Abstract
Peripheral arterial disease is a chronic vascular disease characterized by impaired circulation to the lower extremities. Its most severe stage, known as critical limb ischemia (CLI), puts patients at an increased risk of cardiovascular events, amputation, and death. The objective of this literature review is to describe the burden of disease across a comprehensive set of domains—epidemiologic, clinical, humanistic, and economic—focusing on key studies published in the last decade. CLI prevalence in the United States is estimated to be approximately 2 million and is likely to rise in the coming years given trends in important risk factors such as age, diabetes, and smoking. Hospitalization for CLI patients is common and up to 60% are readmitted within 6 months. Amputation rates are unacceptably high with a disproportionate risk for certain demographic and socioeconomic groups. In addition to limb loss, CLI patients also have reduced life expectancy with mortality typically exceeding 50% by 5 years. Given the poor clinical prognosis, it is unsurprising that the quality of life burden associated with CLI is significant. Studies assessing quality of life in CLI patients have used a variety of generic and disease-specific measures and all document a substantial impact of the disease on the patient’s physical, social, and emotional health status compared to population norms. Finally, the poor clinical outcomes and increased medical resource use lead to a considerable economic burden for national health care systems. However, published cost studies are not comprehensive and, therefore, likely underestimate the true economic impact of CLI. Our summary documents a sobering assessment of CLI burden—a poor clinical prognosis translating into diminished quality of life and high costs for millions of patients. Continued prevention efforts and improved treatment strategies are the key to ameliorating the substantial morbidity and mortality associated with this disease.
Keywords: critical limb ischemia, peripheral arterial disease, burden, amputation, quality of life, economics
Introduction
Peripheral arterial disease (PAD) is a chronic vascular disease characterized by impaired circulation to the lower extremities. While early stages of PAD may be asymptomatic, the hallmarks of its most severe stage, known as critical limb ischemia (CLI), are recurring lower extremity rest pain, ulceration, and gangrene as well as an increased risk of cardiovascular events, amputation, and death.1 Clinical guidelines focus on strategies to promote wound healing and limb salvage as well as medical therapy to prevent cardiovascular ischemic events.2 Revascularization (either surgical or endovascular) is attempted to provide sufficient blood flow to the extremities in at least 50% of the CLI patients and upwards of 90% of the patients in certain interventional centers. Minor or major amputation is utilized when less invasive treatments cannot be used or have failed.
Numerous published studies have provided informative CLI overviews focusing on intervention outcomes or emphasizing one or two elements of disease burden.3–16 However, despite these numerous reviews, a comprehensive landscape assessment of CLI burden describing contemporary studies is lacking. Therefore, the objective of this literature review is to describe the burden of disease across several domains—epidemiologic, clinical, humanistic, and economic—focusing on key studies published since 2007.
Methods
Literature searches of the PubMed and EMBASE databases were undertaken to identify original research studies published from January 2007 through October 2018 assessing the epidemiologic burden (ie, incidence and prevalence), quality of life burden, and economic burden of lower extremity CLI. Non-systematic review studies, editorials, letters, commentaries, or posters presented at scientific meetings were excluded. The 12-year time frame was selected to focus on the most contemporary studies published on the burden of lower extremity CLI. Each search was conducted using controlled vocabulary and limited to studies published in English and involving humans. The preliminary literature searches identified 3,254 potentially relevant studies. Two researchers independently appraised all of the abstracts from the literature searches for potential inclusion in the literature review. Additional studies were identified based on a review of the reference lists from the full-text studies and a grey search of the internet. The definition of CLI is not standardized and often varied across the studies reviewed. Since this was a “pragmatic” burden of illness review, all studies mentioning lower extremity CLI were considered for the review. Studies reporting on related topics such as trends in diagnostic or revascularization techniques or focusing exclusively on treatment outcomes associated with specific CLI treatments that did not contain any information on the burden of CLI were excluded. A total of 73 studies were selected for inclusion in this literature review.
Results
Epidemiologic and clinical burden
Prevalence and incidence
Disease prevalence and incidence are epidemiologic terms that define a population at a single point in time (prevalence) or as the number of new cases that develop in a specified time period (incidence). Together, these two building blocks of epidemiology help to characterize disease burden in terms of the total numbers of patients, establish whether that number (or rate of disease development) may be growing or diminishing, and provide useful comparative information across different populations, whether they be geographically or demographically diverse.
Several recent studies have reported CLI prevalence and incidence in the United States (Table 1). CLI prevalence in the US adult population aged 40 or older is estimated to be 1.28%.17 With approximately 156 million US citizens in this age category,18 this prevalence estimate translates to approximately 2 million total CLI patients in the United States. Prevalence estimates in studies of the US Medicare population range from 0.3% to 2.0%.17,19,20 With a current beneficiary population of approximately 60 million,21 there may be nearly 1.2 million total CLI patients in the US Medicare program.17
Table 1.
CLI prevalence and incidence
| Publication | Country/region | Data source (years) | Population details | Methods | CLI prevalence (N or %) | CLI incidence (annual unless stated) |
|---|---|---|---|---|---|---|
| Baser (2013)19 | United States | 100% Medicare inpatient/outpatient/denominator files (2007–2008) | US Medicare population | ICD-9 and CPT codes used to identify CLI cases | Using ICD-9 codes only: Total—0.23% Using ICD-9 and CPT codes Total—0.54% |
Using ICD-9 codes only: Total—0.20% Fontaine Stage III only—0.05% Fontaine Stage IV only—0.15% Using ICD-9 and CPT codes: Total—0.48% |
| Nehler (2014)17 | United States | MarketScan database (2004–2008) | US population age ≥40 | ICD-9 and CPT codes used to identify CLI cases | 2008 Overall: 1.28% 2008 Commercial: 0.80% 2008 Medicaid: 2.57% 2008 Medicare: 2.01% |
Total CLI: 0.35% |
| Mustapha (2018)20 | United States | Medicare fee-for-service Parts A and B (2011–2015) | Adult US Medicare population | ICD-9 and CPT codes used to identify CLI cases | 2011: 116,031 (0.32%) | Total CLI: 0.26% |
Abbreviations: CLI, critical limb ischemia; CPT, Current Procedural Terminology®; ICD-9, International Classification of Diseases 9th Revision.
CLI prevalence in commercial plans—likely being younger and healthier—are approximately half of Medicare prevalence.17 This is consistent with an observed age gradient and prevalence 2 to 3 times higher in older (age 85+) Medicare patients compared to younger (age 65–69) Medicare patients.19 Annual incidence estimates of total CLI in these same epidemiologic studies range from 0.26% to 0.48%.
Readmission rates
Given the poor prognosis and high morbidity and mortality associated with CLI, patients are commonly hospitalized. In 2016, Agarwal and colleagues quantified the number of CLI hospitalizations in the United States based on an analysis of the US Nationwide Inpatient Sample (NIS), a database that contained discharge data from approximately 8 million hospitalizations during each year of the study period (2003–2011).22 The number of CLI hospitalizations during this time period remained remarkably stable, varying between 325,000 and 375,000 annually.22 This equates to nearly 225 CLI admissions per 1,000,000 US persons aged 40 or older.
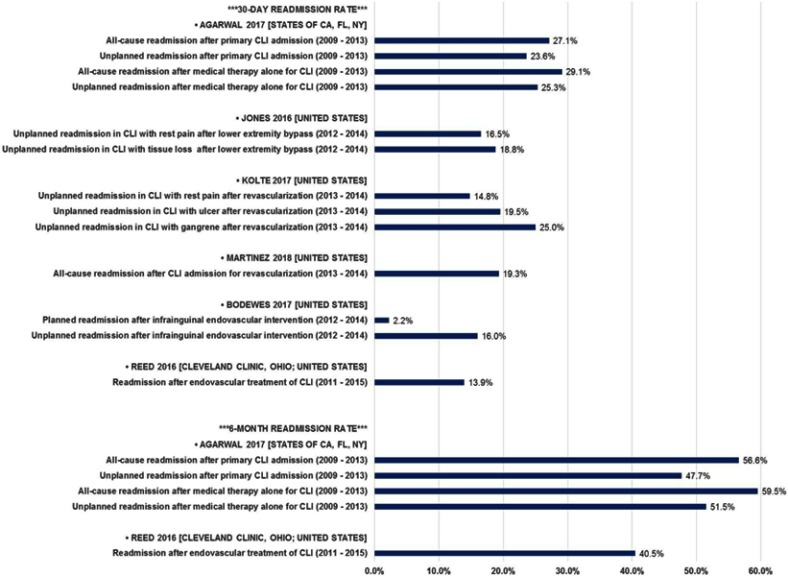
Minimizing hospital readmission is an important goal given the risk hospitalizations pose to patients and the increasing use of readmission rates as a metric on which hospitalization performance may be judged. Historically, readmission rates for CLI patients have been high and, therefore, clinical studies occasionally use readmission as an important outcome measure. Recent studies of CLI readmission rates in the United States (Table 2 and Figure 1) demonstrate that readmission is common within 30 days (approximately 15–30%) of the index hospitalization, with rates approaching 60% at 6 months. Furthermore, a study by Kolte and colleagues (2017) using the NIS database shows an increasing likelihood of readmission with increasing disease severity.23
Table 2.
