Abstract
Several prognostic indices have been employed to predict the outcome of surgical critically ill patients. Among them, acute physiology and chronic health evaluation (APACHE) II, sequential organ failure assessment (SOFA) and simplified acute physiology score (SAPS 3) are widely used. It seems that biological markers such as C-reactive protein (CRP), albumin, and blood lactate levels correlate with the degree of inflammation during the immediate postoperative phase and could be used as independent predictors. The objective of this study is to compare the different predictive values of prognostic indices and biological markers in the outcome of 847 surgical patients admitted to the intensive care unit (ICU) in the postoperative phase.
The patients were divided into survivors (n = 765, 57.4% males, age 61, interquartile range 51–71) and nonsurvivors (n = 82, 57.3% males, age 70, interquartile range 58–79). APACHE II, APACHE II death probability (DP), SOFA, SAPS 3, SAPS 3 DP, CRP, albumin, and lactate were recorded on ICU admission (first 24 hours). The area under the ROC curve (AUROC) and 95% confidence interval (95% CI) were used to measure the index accuracy to predict mortality.
The AUROC and 95% CI for APACHE II, APACHE II DP, SOFA, SAPS 3, SAPS 3 DP, CRP/albumin ratio, CRP, albumin, and lactate were 0.850 (0.824–0.873), 0.855 (0.829–0.878), 0.791 (0.762–0.818), 0.840 (0.813–0.864), 0.840 (0.813–0.864), 0.731 (0.700–0.761), 0.708 (0.676–0.739), 0.697 (0.665–0.728), and 0.601 (0.567–0.634), respectively. The ICU and overall in-hospital mortality were 6.6 and 9.7%, respectively. The APACHE II, APACHE II DP, SAPS 3, SAPS 3 DP, and SOFA scores showed a better performance than CRP/albumin ratio, CRP, albumin, or lactate to predict in-hospital mortality of surgical critically ill patients.
Even though all indices were able to discriminate septic from nonseptic patients, only APACHE II, APACHE II DP, SOFA and to a lesser extent SAPS 3, SAPS 3 DP, and blood lactate levels could predict in the first 24-hour ICU admission surgical patients who have survived sepsis.
Keywords: biological markers, mortality, prognostic index, ROC curve, sepsis
1. Introduction
The treatment provided within a surgical intensive care unit (ICU) should not be restricted to knowledge about procedures, technology, and clinical protocols. Safety, cost-effectiveness and the outcome of the surgical critically ill patient must also be assessed since health costs arise progressively whilst there is resource scarcity.[1,2]
Several classes of inflammation markers have been described: cytokines/chemokines, acute phase proteins (C-reactive protein [CRP] and serum amyloid A), reactive oxygen and nitrogen species, prostaglandins and cyclooxygenase-related factors, and mediators such as transcription factors and growth factors. Among all these markers, the techniques currently available for CRP is easy to perform and present low cost and high analytical sensitivity.[3] The serum lactate level is considered a sensitive marker for sepsis and septic shock and reflects cell-based metabolism. The biological markers such as CRP, albumin, CRP/albumin ratio, and blood lactate levels correlate with the degree of inflammation during the immediate postoperative phase and could be used as independent predictors.[4–7] However, the use of markers of infection can show test results apparently normal in sepsis, especially in patients with a depressed immune response. The prognostic indices can be used for the analysis of a cohort or to a group of specific diseases and can guide health strategies or allocation of resources. Among them, acute physiology and chronic health evaluation (APACHE)[8] sequential organ failure assessment (SOFA),[9] and simplified acute physiology score (SAPS)[10] have been widely used to predict the outcome of the surgical critically ill patients. These scores are more extensive, because of the use of multiple physiological variables from different organic systems. In addition, the use of prognostic indices can be used to not only to predict which patients are likely to develop sepsis but also to predict which patient can survive or not sepsis. The early recognition of patients at risk of developing sepsis allows an appropriate approach that would be started in ICU admission and this may improve outcomes.
Therefore, investigators worldwide have been searching for a paramount prognosticator of the surgical critically ill patient for decades.[11] However, none of these indices have a 100% sensitivity or 100% specificity. The objective of this study is to compare the different predictive values of prognostic indices and biological markers in the outcome of surgical patients admitted to the ICU in the immediate postoperative phase.
2. Methods
2.1. Study design and setting
This retrospective study was conducted in a general 20-bed adult ICU of Sao Francisco Hospital, Ribeirao Preto, Sao Paulo, Brazil. This tertiary ICU admits critically ill adults such as clinical cases or surgical patients. The study protocol was approved by the Research Ethics Committee of Clinics Hospital of Ribeirao Preto Medical School, University of Sao Paulo (Protocol 7076/2016).
