a median number of 27 fractions (range, 11–30 fractions) by the 4-field box technique. Chemotherapy of 5-fluorouracil (5-FU; 425 mg/m2/d) and leucovorin (20 mg/m2/d) was administered intravenously during the first and fifth weeks of radiotherapy. The operations included low anterior resection with colorectal or coloanal anastomosis and abdominoperineal resection. The study was approved by the local ethics committee of the Kosin University Gospel Hospital, Institutional Review Board (No. 2017-06-027-001).
2.2. Specimens
Tumor specimens from all 31 patients were obtained endoscopically (at least 5 × 5 × 5 mm) before the initiation of therapy. The samples were obtained from each patient and were fixed in 10% buffered formalin. The pathologic slides were prepared with hematoxylin–eosin staining and were reviewed by a gastrointestinal pathologist. Selected cases were available for both histological and immunohistochemical analyses with the condition of tumor cellularity higher than 50% of the biopsied tissue volume.
2.3. Methods
2.3.1. Pathologic assessment
All surgical specimens were reviewed by a gastrointestinal pathologist blinded to clinical information. A complete response was reached if viable tumors were not formed and there was a lack of lymph node involvement (pN0).
2.3.2. Immunohistochemistry
Five-micron-thick sections were cut from the paraffin blocks, dewaxed in xylene, and rehydrated through a graded series of ethanol. Antigen retrieval was done by microwave treatment for 20 min at 98°C using 0.01 M citric acid buffer, pH 6.0. Endogenous peroxidase activity was quenched by incubating the sections in 3% hydrogen peroxide for 10 min at room temperature. Nonspecific binding sites were blocked through preincubation with 5% normal goat serum in phosphate-buffered saline for 10 min at room temperature. DAXX (1:200, Sigma-Aldrich, St. Louis, MO), VEGF, p-STAT3, EGFR (1:150–200, Dako, Copenhagen, Denmark), and PD-L1 (1:50, Cell Markers, San Francisco, CA) immunostaining was performed using respective polyclonal antibodies. Antigen–antibody complex was subsequently visualized using the Envision Detection System kit peroxidase/DAB (Dako, Glostrup, Denmark) and counterstained with hematoxylin. Negative controls were used for the tested antibodies and the primary antibody was replaced by either mouse or rabbit nonimmune serum, as appropriate. All of the stained sections were evaluated in a blinded manner without prior knowledge of the patient data. Figure 1 shows an example of molecular marker expression based on immunohistochemistry (×200). To analyze the relationship between the degree of biomarker expression and clinical response, we set scores from 0 to 9 according to the expression of EGFR, DAXX, VEGF, p-STAT3, and PD-L1.
Figure 1.
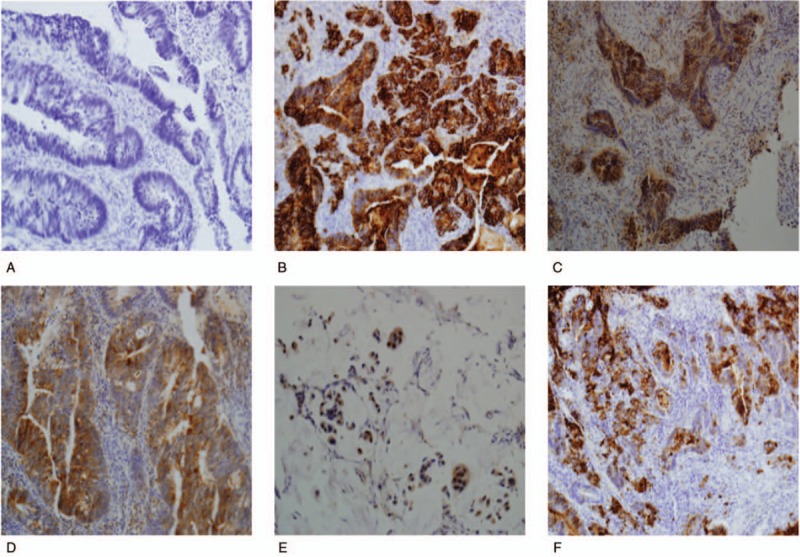
Molecular marker expression based on immunohistochemistry (×200). (A) Negative reaction is seen in rectal adenocarcinoma. (B) EGFR immunostaining shows a positive reaction in rectal adenocarcinoma, moderately-differentiated. (C) DAXX immunostaining shows a positive reaction in rectal adenocarcinoma, well-differentiated. (D) VEGF immunostaining shows a positive reaction in rectal adenocarcinoma, well-differentiated. (E) STAT3 immunostaining shows a positive reaction in rectal adenocarcinoma, mucinous type. (F) PD-L1 immunostaining shows a positive reaction in rectal adenocarcinoma, moderately-differentiated. DAXX = death-domain associated protein, EGFR = epidermal growth factor receptor, PD-L1 = programmed death-ligand 1, STAT3 = signal transducer and activator of transcription 3, VEGF = vascular endothelial growth factor.