CLI readmission rates
| Publication | Country/region | Data source (years) | Population details | Methods | Readmission rate ranges (see figure 1 for detail) |
|---|---|---|---|---|---|
| Reed (2016)70 | Cleveland, OH | Cleveland Clinic (2011–2015) | CLI patients undergoing endovascular treatment | Retrospective analysis of medical records | 30-day: 13.9% 6-month: 40.5% |
| Jones (2016)71 | United States | ACS National Surgical Quality Improvement Program (2012–2014) | CLI patients undergoing open bypass for rest pain or tissue loss | CPT codes to identify cases | 30-day: 16.5–18.8% |
| Agarwal (2017)29 | States of CA, FL, and NY | State inpatient databases (2009–2013) | Patients with a principal diagnosis of CLI | ICD-9 diagnosis and procedure codes used to identify cases and procedures | 30-day: 23.6–29.1% 6-month: 47.7–59.5% |
| Bodewes (2017)72 | United States | ACS National Surgical Quality Improvement Program (2012–2014) | CLI patients undergoing infrainguinal endovascular treatment | CPT codes to identify cases | 30-day: 16% |
| Kolte (2017)23 | United States | Nationwide Readmissions Database (2013–2014) | CLI patients undergoing open or endovascular treatment | ICD-9 diagnosis and procedure codes used to identify cases and procedures | 30-day: 14.8–25.0% |
| Martinez (2018)64 | United States | Nationwide Readmissions Database (2013–2014) | CLI patients undergoing revascularization procedures | ICD-9 diagnosis and procedure codes used to identify cases and procedures | 30-day: 19.3% Overall: 40.4% |
| Masoomi (2018)73 | United States | Nationwide Readmissions Database (2013) | CLI patients with/without revascularization procedures | ICD-9 diagnosis and procedure codes used to identify cases and procedures | 30-day: 5%a |
Notes: aPrimary readmission diagnosis of CLI (rather than all-cause).
Abbreviations: ACS, American College of Surgeons; CLI, critical limb ischemia; CPT, Current Procedural Terminology®; ICD-9, International Classification of Diseases 9th Revision.
Figure 1.
US CLI readmission rates.
Abbreviation: CLI, critical limb ischemia.
Amputation rates and mortality
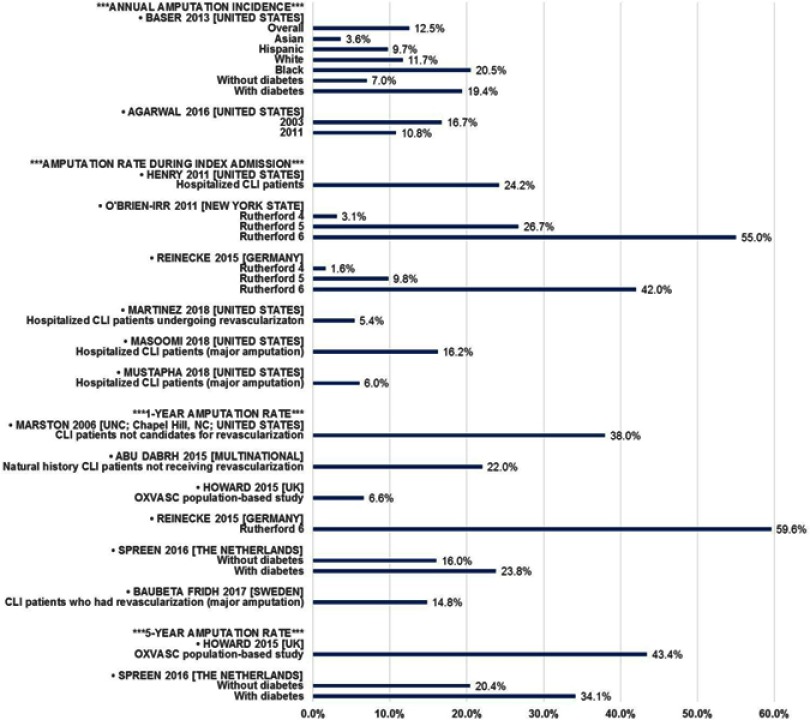
One of the most concerning outcomes for CLI patients is amputation. Recent studies evaluating amputation rates or incidence (terminology varies) are presented in Table 3 and Figure S1. Reviewing the methodologic details for each of these studies demonstrates that there is much heterogeneity in the contemporary literature related to the analysis timeframe (annualized, during hospitalization, or longitudinal evaluation), amputation definition (all types, major amputation only, or unspecified), and populations studied (natural history/untreated, Medicare only, undergoing interventions, all CLI, etc.). Therefore, comparisons and conclusions must be made with caution. Nevertheless, it is clear that, across all studies, amputation rates are unacceptably high—typically exceeding 15–20% at 1 year. The study by Agarwal and colleagues (2016) suggests an association between an increasing focus on limb salvage, greater use of endovascular treatment, and other factors with a decreasing rate of major amputation since 2003 (through 2011 in their study).22 However, there remains ample evidence that there is much room for improvement in terms of reducing the risk of amputation.
Table 3.
CLI amputation rates
| Publication | Country/region | Data source (years) | Population details | Methods | Amputation rate or incidence |
|---|---|---|---|---|---|
| Marstona (2006)74 | Chapel Hill, NC | University of North Carolina Wound Management Center (1999–2005) | CLI patients with unhealed ulcers not candidates for revascularization | Retrospective analysis of prospectively collected data | 1-Year 38% |
| Henry (2011)28 | United States | Nationwide Inpatient Sample (2003–2007) | Hospitalized CLI patients except those with EVT plus amputation | ICD-9 diagnosis and procedure codes used to identify cases and procedures; multivariate regression analyses | During Admission 24.2% Significant Odds Ratiosb Black: 2.15 Native American: 2.00 Low income: 1.12–1.34 Private insurance: 0.74 Medicaid: 1.26 |
| Peacock (2011)65 | Minnesota | State-based hospital claims database (2005–2008) | Patients with lower limb ischemic amputationc | ICD-9 diagnosis and procedure codes used to identify cases and procedures | Age-Adjusted Incidence All: 20.0/100,000 Rural: 21.4/100,000 Urban: 19.6/100,000 |
| O’Brien-Irr (2012)25 | New York State | SPARCS database (2001–2008) | CLI patients undergoing vascular intervention | ICD-9 diagnosis and procedure codes used to identify cases and procedures | During Admission Rutherford 4: 3.1% Rutherford 5: 26.7% Rutherford 6: 55.0% |
| Baser (2013)19 | United States | 100% Medicare inpatient/outpatient/denominator files (2007–2008) | US Medicare population | ICD-9 and CPT codes used to identify CLI cases | Annual Incidence Overall: 12.5% Asian: 3.6% Hispanic: 9.7% White: 11.7% Black: 20.5% DM-: 7.0% DM+: 19.4% |
| Abu Dabrh (2015)3 | Multi-national | Published literature (1986–2013)d | Natural history CLI patients not receiving revascularization | Meta-analysis of 8 RCTs and 5 case series | 1-Year Major amputation: 22% |
| Howard (2015)75 | United Kingdom (Oxfordshire) | Medical records (2002–2012) | CLI patients within the OXVASC study population (no age restriction) | Prospective, population-based study | 1-Year 6.6% 5-Year 43.4% |
| Reinecke (2015)26 | Germany | German health insurer data (2009–2011) | Hospitalized CLI patients | ICD-10 dx and German procedure (OPS) codes to identify cases and procedures | Index Hospitalization Rutherford 4: 1.6% Rutherford 5: 9.8% Rutherford 6: 42.0% 1-Year Rutherford 6: 59.6% 4-Year Rutherford 4: 12.1% Rutherford 5: 35.3% Rutherford 6: 67.3% |
| Agarwal (2016)22 | United States | Nationwide Inpatient Sample (2003–2011) | Hospitalized CLI patients | ICD-9 diagnosis and procedure codes used to identify cases and procedures | Annual Major Amputation 2003: 16.7% 2011: 10.8% |
| Luders (2016)24 | Germany | German health insurer data (2009–2012) | Hospitalized CLI patients (Rutherford 1–3 as reference group) | ICD-10 diagnosis and German procedure (OPS) codes to identify cases and procedures | Hazard Ratio vs Rutherford 1–3 (median follow-up =2.1 years) Rutherford 4: 3.0 Rutherford 5: 9.1 Rutherford 6: 28.3 |
| Spreen (2016)27 | The Netherlands | Pooled patient-level data from the PADI and JUVENTAS trials (2006-unknown) | CLI patients undergoing one of the PADI or JUVENTAS interventions | Analysis of pooled RCT data | Time: Major Amp. (DM-/DM+) 6 months: 11.4%/19.7% 1 year: 16.0%/23.8% 2 years: 17.8%/28.3% 3 years: 18.7%/29.2% 4 years: 20.4%/30.8% 5 years: 20.4%/34.1% |
| Baubeta Fridh (2017)76 | Sweden | Swedish National Quality Register for Vascular Surgery (Swedvasc) (2008–2013) | CLI patients who had a revascularization treatment | Observational cohort study | Time: Major Amputation 6 months: 12.0% 1 year: 14.8% 2 years: 17.2% 3 years: 18.6% |
| Klaphake (2017)31 | The Netherlands | Large teaching hospital (2006–2013) | CLI patients undergoing major amputation | Retrospective analysis of medical records | During Study Period Major Amputation: 26% |
| Martinez (2018)64 | United States | Nationwide Readmissions Database (2013–2014) | CLI patients undergoing revascularization procedures | ICD-9 diagnosis and procedure codes used to identify cases and procedures | During Index Admission Amputation: 5.4% |
| Masoomi (2018)73 | United States | Nationwide Readmissions Database (2013) | CLI patients with/without revascularization procedures | ICD-9 diagnosis and procedure codes used to identify cases and procedures | During Index Admission Major Amputation: 16.2% |
| Mustapha (2018)20 | United States | Medicare fee-for-service Parts A and B (2011–2015) | Adult US Medicare population | ICD-9 and CPT codes used to identify CLI cases | During Index Admission Major Amputation: 6% |
Notes: aAlthough the publication date criteria excluded studies prior to 2007, few papers document the natural history of CLI disease and, therefore, this paper was included within the review; bReference groups in the multivariate logistic regression: Race/ethnicity=white; median income quartiles=Q4 (highest income); payer=Medicare; cUnclear if entire population was CLI; dPublication dates listed; data used in the individual studies may be considerably older.
Abbreviations: Amp, amputation; CLI, critical limb ischemia; CPT, Current Procedural Terminology®; DM, diabetes mellitus; EVT, endovascular treatment; ICD-9/10, International Classification of Diseases 9th/10th Revision; JUVENTAS, Rejuvenating Endothelial Progenitor Cells via Transcutaneous Intra-Arterial Supplementation; OPS, Operationen und Prozedurenschlüssel [German procedure classification]; OXVASC, Oxford Vascular Study; PADI, Percutaneous Transluminal Balloon Angioplasty and Drug-Eluting Stents for Infrapopliteal Lesions in Critical Limb Ischemia; RCT, randomized clinical trial; SPARCS, Statewide Planning and Research Cooperative System.
