Abstract
Chimeric antigen receptor (CAR) T-cell therapy has been transformative for the treatment of B-cell malignancies, with CD19- and CD22-directed CARs being prime examples. However, immunoediting and ensuing antigen loss remain the major obstacle to curative therapy in up to 25% of patients. For example, to achieve the CD19-negative phenotype, malignant cells can pick from a broad array of mechanisms, including focal loss-of-function mutations, dysregulated trafficking to the cell surface, alternative splicing, and lineage switching. In other cases, where resistance is mediated by insufficient antigen density, trogocytosis has been proposed as a possible underlying mechanism. To overcome these barriers, compensatory strategies will be needed, which could include the use of combinatorial CARs, harnessing epitope spreading, and targeting tumor neoantigens.
Keywords: Immunotherapy, immunoediting, chimeric antigen receptors, adoptive T-cell therapy, leukemia, lymphoma, therapeutic resistance, epitope loss, antigen escape, alternative splicing
INTRODUCTION
Hematologic malignancies, especially B-cell leukemias and lymphomas, are generally considered to be “cold” tumors, i.e. to have low mutation burden. Not only does this “coldness” result in the paucity of targetable drivers, but it also makes them largely invisible to the adaptive immune system, trained to recognize non-self antigens. Therefore, immunotherapies for these cancers typically target lineage-, rather than cancer-specific markers. So far, among the surface epitopes, CD19 and CD22 are the most promising targets because of their high expression levels and restricted B-cell expression profile. Successful targeting of these surface markers in the clinic resulted in the recent FDA approval of blinatumomab, a CD19-CD3 bi-specific T-cell engager (BiTE)1 and inotuzumab ozogomycin, a CD22-directed antibody-drug conjugate2. But perhaps the most transformative therapeutics for B-cell malignancies are chimeric antigen receptor (CAR) T-cells, which highlight the promise of this type of immunotherapy in cancer. CAR T-cells (CART) are engineered to express recombinant receptors containing a single-chain variable fragment (scFv) that targets a specific extracellular epitope, an intracellular CD3ζ signaling domain (so-called signal I) and one or more co-stimulatory domains (e.g. 4–1BB or CD28, so-called signals II). scFvs are anchored on the cell surface by short hydrophobic transmembrane domains to ensure formation of proper immunological synapses3.
In 2010, the first adult patient with chronic lymphocytic leukemia (CLL) was successfully treated with a CD19-directed CAR T-cell therapy (CTL019) at the University of Pennsylvania4. Two years later, the first pediatric patient received life-saving CTL019 for B-cell precursor acute lymphoblastic leukemia (B-ALL)5. Subsequent clinical trials using various CD19-directed CARs have shown encouraging results for the treatment of relapsed/refractory leukemias and lymphomas. A phase II ZUMA-1 trial of axicabtagene ciloleucel (YESCARTA®, developed by Kite Pharma) demonstrated a complete response rate of 54% in adult B-cell lymphoma patients mostly within the first month after administration6. In parallel, a phase II ELIANA trial of tisagenlecluecel (KYMRIAH® also known as CTL019, developed by Novartis) in pediatric patients with B-ALL demonstrated an overall remission rate of 81% within 3 months7. Other CD19-directed CAR T-cell trials have yielded similar complete response rates ranging from 60 to 90%8–10. In the last three years, these trials led to accelerated FDA approvals of axicabtagene ciloleucel and tisagenlecluecel for relapsed/refractory B-ALL and B-cell lymphoma. Clinical trials using CD19 CAR T-cells for chronic lymphocytic leukemia (CLL) are ongoing, although lower response rates have thus far precluded FDA approval of CAR T-cell therapies for this disease4,11. CD22-directed CAR T-cells have also shown clinical promise. A recent NIH-based phase I trial in children/adults with relapsed B-ALL treated with an optimized cell dosage of a CD22-directed CAR demonstrated a 73% complete response rate12.
In contrast to CAR T-cell therapies for lymphoid malignancies, CAR T-cell options for the treatment of acute myeloid leukemias have been more limited. Early trials have sought to target CD123 and CD33 but further progress in targeting myeloid markers has been hindered by high toxicity in the myeloid and hematopoietic stem cell compartments13–15.Hence, the subsequent discussion of acquired resistance to CAR T-cell therapies will focus on B-lymphoid malignancies and how selective pressures of potent immunotherapeutics shape their genomes, transcriptomes, and proteomes.
