Abstract
A US army-wide measles outbreak in 1917–18 resulted in more than 95 000 cases and more than 3000 deaths. An outbreak investigation implicated measles and streptococcal co-infections in most deaths, and also characterised a parallel epidemic of primary streptococcal pneumonia in soldiers without measles. For the first time, the natural history and pathogenesis of these diseases was able to be well characterised by a broad-interdisciplinary research effort with hundreds of military and civilian physicians and scientists representing disciplines such as internal medicine, pathology, microbiology, radiology, surgery, preventive medicine, and rehabilitation medicine. A clear conceptualisation of bronchopneumonia resulting from viral–bacterial interactions between pathogens was developed, and prevention and treatment approaches were developed and optimised in real time. These approaches were used in the 1918 influenza pandemic, which began as the measles epidemic waned. The outbreak findings remain relevant to the understanding and medical management of severe pneumonia
Background
The widely known 1918 Spanish influenza pandemic killed thousands of soldiers in US army training camps.1 However, 1 year earlier a less well known respiratory disease epidemic of even higher case-fatality than the Spanish influenza—measles—struck the same camps. The investigation of measles led to medical breakthroughs and created the blueprint for the military response to the 1918 influenza pandemic.
In 1917, measles was a suspected viral disease that developed in 2–3 year cycles in large cities and irregularly in rural areas.2–5 US data for the 1917–19 annual incidence cycle recorded 178 522–529 498 annual hospital admissions from measles and 2316–9944 annual deaths,6 mostly in children under 10 years old. Despite a stable incidence in the US military, between the Civil War (1861–65) and the Spanish–American War (1898), measles mortality dropped from 2∙0 to 0∙32 cases per 1000 troops,7 diminishing its importance before World War 1.
Troop expansion related to World War 1 from 217 272 to 1 538 203 men during 1917 was achieved through enlistment and draft-based mobilisation associated with construction of nearly 40 city-sized mobilisation camps for the army and National Guard throughout the USA (appendix p 1).8,9 The aggregate annual strength of the Army from April 1, 1917, through to 1919 was 4128479 (3922097 enlisted men and 206 382 officers).7,9,10 Some army-wide data only represent enlisted or white enlisted men.
Despite screening (appendix p 2),11 undetected measles exposures before recruits arrived seeded camps with incubating cases in summer 1917, when most camps were not fully operational, leading to widespread epidemics by early October and continuing into 1918 (figure 1 and appendix p 3).9,12 Epidemic descriptions with supporting data for individual camps have been published (appendix p 3).5,6,13,14
Figure 1: Admissions to hospital for selected diagnoses, and number of troops in the US Army.
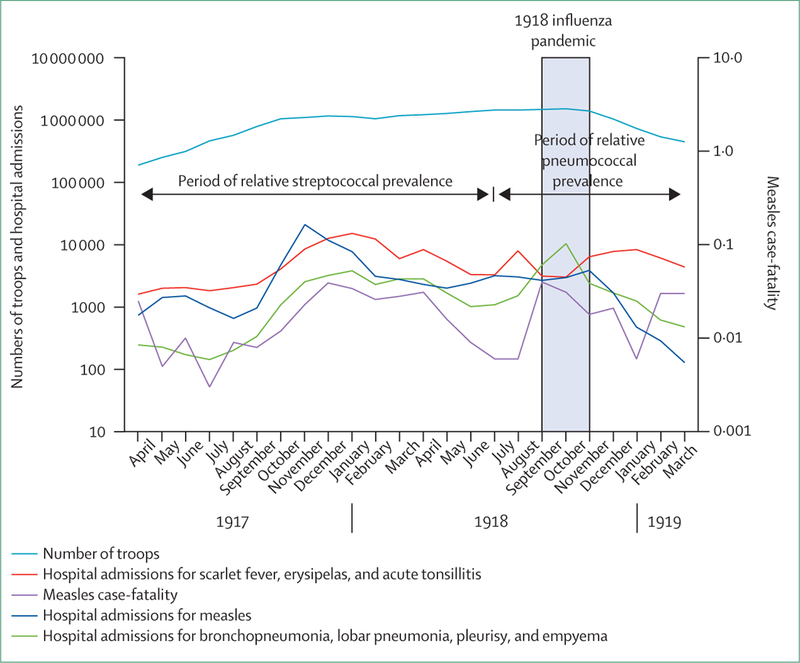
Data for 39 US army mobilisation camps combined, by month, from April 1, 1917, to Dec 31, 1919.9
Army measles epidemiology
For reasons that were initially unclear, measles in 1917–18 produced the highest infection rates in 97 years of continuous military surveillance7 and extreme case-fatality from aggressive bronchopneumonias and other complications. Infection rates were highest in camps whose recruiting areas included rural and remote locales where susceptibility to measles was most common (eg, the south, mid-west, and non-coastal west USA; appendix p 33).13,15,16 Severity statistics were striking:12,17,18 army wide, 95 843 measles cases occurred,10 22 809 (23∙8%) people were admitted to hospital for complications, and 3206 people died (appendix p 6).9,10
Combined camp data show the epidemic peaked in November, 1917, and June to November, 1918, coincident with mobilisation surges (figure 1). Within camps, severe measles developed suddenly, was independent of measles incidence (figure 1), and happened at different dates between camps.13 Bacterial complications after measles infection, including pneumonias, developed in as many as 50% of encamped soldiers, half or more of which were severe or fatal.17–19 Empyema, historically diagnosed in only about 2∙2% of measles-related pneumonias,20 developed in up to 90% of all pneumonia cases.21 The case-fatality of early epidemic empyema reached 90%.8,17,22–35 At Camp Pike (Little Rock, AR) measles is thought to have killed more soldiers than the 1918 influenza pandemic.14
A military pneumonia commission24 documented not only specific postmeasles pneumonias but also an epidemic of severe and fatal primary pneumonia or empyema, or both, in soldiers without measles, which paralleled the measles case-fatality rather than measles incidence (figure 1).9,14,27,35 Despite the extreme severity of the primary bacterial pneumonias, soldiers with measles were ten times more likely to die of secondary bacterial pneumonia or pneumonia with empyema than those without measles. This risk elevation resulted from about three-times higher risk of acquisition of pneumonia or pneumonia with empyema after measles than in people without measles and about three-times higher risk of dying from such a complication when it occurred (appendix).14,22,28,36–40
The effects of bacteria on measles complications
In most army camps, the predominant cause of post-measles pneumonia and of primary pneumonia was Streptococcus haemolyticus, now known as Streptococcus pyogenes,25,37,41–43 typically isolated in pure culture from pneumonias, empyemas, pleural effusions, and from other postmeasles complication sites.5,17,19,22–24,26,38,44–46 Streptococcus pneumoniae type 1, 2, 3, or group 4 (including many previously untypeable pneumococci) predominated in a few camps, but S haemolyticus was common and caused highly fatal superinfections of postmeasles pneumococcal pneumonias.14,22,23,28,29,33,37,47,48 The same phenomenon was noted in autumn 1918, when individuals with postinfluenza pneumococcal pneumonias were often superinfected with streptococci.14,49,50 In at least two camps, high pneumococcal detection rates might have resulted from laboratory misidentification of S haemolyticus as a group 4 pneumococcus.40,51
Epidemics of severe measles and of primary pneumonia with empyema coincided temporally with so-called carriage epidemics of asymptomatic S haemolyticus colonisation.39 Measles and streptococcal-carriage infections predominantly affected newly arrived recruits, therefore greatly increasing their pneumonia risk.52,53 The incidences of streptococcal colonisation in arriving barracked recruits rose in a matter of days from less than 5% to as high as 87∙5%,45,52 and was pronounced in clusters in small group tents (appendix).8,11,12,22,27,29,54 High-incident nasopharyngeal acquisition of streptococcus also occurred in soldiers admitted to open hospital wards for any reason (appendix p 5).11,24,27,53 Streptococcal carriers typically remained colonised for months,10,40,45 and epizootics spread in camp horses and in hospital laboratory research mice and guineapigs.46,55
Epidemiological investigation strongly implicated silent-streptococcal carriage as a risk factor for severe or fatal measles. Camp Wilson (formerly Camp Travis; San Antonio, TX), documented 53 secondary measles pneumonias in 158 streptococcal carriers (34%) versus seven secondary pneumonias in 294 non-carriers (2%), a 15-times difference.19 In an era in which cohort studies were essentially unknown, physicians at Camp Zachary Taylor in Louisville, KY, prospectively cultured 388 sequential patients with measles for nasopharyngeal streptococci on admission to hospital and cohorted them in separate wards on the basis of presence or absence of streptococcal carriage. In the negative ward, patients were recultured daily, and those who became positive for streptococci were transferred to the other ward (figure 2).11,45,56–60 Serious and fatal complications were restricted entirely to patients who were streptococcal carriers, and 96% of minor complications were in streptococcal carriers (table).45
Figure2: Images of the 1917–18 army measles epidemics.