2.3.3. Evaluation immunohistochemistry
Immune reactions were evaluated with the use of a nomogram as reported by Vilkin et al,[33] which incorporates both the proportion of positive cells and staining intensity. The percentage of positive cells was scored as 0 (negative), 1 (1–10%), 2 (11–50%), 3 (51–80%), and 4 (>80%). The intensity of positive staining was scored according to the mean optical density with 4 groups: 0, no staining; 1, weak staining (light yellow); 2, moderate staining (yellow brown); and 3, strong staining (brown). These 2 scores were multiplied together and a final score was assigned as follows: 0 to 1 (negative), 2 to 5 (low-expression), and >6 (high-expression).
2.3.4. Statistical analysis
Statistical evaluations were carried out using SPSS for Windows, Version 24.0 (SPSS, Chicago, IL). To compare variables, the Chi-squared test or Fisher exact test were used for qualitative variables and the Student t test was used for the quantitative variables. Categorical data were analyzed using the Fisher exact or Chi-squared tests. Logistic regression univariate analysis was used to identify variables that predicted a complete response. A P = .05 or less was considered statistically significant.
3. Results
3.1. Patients
A total of 31 patients were included in this study (Table 1). The median age was 53 years (range, 32–68). Of the 31 patients, 21 patients (67.7%) underwent anterior resection, 6 (19.4%) underwent Miles operation, 2 (6.5%) underwent a transabdominal transanal procedure, and 2 (6.5%) underwent Hartmann operation. Assessment of surgical specimens revealed 9 patients with a complete response (29.0%), 1 T1 (3.2%), 6 T2 (19.4%), 12 T3 (38.7%), and 3 T4 (9.7%) with 25 N0 (80.6%), 0 N1 (0.0%), 3 N2 (9.6%), and 3 N3 (9.6%) patients. Only 2 patients (6.5%) showed distant metastasis. Additionally, 4 (12.9%) patients showed perineural invasion and 2 patients (6.5%) showed lymphovascular invasion.
Table 1.
Patients characteristics.
3.2. Biomarker expression
Expression of the 5 tested biomarkers is shown in Figure 2. PD-L1 positivity was revealed in 7 (22.6%) out of 31 patients with 4 (12.9%) at low-expression and 3 (9.7%) at high-expression levels. CR was found in 3 (33.3%) out of 9 patients with 1 (11.1%) low-expression and 2 (22.2) high-expression, and non-CR was found in 4 (18.2%) of 22 with 3 (13.6%) low-expression and 1 (4.6%) high-expression. EGFR positivity was revealed in 22 (71.0%) of 31 patients with 11 (35.5%) low-expression and 11 (35.5%) high-expression. CR was found in 8 (88.9%) of 9 patients with 3 (33.3%) low-expression and 5 (55.6%) high-expression, and non-CR was found in 14 (63.6%) of 22 patients with 8 (36.4%) low-expression and 6 (27.3%) high-expression. VEGF was positivity revealed in 26 (83.9%) of 31 patients with 20 (64.5%) low-expression and 6 (19.4%) high-expression. CR was found in 8 (88.9%) of 9 patients with 5 (55.6%) low-expression and 3 (33.3%) high-expression and non-CR was found in 18 (81.8%) of 22 patients with 15 (68.2%) low-expression and 3 (13.6%) high-expression. p-STAT3 positivity was revealed in 25 (80.6%) of 31 patients with 21 (67.7%) low-expression and 4 (12.9%) high-expression. CR was found in 8 (88.9%) of 9 patients with 6 (66.7%) low-expression and 2 (22.2%) high-expression, and non-CR was found in 17 (77.3%) of 22 patients with 15 (68.2%) low-expression and 2 (9.1%) high-expression. DAXX positivity was revealed in 31 (100%) out of 31 patients with 12 (38.7%) low-expression and 19 (61.3%) high-expression. CR was found in 9 (100%) out of 9 patients with 4 (44.4%) low-expression and 5 (55.6%) high-expression and non-CR was found in 22 (100%) of 22 patients with 8 (36.4%) low-expression and 14 (63.6%) high-expression. Figure 2 shows distribution of biomarker expression.