IMMUNOEDITING BY CAR T-CELLS
The concept of immunoediting was first formulated almost 20 years ago to explain how the host’s immune system controls the spread of pre-malignant cells and how these cells evolve to escape these controls16. It postulated the existence of three distinct ‘E’ phases: elimination (when the immune system surveys for and clears transformed cells), equilibrium (when transformed cells that had evaded the first phase are primed with further editing), and escape (when clone variants shaped by immune pressures expand and become clinically dominant). Recent iterations of the 3Es theory hold that both neoplastic and immune cells are shaped by the process of immunoediting17. And although the original concept dealt primarily with endogenous immunosurveillance, it is now being applied to immunotherapy settings, first and foremost checkpoint inhibition18. However, it stands to reason that CAR T-cell therapy is no exception and could be viewed through the lens of immunoediting as well, in particular during the ‘escape’ phase.
Indeed, despite the impressive rates of initial complete responses to current CAR T-cell therapies for B-cell malignancies, longer term follow-up has demonstrated that a significant proportion of patients do relapse after treatment and often do not respond robustly to re-infusions of the same immunotherapeutic. For example, in the ELIANA trial, the rate of relapse-free survival among patients with an initial response dropped from 80% at 6 months to 59% at 12 months7 In order to design the next generation of immunotherapies with better response rates, more durable remissions, and expanded utility beyond B-cell malignancies, it is imperative to understand how cancer cells evade CAR T-cell immunotherapies, develop resistance, and fuel relapses.
One major paradigm borne out across multiple trials using different CD19 CAR constructs is that escape mechanisms could be either leukemia- or T-cell-intrinsic. In the case of CD19-directed CAR T-cells, patient relapses are often binned into CD19-positive and CD19-negative diseases. In the case of CD19-positive relapses, disease recurrence most often results from the loss of CAR T-cell persistence or T-cell dysfunction19,20. In these scenarios, CAR T-cell failures could be attributed to either intrinsic properties of the engineered T-cells (e.g. the paucity of naïve T-cells for in vitro expansion, the lack of central memory T-cells for lasting antitumor effects, etc.) or factors extrinsic to T-cells (immunologic rejection of CAR T-cells, unfavorable microenvironment, etc.)
As important as these mechanisms are, some trials suggest that the majority of relapsed B-ALLs fall into the CD19-negative category. In the ELIANA trial, out of 22 relapse samples assessed for CD19 status by flow cytometry, at least 15 were CD19-negative. This corresponds to an overall CD19 negative relapse rate of 25% (15 relapses out of 61 initial responders)7. Other B-ALL trials have yielded CD19-negative relapse rates between 7 and 24%8,21,22. However, these rates likely underestimate the true incidence, as lengths of follow-up were highly variable, with a good proportion of patients proceeding to transplant shortly after CAR T-cell administration. CD19-negative clones were also seen in lymphoma patients at relapses following CD19-directed CAR T-cell therapy, albeit with lower prevalence than in B-ALL6. Moreover, at least one CLL patient has been reported to relapse with an aggressive CD19-negative plasmoblastic lymphoma after CAR T-cell therapy23. Finally, blinatumomab therapy is also yielding CD19-negative relapses at rates comparable to CD19-directed CAR T-cells, indicating that antigen escape is not a CAR-dependent mechanism of relapse24,25.
These relapse phenotypes, associated with insufficient levels of cognate epitopes on the surface of escaping cells, are the main subject of this review. We will demonstrate that to achieve the CD19-negative phenotype, malignant B-cells utilize a broad array of mechanisms, including focal mutations, dysregulated trafficking to cell surface, alternative splicing, lineage switching, etc. In contrast, relapses after CD22-directed CAR T-cells occur via downregulation of CD22 rather than total CD22 loss. Thus, understanding the strategies which cells employ to achieve antigen loss or low antigen density is crucial to optimizing future immunotherapy targets and regimens26.
MECHANISMS OF CART-DRIVEN IMMUNOEDITING
Mutational mechanisms of antigen loss
Nonsense/frameshift mutations.
Frameshift mutations are a powerful tool of gene inactivation, especially when premature STOP-codons are inserted close to 5’ ends of open reading frames. These mutations typically result in loss of functional protein either through nonsense-mediated mRNA decay or through significantly truncated polypeptide chains. Thus, when our group began examining CD19 negative B-ALL relapses following CTL019 treatment, it was considered a default mechanism of antigen loss27. Interestingly, only one patient out of four (CHOP133) demonstrated a loss of one copy of chromosome 16 (on which CD19 resides) and a frameshift mutation in exon 2 of CD19 on the other allele. Since the variant allele frequency (VAF) was 100%, this classical two-hit event fully explained the observed total loss CD19 expression by flow cytometry.