(A) Measles isolation ward, Camp Zachary Taylor, Louisville (KY, USA), in winter 1917–18; army isolation principles and practices during the epidemic were often elaborate.11,45,56–59 (B–E) Thousands of surgically thoracotomised soldiers who survived postviral bacterial empyema, especially those associated with Streptococcus haemolyticus, had severe and permanent disabilities.60
Table:
Carriage status of patients with complications associated with incident measles in Levy and Alexander’s Camp Zachary Taylor cohort study45
| Number of patients |
Serious complication* |
Mild Complication† |
All complications | |
|---|---|---|---|---|
| Non-carrier | 89 | 0 | 4 | 4 |
| All carriers combined | 299 | 64 | 98 | 162 |
| Heavily infected‡ | 251 | 62 | 85 | 147 |
| Slightly infected‡ | 48 | 2 | 13 | 15 |
| All measles patients | 388 | 64 | 102 | 166 |
Patients were sent to so-called dirty or clean wards on the basis of the presence or absence of Streptococcus haemolyticus (Streptococcus pyogenes) on admission. Negative patients were recultured daily and transferred if they became positive.
Serious (and fatal) complications included bacterial bronchopneumonia, empyema, meningitis, and peritonitis.
Mild complications included those that were self-limited and medically manageable: otitis media, sinusitis, tonsillitis and peritonsillar abscess, cervical adenitis, bronchitis, and erysipelas. The only complications detected in the 89 non-carriers were two cases of bronchitis and one each of tonsillitis and cervical adenitis.
Positive streptococcal culture plates were prospectively graded as heavily infected and slightly infected (ie, colonised) on the basis of subjective determinations of essentially all plate colonies being of S haemolyticus versus an occasional S haemolyticus colony. Within wards, patients were isolated by the cubicle technique, and isolation precautions were enforced (figure 2).
During the streptococcal-carriage epidemics, incidences of scarlet fever, erysipelas, and acute tonsillitis all rose, peaking from November, 1917, to March, 1918 (figure 1).9,21 After implementation of streptococcal control measures in early 1918,22 pneumonia incidence and measles complications decreased (figure 1).60 Investigators concluded that severe postmeasles pneumonia with or without empyema, primary S haemolyticus pneumonia, and scarlet fever, erysipelas, or tonsillitis, all resulted from epidemics of streptococcal acquisition or carriage related to crowding of people and mass associations during military training.19,25,28,39,40,61,62
Information about other streptococcal complications that might have arisen after the camp streptococcal carriage and scarlet fever epidemics would be of interest for further investigations. Unfortunately, the army data are severely limited by their ability to link streptococcal complications to most other diagnostic categories. The data do not allow the association of streptococcal complications with scarlet fever-associated infections versus non-associated infections. Acute glomerulonephritis was not a specific army diagnostic category, and renal diseases generally were not well represented in any diagnostic category. Presumably haematuria and proteinuria were widely seen in streptococcal infections, but were not deemed to be of great importance for diagnosis, and thus were not categorised. Additionally, rheumatic fever was not a well characterised disease in this era, and was not recorded in army records. However, the 1917–18 winter–spring and the 1918 autumn increases in scarlet fever and in measles case-fatality were associated with corresponding increases in the incidence of acute articular rheumatism. Curiously, the incidences of acute myocarditis and of mitral insufficiency rose and peaked during only one time interval, between November, 1918, and March, 1919 (ie, 1–5 months after the influenza pandemic, with the highest monthly incidence in December, 1918).9 All recruits were screened for cardiac disease before entering into active duty, and thus, recruits with pre-existing mitral insufficiency, heart murmurs, or evidence of rheumatic fever or heart disease, would presumably have been excluded from the Army.
Clinical, pathological, and radiological measles complications
Although uncomplicated camp measles was otherwise clinically unremarkable, complicated cases typically developed early pronounced laryngitis and tracheitis leading to bronchitis, persistent cough, and diffuse moist rales.23,24,62–65 State-of-the-art radiographic facilities were available in most camps.65,66 and army physicians were skilled in clinically diagnosing common low-fatality endemic pneumonia (historically pneumococcal lobar pneumonia).67 In the absence of typical pneumonic signs and symptoms, physicians recognised that laryngo-tracheal and bronchitic signs nevertheless predicted pneumonia development,17,37 and they looked for early lung changes with chest radiography, a relatively new technique still being optimised for non-tuberculosis clinical diagnosis.13
Disappointingly, postmeasles pneumonia was rarely detected by either physical examination or radiography within the first few days of symptom onset in the lower respiratory tract.68 Clinical, radiological, and pathological correlations from hundreds of patients soon explained why pneumonias went underdetected: most severe measles pneumonias were not lobar pneumonias but bronchopneumonias (figure 3),13,14,52,61,63,65 which in early stages of development did not typically produce areas of pulmonary consolidation of sufficient size to be detected by radiography, percussion, assessment for tactile fremitus, or other diagnostic assessments. Only later in the infectious process, when small nodular areas of peribronchial consolidation progressed locally to lobular involvement, or to a picture mimicking that of lobar pneumonia, or to development of large effusions (figure 3),28 did a clinically or radiographically diagnosable disease become apparent. The measles epidemic in the Army was among the first tests of standardised chest radiography in a major respiratory epidemic.
Figure3: Postmeasles streptococcal complications.