2.2. Patients and collected variables
Adult surgical patients (age >18 years old) admitted to ICU during the immediate postoperative phase between 2015 and 2017 were analyzed. Only patients with a set of data (prognostic indices and biological markers) were enrolled in the study. Data concerning the diagnosis upon ICU arrival, comorbidities, demographic profile were documented. The physiological variables (biological markers) CRP (immunoluminometric assay, reference range = 0–10 mg/L), albumin (colorimetric assay, reference range = 3.5–5.5 g/dL), and serum blood lactate (mmol/L) and prognostic indices such as APACHE II, APACHE II death probability (DP) (derived from APACHE II score), SOFA, SAPS 3 DP (derived from SAPS 3 score) were recorded. All data for calculation of the prognostic indices and physiological variables were collected during the first 24 hours after patient admission. The diagnosis of sepsis was defined as life-threatening organ dysfunction caused by a dysregulated host response to infection, according to Sepsis-3 guidelines.[12–14] The calibration of prognostic indices customized equations for South America was provided by manufacturer's software of data bank (Epimed Monitor, Epimed Solutions, Rio de Janeiro, Brazil). However, in this study APACHE II and SAPS 3 calibration were built to improve the performance of the score and it was based upon the comparison between predicted probabilities and observed results which are the basis of Hosmer–Lemeshow goodness of fit test for logistic regression.
2.3. Statistical analysis
Comparison of demographic and clinical data of the patients (survivors and nonsurvivors) was accomplished by employing the 2-sample Wilcoxon rank-sum (Mann–Whitney) test for quantitative variables and Fisher exact test for qualitative variables. Data comparison of APACHE II, APACHE II DP, SOFA, SAPS 3, SAPS 3 DP, and CRP/albumin ratio, CRP, albumin, and lactate of patients survivors and nonsurvivors were analyzed as the median and interquartile range. The capability of each prognostic index and biological markers, and serum blood lactate to predict mortality was analyzed by receiver operating characteristic (ROC) curve. The area under the ROC curve (AUC) and the respective confidence interval (CI) were used as a measure of the overall index accuracy. The comparison among these curves was tested as proposed by DeLong et al.[15] A univariate linear regression was performed adjusted to gender, age, emergency surgery, prognostic indices, and biomarkers to detect which variables were independently associated to the outcome. In order to estimate and interpret survival the of ICU and in-hospital survival time, it was performed a Kaplan–Meier curve for the 2 groups of patients (survivors and nonsurvivors). In order to compare these 2 survival curves, nonparametric log-rank test was used. The significance level was set at P < .05. Statistical analyses were performed using the software Stata v.14.2 (StataCorp, College Station, TX) and MedCalc v.14 (Ostend, Belgium).
3. Results
3.1. Study patients and clinical presentation
Eight hundred forty-seven surgical patients admitted to the ICU of a tertiary care Hospital in the immediate postoperative phase were retrospectively studied. Demographic data and incidence of complications by comparison of groups of patients designated survivors (n = 765; 57.4% males, age of 62 [51–77] years) and nonsurvivors (n = 82; 57.3% males, age of 70 [58–79] years) are reported in Table 1. The ICU and overall in-hospital mortality were 6.6 and 9.7%, respectively. In this study, the Hosmer–Lemeshow test for APACHE II and SAPS 3 were P = .4045 and P = .3897, respectively. These results confirmed a proper calibration and an acceptable discriminatory power for both models. In the general population of the study (n = 847) the median and interquartile range, the AUC and 95% CI and P values for APACHE II, APACHE II DP, SAPS 3, SAPS 3 DP, SOFA, CRP/albumin ratio, CRP, albumin, and lactate for survivors and nonsurvivors are visualized in Figure 1 and Table 2. The pairwise comparison among all predictors is shown in Table 3. The univariate logistic regression showed the following results: gender – Odds ratio (OR) (95% CI) = 0.7380 (0.4184–1.3018) (P = .2941); age – OR (95% CI) = 0.9831 (0.9645–1.0020) (P = .0794); emergency surgery – OR (95% CI) = 1.0382 (0.4951–2.177) (P = .9209); APACHE II – OR (95% CI) = 0.8925 (0.8375–0.9512) (P = .0005); SAPS 3 – OR (95% CI) = 0.9620 (0.9352–0.9896) (P = .0073); SOFA – OR (95%CI) = 0.9293 (0.8227–1.0497) (P = .2379); CRP/albumin – OR (95% CI) = 0.9873 (0.9789–0.9959) (P = .0037); lactate – OR (95% CI) = 0.7995 (0.7030–0.9092) (P = .0007). The comparison of the 2 survival curves, a log-rank test was conducted to determine the occurrence of differences in distribution of survival for both types of patients (survivors and nonsurvivors). The distribution of survival time for these patients, considering the ICU LOS and hospital LOS, were statistically different (P < .0001). The values (in days) for the ICU LOS of mean survival ± standard error and 95% CI were 2.63 ± 0.13 (2.370–2.893) for survivors and 12.32 ± 2.16 (8.082–16.559) for nonsurvivors. For the in-hospital LOS these values were 11.27 ± 0.72 (9.857–12.686) and 31.49 ± 3.18 (25.258–37.773) for survivors versus nonsurvivors, respectively (Fig. 2).