Subsequently, Orlando et al analyzed 12 patients treated with CTL019 who developed surface CD19 (sCD19)-negative relapses28. De novo frameshift mutations mapping to exons 2–5 were found in all 12 relapsed samples, and copy number analysis confirmed loss of heterozygosity (LOH) in 8 of the 9 patients evaluated. As the transmembrane domain of CD19 is encoded in exon 5, none of the resultant short polypeptides could localize to cell surface, even if translated, consistent with the CD19 negativity by flow cytometry.
Missense mutations affecting protein trafficking.
In Sotillo et al, we also described another sCD19-negative relapse (CHOP105) with segmental chromosome loss spanning the CD19 locus on one allele and an in-frame insertion (with 100% VAF) of 3 codons in exon 2 on the other allele. It was not immediately clear why this gain of amino acids would cause loss of sCD19 expression, as measured by surface staining with the FMC63 antibody recognizing the CTL019 epitope29, especially in light of recent studies implicating exon 4-encoded peptides in recognition by CTL01930. Subsequently, we were able to show that the CHOP105-CD19 exon 2 variant cannot be recognized by FMC63 even in permeabilized cells31, attesting to the importance of exon 2-derived amino acid sequences for recognition by CTL019. Of broader significance, however, was the finding that CHOP105-CD19 was not folded properly and thus was retained in the endoplasmic reticulum. Additionally, it has lost its ability to bind to the tetraspanin CD81. As CD81 is critical for proper trafficking of CD19 from the Golgi complex to the plasma membrane32, even counteracting ER retention would be insufficient to deliver CHOP105-CD19 to cell surface for detection by the cognate CAR T-cell31.
Noncoding mutations in splice sites.
In addition to mutations in the CD19 open reading frame, there are at least two instances where DNA alterations affect splice sites. Such mutations, which are likely to cause intron retention, were identified by Orlando et al in the Novartis B-ALL cohort (28, patient #8) and by Delage et al in diffuse large B-cell lymphoma33.
In summary, focal mutations can mediate antigen escape by preventing the full-length protein from being expressed, by conformationally disrupting the epitope region, by interfering with trafficking to the cell surface, and also by deregulating splicing. Based on existing CTL019 studies, the first mechanism (gene inactivation) appears to be more prevalent, at least in the cohorts studied thus far. However, as the following paragraph will show, both deregulated splicing and impaired trafficking can occur in the absence of identifiable cis-acting mutations, highlighting the role of non-mutational mechanisms.
Non-mutational mechanisms of escape
Despite the prevalence of loss-of-function mutations in sCD19-negative CTL019 relapses, there is also experimental evidence that mutations alone do not account for CD19 negativity in all cases. Indeed, Sotillo et al reported that in patient CHOP101, frameshift mutations in CD19 collectively accounted for no more than 50% of CD19 alleles, yet still led to complete loss of sCD19 expression by flow cytometry27. Similarly, in the Novartis cohort of sCD19-negative relapses, quite a few of the reported mutations were subclonal, even after adjusting for tumor content. For example, in patient #5, the VAF of the indel mutation in exon 2 of CD19 was estimated to be 36%. Collectively, these data suggest that other, post-transcriptional or post- translational mechanisms may be at play.
Alternative Splicing.
The subclonal nature of mutations in some sCD19-negative patients informed initial investigation into post-transcriptional regulation of CD19. RNA sequencing followed by the application of the splicing analysis algorithm called MAJIQ34 revealed that in patient CHOP101 skipping of exon 2 led to the emergence at relapse of the alternatively spliced isoform, Δex2 CD19, which lacked the cognate FMC63 epitope27. In fact, this event could essentially bypass frameshift mutations in exon 2, rescuing the transcript from nonsense-mediated decay in patient CHOP133. Of note, while it is possible that the Δex2 CD19 isoform retains some signaling capabilities of CD19, most of it is sequestered in the ER, away from active B-cell receptor complexes where CD19 normally functions.
