Abstract
Neuroimmune system may be involved in the pathological process of bipolar disorder (BD), but the essential association is not fully understood. Accumulating evidence has shown that BD involves the activation of immune cells and the release of inflammatory substances in the central nerve system (CNS). Meanwhile, neuroimmune responses also interact with other hypothesis of the etiology of BD that are widely recognized, such as neurotransmitter systems, neuroendocrine systems, neurotrophic factors, and oxidative stress. Simultaneously, related genes and immune changes in peripheral blood vary with it. Overall, neuroimmunity may play an important role in the pathogenesis of BD, and the inflammatory cytokines, especially interleukin-6 and tumor necrosis factor-alpha, have potential value for the clinical diagnosis and prognosis of BD, as well as predicting the therapeutic effects of drugs. Large-scale studies are needed to extend the evidence on neuroimmunity in BD, and to examine its clinical value for applications such as early prediction and treatment.
Keywords: Neuroimmunity, Inflammation, Cytokines, Bipolar disorder, Translational application
Introduction
Bipolar disorder (BD) is a widespread psychiatric disease affecting > 1% of the world’s population. According to epidemiological research, the lifetime prevalence of the bipolar spectrum is 2%–4%, and it contributes to a significant worldwide disease burden [1].
BD is characterized by both a manic phase and a depressive phase, simultaneously or alternately. The early diagnosis of BD is difficult, as the clinical features can be easily confused with major depressive disorder (MDD). The treatment of unrecognized BD patients might be delayed or they may even be prescribed antidepressants instead of mood stabilizers, which could aggravate a patient’s condition.
Though there is a variety of treatments for BD, it still has high rate of non-response and recurrence, and none of the treatments truly target the disease, as the pathological process has not been fully investigated. Therefore, further studies are required to define the mechanism of BD and modify the current diagnostic system with objective measures as well as to find the specific target for treatment to improve psychiatric practice.
The activation of immune cells induces changes in acute-phase proteins and cytokines. Cytokines are the signaling molecules that regulate the immune response; they can be divided into pro-inflammatory and anti-inflammatory cytokines according to their role in inflammation [2]. Pro-inflammatory cytokines such as interleukin (IL)-6, tumor necrosis factor-alpha (TNF-α), anti-inflammatory cytokines such as IL-4 and IL-10, and pro-inflammatory receptors such as sIL-1RAsIL-6R can suppress the activation of immune response. The new point of view considers the immune response in the central nervous system (CNS) to be a critical part of the pathological process of psychiatric illnesses, including BD, since the activation of immune cells and the release of pro-inflammatory substances may impact on fundamental brain functions such as synaptic transmission, neuroendocrine function, and neuroplasticity [3].
In this review, we focus on the relationship between neuroimmunity and BD. We collected evidence related the shared potential mechanism underlying BD with a view to translational clinical applications.
Neuroimmunity Hypothesis
Neuroimmune responses, embodied as neuroinflammation, includes innate immunological responses in the CNS associated with chemically-induced injury [4]. Studies on the etiology of BD now mainly focus on the immune system, neuroimaging, genetics, blood biochemistry, the endocrine system, and biological rhythms. From these approaches, accumulating evidence has shown that changes in the CNS of BD patients might be related to changes in the immune system—the activation of immune cells and the release of inflammatory substances. Studies of changes in the pro-inflammatory (IL-6 and TNF-α) and anti-inflammatory cytokines in the CNS and peripheral blood of BD patients have reached consistent conclusions, suggesting that neuroimmune system plays an important role in the pathological process of BD [5, 6].
Immune Cells
Microglia, the resident macrophages in CNS tissue, are essential cellular mediators of neuroinflammation and are responsible for homeostasis and synaptic modulation [7]. Since microglia exhibit significant plasticity they can polarize into different phenotypes. When acutely activated, microglia may initiate an activation pathway called M2, diminishing tissue damage and restoring homeostasis [8]. However, if the activation process continues for a longer period, the microglia adopt a pro-inflammatory profile with killing activity, named M1, producing more TNF-α, IL-1β, and High mobility group box 1 protein, leading to tissue inflammation, apoptosis, and impaired neurotransmitter synthesis [9]. Imbalance between the M1 and M2 polarization of microglia may contribute to the pathogenesis of BD [10].
Neuropathological findings have shown evidence of glial alterations in BD. A histological study [11] of the subgenual portion of Brodmann’s area 24 showed a reduced number of glial cells in the brains of patients with BD, especially in those patients with a family history. Rao et al. [12] determined the protein and mRNA levels of excitotoxic and neuro-inflammatory markers in postmortem frontal cortex from 10 BD patients and 10 age-matched controls. The results showed significantly higher levels of astroglial and microglial markers (glial fibrillary acidic protein, inducible nitric oxide synthase, c-fos, and CD11b) in postmortem frontal cortex from BD patients than controls [12]. Jakobsson et al. [13] reported an increase of the level of microglial makers in cerebrospinal fluid (CSF) (monocyte chemoattractant protein-1) and serum (soluble cluster of differentiation 14). YKL-40, a glycoprotein produced by reactive microglia and astrocytes, is also higher both in CSF and serum from patients than controls. These differences remained after controlling for confounding factors. A study [5] using PET scan images after intravenous injection of the radiopharmaceutical (R)-PK11195 was the first to report neuroinflammation in vivo in BD patients. The results showed concentrated microglial activation in the right hippocampus and a non-significant similar trend in the left hippocampus of euthymic patients with bipolar I disorder.
Another important cell type in the neuroimmune system is astrocytes, which are crucial regulators of innate and adaptive immune responses in the injured CNS [14]. Astrocytes are considered to play a controversial role in the regulation of neuroinflammation, depending on timing and context. Astrocyte activity may promote immunosuppression and tissue repair, or on the contrary, exacerbate inflammatory reactions and tissue damage [15, 16]. Meanwhile, astrocytes have been implicated in the regulation of the homeostasis of glutamate, which is the main excitatory neurotransmitter in the CNS. Clearance of excess glutamate from the synaptic cleft can avoid excitotoxicity [17]. Abnormalities in astrocytes may disrupt essential functions, such as blood-brain barrier maintenance, immune defense, neurotransmission, and synapse formation [18–20].
Recent studies have also revealed abnormalities in astrocytes from patients with BD. Toker et al. [21] re-analyzed 15 publicly-available bulk tissue-expression datasets on bipolar disorder, representing various brain regions from eight different cohorts of subjects. They found an increase in the expression profiles of cortical astrocytes and a decrease in the expression profiles of fast-spiking parvalbumin interneurons. No changes in astrocyte expression profiles were found in subcortical regions. Astrocytes can also produce S100-B, a Ca2+-binding protein. Neuropathological analysis [22] has shown a bilateral decrease in the numerical density of S100-B-immunopositive astrocytes in the hippocampal CA1 pyramidal layer of patients with bipolar depression. Another analysis [23] has shown a decrease in S100-B levels in Brodmann area 9 and an increase in Brodmann area 40 in tissues from patients with bipolar disorder. Interestingly, a meta-analysis [24] showed higher levels of peripheral S100-B in patients with bipolar disorder, but it only included two studies and 52 patients.
