Abstract
During early development, the fetal brain undergoes dynamic morphological changes. These changes result from neurogenic events, such as neuronal proliferation, migration, axonal elongation, retraction, and myelination. The duration and intensity of these events vary across species. Comparative assessments of these neurogenic events give us insight into evolutionary changes and the complexity of human brain development. Recent advances in magnetic resonance imaging (MRI), especially ex vivo MRI, permit characterizing and comparing fetal brain development across species. Comparative ex vivo MRI studies support the detection of species-specific differences that occur during early brain development. In this review, we provide a comprehensive overview of ex vivo MRI studies that characterize early brain development in humans, monkeys, cats, as well as rats/mice. Finally, we discuss the current advantages and limitations of ex vivo fetal brain MRI.
Keywords: Fetus, Brain, postmortem, ex vivo, MRI, DTI, HARDI, Tractography
1. Introduction – An overview of brain development assessed by structural ex vivo MRI
1.1. An overview of major histogenic events that occur during early brain development
Prenatal development of the human brain is a complex process. The fetal telencephalic wall can be roughly divided into five transient zones (by the Boulder Committee; Bystron et al., 2008). Superficial to the ventricle towards the pia, these zones are: i) the proliferative zones (ventricular and subventricular zones, VZ/SVZ), ii) intermediate zone (IZ, i.e. the fetal white matter), iii) subplate zone (SP), iv) cortical plate (CP), and v) the marginal zone (MZ). These zones change during gestation as assessed quantitatively (e.g. thickness and volume) and qualitatively (e.g. changes in histological properties). Such changes in the appearance of transient fetal zones reflect the intensity of neurogenic events such as neuronal proliferation, migration, axonal ingrowth and outgrowth, neuronal differentiation, as well as areal specialization (Fig. 1). Towards the end of gestation, all key structural components are established in the fetal brain, and the brain continues to reorganize postnatally.
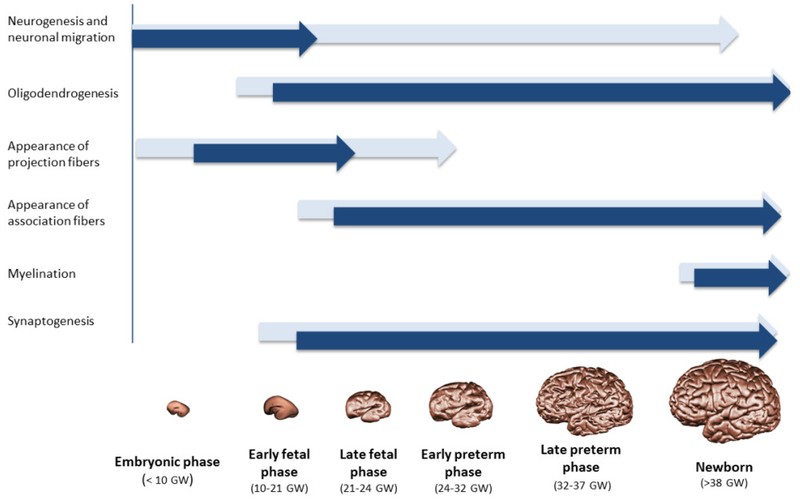
Figure 1.
Summary illustration of the major neurogenic events and their intensities (intensity of the color), which occur during early human brain development. Developmental phases were divided according to classifications of the human brain before 24 GW (Ronan and Müller 2006; Kostovic and Judas 2009; Sidman and Rakic 1973) and after 24 GW (World Health Organization 2012).
Similar to the cerebrum, neuronal proliferation, migration, differentiation, axon growth, synaptogenesis, and pruning also occur during the morphogenesis of the human cerebellum (Sidman and Rakic, 1973; Wang and Zoghbi, 2001; Lavezzi et al., 2006). However, the neurogenic events that occur during cerebellar development continue through the first postnatal year (Altman and Bayer, 1997; Wang and Zoghbi, 2001; Saksena et al., 2008), and occur for longer than those in the cerebrum.
1.2. MRI characteristics of the transient fetal zones
The proliferative zones (VZ, SVZ) contain densely packed cells (neuronal and glial precursors; Rakic, 1973, Kostovic et al., 2002, Radoš et al., 2006). Due to the high density of tightly packed cells, the proliferative zones can be observed from ex vivo MRI as zones with high T1-weighted (T1w) or low T2-weighted (T2w) signal intensities. On diffusion weighted imaging (DWI), these zones are characterized by high fractional anisotropy (FA) (Kostovic et al., 2002, Huang et al., 2006, Radoš et al., 2006, Huang et al., 2009; Huang and Vasung, 2014). The VZ can be discerned from the SVZ only with MRI examinations that have high spatial resolution (e.g. less than 0.2 mm).
The VZ lines the lateral ventricles. The SVZ is initially a thin zone that continues to thicken during mid-fetal stages (Zecevic et al., 2005). Around 13 GW, the SVZ is divided into the inner (cellular) and outer (less cellular) layers by tangential fibers that course between them. On high-resolution MR images, it is easy to recognize this layer of tangential fibers with a low T1w MR signal intensity. However, the identification of the exact borders between the SVZ and other zones (i.e. the VZ and the IZ) remains a challenge. The inner part of the SVZ “blends” with the VZ because they both contain tightly packed cells. On the other hand, the outer part of the SVZ “blends” with the IZ because cells are sparse in both zones. Thus, the delineation of the VZ and the SVZ with ex vivo MRI is not possible with a lower spatial resolution than the thickness of the zone(s) of interest (Vasung et al., 2016).
Axonal pathways (i.e. fetal white matter) span the IZ, which is characterized by a moderate T1w MR signal intensity (Vasung et al., 2016). The IZ is surrounded by the SP zone, which serves as an axonal “waiting compartment” (Kostovic and Rakic, 1990). The IZ and the SP together contain less densely packed neurons, more axonal fibers, and more extracellular matrix compared with the proliferative zones. Compared to the proliferative zones, ex vivo MRI shows that the IZ and SP are characterized by lower or moderate T1w MR signal intensities and higher or moderate T2w MR signal intensities (Kostovic et al., 2002; Radoš et al. 2006; Vasung et al., 2017). Although the SP can be distinguished from the IZ with conventional T1w and T2w MRI, diffusion MRI (dMRI) is superior for characterizing their microstructural compositions. With dMRI, the IZ (composed of compact fiber bundles) has moderate to high FA values, while the SP (containing a high amount of the extracellular matrix and dispersed axonal fibers) consists of low FA values (Fig. 2; Huang et al. 2006; Huang et al., 2009; Wang et al., 2015; Vasung et al., 2017).
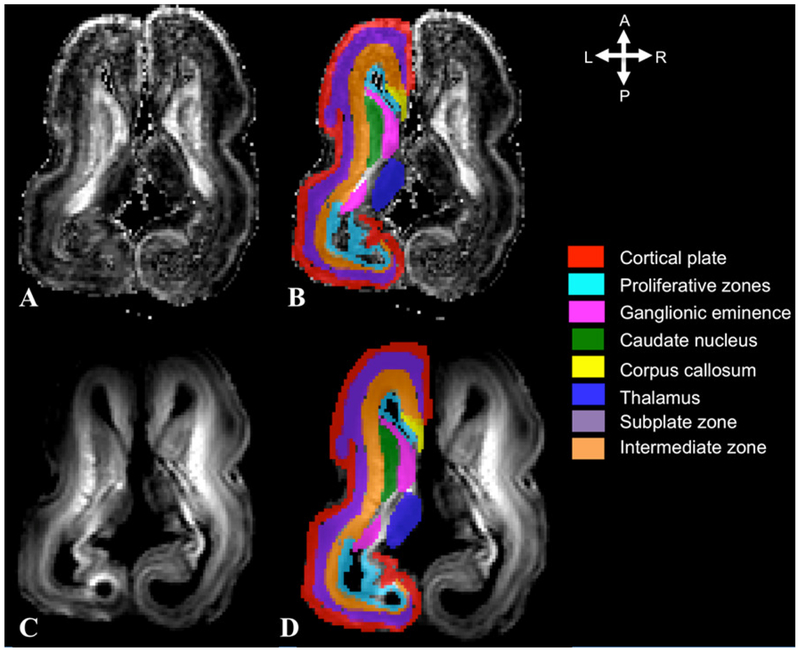
Figure 2. Example of the semi-automatic segmentation of the transient fetal zones during mid-fetal period.
Fractional anisotropy (FA) (A,B) and diffusion weighted MRI (DWI) axial slices (C,D) used for segmentation of the cortical plate (red), proliferative zones (light blue), subplate (purple), intermediate zone (orange), thalamus (dark blue), corpus callosum (yellow), caudate nucleus (green), and ganglionic eminence (GE) (pink). Reproduced from Vasung et al., 2017, with permission.
During the first two trimesters, the CP contains tightly packed post-migratory neurons that are arranged into embryonic columns (Rakic et al. 1988, Jones and Rakic, 2010). During this time, the CP is characterized by high FA values (Fig 3A), high T1w and low T2w MR signal intensities (Huang et al. 2006; Huang et al. 2009; Huang et al. 2012; Kostovic et al., 2002; Radoš et al., 2006). During the last trimester, the dendritic arborizations of the post-migratory CP neurons gradually increase, which results in a gradual decrease of FA values in the CP (McKinstry et al., 2002; Khan et al., 2018; Batalle et al., 2018; Fig 3B).
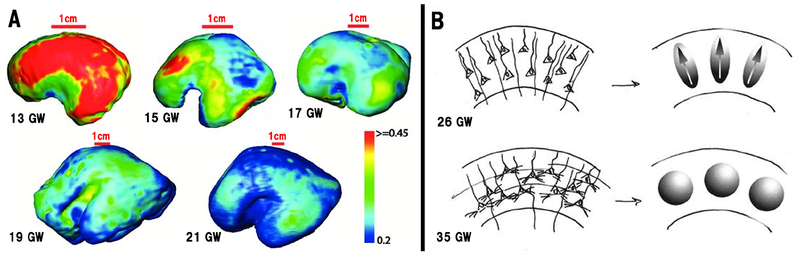
Figure 3. Changes of FA values of the CP during early (A) and the late (B) prenatal development.
(A) FA mapping of the cortical surface of fetal brains at the gestational weeks shown. Color bar indicates FA values in the cortical surface FA maps (Modified from Huang et al., 2012, with permission). (B) A diagram explaining changes in FA values during late prenatal period. Left images are drawings of the cortical structure, while right images are the corresponding diffusion ellipsoids. At 26 GW (upper row), radial glial fibers and pyramidal neurons with prominent, radially oriented apical dendrites are shown. This organization has the effect of restricting water displacement parallel to the cortical surface more than displacement orthogonal to it, resulting in diffusion ellipsoids which are non-spherical with their major axes oriented radially (arrows), which provides high FA values. By 35 GW (lower row), prominent basal dendrites for the pyramidal cells and thalamocortical afferents have been added. This has the effect of restricting water displacement more uniformly in all directions. As a result, the diffusion ellipsoids are spherical, without a preferred orientation, which provides low FA values (Modified from McKinstry et al., 2002, with permission).
Lastly, the MZ is situated directly below the pia. The first synapse formation occurs in the MZ (Molliver et al., 1973). However, it is almost impossible to continuously delineate this zone on MRIs, because the thickness of this zone is often below the typical spatial resolution of ex vivo MRI (e.g. 0.2–1 mm).
1.3. Age-related changes in qualitative MRI characteristics of the transient fetal zones
During prenatal brain development, MR characteristics of the fetal zones (such as FA and MR signal intensities) are not static. Rather, they change with gestational age. It has been suggested that changes in the MR characteristics of fetal zones are related to the intensity of neurogenic events (Kostovic et al., 2002). For example, the high T1w MR signal intensity of the CP, as well as the high FA decrease during the late preterm period (31–36 GW; McKinstry et al., 2002; Ball et al., 2013; Batalle et al., 2018), which coincides with the gradual increase in dendritic arborization of the post-migratory neurons in the CP (Mrzljak et al., 1988; Mrzljak et al., 1991). Thus, it is likely that the increase in dendritic arborization leads to an increase in the distance between the CP neurons restricting the water displacement more uniformly in all directions and resulting in a gradual loss of FA values within the CP (McKinstry et al., 2002).
With ex vivo MRI, the SP can be detected around 15 GW, and reaches its peak thickness between 26 and 32 GW, but then disappears a few months after birth (Kostovic and Rakic, 1990). During the transition from early (25–30 GW) to late preterm (30–36 GW), the SP cannot be identified as a continuous zone. It has been suggested that this change is most likely caused by a relocation of “waiting” axons from the SP to their final destinations in the CP (Kostovic and Judas, 2002, Kostovic and Jovanov-Milosevic, 2006) by the loss of the extracellular matrix and by the reorganization of the fetal white matter into Von Monakow segments (von Monakow, 1905; Kostovic et al., 2014).
1.4. Age-related changes in quantitative MRI characteristics of the transient fetal zones
Similar to the qualitative changes in MRI characteristics of transient fetal zones that are observed during human fetal development, the thickness and volume of fetal zones also change over time. We can consider developmental trajectories of these zones to better understand basic developmental processes (e.g., cell proliferation, migration, axon extension) that occur during human development (Vasung et al., 2016). For example, the surface of the CP increases almost 50 times from 15 to 42 GW, contrasting a very discrete increase in its thickness (sub-millimeter increase, see Fig. 4; Vasung et al., 2016). By 22 GW, the majority of the migrating neurons already reach their final destinations in the CP except in prefrontal regions (Paredes et al., 2016). Thus, before 22 GW, the arrival of the new neurons in the CP might account for the increase in the surface and the thickness of the CP. After 22GW, an increase in the surface of the CP is most likely accounted for by areal differentiation, dendritic arborization, and the ingrowth of axonal fibers (Vasung et al., 2016). Interestingly, although the cortical surface of each lobe grows at differential rates, the percentage of the total cortical surface occupied by the cortical surface of a specific lobe remains constant across gestational ages (Vasung et al., 2016). As another example, the SP peaks in volume and thickness during the axonal “waiting” period (Fig. 4, Kostovic and Judas, 2002; Vasung et al., 2016). As development progresses, the SP wanes and axons invade the CP (Kostovic and Judas, 2002).
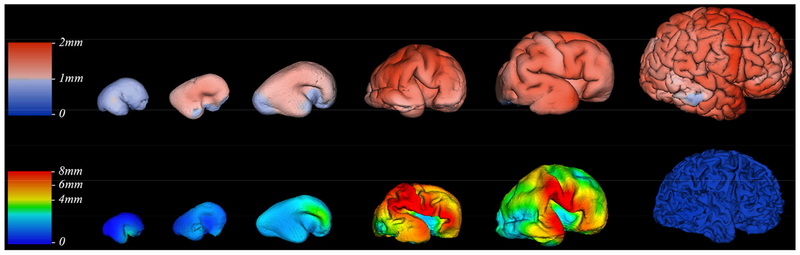
Figure 4. Thickness of transient fetal zones.