Additional studies have demonstrated that CD19 splice isoforms are present in leukemia at the time of diagnosis, suggesting that pressures from CD19-directed therapy can simply select for preexisting treatment-resistant subclones35 rather than require de novo mutations described by other groups28,33. Consistent with this notion, our recent work has identified global dysregulation of splicing as a hallmark of B-ALL, when compared to normal pro-B-cells36. Of high relevance, the list of deregulated splice factors included SRSF3, previously shown to regulate CD19 mRNA splicing and increased inclusion of its exon 227.
Exon 2 is not the only non-constitutive cassette exon in CD19, nor the only one potentially important for immunotherapy. Also increased at relapse was the isoform with skipping of exon 5 and 6. This isoform is predicted to yield a truncated protein without the transmembrane domain, but with a signal peptide and thus with a potential to be secreted. Whether it could act as a soluble decoy for CTL019 remains to be determined.
Epitope Shielding.
While the existence of soluble CD19 isoforms has not been documented, one unusual case of epitope shielding has been described in the literature. In one unique case of post-CTL019 CD19-negative relapse, investigators found that a single leukemic blast had slipped through the T-cell manufacturing process and become positive for both endogenous CD19 and the anti-CD19 CAR37. This allowed the CD19 CAR to bind in cis to the CD19 protein on the same cell, thus shielding the epitope from CD19 CAR T-cells. While this event might be exceedingly rare, it illustrates the power of immunoediting and shows that clonal expansion of a single leukemic cell can underlie therapeutic resistance and subsequent clinical relapses.
Decrease in antigen density.
Loss of the CD19-CD81 interaction described by Bagashev et al31 appears to be a common theme in resistance to immunotherapy. Indeed, Braig et al described a B-ALL patient who developed the sCD19-negative relapse after blinatumomab therapy but had not acquired mutations in either CD19 or CD81. Nevertheless, this leukemia somehow became CD81-negative, as determined by flow cytometry38. This observation led the authors to conclude that loss of sCD19 was due to impaired trafficking to the cells surface.
While the requirement for a chaperone makes CD19 somewhat unique among B-cell differentiation markers, diminished antigen density is emerging as an important immunoediting mechanism, affecting CD19 and CD22 as well as other surface markers. Yu et al have described sequential loss of surface expression of multiple B-cell markers following rounds of CD20, CD30, and finally CD19-directed immunotherapy for primary mediastinal large B-cell lymphoma39. The same group documented significant heterogeneity of surface CD19 and CD22 expression in B-ALL40.
Diminished sCD22 expression has particularly important clinical implications, since CD22-directed CAR T-cells have become another viable treatment option for many patients with CD19-negative relapses. For example, in the initial phase I trials conducted at the NIH by Fry et al, 8 out of 12 patients relapsed within 1 year after receiving CD22 CARs26. Interestingly, 7 out of these 8 patients did not sustain complete antigen loss but instead demonstrated downregulation of CD22 surface expression. This occurred in the absence of any acquired CD22 mutations or changes in mRNA levels, suggesting that surface expression could be regulated at the post-transcriptional level or through generation of alternative isoforms.
Most recently, Hamieh et al published an interesting report illustrating the mechanism by which antigen site density may be decreased41. Using in vivo models to mimic CAR T-cell therapy relapse, they showed that CD19 downregulation can happen through CAR-mediated trogocytosis, a process through which lymphocytes extract surface molecules from the antigen-presenting cells conjugated through the immunological synapse42. Of note, this phenomenon was observed with multiple anti-CD19, anti-CD22, anti-B-cell maturation antigen (BCMA), and anti-mesothelin CARs, demonstrating a pervasive role of trogocytosis in downregulating surface expression even in CAR T-cell therapies that ultimately develop complete antigen loss as a major form of resistance. Interestingly, trogocytosis brought about not only diminished antigen density, but also “fratricide” T-cell killing and hastened T-cell exhaustion. While T-cell-specific inhibitory effects could be partially ameliorated by increasing the ratio of CAR T-cell-to-leukemia cells to facilitate cooperative killing, trogocytosis could be an important connection between tumor cell- and T-cell-intrinsic mechanisms of resistance.
Lineage switching.