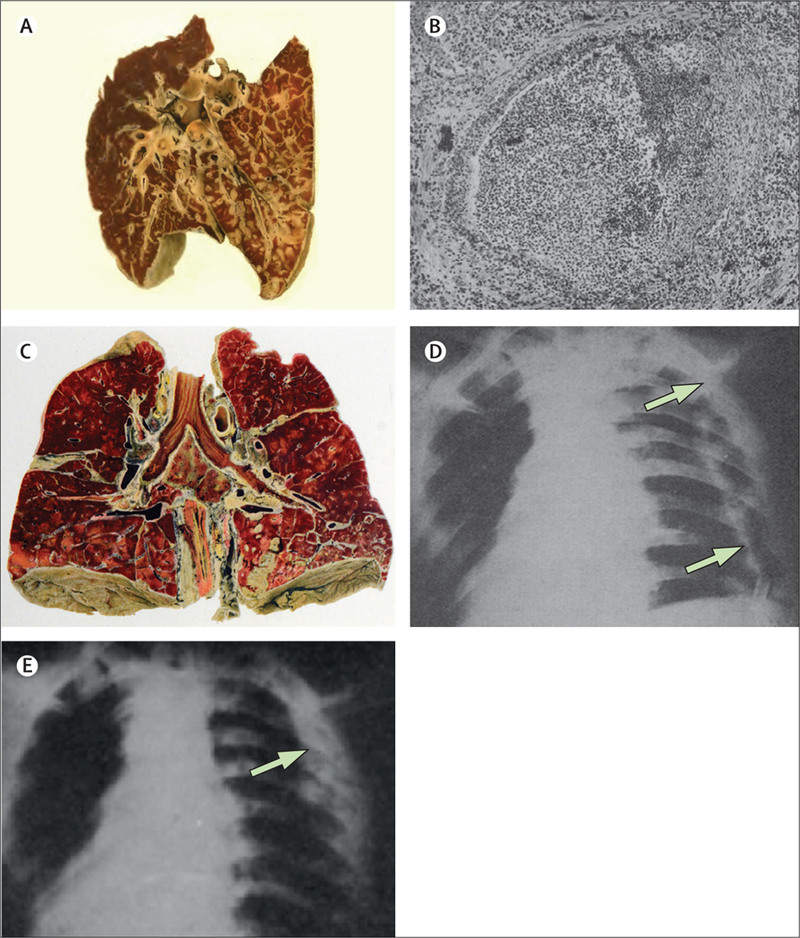
(A) Typical streptococcal bronchopneumonia,61 with early stage micronodular consolidation extending along and around the bronchioles, and sometimes extending to the lung periphery to break out as subpleural abscesses potentially leading to empyemas. Near universal early laryngitis and tracheitis suggested infectious spread in a distal direction along the bronchial tree. (B) Bilateral postmeasles streptococcal bronchopneumonia.28 A bronchus is filled with purulent exudate, with transmural inflammation and necrosis of the bronchial wall (right side of bronchus). In the pulmonary tissue, bacteria were concentrated more in the interstitium than in alveoli,63,64 and pathologists claimed “an apparent inability [of Streptococcus haemolyticus] to enter the tissue proper.”13 (C) Interstitial bronchopneumonia with bilateral empyema and fibrinous pericarditis due to S haemolyticus.62 (D) Patient with air-filled empyema cavity, its walls indicated by arrows, and a tube entering the cavity at the bottom. (E) The same patient treated with repeated drainage followed by irrigation with Dakin’s solution; after negative pressure suction, the thickened visceral pleura lies against the chest wall.31
Autopsy pathology and post-mortem bacteriology
In 1917, bronchopneumonia was understood best as a post-mortem pathological diagnosis rather than as a clinical description, and its association with epidemic viral respiratory disease was not fully appreciated. The preeminent pathologist of the camp epidemics, Johns Hopkins’ William MacCallum (1874–1944), coined the term interstitial bronchopneumonia to describe 1917 measles (and later, 1918 influenza) autopsy findings (figure 3).14,24,28,63,69 In most of these measles autopsies, S haemolyticus was the predominant organism,19,23,24,28,38,51 causing a breakdown of bronchial and bronchiolar walls along the respiratory tree, purulent intraluminal exudate, streptococcal pulmonary lymphangitis with lymphatic spread, early pleuritis, pleural effusions, and empyemas. These empyemas tended to loculate, to lead to pericarditis, and to cause sepsis (figure 3). 13,14,40
With or without empyema, postmeasles pneumonia deaths were caused by cardiac failure, septicaemia or, in cases with early rapid progression of pneumonia, by severe diffuse cyanotic respiratory distress (as would later be seen in the 1918–19 influenza pandemic).49 These clinical and pathological features seem to correspond to what would now be called acute respiratory distress syndrome,70 and apparently represented suffocation from diffuse lung oedema, or diffuse bronchiolar obstruction, or both.36 Retrospectively, highly similar but not fully appreciated, postmeasles bronchopneumonias had been described as long ago as the US Civil War,71 if not earlier,64,72 and had also been noted for years in sporadic paediatric measles deaths. These bronchopneumonias showed similar streptococcal pathological changes and seem to have been associated with previous respiratory viral infections,13,18 presumably including seasonal influenza and other viruses. The importance of secondary bacterial infections in severe disease progression led one observer to state, “no patient died of measles”.16
Prevention and infection control of measles
In recognition of the aetiological link between naso-pharyngeal-streptococcal carriage and postmeasles and primary pneumonia, all army camps strengthened infection control practices. Some of these measures included longer and more stringent isolation of arriving recruits than previously; policing to prevent congregation in barracks, canteens, theatres, Young Men’s Christian Associations, and elsewhere; head-to-foot staggering of adjacent barrack cots; continual wet mopping and disinfection of floors, walls, tables, door knobs; repeated (and questionably efficacious)10,45,73 throat gargling or spraying with disinfectant solutions; improved barracks ventilation; tent-exhaust modifications and daytime breakdown of tents.6,11,39,53,56–58 In hospital wards, where high streptococcal carriage was sometimes found in ward personnel,11,40 all staff wore gowns and gauze masks, changed them, and washed hands with soap, water or disinfectants between contact with patients, which were uncommon practices before 1917. Some wards mandated that staff wear rubber gloves (figure 2). Patients were placed in cubicle isolation, hanging bed sheets from wires strung around the bed, and were required to wear masks (figure 2). Cubicle sheets were sometimes periodically sprayed with disinfectants. Masked patients were only allowed into lavatories one at a time under attendant supervision. Patients with measles and pneumonia were isolated on separate wards from those without pneumonia.10 Streptococcal vaccines were quickly produced and given, but without clear benefit.10,28,69,73–75
In anecdotal before and after observations, general belief was that logistic’ sanitary’ and nursing interventions reduced bacterial carriage.5,11,16,22,37,52,53,56–58,76,77 By the time of the onset of the influenza pandemic (September and October’ 1918)’ streptococcal carriage and diseases had both greatly decreased in incidence. Streptococci caused a significantly lower percentage of postinfluenza pneumonias in 1918 than had been the case with postmeasles pneumonias.23,49,50 However’ the efficacy of streptococcal control measures was never proven scientifically.
Treatment
In the preantibiotic era’ pneumonia treatment was directed at the prevention and prompt management of complications’ including treatment with digitalis and cardiac stimulants (caffeine’ camphor’ and epinephrine) to prevent pneumonia-associated cardiac failure.17 Camphor was also used for coryza’ and camphor or codeine for cough’ with morphine for cough or pain,23,36 and drainage of or operation on empyemas.10,11,34,59,60,78,79 Antistreptococcal hyperimmune horse serums were given intravenously or intrapleurally’ with little or no benefit.10,28,33,36,55 Radiology assumed an increasingly important role in pneumonia management and particularly in empyema management.18,21–23,31,35,65
The streptococcal empyema epidemic was studied on an emergency basis by an Army Empyema Commission,34 whose recommendations greatly improved management and probably saved many lives.34,59,60,76 Early thoracotomy for effusion or empyema caused sudden cardiac deaths, seemingly by producing ipsilateral lung collapse that could not be immediately compensated by diseased contralateral lungs.11,18–21,23,27–37,56,59,60 The results from clinical and radiological studies showed the natural course of S haemolyticus pleural effusions and empyemas and their optimum medical and surgical management, including cannulation, aspiration, or irrigation of empyema cavities with rubber tubes and repeated instillation of Dakin’s solution;31,77,80,81 and non-aggressive periodic or daily tapping of early effusions as fluids gradually changed from straw-coloured or rose-coloured to frank pus, at which time safe thoracotomy was possible.11,76 Empyema management became an important part of surgical practice during the rest of the war; recovery usually extended over many months and left soldiers weakened, emaciated, and often permanently disabled (figure 2).60 The number of patients with postmeasles and postinfluenza empyema who had thoracotomies eventually rose into the thousands, creating a substantial public health and disability issue in the military.59,60
Discussion
The response to the 1917–18 measles epidemic was a striking moment in time when medicine became recognisably modern. This period is notable for integration of comprehensive findings from multidisciplinary investigations to control an explosive epidemic in so-called real time. The broader understanding derived from such correlative information stimulated both clinical and basic research, and improved science-based clinical management (eg, infection control, bacteriological diagnosis, and medical, surgical, and radiological management of empyemas). As a result of caring for patients in this epidemic, many medical disciplines were transformed in the process. In hindsight, the experience of the measles epidemic was important in the control of the influenza pandemic that followed, the response to which closely copied successful features of the response to the measles epidemic.10
The medical literature on military measles epidemics, including severe and complicated cases, goes back to the early 19th century, especially in the French and Russian military literature (accessible in standard medical literature indices). Although occasional cases or outbreaks of complicated measles associated with streptococcal infections had been reported in the past, and were again reported during World War 1, no other severe measles epidemics of similar scale were reported by other militaries worldwide.