Table 1.
Demographic and clinical characteristics of surgical critically ill patients survivors and nonsurvivors.

Figure 1.
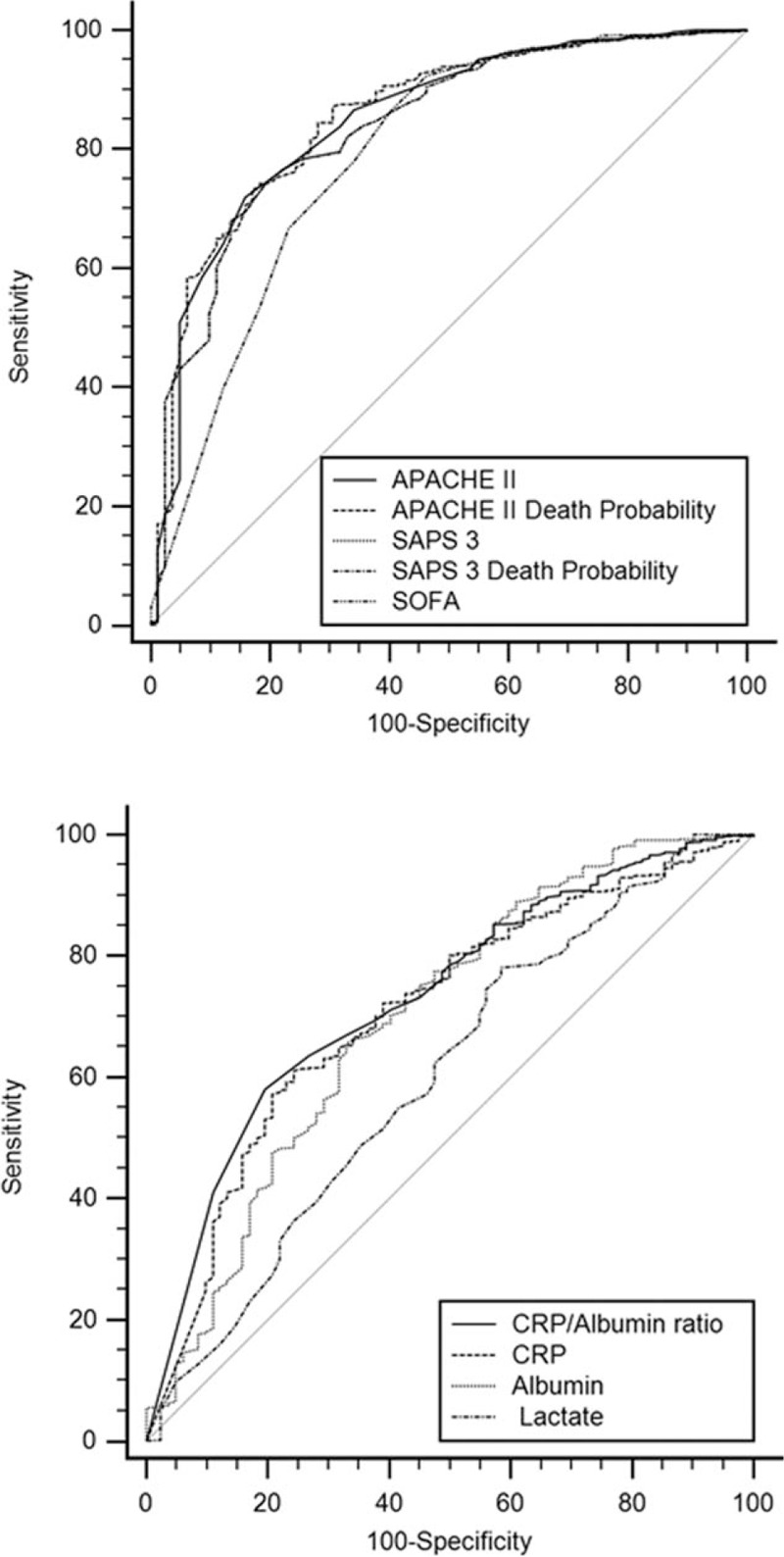
Comparison of ROC curves of APACHE II, APACHE II death probability, SAPS 3, SAPS 3 death probability, SOFA, CRP/albumin ratio, CRP, albumin and lactate of surgical patients. APACHE = acute physiology and chronic health evaluation, CRP = C-reactive protein, ROC = receiver operating characteristic, SAPS = simplified acute physiology score.