Figure 2.
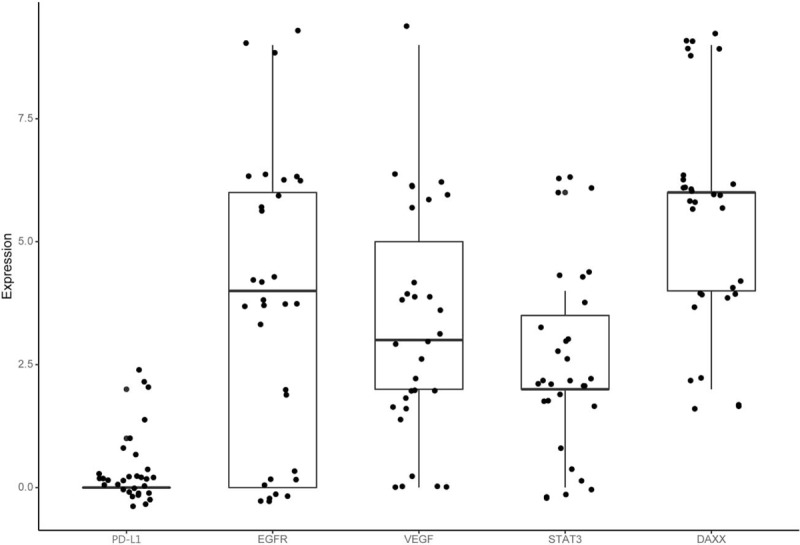
Biomarker expression. DAXX = death-domain associated protein, EGFR = epidermal growth factor receptor, PD-L1 = programmed death-ligand 1, STAT3 = signal transducer and activator of transcription 3, VEGF = vascular endothelial growth factor.
The mean serum prechemoradiotherapy CEA level was 11.51 ± 15.061 for all patients, of which CR level was 16.71 ± 23.398 and non-CR was 9.39 ± 9.958. The mean serum prechemoradiotherapy pre-CRT CA19-9 level was 50.618 ± 127.52 for all patients, of which CR level was 16.71 ± 23.398 and non-CR was 63.032 ± 150.299.
3.3. Correlations between the 5 biomarkers and CR
Correlations between the 5 biomarkers and CR are shown in Table 2. Logistic regression analysis was used to identify factors independently associated with CR in rectal cancer; VEGF expression was significantly correlated with CR (P = .01), and only VEGF expression was found to be a significant independent predictive factor (95% confidence interval 1.10–2.85, P = .01). When the patient population was classified according to CR criteria, 4 markers (PD-L1, DAXX, p-STAT3, and EGFR) failed to affect the probability of CR. Regarding the correlation of clinicopathologic parameters and biomarker expression, VEGF expression was significantly associated with p-STAT3 expression (P = .005).
Table 2.
Univariate analysis of biomarkers predict for complete response.