Measles research findings characterised the natural history and pathogenesis of an important epidemic disease and therefore helped control it. Autopsy documentation of asynchronous changes representing different stages of the disease process within and between patients strongly implied a natural history of infection. The process started with nasopharyngeal viral infection that spread directly down the respiratory tract to cause viral laryngitis or tracheitis with hoarseness, aphonia, and barking cough, after which infection often proceeded into the bronchi and bronchioles, removing the epithelium of goblet and ciliated cells, and impairing mucociliary clearance. Unless patients carried pneumopathogenic nasopharyngeal bacteria such as streptococci or pneumococci, repair of such epithelial damage occurred quickly, and the patient recovered in a week or so. If, however, nasopharyngeal pneumopathogenic bacteria were present, they could then spread through the direct bronchial or bronchiolar extension, adhering to and destroying basal cells and peribronchiolar tissue along the way, leading to early bronchopneumonia, progressing to consolidation, clinically and radiographically simulating lobular and lobar-like pneumonias. Streptococcal empyema could develop at any point in the process, either via bronchiolar or retrograde lymphatic extension.64 The bronchopneumonic pattern was thought to suggest immune resistance52,72,82 (a term apparently used non-specifically); empyema became recognised as a result not of pneumonia severity, but of rapid bronchiolar disease progression.36 Many empyema patients had no clinical or radiological evidence of pneumonia at all. Such a sequence of events is consistent with the anatomical pattern of bronchopneumonia, in which early lesions were bilateral, diffuse, peribronchiolar, and without substantial early radiographic or clinical findings. Measles pneumonia came to be viewed as an active process rather than a diagnostic entity, a series of interacting events involving virus, bacteria, and hosts that show the distal progression of infection.18,27,28,36,38,51,61,63,72,74,83–87 These army studies showed that unlike lobar pneumonia, which might develop through aspiration or haematogenous bacterial spread, bronchopneumonia and empyema were both manifestations of severe pan-bronchitis caused by interactions between virus and bacteria.
Understanding viral-bacterial co-pathogenesis proved crucial months later when the influenza pandemic caused thousands of post-influenza bacterial pneumonias similar or identical in their epidemiology, pathogenesis, and their pathological, bacteriological, and radiological features, to postmeasles complications (panel). Pathological studies of both epidemics led some observers to judge them as a single epidemic entity featuring a shared natural history of development, one in which pneumopathogenic bacteria spread distally along epithelially denuded airways to initiate bronchial, pulmonary, and pleural disease:62,63,85 such inciting damage was thought of as a generic effect of cytolytic viral respiratory agents, in one year measles, and in the next influenza.48,52,56,69,72,82–84,90–92
The lessons learned from both epidemics might still be relevant. Both showed the importance of viral and bacterial copathogenesis, pointing to common pathogenic pathways that constitute potential targets for diagnosis, prevention, and treatment. For example, in the modern era many, if not most, pandemic influenza deaths still result from secondary bacterial pneumonia,50,93–95 although in the antibiotic era Staphylococcus aureus supplanted streptococci and pneumococci in importance.93,94 We suspect that many or most seasonal postinfluenza pneumonias arise from similar mechanisms,50,94 and that some other severe endemic pneumonias result from analogous bacterial interactions with cytolytic viruses such as parainfluenza virus type 3, respiratory syncytial virus, or human metapneumovirus.94
Presently, tens of thousands of annual deaths in the USA result from secondary pneumonias,96 with more than a million annual hospital admissions, 20–40% of which are complicated by empyemas.97,98 These data show the need for better understanding of how such processes occur and how they can be prevented. If they result from copathogenc mechanisms involving epidemic or endemic viruses and pneumopathogenic nasopharyngeal bacterial flora, as we believe, greater physician awareness and more aggressive, targeted prevention and treatment is crucial, including appropriate early use of antivirals and antibiotics. This is especially needed when signs and symptoms of viral inflammation of the lower-respiratory tract raise the possibility of impending progression to bronchopneumonia. The sobering observation that before becoming clinically obvious, many bronchopneumonias in 1917–18 had already progressed to a point where recovery was unlikely, suggests that to be effective, aggressive antibiotic treatment must begin at the earliest possible stage and that a critical need exists for early biomarkers and point-of-care diagnostics of sufficient sensitivity to detect early stages of this process. Routine sputum and nasopharyngeal bacterial cultures might also be helpful in detecting pneumopathogenic bacteria and guiding antibiotic treatment, in addition to early invasive diagnostic procedures.
In 1917–18, pneumonia severity was widely believed to be a result of new virulent streptococcal strains, perhaps somehow enhanced in their virulence by high-incident spread in crowded places with soldiers susceptible to infection.11,16,17,25,63,65,86,91,99,100 Until bacterial genomes from the 1917–18 epidemics are studied, this hypothesis cannot be investigated. However, some communities surrounding affected army bases experienced streptococcal epidemics of unprecedented severity, which were attributed to exported camp strains.25,86,100 Importantly in 1911, the USA began to experience widespread epidemics of severe or fatal septic sore throats caused by human-derived streptococci from milk from cows with garget (inflamed udders) and national winter epidemics of similarly severe streptococcal disease unassociated with milk. Both epidemics were widely attributed to new pathogenic strains of S haemolyticus.25,101 In the modern era, severe streptococcal epidemics have been linked to pathogenic bacterial clones (eg, M59 outbreaks in Montana and Wyoming102 and horizontally acquired streptococcal M1 genetic virulence factors).103
Historical data also support sudden appearance and spread of streptococci causing extremely high scarlet-fever mortality.104 In this context, one of the most puzzling unsolved medical mysteries of the 19th century is the occasional unexpected appearance of highly fatal measles epidemics, especially in indigenous native populations and in the poor communities, such as the infamous 1875 outbreak in Fiji, known as the virgin soil epidemic, which killed 30% of all native Fijians in a few weeks.105,106 The Fiji epidemic became a eugenics-era metaphor for the notion that infectious diseases routinely select against indigenous populations—the high Fijian mortality was widely ascribed to genetic selection of measles against “a primitive Oriental people.”106 A British commission107 on this topic concluded that Fijians had “premature civilisation” and “degeneracy of the people as a race” (Fijians are actually an eclectic community of Melanesians and Polynesians), prompting a British policy to import workers from the Indian subcontinent to (hopefully) interbreed with Fijians and therefore “revitalize” their “race”.