Table 2.
APACHE II, APACHE II death probability (DP), SAPS 3, SAPS 3 death probability, SOFA, C-reactive protein (CPR)/albumin ratio, CRP, albumin, and lactate area under the curve (AUC) of receiver-operating characteristic curve (ROC), and respective confidence interval (CI) values.
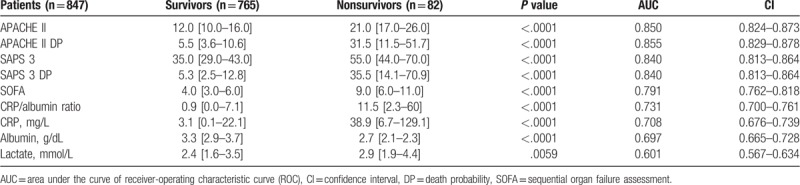
Table 3.
Pairwise comparison of ROC curves for the different variables studied[15].
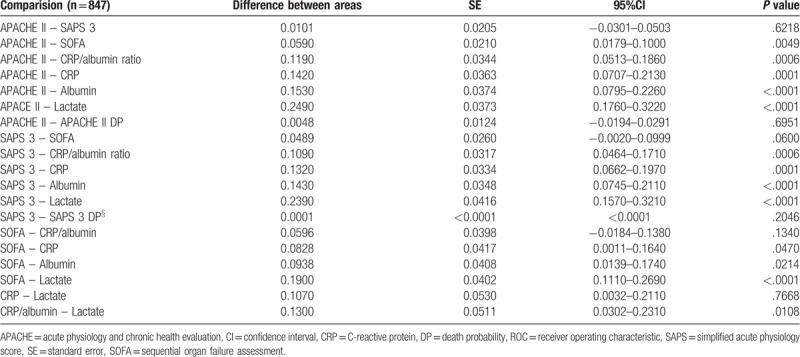
Figure 2.

Survival analysis for the ICU outcome and in-Hospital outcome for survivors and nonsurvivors patients. ICU = intensive care unit.
3.2. Study population of septic and nonseptic patients
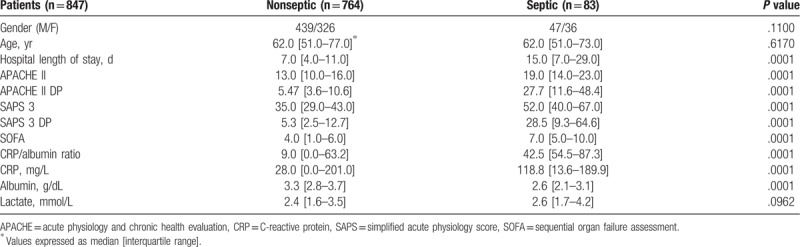
All patients (n = 847) in the postoperative period were studied. Demographic data, prognostic indices, and biological markers were collected on admission to ICU and the patients were designated as nonseptic (n = 764; 57.4 males, age 62 [51–77] years) and septic (n = 83; 56.6 males, age 62 [51–73] years). The comparison of data concerning demographic, prognostic indices and biological markers and its respective P values for septic and nonseptic patients are shown in Table 4.
Table 4.
Characteristics of surgical critically ill septic and nonseptic patients.