An important role of T cells in mood disorders has also been noted in the CNS of patients. There are different subsets of T cells with diverse functions that can be distinguished by CD protein expression, by chemokine receptor expression, and by the cytokines they produce. Activated microglia function as antigen cell-mediated specific antigens, and can activate T lymphocytes. Therefore, microglia are most likely to participate in functional maintenance after the activation of T cells in the CNS. Astrocytes also have immune-regulatory functions. Different states (resting/activation) of astrocytes can regulate T cell proliferation and apoptosis, and affect the secretion of T cell cytokines such as Th1 and Th2 cytokines which may play an important role in the process of CNS neuroimmune disease [25]. Poletti et al. [26] focused on the relationship between circulating levels of T helper cells and structural and functional brain imaging in depressed bipolar patients. They detected Th1, Th2, Th17, Th22, and T regulatory cells in 25 in-patients with major depressive episodes during the course of BD-I, and 21 healthy controls. The results showed that T cell-mediated immune activation has a consistently relationship with mood disorders, especially for Th17 cells. Immune activation maintains the functional and structural integrity of the brain [26].
Pro-inflammatory Substances
Pro-inflammatory substances, induced by the activation of immune cells, have an adverse impact on neuroregeneration in CNS and affect the resilience of several important brain areas such as the prefrontal cortex and hippocampus, which may contribute to the progression of illness [27].
The immune-related protein concentrations in the CSF have been explored to test the possible mechanisms in bipolar patients. Isgren et al. [28] have shown higher CSF concentrations of IL-8, monocyte chemoattractant protein 1 (MCP-1/CCL-2), chitinase-3-like protein1 (CHI3L1/YKL-40), and neurofilament light chain (NF-L) in euthymic patients with BD compared with healthy controls (HCs). Isgren et al. [29] then carried out a longitudinal study to investigate whether CSF markers of neuroinflammation and neuronal injury can predict the clinical outcomes in patients with BD and found negative results. The CSF of 77 patients were analyzed at baseline and then followed for 6 years–7 years. The results showed that YKL-40 concentrations were negatively associated with manic/hypomanic episodes and with the occurrence of psychotic symptoms. Clinical outcomes were not associated with the concentrations of IL-8 and NF-L. High concentrations of these selected CSF markers of neuroinflammation and neuronal injury at baseline were not consistently associated with poor clinical outcomes in this prospective study. This negative result needs more verification in larger prospective studies [29]. A meta-analysis [30] compared aberrant CSF levels of cytokine in patients with schizophrenia, BD, and depression. The results showed that the CSF level of IL-1β was significantly increased in patients with BD versus controls, and IL-8 was increased at the trend level. There was no significant difference for IL-6. Between-study heterogeneity was significant for IL-1β, IL-6, and IL-8. These results have consistently shown elevated cytokine levels in BD patients.
In addition to CSF, there is already positive evidence of changes in serum cytokines levels in patients with BD (Table 1). In one study [31] including 300 blood samples from 97 patients with BD and 133 blood samples from 72 HCs, the serum IL-6, IL-8, IL-18, TNF-α, and high sensitivity C-reactive protein (hsCRP) were measured together with leukocyte and neutrophil counts. After adjusting for confounders, the results showed that the leukocyte and neutrophil counts were both higher in patients with BD than in HCs. Serum IL-6, TNF-α, and hsCRP levels were found to be positively associated with leukocyte and neutrophil counts. Similarly, in a preliminary study [32] aimed to investigate the activation pattern of T lymphocyte populations and immune checkpoint inhibitors on immunocytes in patients with bipolar II disorder depression or major depression, the blood cell count of T lymphocyte subsets and the plasma levels of cytokines (IL-2, IL-4, IL-6, IL-10, TNF-α, and interferon (IFN)-γ) were selectively investigated. As a result, the plasma level of IL-6 was found to be elevated in patients with BD and MD. Changes of cytokines in patients with BDs of different features varied in degree, and were related to the course of disease and the characteristics of symptoms and may alter after medication [33]. Blood-brain barrier disruption may explain the connection between changes in peripheral inflammatory markers and the neuroinflammation in the pathophysiology of BD [34]. During the inflammatory process, the release of cytokines might induce permeability of blood-brain barrier, and consequently allow the entry of peripheral pro-inflammatory mediators into the brain, constituting a bidirectional transmission of these inflammatory substances [35].
Table 1.
BD and serum levels of cytokines: summary of two new studies.
| Author, year | Title | Sample | Study design | Assessments | Main findings |
|---|---|---|---|---|---|
| Munkholm et al. (2018) | Leukocytes in peripheral blood from patients with BD-trait and state alterations and association with levels of cytokines and C-reactive protein | n = 169 (97 BD patients and 72 HC) | Prospective cohort | IL-6, IL-8, IL-18, TNF-α, hsCRP, together with leukocytes and neutrophils in blood |
Leukocyte and neutrophil counts both higher in patients with BD than HCs. Serum IL-6, TNF-α, and hsCRP levels positively associated with leukocyte and neutrophil counts Plasma level of IL-6 increased in patients with BD and MD |
| Wu et al. (2017) | Circulating T lymphocyte subsets, cytokines, and immune checkpoint inhibitors in patients with bipolar II or major depression: a preliminary study | n = 65 (23 BD patients, 22 MD patients, and 20 HCs) | Prospective cohort | T lymphocyte subsets and IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ in blood |
Relationship with Neurotransmitter Systems
Strong evidence suggests that inflammation induces changes in the serotonin, dopamine, and glutamate systems in brain regions, which may result in mental disorders and other related behavioral changes [36, 37]. Meanwhile, peripherally-administered cytokine also activates a CNS inflammatory response in humans that interacts with monoamine (serotonin) metabolism, which is associated with depression [38]. Although many studies have demonstrated reciprocal relationships between neurotransmitter systems and immune-inflammatory pathways that occur in CNS, studies linking neurotransmitters and cytokines are still limited concerning BD.
Serotonin cell bodies originating within the dorsal raphe (DR) play a major role in the regulation of behavioral features characteristic of mania. Howerton et al. [39] found that inflammation within the DR, but not the prefrontal cortex, results in a profound display of manic-like behavior in mice, characterized by increased stress-induced locomotion and responsivity, and reduced risk-aversion/fearfulness, which are rescued by acute treatment with a selective serotonin reuptake inhibitor. Microarray analysis of the DR has revealed a dramatic increase in immune-related genes, and dysregulation of genes important in GABAergic, glutamatergic, and serotonergic neurotransmission. This result suggests that serotonin dysregulation stemming from neuroinflammation in the DR underlies manic-like behaviors [39]. Activation of the kynurenine pathway is one of the mechanisms by which inflammation can induce depression. It involves multiple pathways including interference with the bioavailability of tryptophan central to the synthesis of the neurotransmitter serotonin [40]. Mukherjee et al. [41] examined the effect of neopterin, a marker of cellular immune activation, and kynurenine (KYN), an inflammatory byproduct of the serotonin pathway, on the association between total sleep time and the severity of depression in 21 symptomatic BD participants. The results showed that the serotonin precursor tryptophan (TRP) is significantly lower in BD participants than in HCs. KYN, TRP, and the KYN/TRP ratio are associated with the severity of depression when total sleep time and body mass index (BMI) are included in the model. The KYN/TRP ratio trends towards a negative association with mania symptoms, controlling for BMI and total sleep time, in acutely symptomatic BD participants. However, Neopterin is not associated with sleep or mood severity. No significant differences were found in sleep phenotype or biomarkers after the usual clinical care [41].