The US army measles epidemics offer an alternative explanation for such unexpectedly fatal measles; that it might have resulted from bacterial copathogenesis (possibly among a population with vitamin A deficiency108). Hints of this possibility were noted several years before the army epidemics (eg, in paediatric and other civilian populations,109–115 in Ellis Island data showing high measles mortality in immigrant children with concomitant scarlet fever,116 and in 1914 data from Scottish Highland military recruits showing elevated measles mortality in units with one or more cases of scarlet fever).117 The 1875 Fiji epidemic was caused by measles imported on a ship of several hundred people arriving from Sydney, NSW, Australia, at a time when Sydney had a scarlet fever epidemic.105 Neither Fijians who escaped measles infection in 1875, nor descendants of either individuals who did not survive the measles outbreak or survivors have since been proved susceptible to measles complications, apparently debunking a central tenet of the virgin soil explanation of measles mortality. Nevertheless, the possible association in the army epidemics of highly pathogenic streptococcal strains, including strains that could have been copathogenic in the context of measles infection, has not been ruled out. With rapid whole-genome sequencing of multiple streptococcal strains now practical,102,103 and with growing knowledge of streptococcal genetics and evolution, further investigation might be possible if suitable archival specimens can be identified.
The role of women in supporting 1917 epidemic responses is rarely acknowledged. Because many male physicians and researchers volunteered for the war effort, female scientists assumed greater leadership roles on the home front than they had previously, and many excelled, such as The Rockefeller’s Martha Wollstein (1868–1939) and Rush Medical College’s Ruth Tunnicliff (1876–1946), whose streptococcal and bronchopneumonia works influenced measles epidemic investigators.115,118,119 Tunnicliff also developed the first measles immune serum. The work of Wollstein and others was carried on by the Rockefeller’s Rebecca Craighill Lancefield (1895–1981), whose streptococcal typing system is still used almost a century after it was first developed. Within and outside of the military, female nurses provided care and public health services, assumed great clinical responsibilities, and undoubtedly helped reduce the mortality of the measles and influenza epidemics.31,120,121
The physicians and scientists who investigated the 1917–18 US army measles epidemics clearly understood that they were witnessing an important and unprecedented event; many went to great lengths to record information about it so that physicians of the future might be able to understand what they could not. The efforts of these men and women have left us with a rich medical record of thousands of pages of published manuscripts, thousands more pages of primary records, hundreds of radiographs, and an unknown number of autopsy tissue specimens potentially containing nucleic acids representing viral, bacterial, and host-response genes. The epidemic is an important chapter in the past century of medicine. Answers to many remaining questions are undoubtedly waiting to be revealed.
Among the important questions that might be answered if archival pathological specimens can be identified, are the genomic nature and variability of measles viruses and of streptococci, including the presence or absence of known streptococcal virulence factors, and evidence for copathogenic mechanisms that might be studied experimentally in an (as yet unestablished) animal model, ideally a primate model. That the 1917 army measles epidemics and the 1918 army influenza epidemics produced secondary epidemics of bronchopneumonia that were highly similar in their clinical appearance, radiological findings, bacteriology, pathology, and histology, strongly suggests shared pathogenic mechanisms that are not specific to any single infectious agent acting alone. This observation is extremely important because it has implications for pneumonias nowadays. These epidemic investigations will hopefully stimulate research on the causes and pathogenic mechanisms of such pneumonias, including attempts to understand, detect, and diagnose early events in the natural history of the disease process, and to develop effective treatments to reduce disease progression before illness becomes severe.
Supplementary Material
Panel:
Clinical, epidemiological, and pathological similarities between the 1917–18 measles and the 1918–19 influenza epidemics in 39 US army camps6,10,13,21,24,28,30,34,37–39,45,51,53,62,63,70,72,82,88
| Shared epidemiological fi ndings |
| • Epidemic of high incidence with respiratory and fomite spread |
| • High incidence of secondary bacterial pneumonia |
| • Mean of 10 days from illness onset to pneumonia death37,38,89 |
| • High incidence of postpneumonia empyema |
| • Severe disease statistically associated with nasopharyngeal carriage of bacterial pathogens |
| • Acquisition of bacterial pathogen carriage both before and after viral disease onset*37,38,89 |
| Shared clinical fi ndings |
| • Acute laryngotracheobronchitis associated with resultant lung complications |
| • Rapid progression of pneumonia to empyema or septic shock |
| • Death by sepsis, cardiac failure, acute respiratory distress syndrome, with or without heliotrope cyanosis |
| Shared pathological and bacteriological fi ndings |
| • Strong predominance of bronchopneumonia over lobar pneumonia |
| • Bronchopneumonia usually bilateral |
| • Bronchopneumonia often ends in septic shock, empyema, cardiac failure, or acute respiratory distress syndrome |
| • Evidence of desquamative laryngotracheobronchitis and pan-bronchitis at autopsy |
| • Peri-bronchial pattern of lesions evident in early or less involved areas of lung |
| • Very extensive neutrophil infi ltration, especially in pneumococcal cases† |
| • Extensive necrosis, vasculitis, and haemorrhage in involved lung tissue† |
| • Asynchronous changes throughout lung |
| • Evidence of repair of (viral) injury |
| • Hyaline membranes and ductal dilatation |
| • Single or mixed bacterial infection with pneumonia pathogens‡ |
| • Pneumonia pathogens in fatal cases identical to those causing camp carriage epidemics‡ |
| • Bacillus (Haemophilus) infl uenzae often found but usually not considered pathogenic |
| Distinct pathological and bacteriological fi ndings |
| • Warthin-Finkeldey cells in lungs only in 1917–18 measles outbreak |
In 1917, pathologists suspected that both influenza and measles were viral.
In measles streptococcal bronchopneumonias, interstitial inflammation was relatively more common and alveolar neutrophilic infiltration less common than in many pandemic influenza bronchopneumonias. Streptococcal bronchopneumonias were also more likely to be haemorrhagic.24,38,39,63
Mixed bacterial infections at autopsy were common in both post-influenza pneumonia (20%)50 and postmeasles pneumonia, although standardised bacteriological data for measles were not collected. The existing measles autopsy data, sparse and often of poor quality, nevertheless suggest that most postmeasles pneumonias were caused by streptococci with or without pneumococci—eg, data from Cumming and colleagues19 on 18 measles pneumonia autopsies, which show pure streptococci in 15, and streptococci-positive pneumococci in three.21 Other bacteria were found less often.
Search strategy and selection criteria
We did a systematic historical examination of published literature relating to army-wide measles outbreaks and related subjects such as “empyema”, “pneumonia”, “streptococcal infection”, and other topics discussed in the text, with The Index Catalog of the Library of the Surgeon General’s Office, The Index Medicus, and electronic and non-electronic catalogues at the US National Library of Medicine, and US National Institutes of Health. We assessed all relevant publications in the available literature, which were in the English, French, German, Italian, and Russian languages. We searched for references published from 1917 to 2014.
Acknowledgments
This research was supported by the intramural research funds of the US National Institutes of Health and the National Institute of Allergy and Infectious Diseases. We thank James Musser for sharing information about streptococcal phylogenetics and pathogenesis, Daniel Mollura for discussing with us radiological aspects of army medicine of the 1917–19 era, Ken Maguire (Camp Zachary Taylor Historical Society) for providing photographic images related to the epidemics and for research support. We thank Jeffrey Reznick, the staff of the History of Medical Division, National Library of Medicine, and Betty Murgolo and the Document Delivery team at the National Institute of Health Library.
Footnotes
Declaration of interests
We declare no competing interests.