3.3. Study population of patients who survived or not sepsis
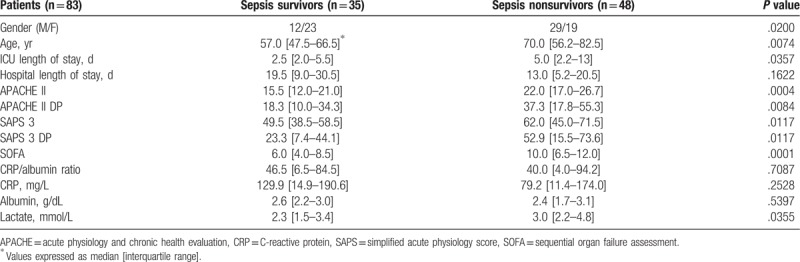
All patients (n = 83) with sepsis in the postoperative period were studied. Demographic data, prognostic indices, and biological markers were collected on admission to ICU and the patients were designated as sepsis survivors (n = 35; 34.3 males, age 57.0 [47.5–66.5] years) and sepsis nonsurvivors (n = 48; 60.4 males, age 70.0 [56.2–82.5] years). The comparison of data of demographic, prognostic indices, and biological markers for patients septics who have survived or not sepsis are summarized in Table 5. APACHE II (P = .0004), APACHE II DP (P = .0084), SOFA (P = .0001) and to a lesser extent SAPS 3 (P = .0117), SAPS 3 DP (P = .0117), and blood lactate levels (P = .0355) could predict the surgical patients who have survived sepsis.
Table 5.
Characteristics of surgical critically ill patients with sepsis survivors and nonsurvivors.
4. Discussion
Although the purpose of an ICU is to provide the best treatment possible so that patients will have a more satisfactory prognosis, it must be economically feasible. This is because healthcare resources worldwide are becoming scarce over time. In this context, a number of studies have stated that the optimization of the treatment delivered to patients admitted to an ICU should not be restricted to procedures and clinical protocols. ICU programs such as risk management, performance, and early outcome prediction, preferably in the first 24 hours of patient's admission, should be also considered. Some of these systems employed for the management of ICU complications after major surgery and mortality prediction are APACHE II, SAPS 3, and SOFA prognostic indices. Therefore, it seems, theoretically, that these scores could predict better the outcome than 1 variable alone since these indices include several physiologic parameters in their equations. Thus, it should be considered the simplicity of SOFA. This score has only 6 variables, whereas APACHE II and SAPS 3 have 14 and 20 variables, respectively. However, some other physiological variables, that is, biological markers (CRP, albumin, and lactate) could play a significant role as independent factors to predict mortality in surgical critically ill patients. In this sense, all scores could be usually used as predictors of clinical deterioration and/or for the diagnosis of surgical complications.
CRP is the variable most available to detect postoperative inflammation and the most used biomarker of infection in ICU patients.[16,17] Indeed, the fate of CRP acting as a biological biomarker following operation is already known. CRP was first described in 1930 by William Tillett and Thomas Francis.[18] These authors isolated a third serologic “fraction C” from patients infected with pneumococcus that was distinct from previously known capsular polysaccharide and nucleoprotein fractions detectable by specific antibody response. CRP is an acute phase protein synthesized by the liver, which levels rise in response to inflammation. CRP is relevant not only as a parameter of clinical deterioration and decision making in the diagnosis or intervention procedures strategies but also in the early detection of inflammation and infection after major abdominal surgery of patients admitted to ICU. The CRP normal level (0–8 mg/L) is known to rise in response to the initial surgical stress due to tissue damage, reaching a peak in 48 to 72 hours in the postoperative phase and decreasing soon after.[19,20] However, its levels could remain high if complications persist. Several studies have tried to target the cutoff point to CRP for postoperative complications or even sepsis.[21–23] A systematic review including 1427 patients in 7 studies was performed to evaluate the predictive power of CRP levels at D3 in the postoperative period of major abdominal surgeries. It was concluded that 20% of patients had postoperative infection complications after major abdominal surgeries.[2] In another similar investigation, Gans et al[24] observed in 2215 patients of 16 studies that infections complications after major abdominal surgery are very unlikely in patients with a CRP below 159 mg/L on D3. These findings could be helpful to select patients for safe and early hospital discharge and prevent the overuse of imaging. Conversely, early clinical complications may be indistinguishable of the postoperative inflammatory response to the surgical traumatic procedure.[25] In fact, CRP tends to normalize in patients without postoperative complications because of its half-life of 19 hours.[26]
Nevertheless, some data are controversial. Meyer et al[27] stated that an increase in CRP is a poor parameter for early detection of complications in critically ill surgical patients (OR = 0.983, 95% CI = 0.932–1.036). These authors have still found that CRP had not a satisfactory performance as a predictor of survival in the ICU during the early course of admission, neither has CRP a significant relationship between increased levels and surgical complications. In order to corroborate these findings, Castelli et al[28] could not be able to demonstrate increased levels of CRP in patients with trauma when septic complications were diagnosed.