A study [42] including 20 patients with euthymic BD and 20 HCs used single-photon emission computed tomography with the radiotracer 123I ADAM for serotonin transporter (SERT) imaging reported that SERT availability is significantly lower in the midbrain and caudate of patients with BD than in HCs, but not in the thalamus and putamen. SERT availability is significantly associated with peripheral IL-10 in the thalamus, but not in the midbrain, caudate, or putamen [42]. Similarly, Chou et al. [43] measured seven cytokines (TNF-α, IFN-γ, IL-1α, IL-1β, IL-4, IL-6, and IL-10) and the availability of SERT in regions of interest (midbrain, thalamus, putamen, and caudate) of 28 BD type I patients and 28 HCs. They found that SERT availability in the midbrain and caudate was significantly lower in BD than in HCs. IL-1β was significantly lower, whereas IL-10 was significantly higher in BD than HCs. Multiple linear regression analyses revealed associations between the cytokines IL-1α, IL-1β, IL-6 and SERT availability in the midbrain but not in the thalamus, putamen, and caudate. Furthermore, linear mixed effect analyses demonstrated that these associations did not differ between HCs and BD patients. The result indicated that significant associations between cytokines and SERT availability may explain the role of cytokines in mood regulation, but it may be not specific to BD [43].
Relationship with Neuroendocrine Systems
The hypothalamic-pituitary-adrenal (HPA) axis is an important part of the neuroendocrine system, as well as one of the main systems involved in the response to stress. The hippocampus, amygdala, and prefrontal cortex undergo stress-induced structural remodeling, which alters behavioral and physiological responses [44]. Cortisol, the main by-product of the HPA axis, has both adaptive and maladaptive effects on these brain regions, which could influence cognitive and emotional functioning [45, 46].
As BD is closely connected to psychosocial stress, it is not surprising that BD patients display abnormalities of HPA-axis function, which has been tied to the immune system. During chronic glucocorticoid exposure induced by chronic stress, changes in both innate and adaptive immune responses during aging have been reported [47]. This may be the underlying mechanism in the pathogenesis of BD since BD can be considered as a model of accelerated aging because it has been associated with shortened telomeres, higher cytomegalovirus IgG titers, and the expansion of senescent and regulatory T cells [48].
The immune system could also influence the HPA axis to contribute to the pathology of BD. Pro-inflammatory cytokines, such as IFN, TNF, and IL-6 significantly up-regulate the activity of the HPA axis, leading to an increase in systemic cortisol levels [49]. However, with chronic inflammation, HPA activation may cause harmful effects by chronic hypercortisolemia, which downregulates the synthesis of glucocorticoid receptors, translocation and sensitivity in the pituitary and hypothalamus, and effectively inhibits the negative feedback loop of the HPA axis [50–52]. The negative feedback loop leads to further impairments in mood and cognition with increasingly elevated cortisol levels. Impaired cortisol suppression is a strong predictor of mood disorder [53].
Murri and colleagues [54] conducted a meta-analysis that included 41 studies showing that BD is consistently associated with significantly increased levels of cortisol (basal and post-dexamethasone) and adrenocorticotropic hormone. A recent animal study [55] using a mouse model of mania induced by paradoxical sleep deprivation showed that lithium treatment prevents the alteration of HPA-axis activation and cytokine levels in the periphery and brain. In this study, male C57 mice were treated with saline or lithium for 7 days. Immediately after the sleep deprivation protocol, the locomotor activity was evaluated and serum and brain samples were extracted to evaluate the circulating levels of corticosterone and adrenocorticotropic hormone. Experimental results in mice suggest a similar mechanism of the therapeutic effect of lithium in humans and indicated changes in the HPA axis in bipolar patients.
Relationship with Neurotrophic Factors
Brain-derived neurotrophic factor (BDNF), a neurotrophic maker, is considered to play an important role in nerve regeneration and neuroplasticity, as well as to provide conditions indispensable for the proper functioning of nerve cells. Current evidence indicates decreased BDNF levels in BD patients compared to HCs and correlations between BDNF and the severity of symptoms/mood episodes. The results, though, appear conflicting [56]. The interactions between neurotrophic signaling and the immune system in BD are worth exploring as they may both be involved in the underlying pathological mechanisms relevant to disease activity and neuroprogression. A prospective 6–12-month follow-up study [57] reported no significant difference in BDNF and inflammatory markers (hsCRP, IL-1β, IL-6, IL-8, IL-18, and TNF-α) between overall BD patients and HCs, while the hsCRP levels were lower in depressive episodes than in euthymic and manic states after adjustment. However, BDNF and other inflammatory markers did not vary according to affective state. Another prospective case-control study [58] also investigated the changes of BDNF according to mood states and their relationships with inflammatory markers. Similarly, no significant differences were found in BDNF between BD patients and HCs, and neither BDNF nor inflammatory markers were correlated with the severity of symptoms. In addition, this study presented methodological limitations that may influence the analysis of biomarkers. On the contrary, to further verify the potential roles of neurotrophic and inflammatory markers, Wang et al. [59] recruited drug-naïve patients with subthreshold BD and BD-II undergoing 12 weeks of treatment and measured their BDNF and cytokines (TNF-α, TGF-β, IL-6, IL-8, and IL-1β). The result showed that the subthreshold BD group had significantly lower levels of BDNF and TGF-β, which was different from the changes in BD-II patients. This finding supports a role of neurotrophic and inflammatory markers in the clinical severity and prognosis of BD.
A few studies have focused on the link between BD and cardiovascular disease and then explored whether BDNF and inflammatory cytokines underlie the potential mechanism. A case-control study [60] found that IL-6 increased in symptomatic and asymptomatic BD patients versus HCs. Furthermore, in symptomatic BD patients, lower BDNF was associated with greater mean carotid intima media thickness, suggesting a potential interaction between neurotrophic/inflammatory biomarkers and atherosclerosis proxies as well as promising prevention strategies against cardiovascular disease in BD.
Relationship with Oxidative Stress
Activated oxidative and nitrosative stress pathways and a pro-atherogenic lipid profile have been found in both mood disorders and metabolic syndrome. Under these conditions, the levels of pro-inflammatory cytokines, lipid peroxidation biomarkers, including malondialdehyde and atherogenic indices are increased, as well as the levels of antioxidants, such as HDL-c [61, 62]. These results suggest there may be a shared immune-inflammatory, oxidative and nitrosative stress, and metabolic pathways underpinning metabolic syndrome and BD. In a study [63] investigating potential differences in cytokines, oxidative stress, and telomere length markers between patients with BD and HCs, the serum levels of IL-6, IL-10, TNF-α, and 3-nitrotyrosine and the activity of glutathione peroxidase, glutathione reductase, and glutathione S-transferase were measured. The results showed higher levels of inflammatory cytokines such as IL-6 and IL-10 than in controls, and those of IL-6 and CCL24 than in their siblings.. The increased cytokine levels and the decreased levels of oxidative substances suggest that there might be a connection between oxidative stress and inflammation.
Relevant studies of BD mainly focus on the relationship between oxidative stress and the immune system but few in the further correlations in the CNS. In a community study [64] aiming to explore the relationship between oxidative stress, inflammatory cytokines, and circadian preferences in BD patients, serum IL-6, Il-10, TNF-α were measured and thiobarbituric acid-reactive substances (TBARS), uric acid and protein carbonyl content were used to assess oxidative stress. This study recruited 215 participants. In the bipolar group, there was a greater change in biological rhythms than in the major depression and control groups. Decreased levels of IL-6 and TNF-α and lower TBARS levels were found in patients with BD who were active at night and had a reversed day/night cycle. These results suggested that lower serum levels of IL-6, TNF-α, and TBARS were associated with evening preference in the BD group. From this study, it is evident that oxidative stress may affect the immune system through reactive oxygen species (ROS) and reactive nitrogen species (RNS), thus affecting the CNS, which provides the new idea that the surrounding systems related to the neuroimmune system may also participate in the pathological mechanism underlying BD (Figs. 1 and 2), and this may have value for further investigations in BD patients.