Contributor Information
David M Morens, Office of the Director and Viral Pathogenesis and Evolution Section, Laboratory of Infectious Diseases.
Jeffery K Taubenberger, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
References
- 1.Byerly CR. Fever of war: the Influenza epidemic in the US Army during World War I. New York: New York University Press, 2005. [Google Scholar]
- 2.Parkes LC. Measles. In: Parkes LC. Hygiene and Public Health, 4th edn. Philadelphia: P Blakiston, Sons & Co, 1895: 436–39. [Google Scholar]
- 3.Hektoen L Experimental measles: a review. J Am Med Assoc 1919; 72:177–80. [Google Scholar]
- 4.Anderson JF, Goldberger J. Experimental measles in the monkey; a preliminary note. Public Health Rep 1911; 26: 847–48. [PubMed] [Google Scholar]
- 5.Vedder EB. The epidemiology of the sputum-borne diseases and its relation to the health of the national forces. Mil Surg 1919; 44: 123–53. [Google Scholar]
- 6.Hall MW. Inflammatory diseases of the respiratory tract (bronchitis; influenza; bronchopneumonia; lobar pneumonia) In: Ireland MW, Siler JF, eds. The Medical Department of the United States Army in the World War. Volume IX. Communicable and other diseases. Washington DC: US Government Printing Office, 1928: 61–170. [Google Scholar]
- 7.Love AG. A brief summary of the viral statistics of the US Army during the World War. Mil Surg 1922; 51: 139–68. [Google Scholar]
- 8.Soper GA. Health of the troops in the United States during 1918. Mil Surg 1919; 45: 377–98. [Google Scholar]
- 9.Love AG, Ireland MW, eds. The Medical Department of the United States Army in the World War Volume XV Statistics.Chapter II. Medical and casualty statistics. Washington DC: US Government Printing Office, 1925. [Google Scholar]
- 10.Michie HC, Lull GE. Measles. In: Ireland MW, Siler JF, eds. Communicable and other diseases. Measles. Statistical considerations In: Ireland MW, Siler JF, eds. The Medical Department of the United States Army in the World War. Volume IX. Communicable and other diseases. Chapter XII. Washington DC: US Government Printing Office, 1928: 409–50. [Google Scholar]
- 11.Hamburger WW, Fox H. A study of the epidemics of pneumococcus and Streptococcus infections, and measles, at Camp Zachary Taylor, Kentucky, autumn, 1917, to summer, 1918. Med Clin North Am 1918; 2: 321–78. [Google Scholar]
- 12.Duncan LC. An epidemic of measles and pneumonia in the 31st Division, Camp Wheeler, Ga. Mil Surg 1918; 42: 123–38. [Google Scholar]
- 13.Vaughan VC, Palmer GT. Communicable diseases in the National Guard and National Army of the United States during the six months from September 29, 1917, to March 29, 1918. J Lab Clin Med 1918; 3: 635–718. [Google Scholar]
- 14.Callendar GR. Pathology of the acute respiratory diseases. I. In camps in the United States In: Ireland MW, Callendar GR, Coupal JF, eds. The Medical Department of the United States Army in the World War. Volume XII. Pathology of the acute respiratory diseases, and of gas gangrene following war wounds. Washington DC: US Government Printing Office, 1929: 7–186. [Google Scholar]
- 15.Love AG, Davenport CB. Immunity of city-bred recruits. Arch Intern Med 1919; 24: 129–53. [Google Scholar]
- 16.Fox H History of measles at Camp Zachary Taylor, Kentucky, summer of 1917 to winter of 1919. Mil Surg 1919; 45: 185–99. [Google Scholar]
- 17.Hamburger WW, Mayers LH. Pneumonia and empyema at Camp Zachary Taylor, KY. J Am Med Assoc 1918; 70: 915–18. [PMC free article] [PubMed] [Google Scholar]
- 18.Greenway JC, Boettiger C, Colwell HS. Pneumonia and some of its complications at Camp Bowie. Arch Intern Med 1919; 24: 1–34. [Google Scholar]
- 19.Cumming JG, Spruit CB, Lynch C. The pneumonias: streptococcus and pneumococcus groups. J Am Med Assoc 1918; 70: 1066–70. [Google Scholar]
- 20.Osler W, McCrae T. The principles and practice of medicine. New York: D Appleton & Co, 1914. [Google Scholar]
- 21.Clendening L Reinfection with Streptococcus hemolyticus in lobar pneumonia, measles and scarlet fever and its prevention. Am J Med Sci 1918; 56: 575–86. [Google Scholar]
- 22.Beals LS, Zimmerman BF, Marlow SB. Acute respiratory diseases among troops with especial reference to empyema. J Infect Dis 1918; 23: 475–92. [Google Scholar]
- 23.Brooks H, Cecil RL. A study of 80 cases of empyema at Camp Upton. Arch Intern Med 1918; 22: 269–89. [Google Scholar]
- 24.Cole R, MacCallum WG. Pneumonia at a base hospital. J Am Med Assoc 1918; 70: 1146–56. [Google Scholar]
- 25.Gay FP. Recent aspects of streptococcus infection. J Lab Clin Med 1918; 3: 721–57. [Google Scholar]
- 26.Irons EE, Marine D. Streptococcal infections following measles and other diseases. J Am Med Assoc 1918; 70: 687–88. [Google Scholar]
- 27.Miller JL, Lusk FB. Epidemic of Streptococcus pneumonia and empyema at Camp Dodge, Iowa. J Am Med Assoc 1918; 71: 702–04. [Google Scholar]
- 28.Stone WJ, Phillips BG, Bliss WP. A clinical study of pneumonia based on eight hundred and seventy-one cases. Arch Intern Med 1918; 22: 409–39. [Google Scholar]
- 29.Gray H Pneumonia and empyema. Boston Med Surg J 1919; 180: 448–51. [Google Scholar]
- 30.Opie EL, Freeman AW, Blake FG, Small JC, Rivers TM. Pneumonia at Camp Funston. Report to the Surgeon General. J Am Med Assoc 1919; 72: 108–16. [Google Scholar]
- 31.Mozingo AE. The surgical treatment of empyema by a closed method. J Am Med Assoc 1918; 71: 2062–68. [Google Scholar]
- 32.Berry FB. Historical note. In: Coates JB Jr, Berry FB, McFetridge EM, eds. Medical Department, United States Army. Surgery in World War II. Thoracic surgery. Volume I Washington, DC: Office of the Surgeon General, Department of the Army, 1963: 3–49. [Google Scholar]
- 33.Stone WJ. Pericarditis as a complication of pneumonia, based on three hundred necropsies. J Am Med Assoc 1919; 73: 254–58. [Google Scholar]
- 34.Dunham EK, Graham EA, Mitchell JF, et al. Empyema Commission. Cases of empyema at Camp Lee, VA. Preliminary report by the Empyema Commission. J Am Med Assoc 1918; 71: 366–73. [Google Scholar]
- 35.Bloedorn WA. Broncho-pneumonia, with special reference to incidence and diagnosis. Mil Surg 1922; 51: 646–56. [Google Scholar]
- 36.Elwyn H Pneumonia at Camp Greene: a few considerations from a clinical standpoint. South Med J 1918; 11: 780–85. [Google Scholar]
- 37.Vaughan WT, Schnabel TG. Pneumonia and empyema at Camp Sevier. Arch Intern Med 1918; 22: 440–65. [Google Scholar]
- 38.Lucke B Postoperative findings in measles, bronchopneumonia and other infections. J Am Med Assoc 1918; 70: 2006–11. [Google Scholar]
- 39.MacCallum WG, Welch WH, Litchfield L, et al. Pathology of the epidemic streptococcus bronchopneumonia in the Army Camps. J Am Med Assoc 1918; 71: 704–10. [Google Scholar]