Serum albumin is a negative acute phase maintenance protein that is rapidly downregulated by inflammatory signals.[5,29] Postoperative decreases of serum albumin can be used as a marker of the surgical stress response and early predictor in the clinical outcome after major surgery. Early identification of patients at risk may improve the outcome. The mechanisms of early postoperative albumin decrease are caused by altered metabolism, blood loss/dilution, and redistribution in the third space due to capillary leakage. Nevertheless, these alterations appear to be related to the extent of systemic inflammatory response.[30] Labgaa et al[31] demonstrated in a cohort of 138 patients after major abdominal surgery, the logistic regression of univariate, and multivariate analysis for predictors of postoperative complications that a serum albumin drop greater than 10 g/L in the first postoperative day has an OR of 6.89 [95% CI = 2.96–16.14].
The CRP to albumin ratio is being used as a prognostic score to assess outcomes in patients with cancer, inflammation, and sepsis. More recently, this ratio has been employed to predict postoperative complications of abdominal surgeries.[32] The authors of this investigation evaluated the prognostic value of CRP/albumin on day 3 in 214 patients submitted to abdominal surgeries and showed that 33.6% of these patients had postoperative complications and patients with a CRP/albumin ratio >2.0 suffered more (49.3 vs 22.1%, P < 0.05). Thus, the surgical site infections were 21.1 versus 4.85% of the patients (P < 0.001). It was concluded that the bigger the CRP/albumin ratio, the higher is the probability of postoperative complications. Ranzani et al[22] studied the capabilities of CRP/albumin to predict 90-day mortality in 334 patients. The AUROC of CRP, albumin, and CRP/albumin in admission were 0.50, 0.621, and 0.612, respectively. These authors showed that CRP/albumin ratio >2 presented the highest sensibility and sensitivity in the prediction of 90-day mortality in patients with sepsis/septic shock. The use of a ratio between CRP and albumin would provide a variable capable of merging the information provided by CRP and albumin into an index that correlated positively with infection, that is, a higher ratio indicates higher inflammation.[33]
Lactate has the classic status to be an independent predictor of the outcome of surgical critically ill patients.[34,35] However, Meyer et al[36] have concluded that lactate levels seem to be a poor marker for the diagnosis of complications in surgical ICU patients. Likewise, Filho et al[37] conducted a retrospective cohort study including 443 patients admitted to an ICU with severe sepsis or septic shock from the emergency department. These authors pointed out that the initial blood lactate level of more than 2.5 mmol/L was the best threshold to predict 28-day mortality among these patients. In our study, we did not find significant statistical differences in the lactate level between survivors and nonsurvivors. Nonetheless, lactate was statistically different between patients who have survived or not sepsis.
Although several studies have shown increasing CRP levels indicating inflammation in the general ICU population as we described previously, our study compared and evaluated the performance of different prognostic indices such as APACHE II, APACHE II DP, SAPS 3, SAPPS 3 DP, SOFA, CRP/albumin ratio, and lactate in surgical patients in the immediate postoperative phase. Our date was not able to demonstrate whether or not the biomarkers studied are suitable for use as prognostic indices. On the other hand, the prognostic indices could predict better the outcome of surgical critically ill patients, with special attention to APACHE II, APACHE II DP, SOFA, SAPS 3, and SAPS 3 DP that could distinguish patients who have survived or not sepsis.
This study has some limitations. It is a study carried out in a single center, which limits the extent of the results to other populations and the protocol design was observational and retrospective. In addition, there is a lack of information on the amount of blood and albumin administered or the perioperative phase. However, through this study, we would like to deliver a message to anesthesiologists, intensivists, and surgeons, about the use of APACHE II and SOFA as prognostic index to explore not only outcome of surgical critically ill patient used in the traditional postoperative approach but also their use to predict patients who have survived sepsis at ICU admission.
5. Conclusions
The data presented in this study pointed out a good performance of all prognostic indices in predicting septic and nonseptic surgical critically ill patients, and therefore APACHE II, APACHE II DP, and SOFA collected in the first 24 hours of ICU admission predicted better and to a lesser extent SAPS 3, SAPS 3 DP, and lactate the patients who have survived sepsis. In contrast, CRP, albumin, CRP/albumin ratio, and lactate performances at ICU admission were modest, which limits their use as sole markers of outcome. The present study gives some insight into this issue. Further studies ideally multicentric and prospective with a larger cohort will be needed to corroborate these findings.