Thickness of cortical plate (upper row) and SP (bottom row) measured in millimeters (color coded bars) at 15, 18, 20, 26, 32, and 42 GW (left to right). Reproduced from Vasung et al., 2016, with permission.
From 15 to 22 GW, the relative volume of the proliferative zones (i.e. the percentage of the hemispheric volume occupied by these zones) decreases exponentially (Vasung et al., 2016), which coincides with the cessation of neurogenesis. Thus, the quantitative and qualitative MRI characteristics of the fetal transient zones most likely reflect the changes in neurogenic events that occur within these zones. However, the detailed information on neuronal migration and axonal outgrowth/ingrowth cannot be characterized with MR measurements of the fetal transient zones. Therefore, we will discuss the potential, advances, and limitations of ex vivo dMRI, dMRI tractography, T1w, and T2w MRI, to characterize in detail, the dynamic processes that occur during fetal brain development.
2. Technological considerations of Ex Vivo fetal MRI
2.1. Advantages of the ex vivo MRI compared to in vivo MRI
Ex vivo MRI provides structural and diffusion images of the specimen scanned at high spatial resolution (e.g. 500um or higher) and high signal-to-noise ratio (SNR). The high SNR and spatial resolution are more easily achieved by using high-field strength MRI scanners and using custom-made radio-frequency (RF) coils that more tightly fit the specimen. These coils can be single-channel surface or volume coils (for small specimens) or multi-channel phased-array coils (for large specimens). In addition, compared to in vivo MRI where scan times are limited by the subject’s ability to remain still during a typical exam of less than 1.5 hours long, ex vivo MR scan times often take many hours to boost SNR, and are limited almost only by the MR scanner’s availability and the costs for its use. Therefore, both structural and diffusion ex vivo MRI scans can detect detailed information about tissue properties that go unseen on in vivo imaging.
Aside from the lower spatial resolution, the quality of in vivo MR images may be compromised by artifacts due to subject’s motion. Strong susceptibility effects are typically observed around ears and nasal cavities (i.e. in areas with air-tissue boundaries). Thus, it is sometimes difficult to identify subtle cortical abnormalities or abnormal fiber pathways from in vivo MRI. Ex vivo MRI does not suffer from motion artifacts, and susceptibility artifacts can be mitigated by proper and careful preparation of the specimen (i.e. avoiding the creation of air bubbles). Therefore, careful assessment of high-quality ex vivo MR images in combination with histology can provide a basis for detection of subtle cortical abnormalities using T1w and T2w MRI, and abnormal development of fiber pathways using dMRI. These findings can be used to guide the detection of cortical abnormalities or abnormal fiber pathways in vivo. Similarly, if cortical abnormalities or abnormal fiber pathways are detected in vivo, then ex vivo MRI studies along with histopathology analyses can be used to confirm these findings. For example, when we see an abnormal fiber pattern in vivo, and if ex vivo dMRI tractography also detects the same fiber pattern, the in vivo pathways would likely correspond to “real” pathways and would not likely be artifacts due to noise and/or dMRI tractography algorithms.
Finally, spatiotemporal changes in anatomy of the human fetal brain as assessed from ex vivo MRI results are the product of complex processes that span spatiotemporal changes in gene expression and epigenetic modifications during development (Huang et al., 2013). Spatiotemporal changes in gene expression do vary across species, and it would be of interest to investigate the relationship between spatiotemporal changes in gene expression and diffusion MR tractography to better understand basic developmental processes that occur during human brain development (for review, see e.g. Judaš, 2011; Kang et al., 2011; Pletikos et al., 2014; Charvet et al., 2018). Combining ex vivo MRI with genetic and histological analyses is the first step towards a better understanding of the impact that gene expressions can have on regional morphometry and fiber growth/retraction.
2.2. Advances in ex vivo Structural MRI
As mentioned above, numerous neurogenic processes occur during prenatal brain development. These processes are under strong genetic influences (Pletikos et al., 2014), and may be associated with MR signal intensity or dMRI measurements (Kostovic et al., 2002; McKinstry et al., 2002; Wang et al., 2015; Lefèvre et al., 2016). The majority of in vivo fetal MR imaging protocols use single-shot T2w MRI sequences (e.g. Prayer et al., 2006), as they are more robust to fetal motion. However, the single-shot T2w scans tend to be inferior to multi-shot turbo-spin-echo (TSE) scans, which are often performed postnatally. Moreover, direct employment of the TSE protocol used in vivo may not be optimal to characterize transient zones found in ex vivo fetal brains. This is especially the case during the last phases of fetal development, which is most likely due to differences in tissue properties between in vivo and ex vivo brains given that ex vivo brain are fixed. One way to deal with this issue is to acquire T2 maps on postmortem samples to infer the best imaging parameters of the TSE that would maximize the T2 contrast. In this review, we focus on T1w MRI and dMRI properties of the developing ex vivo fetal brain.
2.3. Advances in ex vivo Diffusion MRI
dMRI is useful for detecting microstructural diffusion properties in tissue. Using tractography techniques, we can identify pathways in the entire brain mantle in a 3-D manner. High-angular resolution diffusion MRI (HARDI) (Tuch et al., 2002, 2003) with optimal scanning parameters (such as e.g. high b values) theoretically provides exceptional angular resolutions of fiber pathways that traverse long distances throughout the brain, and disambiguating fiber trajectories at points of intersection (crossing fibers). Given that many imaging voxels likely contain multiple fiber orientations, the use of HARDI is an important solution for finding accurate pathways in the brain. However, although limitations have been increasingly reported for traditional diffusion tensor imaging (DTI) tractography, it is still useful depending on the pathways of interest and the goal of the research study (e.g. Song L et al., 2017).
Spatiotemporal developmental time courses of the maturation of the fornix, cingulum bundle, corpus callosal and thalamocortical pathways, inferior longitudinal, inferior fronto-occipital, uncinate, and arcuate fasciculi, as well as insular, cerebellar, and many other pathways in human fetal ages have been described with the use of HARDI (Takahashi et al., 2012, 2014; Song et al., 2015; Wilkinson et al., 2016; Vasung et al., 2017; Das and Takahashi, 2017). Although ex vivo dMRI has produced an exceptional resolution of fiber pathways, it is possible to achieve comparable results using in vivo imaging (e.g. Xu et al., 2014). Specifically, we imaged in vivo and ex vivo human fetal brains, and found that radial coherence of the cerebral cortex coincides with the presence of radial glial fibers (Xu et al., 2014). The transformation of these radial glia into astrocytes also coincides with the emergence of cortico-cortical coherence identified with HARDI tractography. Importantly, this transition was viewed in both ex vivo and in vivo brains at the same timing in the studied brain region. Reproducibility between ex- and in vivo results were also reported by several groups (Edlow et al., 2012; Wedeen et al., 2012). Such accurate, high-resolution ex vivo tractography data provides an excellent reference for in vivo tractography. Information regarding the degree to which in vivo results can reproduce ex vivo results will be important for clinical interpretation of this technique.
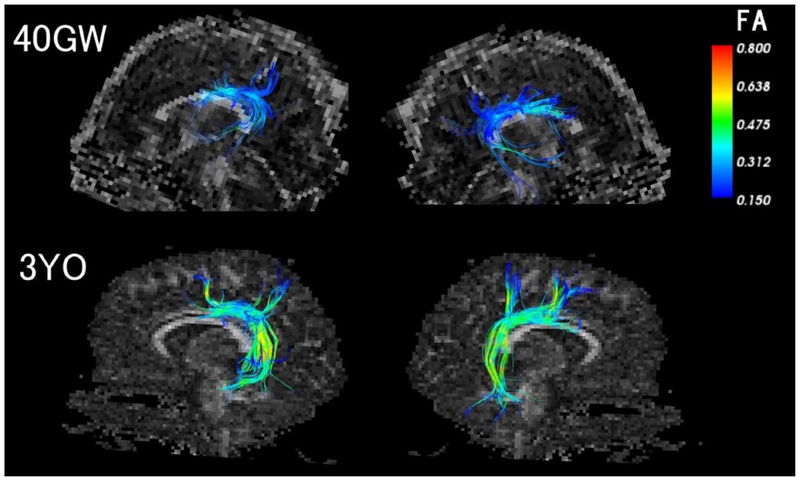
The majority of the current diffusion MR tractography studies use a thresholding system based on FA (typically a pre-set FA value, e.g. 0.2) to terminate fibers in the gray matter. However, areas with low myelin and areas that span crossing fibers in the developing brain have low FA values, which may potentially incorrectly terminate pathways in select brain regions. We have reconstructed fibers without setting FA thresholds, and successfully detected immature emerging fiber pathways across a number of studies (e.g. Takahashi et al., 2012, 2014). For example, we reported that the majority of the arcuate fasciculus pathways at 40 GW had low FA values (i.e., FA values lower than 0.2; Fig. 5; Wilkinson et al., 2017). Tractography based on FA values could cause errors in reconstructing the arcuate fasciculus during prenatal and postnatal development. At 3 years old and later, peripheral arcuate pathways showed FA values lower than 0.2 (Fig. 5; Wilkinson et al., 2017). Therefore, it is critical to consider the use of an FA threshold to accurately detect full length/volume of developing pathways.
Figure 5. Color-coded fractional anisotropy (FA) values on arcuate fasciculus in 40 GW and 3 years old subjects.
Note that regions with FA values lower than 0.15–0.2 are observed in the majority (40 GW) and peripheral regions (3 years old) of the arcuate fasciculus that cannot be detected with popular DTI analysis methods with an FA threshold (Modified from Wilkinson et al., 2017, with permission). In addition to other technical issues including the quality of MRI scan acquisition and analysis, thresholding parameters based on FA values are important factors when performing tractography.
3. Neuronal Migration Pathways
3.1. Radial and tangential migration pathways in humans.
Two critical processes occur in the second and third trimesters: migration of neurons and development of axonal connections, along with considerable growth of the brain (Rakic et al., 1972; Caviness et al., 1996; Volpe, 2008). During corticogenesis, neuronal subtypes differ in both their origins and migrational trajectories. Future excitatory glutamatergic projection neurons originate from the proliferative dorsopallial VZ/SVZ, and migrate along radial glial fascicles to the CP (Rakic, 1972; Rakic, 1988; Bystron et al., 2008). Previous research used vimentin immuhistochemistry to identify radial glial cells in the developing human visual cortex at successive stages of development (i.e., GW 14, 19, 36, and 79: post-term). The authors found that the number of radial glial cells in the visual cortex decreased after 36 GW, and was concomitant with a loss of radial structure (Honig et al., 1996). However, that study had a large sampling gap between 19 GW and 36 GW, an interval of particular interest in the field of normal brain development as well as preterm brain injury. Moreover, their analyses were focused on the primary visual cortex, and it is not clear whether these observations hold across the entire developing cortex. The other, major migratory routes for neurons are mainly from the ganglionic eminence (GE) that course tangential to the CP, containing future inhibitory GABAergic interneurons (Wonders et al., 2006; see also 3.2.). Altered neuronal migration has been implicated in a range of neurological and psychiatric disorders (e.g. Gressens, 2006; Volpe et al., 2009; Benes et al., 2001; Marín et al., 2001, 2003). Given the clear distinction between murine and primate migratory patterns, and increasing evidence suggesting that certain aspects of neuronal migration are unique to human corticogenesis (e.g. Letinic et al., 2001), there is a clear necessity to investigate migratory patterns directly in the human brain. However, due to limitations inherent to microscopic studies, only small regions of migratory streams can be investigated through histological assessment alone. A systematic assessment of the migratory streams present at any fetal stage is still lacking.
Traditionally, dMR tractography has been used to detect white matter (axonal) pathways of adult brains. However, since dMRI detects directions of water diffusivity in the tissue, it is likely to also detect glial scaffoldings for neuronal migration in fetal ages. A few groups have imaged diffusion coherence of the migration pathways and changes in the radial organization of the CP by using conventional DTI (e.g. Huang et al., 2009; Huang et al., 2013; Yu et al., 2016; Song et al., 2017). Figure 6 demonstrates that the HARDI tractography technique (Tuch et al., 2003; Wedeen et al., 2008) can clearly identify pathways likely related to radial neuronal migration (a part of the data shown in Fig. 6 was used in Takahashi et al., 2012). Recently, one of our research studies (Xu et al., 2014) compared HARDI tractography with immunomarkers to identify radial glial fibers, axons, and blood vessels across many developmental time points in human fetuses. We showed that, at mid-gestation, radial coherence is concomitant with the presence of radial glial fibers, At 30–31 GW, the transition from HARDI-defined radial pathways to cortico-cortical pathways began simultaneously with the transformation of radial glial fibers to astrocytes. By term, both radial HARDI pathways and radial glial fibers had disappeared (Fig. 6D). With histology, white matter axons were radial, tangential, and oblique over the second half of gestation, whereas penetrating blood vessels were consistently radial. Thus, we concluded that radial HARDI pathways in the fetal white matter likely reflects a composite of radial glial fibers, penetrating blood vessels, and radial axons of which its transient expression most closely matches that of radial glial fibers (Xu et al., 2014).
Figure 6. Radial coherence of the telencephalic fetal wall.