Another important mechanism of antigen loss occurs through lineage switching, in which the selective pressure of B-cell marker-directed immunotherapy causes B-ALL to undergo re-programming into a myeloid leukemia expressing none of the B-lineage markers. In humans, lineage switching is most commonly associated with B-ALL or mixed phenotype acute leukemias (MPAL) harboring MLL rearrangements (MLL-r). Prior to the advent of CAR T-cell therapies, it was thought to be a very rare event associated with chemotherapy43,44. However, in a Seattle-based CD19-directed CAR T-cell trial, 2 out of 7 patients with MLL-r B-ALL demonstrated lineage switching at the time of relapse21; similar results were reported by Jacoby et al45. At the cellular level, lineage switching in CD19 CAR T-cell treated patients could be due to either outgrowth of pre-existing CD19-negative myeloid subclones or active reprogramming of differentiated lymphoid cells into myeloid blasts at time of relapse. While the underlying mechanism remains to be elucidated, the existence of well-documented mouse models of lineage infidelity46,47 argues in favor of the second scenario.
THERAPEUTIC IMPLICATIONS AND FUTURE PERSPECTIVES
Surface antigen loss and/or decreased expression have emerged as major mechanisms whereby B-cell malignancies evade CAR T-cell detection (Figure 1). Since immunoedited malignant cells fuel clinical relapses, antigen escape clearly poses a major obstacle to latter-day immunotherapies. There is still much that is poorly understood about resistance to CAR T-cell therapies. On the one hand, some anticipated mechanisms of resistance thus far have not borne out. One salient example is the apparent lack of epigenetic silencing of the target gene, be that CD19 or CD22. Furthermore, there has been no evidence so far for inherent or acquired cross-resistance to immunotherapy. For example, prior exposure to CD19 CAR T therapy appears to have no effect on sensitivity to CD22 CAR T-cells26. One the other hand, new resistance mechanisms continue to be discovered and undoubtedly will be more fully elucidated over time.
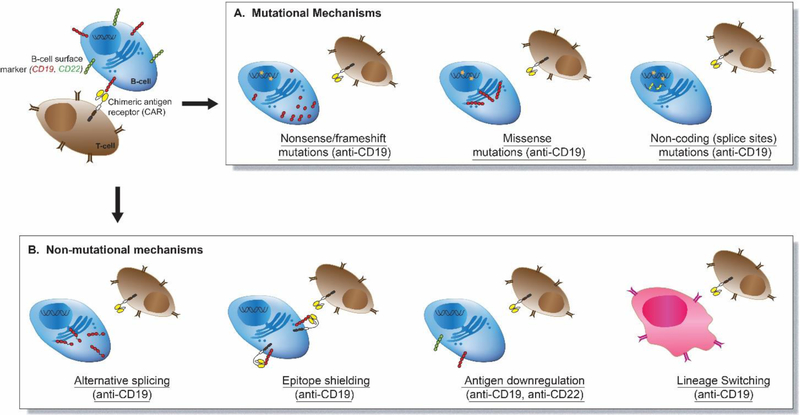
Figure 1. Mechanisms of CAR T cell -driven immunoediting in B-cell leukemia.
CAR T-cell therapies involve the use of genetically engineered T-cells expressing chimeric antigen receptor (CAR) which target B-cells surface markers such as CD19 (red) or CD22 (green). A. Focal mutations in the CD19 gene (orange stars) are known to result in antigen loss. Nonsense mutations lead to expression of non-functional truncated protein; missense mutations mediate antigen escape via retention of the misfolded protein in endoplasmic reticulum; splice site mutations cause deleterious alterations in splicing such as intron retention. B. Non-mutational mechanisms such as alternative splicing, epitope shielding, antigen downregulation, and lineage switching have also been shown to cause acquired resistance to CAR T-cells.
In the future, there is also hope that certain patients might be good candidates for receiving CAR T-cells as an upfront therapy, thereby decreasing toxicities associated with extended chemotherapy regimens and potentially eliminating the need for hematopoietic stem cell transplants. Biomarkers that predict for decreased risk of CAR T-cell relapse would be useful in selecting such candidates. At present, it is still unclear what can be done to anticipate, circumvent, or better yet prevent antigen escapes.
The apparent preservation of cognate antigen expression in at least some patients raises hopes that mutant CD19 isoforms could be targeted with alternative CAR T-cells. However, at least in the case of CD19, this would require additional effort to rescue the mutated or mis-spliced proteins from ER retention and to facilitate their intracellular trafficking. The downregulation of CD22 as a mechanism of resistance also suggests that B-cells may not need complete epitope loss to resist CAR T-cell killing. Rather, there might be a threshold of antigen density below which CARs are ineffective at recognizing the cognate epitope. This has critical implications for CAR T-cell trial design. If targeted antigens are not adequately expressed on the cell surface, inhibition of trogocytosis (by yet to be identified means) or promoting trafficking to the plasma membrane (via the use of chemical chaperones) could be considered as potential adjuvant therapies.