Amputation rates vary primarily by disease severity. Luders et al (2016) demonstrated that CLI patients have a much higher risk of amputation relative to less severe PAD patients (Rutherford 1–3).24 Within CLI, a clear gradient of higher amputation risk with higher disease severity exists both at initial hospitalization (3.1%, 26.7%, and 55.0% for Rutherford 4, 5, and 6, respectively)25 and through longer-term (4 year) follow-up (12.1%, 35.3%, and 67.3% for Rutherford 4, 5, and 6, respectively).26
Amputation rates also vary by the presence of comorbid conditions. For example, diabetes is a major risk factor associated with higher amputation rates. Studies by Baser (2013) and Spreen (2016) suggest that the probability of amputation is at least 50% higher in CLI patients with diabetes versus those without this comorbidity.19,27
Other disparities in amputation risk are apparent that may have demographic and socioeconomic underpinnings. While race/ethnicity, income, and insurance status/type may have some association with diabetes status and/or disease severity, these factors have been shown to be important in amputation. For example, in a study by Henry and colleagues,28 statistically significantly higher odds of amputation during hospitalization were shown for:
Black and Native American CLI patients (Odds ratios (OR)=2.15 and 2.00, respectively; reference = white patients);
Low-income patients (ORs =1.12–1.34; reference = highest income quartile); and
Medicaid patients (OR=1.26; reference = Medicare) [Note: patients with private insurance were shown to have lower odds of amputation—OR =0.74].
Similarly, the Baser 2013 study19 also evaluated the annual incidence of amputation in different races in the US Medicare program during the years 2007–2008. The researchers quantified an annual amputation incidence of 20.5% for black CLI patients, approximately double the incidence observed in white and Hispanic CLI patients. The finding of racial disparity in amputation frequency was corroborated in research conducted by Mustapha and colleagues using more recent (2011–2015) Medicare data. In their study, blacks more frequently had major amputation than whites (10% vs 4%; P<0.001), which, according to the authors, was only partially explained by differences in patient characteristics (eg, a higher prevalence of gangrene in blacks vs whites—36% vs 22%; P<0.001).20
Not only is amputation a major concern of CLI patients because of limb loss, but also because of the elevated mortality associated with amputation. Studies in the United States and Germany have explored this issue in patients hospitalized for CLI29 or PAD.30 Major amputation was a statistically significant risk factor for in-hospital mortality in both studies with odds ratios of 2.81 (relative to CLI patients without major amputation)29 and 6.69 (relative to any PAD patients without major amputation).30
Longer-term mortality also appears elevated in patients undergoing amputation. In a US Medicare population study, 1-year mortality post-CLI diagnosis was 30.3% in patients without nontraumatic amputation and 40.4% in patients with nontraumatic amputation.19 In a study of elderly (>70 years of age) Dutch CLI patients, mortality rates at 1, 3, and 5 years were 27%, 49%, and 61%, respectively, in those treated without amputation.31 However, in the elderly CLI patients undergoing amputation, mortality rates at the same timepoints were 44%, 66%, and 85%, respectively, indicating substantially worse survival outcomes.31
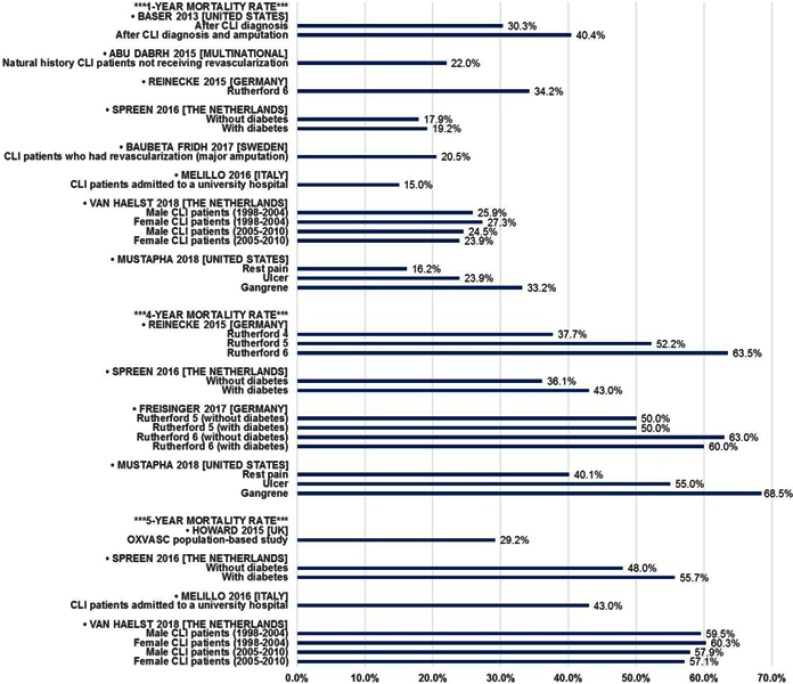
Long-term mortality rates
As discussed earlier, amputation is associated with poor survival outcomes; however, the long-term prognosis for all CLI patients also is unfavorable. Using a variety of methods and data sources, many researchers have quantified mortality in CLI patients (Table 4 and Figure 2). One-year mortality ranges from 15% to 40%, depending on a variety of factors. In addition to the aforementioned impact of amputation, increasing disease severity and diabetes are associated with increased mortality. Over time horizons of 4 to 5 years, mortality commonly exceeds 50%, especially in patients with more severe disease (Rutherford 5 [ulcer] or Rutherford 6 [gangrene]). However, the evidence suggests that the impact of diabetes is attenuated with longer-term follow-up. Studies by Spreen (2016) and Freisinger (2017) in the Netherlands and Germany, respectively, show no mortality difference by diabetes status32 or minor differences that are not statistically significant.27,32
Table 4.
Long-term CLI mortality rates
| Publication | Country/region | Data source (years) | Population details | Methods | Long-term mortality |
|---|---|---|---|---|---|
| Baser (2013)19 | United States | 100% Medicare inpatient/outpatient/denominator files (2007–2008) | US Medicare population | ICD-9 and CPT codes used to identify CLI cases | 1-Year After CLI dx: 30.3% After CLI dx + amp: 40.4% |
| Soga (2014)77 | Japan | Prospective, multicenter (n=17) database (2004–2011) | CLI patients undergoing endovascular treatment | Retrospective analysis of medical records | 2-Year 41% |
| Abu Dabrh (2015)3 | Multi-national | Published literature (1986–2013)a | Natural history CLI patients not receiving revascularization | Meta-analysis of 8 RCTs and 5 case series | 1-Year 18%-22%b |
| Howard (2015)75 | United Kingdom (Oxfordshire) | Medical records(2002–2012) | CLI patients within the OXVASC study population (no age restriction) | Prospective, population-based study | 30-Day 7.4% 5-Year 29.2% |
| Reinecke (2015)26 | Germany | German health insurer data (2009–2011) | Hospitalized CLI patients | ICD-10 diagnosis and German procedure (OPS) codes to identify cases and procedures | 1-Year Rutherford 6: 34.2% 4-Year Rutherford 4: 37.7% Rutherford 5: 52.2% Rutherford 6: 63.5% |
| Luders (2016)24 | Germany | German health insurer data (2009–2012) | Hospitalized CLI patients (Rutherford 1–3 as reference group) | ICD-10 diagnosis and German procedure (OPS) codes to identify cases and procedures | Hazard Ratio vs Rutherford 1–3 (median follow-up =2.1 years) Rutherford 4: 2.01 Rutherford 5: 2.46 Rutherford 6: 3.59 |
| Spreen (2016)27 | The Netherlands | Pooled patient-level data from the PADI and JUVENTAS trials (2006-unknown) | CLI patients undergoing one of the PADI or JUVENTAS interventions | Analysis of pooled RCT data | Time Point: Mort. (DM-/DM+) 6 months: 8.0%/12.8% 1 year: 17.9%/19.2% 2 years: 25.6%/32.0% 3 years: 31.3%/38.1% 4 years: 36.1%/43.0% 5 years: 48.0%/55.7% |
| Baubeta Fridh (2017)76 | Sweden | Swedish National Quality Register for Vascular Surgery (Swedvasc) (2008–2013) | CLI patients who had a revascularization treatment | Observational cohort study | Time Point: Mortality 6 months: 13.5% 1 year: 20.5% 2 years: 31.7% 3 years: 41.4% |
| Freisinger (2017)32 | Germany | German health insurer data (2009–2011) | Hospitalized CLI patients with tissue loss | ICD-10 diagnosis and German procedure (OPS) codes to identify cases and procedures | 4-Year (Rutherford 5) Without diabetes: 50% With diabetes: 50% 4-Year (Rutherford 6) Without diabetes: 63% With diabetes: 60% |
| Melillo (2016)78 | Italy | University hospital (Years not stated; 5–15 year follow-up) | CLI patients consecutively admitted to medical or surgical wards | Retrospective case-control study | Time Point: Mortality 1 year: 15% 2 years: 24% 5 years: 43% |
| van Haelst (2018)79 | The Netherlands | Hospital Discharge Register; Population Register; Cause of Death Register (1998–2010) | CLI patients not admitted in prior 3 years | ICD-9 and ICD-10 codes used to identify CLI cases | Time Point: Mort. (Male/Female) 1 yr (1998–2004): 25.9%/27.3% 1 yr (2005–2010): 24.5%/23.9% 5 yrs (1998–2004): 59.5%/60.3% 5 yrs (2005–2010): 57.9%/57.1% |
| Mustapha (2018)20 | United States | Medicare fee-for-service Parts A and B (2011–2015) | Adult US Medicare population | ICD-9 and CPT codes used to identify CLI cases | 1-Year Rest pain: 16.2% Ulcer: 23.9% Gangrene: 33.2% 4-Year Rest pain: 40.1% Ulcer: 55.0% Gangrene: 68.5% |
Notes: aPublication dates listed; data used in the individual studies may be considerably older; bWith (22%) and without (18%) one study with substantially longer follow-up; 1-year mortality based on the median follow-up of the included studies being 1 year.