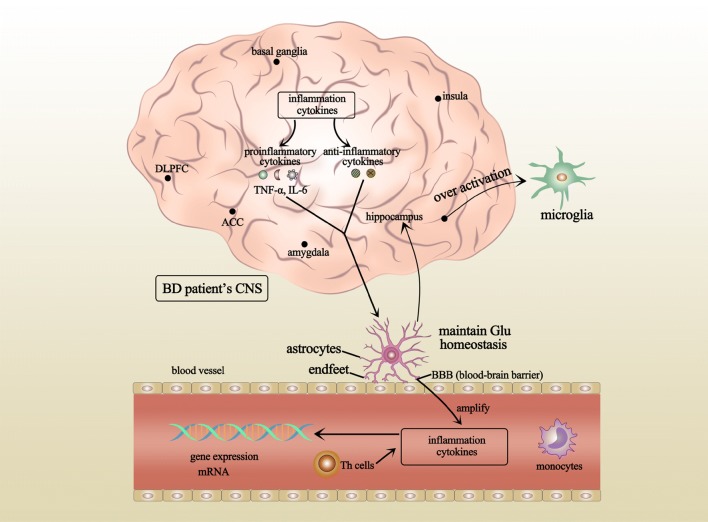
Fig. 1.
Neuroimmunology and BD. Several brain regions in BD patients, such as the amygdala, insula, and ACC, have innate immune alterations. Glial cells include microglia and astrocytes. The over-activation of microglia in the hippocampus of BD patients has been reported. Astrocytes can maintain Glu homeostasis and prevent excitotoxicity in the brain. These immune changes in the CNS also occur in astrocytes and are further characterized by changes in peripheral blood cells in BD patients through the blood-brain barrier. Inflammatory cytokines in the CNS include pro-inflammatory and anti-inflammatory cytokines. These cytokines serve as an index that is mainly measured in the blood of BD patients to reflect changes in immunity. In addition, the gene expression of cytokines may also change. The changes in the nervous system and immune system may be linked to each other and other systems, enabling the pathological mechanisms in patients with BD. CNS, central nervous system; ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; Glu, glutamic acid; ROS, reactive oxygen species; RNS, reactive nitrogen species.
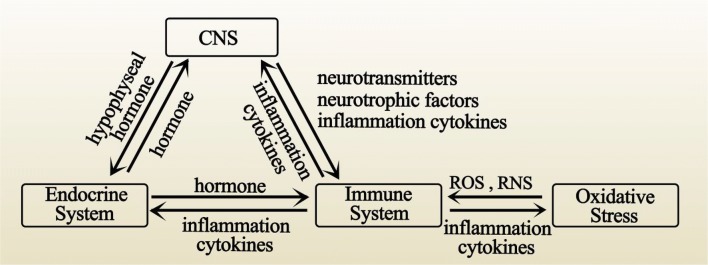
Fig. 2.
Interrelationship among systems in BD patients. The CNS, endocrine system and immune system are interrelated by multiple substances together forming the possible neuroinflammatory change in BD patients. The immune system also interacts with oxidative stress.
Genetic Association Studies
General Susceptibility
BD is a highly heritable and heterogeneous mental disease. Some immuno-related genes have been proposed to confer susceptibility to BD, but few studies focused on the same target gene. Ozdemircan et al. [65] reported that COX-2 gene variants are positively associated with the development of BD-I. According to their findings, the COX-2-1195A-GAA genotype may promote the development of BD-I, and the COX-2-1195A-GG genotype could be protective against BD-I. Another genetic association study [66] focused on the variable number of tandem repeat polymorphisms of the IL-1Ra gene (IL1RN). IL1RN plays an important role in regulating the activity of IL-1α and IL-1β. The study showed that the IL1RN*1/2 genotype is more prevalent in BD patients than in HCs, and it could increase the risk of BD according to the results of multiple logistic regression analysis. Further stratification analysis also revealed that carrying IL1RN*1/2 could lengthen the disease course. Yoon et al. [67] reported that the T allele of IFN-γ +874A/T is more frequent in BD patients than controls, and they also found that the distribution of three genotypes of IFN-γ vary according to the severity of manic symptoms. Monocyte chemoattractant protein 1 (MCP-1) is also a cytokine functioning in innate immune reactions. A case-control study [68] genotyped individuals for the A-2518G polymorphism of the MCP-1 gene and then explored genotypic and allelic associations between BD patients and controls. The results suggested that MCP-1 gene may confer susceptibility to BD. Besides, different genotype and allele distributions of TNF-α, IFN-γ, IL-10, and IL-1 polymorphisms have also reported in BD patients [69, 70].
Brain Function
IL-1 has been suggested to have an underlying effect on the pathogenesis of psychiatric diseases as well as cognitive function [71, 72]. A study with a small sample that aimed to analyze the impact of IL-1 polymorphism on brain morphology in BD found that the A -511C/T polymorphism (rs16944) of the IL-1B gene is associated with whole-brain gray matter (GM) deficits and left dorsolateral prefrontal cortex GM deficits, indicating that IL-1 gene variants facilitate the GM deficits in BD patients that may induce the brain dysfunction. Meanwhile, IL-1β has also been proposed to be the candidate gene that contributes to alteration of brain functions [70]. These results supported a role of cytokines in the progress of the disease, but further studies with large sample are required to confirm the results.
Treatment Response
Current evidence indicates that neuroimmunity may play an important role in drug efficacy in BD patients, as patients with higher cytokine levels have a lower response rate [73]. Calati and colleagues [74] explored whether the single-nucleotide polymorphisms of two genes involved in neuroplasticity and the inflammatory response [mitogen activated protein kinase 1, MAPK1 (rs3810608, rs6928, rs13515 and rs8136867), and the cyclic AMP responsive element binding protein 1, CREB1 (rs889895, rs6740584, rs2551922 and rs2254137)] are associated with the treatment response in MDD and BD. A higher MAPK1 rs8136867 AG genotype was reported in the whole sample (MDD and BD), suggesting that immuno-related gene variation is associated with treatment remission. Amare et al. [75] conducted a genome-wide association study including 2586 BD patients and calculated the polygenic score for schizophrenic BD in order to investigate the association between the genetic overlap and treatment response to lithium. The results showed that patients with a lower polygenic load for schizophrenia had a higher response rate to lithium. Furthermore, 15 genetic loci that may have overlap effects on the response to lithium treatment and susceptibility to schizophrenia were identified, and these loci were associated with the HLA antigen complex and inflammatory cytokines from functional pathway and network analysis.
Gene Expression of Inflammatory Cytokines
Most studies have focused on cytokine proteins, but gene expression is key for a cell to execute its function. Studies on gene expression in peripheral blood cells showed the presence of increased messenger RNA (mRNA) expression of pro-inflammatory mediators in patients with mood disorders [76]. A systematic review [77] has shown that the expression and polymorphism of genes are directly related to the level of cytokines.