Another idea to overcome antigen escape that has gained considerable traction is the design of combinatorial CARs, which concurrently target multiple antigens. Several phase I trials are underway investigating the combinations of CD19- and CD22-directed CAR T-cell therapy. The therapies can be combined at the level of CAR specificity (“tandem CAR”), vector design (“bicistronic CAR”), CAR T-cell manufacturing (“co-transduction”), or infusion (“co-administration”)48. In addition to CD19 and CD22, other studies are examining various combinations of CD19, CD20, CD22, and CD123 for B-ALL. Combinatorial CARs targeting both CD19 and CD123 may be especially well-suited for MLL-r leukemias as CD123 is expressed on both lymphoid and myeloid blasts49. Further combinations include a trivalent CAR to target CD19, CD20, and CD2250. However, targeting multiple antigens simultaneously still entails the theoretical risk of relapse with multiple antigen-negative disease, which could quickly exhaust other antigen-directed therapy options.
In light of that risk, exploiting “epitope spreading” has been gaining momentum as an alternative strategy to minimizing resistance. Epitope spreading is the phenomenon whereby initial epitope-directed immune responses prime T-cells to induce recognition of closely related but a more diversified set of epitopes, some of which may even reside on other proteins51. This concept has already been shown to aid responses to immunotherapy in melanoma and pancreatic cancer52,53, but thus far, investigators have not been successful at harnessing the power of epitope spreading to induce wider anti-tumor immune responses in B-ALL. This could reflect the overall lower immunogenicity of B-ALL or the immunosuppressive state from the lymphodepletion prior to CAR T-cell administration. However, it is possible that by combining CAR T-cell therapies with other immunotherapies one would boost the anti-tumor immune response to the points where multiple antigens can be recognized54.
As promising new CAR T-cell therapies are translated beyond the realm of hematologic malignancies, the paradigm of antigen escape is likely to persist. Solid tumors have a marked degree of heterogeneity in tumor antigen expression and lack highly expressed and highly conserved cell markers, such as CD19 and CD22. Therefore, the threshold for developing antigen escape is presumably much lower for solid tumors. Indeed, antigen loss has also been demonstrated using CAR T-cell therapies against surface epitopes such has BCMA for multiple myeloma and EGFRvIII for glioblastoma multiforme55,56. Thus, lessons learned from studying resistance mechanisms in hematologic malignancies should benefit CAR T-cell therapies for cancers beyond leukemias and lymphomas. Additionally, given the prevalence of antigen escape, there is a pressing need to maintain robust pipelines for discovery and validation of new immunotherapy targets.
In order to mitigate antigen loss and on-target/off-tumor effects, a guiding principle in target selection is finding an antigen that is highly expressed and ubiquitous across cancer cells, near- absent in normal tissues, and functionally important to cancer maintenance to minimize easy disposal or immunoediting of the antigen11. In reality, various trade-offs are made to accommodate these principles, and antigen escape remains a recurrent problem. A potential long-term solution to this problem is the targeting of tumor neoantigens. Unlike traditional lineage markers like CD19 or CD22, neoantigens hold the promise of minimizing toxicity given their absence, by definition, in normal tissues. Although there has always been significant interest in mutation-driven neoantigens, another useful source of neoantigens might be alternative splicing prevalent in many tumor types57. While most effort in that area has been directed toward intracellular neoantigens, the requirement for MHC-restricted presentation sharply reduces the repertoire of suitable epitopes58. Thus, one might consider focusing on proteins with alternatively spliced extracellular domains which could be targeted by CAR T-cells and antibody-drug conjugates with the selectivity current immunotherapeutics do not possess.
ACKNOWLEDGEMENTS
Relevant research in our laboratory was supported by grants from the NIH (U01 CA232563), V Foundation for Cancer Research (T2018–014), and William Lawrence and Blanche Hughes Foundation. MA and ATT also acknowledge support by St. Baldrick’s- Stand Up to Cancer Pediatric Dream Team Translational Research Grant (SU2C-AACR-DT-27–17).
Footnotes
CONFLICT OF INTEREST STATEMENT
ATT has an interest in intellectual property “Discovery of CD19 Spliced Isoforms Resistant to CART-19.” This interest does not meet the definition of a reviewable interest under Children’s Hospital of Philadelphia’s (CHOP’s) conflict of interest policy and is therefore not a financial conflict of interest. Furthermore, this intellectual property is held by CHOP and has not been licensed or otherwise commercialized to date. However, should this technology be commercialized in the future, ATT would be entitled to a share of royalties earned by CHOP per its patent policy.