Abbreviations: Amp, amputation; CLI, critical limb ischemia; CPT, Current Procedural Terminology®; DM, diabetes mellitus; ICD-9/10, International Classification of Diseases 9th/10th Revision; JUVENTAS, Rejuvenating Endothelial Progenitor Cells via Transcutaneous Intra-Arterial Supplementation; OPS, Operationen und Prozedurenschlüssel [German procedure classification]; OXVASC, Oxford Vascular Study; PADI, Percutaneous Transluminal Balloon Angioplasty and Drug-Eluting Stents for Infrapopliteal Lesions in Critical Limb Ischemia; RCT, randomized clinical trial; yr(S), year(S).
Figure 2.
Long-term CLI mortality rates.
Abbreviations: CLI, critical limb ischemia; OXVASC, Oxford Vascular Study.
Quality of life burden
The quality of life burden in CLI patients is substantial. Patients with CLI suffer from ischemic rest pain, non-healing ulcers, and/or symptomatic gangrene.1,12 This debilitating disease causes dependency on caregivers for support, the need for permanent local wound treatment, and the chronic use of pain-relieving medications.11,33 Further, as discussed earlier, there is a significant risk (and fear) of major amputation and a high rate of mortality.11 Additionally, patients with CLI often suffer from one or more comorbidities such as coronary artery disease, dementia, cerebrovascular disease, and diabetes.15,34 All of these factors severely impair a patient’s quality of life.
Most of the studies published in the past twelve years assessing quality of life in CLI patients have examined the change in quality of life (ie, improvement) after an intervention (ie, endovascular procedures, surgical revascularization, etc.) in comparison to baseline quality of life scores. Only a few contemporary studies have directly assessed the quality of life burden of CLI in a non-interventional setting.14,35 Although these intervention studies were not designed to compare baseline quality of life scores in CLI patients to healthy people in the community, they may provide useful information on the magnitude of quality of life burden in CLI patients who are candidates for medical intervention. To determine the quality of life burden in CLI patients, this review primarily focused on comparing the baseline quality of life scores of CLI patients reported from interventional studies to the quality of life scores of healthy people reported from studies of community-based norms.
Instruments for assessing quality of life in CLI patients
Studies examining the quality of life in CLI patients have used a combination of generic quality of life questionnaires (ie, SF-36) and disease-specific measures (ie, VascuQOL) (Table 5). Generic instruments are applicable to CLI populations because they address multidimensional domains of quality of life relevant to CLI patients such as mental health, physical functioning, bodily pain, emotional health, commonly performed daily activities, and social functioning.11 The SF-36 is the most frequently used generic quality of life assessment tool in CLI studies. Other generic quality of life measures that have been used in CLI studies include the Nottingham Health Profile (NHP), the World Health Organization Quality of Life Assessment Instrument (WHOQOL-100 and WHOQOL BREF), and the EuroQol-5D. The EuroQol-5D has been used in CLI studies to derive health state utilities.
Table 5.
Commonly used generic and disease-specific quality of life instruments in CLI studies
| QOL instrument | Abbreviation | Domains | Scoring |
|---|---|---|---|
| Short-Form 36 or RAND Short-Form 36 | SF-36 or RAND-36 | 36 items assessing 8 health-related concepts including physical functioning, bodily pain, general health, vitality, social functioning, mental health, and emotional well-being. The measure also yields psychometrically-based physical and mental health summary scores. | 0 to 100 |
| Short-Form 6 Dimension | SF-6D | The SF-6D health index is designed for calculating Quality Adjusted Life Years (QALYs), which are used to estimate the cost-effectiveness of health interventions. | 0 to 1 |
| EuroQol-5D (Utility Measure) EuroQol-Visual Analog Scale |
EQ-5D EQ-VAS |
A descriptive system of 5 dimensions including mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Visual analog scale from 0 to 100. |
EQ-5D Items: 0–1 VAS: 0–100 |
| Nottingham Health Profile | NHP | 38 statements addressing 6 health-related concepts including energy, pain, emotional reaction, sleep, social isolation, and physical mobility. | 0–100 |
| FLeQKI [German] | FLeQKI [German] | 35 items addressing 7 domains including comorbidity, physical pain, physical functioning, physical state, social functioning, mental health, and therapy-induced limitations. | |
| Vascular Quality of Life Questionnaire | VascuQOL-25 VascuQOL-6 |
25 questions assessing 5 domains including pain, symptoms, activities, social well-being, and emotional well-being. 6 items assessing the same 5 domains listed above. |
1–7 6–24 |
| Walking Impairment Questionnaire | WIQ | 16 questions assessing 4 domains including symptoms, distance, speed, and climbing. | 0–100 |
| World Health Organization Quality of Life Assessment Instrument | WHOQOL-100 WHOQOL-BREF |
100 questions assessing physical health, psychological health, social relationships, and environment. 26 questions assessing 4 domains including physical health, psychological health, social relationships, and environment. |
0–100 0–100 |
Disease-specific quality of life instruments are applicable to CLI populations because they address the specific limitations experienced by the patient, making them more sensitive to detect clinically relevant changes in health status in response to disease progression or treatment.36 However, a disease-specific quality of life measure for CLI has yet to be developed. Consequently, studies examining the quality of life in CLI patients have relied on disease-specific measures that have previously been developed for PAD patients. The Vascular Quality of Life Questionnaire (VascuQOL) is a PAD-specific measure that has been validated in CLI patients and used in several CLI studies.37 Ideally, studies examining quality of life in patients with CLI should include both generic and disease-specific measures.
Assessments of quality of life burden in CLI patients
Several studies have reported baseline SF-36 scores in CLI patients from clinical trials of various revascularization procedures (Table 6).14,38–48 In most of these studies, low quality of life baseline SF-36 scores were consistently observed in the physical health domains, including physical functioning, role physical functioning, and bodily pain compared to US community-based norms. The population norms in the United States for each of the SF-36 domains are as follows: physical functioning, 50.7; role physical, 49.5; bodily pain, 50.6; general health, 50.1; vitality, 53.7; social functioning, 51.4; role emotional, 51.4; mental health, 54.3; physical component summary, 49.2; and mental component summary, 53.8.49
Table 6.
Baseline SF-36/RAND-36 quality of life scores (0–100) for patients with CLI
| Publication | Instrument | PF | RP | BP | GH | VT | SF | RE | MH | PCS | MCS | CLI Population | Procedure(s) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Burt (2010)38 | SF-36 | – | – | – | – | – | – | – | – | 31.1 | 57.6 | 100% | Autologous peripheral blood cell implantation |
| Deutschmann (2007)39 | SF-36 | 24.8 | 5.6 | 13.9 | 50a | 28.1 | 70a | 28.5 | 46a | – | – | 52% | Percutaneous transluminal angioplasty |
| Engelhardtb (2008)40 | SF-36 | 25 | 12 | 21 | 56 | 41 | 50 | 38 | 56 | 100% | Infrageniculate bypass surgery | ||
| Forbes (2010)41 | SF-36 SF-36 |
22.7 23.0 |
10.3 13.1 |
30.4 32.0 |
49.1 48.0 |
34.2 36.3 |
40.6 44.2 |
31.5 36.3 |
58.9 60.1 |
100%c 100%c |
Angioplasty Bypass surgery |
||
| Keeling (2008)42 | SF-36 | 35.6 | 16.7 | 39.3 | 60.4 | 44.4 | 71.8 | 55.6 | 75.5 | – | – | 44% | Percutaneous transluminal angioplasty |
| Kumar (2011)43 | RAND-36 | 20.9 | 21.1 | 31.7 | 28.9 | 21.8 | 28.6 | 23.5 | – | – | – | 100% | Various revascularization procedures |
| Landry (2014)44 | SF-36 | 30 | 4 | 25 | 49 | 42 | 47 | 57 | 69 | – | – | 100% | Lower extremity bypass surgery |
| Peeters Weemd (2016)45 | SF-36 | 33 | 38 | 45 | 38 | 48 | 41 | 45 | 47 | 35 | 49 | 100%d | Autologous bone marrow cell therapy |
| Shigematsu (2010)46 | SF-36 | 24.4 22.9 |
28.7 2.1 |
32.1 30.1 |
38.4 36.8 |
43.1 45.0 |
50.5 45.8 |
30.9 11.1 |
50.6 59.0 |
100% | Hepatocyte growth factor plasmid No treatment |
||
| Sprengers (2010)14 | SF-36 | 28 | 34.4 | 32.9 | 39.7 | 44.8 | 36.9 | 39.5 | 42.6 | 30.8 | 45.9 | 100% | Bone marrow cell therapy |
| van Hattume (2011)47 | RAND-36 | 49 | 38 | 59 | 55 | 55 | 65 | 56 | 68 | 37 | 48 | 28% | Bypass surgery |
| Wohlgemuth (2008)48 | SF-36 | 40.7 | 26.9 | 17.6 | 29.3 | 55.6 | 70.2 | 51.9 | 62.3 | 70% | Bypass surgery | ||
| Maglinte (2012)49 | SF-36 | 50.7 | 49.5 | 50.6 | 50.1 | 53.7 | 51.4 | 51.4 | 54.3 | 49.2 | 53.8 | - | US population norms |
Notes: aBaseline SF-36 scores estimated from figure #3 in Deutschmann 2007;39 bA weighted average of the baseline SF-36 scores for the three patient subgroups (Non-Diabetes, Diabetes, and Secondary Amputation) from Table #3 in Engelhardt 2008;40 cCLI population defined as “severe lower limb ischemia” in the Forbes 201041 study (BASIL trial); dFor the purpose of this analysis, the CLI patient population from the Peeters Weem 201645 study was defined as patients with “no option severe limb ischemia”; baseline SF-36 scores estimated from figure #2 in Peeters Weem 2016;45 eFor the purposes of this analysis, the SF-36 scores presented in the table earlier are from Table II in the van Hattum 201147 study for the Bypass and Oral Anticoagulants or Aspirin (BOA) Study group.
Abbreviations: BP, Bodily Pain; CLI, critical limb ischemia; GH, General Health; MCS, Mental Component Summary; MH, Mental Health; PCS, Physical Component Summary; PF, Physical Functioning; RAND-36, 36-item Short-Form Health Survey; RE, Role Emotional; RP, Role Physical; SF, Social Functioning; SF-36, 36-item Short-Form Health Survey; VT, Vitality.