4. Discussion
Although surgical resection has been the primary treatment for localized rectal cancers, additional treatment prior to surgery for advanced cases has been required. It is now evident that preoperative CRT is not equally beneficial for all patients with locally advanced rectal cancer who are routinely recommended to receive preoperative combined modality therapy.[2–4] Some patients have a minimal response to preoperative therapy, whereas others have no detectable tumor cells in the surgical specimen. The ability to predict tumor response before treatment may significantly impact the selection of patients for preoperative CRT as well as the potential to modify the postoperative treatment plan. Therefore, the identification of biomarkers to predict the response for preoperative CRT has emerged. Several candidates as predictive CRT biological markers have been suggested; however, the results were often inconclusive and although gene-expression signatures associated with different clinical variables have been proposed, the clinical impact remains poor.[7,12–16]
This study aimed to identify biomarkers that predict CR in preoperative biopsied specimens from patients with locally advanced rectal cancer treated with preoperative CRT, including molecules involved in angiogenesis (VEGF), apoptosis (DAXX, p-STAT3), tumor cell proliferation (EGFR), and immune response (PD-L1). We found that VEGF expression provides an anticipating CR target for rectal cancer with preoperative CRT. VEGF expression was the only factor with an independent predictive value for CR after CRT in rectal cancer. Hur et al[34] reported that VEGF mRNA expression was not a predictive biomarker of CR and Qiu et al[14] also reported pretreatment VEGF level was unrelated to histological response. However, Zlobec et al[19] found a significant association between the radiation response of rectal cancer and VEGF expression, where loss of VEGF and positive EGFR were predictive of complete pathologic response in patients undergoing preoperative radiotherapy. In this study, VEGF and EGFR showed a negative correlation with CR; however, the loss of VEGF and positive EGFR were not significantly independent predictive factors for CR.
The expression of apoptosis factors, such as bax, p53, and survivin in rectal cancer specimens has also been shown to predict tumor responses to CRT in several investigations.[20–22] In this study, DAXX, which has a role in apoptosis, was not related to the response of preoperative CRT. This discordant result might be due to different roles based on tissue or tumor type. In ovarian cancer, DAXX has been shown to promote ovarian cancer cell proliferation and chemoresistance in ovarian cancer cells.[29,35]
In this study, p-STAT3 expression was not related to CR. Because p-STAT3 is constitutively activated in colon cancer-initiating cells,[31] p-STAT3 was assumed to be a biomarker predicting CRT. There is no exploration for the relationship of DAXX or p-STAT3 and the response to chemoradiotherapy. In this study, EGFR expression was not correlated with CR, although theoretically EGFR is supposed to be correlated with CRT response. Combined VEGF and EGFR (loss of VEGF and positive EGFR) can be predictive of CR.[19] One report suggested that EGFR is a predictor of tumor response to preoperative radiotherapy.[36] If the appropriate cases could be added to this study, the results might show this to be a significant predictor for CR. In this study, conflicting results or discrepancies with previous reports probably reflect differences in study design, patient selection, sample size, scoring molecular marker positivity, and patient cohort including stage at presentation, regimen of chemotherapy, and dose of radiation administered among different studies. There are also limitations to this study. First, although CR was the study end point in this analysis, other possible study end points such as TNM downstaging, could have been significant confounding variables because there is extensive heterogeneity in non-CR tumors. Second, assessing whether the response by molecular markers predicts long-term recurrence and/or survival rates was not evaluated, although the response after preoperative CRT seems to be a strong prognostic factor. Third, limitation of this study is the small sample size. To address these limitations, continued efforts will include the collection of specimens from additional patients to further validate these results.
5. Conclusions
It was confirmed that VEGF expression from diagnostic biopsy specimens before surgery was a factor associated with complete response after a preoperative chemoradiotherapy. The expression of the biomarker from the preoperative biopsy specimen which is associated with the response of preoperative chemoradiotherapy is not yet well known. In conclusion, although there is a restriction of small sample size, our finding suggested that this study can be foundation for a larger further study for biomarkers which can predict neoadjuvant therapy response of specimens obtained for diagnosis before surgery.
Acknowledgments
Authors thank Nayoung Kim for her contribution in statistical methods.
Author contributions
Conceptualization: Tae Sig Jeung, HeeKyung Chang.
Data curation: Seung-Hyun Lee.
Formal analysis: Jesang Yu, HeeKyung Chang.
Investigation: Jesang Yu, HeeKyung Chang.
Methodology: Jesang Yu, HeeKyung Chang.
Project administration: Tae Sig Jeung, HeeKyung Chang.
Writing – original draft: Jesang Yu, HeeKyung Chang.
Writing – review & editing: Jesang Yu, HeeKyung Chang.
Jesang Yu orcid: 0000-0002-0469-2660.