Pathways coursing through a sagittal slice at 17 GW (A), 20 GW (B), 31 GW (C), and 40 GW (D). (Modified from Takahashi et al., 2012, with permission)
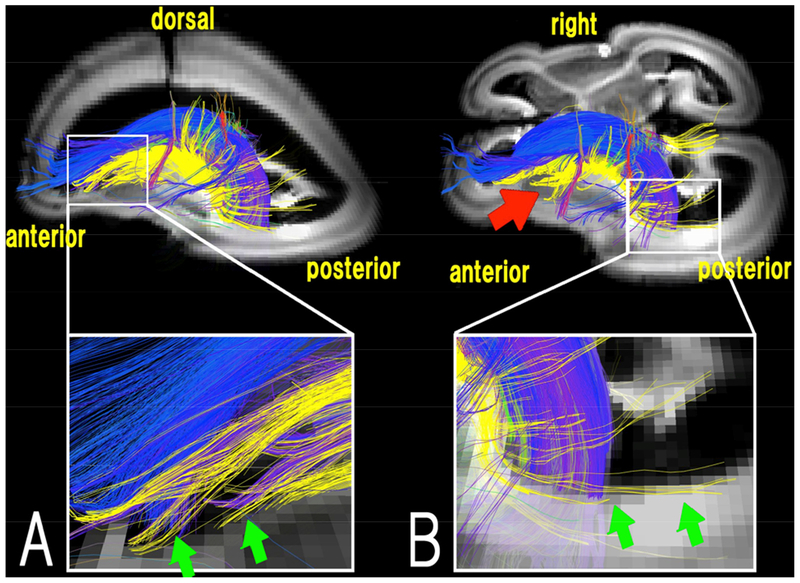
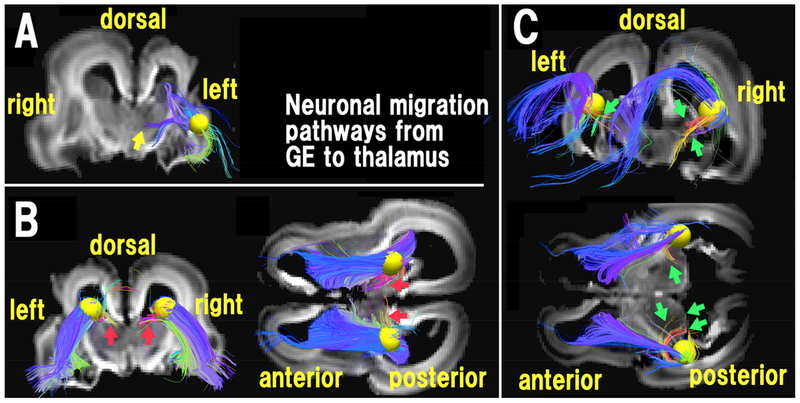
We have recently further differentially identified radial pathways between different fetal transient zones (Vasung et al., 2017), as well as pathways likely originating from the VZ to the insular and surrounding cortices (Das and Takahashi 2017) and the thalamus (Fig. 7; Wilkinson et al., 2016). Yellow pathways in Figure 7 were identified as pathways from or to the thalamus, which can be seen originating from the VZ. These results are the first to clearly show, in a 3-D manner, tractography streamlines stretching from proliferative zones to subcortical nuclei in humans, which most likely correspond to neuronal migration pathways. In addition, we have also identified tractography streamlines that likely reflect neuronal migration pathways from the GE (Fig. 8; Wilkinson et al., 2016; see also Miyazaki et al., 2016), and those within the cerebellum (Takahashi et al., 2014).
Figure 7. Thalamic pathways.
Oblique sagittal (A) and oblique axial (B) views of a 17 GW old brain. Pathways from/to the thalamus are shown in yellow and pathways linked to the ganglionic eminence (GE) are color-coded based on the standard RGB code to show the spatial locations of terminal regions of each pathway (red: right-left, green: dorsal-ventral, blue: anterior-posterior). Red arrow: pathways from/to the thalamus running vertically from the GE, towards the surface of the brain. (Modified from Wilkinson et al., 2016, with permission)
Figure 8. Three distinct diffusion MR tractography pathways between the GE and the thalami at 18 GW.
(A) Pathways running through a yellow sphere placed in an anterior ganglionic eminence (GE) region. The anterior GE region was connected to middle regions of the thalami (yellow arrow). (Modified from Wilkinson et al., 2016). (B) Pathways running through yellow spheres placed in a dorsal GE region. Coronal (left) and axial (right) views are shown. The dorsal GE region was connected to dorsal superficial regions of the thalami (red arrows). (C) Pathways through yellow spheres placed in mid-posterior GE regions. The mid-posterior GE regions were connected to posterior and middle parts of the thalami, corresponding to the ventral complex (green arrows).
It is possible to resolve cerebellar pathways more accurately using size-optimized coils at higher spatial resolution. Erroneous fiber pathways tend to be detected in the cerebellum when scanned using a large coil for a whole brain with the cerebrum attached (Takahashi et al., 2014). After dissecting the cerebellum from the cerebrum, SNR, spatial resolution and diffusion MR tractography outcomes significantly improved using a size-optimized coil for each cerebellum-only sample (Takahashi et al., 2014), identifying longer, more coherent tractography pathways. Neuronal migration-related radial pathways in the cerebellum at 17 GW were also clearly identified with the size-optimized coils, which disappeared by 38 GW, being replaced by horizontal pathways in the cerebellar cortex (Takahashi et al., 2014).
3.2. Developmental processes accounting for the expansion of GABAergic and pyramidal neuron numbers in primates
During brain development, pyramidal neurons and some GABAergic neurons situated in the adult cortex are generated, from distinct regions of the telencephalon (Lavdas et al., 1999; Ang et al., 2003; Letinic et al., 2002; Nery et al., 2002; Fig. 9). Pyramidal neurons arise from the developing cortical proliferative pool and migrate to the CP along radial glia according to the well-known ‘inside out’ sequence (Rakic, 1974, 2003), which can be visualized with ex vivo dMR scans (Takahashi et al., 2011, 2012). In mice and primates, many GABAergic neurons originate from the GE and migrate to various layers of the developing cerebral cortex (Lavdas et al., 1999; Ang et al., 2003; Letinic et al., 2002; Nery et al., 2002). Interestingly, a recent study showed that the migration of GABAergic interneurons in humans extends into the postnatal period (Paredes et al., 2016). Whether GABAergic neuron migration extends for an unusually long time in humans compared to rodents is an open question. Diffusion and structural MRI scans can be used to track the maturation of future GABAergic and pyramidal neurons during neurogenesis, their migration to the CP (Takahashi et al., 2011, 2012), and compare the timing of neuron production in humans relative to other species (Fig. 9).
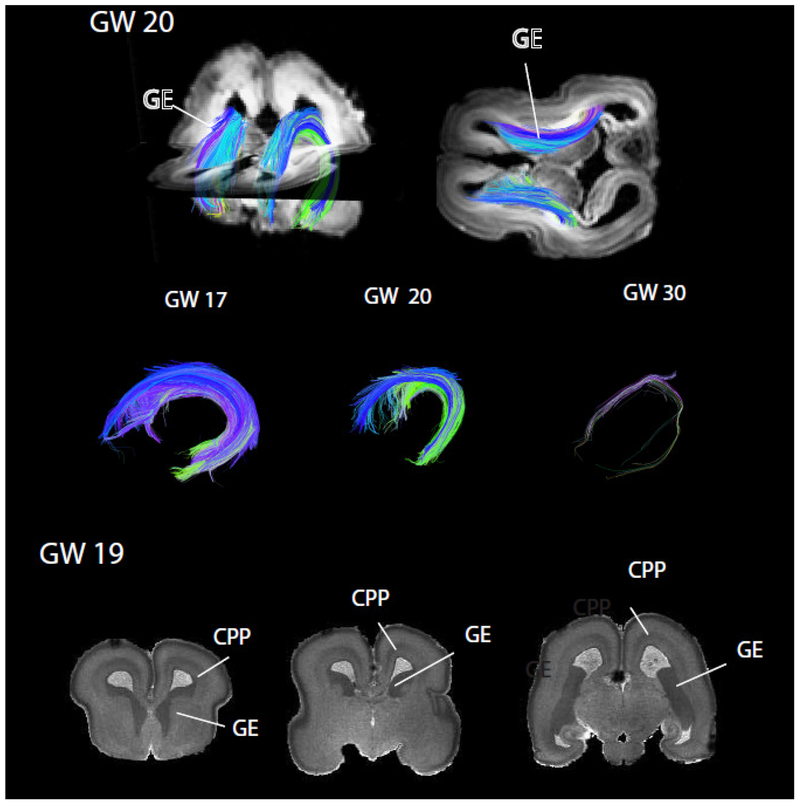
Figure 9. Diffusion MR tractography of the ganglionic eminence (GE) from gestational week (GW) 17 to 30 in humans.
The GE wanes between GW 20 and 30. Coronal planes from a structural T1w MRI scans of a human at GW19 show cell dense regions consisting of the GE and cortical proliferative pool (CPP). Comparing the growth of these two proliferative pools throughout development in humans, macaques, and mice permits identifying evolutionary changes in neurogenesis timing across species. The structural MRI scan is made available by the Allen Institute for Brain Science (Miller et al., 2014), and is available at: http://download.alleninstitute.org/brainspan/MRI_DTI_data_for_prenatal_specimens/. Image credit: Allen Institute.
The GE and cortical proliferative pool are known to contain proliferative cells in primates as in rodents even though the cortical proliferative pool of primates is expanded relative to rodents (Kriegstein et al., 2006; Lukaszewicz et al., 2005; Workman et al., 2013; Charvet et al., 2017b). Throughout development, progenitor pools grow, peak and wane as the cells exit the cell cycle (Charvet et al., 2017b, Vasung et al., 2016). Mitotic markers are expressed in the GE and cortical proliferative pool throughout development (Lukaszewicz et al., 2005; Charvet and Striedter, 2008; Cheung et al., 2009; Del Bigio, 2011; Chen et al., 2017). In mice, the GE grows and peaks in size at approximately embryonic day (E) 15.5, after which the GE wanes as cells exit the cell cycle and migrate to their final destination. A recent study used single cell RNA sequencing from the mouse medial GE at several stages of embryonic development and showed that the number of immature neurons increased significantly from E15.5 to E17.5 in the medial GE (Chen et al., 2017). The increase in post-proliferative neurons in the medial GE coincides with the decline in GE size in mice (Charvet et al., 2017b). Ex vivo diffusion MR scans of human fetuses are instrumental in identifying whether neurogenesis is protracted in humans relative to other mammals.
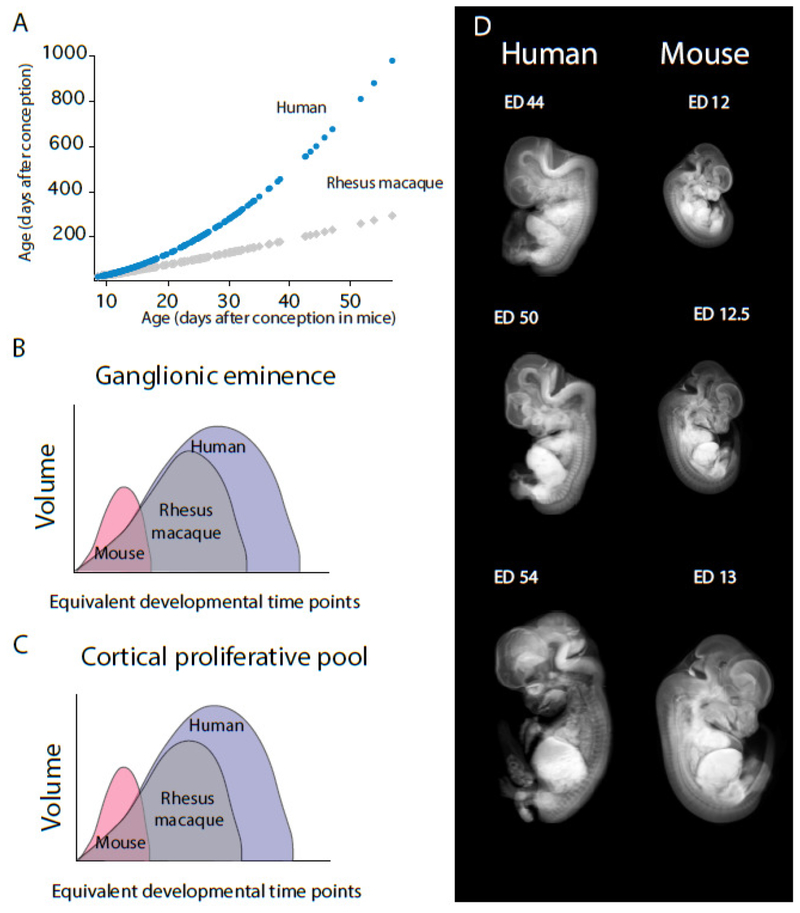
A comparative analysis of growth trajectories of the GE and cortical proliferative pool requires a comparison of equivalent developmental time points between species given inter-species variation in overall developmental schedules. The timing of maturational milestones can be used to identify equivalent development time points across a broad range of species, including humans, macaques, and mice (Clancy et al., 2001; Workman et al., 2013; Charvet et al., 2017b; Charvet and Finlay, 2018, Charvet et al., 2018; Fig. 10). For instance, the timing of developmental transformations in macaque and humans is plotted against equivalent developmental transformations observed in mice, which permits identifying corresponding ages across species (Fig. 10). Once overall changes in developmental schedules are controlled for, the study showed that the GE and cortical proliferative pool grow for much longer than expected in humans and macaques relative to the timing of other developmental events (Charvet et al., 2017b; Fig. 10). High angular resolution diffusion MR scans confirm the growth trajectories of the GE in a different set of human fetuses (Fig. 9). Accordingly, humans and macaques extend isocortical and GABAergic neurogenesis for longer than expected considering the timing of other developmental transformations. The consequence of extending the duration of neurogenesis is that pyramidal and GABAergic neurons are amplified in the primate cortex. A concomitant increase in the number of pyramidal and GABAergic neurons may be necessary for the cortex to remain functional in primates.
Figure 10. Extended duration of cortical and GE neurogenesis in primates versus rodents.
(A) The timing of developmental transformations (expressed as age in days after conception in macaques and humans) is regressed against equivalent developmental transformations found in mice (Workman et al., 2013; Charvet et al., 2017b, Charvet and Finlay, 2018; Charvet et al., 2018). The timing of developmental transformations is instrumental in identifying corresponding ages across species. Such an approach permits identifying variation in the timing of select developmental processes after controlling for variation in developmental schedules across species. Using this approach, Charvet et al. (2017a) found that the ganglionic eminence (GE) (B) and cortical proliferative pool (C) grow for significantly longer in humans and macaques once overall differences in the duration of developmental schedules are controlled for (see Workman et al., 2013; Charvet et al., 2017a; Charvet and Finlay, 2018; Charvet et al., 2018). (D) Examples of equivalent developmental time points are shown from reconstructed structural MRI scans and micro CT-scans. Micro CT-scans of prenatal mice are from Wong et al., 2012. Structural MR scans of prenatal humans are from the multi-dimensional human embryo project (http://embryo.soad.umich.edu/index.html).
The maturation of the GE and cortical proliferative pool is protracted in primates relative to mice and the timing of post-neurogenetic maturation of GABAergic and pyramidal neurons is conserved in primates as in rodents. The timing of tract formation and the time course in the emergence of genes and proteins expressed by GABAergic and pyramidal neurons show no evidence of a delay in primates relative to the timing of other events (Charvet et al., 2017b). The dissociation between protracted timing of neurogenesis and the conserved timing of post-neurogenetic maturation could facilitate cross-synaptic maturation across brain regions and explain structural differences during brain development between primates and rodents. For instance, it has been suggested that the emergence of tracts coursing between the inner and outer SVZ during fetal development is unique to primates and to some extent carnivores (Smart et al., 2002). Accordingly, the outer SVZ would represent a relatively distinct progenitor pool population in primate evolution. Given that the timing of post-neurogenetic maturation is decoupled from neurogenesis timing, we suggest that the formation of tracts coursing through the inner and outer SVZ in primate development could emerge as a result of the extension in cortical cell proliferation coupled with the conserved timing of tract formation. Tracts coursing between the inner and outer subventricular might appear unusual in primates because neurogenesis extends during the growth of tracts whereas neurogenesis largely wanes during these equivalent developmental time points in mice.