- 40.Cumming JG, Spruit CB, Aten EJ. Streptococcus pneumonia. J Am Med Assoc 1919; 72: 704–07 [Google Scholar]
- 41.Holman WL, Avery OT, Kinsella RA, Brown JH. Recommendations of the Committee on a standard routine method for the isolation and infectious disease of hemolytic Streptococci from throats, sputa, and pathologic exudates. J Lab Clin Med 1917–1918; 3: 618–21. [Google Scholar]
- 42.Arnold L Classification of streptococcus. I. Streptococci isolated from normal throats, classified by sugar fermentations. J Lab Clin Med 1920; 5: 587–90 [Google Scholar]
- 43.Arnold L Classification of streptococcus. II. Streptococci isolated from influenza throats, classified by sugar fermentations. J Lab Clin Med 1920; 5: 591–92 [Google Scholar]
- 44.Thomas HM. Pneumonia at Camp Meade. J Am Med Assoc 1918; 71: 1307–10. [Google Scholar]
- 45.Levy RL, Alexander HL. The predisposition of Streptococcus carriers to the complications of measles: results of separation of carriers from non-carriers at a base hospital. J Am Med Assoc 1918; 70: 1827–30. [Google Scholar]
- 46.Keegan JJ. The pathology of epidemic pneumonia in mice and guinea-pigs. Arch Intern Med 1920; 26: 570–93. [Google Scholar]
- 47.Gray H Pneumonia and empyema. Boston Med Surg J 1919; 180: 351–54. [Google Scholar]
- 48.Pilot I, Davis DJ. Hemolytic streptococci in the faucial tonsil and their significance as secondary invaders. J Infect Dis 1919; 24: 386–99. [Google Scholar]
- 49.Jordan EO. Epidemic influenza: a survey. Chicago: American Medical Association, 1927. [Google Scholar]
- 50.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 2008; 198: 962–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Opie EL, Blake FG, Small JC, Rivers TM. The pathology and bacteriology of pneumonia following measles In: Opie EL, Blake FG, Small JC, Rivers TM, eds. Epidemic respiratory disease. The pneumonias and other infections of the respiratory tract accompanying influenza and measles. Chapter VI. St Louis: CV Mosby, 1921: 334–58. [Google Scholar]
- 52.McNabb PE. Post-mortem pneumonia. Mil Surg 1922; 51: 313–26. [Google Scholar]
- 53.Small JC. Secondary infection in the ward treatment of measles In: Opie EL, Blake FG, Small JC, Rivers TM, eds. Epidemic respiratory disease. The pneumonias and other infections of the respiratory tract accompanying influenza and measles. Chapter V. St Louis: CV Mosby, 1921: 282–333. [Google Scholar]
- 54.Sailer J, Hall MW, Wilson RL, McCoy C. A study of pneumococcus carriers. Arch Intern Med 1919; 24: 600–10. [Google Scholar]
- 55.Alexander HL. Hemolytic streptococcus causing severe infections at Camp Zachary Taylor, KY. J Am Med Assoc 70: 775–76. [Google Scholar]
- 56.Capps JA. The limitation and control of streptococcus and other respiratory infections. War Med 1918; 2: 571–87. [PMC free article] [PubMed] [Google Scholar]
- 57.Capps JA. A new adaptation of the face mask in the control of contagious disease. J Am Med Assoc 1918; 70: 910–10. [PMC free article] [PubMed] [Google Scholar]
- 58.Capps JA, McLester JS, Lichty JA, et al. Measures for the prevention and control of respiratory infections in military camps. J Am Med Assoc 1918; 71: 448–51 [Google Scholar]
- 59.Dunham EK, Graham EA, Mitchell JF, et al. Empyema Commission. Cases of empyema at Camp Lee, VA. Preliminary report by the Empyema Commission. J Am Med Assoc 1918; 71: 443–48. [Google Scholar]
- 60.Dunham EK. Section I. Empyema In: Ireland MW, ed. The Medical Department of the United States Army in the World War. Volume XI. Surgery. Chapter II. Washington DC: US Government Printing Office, 1924: 33–392. [Google Scholar]
- 61.Lucke B, Rea MH. A study of the hemolytic streptococci in the throat and in empyema. J Infect Dis 1919; 24: 533–46. [Google Scholar]
- 62.Callendar GR. Pathology of the acute respiratory diseases. III. Pathological anatomy In: Ireland MW, Callendar GR, Coupal JF, eds. The Medical Department of the United States Army in the World War. Volume XII. Pathology of the acute respiratory diseases, and of gas gangrene following war wounds. Chapter I. Washington DC: US Government Printing Office, 1929: 196–387. [Google Scholar]
- 63.MacCallum WG. Pathology and epidemic pneumonia in camps and cantonments. Med Rec 1919; 95: 776–84. [Google Scholar]
- 64.Vaughan VC. Pathology of the pneumonia in the United States Army camps during the winter of 1917–1918. J Clin Lab Med 1919; 4: 745–50. [Google Scholar]
- 65.Davis EL. A roentgen study of one thousand chests, at Camp Devens, Mass. J Am Med Assoc 1918; 70: 1525–29. [Google Scholar]
- 66.Mollura DJ, Morens DM, Taubenberger JK, Bray M. The role of radiology in influenza: novel H1N1 and lessons learned from the 1918 pandemic. J Am Coll Radiol 2010; 7: 690–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leen TF. Recent treatment of lobar pneumonia. Boston Med Surg J 1918; 178: 251–55. [Google Scholar]
- 68.Gray H Pneumonia and empyema. Boston Med Surg J 1919; 180: 330–34. [Google Scholar]
- 69.Lucke B Pathology and bacteriology of pneumonia. Pennsylvania Med J 1920; 23: 369–71. [Google Scholar]
- 70.Short KR, Kroeze EJBV, Fouchier RAM, Kuiken T. Pathogenesis of influenza-induced acute respiratory distress syndrome. Lancet Infect Dis 2014; 14: 57–69. [DOI] [PubMed] [Google Scholar]
- 71.Bartholow R Synopsis of a report on camp measles, based upon an analysis of one hundred cases, made to the Surgeon-General. Am Med Times 1864; 8: 242–44. [Google Scholar]
- 72.MacCallum WG. Pathological anatomy associated with influenza; 23 plates. Johns Hopkins Hosp Rep 1921; 20: 149–249. [Google Scholar]
- 73.Fox H, Hamburger WW. The streptococcus epidemic at Camp Zachary Taylor, KY. J Am Med Assoc 1918; 70: 1758–60. [Google Scholar]
- 74.Schorer EH, Clark FD, Sanderson R, Dickson JD, Huntoon FM. Pneumonia and empyema in the late winter of 1917–1918. Med Rec 1919; 95: 673–80. [Google Scholar]
- 75.Chien Y-W, Klugman KP, Morens DM. Efficacy of whole-cell killed bacterial vaccines in preventing pneumonia and death during the 1918 influenza pandemic. J Infect Dis 2010; 202: 1639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anonymous. Hemolytic streptococci and the tonsils. J Am Med Assoc 1919; 72: 1295–96. [Google Scholar]
- 77.Sellards AW. A census of susceptibility to measles and its relation to quarantine procedures. Mil Surg 1919; 45: 562–65. [Google Scholar]
- 78.McKenna H Operation for empyema. A preliminary report covering an observation on one hundred and 50-five cases. J Am Med Assoc 1918; 71: 743–45. [Google Scholar]
- 79.Stevens FA. The effects of irrigation with surgical solution of chlorinated soda. J Am Med Assoc 1924; 83: 1495–97. [Google Scholar]