Footnotes
Abbreviations: AV = anal verge, CA19-9 = cancer antigen 19-9, CEA = carcinoembryonic antigen, CI = confidence interval, CR = complete response, CRT = chemoradiation therapy, DAXX = death-domain associated protein, EGFR = epidermal growth factor receptor, LAR = low anterior resection, PD-L1 = programmed death-ligand 1, STAT3 = signal transducer and activator of transcription 3, TATA = transabdominal transanal resection, VEGF = vascular endothelial growth factor.
The authors have no conflicts of interest to disclose.
References
- [1].Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- [2].Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg 2004;240:711–7. discussion 717-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Huh JW, Jung EJ, Park YA, et al. Preoperative chemoradiation followed by transanal excision for rectal cancer. J Surg Res 2008;148:244–50. [DOI] [PubMed] [Google Scholar]
- [4].Kim CJ, Yeatman TJ, Coppola D, et al. Local excision of T2 and T3 rectal cancers after downstaging chemoradiation. Ann Surg 2001;234:352–8. discussion 358-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kurt A, Yanar F, Asoglu O, et al. Low Mmp 9 and VEGF levels predict good oncologic outcome in mid and low rectal cancer patients with neoadjuvant chemoradiation. BMC Clin Pathol 2012;12:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yan H, Wang R, Yu J, et al. Predictive value of Smac, VEGF and Ki-67 in rectal cancer treated with neoadjuvant therapy. Oncol Lett 2010;1:641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bertolini F, Bengala C, Losi L, et al. Prognostic and predictive value of baseline and posttreatment molecular marker expression in locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. Int J Radiat Oncol Biol Phys 2007;68:1455–61. [DOI] [PubMed] [Google Scholar]
- [8].Hou G, Song R, Yang J, et al. Treatment effect of conversion therapy and its correlation with VEGF expression in unresectable rectal cancer with liver metastasis. Oncol Lett 2018;16:749–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kim HJ, Bae SB, Jeong D, et al. Upregulation of stromal cell-derived factor 1alpha expression is associated with the resistance to neoadjuvant chemoradiotherapy of locally advanced rectal cancer: angiogenic markers of neoadjuvant chemoradiation. Oncol Rep 2014;32:2493–500. [DOI] [PubMed] [Google Scholar]
- [10].Maretto I, Pomerri F, Pucciarelli S, et al. The potential of restaging in the prediction of pathologic response after preoperative chemoradiotherapy for rectal cancer. Ann Surg Oncol 2007;14:455–61. [DOI] [PubMed] [Google Scholar]
- [11].Huh JW, Min JJ, Lee JH, et al. The predictive role of sequential FDG-PET/CT in response of locally advanced rectal cancer to neoadjuvant chemoradiation. Am J Clin Oncol 2012;35:340–4. [DOI] [PubMed] [Google Scholar]
- [12].Negri FV, Campanini N, Camisa R, et al. Biological predictive factors in rectal cancer treated with preoperative radiotherapy or radiochemotherapy. Br J Cancer 2008;98:143–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Smith FM, Reynolds JV, Kay EW, et al. COX-2 overexpression in pretreatment biopsies predicts response of rectal cancers to neoadjuvant radiochemotherapy. Int J Radiat Oncol Biol Phys 2006;64:466–72. [DOI] [PubMed] [Google Scholar]
- [14].Qiu H, Sirivongs P, Rothenberger M, et al. Molecular prognostic factors in rectal cancer treated by radiation and surgery. Dis Colon Rectum 2000;43:451–9. [DOI] [PubMed] [Google Scholar]
- [15].Garcia-Aguilar J, Chen Z, Smith DD, et al. Identification of a biomarker profile associated with resistance to neoadjuvant chemoradiation therapy in rectal cancer. Ann Surg 2011;254:486–92. discussion 492-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lin JT, Chang TH, Chang CS, et al. Prognostic value of pretreatment CD44 mRNA in peripheral blood of patients with locally advanced head and neck cancer. Oral Oncol 2010;46:e29–33. [DOI] [PubMed] [Google Scholar]