In a study to detect the markers of adaptive and innate immunity in postmortem brains from teenage and adult suicide victims and cytokine gene expression, there was an increase in the protein and mRNA expression of Toll-like receptors in the prefrontal cortex of adult depressed suicide victims and normal controls. It has been reported that the mRNA and protein expression of TNF-α, IL-1β, IL-6 and some specific membrane-bound receptors, such as IL1R1, TNFR1, and IL1RA, are significantly increased in both MDD and BPD patients [78]. A study [79] analyzing the expression levels of IFNγ-AS1 long non-coding RNA, IFNγ and IL-1β mRNAs in the blood cells of 27 schizophrenia patients, 30 bipolar patients, and 32 HCs showed a significantly decrease in IFNγ-AS1 expression and the transcript levels of IL-1β in BD patients compared with controls. All these studies indicated that gene expression of inflammatory cytokines play a role in BD.
Complement levels and complement expression levels are also related to BD according to a study [80] measuring serum C4, factor B, sC5b-9, and mRNA expression levels of C1q, C4, factor B, and CD55 in 22 chronic BD patients, 24 first-episode BD patients, and 19 HCs .A significant decrease in serum complement levels was found in chronic BD patients. The mRNA expression levels of C1q, C4, and factor B in peripheral blood mononuclear cells were significantly elevated, while the mRNA expression level of the complement inhibitor CD55 was significantly reduced, which was possibly a compensatory mechanism for excessive activation of the complement cascade and subsequent overconsumption of the complement system in BD patients.
The results of monocyte pro-inflammatory gene activation studies suggest that gene alterations in monocytes might be involved in BD. In one study [81], monocyte immune gene activation was determined in 97 well-controlled, largely euthymic BD patients. Compared to HCs, the pro-inflammatory genes for inflammation-related cytokines/factors were downregulated, and the glucocorticoid receptor α (GRα) and the hepatocyte growth factor (HGF) genes were upregulated in the monocytes of the BD patients.
Changes in peripheral blood markers can modulate M1/M2 polarization in BD. An innovative study [82] supported this by investigating the phenotype of cultured macrophages treated with the serum from BD patients. In this study, 18 patients with BD and five healthy individuals were included and the gene expression of selected M1 and M2 markers were assessed. Chemokine (C-X-C motif) ligand (CXCL) is a chemokine subfamily that mainly attract neutrophils to the site of infection and play an important role in inflammatory response. Compared to the euthymic group, macrophages treated with serum from patients in an acute episode displayed down-regulation of CXCL9 and CXCL10 expression.
A large gene study, which involved a genome-wide association study of 36,989 individuals with schizophrenia and genotype data from patients with BD from the Consortium on Lithium Genetics, demonstrated that genetic variants in inflammatory cytokines such as TNF, IL-4, and IFN-γ play a role the in treatment response to lithium in BPAD. A further genetic perspective is that cytokine levels may be used as markers for patients with BD [83].
Though a large proportion of studies focused on cytokines themselves, polymorphisms and their genes expression are the key that influences cytokine levels the execution of their functions. The study of gene expression in patients with BD currently includes some pro-inflammatory factors, their receptors, complement factors, and monocytes. When the macrophages of normal people was exposed to the blood of patients with BD in vitro, the expression of macrophage-related genes was significantly altered, which further confirmed this view [82]. Genome-wide association analysis also found that the mutation of cytokine genes had a underlying effect on the treatment of bipolar patients. To sum up, the changes of cytokines in bipolar patients cannot be ignored at the level of their genes. Further specific studies are needed to confirm these changes.
Translational Application
The basic research on the neuroimmunological mechanism in patients with BD has been relatively deep and specific, but its clinical translation remain inadequate and need further meticulous studies. Some of its mechanisms have been revealed in studies of mood stabilizers. At the same time, clinical trials using anti-inflammatory treatment have shown a certain efficacy. Measuring the serum levels of these neuroinflammatory factors and using them to predict the risk of disease and the efficacy of antidepressant drugs in patients is worth further investigation.
Risk of Disease
Peripheral immune-inflammatory imbalances have been documented in BD, but the immunological changes prior the onset of disease have rarely been studied. Hayes et al. [84] conducted a longitudinal birth cohort study to examine the role of inflammatory pathways in the pathophysiology of mania. The study found that higher levels of IL-6 in childhood predict the hypomanic symptoms in young adulthood, while the levels of C-reactive protein and atopic disorders including asthma and eczema had no significant relevance. Current data [85] reflect that increased pro-inflammatory cytokines, especially IL-6, appear in the early stages of psychosis, including at-risk stages. This preliminary discovery has raised a hypothesis on the association between immune inflammatory changes and the risk for BD patients with psychotic symptoms. A case-control study [86] found no definitive link between high levels of prenatal maternal cytokines and a high risk for BD in the offspring, and the results for most cytokines were approximately the same when analyses were restricted to BD patients with psychotic features, indicating that the effect of maternal serum cytokine levels may be modified by additional exposure. Large cohort studies are needed to further explore the value of cytokines in predicting the onset of BD, and this may also elucidate the mediation effect of immuno-inflammation responses.
Predictors of Antidepressant Response
Although emerging evidence has shown that cytokine levels could predict the response to antidepressant drugs in depression [87], few studies have explored their clinical value in BD treatment. Activation of the immune response and inflammation cascades could impact synaptic transmission, neuroplasticity, and white matter integrity. Indeed, they may be involved in the progression and staging of BD, influencing the response rate or prognosis. A clinical trial [73] evaluated 15 immune-regulating compounds in BD patients who were suffering a depressive episode at baseline and then used total sleep deprivation and light therapy as treatments to identify the proportion who responded. The study found that five highly-intercorrelated compounds (IL-8, MCP-1, IFN-γ, IL-6, and TNF-α) were significantly higher in non-responders than responders, corrected for multiple comparisons, indicating that high levels of pro-inflammatory substances may be associated with a poor response to antidepressant treatment. Independent replication in larger samples would be of interest to confirm these results.
Involvement in the Mechanism of Action of Mood Stabilizers
Mood stabilizers are widely recommended for BD patients both in the manic and depression phases, and they are also used in maintenance treatment. The mood stabilizers include lithium with anti-manic effects, and antiepileptic drugs such as valproate, lamotrigine, and carbamazepine. Though they all have proven therapeutically effective according to clinical trials and conditions, we still have not found the biological basis of ‘mood stabilization’, as the specific treatment target has not been revealed. Mood stabilizers have complicated modes of action, and there are subtle differences between them. A number of studies have shown that they have shared actions on signaling pathways, which deserve further exploration to develop an actual ‘mood stabilizer’.
Neuroimmunity has been proposed to play an important role in BD pathology. There is also abundant evidence that elevated pro-inflammatory cytokine concentrations and an increased immune response are affected by mood stabilizers, and this could be one of the crucial mechanisms behind their benefits.
A systematic review [88] including 17 articles explored the effects of mood stabilizers on cytokines, showing an activation of pro-inflammatory responses in medication-free BD patients during the manic phase. Long-term use of lithium could alter this increase of cytokines while short-term use lacks data. Two studies reported no effect of valproate and no studies have been found on carbamazepine and lamotrigine.
Lithium has multiple effects, including mediating immuno-inflammatory responses, on different biological and biochemical pathways [89]. It has been reported that lithium alters the inflammation systems by decreasing the level of pro-inflammatory cytokines as anti-inflammatory agents, and mitigate the mania-like behavior induced by paradoxical sleep deprivation in mice [90].