Acknowledgments
We are thankful to Mr. Ronaldo Vicente Martins for his technical assistance and the Fundação de Amparo ao Ensino, Pesquisa e Assistência (FAEPA) of Clinics Hospital, Ribeirão Preto Medical School, University of São Paulo, Brazil for financial support.
Author contributions
Conceptualization: Anibal Basile-Filho.
Data curation: Edson Antonio Nicolini, Roosevelt Santos Nunes.
Formal analysis: Mayra Gonçalves Menegueti, Marcus Antonio Ferez.
Methodology: Anibal Basile-Filho.
Project administration: Anibal Basile-Filho.
Software: Maria Auxiliadora-Martins.
Supervision: Anibal Basile-Filho.
Validation: Mayra Gonçalves Menegueti.
Visualization: Lorena Aparecida de Brito Rodrigues.
Writing – original draft: Anibal Basile-Filho.
Writing – review and editing: Alessandra Fabiane Lago.
Footnotes
Abbreviations: APACHE = acute physiology and chronic health evaluation, AUC = area under the ROC curve, CI = confidence interval, CRP = C-reactive protein, ICUs = intensive care units, ROC = receiver operating characteristic, SAPS 3 = simplified acute physiology score, SOFA = sequential organ failure assessment.
Data available on request from the authors. The data that support the findings of this study are available from the first author AB-F upon reasonable request.
The Fundação de Amparo ao Ensino, Pesquisa e Assistência (FAEPA-HCRP) provided financial support for research assistance, statistical analysis, and publication charges.
The authors have no conflicts of interest to disclose.
References
- [1].Basile-Filho A, Menegueti MG, Auxiliadora-Martins M, et al. Why the surgical patients are so critical in their intensive care unit arrival. Acta Cir Bras 2013;28Suppl 1:48–53. [DOI] [PubMed] [Google Scholar]
- [2].Vonlanthen R, Slankamenac K, Breitenstein S, et al. The impact of complications on costs of major surgical procedures: a cost analysis of 1200 patients. Ann Surg 2011;254:907–13. [DOI] [PubMed] [Google Scholar]
- [3].Brenner DR, Scherer D, Muir K, et al. A review of the application of inflammatory biomarkers in epidemiologic cancer research. Cancer Epidemiol Biomarkers Prev 2014;23:1729–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhang Z, Ni H. C-reactive protein as a predictor of mortality in critically ill patients: a meta-analysis and systematic review. Anaesth Intensive Care 2011;39:854–61. [DOI] [PubMed] [Google Scholar]
- [5].Hübner M, Mantziari S, Demartines N, et al. Postoperative albumin drop is a marker for surgical stress and a predictor for clinical outcome: a pilot study. Gastroenterol Res Pract 2016;2016:8743187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vincent JL, Donadello K, Schmit X, et al. Biomarkers in the critically ill patient: C-reactive protein. Crit Care Clin 2011;27:241–51. [DOI] [PubMed] [Google Scholar]
- [7].Li S, Peng K, Liu F, et al. Changes in blood lactate levels after major elective abdominal surgery and the association with outcomes: a prospective observational study. J Surg Res 2013;184:1059–69. [DOI] [PubMed] [Google Scholar]
- [8].Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818–29. [PubMed] [Google Scholar]
- [9].Vincent JL, Moreno R, Takala J, et al. The SOFA. (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of European society of intensive care medicine. Intensive Care Med 1996;22:707–10. [DOI] [PubMed] [Google Scholar]
- [10].Moreno RP, Metnitz PG, Almeida E, et al. SAPS 3 Investigators. From evaluation of the patient to evaluation of the intensive care unit. Part 2: development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med 2005;31:1345–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Meyer ZC, Schreinemakers JM, de Waal RA, et al. Searching for predictors of surgical complications in critically ill surgery patients in the intensive care unit: a review. Surg Today 2015;45:1091–101. [DOI] [PubMed] [Google Scholar]
- [12].Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016;315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Shankar-Hari M, Phillips GS, Levy ML, et al. Sepsis definitions task force: developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016;315:775–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016;315:762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the area under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45. [PubMed] [Google Scholar]
- [16].Povoa P, Coelho L, Almeida E, et al. C-reactive protein as a marker of infection in critically ill patients. Clin Microbiol Infect 2005;11:101–18. [DOI] [PubMed] [Google Scholar]