REFERENCES
- 1.Topp MS, Gokbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol 2015;16:57–66. [DOI] [PubMed] [Google Scholar]
- 2.Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med 2018;379:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011;365:725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013;368:1509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017;377:2531–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 2018;378:439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JH, Riviere I, Gonen M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med 2018;378:449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015;385:517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014;6:224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pegram HJ, Smith EL, Rafiq S, Brentjens RJ. CAR therapy for hematological cancers: can success seen in the treatment of B-cell acute lymphoblastic leukemia be applied to other hematological malignancies? Immunotherapy 2015;7:545–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nature Med 2017: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mardiros A, Forman SJ, Budde LE. T cells expressing CD123 chimeric antigen receptors for treatment of acute myeloid leukemia. Curr Opin Hematol 2015;22:484–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tasian SK. Acute myeloid leukemia chimeric antigen receptor T-cell immunotherapy: how far up the road have we traveled? Ther Adv Hematol 2018;9:135–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim MY, Yu KR, Kenderian SS, et al. Genetic inactivation of CD33 in hematopoietic stem cells to enable CAR T cell immunotherapy for acute myeloid leukemia. Cell 2018;173:1439–53 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002;3:991–8. [DOI] [PubMed] [Google Scholar]
- 17.Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases--elimination, equilibrium and escape. Curr Opin Immunol 2014;27:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol 2019;16:151–67. [DOI] [PubMed] [Google Scholar]
- 19.Fraietta JA, Lacey SF, Orlando EJ, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med 2018;24:563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fraietta JA, Nobles CL, Sammons MA, et al. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature 2018;558:307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gardner R, Wu D, Cherian S, et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood 2016;127:2406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 2016;126:2123–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans AG, Rothberg PG, Burack WR, et al. Evolution to plasmablastic lymphoma evades CD19-directed chimeric antigen receptor T cells. Br J Haematol 2015;171:205–9. [DOI] [PubMed] [Google Scholar]
- 24.Zugmaier G, Gokbuget N, Klinger M, et al. Long-term survival and T-cell kinetics in relapsed/refractory ALL patients who achieved MRD response after blinatumomab treatment. Blood 2015;126:2578–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Topp MS, Gokbuget N, Zugmaier G, et al. Phase II Trial of the Anti-CD19 Bispecific T Cell-Engager Blinatumomab Shows Hematologic and Molecular Remissions in Patients With Relapsed or Refractory B-Precursor Acute Lymphoblastic Leukemia. J Clin Oncol 2014;32:4134–40. [DOI] [PubMed] [Google Scholar]
- 26.Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med 2018;24:20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sotillo E, Barrett DM, Black KL, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov 2015;5:1282–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orlando EJ, Han X, Tribouley C, et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nature Medicine 2018. [DOI] [PubMed] [Google Scholar]
- 29.Nicholson IC, Lenton KA, Little DJ, et al. Construction and characterisation of a functional CD19 specific single chain Fv fragment for immunotherapy of B lineage leukaemia and lymphoma. Mol Immunol 1997;34:1157–65. [DOI] [PubMed] [Google Scholar]
- 30.Sommermeyer D, Hill T, Shamah SM, et al. Fully human CD19-specific chimeric antigen receptors for T-cell therapy. Leukemia 2017;31:2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bagashev A, Sotillo E, Tang C-HA, et al. CD19 alterations emerging after CD19-directed immunotherapy cause retention of the misfolded protein in the endoplasmic reticulum. Mol Cell Biol 2018;38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shoham T, Rajapaksa R, Boucheix C, et al. The tetraspanin CD81 regulates the expression of CD19 during B cell development in a postendoplasmic reticulum compartment. J Immunol 2003;171:4062–72. [DOI] [PubMed] [Google Scholar]