The inhibition of glycogen synthase kinase (GSK-3) by lithium has long been known [91]. GSK-3, an immunoregulatory molecule, acts indirectly on the inflammatory immune response by altering its downstream molecules such as nuclear factor-κB and cAMP response element binding protein, which might explain the anti-inflammatory effect of lithium. Valproic acid inhibits histone deacetylase and GSK-3, and this could also modulate inflammation [92]. One study compared their regulatory effects on immuno-inflammatory responses and the results showed their differential modulation of dendritic cells to mediate a distinct T cell response. The study also provided evidence of complementary effects of valproic acid and lithium [93]. There is other access for mood stabilizers to participate in the common pathogenic pathway of the immune response and BD, such as decreasing the permeability of the blood-brain barrier and acting on microglial cells [94, 95]. Investigating the immune modulation effects of mood stabilizers may contribute to the development of new targeted drugs.
Involvement in Immunoregulation Treatment
Consistent with previous studies that showed activation of immune-inflammatory response in BD, clinical studies have demonstrated the therapeutic effect of immunoregulation/anti-inflammatory treatment. Current evidence is mainly focused on the treatment of bipolar depression. A recent meta-analysis [96] reported moderate but significant overall efficacy of anti-inflammatory agents in the adjunct treatment of bipolar depression, but only N-acetylcysteine was independently found to have an antidepressant effect. This reduction in severity of depression was not associated with manic induction. A randomized double-blind trial [97] has shown that the adjunct use of aspirin or minocycline may be associated with a higher response rate in the treatment of bipolar depression, and patients with better clinical outcomes had a significantly greater decrease in IL-6 levels during the 6-week period. However, neither adjunct aspirin nor minocycline reduced the severity of depression. Another pilot study [98] suggested that minocycline has anti-depressive effects in bipolar depression probably by decreasing the pro-inflammatory cytokine levels. Few studies, though, have found adjunct therapeutic effects of celecoxib in the treatment of acute manic episodes [99]. Some exploratory research concerns other anti-inflammatory therapies, such as using TNF-α inhibitors and taking probiotics. Relevant scientific evidence for their benefits is insufficient [3]. Many new strategies that have not been studied but normalize the neuroimmunity pathways like 1-methyl-D,L-tryptophan inhibitors, glucagon-like peptide-1 receptor agonists, or agonists of the nicotinic alpha-7 receptor require further verification of their promise in BD therapy. Exploring potential targets of interest in the treatment of BD may help to improve the current treatment [7].
With emerging relevant evidence, currently, cognitive impairment is recognized as a clinical trait of BD. This dysfunction appears in all phases of the disease, and it may have extensive connections with activation of the immune system. Immune regulation has been proposed as a potential choice to improve the cognitive function of BD patients among numerous tentative treatment methods which have significant but moderate efficacy [100]. However, related efficacy outcomes have not yet been reported.
Conclusions
BD is a highly relapsing disease in which the mechanism is still unclear. The fundamental exploration of neuroimmunity has been quite detailed. It is urgent to translate its research results into clinical applications to embody its value. In this review, we comprehensively describe the changes in the CNS and peripheral immune system in patients with BD as well as their relationship with the surrounding systems (Figs. 1 and 2), and the present status of their translation to the clinic. Currently, the number of relevant clinical studies remains small, and the sample size of studies which have already been carried out is still small. Exploring neuroimmunity as a potential therapeutic target for bipolar disorder deserves further exploration and validation.
Acknowledgements
This review was supported by the National Basic Research Development Program of China (2016YFC1307100), the Shanghai Mental Health Centre Clinical Research Center Special Project for Big Data Analysis (CRC2018DSJ01-1), the Sanming Project of Medicine in Shenzhen City (SZSM201612006), the National Natural Science Foundation of China (91232719 and 81771465), and the National Key Clinical Disciplines at Shanghai Mental Health Centre (OMA-MH, 2011-873).
Contributor Information
Jun Chen, Email: doctorcj2010@gmail.com.
Yiru Fang, Email: yirufang@aliyun.com.
References
- 1.Grande I, Berk M, Birmaher B, Vieta E. Bipolar disorder. Lancet. 2016;387:1561–1572. doi: 10.1016/S0140-6736(15)00241-X. [DOI] [PubMed] [Google Scholar]
- 2.Panaro MA, Aloisi A, Nicolardi G, Lofrumento DD, Nuccio FD, Pesa VL, et al. Radio electric asymmetric conveyer technology modulates neuroinflammation in a mouse model of neurodegeneration. Neurosci Bull. 2018;34:270–282. doi: 10.1007/s12264-017-0188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenblat JD, Mcintyre RS. Bipolar Disorder and Immune Dysfunction: Epidemiological Findings, Proposed Pathophysiology and Clinical Implications. Brain Sciences. 2017;7:144. doi: 10.3390/brainsci7110144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Callaghan JP, Sriram K, Miller DB. Defining “neuroinflammation”. Ann N Y Acad Sci. 2008;1139:318–330. doi: 10.1196/annals.1432.032. [DOI] [PubMed] [Google Scholar]
- 5.Pinto JV, Passos IC, Librenza-Garcia D, Marcon G, Schneider MA, Conte JH, et al. Neuron-glia interaction as a possible pathophysiological mechanism of bipolar disorder. Curr Neuropharmacol. 2018;16:519–532. doi: 10.2174/1570159X15666170828170921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felger JC. Imaging the role of inflammation in mood and anxiety-related disorders. Curr Neuropharmacol. 2018;16:533–558. doi: 10.2174/1570159X15666171123201142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ascoli BM, Gea LP, Colombo R, Barbe-Tuana FM, Kapczinski F, Rosa AR. The role of macrophage polarization on bipolar disorder: Identifying new therapeutic targets. Aust N Z J Psychiatry. 2016;50:618–630. doi: 10.1177/0004867416642846. [DOI] [PubMed] [Google Scholar]
- 8.Franco R, Fernandez-Suarez D. Alternatively activated microglia and macrophages in the central nervous system. Prog Neurobiol. 2015;131:65–86. doi: 10.1016/j.pneurobio.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakagawa Y, Chiba K. Diversity and plasticity of microglial cells in psychiatric and neurological disorders. Pharmacol Ther. 2015;154:21–35. doi: 10.1016/j.pharmthera.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao JS, Harry GJ, Rapoport SI, Kim HW. Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol Psychiatry. 2010;15:384–392. doi: 10.1038/mp.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jakobsson J, Bjerke M, Sahebi S, Isgren A, Ekman CJ, Sellgren C, et al. Monocyte and microglial activation in patients with mood-stabilized bipolar disorder. J Psychiatry Neurosci. 2015;40:250–258. doi: 10.1503/jpn.140183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colombo E, Farina C. Astrocytes: Key Regulators of Neuroinflammation. Trends in Immunology. 2016;37:608–620. doi: 10.1016/j.it.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Drogemuller K, Helmuth U, Brunn A, Sakowicz-Burkiewicz M, Gutmann DH, Mueller W, et al. Astrocyte gp130 expression is critical for the control of Toxoplasma encephalitis. J Immunol. 2008;181:2683–2693. doi: 10.4049/jimmunol.181.4.2683. [DOI] [PubMed] [Google Scholar]