4. Axonal Development.
4.1. Axonal pathways in humans
From histological observations, the thalamic afferent fibers grow into the CP at 26–32 GW, while the cortico-cortical pathways grow into the CP around 32–38 GW, i.e. after they “wait” in the SP for an extended time (Kostovic and Jovanov-Milosevic, 2006; Kostovic et al., 2010; see also Fig. 11). Therefore, the majority of the axonal trajectories are already formed prior to these gestational ages. Although the relative timing of development of these pathways is known, we still do not know the regional variation in their development throughout the brain. Conventional structural MRI and DTI have detected projection pathways appearing at 22–26 GW (for review see Kostovic and Jovanov-Milosevic, 2006). DTI has been used to study developing white matter properties in both the animal (Zhang et al. 2003, 2005; Huang et al. 2006, 2008; Kroenke et al. 2007; D’Arceuil et al. 2008; Kuo et al. 2008) and the human fetus (Huang et al. 2009). Some research studies using diffusion MR tractography (Zhang et al. 2003; Huang et al. 2006, 2009; Dubois et al., 2006; D’Arceuil et al. 2008) have shown the development of the major, thick white matter pathways. However, these studies have all used DTI with its limitations. Thus, they could not display the full lengths of fiber pathways and none of these studies were able to describe the precise time-courses of the many brain pathways.
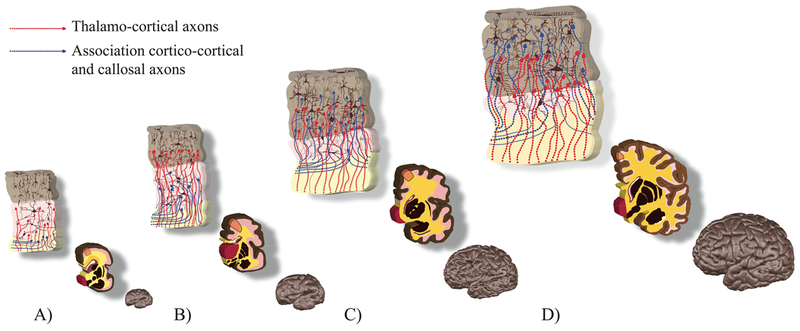
Figure 11. Summary illustration of transient fetal zones at 27 (A), 32 (B), 36 (C) and 42 (D) GW.
Upper and middle row: the cortical plate in brown, the subplate in pink, the intermediate zone in yellow. Major histogenetic events (upper row) are orange insets from the middle row: neurons in brown, thalamo-cortical axons in red, cortico-cortical and callosal axons in blue. Associated changes in cortical morphology (gyrification and surface growth) can be seen in the bottom row. Dendritic arborisation of cortical and subplate neurons is painted according to (Mrzljak et al., 1988, 1992). Development of cortical connectivity was illustrated according to the work of Kostovic and group (for reviews see (Kostovic and Jovanov-Milosevic, 2006; Kostovic and Vasung, 2009; Vasung et al., 2010)). Upper and middle rows were painted using the template coronal slices of the brains used in Vasung et al., 2016.
Studying human fetal brain connectivity remains a challenging task. The majority of information describing the development of fetal connectivity is from axonal tracing or histological studies. However, there are only a few studies employing tracing techniques, as these require short postmortem intervals on already limited fetal material. Similarly, standard techniques of histological fiber staining do not stain fibers during the early developmental phases. Therefore, descriptions of fiber development employing histology have relied on the histological staining of neurons (pale areas denote areas of developing fiber pathways, e.g. see Vasung et al., 2010) or histological techniques that stain transient chemical properties of these fibers (e.g Kostovic and Goldman-Rakic, 1983). Lastly, unlike in the adult brain, directionality of fiber growth in the fetal brain can be deducted from histological stained sections, as chemical properties of these fibers change gradually from their origin towards the target.
The first connections that develop in the fetus belong to the major projection pathways (His, 1904, Hochstetter, 1919). Basal forebrain axons, arising from the magnocellular cholinergic neurons (Kostovic, 1986), are observed as early as 10–11 GW as they course through the external capsule. During this time, the thalamo-cortical axons span the telencephalon-diencephalic junction, and are evident by cholinesterase staining (Kostovic and Goldman-Rakic, 1983). Thus, as early as 12 GW, one can reliably identify components of the external and internal capsule in the fetal brain. Aside from projection fiber pathways, the existence of the fornix, most likely containing septo-hippocampal fiber pathways (Kostovic et al., 1980), has also been reported at this stage (His, 1904, Hochstetter, 1919). At this stage, pyramidal neurons, whose axons will form association fibers, have not yet migrated (Rakic, 1974).
Thirteen GW marks an important phase in the developing fetal brain. At this time, the SP can be identified in histological sections (Molliver et al., 1973, Kostovic and Rakic, 1990). However, SP can be detected on high-resolution structural MRI within 15 GW (Kostovic et al., 2002). During the following weeks there is increased growth of the efferent fiber pathways as some of the lower layer neurons of the cerebral cortex might have already reached their destinations. There are several DTI-based findings during this period. The largest fiber pathway at this stage seems to be the corpus callosum (Vasung et al., 2010). In addition, thick cortico-pontine pathways, as well as limbic fiber pathways (Vasung et al., 2010), are evident from tractography reconstructions.
From 20 to 26 GW the volume of the SP zone, axonal “waiting compartment” (Kostovic and Rakic, 1990, Kostovic et al., 2014), continues to increase with age. This period is marked by the massive growth of the axonal bundles that span the cortex. Using DTI and HARDI, our own (Takahashi et al., 2012; Wilkinson et al., 2016; Vasung et al., 2017) and another (Huang et al., 2006, 2009) group has successfully identified the following projection fiber pathways: cortico-ponto-cerebellar and fibers related to pons, basal ganglia fibers (belonging to the fasciculus subcallosus-Muratoff bundle or external capsule), thalamic, and basal forebrain fibers. However, at this stage these fibers are seen mostly approaching or terminating within the SP (Vasung et al., 2017). The fetal telencephalon also displays strong radial coherence at this stage (Takahashi et al., 2012, Vasung et al., 2017). In parallel to the ongoing process of neurogenesis, some of the association fiber pathways, which are still in formation, can be recognized at these ages (e.g. inferior longitudinal fasciculus, fronto-occipital fasciculus, middle longitudinal fasciculus, and vertical occipital fasciculus) (Takahashi et al., 2012; Song et al., 2015; Vasung et al., 2017; e.g. Figs. 6, 11).
From 26 GW onwards, the thalamic afferent fibers that were “waiting in the SP”, relocate to the CP (Kostovic and Jovanov-Milosevic, 2006). This thalamo-cortical relocation has been confirmed by a DiI tracing study in the fetal brain (Hevner, 2000). This period is also marked by an exponential increase in white matter volume (Vasung et al., 2016), which is most likely caused by rapid outgrowth of long and short associational cortico-cortical pathways (Huang et al., 2006, Huang et al., 2009, Takahashi et al., 2012, Vasung et al., 2017; e.g. Figs. 6, 11). Similarly, during this period the corpus callosum continues to grow and develop (Vasung et al., 2017), while the telencephalon gradually loses its radial coherence (Takahashi et al., 2012, Vasung et al., 2017).
Although we are on the verge of identifying the spatio-temporal sequence of gradual maturation in fibers of the fetus, it should be noted that most of these findings come from DTI imaging which suffers from numerous limitations (Jones et al., 2013, Maier-Hein et al., 2016). Thus, until we have a “ground truth” for diffusion and tractography models (i.e. polarized light imaging, Palm et al., 2010), we should be cautious in interpreting these results, and should rely on knowledge from histology.
4.2. Evolutionary changes in cortical connectivity patterns across mammals: what is special about primates?
There are a number of differences in brain connectivity patterns between primates and other mammals. For instance, diffusion MR scans and tract-tracers have identified cross-cortical projections between auditory and frontal regions of the cortex called the arcuate fasciculus in primates (Deacon, 1992; Rilling et al., 2008; Schmahmann and Pandya, 2009). The arcuate fasciculus has been observed in Old World monkeys, apes, and humans but has not been observed in other mammals (Schmahmann and Pandya, 2009). Notably, the arcuate fasciculus is expanded in humans relative to other primates, which suggests that the arcuate fasciculus evolved within the primate lineage, and that it may have been expanded to process information related to language. Species differences in gene expression patterns can also be used to identify evolutionary changes in connectivity patterns (Hof et al., 1995; Zeng et al., 2012; Krienen et al., 2016; Charvet et al., 2018) because neurons can be classified according to the genes that they express. This approach is indirect in that it provides no information as to the precise terminations of cell types. Thus, there are a number of complimentary venues through which to study evolutionary changes in connectivity patterns.
For the most part, evolutionary changes in connectivity patterns have been challenging to identify because of the difficulty in quantifying connectivity patterns, and resolving the precise terminations of tracts. Combining results from diffusion MR tractography of ex vivo brains, gene expression, and stereological methods across cell types can be used to identify evolutionary changes in cross-cortical connectivity patterns in adulthood and the developmental processes that generate them (Charvet et al., 2018). A focus on human as well as non-human primates is especially valuable because the study of the non-human primate brain yields insight into the evolutionary history and the context in which the human brain evolved.
Cortical neurons exhibit stereotypical patterns of projections, which vary according to their position across the depth of the cortex and the genes that they express (DeFelipe et al., 2002; 2013; Hevner et al., 2003; Molnár and Cheung, 2006; Molyneaux et al., 2007). Tract-tracing studies have shown that superficial layers (i.e., layers II-III) preferentially project within and across cortical regions (Fitzpatrick and Imig, 1980; Foster et al., 1981; Joschko et al., 1987; Coogan and Burkhalter, 1990). Layer IV neurons preferentially project locally whereas many layer V-VI neurons preferentially project to subcortical structures (Jones and Burton, 1976; Kawamura 1973a, b, c; Barbas, 1986; Striedter 2005; Schmahmann and Pandya 2009). Within each of these layers, cortical neurons can further be classified according to the neurotransmitters that they express (DeFelipe et al., 2013). Many cortical pyramidal neurons project over long distances through the cortical white matter to target cortical or subcortical structures. In contrast, cortical GABAergic neurons are inhibitory and typically form local circuits within the grey matter of the isocortex. The position of a neuron across the cortical depth can be used to predict its projection patterns and the suite of genes that it expresses.
There is extensive conservation in stereotypical projection patterns across mammals though there are notable differences in the population of these cell types. For example, primates exhibit a well-defined layer IV and relatively thick upper layers compared with many other mammals (Hustler et al., 2005; Fig. 12). A previous study compared layers II-IV neurons across a broad range of primate (e.g., Old World, New World, lemurs, prosimians) and non-primate species (e.g., rodents, carnivores, and Afrotheria). After controlling for brain size, primates possess disproportionately more layer II-IV neurons compared with rodents and other mammals (Charvet et al., 2017a). There is only a slight relative increase in the percentage of GABAergic interneurons in the cortex of primates relative to rodents such that the amplification of layers II-IV neurons in primates is concomitant with an absolute increase in GABAergic interneuron and pyramidal neurons relative to many other mammals (Beaulieu et al., 1992, 1993; Džaja et al., 2014). Given that layers II-IV consist of GABAergic and pyramidal neurons, the human and non-human primate cortex should show major differences in cortico-cortical circuits relative to other species.
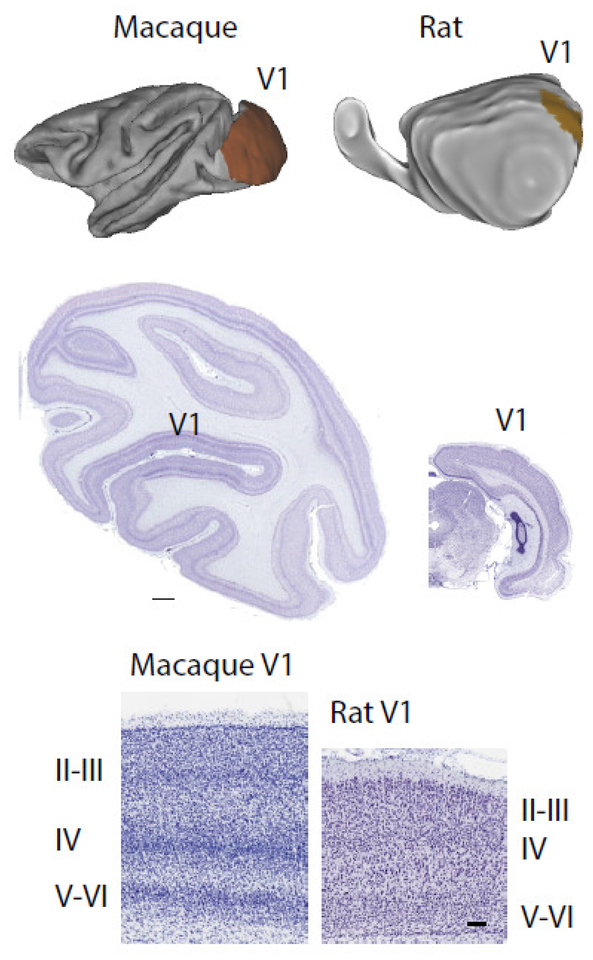
Figure 12. Structural differences between the primary visual cortex of macaques and rats.
Top panel shows the cortex of a macaque and a rat, and the primary visual cortex is highlighted in both species. Coronal sections through the cortex of a macaque and rat show that the primary visual cortex of the macaque possesses a well-defined layer IV and relatively thick upper layers compared with rats. Images of Nissl-stained sections were taken from brainmaps.org. Scale bar of the middle panel: 1 mm. Scale bar of the lower panel: 100 μm.