Sprengers and colleagues (2010) assessed quality of life using the SF-36 in 47 patients with “no-option” CLI using baseline data from the JUVENTAS trial, a randomized clinical trial examining the effects of bone marrow mononuclear cells in CLI patients.14 “No-option” CLI was defined as patients with no surgical or endovascular options for revascularization.14 The results of the study showed that patients with no-option CLI reported baseline SF-36 quality of life scores well below the general population quality of life scores on every health dimension of the SF-36 (Table 6).14 Similarly, Forbes and colleagues (2010) examined quality of life in 452 patients with CLI undergoing either angioplasty or bypass surgery from the BASIL trial and found that baseline SF-36 scores were well below the general population norms in both treatment groups for all the physical health domains, including physical functioning (22.7 and 23), role physical (10.3 and 13.1), and bodily pain (30.4 and 32) reflecting a substantial quality of life burden in these patients (Table 6).41
The EuroQol-5D and the EuroQol VAS (visual analog scale) have been used in several CLI studies to derive health state utilities (Table 7).35,41,47,50–53 The EuroQol-5D index population norm in the United States is 0.825 (on a 0 to 1 scale) and the EuroQol VAS population norm is 80 (on a 0 to 100 scale).54 A study by Pisa and colleagues (2012) was one of the few recently published studies which assessed the impact of CLI on a patient’s quality of life that was not derived from an interventional study.35 In this study, 200 patients with CLI were interviewed to assess the impact of CLI on health status using the EuroQol-5D, VAS, and other utility measures.35 The mean calculated EuroQol-5D and VAS scores for CLI patients were 0.56 and 56, respectively, indicating an impaired health status when compared to US population norms (Table 7).35
Table 7.
Baseline EuroQol-5D utility scores and VAS scores for patients with CLI
| Publication | Instrument | Baseline score | CLI population | Procedures |
|---|---|---|---|---|
| EuroQol-5D | ||||
| Egberg (2010)52 | EQ-5D | 0.54 | 15% | Percutaneous transluminal angioplasty |
| Forbes (2010)41 | EQ-5D EQ-5D SF-6D SF-6D |
0.26 0.28 0.53 0.54 |
100%a 100%a 100%a 100%a |
Angioplasty Bypass surgery Angioplasty Bypass surgery |
| Pisa (2012)35 | EQ-5D | 0.56 | 100% | NA |
| van Hattum (2011)47 | EQ-5D | 0.68 | 28% | Bypass surgery |
| Szende (2014)54 | EQ-5D | 0.825 | – | US population norm |
| VAS | ||||
| Bague (2017)50 | VAS for EQ-5D | 65.8 | 13% | Endovascular |
| Brother (2015)51 | VAS | 45.6 | 100% | Open surgery or endovascular treatment |
| Forbes (2010)41 | VAS for EQ-5D VAS for EQ-5D |
53 55 |
100%a 100%a |
Angioplasty Bypass surgery |
| Klepanec (2012)53 | VAS for EQ-5D | 51 | 100% | Intramuscular delivery of autologous bone marrow cells |
| Pisa (2012)35 | VAS for EQ-5D | 56 | 100% | NA |
| van Hattum (2011)47 | VAS for EQ-5D | 66 | 28% | Bypass surgery |
| Szende (2014)54 | VAS for EQ-5D | 80 | – | US population norm |
Note: aCLI population defined as “severe lower limb ischemia” for Forbes 201041 (BASIL trial).
Abbreviations: CLI, critical limb ischemia; EQ-5D, EuroQol-5 Dimension; NA, not applicable; SF-6D, Short-Form Six-Dimension; VAS, visual analog scale.
More recently, Steunenberg and colleagues (2018) assessed quality of life in elderly CLI patients undergoing endovascular, surgical, or conservative therapy using the WHOQOL-BREF questionnaire.33 General population norms for the four WHOQOL-BREF domains are 73.5 for the physical health domain, 70.6 for psychological well-being domain, 71.5 for the social relationships domain, and 75.1 for the environment domain.55 The baseline WHOQOL-BREF scores for the CLI patients in the Steunenberg study were well below the general population norms for all four domains including physical health (10.9–11.6), psychological well-being (14.0–14.2), social relationships (15.4–15.5), and the environment (15.4–15.6) reflecting a substantial quality of life burden in these patients.33
Several studies have reported baseline VascuQOL in CLI patients from clinical trials of various revascularization procedures and medical therapies.37,41,44,56–60 In all of these CLI intervention studies, low baseline VascuQOL scores were consistently observed indicating that patients have an impaired quality of life at baseline prior to undergoing an intervention. For example, Forbes and colleagues (2010) evaluated quality of life using the VascuQOL-25 (scoring from 1 to 7) in 452 CLI patients enrolled in the BASIL trial and found low pre-operative baseline scores in both the angioplasty group (2.8) and bypass surgery group (2.9).41 Similarly, Landry and colleagues (2014) evaluated quality of life in 18 CLI patients undergoing lower extremity bypass surgery and found a low VascuQOL-25 score of 2.9 at baseline indicating a substantial quality of life burden in these patients.44
Economic burden
National CLI cost
A complete understanding of the burden of CLI would benefit from a comprehensive estimate of the national cost of the disease. This type of data is lacking in the contemporary CLI literature. Recent studies estimating the national economic burden of CLI have focused on the costs of hospitalized patients, which undoubtedly comprises a substantial portion of the CLI economic burden; however, no comprehensive accounting (beyond hospitalization) of national costs over a longer term has been published recently. An additional limitation of the data in the published literature is that estimates often have focused solely on the hospitalization costs of a subgroup of CLI patients (those undergoing surgical or endovascular revascularization) likely leaving a large number of CLI-related hospitalizations unexplored.
Despite these limitations, three studies do report national cost estimates for CLI.23,61,62 Sachs et al (2011) used the NIS (1999–2007) to estimate the costs of inpatient procedures (angioplasty or bypass) in CLI patients in the United States.61 The results of this study showed a 50% increase in the total inpatient costs from 2001 ($579 million) to 2007 ($870 million).61 An analysis by Kolte and colleagues (2017) using the Nationwide Readmissions Database (2013–2014) estimated the inpatient costs for open or endovascular treatment of CLI patients to be approximately $4.2 billion with an additional $625 million spent for 30-day unplanned readmissions.23 Finally, Malyar and colleagues (2013) examined all hospitalizations in Germany in 2007 and 2009 to estimate the inpatient costs for all CLI patients and found that total hospital reimbursement for CLI patients exceeded €1.1 billion in 2007 and €1.3 billion in 2009.62
A recent longitudinal analysis by Mustapha and colleagues (2018) of US Medicare data over the time period 2011 (for the index CLI diagnosis) to September 2015 offers a more comprehensive accounting of CLI costs than those studies focused solely on hospitalization.20 In unmatched and propensity-score-matched patient samples, Mustapha estimated a cost per CLI patient over the 4-year follow-up period of $93,800 and $117,800 for the 2 samples, respectively.20 The majority (~62–68%) of the cost was attributable to inpatient admissions with the remainder approximately equally split between hospital outpatient and physician/supplier costs. Although not an overall national estimate, the authors did calculate an annual cost to Medicare of ~$12 billion attributable to incident CLI cases. If considering other published epidemiologic data as well as non-Medicare patients, the total US cost of CLI likely would be several times higher than Mustapha’s conservative estimate.
Index CLI hospitalization cost
Since hospitalization comprises a large percentage of total CLI cost, research evaluating hospitalization cost at the individual patient level is particularly notable. US and German studies have documented the hospital costs of index CLI procedures using large national databases (Table 8). The US studies provide information on the hospital cost trajectory over a decade, whereas the German studies explore the impact of disease severity and co-morbidity on costs.
Table 8.
Index CLI hospitalization costs
| Publication | Country/region | Data source (years) | Currency | Population details | Methods | Total cost |
|---|---|---|---|---|---|---|
| Reinecke (2015)26 | Germany | German health insurer data (2009–2011) | Euro | Hospitalized CLI patients | ICD-10 dx and German procedure (OPS) codes to identify cases and procedures | Mean Cost 2011: €5,316 (Rutherford 4) 2011: €6,021 (Rutherford 5) 2011: €8,461 (Rutherford 6) |
| Agarwal (2016)22 | United States | Nationwide Inpatient Sample (2003–2011) | 2015 USD | Hospitalized CLI patients | ICD-9 diagnosis and procedure codes used to identify cases and procedures | Mean Cost 2003: ~$23,000 2007: ~$23,000 2011: ~$23,000 |
| Dua (2016)63 | United States | Nationwide Inpatient Sample (2001–2011) | 2015 USD | Elective admissions to treat CLI | ICD-9 diagnosis and procedure codes used to identify cases and procedures | Median Cost 2001: $12,568 2004: $15,588 2007: $17,964 2011: $20,587 |
| Freisinger (2017)32 | Germany | German health insurer data (2009–2011) | Euro | Hospitalized CLI patients with tissue loss | ICD-10 dx and German procedure (OPS) codes to identify cases and procedures | Mean Cost Rutherford 5 2011: €5,975 (with DM) 2011: €6,058 (without DM) Rutherford 6 2011: €8,860 (with DM) 2011: €8,080 (without DM) |
| Martinez (2018)64 | United States | Nationwide Readmissions Database (2013–2014) | USD | CLI patients undergoing revascularization procedures | ICD-9 diagnosis and procedure codes used to identify cases and procedures | Mean Cost Index admission: $29,148 Readmission: $17,681 |
Abbreviations: CLI, critical limb ischemia; DM, diabetes mellitus; ICD-9/10, International Classification of Diseases 9th/10th Revision; OPS, Operationen und Prozedurenschlüssel [German procedure classification]; USD, US dollar.