- 16.Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol. 2007;8:247–256. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- 17.Papa M, De LC, Petta F, Alberghina L, Cirillo G. Astrocyte–neuron interplay in maladaptive plasticity. Neurosci Biobehav Rev. 2014;42:35–54. doi: 10.1016/j.neubiorev.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Correale J, Farez MF. The role of astrocytes in multiple sclerosis progression. Front Neurol. 2015;6:180. doi: 10.3389/fneur.2015.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- 20.Domingues HS, Portugal CC, Socodato R, Relvas JB. Oligodendrocyte, astrocyte, and microglia crosstalk in myelin development, damage, and repair. Front Cell Dev Biol. 2016;4:71. doi: 10.3389/fcell.2016.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toker L, Mancarci BO, Tripathy S, Pavlidis P. Transcriptomic evidence for alterations in astrocytes and parvalbumin interneurons in subjects with bipolar disorder and schizophrenia. Biol Psychiatry. 2018;84:787–796. doi: 10.1016/j.biopsych.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomasz G, Schroeter ML, Wiebke L, Hans-Gert B, Henrik D, Kolja S, et al. S100B-immunopositive astrocytes and oligodendrocytes in the hippocampus are differentially afflicted in unipolar and bipolar depression: a postmortem study. Journal of Psychiatric Research. 2013;47:1694–1699. doi: 10.1016/j.jpsychires.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Dean B, Gray L, Scarr E. Regionally specific changes in levels of cortical S100beta in bipolar 1 disorder but not schizophrenia. Aust N Z J Psychiatry. 2006;40:217–224. doi: 10.1080/j.1440-1614.2006.01777.x. [DOI] [PubMed] [Google Scholar]
- 24.da Rosa MI, Simon C, Grande AJ, Barichello T, Oses JP, Quevedo J. Serum S100B in manic bipolar disorder patients: Systematic review and meta-analysis. J Affect Disord. 2016;206:210–215. doi: 10.1016/j.jad.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 25.Dionisio-Santos DA, Olschowka JA, O’Banion MK. Exploiting microglial and peripheral immune cell crosstalk to treat Alzheimer’s disease. J Neuroinflammation. 2019;16:74. doi: 10.1186/s12974-019-1453-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poletti S, De WH, Mazza E, Wijkhuijs AJ, Locatelli C, Aggio V, et al. Th17 cells correlate positively to the structural and functional integrity of the brain in bipolar depression and healthy controls. Brain Behav Immun. 2017;61:317–325. doi: 10.1016/j.bbi.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 27.Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isgren A, Jakobsson J, Palsson E, Ekman CJ, Johansson AG, Sellgren C, et al. Increased cerebrospinal fluid interleukin-8 in bipolar disorder patients associated with lithium and antipsychotic treatment. Brain Behav Immun. 2015;43:198–204. doi: 10.1016/j.bbi.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Isgren A, Sellgren C, Ekman CJ, Holménlarsson J, Blennow K, Zetterberg H, et al. Markers of neuroinflammation and neuronal injury in bipolar disorder: relation to prospective clinical outcomes. Brain Behav Immun. 2017;65:195–201. doi: 10.1016/j.bbi.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Wang AK, Miller BJ. Meta-analysis of Cerebrospinal Fluid Cytokine and Tryptophan Catabolite Alterations in Psychiatric Patients: Comparisons Between Schizophrenia, Bipolar Disorder, and Depression. Schizophr Bull. 2018;44:75–83. doi: 10.1093/schbul/sbx035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munkholm K, Jacoby AS, Lenskjold T, Bruunsgaard H, Vinberg M, Kessing LV. Leukocytes in peripheral blood in patients with bipolar disorder – trait and state alterations and association with levels of cytokines and C-reactive protein. Psychiatry Res. 2018;261:383–390. doi: 10.1016/j.psychres.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 32.Wu W, Zheng YL, Tian LP, Lai JB, Hu CC, Zhang P, et al. Circulating T lymphocyte subsets, cytokines, and immune checkpoint inhibitors in patients with bipolar II or major depression: a preliminary study. Scientific Reports. 2017;7:40530. doi: 10.1038/srep40530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Modabbernia A, Taslimi S, Brietzke E, Ashrafi M. Cytokine alterations in bipolar disorder: a meta-analysis of 30 studies. Biol Psychiatry. 2013;74:15–25. doi: 10.1016/j.biopsych.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21:1696–1709. doi: 10.1038/mp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benedetti F, Mazza E, Poletti S, Bollettini I, Locatelli C, Colombo C, et al. Inflammatory cytokines influence measures of white matter integrity in bipolar disorder. Neurol Psychiatry Brain Res, 2016: 1–2. [DOI] [PubMed]
- 36.Haroon E, Miller AH, Sanacora G. Inflammation, Glutamate, and Glia: A Trio of Trouble in Mood Disorders. Neuropsychopharmacology. 2017;42:193–215. doi: 10.1038/npp.2016.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song C, Merali Z, Anisman H. Variations of nucleus accumbens dopamine and serotonin following systemic interleukin-1, interleukin-2 or interleukin-6 treatment. Neuroscience. 1999;88:823–836. doi: 10.1016/S0306-4522(98)00271-1. [DOI] [PubMed] [Google Scholar]
- 38.Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, Woolwine BJ, et al. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol Psychiatry. 2009;65:296–303. doi: 10.1016/j.biopsych.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howerton AR, Roland AV, Bale TL. Dorsal raphe neuroinflammation promotes dramatic behavioral stress dysregulation. J Neurosci. 2014;34:7113–7123. doi: 10.1523/JNEUROSCI.0118-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arnone D, Saraykar S, Salem H, Teixeira AL, Dantzer R, Selvaraj S. Role of Kynurenine pathway and its metabolites in mood disorders: A systematic review and meta-analysis of clinical studies. Neurosci Biobehav Rev. 2018;92:477–485. doi: 10.1016/j.neubiorev.2018.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mukherjee D, Krishnamurthy VB, Millett CE, Reider A, Can A, Groer M, et al. Total sleep time and kynurenine metabolism associated with mood symptom severity in bipolar disorder. Bipolar Disord. 2018;20:27–34. doi: 10.1111/bdi.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsu JW, Lirng JF, Wang SJ, Lin CL, Yang KC, Liao MH, et al. Association of thalamic serotonin transporter and interleukin-10 in bipolar I disorder: a SPECT study. Bipolar Disord. 2014;16:241–248. doi: 10.1111/bdi.12164. [DOI] [PubMed] [Google Scholar]
- 43.Chou YH, Hsieh WC, Chen LC, Lirng JF, Wang SJ. Association between the serotonin transporter and cytokines: Implications for the pathophysiology of bipolar disorder. J Affect Disord. 2016;191:29–35. doi: 10.1016/j.jad.2015.10.056. [DOI] [PubMed] [Google Scholar]