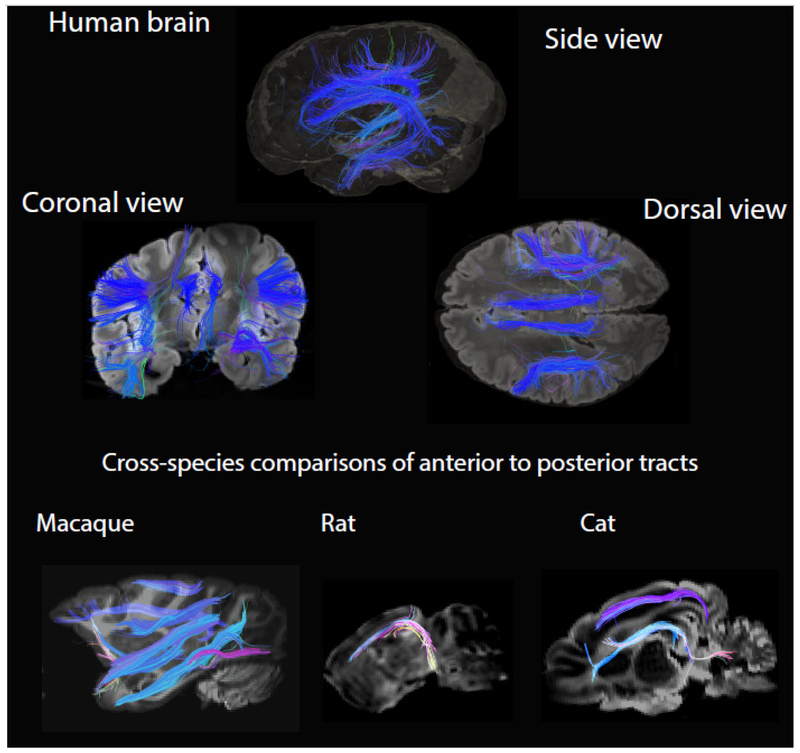
Ex vivo diffusion MR scans can be used to visualize tracts across the entire brain of any species (Takahashi et al., 2010; Wedeen et al., 2012; Calabrese et al., 2015; Feng et al., 2017). In the white matter of the cortex, diffusion MR scans show projections of pyramidal neurons, but not those of locally projecting GABAergic interneurons that remain within the grey matter of the cortex. Tracts within the white matter of the cortex might relay information within the cortex or between subcortical and cortical structures. The direction/orientation of a diffusion MRI-based tract can be used to distinguish projections that relay information within the cortex from those that relay information between the cortical and subcortical structures. In the white matter, tracts coursing across the dorsal to ventral direction have a tendency to form projections between cortical and sub-cortical structures (e.g., corticocpsinal tract, thalamo-cortical tracts) and to some extent cross-cortically. In contrast, pathways coursing along the anterior to posterior direction in the cortical white matter have a strong tendency to project cross-cortically (Mori et al., 2002). Concomitant with an increase of neurons in layers II-IV, primates possess an increase in anterior to posterior cortico-cortical tracts compared with rodents and other mammalian species (Charvet et al., 2017a; Fig. 13). That is, the human and non-human primates cross-cortical connectivity patterns differ from that of other examined mammals.
Figure 13. Ex vivo high angular resolution diffusion MR tractography of a human, macaque, rat, and cat brain.
The human brain possesses an extensive number of anterior to posterior cross-cortically projecting tracts. Other primates such as the rhesus macaque also possess many anterior to posterior cortical tracts compared with other mammals such as rats and cats (Charvet et al., 2017b).
Previous studies have examined species differences in gene expression across cortical layers of primates and rodents. Some genes are expressed primarily by long range projecting neurons in the cortex (Hof et al., 1995; Voelker et al., 2004). For instance, humans possess a relative increase in Neurofilament heavy polypeptide (NEFH) expression and other synaptic-related genes in layers III of the primary visual cortex compared with mice (Zeng et al., 2012). The observation that NEFH is preferentially expressed in long range projecting neurons, and that its expression is amplified in layers III of the human primary visual cortex suggests that humans possess an amplification of long-range cortico-cortical projecting neurons (Hof et al., 1995; Zeng et al., 2012; Charvet et al., 2018). Interestingly, a relative increase in NEFH expression is also observed in layer III of macaques compared with mice, suggesting that the increase in layer III NEFH expression may be a feature shared among human and non-human primates (Bakken et al., 2016). Combining comparative analyses of neuron numbers, diffusion MR tractography, and gene expression patterns across species demonstrates that humans and non-human primates deviate from other mammals in possessing increased long range cross-cortically projecting neurons.
5. Limitations
Ex vivo MRI allows us to visualize fiber pathways with extremely high-resolution tissue structures. Ex vivo structural MRI provides a means to identify detailed morphological changes whereas ex vivo diffusion MRI provides an unprecedented perspective with which to study the development of fiber pathways. However, we are still not able to directly distinguish which tractography pathways represents axons, glial fibers, or aligned cell components, because the current structural and diffusion MRI techniques do not produce direct measures of the tissue. To overcome this limitation, integrating analyses from MR methods with histology or gene expression data improves our understanding of the basic developmental processes occurring during human fetal brain development. However, histology has a different type of limitation, which precludes the study of global fiber connections, and therefore, direct correlation of high-resolution diffusion MRI and histology to determine what tractography can and cannot detect and to what extent it is currently informative when assessing MRI results.
Since the presence of any microscopic neuropathology cannot be determined prospectively before scanning, pathology of MRI-scanned cases should be carefully assessed after the scans to better correlate MRI and histopathology findings. Neurologically healthy brain specimens are valuable not only as controls, but also because there is still a significant lack of knowledge about typical human brain development. One obvious problem is that “normal” infants do not die, and natural disease in autopsied cases may introduce some error when interpreting normal human brain development.
Postmortem interval (PMI) and the time in fixative (TIF, e.g. 4% formalin) are potential factors that could affect results from ex vivo MRI examinations. Specimens with shorter PMI and TIF are thought to be better to minimize confounding variables. In fact, the potential for ex vivo MRI techniques to detect fine tissue properties including reconstructed fiber pathways has at the same time the potential to detect false positives. This problem is typically prominent when the condition of imaged specimens is not optimal as a result of long PMI and/or TIF. However, we have experience in the use of specimens with long PMI and TIF, and have obtained results with high spatial resolution and SNR (e.g. Wang et al., 2015).
In addition, as a common issue both in in- and ex vivo fetal MRI, the age of the fetuses and prematurely born infants is most commonly estimated on the basis of their crown–rump lengths (CRL) (O’Rahilly and Müller, 1984) and pregnancy records. In order to provide accurate and unbiased age estimation of fetuses and of prematurely born infants, it is common in the field of anatomy to express the age as weeks from conception (post-conceptional week [PCW], typically gestational weeks [GW] minus 2 weeks) (Olivier and Pineau, 1962), if available. However, given that the majority of researchers in neuroimaging use gestational weeks, for the purpose of this article we have reported major histogenic findings using GW for age.
6. Future directions
6.1. Large-scale data analyses
Pattern recognition technologies are emerging as important techniques for the analysis of ex vivo fetal MRI examinations. MRI has been shown to have the potential to play an important role in the analysis of post mortem fetuses, as some conditions are easier to identify from ex vivo MRI than through traditional autopsy (Thali et al., 2003). Previous work analyzing the potential of post mortem MRI for the assessment of the cause of fetal death has demonstrated that MRI can potentially play an assistive role (Thayyil et al., 2013). This post-mortem analysis was normally performed manually (i.e. in the absence of advanced pattern recognition tools) which puts considerable burden on the radiologists and pathologists involved in the research. Pattern recognition technologies can potentially play an important role in these types of studies by facilitating histological validation and automating the extraction of anatomical measurements from both MRI and microscopic resected tissue.
The majority of pattern recognition technologies developed for fetal MRI to date have focused on in vivo imaging (Clouchoux et al., 2012; Dittrich et al., 2014; Gholipur et al., 2017; Habas et al., 2010; Kuklisova-Murgasova et al., 2011; Serag et al., 2012; Wright et al., 2015; see also Levman and Takahashi, 2015), using a variety of registration and regression technologies in order to establish a baseline atlas of expected development of the fetal brain. Reviews of this specific topic are available (Clouchoux et al., 2012b; Makropoulos et al., 2017). Although the main research thus far has focused on in vivo imaging, such contributions have considerable potential towards being adapted for applications in an ex vivo MRI. Considerable research has focused on ex vivo MRI based modeling of the medial temporal cortices and associated structures, as summarized in a review article (Augustinack et al., 2013), analysis of which demonstrates the potential to support probabilistic brain mapping based on ex vivo MRI contrast, validated to histology and subsequently aligned to in vivo imaging. Related work has demonstrated the feasibility of using such technologies for ex vivo brain analysis and has demonstrated that myelin and Nissl staining relate to contrast in ex vivo MRI (Augustinack et al., 2014).
Recent work has demonstrated the feasibility of adapting the types of technologies relied upon in automated in vivo brain imaging analysis to post mortem fetal brains (Zhan et al., 2013). In this application, 34 ex vivo brain specimens were imaged with a 7T MRI scanner, with samples ranging in gestational age from 15 to 22 GW. The technology developed incorporated automated morphometrics, tensor-based morphometry and surface modeling techniques for data analysis. These techniques support the assessment age-specific shape changes during gestation, including the development of the lateral ventricles, the Sylvian fissure and local surface measurements, which help to inform researchers as to the natural patterns of brain growth. Previous work has also demonstrated the feasibility of measuring cortical folding from ex vivo fetal MRI examinations (Batchelor et al., 2002), research that has demonstrated sensitivity to anatomical variability as well as the quantification of differences between normal and abnormal fetal brains.
Ex vivo technologies have the potential to not only support autopsies that otherwise would be performed manually (Thayyil et al., 2013), but investigations that make use of such technologies have the potential to identify abnormal trajectories of brain growth that may be associated with extremely early stage development of neurological disorders, such as autism. However, there are many challenges to overcome when making such pattern recognition technologies widely available and useful, including the need for reliable data analysis techniques that can handle the large-scale deformation differences observed in ex vivo specimens relative to that observed in vivo, contrast differences (Augustinack et al., 2014), challenges associated with ex vivo ventricular bleeds without dilation (Thayyil 2011; Thayyil et al. 2013) and more.
Although the majority of the related work in this field has been focused on in vivo fetal MRI, pattern recognition technologies are developing towards playing an important role in ex vivo imaging of the fetal brain with MRI. It is anticipated that with time, such techniques will play a crucial role in histological validation, automated distributed measurement extraction, identification of anatomical abnormalities associated with neurodevelopmental disorders, as well as towards providing assistance in pathological analyses (by providing automated quantified measurements from MRI (e.g. Levman et al., 2017) to support microscopic cellular analysis), including diagnostic decision support systems for pathologists.
6.2. Translation of ex vivo results to in vivo application
Some human brain banks (Kretschmann et al., 1979; Kostovic et al., 1991; Judaš et al., 2011) offer an indisputable source of information on histological correlates of these neuroimaging findings, which are in turn, often challenging to interpret. Nevertheless, the only systematic correlation and co-registration of histology and MRI images exist only for the adult human brain (Amunts et al., 2013). Moreover, the definition of transient fetal zones on MRI images relies on scattered published histological slices that do not cover the entire brain (Kostovic and Goldman-Rakic, 1983; Kostovic and Rakic, 1990; Kostovic et al., 2002; Rados et al., 2006; Kostovic and Vasung, 2009), or on several available atlases covering the entire brain but relying on descriptions that are appropriate for the developing rodent brain (Bayer and Altman, 2003, 2005; Griffiths, 2010). Thus, to our knowledge, there is no study that systematically investigates the MRI-histological correlation of the human fetal brain across the entire telencephalon.
Ex vivo assessment of MR signal intensities, DTI parameters, and tractography-reconstructed pathways of the transient fetal zones are crucial for translation on in vivo subjects. However, only a correlation between ex vivo histology and MRI can provide sufficient information to offer proper biological interpretation of the in vivo MRI findings. Despite its lower resolution compared to ex vivo MRI, and despite unpredictable fetal motion, researches have already successfully captured in vivo structural and diffusion MRI of the fetal brain (Girard et al., 1995, Garel et al., 2001, Glenn and Barkovich, 2006, Glenn and Barkovich, 2006, Rutherford, 2009, Habas et al., 2010, Gholipour et al., 2014, Jakab et al., 2014, Mitter et al., 2015). Therefore, with new MR image acquisition and reconstruction techniques (e.g. Alexander et al., 2017; Hamilton et al., 2017; Liu et al., 2017; Poser and Setsompop, 2018), a detailed high resolution histological and MRI ex vivo atlases of the developing fetal brain (Kostovic et al., 2002, Huang et al., 2006, 2009, Vasung et al., 2010, 2016, 2017; Takahashi et al., 2012, Xu et al., 2014, Wang et al., 2015, Wilkinson et al., 2016, Das and Takahashi, 2017) can offer valuable information for interpretation of in vivo fetal MRI findings, and can identify areas where technical improvements in MRI acquisition, reconstruction, or analysis are still necessity.
Advances in techniques and interpretations of in vivo fetal MRI directly contribute to progress in fetal treatments such as fetal surgeries (Moon-Grady et al., 2017, Moldenhauer et al., 2017) and gene therapies (Witt et al., 2017, Massaro et al., 2018). Quantitative MRI analysis is crucial for early diagnoses and assessment of therapeutic effects as well as treatment side effects, especially when targeting neurological disorders. Since neuronal migration and cortical gyrification during brain development are closely associated with spatiotemporal gene expression patterns (Meyer, 2007, Huang et al., 2012), especially in gene therapies, we need to pay attention to potential risks to the modulation of brain morphology due to unexpected alteration of gene expression patterns. Detailed ex vivo fetal MRI observations could assist interpretations of atypical morphological findings in various in vivo fetal MRI scans including these fetal therapies.
In recent years, advances in neonatal care led to increased survival of prematurely born infants. The level of prematurity and the degree of severity of identifiable brain lesions have been associated with smaller brain volumes, smaller volumes of the gray and white matter at term equivalent age, childhood and adolescence (for review see Ment et al., 2009; Ferriero, 2016; Malhotra et al., 2017). Imaging biomarkers are starting to serve as a useful tool for identification of cognitive disabilities that these children display later in their life (Kwon et al., 2014). However, we are still far from accurate neurobiological interpretation of these biomarkers. Moreover, as the regional trajectories of human fetal brain growth during preterm development remain unknown, it is difficult to assess to what extent these trajectories are altered following preterm birth. To this end, the failure of brain maturation following preterm birth (Kinney and Volpe, 2018) indicates that the maturation trajectories of certain transient fetal zones might be altered. Therefore, comprehensive ex vivo MR and histological analyses dedicated to the assessment of transient fetal zones following premature birth are needed.
7. Conclusion
Ex vivo fetal brain MRI has great potential to resolve detailed tissue structures such as tissue properties that reflect various neurogenic processes including neuronal migration and emergence of axonal pathways in fetal transient zones. Depending on the condition of postmortem brain specimens, MRI scan protocols and data processing procedures (e.g. no FA thresholds), it is possible that ex vivo MRI provides false positive results. However, careful assessments with histopathology techniques and large-scale scans/analyses would minimize the effect of such confounders. In fact, current in vivo MRI examinations have a greater risk of false positives, and ex vivo MRI results with careful consideration will likely be able to serve as a great reference to in vivo MRI findings on fetal brain development and disorders.