- [17].Relf M, LeJeune S, Scott PA, et al. Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor beta-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res 1997;57:963–9. [PubMed] [Google Scholar]
- [18].Toi M, Atiqur Rahman M, Bando H, et al. Thymidine phosphorylase (platelet-derived endothelial-cell growth factor) in cancer biology and treatment. Lancet Oncol 2005;6:158–66. [DOI] [PubMed] [Google Scholar]
- [19].Zlobec I, Steele R, Compton CC. VEGF as a predictive marker of rectal tumor response to preoperative radiotherapy. Cancer 2005;104:2517–21. [DOI] [PubMed] [Google Scholar]
- [20].Chang HJ, Jung KH, Kim DY, et al. Bax, a predictive marker for therapeutic response to preoperative chemoradiotherapy in patients with rectal carcinoma. Hum Pathol 2005;36:364–71. [DOI] [PubMed] [Google Scholar]
- [21].Kandioler D, Zwrtek R, Ludwig C, et al. TP53 genotype but not p53 immunohistochemical result predicts response to preoperative short-term radiotherapy in rectal cancer. Ann Surg 2002;235:493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rodel F, Hoffmann J, Distel L, et al. Survivin as a radioresistance factor, and prognostic and therapeutic target for radiotherapy in rectal cancer. Cancer Res 2005;65:4881–7. [DOI] [PubMed] [Google Scholar]
- [23].Kojima M, Ishii G, Atsumi N, et al. CD133 expression in rectal cancer after preoperative chemoradiotherapy. Cancer Sci 2010;101:906–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Speake WJ, Dean RA, Kumar A, et al. Radiation induced MMP expression from rectal cancer is short lived but contributes to in vitro invasion. Eur J Surg Oncol 2005;31:869–74. [DOI] [PubMed] [Google Scholar]
- [25].Diez M, Ramos P, Medrano MJ, et al. Preoperatively irradiated rectal carcinoma: analysis of the histopathologic response and predictive value of proliferating cell nuclear antigen immunostaining. Oncology 2003;64:213–9. [DOI] [PubMed] [Google Scholar]
- [26].Willett CG, Warland G, Hagan MP, et al. Tumor proliferation in rectal cancer following preoperative irradiation. J Clin Oncol 1995;13:1417–24. [DOI] [PubMed] [Google Scholar]
- [27].Spano JP, Fagard R, Soria JC, et al. Epidermal growth factor receptor signaling in colorectal cancer: preclinical data and therapeutic perspectives. Ann Oncol 2005;16:189–94. [DOI] [PubMed] [Google Scholar]
- [28].Krasinskas AM. EGFR signaling in colorectal carcinoma. Patholog Res Int 2011;2011:932932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tzeng SL, Cheng YW, Li CH, et al. Physiological and functional interactions between Tcf4 and Daxx in colon cancer cells. J Biol Chem 2006;281:15405–11. [DOI] [PubMed] [Google Scholar]
- [30].Inaguma S, Lasota J, Wang Zv. Clinicopathologic profile, immunophenotype, and genotype of CD274 (PD-L1)-positive colorectal carcinomas. Mod Pathol 2017;30:278–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lin L, Liu A, Peng Z, et al. STAT3 is necessary for proliferation and survival in colon cancer-initiating cells. Cancer Res 2011;71:7226–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer 2015;15:409–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Vilkin A, Leibovici-Weissman Y, Halpern M, et al. Erratum to: “Immunohistochemistry staining for mismatch repair proteins: the endoscopic biopsy material provides useful and coherent results” [Hum Pathol 2015;46:1705-1711]. Hum Pathol 2017;67:229. [DOI] [PubMed] [Google Scholar]
- [34].Hur H, Tulina I, Cho MS, et al. Biomarker-based scoring system for prediction of tumor response after preoperative chemoradiotherapy in rectal cancer by reverse transcriptase polymerase chain reaction analysis. Dis Colon Rectum 2016;59:1174–82. [DOI] [PubMed] [Google Scholar]
- [35].Pan WW, Zhou JJ, Liu XM, et al. Death domain-associated protein DAXX promotes ovarian cancer development and chemoresistance. J Biol Chem 2013;288:13620–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Giralt J, Eraso A, Armengol M, et al. Epidermal growth factor receptor is a predictor of tumor response in locally advanced rectal cancer patients treated with preoperative radiotherapy. Int J Radiat Oncol Biol Phys 2002;54:1460–5. [DOI] [PubMed] [Google Scholar]