Agarwal (2016) and Dua (2016) conducted separate analyses using the NIS over similar time periods.22,63 The key difference in the studies was the inclusion criteria which yielded somewhat different patient populations. Agarwal included all adult hospitalizations with a CLI diagnosis whereas Dua excluded any emergent procedures or primary amputations without a prior revascularization, focusing only on patients with elective procedures. Over a 9-year period (2003–2011) with over 640,000 CLI admissions, Agarwal found that the mean hospitalization cost was consistent and unchanged—approximately $23,000 in each year.22 In patients treated electively for CLI, Dua reported a very different result—a 63% increase in the median hospitalization cost from 2001 ($12,568) to 2011 ($20,587).63 While the median cost would be expected to be lower than the mean, and the exclusion of costly emergent procedures and amputations also would explain a lower average cost in the Dua study, the difference in the cost trajectories between the two studies is difficult to reconcile.
More recently, a study by Martinez and colleagues (2018) extended the hospitalization cost findings to 2013/2014 using the Nationwide Readmissions Database (NRD), which included data from nearly 15 million admissions in 22 US states.64 The results of the study showed a mean index admission cost for CLI of $29,148, as well as a 30-day readmission cost of $17,681.64 This latter mean estimate is greater than the median 30-day readmission cost of $12,419 reported by Kolte et al (2017) from their analysis of the 2013/14 NRD.23
German researchers have employed the BARMER GEK database (the largest public health insurer in the country) to quantify the index hospitalization cost for CLI patients. In two studies, both using data from 2009 to 2011, results documented the higher admission cost associated with increasing disease severity.26,32 Interestingly, index admission costs were similar between CLI patients with and without diabetes, although analyses of inpatient follow-up cost post-discharge noted higher costs for diabetics.32
Amputation cost
Although evidence suggests that amputation rates may be declining,22 the cost associated with amputation and its elevated morbidity and mortality is still an important component of the overall economic burden of CLI. A few recent studies have quantified amputation-related costs. Hospitalization costs for an amputation may be the easiest to estimate given no requirement for longer follow-up, yet do not appear to have been studied (published) in the CLI population since 2012. Mean or median hospital costs in the 2008/2009 time period have been estimated to range from approximately $10,000 to nearly $30,000 with much of the variability due to the level of amputation required (minor vs major).65,66
Given the challenges in studying patients for periods of time exceeding 1–2 years, especially with the high mortality experienced by this population, the relative lack of contemporary long-term amputation cost studies is understandable. Furthermore, the lone study calculating costs over a 10-year period relies on economic modeling for its cost estimation.66 The authors reported a 10-year expected cost (discounted to net present value) of a primary amputation strategy in Rutherford 5 CLI patients as $78,958.66
The most recent study documenting the costs of amputation used sophisticated statistical modeling of the US MarketScan database (2006–2014) to highlight the economic burden associated with amputation.67 The analysis evaluated the monthly cost per patient after the first CLI diagnosis and compared those costs between patients who underwent a major amputation and those that did not. The authors included a broad array of resources (eg, inpatient, outpatient, labs, etc.) and calculated two incremental costs—one that included all resources except pharmacy and another that included pharmacy costs. The results suggested that CLI patients with a major amputation had costs approximately $5,000 per month higher than those that did not require a major amputation. When adding pharmacy costs, the incremental cost increased to approximately $6,000 per month.67
Documenting the economic burden of amputation contributes to an understanding of the overall cost of CLI. However, drawing definitive conclusions about amputation costs based on the most recently published studies is challenging. Differences in study design and robustness, populations and data sources, medical (and non-medical) resources considered, and other factors all contribute to a wide range of cost estimates.
Discussion
CLI is a serious condition that affects millions of patients globally. Its poor prognosis (in terms of both morbidity and mortality) suggests the impact of the disease is substantial. Furthermore, with increasing PAD prevalence and advancing age in many populations, coupled with the growth in other CLI risk factors such as diabetes (globally)68 and smoking (in many countries),69 there seems little reason for optimism that the number of CLI patients will decrease in the near future. Therefore, a comprehensive understanding of disease burden is warranted.
The clinical consequences of CLI, especially excess mortality and the high risk of amputation, are particularly concerning. That these outcomes appear to have a differential racial and socioeconomic impact only adds to their gravity. Other consequences of CLI include ischemic rest pain, non-healing ulcers, symptomatic gangrene, dependency on caregivers for support, the need for permanent local wound treatment, and the chronic use of pain-relieving medications. All of these factors significantly impair the quality of life for CLI patients by imposing a substantial burden on a patient’s emotional, social, and physical well-being. In addition to its humanistic impact, CLI morbidity and treatment pose significant financial challenges. At a national level, the disease results in expenditures of billions of dollars and euros in the United States and European countries annually. These expenses appear to be driven largely by hospitalization costs and secondarily by the costs of amputation. Extremely high readmission rates only serve to compound the economic burden.
Our review of the literature related to the burden of CLI has several limitations that should be mentioned. First, many studies, although recently published, utilize data from large databases that may report results from over a decade ago. This data lag may reduce the relevance of even contemporarily published research. Second, our review was not intended to evaluate or recommend specific CLI treatment strategies or individual products or devices and, therefore, non-treatment specific studies or large database studies were preferred over individual clinical trials or single-center studies. This preference, however, was relaxed for the assessment of quality of life burden. The examination of the quality of life burden in CLI patients was based primarily on baseline data from CLI interventional studies as there is a scarcity of contemporary quality of life burden of illness studies in CLI patients. These intervention studies were not designed to compare baseline quality of life scores in CLI patients to healthy people in the community but may provide useful information on the magnitude of quality of life burden in CLI patients. Third, a CLI-specific quality of life measure has yet to be developed. Consequently, studies examining the quality of life in CLI patients have used a combination of generic quality of life questionnaires (ie, SF-36) and disease-specific measures (ie, VascuQOL) that have previously been used to measure quality of life in PAD patients. Finally, for a variety of reasons, comprehensive (in terms of both medical and non-medical/indirect costs) and long-term economic analyses are lacking in CLI patients. This necessarily renders our understanding of the economics associated with the disease as incomplete and the economic burden of CLI underestimated.
By necessity, our research effort explicitly focused on summarizing CLI burden yet the search process yielded several observations related to the published literature that should be addressed. For example, despite our comprehensive search strategies, nearly all recent peer-reviewed CLI epidemiologic research is limited to the US perspective. Several abstracts and non-peer-reviewed presentations were identified that described CLI epidemiology in other countries, but these lacked the detail necessary for inclusion in our review. Also, numerous potentially relevant studies discussed trends in utilization of diagnostic modalities and revascularization (or other) treatment approaches, clinical outcomes associated with interventions, or potential demographic/socioeconomic disparities related to the disease without explicit information pertaining to CLI burden. These topics are important areas for continued research and synthesis, warranting a similar comprehensive assessment and literature review.
Conclusion
This literature review is unique in that it provides a broad perspective of the burden of CLI while focusing on the most recent key publications. Our summary documents a sobering assessment of CLI burden—a poor clinical prognosis translating into diminished quality of life and high costs for millions of patients. Continued prevention efforts and improved treatment strategies are the key to ameliorating the substantial morbidity and mortality associated with this disease.
Disclosure
Steve Duff is an employee of Veritas Health Economics Consulting which was contracted to conduct the literature review. Michael S Mafilios is an employee of Health Economics Associates which was contracted to conduct the literature review. Prajakta Bhounsule and James T Hasegawa are employees of Abbott Vascular. The authors report no other conflicts of interest in this work.
Supplementary material
CLI amputation rates.
Abbreviations: CLI, critical limb ischemia; OXVASC, Oxford Vascular Study.
References
- 1.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG; TASC II Working Group. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45(Suppl S):S5–S67. doi: 10.1016/j.jvs.2006.12.037 [DOI] [PubMed] [Google Scholar]
- 2.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2017;69(11):e71–e126. doi: 10.1016/j.jacc.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 3.Abu Dabrh AM, Steffen MW, Undavalli C, et al. The natural history of untreated severe or critical limb ischemia. J Vasc Surg. 2015;62(6):1642–1651.e1643. doi: 10.1016/j.jvs.2015.07.065 [DOI] [PubMed] [Google Scholar]
- 4.Alabi O, Roos M, Landry G, Moneta G. Quality-of-life assessment as an outcomes measure in critical limb ischemia. J Vasc Surg. 2017;65(2):571–578. doi: 10.1016/j.jvs.2016.08.097 [DOI] [PubMed] [Google Scholar]
- 5.Alonso A, Garcia LA. The costs of critical limb ischemia. Endovasc Today. 2011;32–36. [Google Scholar]
- 6.Barshes NR, Belkin M, MOVIE Study Collaborators. Framework for the evaluation of “value” and cost-effectiveness in the management of critical limb ischemia. J Am Coll Surg. 2011;3(4):552–566. doi: 10.1016/j.jamcollsurg.2011.07.011 [DOI] [PubMed] [Google Scholar]
- 7.Biancari F. Meta-analysis of the prevalence, incidence and natural history of critical limb ischemia. J Cardiovasc Surg. 2013;54:663–669. [PubMed] [Google Scholar]
- 8.Dua A, Lee CJ. Epidemiology of peripheral arterial disease and critical limb ischemia. Tech Vasc Interv Radiol. 2016;19(2):91–95. doi: 10.1053/j.tvir.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 9.Farber A, Eberhardt RT. The current state of critical limb ischemia: a systematic review. JAMA Surg. 2016;151(11):1070–1077. doi: 10.1001/jamasurg.2016.2018 [DOI] [PubMed] [Google Scholar]
- 10.Gulati A, Garcia L, Acharji S, et al. Epidemiology of chronic critical limb ischemia In: Dieter RS, editor. Critical Limb Ischemia. Switzerland: Springer International Publishing Switzerland; 2017:9–14. [Google Scholar]
- 11.Lawall H, Zemmrich C, Bramlage P, Aann B. Health related quality of life in patients with critical limb ischemia. Vasa. 2012;41(2):78–88. doi: 10.1024/0301-1526/a000169 [DOI] [PubMed] [Google Scholar]
- 12.Monaro S, West S, Gullick J. An integrative review of health-related quality of life in patients with critical limb ischaemia. J Clin Nurs. 2016;26:2826–2844. doi: 10.1111/jocn.13623 [DOI] [PubMed] [Google Scholar]
- 13.Shishehbor MH, White CJ, Gray BH, et al. Critical limb ischemia: an expert statement. J Am Coll Cardiol. 2016;68(18):2002–2015. doi: 10.1016/j.jacc.2016.04.071 [DOI] [PubMed] [Google Scholar]
- 14.Sprengers RW, Teraa M, Moll FL, de Wit GA, van der Graaf Y, Verhaar MC, JUVENTAS Study Group; SMART Study Group. Quality of life in patients with no-option critical limb ischemia underlines the need for new effective treatment. J Vasc Surg. 2010;52(4):843–849. doi: 10.1016/j.jvs.2010.04.057 [DOI] [PubMed] [Google Scholar]
- 15.Steunenberg SL, Raats JW, Te Slaa A, de Vries J, van der Laan L. Quality of life in patients suffering from critical limb ischemia. Ann Vasc Surg. 2016;36:310–319. doi: 10.1016/j.avsg.2016.05.087 [DOI] [PubMed] [Google Scholar]
- 16.Uccioli L, Meloni M, Izzo V, Giurato L, Merolla S, Gandini R. Critical limb ischemia: current challenges and future prospects. Vasc Health Risk Manag. 2018;14:63–74. doi: 10.2147/VHRM.S125065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nehler MR, Duval S, Diao L, et al. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. J Vasc Surg. 2014;60(3):686–695. doi: 10.1016/j.jvs.2014.03.290 [DOI] [PubMed] [Google Scholar]
- 18.United States Census Bureau, Population Division. Annual Estimates of the Resident Population by Single Year of Age and Sex for the United States: April 1, 2010 to July 1, 2017. Release date June 2018.