- 33.Delage L, Manzoni D, Quinquenet C, et al. Molecular analysis of a CD19-negative diffuse large B-cell lymphoma. Haematologica 2019;104:e114–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaquero-Garcia J, Barrera A, Gazzara MR, et al. A new view of transcriptome complexity and regulation through the lens of local splicing variations. eLife 2016;5:e11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fischer J, Paret C, El Malki K, et al. CD19 isoforms enabling resistance to CART-19 immunotherapy are expressed in B-ALL patients at initial diagnosis. J Immunother 2017;40:187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Black KL, Naqvi AS, Asnani M, et al. Aberrant splicing in B-cell acute lymphoblastic leukemia. Nucl Acids Res 2018;46:11357–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruella M, Xu J, Barrett DM, et al. Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nature Medicine 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braig F, Brandt A, Goebeler M, et al. Resistance to anti-CD19/CD3 BiTE in acute lymphoblastic leukemia may be mediated by disrupted CD19 membrane trafficking. Blood 2017;129:100–4. [DOI] [PubMed] [Google Scholar]
- 39.Yu H, Sotillo E, Harrington C, et al. Repeated loss of target surface antigen after immunotherapy in primary mediastinal large B cell lymphoma. Am J Hematol 2016;92:E11–E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenthal J, Naqvi AS, Luo M, et al. Heterogeneity of surface CD19 and CD22 expression in B lymphoblastic leukemia. Am J Hematol 2018. [DOI] [PubMed] [Google Scholar]
- 41.Hamieh M, Dobrin A, Cabriolu A, et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature 2019;568:112–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joly E, Hudrisier D. What is trogocytosis and what is its purpose? Nat Immunol 2003;4:815. [DOI] [PubMed] [Google Scholar]
- 43.Rossi JG, Bernasconi AR, Alonso CN, et al. Lineage switch in childhood acute leukemia: an unusual event with poor outcome. Am J Hematol 2012;87:890–7. [DOI] [PubMed] [Google Scholar]
- 44.Rayes A, McMasters RL, O’Brien MM. Lineage switch in MLL-rearranged infant leukemia following CD19-directed therapy. Pediatr Blood Cancer 2016;63:1113–5. [DOI] [PubMed] [Google Scholar]
- 45.Jacoby E, Nguyen SM, Fountaine TJ, et al. CD19 CAR immune pressure induces B-precursor acute lymphoblastic leukaemia lineage switch exposing inherent leukaemic plasticity. Nat Commun 2016;7:12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu D, Allman D, Goldscmidt M, Atchison M, Monroe JG, Thomas-Tikhonenko A. Oscillation between B-lymphoid and myeloid lineages in Myc-induced hematopoietic tumors following spontaneous silencing/reactivation of the EBF/Pax5 pathway. Blood 2003;101:1950–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Busslinger M, Nutt SL, Rolink AG. Lineage commitment in lymphopoiesis. Curr Opin Immunol 2000;12:151–8. [DOI] [PubMed] [Google Scholar]
- 48.Majzner RG, Mackall CL. Tumor antigen escape from CAR T-cell therapy. Cancer Discov 2018;8:1219–26. [DOI] [PubMed] [Google Scholar]
- 49.Ruella M, Barrett DM, Kenderian SS, et al. Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J Clin Invest 2016;126:3814–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fousek K, Watanabe J, George A, et al. Targeting primary pre-B cell acute lymphoblastic leukemia and CD19-negative relapses using trivalent CAR T cells. Blood 2017;130:4614. [Google Scholar]
- 51.Bollard CM, Gottschalk S, Torrano V, et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J Clin Oncol 2014;32:798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beatty GL, O’Hara MH, Lacey SF, et al. Activity of Mesothelin-Specific Chimeric Antigen Receptor T Cells Against Pancreatic Carcinoma Metastases in a Phase 1 Trial. Gastroenterology 2018;155:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chapuis AG, Roberts IM, Thompson JA, et al. T-Cell Therapy Using Interleukin-21-Primed Cytotoxic T-Cell Lymphocytes Combined With Cytotoxic T-Cell Lymphocyte Antigen-4 Blockade Results in Long-Term Cell Persistence and Durable Tumor Regression. J Clin Oncol 2016;34:3787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Majzner RG, Heitzeneder S, Mackall CL. Harnessing the immunotherapy revolution for the treatment of childhood cancers. Cancer Cell 2017;31:476–85. [DOI] [PubMed] [Google Scholar]
- 55.O’Rourke DM, Nasrallah MP, Desai A, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ali SA, Shi V, Maric I, et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood 2016;128:1688–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Slansky JE, Spellman PT. Alternative Splicing in Tumors - A Path to Immunogenicity? N Engl J Med 2019;380:877–80. [DOI] [PubMed] [Google Scholar]
- 58.Kahles A, Lehmann KV, Toussaint NC, et al. Comprehensive analysis of alternative splicing across tumors from 8,705 patients. Cancer Cell 2018;34:211–24 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]