Acknowledgements
This work was supported by the Eunice Shriver Kennedy National Institute of Child Health and Human Development (NICHD) R01HD078561 and R21HD069001 (ET), National Institute of Mental Health (NIMH) R21MH118739 (ET), and the National Institute of Neurological Disorders and Stroke (NINDS) R03NS091587 and R03NS101372 (ET). Swiss National Science Foundation grant No:P300PB_167804 (LV), Natural Science and Engineering Research Council of Canada’s Canada Research Chair grant (231266), Canada Foundation for Innovation and Nova Scotia Research and Innovation Trust infrastructure grant (R0176004; JL), and NIGMS grant (5P20GM103653; CJC) also supported this work. The content of the manuscript is the view of the authors and does not necessarily represent the official views of the Eunice Shriver Kennedy NICHD, NIMH, NINDS, or NIH. The data acquired in this study form a part of the BrainSpan Atlas of the Developing Human Brain. We are grateful to Dr. Brad Smith, University of Michigan for extended access to prenatal human MRI scans available at http://embryo.soad.umich.edu. Imaging was performed at the Center for In-Vivo Microscopy, Duke University and was funded by NIH N01-HD-6–3257 P/G F003637. A structural MRI scan of a human fetus at gestational week was downloaded from the Allen brain atlas website. Structural MRI scans are made available by the Allen Institute for Brain Science (Miller et al., 2014), which are available at: (http://download.alleninstitute.org/brainspan/MRI_DTI_data_for_prenatal_specimens/).
Abbreviations:
- CP
cortical plate
- CRL
crown–rump length
- DTI
diffusion tensor MRI
- DWI
diffusion weighted images
- FA
fractional anisotropy
- GE
ganglionic eminence
- GW
gestational weeks
- HARDI
high-angular resolution diffusion MRI
- IZ
intermediate zone
- MR
magnetic resonance
- MRI
magnetic resonance imaging
- PMI
postmortem interval
- SP
subplate
- SVZ
subventricular zone
- T1w
T1-weighted
- T2w
T2-weighted
- TIF
time in fixative
- VZ
ventricular zone
REFERENCES
- Alexander DC, Zikic D, Ghosh A, Tanno R, Wottschel V, Zhang J, Kaden E, Dyrby TB, Sotiropoulos SN, Zhang H, Criminisi A. Image quality transfer and applications in diffusion MRI. Neuroimage. 2017. May 15;152:283–298. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. 1997. Development of the cerebellar system: in relation to its evolution, structure, and functions. CRC. [Google Scholar]
- Ang ES, Haydar TF, Gluncic V, Rakic P. 2003. Four-dimensional migratory coordinates of GABAergic interneurons in the developing mouse cortex. J Neurosci 23, 5805–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustinack JC, van der Kouwe AJ, Fischl B 2013. Medial temporal cortices in ex vivo magnetic resonance imaging. J Comp Neurol 521(18):4177–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustinack JC, Magnain C, Reuter M, van der Kouwe AJ, Boas D, Fischl B 2014. MRI parcellation of ex vivo medial temporal lobe. NeuroImage 93(Pt 2):252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakken Trygve E., Miller Jeremy A., Ding Song-Lin, Sunkin Susan M., Smith Kimberly A., Ng Lydia, Szafer Aaron et al. 2016. Comprehensive transcriptional map of primate brain development. Nature 535:7612: 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball Gareth, Boardman James P., Aljabar Paul, Pandit Anand, Arichi Tomoki, Merchant Nazakat, Rueckert Daniel, Edwards A. David, and Counsell Serena J.. 2013. “The Influence of Preterm Birth on the Developing Thalamocortical Connectome.” Cortex; a Journal Devoted to the Study of the Nervous System and Behavior 49 (6): 1711–21. [DOI] [PubMed] [Google Scholar]
- Barbas H 1986. Pattern in the laminar origin of corticocortical connections. J Comp Neurol 252:415–422. [DOI] [PubMed] [Google Scholar]
- Batalle Dafnis, Jonathan O’Muircheartaigh Antonios Makropoulos, Kelly Christopher J., Dimitrova Ralica, Hughes Emer J., Hajnal Joseph V., et al. 2019. “Different Patterns of Cortical Maturation before and after 38 Weeks Gestational Age Demonstrated by Diffusion MRI in Vivo.” NeuroImage 185 (January): 764–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor PG, Castellano Smith AD, Hill DL, Hawkes DJ, Cox TC, Dean AF. 2002. Measures of cortical folding applied to the development of the human fetal brain. IEEE Transactions on Medical Imaging 21(8):953–965. [DOI] [PubMed] [Google Scholar]
- Bayer Shirley A., and Altman Joseph. 2005. The Human Brain during the Second Trimester (Atlas of the Human Central Nervous System Development). CRC Press, Indianapolis. [Google Scholar]
- Beaulieu C 1993. Numerical data on neocortical neurons in adult rat, with special reference to the GABA population. Brain Res 609, 284–92. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, Kisvarday Z, Somogyi P, Cynader M, Cowey A. 1992. Quantitative distribution of GABA-immunopositive and -immunonegative neurons and synapses in the monkey striate cortex (area 17). Cereb Cortex 2:295–309. [DOI] [PubMed] [Google Scholar]
- Benes FM, Berretta S. 2001. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacol. 25(1):1–27. [DOI] [PubMed] [Google Scholar]
- Bystron I, Blakemore C, Rakic P. 2008. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci. 9(2): 110–122. [DOI] [PubMed] [Google Scholar]
- Bystron I, Blakemore C, Rakic P. 2008. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci. 9(2):110–22. [DOI] [PubMed] [Google Scholar]
- Calabrese E, Badea A, Coe CL, Lubach GR, Shi Y, Styner MA, Johnson GA. 2015. A diffusion tensor MRI atlas of the postmortem rhesus macaque brain. NeuroImage, 117:408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness VS, Kennedy DN, Bates J, Makris N. 1996. The developmental neuroimaging: Mapping the development of brain and behaviour. New York: Academic Press, p. 3–14. [Google Scholar]
- Charvet CJ, Striedter GF. 2008. Developmental species differences in brain cell cycle rates between northern bobwhite quail (Colinus virginianus) and parakeets (Melopsittacus undulatus): implications for mosaic brain evolution. Brain Behav Evol. 72(4):295–306. [DOI] [PubMed] [Google Scholar]
- Charvet CJ, Cahalane DJ, Finlay BL. 2015. Systematic, cross-cortex variation in neuron numbers in rodents and primates. Cereb Cortex 25:147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charvet CJ, Reep RL, Finlay BL. 2016. Evolution of cytoarchitectural landscapes in the mammalian isocortex: Sirenians (Trichechus manatus) in comparison with other mammals. J Comp Neurol 524:772–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charvet CJ, Hof PR, Raghanti MA, van der Kouwe AJ, Sherwood CC, Takahashi E. 2017a. Combining diffusion MR tractography with stereology highlights increased cross-cortical integration in primates. J Comp Neurol 525, 1075–1093. doi 10.1002/cne.24115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charvet CJ, Šimić G, Kostović I, Knezović V, Vukšić M, Leko MB, Takahashi E, Sherwood CC, Wolfe MD and Finlay BL, 2017b. Coevolution in the timing of GABAergic and pyramidal neuron maturation in primates. Proc. R. Soc. B, 284(1861), 20171169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charvet CJ, Stimpson CD, Kim YD, Raghanti MA, Lewandowski AH, Hof PR, Gómez-Robles A, Krienen FM and Sherwood CC, 2017c. Gradients in cytoarchitectural landscapes of the isocortex: Diprotodont marsupials in comparison to eutherian mammals. J Comp Neurol, 525(8), 1811–1826. [DOI] [PubMed] [Google Scholar]
- Charvet CJ, Finlay BL. 2018. Comparing Adult Hippocampal Neurogenesis Across Species: Translating Time to Predict the Tempo in Humans. Front Neurosci. 12:706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charvet C, Palani A, Kabaria P, Takahashi E. 2018. Evolution of connections and cell types: integrating diffusion MR tractography with gene expression data highlights increased cortico-cortical projections in primates. bioRxiv, 493593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AF, Kondo S, Abdel-Mannan O, Chodroff RA, Sirey TM, Bluy LE, Webber N, DeProto J, Karlen SJ, Krubitzer L, Stolp HB. 2009. The subventricular zone is the developmental milestone of a 6-layered neocortex: comparisons in metatherian and eutherian mammals. Cereb Cortex. 20(5):1071–81. [DOI] [PubMed] [Google Scholar]
- Chen YJJ, Friedman BA, Ha C, Durinck S, Liu J, Rubenstein JL, Seshagiri S, Modrusan Z 2017. Single-cell RNA sequencing identifies distinct mouse medial ganglionic eminence cell types. Scientific Reports, 7, 45656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL (2001) Translating developmental time across mammalian species. Neuroscience 105:7–17. [DOI] [PubMed] [Google Scholar]
- Clouchoux C, Kudelski D, Gholipour A, Warfield SK, Viseur S, Bouyssi-Kobar M, Mari JL, Evans AC, du Plessis AJ, Limperopoulos C 2012. Quantitative in vivo MRI measurement of cortical development in the fetus. Brain Struct Funct 217(1):127–139. [DOI] [PubMed] [Google Scholar]
- Clouchoux C, Limperopoulos C 2012. Novel applications of quantitative MRI for the fetal brain. Pediatric Radiology 42(S1):24–32. [DOI] [PubMed] [Google Scholar]
- Coogan TA, Burkhalter A. 1990. Conserved patterns of cortico-cortical connections define areal hierarchy in rat visual cortex. Exp brain Res. 80(1):49–53. [DOI] [PubMed] [Google Scholar]
- D’Arceuil H, Liu C, Levitt P, Thompson B, Kosofsky B, de Crespigny A, 2008. Threedimensional high-resolution diffusion tensor imaging and tractography of the developing rabbit brain. Dev. Neurosci 30, 262–275. [DOI] [PubMed] [Google Scholar]
- Das A, Takahashi E. 2017. Neuronal Migration and Axonal Pathways Linked to Human Fetal Insular Development Revealed by Diffusion MR Tractography. Cereb Cortex 31:1–9. doi: 10.1093/cercor/bhx224. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich E, Riklin Raviv T, Kasprian G, Donner R, Brugger PC, Prayer D, Langs G 2014. A spatio-temporal latent atlas for semi-supervised learning of fetal brain segmentations and morphological age estimation. Medical image analysis 18, 9–21. [DOI] [PubMed] [Google Scholar]
- Džaja D, Hladnik A, Bičanić I, Baković M, Petanjek Z. 2014. Neocortical calretinin neurons in primates: increase in proportion and microcircuitry structure. Front Neuroanat 8, 103. doi: 10.3389/fnana.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon TW. Cortical connections of the inferior arcuate sulcus cortex in the macaque brain. Brain Res 1992. February 21;573(1):8–26. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Alonso-Nanclares L and Arellano JI 2002. Microstructure of the neocortex: comparative aspects. J Neurocyt, 31(3–5), pp.299–316. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Anderson S, López-Cruz PL, Benavides-Piccione R, Bielza C, Larrañaga P, Burkhalter A, Cauli B, Fairén A, Feldmeyer D, Fishell G, Fitzpatrick D, Freund TF, González-Burgos G, Hestrin S, Hill S, Hof PR, Huang J, Jones EG, Kawaguchi Y, Kisvárday Z, Kubota Y, Lewis DA, Marín O, Markram H, McBain CJ, Meyer HS, Monyer H, Nelson SB, Rockland K, Rossier J, Rubenstein JL, Rudy B, Scanziani M, Shepherd GM, Sherwood CC, Staiger JF, Tamás G, Thomson A, Wang Y, Yuste R, Ascoli GA. 2013. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat Rev Neurosci 14(3), 202–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bigio MR. 2011. Cell proliferation in human ganglionic eminence and suppression after prematurity-associated haemorrhage. Brain 134, 1344–1361. [DOI] [PubMed] [Google Scholar]
- Dubois J, Hertz-Pannier L, Dehaene-Lambertz G, Cointepas Y, Le Bihan D. 2006. Assessment of the early organization and maturation of infants’ cerebral white matter fiber bundles: a feasibility study using quantitative diffusion tensor imaging and tractography. Neuroimage 30:1121–1132. [DOI] [PubMed] [Google Scholar]
- Edlow BL, Takahashi E, Wu O, Benner T, Dai G, Bu L, Grant PE, Greer DM, Greenberg SM, Kinney HC, Folkerth RD. 2012. Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. J Neuropathol Exp Neurol 71: 531–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Jeon T, Yu Q, Ouyang M, Peng Q, Mishra V, Pletikos M, Sestan N, Miller MI, Mori S, Hsiao S. 2017. Population-averaged macaque brain atlas with high-resolution ex vivo DTI integrated into in vivo space. Brain Struct Funct 222(9):4131–4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferriero DM. The Vulnerable Newborn Brain: Imaging Patterns of Acquired Perinatal Injury. Neonatology 2016;109(4):345–51. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick KA, Imig TJ. 1980. Auditory cortico-cortical connections in the owl monkey. J Comp Neurol 192(3):589–610. [DOI] [PubMed] [Google Scholar]
- Foster RE, Donoghue JP, Ebner FF. 1981. Laminar organization of efferent cells in the parietal cortex of the Virginia opossum. Experimental brain research 43(3):330–6. [DOI] [PubMed] [Google Scholar]
- Garel C, Chantrel E, Brisse H, Elmaleh M, Luton D, Oury JF, et al. 2001. Fetal cerebral cortex: normal gestational landmarks identified using prenatal MR imaging. AJNR Am J Neuroradiol 22(1):184–9. [PMC free article] [PubMed] [Google Scholar]
- Gholipur A, Rollins CK, Velasco-Annis C, Ouaalam A, Akhondi-Asl A, Afacan O, Ortinau CM, Clancy S, Limperopoulos C, Yang E, Estroff JA, Warfield SK. 2017. A normative spatiotemporal MRI atlas of the fetal brain for automatic segmentation and analysis of early brain growth. Scientific Reports 7:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholipour A, Estroff JA, Barnewolt CE, Robertson RL, Grant PE, Gagoski B, Warfield SK, Afacan O, Connolly SA, Neil JJ, Wolfberg A, Mulkern RV. 2014. Fetal MRI: A Technical Update with Educational Aspirations. Concepts Magn Reson Part A Bridg Educ Res 43(6):237–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard N, Raybaud C, Poncet M. 1995. In vivo MR study of brain maturation in normal fetuses. AJNR Am J Neuroradiol 16(2):407–13. [PMC free article] [PubMed] [Google Scholar]
- Glenn O, Barkovich A. 2006. Magnetic resonance imaging of the fetal brain and spine: an increasingly important tool in prenatal diagnosis, part 1. American Journal of Neuroradiology 27(8):1604–11. [PMC free article] [PubMed] [Google Scholar]
- Glenn OA, Barkovich J. 2006. Magnetic resonance imaging of the fetal brain and spine: an increasingly important tool in prenatal diagnosis: part 2. AJNR Am J Neuroradiol 27(9):1807–14. [PMC free article] [PubMed] [Google Scholar]
- Gressens P 2006. Pathogenesis of migration disorders. Curr Opin Neurol 19(2):135–40. [DOI] [PubMed] [Google Scholar]
- Habas PA, Kim K, Corbett-Detig JM, Rousseau F, Glenn OA, Barkovich AJ, et al. 2010. A spatiotemporal atlas of MR intensity, tissue probability and shape of the fetal brain with application to segmentation. Neuroimage 53(2):460–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J, Franson D, Seiberlich N. Recent advances in parallel imaging for MRI. Prog Nucl Magn Reson Spectrosc 2017. August;101:71–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevner RF. 2000. Development of connections in the human visual system during fetal mid-gestation: a Dil-tracing study. J Neuropathol Exp Neurol. 59(5):385–92. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Daza RA, Rubenstein JL, Stunnenberg H, Olavarria JF, Englund C. 2003. Beyond laminar fate: toward a molecular classification of cortical projection/pyramidal neurons. Dev Neurosci 25(2–4):139–51. [DOI] [PubMed] [Google Scholar]
- His W 1904. Die Entwickelung des menschlichen Gehirns während der ersten Monate: Untersuchungsergebnisse: Hirzel. [Google Scholar]
- Hochstetter F. Beitr? ge zur Entwicklungsgeschichte des menschlichen Gehirns: Рипол Классик. 1919.