- 19.Baser O, Verpillat P, Gabriel S, Wang L. Prevalence, incidence, and outcomes of critical limb ischemia in the US Medicare population. Vasc Dis Manag. 2013;10(2):26–36. [Google Scholar]
- 20.Mustapha JA, Katzen BT, Neville RF, et al. Determinants of long-term outcomes and costs in the management of critical limb ischemia: a population-based cohort study. J Am Heart Assoc. 2018;7(16):e009724. doi: 10.1161/JAHA.118.008528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Medicare and Medicaid Services; Office of the Actuary. CMS Program Data – populations. July 2018 Version Available from: https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/cms-fast-facts/index.html. Accessed January8, 2019.
- 22.Agarwal S, Sud K, Shishehbor MH. Nationwide trends of hospital admission and outcomes among critical limb ischemia patients: from 2003-2011. J Am Coll Cardiol. 2016;67(16):1901–1913. doi: 10.1016/j.jacc.2016.02.040 [DOI] [PubMed] [Google Scholar]
- 23.Kolte D, Kennedy KF, Shishehbor MH, et al. Thirty-day readmissions after endovascular or surgical therapy for critical limb ischemia: analysis of the 2013 to 2014 Nationwide Readmissions Databases. Circulation. 2017;136(2):167–176. doi: 10.1161/CIRCULATIONAHA.117.027625 [DOI] [PubMed] [Google Scholar]
- 24.Luders F, Bunzemeier H, Engelbertz C, et al. CKD and acute and long-term outcome of patients with peripheral artery disease and critical limb ischemia. Clin J Am Soc Nephrol. 2016;11(2):216–222. doi: 10.2215/CJN.05600515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Brien-Irr MS, Harris LM, Dosluoglu HH, Dryjski ML. Procedural trends in the treatment of peripheral arterial disease by insurer status in New York State. J Am Coll Surg. 2012;215(3):311–321. doi: 10.1016/j.jamcollsurg.2012.05.033 [DOI] [PubMed] [Google Scholar]
- 26.Reinecke H, Unrath M, Freisinger E, et al. Peripheral arterial disease and critical limb ischaemia: still poor outcomes and lack of guideline adherence. Eur Heart J. 2015;36(15):932–938. doi: 10.1093/eurheartj/ehv006 [DOI] [PubMed] [Google Scholar]
- 27.Spreen M, Gremmels H, Teraa M, et al. Decreased limb survival in diabetic patients with critical limb ischemia compared with non-diabetic patients. Cardiovasc Intervent Radiol. 2016;39(3 Supplement 1):S305. [Google Scholar]
- 28.Henry AJ, Hevelone ND, Belkin M, Nguyen LL. Socioeconomic and hospital-related predictors of amputation for critical limb ischemia. J Vasc Surg. 2011;53(2):330–339.e1. doi: 10.1016/j.jvs.2010.08.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agarwal S, Pitcavage JM, Sud K, Thakkar B. Burden of readmissions among patients with critical limb ischemia. J Am Coll Cardiol. 2017;69(15):1897–1908. doi: 10.1016/j.jacc.2017.02.040 [DOI] [PubMed] [Google Scholar]
- 30.Malyar NM, Freisinger E, Meyborg M, et al. Low rates of revascularization and high in-hospital mortality in patients with ischemic lower limb amputation: morbidity and mortality of ischemic amputation. Angiology. 2016;67(9):860–869. doi: 10.1177/0003319715626849 [DOI] [PubMed] [Google Scholar]
- 31.Klaphake S, de Leur K, Mulder PGH, et al. Mortality after major amputation in elderly patients with critical limb ischemia. Clin Interv Aging. 2017;12:1985–1992. doi: 10.2147/CIA.S137570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freisinger E, Malyar NM, Reinecke H, Lawall H. Impact of diabetes on outcome in critical limb ischemia with tissue loss: a large-scaled routine data analysis. Cardiovasc Diabetol. 2017;16(1):41. doi: 10.1186/s12933-017-0624-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steunenberg SL, de Vries J, Raats JW, et al. Quality of life and mortality after endovascular, surgical, or conservative treatment of elderly patients suffering from critical limb ischemia. Ann Vasc Surg. 2018;51:95–105. doi: 10.1016/j.avsg.2018.02.044 [DOI] [PubMed] [Google Scholar]
- 34.Steunenberg SL, Te Slaa A, Ho GH, Veen EJ, de Groot HG, van der Laan L. Dementia in patients suffering from critical limb ischemia. Ann Vasc Surg. 2017;38:268–273. doi: 10.1016/j.avsg.2016.05.136 [DOI] [PubMed] [Google Scholar]
- 35.Pisa G, Reinhold T, Obi-Tabot E, Bodoria M, Brüggenjürgen B. Critical limb ischemia and its impact on patient health preferences and quality of life-an international study. Int J Angiol. 2012;21(3):139–146. doi: 10.1055/s-0032-1324738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nordanstig J, Wann-Hansson C, Karlsson J, et al. Vascular Quality of Life Questionnaire-6 facilitates health-related quality of life assessment in peripheral arterial disease. J Vasc Surg. 2014;59(3):700–707. doi: 10.1016/j.jvs.2013.08.099 [DOI] [PubMed] [Google Scholar]
- 37.Jens S, Conijn AP, Frans FA, et al. Outcomes of infrainguinal revascularizations with endovascular first strategy in critical limb ischemia. Cardiovasc Intervent Radiol. 2015;38(3):552–559. doi: 10.1007/s00270-014-0955-5 [DOI] [PubMed] [Google Scholar]
- 38.Burt RK, Testori A, Oyama Y, et al. Autologous peripheral blood CD133+ cell implantation for limb salvage in patients with critical limb ischemia. Bone Marrow Transplant. 2010;45(1):111–116. doi: 10.1038/bmt.2009.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deutschmann HA, Schoellnast H, Temmel W, et al. Endoluminal therapy in patients with peripheral arterial disease: prospective assessment of quality of life in 190 patients. AJR Am J Roentgenol. 2007;188(1):169–175. doi: 10.2214/AJR.05.1408 [DOI] [PubMed] [Google Scholar]
- 40.Engelhardt M, Bruijnen H, Scharmer C, Wohlgemuth WA, Willy C, Wölfle KD. Prospective 2-years follow-up quality of life study after infrageniculate bypass surgery for limb salvage: lasting improvements only in non-diabetic patients. Eur J Vasc Endovasc Surg. 2008;36(1):63–70. doi: 10.1016/j.ejvs.2008.01.026 [DOI] [PubMed] [Google Scholar]
- 41.Forbes JF, Adam DJ, Bell J, et al.; BASIL trial Participants. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: health-related quality of life outcomes, resource utilization, and cost-effectiveness analysis. J Vasc Surg. 2010;51(5Suppl):43S–51S. doi: 10.1016/j.jvs.2010.01.076 [DOI] [PubMed] [Google Scholar]
- 42.Keeling AN, Naughton PA, O’Connell A, Lee MJ. Does percutaneous transluminal angioplasty improve quality of life? J Vasc Interv Radiol. 2008;19(2 Pt 1):169–176. doi: 10.1016/j.jvir.2007.09.003 [DOI] [PubMed] [Google Scholar]
- 43.Kumar BN, Gambhir RP. Critical limb ischemia-need to look beyond limb salvage. Ann Vasc Surg. 2011;25(7):873–877. doi: 10.1016/j.avsg.2011.05.020 [DOI] [PubMed] [Google Scholar]
- 44.Landry GJ, Esmonde NO, Lewis JR, et al. Objective measurement of lower extremity function and quality of life after surgical revascularization for critical lower extremity ischemia. J Vasc Surg. 2014;60(1):136–142. doi: 10.1016/j.jvs.2014.01.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peeters Weem SM, Teraa M, Den Ruijter HM, de Borst GJ, Verhaar MC, Moll FL. Quality of life after treatment with autologous bone marrow derived cells in no option severe limb ischemia. Eur J Vasc Endovasc Surg. 2016;51(1):83–89. doi: 10.1016/j.ejvs.2015.09.010 [DOI] [PubMed] [Google Scholar]
- 46.Shigematsu H, Yasuda K, Iwai T, et al. Randomized, double-blind, placebo-controlled clinical trial of hepatocyte growth factor plasmid for critical limb ischemia. Gene Ther. 2010;17(9):1152–1161. doi: 10.1038/gt.2010.51 [DOI] [PubMed] [Google Scholar]
- 47.van Hattum ES, Tangelder MJ, Lawson JA, Moll FL, Algra A. The quality of life in patients after peripheral bypass surgery deteriorates at long-term follow-up. J Vasc Surg. 2011;53(3):643–650. doi: 10.1016/j.jvs.2010.09.021 [DOI] [PubMed] [Google Scholar]
- 48.Wohlgemuth WA, Safonova O, Engelhardt M, Freitag M, Wölfle K, Kirchhof K. Improvement of the quality of life concerning the health of patients with peripheral arterial disease (PAD) after successful bypass surgery. Vasa. 2008;37(4):338–344. doi: 10.1024/0301-1526.37.4.338 [DOI] [PubMed] [Google Scholar]
- 49.Maglinte GA, Hays RD, Kaplan RM. US general population norms for telephone administration of the SF-36v2. J Clin Epidemiol. 2012;65(5):497–502. doi: 10.1016/j.jclinepi.2011.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bague N, Julia P, Sauguet A, et al. Femoropopliteal in-stent restenosis repair: midterm outcomes after paclitaxel eluting balloon use (PLAISIR Trial). Eur J Vasc Endovasc Surg. 2017;53(1):106–113. doi: 10.1016/j.ejvs.2016.10.002 [DOI] [PubMed] [Google Scholar]