- 80.Odell JA. Management of empyema thoracis. J R Soc Med 1994; 87: 466–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gorgas WC. Empyema in base hospitals. War Surg Med 1918; 1: 1–40. [Google Scholar]
- 82.MacCallum WM. Pathology of the pneumonia following influenza. J Am Med Assoc 1919; 72: 720–23. [Google Scholar]
- 83.MacCallum WG. Pathological studies in the recent epidemics of pneumonia. Trans South Surg Assoc 1919; 31: 180–92. [Google Scholar]
- 84.Opie EL, Blake FG, Rivers TM. The pathology and bacteriology of pneumonia following influenza. Chapter IV In: Opie EL, Blake FG, Small JC, Rivers TM, eds. Epidemic respiratory disease. The pneumonias and other infections of the respiratory tract accompanying influenza and measles. St Louis: CV Mosby, 1921: 107–281. [Google Scholar]
- 85.Glomset DJ. The pathology of the streptococcus infection of the lungs. J Iowa State Med Soc 1919; 9: 143–47 [Google Scholar]
- 86.Cole R Etiology of pneumonia. Med Rec 1919; 95: 36–37 [Google Scholar]
- 87.Gray H Pneumonia and empyema. Boston Med Surg J 1919; 180: 265–69. [Google Scholar]
- 88.Gray H Pneumonia and empyema. Boston Med Surg J 1919; 180: 305–07 [Google Scholar]
- 89.Rackemann FM, Brock S. The epidemiology of influenza at Camp Merritt, NJ. Arch Intern Med 1919; 23: 582–602. [Google Scholar]
- 90.Brundage JF, Shanks GD. Deaths from bacterial pneumonia during 1918–19 influenza pandemic. Emerg Infect Dis 2008; 14: 1193–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Blanton WB, Burhans CW, Hunter OW. Studies in streptococcal infections at Camp Custer, Michigan. J Am Med Assoc 1919; 73: 1520–24. [Google Scholar]
- 92.Cumming JG, Spruit CB. The transmission of the pneumonia producing group of organisms. Mil Surg 1920; 46: 391–402. [Google Scholar]
- 93.Gill JR, Sheng Z-M, Ely SF, et al. Pulmonary pathologic findings of fatal 2009 pandemic influenza A/H1N1 viral infections. Arch Pathol Lab Med 2010; 134: 235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chertow DS, Memoli MJ. Bacterial coinfection in influenza: a grand rounds review. JAMA 2013; 309: 275–82. [DOI] [PubMed] [Google Scholar]
- 95.Kash JC, Walters KA, Davis AS, et al. Lethal synergism of 2009 pandemic H1N1 influenza virus and Streptococcus pneumoniae coinfection is associated with loss of murine lung repair responses. MBio 2011; 2: e00172–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289: 179–86. [DOI] [PubMed] [Google Scholar]
- 97.Light RW. Parapneumonic effusions and empyema. Proc Am Thorac Soc 2006; 3: 75–80. [DOI] [PubMed] [Google Scholar]
- 98.Bender JM, Ampofo K, Sheng X, Pavia AT, Cannon-Albright L, Byington CL. Parapneumonic empyema deaths during past century, Utah. Emerg Infect Dis 2009; 15: 44–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Small AA. Pneumonia at a base hospital. Observations in one thousand one hundred cases at Camp Pike, Ark. J Am Med Assoc 1918; 71: 700–02. [Google Scholar]
- 100.Bierring WL, Luginbuhl CB, Burt CW. Streptococcus pneumonia and empyema. An infection affecting eight members of one family with seven deaths. J Am Med Assoc 1918; 71: 1475. [Google Scholar]
- 101.Cummings HH, Canfield RB, Blair W, et al. The role of the Streptococcus in the recent epidemic of acute respiratory infection. Trans Clin Soc University Michigan 1917; 8: 91–101. [Google Scholar]
- 102.Brown CC, Olsen RJ, Fittipaldi N, et al. Spread of virulent group A Streptococcus type emm59 from Montana to Wyoming, USA. Emerg Infect Dis 2014; 20: 679–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nasser W, Beres SB, Olsen RJ, et al. Evolutionary pathway to increased virulence and epidemic group A Streptococcus disease derived from 3,615 genome sequences. Proc Natl Acad Sci USA 2014; 111: E1768–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Katz AR, Morens DM. Severe streptococcal infections in historical perspective. Clin Infect Dis 1992; 14: 298–307. [DOI] [PubMed] [Google Scholar]
- 105.Morens DM. Measles in Fiji, 1875. Thoughts on the history of emerging infectious diseases. Pacific Health Dialog 1998; 5: 119–28. [Google Scholar]
- 106.Vaughan V, Vaughan HF, Palmer GT. Measles. Morbilli; Rubeola. In: Vaughan VC, Vaughan HF, Palmer GT, eds. Epidemiology and public health A text and reference book for physicians, medical students and health workers. Volume I. Respiratory infections. Chapter VI. St Louis: CV Mosby Company, 1922: 144–78. [Google Scholar]
- 107.Corney BG, Stewart J, Thomson B. Report of the Commission appointed to inquire into the decrease of the native population, with appendices Suva: Edward John March, US Government Printer, 1896. [Google Scholar]
- 108.Hussey GD, Klein M. A randomized, controlled trial of vitamin A in children with severe measles. N Engl J Med 1990; 323: 160–64. [DOI] [PubMed] [Google Scholar]
- 109.Koplik H Empyema in infants and children: its frequency, etiology, symptomatology and prognosis. Med Newsl (Lond) 1902; 81: 481–83. [Google Scholar]
- 110.Anthony BH. Some characteristics of the streptococci found in scarlet fever. J Infect Dis 1909; 6: 332–38. [Google Scholar]
- 111.Lorey A Bakteriologishe Untersuchungen bei Masern. Zeitschrift für Hygiene 1909; 63: 135–50. [Google Scholar]
- 112.Eyre J The bacteriology of bronchopneumonia—a statistical analysis. J Bacteriol Pathol 1910; 14: 160–63. [Google Scholar]
- 113.Thursfield H Report on an enquiry into the causes of death in measles In: 42nd Annual Report of the [London] Local Government Board, 1912–13. Supplement containing the Report of the Medical Officer for 1912–1913. Appendix B, No 2. London: His Majesty’s Stationery Office, 1914: 358–74. [Google Scholar]
- 114.Hektoen L The bacteriology of measles. J Am Med Assoc 1918; 71: 1201–05. [Google Scholar]
- 115.Wollstein M The bacteriology of broncho-and lobular pneumonia in infancy. J Exp Med 1905; 6: 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wilson JG. Some important factors affecting the incidence of measles and the fatality therefrom. Arch Pediatr 1916; 33: 261–72. [Google Scholar]
- 117.Kinnear W Epidemic of measles in the Highland Division at Bedford, 1914–15. Edinburgh Med J 1923; 30: 593–99. [Google Scholar]
- 118.Wollstein M, Meltzer SJ. The character of the pneumonic lesions produced by intrabronchial insufflation of virulent streptococci. J Exp Med 1913; 18: 548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tunnicliff R On the group specificness of antibodies in antistreptococcus serum. J Infect Dis 1922; 31: 373–81. [Google Scholar]
- 120.Byerly CR. Postmortem In: Byerly CR. New York: New York University Press, 2005: 125–52. [Google Scholar]
- 121.Keeling AW. “Alert to the necessities of the emergency”: US nursing during the 1918 influenza pandemic. Public Health Rep 2010; 125 (suppl 3): 105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