- Hof PR, Nimchinsky EgA, Morrison JH. 1995. Neurochemical phenotype of corticocortical connections in the macaque monkey: quantitative analysis of a subset of neurofilament protein-immunoreactive projection neurons in frontal, parietal, temporal, and cingulate cortices. J Comp Neurol 362:109–133. [DOI] [PubMed] [Google Scholar]
- Honig LS, Herrmann K, Shatz CJ. 1996. Developmental changes revealed by immunohistochemical markers in human cerebral cortex. Cereb Cortex, 6, 794–806. [DOI] [PubMed] [Google Scholar]
- Huang H, Jeon T, Sedmak G, Pletikos M, Vasung L, Xu X, Yarowsky P, Richards LJ, Kostovic I, Sestan N, Mori S. Coupling diffusion imaging with histological and gene expression analysis to examine the dynamics of cortical areas across the fetal period of human brain development. Cereb Cortex 2013. November;23(11):2620–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Vasung L, 2014. Gaining insight of fetal brain development with diffusion MRI and histology. Int J Dev Neurosci 32, 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Zhang J, Wakana S, Zhang W, Ren T, Richards L, Yarowsky P, Donohue P, Graham E, van Zjil PCM, Mori S 2006. White and gray matter development in human fetal, newborn and pediatric brains. NeuroImage 33, 27–38. [DOI] [PubMed] [Google Scholar]
- Huang H, Yamamoto A, Hossain MA, Younes L, Mori S 2008. Quantitative cortical mapping of fractional anisotropy in developing rat brains. J. Neurosci 28, 1427–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Xue R, Zhang J, Ren T, Richards L, Yarowsky P, Miller MI, Mori S 2009. Anatomical characterization of human fetal brain development with diffusion tensor magnetic resonance imaging. J. Neurosci 29, 4263–4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Wah IY, Pooh RK, Choy KW. 2012. Molecular genetics in fetal neurology. Semin Fetal Neonatal Med 17(6):341–6. [DOI] [PubMed] [Google Scholar]
- Jakab A, Schwartz E, Kasprian G, Gruber GM, Prayer D, Schopf V, et al. 2014. Fetal functional imaging portrays heterogeneous development of emerging human brain networks. Front Hum Neurosci 8:852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Knösche TR, Turner R. 2013. White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage 73:239–54. [DOI] [PubMed] [Google Scholar]
- Jones EG, Burton H. 1976. Areal differences in the laminar distribution of thalamic afferents in cortical fields of the insular, parietal and temporal regions of primates. J Comp Neurol 168(2):197–247. [DOI] [PubMed] [Google Scholar]
- Jones EG., Rakic P., 2010. Radial columns in cortical architecture: it is the composition that counts. Cereb Cortex 20, 2261–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joschko MA, Sanderson KJ. 1987. Cortico-cortical connections of the motor cortex in the brushtailed possum (Trichosurus vulpecula). J Anat 150:31. [PMC free article] [PubMed] [Google Scholar]
- Kang Hyo Jung, Yuka Imamura Kawasawa Feng Cheng, Zhu Ying, Xu Xuming, Li Mingfeng, Sousa André M. M., et al. 2011. “Spatio-Temporal Transcriptome of the Human Brain.” Nature 478 (7370): 483–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura K 1973a. Corticocortical fiber connections of the cat cerebrum. III. The occipital region. Brain Res 41–60. [DOI] [PubMed] [Google Scholar]
- Kawamura K 1973b. Corticocortical fiber connections of the cat cerebrum. I. The temporal region. Brain Res 51:1–21. [DOI] [PubMed] [Google Scholar]
- Kawamura K 1973c. Corticocortical fiber connections of the cat cerebrum. II. The parietal region. Brain Res 51: 23–40. [DOI] [PubMed] [Google Scholar]
- Kawamura K, Otani K. 1970. Corticocortical fiber connections in the cat cerebrum: the frontal region. J Comp Neurol 139:423–448. [DOI] [PubMed] [Google Scholar]
- Kelava I, Reillo I, Murayama AY, Kalinka AT, Stenzel D, Tomancak P, Matsuzaki F, Lebrand C, Sasaki E, Schwamborn JC and Okano H 2011. Abundant occurrence of basal radial glia in the subventricular zone of embryonic neocortex of a lissencephalic primate, the common marmoset Callithrix jacchus. Cereb cortex, 22(2), pp.469–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC, Volpe JJ, 2018. Encephalopathy of Prematurity: Neuropathology Volpe’s Neurology of the Newborn (Sixth Edition). Elsevier, pp. 389–404. [Google Scholar]
- Kostovic I 1986. Prenatal development of nucleus basalis complex and related fiber systems in man: a histochemical study. Neuroscience 17(4):1047–77. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Goldman-Rakic PS. 1983. Transient cholinesterase staining in the mediodorsal nucleus of the thalamus and its connections in the developing human and monkey brain. J Comp Neurol 219(4):431–47. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Jovanov-Milosevic N. 2006. The development of cerebral connections during the first 20–45 weeks’ gestation. Semin Fetal Neonatal Med 11(6):415–22. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Jovanov-Milosevic N, Rados M, Sedmak G, Benjak V, Kostovic-Srzentic M, et al. 2014. Perinatal and early postnatal reorganization of the subplate and related cellular compartments in the human cerebral wall as revealed by histological and MRI approaches. Brain Struct Funct 219(1):231–53. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Judas M. 2002. Correlation between the sequential ingrowth of afferents and transient patterns of cortical lamination in preterm infants. Anat Rec 267(1):1–6. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Judas M. 2009. Early Development of Neuronal Circuitry of the Human Prefrontal Cortex. Cogn Neurosci 1: 29–47. [Google Scholar]
- Kostovic I, Judas M. 2010. The development of the subplate and thalamo-cortical connections in the human foetal brain. Acta Paediatrica 99(8):1119–27. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Judas M, Rados M, Hrabac P. 2002. Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb Cortex 12(5):536–44. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Kracun I, Kelovic Z. The development of “cholinergic”reaction in the septal area of the human fetus. Verh Anat Ges 1980;74:729–32. [Google Scholar]
- Kostovic I, Rakic P. 1990. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol 297(3):441–70. [DOI] [PubMed] [Google Scholar]
- Krienen FM, Yeo BT, Ge T, Buckner RL, Sherwood CC. 2016. Transcriptional profiles of supragranular-enriched genes associate with corticocortical network architecture in the human brain. Proc Natl Acad Sci U S A. 113(4):E469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, Noctor S & Martínez-Cerdeño V 2006. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat. Rev. Neurosci, 7, 883–890. [DOI] [PubMed] [Google Scholar]
- Kroenke CD, Van Essen DC, Inder TE, Rees S, Bretthorst GL, Neil JJ. 2007. Microstructural changes of the baboon cerebral cortex during gestational development reflected in magnetic resonance imaging diffusion anisotropy. J Neurosci 27, 12506–12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuklisova-Murgasova M & Aljabar P et al. 2011A dynamic 4D probabilistic atlas of the developing brain. NeuroImage 54, 2750–2763. [DOI] [PubMed] [Google Scholar]
- Kunz N, Zhang H, Vasung L, O’Brien KR, Assaf Y, Lazeyras F, Alexander DC, Huppi PS, 2014. Assessing white matter microstructure of the newborn with multi-shell diffusion MRI and biophysical compartment models. Neuroimage 96, 288–299. [DOI] [PubMed] [Google Scholar]
- Kuo L-W, Chen J-H, Wedeen VJ, Tseng WY 2008. Optimization of diffusion spectrum imaging and q-ball imaging on clinical MRI system. NeuroImage 41, 7–18. [DOI] [PubMed] [Google Scholar]
- Lavezzi AM, Ottaviani G, Terni L, Matturri L 2006. Histological and biological developmental characterization of the human cerebellar cortex. Int. J. Dev. Neurosci 24, 365–371. [DOI] [PubMed] [Google Scholar]
- Lavdas AA, Grigoriou M, Pachnis V, Parnavelas JG. 1999. The medial ganglionic eminence gives rise to a population of early neurons in the developing cerebral cortex. J Neurosci 19, 7881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefèvre J, Germanaud D, Dubois J, Rousseau F, de Macedo Santos I, Angleys H, Mangin JF, Hüppi PS, Girard N, De Guio F. Are Developmental Trajectories of Cortical Folding Comparable Between Cross-sectional Datasets of Fetuses and Preterm Newborns? Cereb Cortex 2016. July;26(7):3023–35 [DOI] [PubMed] [Google Scholar]
- Letinic K, Rakic P. 2001. Telencephalic origin of human thalamic GABAergic neurons. Nat Neurosci 4(9): 931–936. [DOI] [PubMed] [Google Scholar]
- Letinic K, Zoncu R, Rakic P. 2002. Origin of GABAergic neurons in the human neocortex. Nature 417, 645–649. doi: 10.1038/nature00779 [DOI] [PubMed] [Google Scholar]
- Levman J, MacDonald P, Lim AR, Takahashi E. 2017. A Pediatric Structural MRI Analysis of Healthy Brain Development from Newborns to Adults. Human Brain Mapping September 12. doi: 10.1002/hbm.23799. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levman J, Takahashi E. 2015. Multivariate analyses applied to fetal, neonatal and pediatric MRI of neurodevelopmental disorders. Nueroimage: Clinical 9:532–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Buch S, Chen Y, Choi HS, Dai Y, Habib C, Hu J, Jung JY, Luo Y, Utriainen D, Wang M, Wu D, Xia S, Haacke EM. Susceptibility-weighted imaging: current status and future directions. NMR Biomed 2017. April;30(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukaszewicz A, Savatier P, Cortay V, Giroud P, Huissoud C, Berland M, Kennedy H, Dehay C. 2005. G1 phase regulation, area-specific cell cycle control, and cytoarchitectonics in the primate cortex, Neuron 47:353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier-Hein K, Neher P, Houde J-C, Cote M-A, Garyfallidis E, Zhong J, et al. 2016. Tractography-based connectomes are dominated by false-positive connections. bioRxiv 084137. [Google Scholar]
- Makropoulos A Counsell SJ, Rueckert D. 2017. A review on automatic fetal and neonatal brain MRI segmentation. NeuroImage June 28 pii: S1053–8119(17) 30545–1. doi: 10.1016/j.neuroimage.2017.06.074. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Malhotra A, Fahey MC, Davies-Tuck M, Wong F, Carse E, Whiteley G, Ditchfield M. Comparison of preterm and term equivalent age MRI for the evaluation of preterm brain injury. J Perinatol 2017. July;37(7):864–868. [DOI] [PubMed] [Google Scholar]
- Marín O, Rubenstein JL. 2001. A long, remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci 2(11): 780–790. [DOI] [PubMed] [Google Scholar]
- Marín O, Rubenstein JLR. 2003. Cell migration in the forebrain. Annu Rev Neurosci 26: 441–483. [DOI] [PubMed] [Google Scholar]
- Massaro G, Mattar CNZ, Wong AMS, et al. 2018. Fetal gene therapy for neurodegenerative disease of infants. Nat Med 24(9):1317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinstry RC, Mathur A, Miller JH, Ozcan A, Snyder AZ, Schefft GL, et al. 2002. Radial organization of developing preterm human cerebral cortex revealed by non-invasive water diffusion anisotropy MRI. Cereb Cortex 12(12):1237–43. [DOI] [PubMed] [Google Scholar]
- Ment Laura R., Hirtz Deborah, and Hüppi Petra S.. 2009. “Imaging Biomarkers of Outcome in the Developing Preterm Brain.” Lancet Neurology 8 (11): 1042–55. [DOI] [PubMed] [Google Scholar]
- Meyer G 2007. Genetic control of neuronal migrations in human cortical development. Adv Anat Embryol Cell Biol 189:1 p preceding 1, 1–111. [PubMed] [Google Scholar]
- Miller JA, Ding SL, Sunkin SM, Smith KA, Ng L, Szafer A, Ebbert A, Riley ZL, Aiona K, Arnold JM, Bennet C. 2014. Transcriptional landscape of the prenatal human brain. Nature 508(7495):199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitter C, Jakab A, Brugger PC, Ricken G, Gruber GM, Bettelheim D, Scharrer A, Langs G, Hainfellner JA, Prayer D, Kasprian G. 2015. Validation of in utero tractography of human fetal commissural and internal capsule fibers with histological structure tensor analysis. Front Neuroanat 9:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki Y, Song JW, Takahashi E. 2016. Asymmetry of radial and symmetry of tangential neuronal migration pathways in developing human fetal brains. Front. Neuroanat 10:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldenhauer JS, Adzick NS. 2017. Fetal surgery for myelomeningocele: After the Management of Myelomeningocele Study (MOMS). Semin Fetal Neonatal Med 22(6):360–6. [DOI] [PubMed] [Google Scholar]
- Molliver ME, Kostovic I, Van der Loos H. 1973. The development of synapses in cerebral cortex of the human fetus. Brain Res 50(2):403–7. [DOI] [PubMed] [Google Scholar]
- Mori S, Kaufmann WE, Davatzikos C, Stieltjes B, Amodei L, Fredericksen K, Pearlson GD, Melhem ER, Solaiyappan M, Raymond GV, Moser HW. 2002. Imaging cortical association tracts in the human brain using diffusion-tensor-based axonal tracking. Magn Reson Med 47(2):215–23. [DOI] [PubMed] [Google Scholar]
- Molnár Z, Cheung AF. 2006. Towards the classification of subpopulations of layer V pyramidal projection neurons. Neuroscience research 55(2):105–15. [DOI] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD. 2007. Neuronal subtype specification in the cerebral cortex. Nature reviews. Neuroscience 8(6):427. [DOI] [PubMed] [Google Scholar]