- 51.Brothers TE. Failure of patients with peripheral arterial disease to accept the recommended treatment results in worse outcomes. Ann Vasc Surg. 2015;29(2):244–259. doi: 10.1016/j.avsg.2014.08.006 [DOI] [PubMed] [Google Scholar]
- 52.Egberg L, Mattiasson AC, Ljungström KG, Styrud J. Health-related quality of life in patients with peripheral arterial disease undergoing percutaneous transluminal angioplasty: a prospective one-year follow-up. J Vasc Nurs. 2010;28(2):72–77. doi: 10.1016/j.jvn.2010.02.001 [DOI] [PubMed] [Google Scholar]
- 53.Klepanec A, Mistrik M, Altaner C, et al. No difference in intra-arterial and intramuscular delivery of autologous bone marrow cells in patients with advanced critical limb ischemia. Cell Transplant. 2012;21(9):1909–1918. doi: 10.3727/096368912X636948 [DOI] [PubMed] [Google Scholar]
- 54.Szende A, Janssen B, Cabases J, editors. Self-Reported Population Health: An International Perspective Based on EQ-5D. EuroQol Group. London, UK: Springer Open; 2014. [PubMed] [Google Scholar]
- 55.Hawthorne G, Herrman H, Murphy B. Interpreting the WHOQOL-BREF: preliminary population norms and effect sizes. Soc Indic Res. 2006;77(1):37–59. doi: 10.1007/s11205-005-5552-1 [DOI] [Google Scholar]
- 56.Corriere MA, Goldman MP, Barnard R, et al. Cumulative number of treatment interventions predicts health-related quality of life in patients with critical limb ischemia. Ann Vasc Surg. 2017;44:41–47. [DOI] [PubMed] [Google Scholar]
- 57.Frans FA, Met R, Koelemay MJ, et al. Changes in functional status after treatment of critical limb ischemia. J Vasc Surg. 2013;58(4):957–965.e1. doi: 10.1016/j.jvs.2013.04.034 [DOI] [PubMed] [Google Scholar]
- 58.Frans FA, Nieuwkerk PT, Met R, et al. Statistical or clinical improvement? Determining the minimally important difference for the vascular quality of life questionnaire in patients with critical limb ischemia. Eur J Vasc Endovasc Surg. 2014;47(2):180–186. doi: 10.1016/j.ejvs.2013.10.012 [DOI] [PubMed] [Google Scholar]
- 59.Murphy MP, Lawson JH, Rapp BM, et al. Autologous bone marrow mononuclear cell therapy is safe and promotes amputation-free survival in patients with critical limb ischemia. J Vasc Surg. 2011;53(6):1565–1574. doi: 10.1016/j.jvs.2011.01.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nordanstig J, Pettersson M, Morgan M, Falkenberg M, Kumlien C. Assessment of minimum important difference and substantial clinical benefit with the Vascular Quality of Life Questionnaire-6 when evaluating revascularisation procedures in peripheral arterial disease. Eur J Vasc Endovasc Surg. 2017;54(3):340–347. doi: 10.1016/j.ejvs.2017.06.022 [DOI] [PubMed] [Google Scholar]
- 61.Sachs T, Pomposelli F, Hamdan A, Wyers M, Schermerhorn M. Trends in the national outcomes and costs for claudication and limb threatening ischemia: angioplasty vs bypass graft. J Vasc Surg. 2011;54(4):1021–1031. doi: 10.1016/j.jvs.2011.03.281 [DOI] [PubMed] [Google Scholar]
- 62.Malyar N, Fürstenberg T, Wellmann J, et al. Recent trends in morbidity and in-hospital outcomes of in-patients with peripheral arterial disease: a nationwide population-based analysis. Eur Heart J. 2013;34(34):2706–2714. doi: 10.1093/eurheartj/eht288 [DOI] [PubMed] [Google Scholar]
- 63.Dua A, Desai SS, Patel B, et al. Preventable complications driving rising costs in management of patients with critical limb ischemia. Ann Vasc Surg. 2016;33:144–148. doi: 10.1016/j.avsg.2015.11.026 [DOI] [PubMed] [Google Scholar]
- 64.Martinez RA, Shnayder M, Parreco J, et al. Nationally representative readmission factors in patients with claudication and critical limb ischemia. Ann Vasc Surg. 2018;52:96–107. doi: 10.1016/j.avsg.2018.03.011 [DOI] [PubMed] [Google Scholar]
- 65.Peacock JM, Keo HH, Duval S, et al. The incidence and health economic burden of ischemic amputation in Minnesota, 2005-2008. Prev Chronic Dis. 2011;8(6):A141. [PMC free article] [PubMed] [Google Scholar]
- 66.Barshes NR, Chambers JD, Cohen J, Belkin M. Model To Optimize Healthcare Value in Ischemic Extremities 1 (MOVIE) Study Collaborators. Cost-effectiveness in the contemporary management of critical limb ischemia with tissue loss. J Vasc Surg. 2012;56(4):1015–1024. doi: 10.1016/j.jvs.2012.02.069 [DOI] [PubMed] [Google Scholar]
- 67.Armstrong EJ, Ryan MP, Baker ER, Martinsen BJ, Kotlarz H, Gunnarsson C. Risk of major amputation or death among patients with critical limb ischemia initially treated with endovascular intervention, surgical bypass, minor amputation, or conservative management. J Med Econ. 2017;20(11):1148–1154. doi: 10.1080/13696998.2017.1361961 [DOI] [PubMed] [Google Scholar]
- 68.World Health Organization. Global report on diabetes 2016. Availbale from: https://www.who.int/diabetes/global-report/en/. Accessed January15, 2019.
- 69.World Health Organization Global Health Observatory data repository. Tobacco use: data by country. Available from: http://apps.who.int/gho/data/node.main.65. Accessed January15, 2019.
- 70.Reed GW, Raeisi-Giglou P, Kafa R, Malik U. Hospital readmissions following endovascular therapy for critical limb ischemia: associations with wound healing, major adverse limb events, and mortality. J Am Heart Assoc. 2016;5(5):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jones CE, Richman JS, Chu DI, Gullick AA, Pearce BJ, Morris MS. Readmission rates after lower extremity bypass vary significantly by surgical indication. J Vasc Surg. 2016;64(2):458–464. doi: 10.1016/j.jvs.2016.03.422 [DOI] [PubMed] [Google Scholar]
- 72.Bodewes TC, Soden PA, Ultee KH, et al. Risk factors for 30-day unplanned readmission following infrainguinal endovascular interventions. J Vasc Surg. 2017;65(2):484–494. doi: 10.1016/j.jvs.2016.08.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Masoomi R, Shah Z, Quint C, et al. A nationwide analysis of 30-day readmissions related to critical limb ischemia. Vascular. 2018;26(3):239–249. doi: 10.1177/1708538117727955 [DOI] [PubMed] [Google Scholar]
- 74.Marston WA, Davies SW, Armstrong B, et al. Natural history of limbs with arterial insufficiency and chronic ulceration treated without revascularization. J Vasc Surg. 2006;44:108–114. doi: 10.1016/j.jvs.2006.03.026 [DOI] [PubMed] [Google Scholar]
- 75.Howard DP, Banerjee A, Fairhead JF, Hands L, Silver LE, Rothwell PM. Population-based study of incidence, risk factors, outcome, and prognosis of ischemic peripheral arterial events: implications for prevention. Circulation. 2015;132(19):1805–1815. doi: 10.1161/CIRCULATIONAHA.115.016424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baubeta Fridh E, Andersson M, Thuresson M, et al. Amputation rates, mortality, and pre-operative comorbidities in patients revascularised for intermittent claudication or critical limb ischaemia: a population based study. Eur J Vasc Endovasc Surg. 2017;54:480–486. doi: 10.1016/j.ejvs.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 77.Soga Y, Iida O, Takahara M, et al. Two-year life expectancy in patients with critical limb ischemia. JACC Cardiovasc Interv. 2014;7(12):1444–1449. doi: 10.1016/j.jcin.2014.06.018 [DOI] [PubMed] [Google Scholar]
- 78.Melillo E, Micheletti L, Nuti M, et al. Long-term clinical outcomes in critical limb ischemia—a retrospective study of 181 patients. Eur Rev Med Pharmacol Sci. 2016;20:502–508. [PubMed] [Google Scholar]
- 79.van Haelst STW, Koopman C, Den Ruijter HM, et al. Cardiovascular and all-cause mortality in patients with intermittent claudication and critical limb ischaemia. BJS. 2018;105:252–261. doi: 10.1002/bjs.10657 [DOI] [PubMed] [Google Scholar]
- 80.Wohlgemuth WA, Olbricht W, Klarmann S, et al. [Disease-specific Questionnaire for Quality of Life in Patients with Peripheral Arterial Occlusive Disease in the Stage of Critical Ischemia (FLeQKI)–methodical development of a specific measuring instrument and psychometric evaluation of its validity and reliability (Part 1)]. Rofo. 2007;179(12):1251–1257. doi: 10.1055/s-2007-963515 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CLI amputation rates.
Abbreviations: CLI, critical limb ischemia; OXVASC, Oxford Vascular Study.