- [17].Lelubre C, Anselin S, Zouaoui Boudjeltia K, et al. Interpretation of C-reactive protein concentrations in critically ill patients. Biomed Res Int 2013;2013:124021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Francis T, Tillett WS. Cutaneous reactions in pneumonia. The development of antibodies following the intradermal injection of type-specific polysaccharide. J Exp Med 1930;52:573–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kragsbjerg P, Holmberg H, Vikerfors T. Serum concentrations of interleukin-6, tumour necrosis factor-alpha, and C-reactive protein in patients undergoing major operations. Eur J Surg 1995;161:17–22. [PubMed] [Google Scholar]
- [20].Ticinesi A, Lauretani F, Nouvenne A, et al. C-reactive protein (CRP) measurement in geriatric patients hospitalized for acute infection. Eur J Intern Med 2017;37:7–12. [DOI] [PubMed] [Google Scholar]
- [21].Welsch T, Frommhold K, Hinz U, et al. Persisting elevation of C-reactive protein after pancreatic resections can indicate developing inflammatory complications. Surgery 2008;143:20–8. [DOI] [PubMed] [Google Scholar]
- [22].Ranzani OT, Zampieri FG, Forte DN, et al. C-reactive protein/albumin ratio predicts 90-day mortality of septic patients. PLoS One 2013;8:e59321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kim MH, Ahn JY, Song JE, et al. The C-reactive protein/albumin ratio as an independent predictor of mortality in patients with severe sepsis or septic shock treated with early goal-directed therapy. PLoS One 2015;10:e0132109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gans SL, Atema JJ, van Dieren S, et al. Diagnostic value of C-reactive protein to rule out infectious complications after major abdominal surgery: a systematic review and meta-analysis. Int J Colorectal Dis 2015;30:861–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bianchi RA, Silva NA, Natal ML, et al. Utility of base deficit, lactic acid, microalbuminuria, and C-reactive protein in the early detection of complications in the immediate postoperative evolution. Clin Biochem 2004;37:404–7. [DOI] [PubMed] [Google Scholar]
- [26].Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest 2003;111:1805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Meyer ZC, Schreinemakers JM, Mulder PG, et al. The role of C-reactive protein and the SOFA score as parameter for clinical decision making in surgical patients during the intensive care unit course. PLoS One 2013;8:e55964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Castelli GP, Pognani C, Cita M, et al. Procalcitonin as a prognostic and diagnostic tool for septic complications after major trauma. Crit Care Med 2009;37:1845–9. [DOI] [PubMed] [Google Scholar]
- [29].Sang BH, Bang JY, Song JG, et al. Hypoalbuminemia within two postoperative days is an independent risk factor for acute kidney injury following living donor liver transplantation: a propensity score analysis of 998 consecutive patients. Crit Care Med 2015;43:2552–61. [DOI] [PubMed] [Google Scholar]
- [30].Fleck A, Raines G, Hawker F, et al. Increased vascular permeability: a major cause of hypoalbuminaemia in disease and injury. Lancet 1985;1:781–4. [DOI] [PubMed] [Google Scholar]
- [31].Labgaa I, Joliat GR, Kefleyesus A, et al. Is postoperative decrease of serum albumin an early predictor of complications after major abdominal surgery? A prospective cohort study in a European centre. BMJ Open 2017;7:e013966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Karayiannis D, Bouloubasi Z, Baschali A, et al. Postoperative C-reactive protein to albumin ratio as a diagnostic tool for predicting complications after abdominal surgery. Clin Nutr ESPEN 2018;24:176. [Google Scholar]
- [33].Fairclough E, Cairns E, Hamilton J, et al. Evaluation of a modified early warning system for acute medical admissions and comparison with C-reactive protein/albumin ratio as a predictor of patient outcome. Clin Med 2009;9:30–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].McNelis J, Marini CP, Jurkiewicz A, et al. Prolonged lactate clearance is associated with increased mortality in the surgical intensive care unit. Am J Surg 2001;182:481–5. [DOI] [PubMed] [Google Scholar]
- [35].Basile-Filho A, Nicolini EA, Auxiliadora-Martins M, et al. The use of perioperative serial blood lactate levels, the APACHE II and the postoperative MELD as predictors of early mortality after liver transplantation. Acta Cir Bras 2011;26:535–40. [DOI] [PubMed] [Google Scholar]
- [36].Meyer ZC, Schreinemakers JM, Mulder PG, et al. Determining the clinical value of lactate in surgical patients on the intensive care unit. J Surg Res 2013;183:814–20. [DOI] [PubMed] [Google Scholar]
- [37].Filho RR, Rocha LL, Correa TD, et al. Blood lactate levels cutoff and mortality prediction in sepsis-time for a reappraisal? A retrospective cohort study. Shock 2016;46:480–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