- Moon-Grady AJ, Baschat A, Cass D, et al. 2017. Fetal Treatment 2017: The Evolution of Fetal Therapy Centers – A Joint Opinion from the International Fetal Medicine and Surgical Society (IFMSS) and the North American Fetal Therapy Network (NAFTNet). Fetal Diagn Ther 42(4):241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrzljak L, Uylings H, Kostovic I, van Eden CG. 1988. Prenatal development of neurons in the human prefrontal cortex: I. A qualitative Golgi study. J Comp Neurol 271(3):355–86. [DOI] [PubMed] [Google Scholar]
- Mrzljak L, Uylings HB, Van Eden GG, Judáš M. 1991. Neuronal development in human prefrontal cortex in prenatal and postnatal stages. Prog Brain Res 85:185–222. [DOI] [PubMed] [Google Scholar]
- Nery S, Fishell G, Corbin JG. 2002. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nature Neurosci 5, 1279–87. doi: 10.1523/JNEUROSCI.0604-09.2009 [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Berger B, Esclapez M. 2009a. Origins of cortical GABAergic neurons in the cynomolgus monkey. Cereb Cortex 19(2): 249–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petanjek Z, Kostović I, Esclapez M. 2009b. Primate-specific origins and migration of cortical GABAergic neurons. Front Neuroanat 3:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm C, Axer M, Gräßel D, Dammers J, Lindemeyer J, Zilles K, et al. 2010. Towards ultra-high resolution fibre tract mapping of the human brain–registration of polarised light images and reorientation of fibre vectors. Front Hum Neurosci 4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes MF, James D, Gil-Perotin S, Kim H, Cotter JA, Ng C, Sandoval K, Rowitch DH, Xu D, McQuillen PS, Garcia-Verdugo JM. 2016. Extensive migration of young neurons into the infant human frontal lobe. Science 354(6308):aaf7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletikos M, Sousa AM, Sedmak G, Meyer KA, Zhu Y, Cheng F, et al. 2014. Temporal specification and bilaterality of human neocortical topographic gene expression. Neuron 81(2):321–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poser BA, Setsompop K. Pulse sequences and parallel imaging for high spatiotemporal resolution MRI at ultra-high field. Neuroimage. 2018. Mar;168:101–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prayer D, Kasprian G, Krampl E, Ulm B, Witzani L, Prayer L, et al. 2006. MRI of normal fetal brain development. Eur J Radiol 57(2):199–216. [DOI] [PubMed] [Google Scholar]
- Radoš M, Judaš M, Kostović I. 2006. In vitro MRI of brain development. Eur J Radiol 57(2):187–98. [DOI] [PubMed] [Google Scholar]
- Rakic P 1972. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol 145(1): 61–83. [DOI] [PubMed] [Google Scholar]
- Rakic P 1988. Specification of cerebral cortical areas. Science 241(4862): 170–176. [DOI] [PubMed] [Google Scholar]
- Rakic P 1974. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science 183(4123):425–7. [DOI] [PubMed] [Google Scholar]
- Rakic P 2003. Developmental and evolutionary adaptations of cortical radial glia. Cereb cortex, 13(6), 541–549. [DOI] [PubMed] [Google Scholar]
- Rakic P 1974. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science, 183(4123), 425–427. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Glasser MF, Preuss TM, Ma X, Zhao T, Hu X, Behrens TE. 2008. The evolution of the arcuate fasciculus revealed with comparative DTI. Nat Neurosci 11(4):426–8. doi: 10.1038/nn2072. [DOI] [PubMed] [Google Scholar]
- Ronan RO, Müller F. 2006. The Embryonic Human Brain: An Atlas Of Developmental Stages John Wiley & Sons. [Google Scholar]
- Rutherford MA. 2009. Magnetic resonance imaging of the fetal brain. Current opinion in obstetrics and gynecology 21(2):180–6. [DOI] [PubMed] [Google Scholar]
- Saksena S, Husain N, Das V, Pradhan M, Trivedi R, Srivastava S, Malik GK, Rathore RK, Sarma M, Pandey CM, Gupta RK. 2008. Diffusion tensor imaging in the developing human cerebellum with histologic correlation. Int J Dev Neurosci 26(7):705–11. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya D. 2009. Fiber pathways of the brain. Oxford Univ Press USA. [Google Scholar]
- Serag A, Aljabar P, Ball G, Counsell SJ, Boardman JP, Rutherford MA, Edwards AD, Hajnal JV, Rueckert D. 2012. Construction of a consistent high-definition spatio-temporal atlas of the developing brain using adaptive kernel regression. NeuroImage 59, 2255–2265. [DOI] [PubMed] [Google Scholar]
- Shitamukai A, Konno D, Matsuzaki F. 2011. Oblique radial glial divisions in the developing mouse neocortex induce self-renewing progenitors outside the germinal zone that resemble primate outer subventricular zone progenitors, J Neurosci 31:3683–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman RL, Rakic P. 1973. Neuronal migration, with special reference to developing human brain: a review. Brain Res 62(1): 1–35. [DOI] [PubMed] [Google Scholar]
- Smart IH, Dehay C, Giroud P, Berland M & Kennedy H 2002. Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb. Cortex 12:37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JW, Mitchell PD, Kolasinski J, Ellen Grant P, Galaburda AM, Takahashie E. 2015. Asymmetry of white matter pathways in developing human brains. Cereb Cortex 25:2883–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Mishra V, Ouyang M, Peng Q, Slinger M, Liu S, Huang H, 2017. Human Fetal Brain Connectome: Structural Network Development from Middle Fetal Stage to Birth. Front Neurosci 11, 561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striedter GF 2005. Principles of Brain Evolution Sinauer, Sunderland, MA. [Google Scholar]
- Takahashi E, Dai G, Wang R, Ohki K, Rosen GD, Galaburda AL, Grant PE, Wedeen VJ. 2010. Development of cerebral fiber pathways in cats revealed by diffusion spectrum imaging. Neuroimage: 49:1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi E, Dai G, Rosen GD, Wang R, Ohki K, Folkerth RD, Galaburda AL, Wedeen VJ, Grant PE. 2011. Developing neocortex organization and connectivity in cats revealed by direct correlation of diffusion tractography and histology. Cereb Cortex 21:200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi E, Folkerth RD, Galaburda AL, Grant PE. 2012. Emerging cerebral connectivity in the human fetal brain: An MR tractography study. Cereb Cortex: 22:455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi E, Hayashi E, Schmahmann JD, Grant PE. 2014. Development of cerebellar connectivity in human fetal brains revealed by high angular resolution diffusion tractography. Neuroimage 96:326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuch DS, Reese TG, Wiegell MR, Makris N, Belliveau JW, Wedeen VJ. 2002. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med 48:577–82. [DOI] [PubMed] [Google Scholar]
- Tuch DS, Reese TG, Wiegell MR, Wedeen VJ. 2003. Diffusion MRI of complex neural architecture. Neuron 40, 885–895. [DOI] [PubMed] [Google Scholar]
- Thali MJ, Yen K, Schweitzer W, Vock P, Boesch C, Ozdoba C, Schroth G, Ith M, Sonnenschein M, Doernhoefer T, Yen K, Schweitzer W, Vock P, Boesch C, Ozdoba C, Schroth G, Ith M, Sonnenschein M, Doernhoefer T. 2003. Virtopsy, a new imaging horizon in forensic pathology: virtual autopsy by postmortem multislice computed tomography (MSCT) and magnetic resonance imaging (MRI) -- a feasibility study. J Forensic Sci 48(2):386–403. [PubMed] [Google Scholar]
- Thayyil S 2011. Less invasive autopsy: an evidenced based approach. Arch Dis Child 96:681–687. [DOI] [PubMed] [Google Scholar]
- Thayyil S, Sebire NJ, Chitty LS, Wade A, Chong WK, Olsen O, Gunny RS, et al. 2013. Post-mortem MRI versus conventional autopsy in fetuses and children: a prospective study. The Lancet 382(9888):223–233. [DOI] [PubMed] [Google Scholar]
- Vasung L, Huang H, Jovanov-Milosevic N, Pletikos M, Mori S, Kostovic I. 2010. Development of axonal pathways in the human fetal fronto-limbic brain: histochemical characterization and diffusion tensor imaging. J Anat 217(4):400–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasung L, Huang H, Jovanov-Milošević N, Pletikos M, Mori S, Kostović I. 2010. Development of axonal pathways in the human fetal fronto-limbic brain: histochemical characterization and diffusion tensor imaging. J Anat 217(4):400–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasung L, Lepage C, Rados M, Pletikos M, Goldman JS, Richiardi J, et al. 2016. Quantitative and Qualitative Analysis of Transient Fetal Compartments during Prenatal Human Brain Development. Front Neuroanat 10:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasung L, Raguz M, Kostovic I, Takahashi E. 2017. Spatiotemporal Relationship of Brain Pathways during Human Fetal Development Using High-Angular Resolution Diffusion MR Imaging and Histology. Front Neurosci 11:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker CC, Garin N, Taylor JS, Gähwiler BH, Hornung JP, Molnár Z. 2004. Selective neurofilament (SMI-32, FNP-7 and N200) expression in subpopulations of layer V pyramidal neurons in vivo and in vitro. Cereb Cortex 14(11):1276–86. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. 2008. Neurology of the newborn. 5 ed. Philadephia: Elsevier Science [Google Scholar]
- Volpe JJ. 2009. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol 8(1):110–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Monakow C. Gehirnpathologie: Alfred Hölder. 1905.
- Wang R, Dai G, Takahashi E. 2015. High Resolution MRI Reveals Detailed Layer Structures in Early Human Fetal Stages: In Vitro Study with Histologic Correlation. Front Neuroanat 9:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang VY, Zoghbi HY 2001. Genetic regulation of cerebellar development. Nat. Rev. Neurosci 2:484–491. [DOI] [PubMed] [Google Scholar]
- Wedeen VJ, Rosene DL, Wang R, Dai G, Mortazavi F, Hagmann P, et al. 2012. The geometric structure of the brain fiber pathways. Science 335(6076):1628–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedeen VJ, Wang RP, Schmahmann JD, Benner T, Tseng WY, Dai G, et al. 2008. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage 41(4):1267–77. [DOI] [PubMed] [Google Scholar]
- Wilkinson M, Kane T, Wang R, Takahashi E. 2016. Migration Pathways of Thalamic Neurons and Development of Thalamocortical Connections in Humans Revealed by Diffusion MR Tractography. Cereb. Cortex Dec. 2, doi: 10.1093/cercor/bhw339 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt R, MacKenzie TC, Peranteau WH. 2017. Fetal stem cell and gene therapy. Semin Fetal Neonatal Med 22(6):410–4. [DOI] [PubMed] [Google Scholar]
- Wonders CP, Anderson SA. 2006. The origin and specification of cortical interneurons. Nat Rev Neurosci 7(9): 687–696. [DOI] [PubMed] [Google Scholar]
- Wong MD, Dorr AE, Walls JR, Lerch JP, Henkelman RM. 2012. A novel 3D mouse embryo atlas based on micro-CT. Development 139(17):3248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman AD, Charvet CJ, Clancy B, Darlington RB, Finlay BL. 2013. Modeling transformations of neurodevelopmental sequences across mammalian species. J Neurosci 33:7368–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright R, Makropoulos A, Kyriakopoulou V, Patkee PA, Koch LM, Rutherford MA, Hajnal JV, Rueckert D, Aljabar P. 2015. Construction of a fetal spatio-temporal cortical surface atlas from in utero MRI: Application of spectral surface matching. NeuroImage 120, 467–480. [DOI] [PubMed] [Google Scholar]
- Xu G, Takahashi E, Folkerth RD, Haynes RL, Volpe JJ, Grant PE, Kinney HC. 2014. Radial coherence of diffusion tractography in the cerebral white matter of the human fetus: neuroanatomic insights. Cereb Cortex 24(3):579–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Ouyang A, Chalak L, Jeon T, Chia J, Mishra V, Sivarajan M, Jackson G, Rollins N, Liu S, Huang H, 2016. Structural Development of Human Fetal and Preterm Brain Cortical Plate Based on Population-Averaged Templates. Cereb Cortex 26, 4381–4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecevic N, Chen Y, Filipovic R. 2005. Contributions of cortical subventricular zone to the development of the human cerebral cortex. J Comp Neurol 491(2):109–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Shen EH, Hohmann JG, Oh SW, Bernard A, Royall JJ, Glattfelder KJ, Sunkin SM, Morris JA, Guillozet-Bongaarts AL, Smith KA, Ebbert AJ, Swanson B, Kuan L, Page DT, Overly CC, Lein ES, Hawrylycz MJ, Hof PR, Hyde TM, Kleinman JE, Jones AR. 2012. Large-scale cellular-resolution gene profiling in human neocortex reveals species-specific molecular signatures. Cell 149:483–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan J, Dinov ID, Li J, Zhang Z, Hobel S, Shi Y, Lin X, Zamanyan A, Feng L, Teng G, Fang F, Tang Y, Zang F, Toga AW, Liu S. 2013. Spatial–temporal atlas of human fetal brain development during the early second trimester. Neuroimage 82:115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Richards LJ, Yarowsky P, Huang H, van Zijl PCM, Mori S. 2003. Threedimensional anatomical characterization of the developing mouse brain by diffusion tensor microimaging. NeuroImage 20:1639–1648. [DOI] [PubMed] [Google Scholar]
- Zhang J, Chen Y, Hardwick JM, Miller MI, Plachez C, Richards LJ, Yarowsky P, van Zijl PCM, Mori S. 2005. Magnetic resonance diffusion tensor microimaging reveals a role for Bcl-x in brain development and homeostasis. J. Neurosci 25:1881–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]