- 44.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 45.Andela CD, van Haalen FM, Ragnarsson O, Papakokkinou E, Johannsson G, Santos A, et al. MECHANISMS IN ENDOCRINOLOGY: Cushing’s syndrome causes irreversible effects on the human brain: a systematic review of structural and functional magnetic resonance imaging studies. Eur J Endocrinol. 2015;173:R1–14. doi: 10.1530/EJE-14-1101. [DOI] [PubMed] [Google Scholar]
- 46.Sonino N, Fava GA. Psychiatric disorders associated with Cushing’s syndrome. Epidemiology, pathophysiology and treatment. CNS Drugs 2001, 15: 361–373. [DOI] [PubMed]
- 47.Moisés E. Bauer JCMM, Luz C The role of stress factors during aging of the immune system. Ann N Y Acad Sci. 2009;1153:139–152. doi: 10.1111/j.1749-6632.2008.03966.x. [DOI] [PubMed] [Google Scholar]
- 48.Bauer ME, Wieck A, Petersen LE, Baptista TS. Neuroendocrine and viral correlates of premature immunosenescence. Ann N Y Acad Sci. 2015;1351:11–21. doi: 10.1111/nyas.12786. [DOI] [PubMed] [Google Scholar]
- 49.Beishuizen A, Thijs LG. Endotoxin and the hypothalamo-pituitary-adrenal (HPA) axis. J Endotoxin Res. 2003;9:3–24. doi: 10.1179/096805103125001298. [DOI] [PubMed] [Google Scholar]
- 50.Young AH. The Effects of HPA Axis Function on Cognition and Its Implications for the Pathophysiology of Bipolar Disorder. Harv Rev Psychiatry. 2014;22:331–333. doi: 10.1097/HRP.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 51.Réus GZ, Fries GR, Stertz L, Badawy M, Passos IC, Barichello T, et al. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience. 2015;300:141–154. doi: 10.1016/j.neuroscience.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 52.Spijker AT, Van Rossum EFC. Glucocorticoid Sensitivity in Mood Disorders. Neuroendocrinology. 2012;95:179–186. doi: 10.1159/000329846. [DOI] [PubMed] [Google Scholar]
- 53.Cowen PJ. Not fade away: the HPA axis and depression. Psychol Med. 2010;40:1–4. doi: 10.1017/S0033291709005558. [DOI] [PubMed] [Google Scholar]
- 54.Belvederi MM, Prestia D, Mondelli V, Pariante C, Patti S, Olivieri B, et al. The HPA axis in bipolar disorder: Systematic review and meta-analysis. Psychoneuroendocrinology. 2016;63:327–342. doi: 10.1016/j.psyneuen.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 55.Valvassori SS, Resende WR, Dal-Pont G, Sangaletti-Pereira H, Gava FF, Peterle BR, et al. Lithium ameliorates sleep deprivation-induced mania-like behavior, hypothalamic-pituitary-adrenal (HPA) axis alterations, oxidative stress and elevations of cytokine concentrations in the brain and serum of mice. Bipolar Disord. 2017;19:246–258. doi: 10.1111/bdi.12503. [DOI] [PubMed] [Google Scholar]
- 56.Wu R, Fan J, Zhao J, Calabrese JR, Gao K. The relationship between neurotrophins and bipolar disorder. Expert Rev Neurother. 2014;14:51–65. doi: 10.1586/14737175.2014.863709. [DOI] [PubMed] [Google Scholar]
- 57.Jacoby AS, Munkholm K, Vinberg M, Pedersen BK, Kessing LV. Cytokines, brain-derived neurotrophic factor and C-reactive protein in bipolar I disorder - Results from a prospective study. J Affect Disord. 2016;197:167–174. doi: 10.1016/j.jad.2016.03.040. [DOI] [PubMed] [Google Scholar]
- 58.van den Ameele S, Coppens V, Schuermans J, De Boer P, Timmers M, Fransen E, et al. Neurotrophic and inflammatory markers in bipolar disorder: A prospective study. Psychoneuroendocrinology. 2017;84:143–150. doi: 10.1016/j.psyneuen.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 59.Wang TY, Lee SY, Chen SL, Chang YH, Wang LJ, Chen PS, et al. Comparing clinical responses and the biomarkers of BDNF and cytokines between subthreshold bipolar disorder and bipolar II disorder. Sci Rep. 2016;6:27431. doi: 10.1038/srep27431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hatch JK, Scola G, Olowoyeye O, Collins JE, Andreazza AC, Moody A, et al. Inflammatory Markers and Brain-Derived Neurotrophic Factor as Potential Bridges Linking Bipolar Disorder and Cardiovascular Risk Among Adolescents. J Clin Psychiatry. 2017;78:e286–e293. doi: 10.4088/JCP.16m10762. [DOI] [PubMed] [Google Scholar]
- 61.Nunes SO, Lg PDM, Mr PDC, Barbosa DS, Vargas HO, Berk M, et al. Atherogenic index of plasma and atherogenic coefficient are increased in major depression and bipolar disorder, especially when comorbid with tobacco use disorder. J Affect Disord. 2015;172:55–62. doi: 10.1016/j.jad.2014.09.038. [DOI] [PubMed] [Google Scholar]
- 62.Lgp DM, Sov N, Anderson G, Vargas HO, Barbosa DS, Galecki P, et al. Shared metabolic and immune-inflammatory, oxidative and nitrosative stress pathways in the metabolic syndrome and mood disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:34–50. doi: 10.1016/j.pnpbp.2017.04.027. [DOI] [PubMed] [Google Scholar]
- 63.Vasconcelos-Moreno MP, Fries GR, Gubert C, Dos Santos BT, Fijtman A, Sartori J, et al. Telomere length, oxidative stress, inflammation and BDNF levels in siblings of patients with bipolar disorder: implications for accelerated cellular aging. Int J Neuropsychopharmacol. 2017;20:445–454. doi: 10.1093/ijnp/pyx001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mondin TC, Cardoso TDA, Moreira FP, Wiener C, Oses JP, Souza LDDM, et al. Circadian preferences, oxidative stress and inflammatory cytokines in bipolar disorder: A community study. J Neuroimmunol. 2016;301:23–29. doi: 10.1016/j.jneuroim.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 65.Ozdemircan A, Dasdemir S, Kucukali CI, Bireller ES, Ozturk H, Cakmakoglu B. COX-2 gene variants in bipolar disorder-I. Psychiatr Danub. 2015;27:385–389. [PubMed] [Google Scholar]
- 66.Rafiei A, Hosseini SH, Taheri M, Hosseni-khah Z, Hajilooi M, Mazaheri Z. Influence of IL-1RN intron 2 variable number of tandem repeats (VNTR) polymorphism on bipolar disorder. Neuropsychobiology. 2013;67:116–121. doi: 10.1159/000346112. [DOI] [PubMed] [Google Scholar]
- 67.Yoon HK, Kim YK. The T allele of the interferon-gamma +874A/T polymorphism is associated with bipolar disorder. Nord J Psychiatry. 2012;66:14–18. doi: 10.3109/08039488.2011.593045. [DOI] [PubMed] [Google Scholar]
- 68.Altamura AC, Mundo E, Cattaneo E, Pozzoli S, Dell’osso B, Gennarelli M, et al. The MCP-1 gene (SCYA2) and mood disorders: preliminary results of a case-control association study. Neuroimmunomodulation. 2010;17:126–131. doi: 10.1159/000258696. [DOI] [PubMed] [Google Scholar]
- 69.Clerici M, Arosio B, Mundo E, Cattaneo E, Pozzoli S, Dell’osso B, et al. Cytokine polymorphisms in the pathophysiology of mood disorders. CNS Spectr. 2009;14:419–425. doi: 10.1017/S1092852900020393. [DOI] [PubMed] [Google Scholar]
- 70.Papiol S, Rosa A, Gutierrez B, Martin B, Salgado P, Catalan R, et al. Interleukin-1 cluster is associated with genetic risk for schizophrenia and bipolar disorder. J Med Genet. 2004;41:219–223. doi: 10.1136/jmg.2003.012914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lotrich FE, Butters MA, Aizenstein H, Marron MM, Reynolds CF, 3rd, Gildengers AG. The relationship between interleukin-1 receptor antagonist and cognitive function in older adults with bipolar disorder. Int J Geriatr Psychiatry. 2014;29:635–644. doi: 10.1002/gps.4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fillman SG, Weickert TW, Lenroot RK, Catts SV, Bruggemann JM, Catts VS, et al. Elevated peripheral cytokines characterize a subgroup of people with schizophrenia displaying poor verbal fluency and reduced Broca’s area volume. Mol Psychiatry. 2016;21:1090–1098. doi: 10.1038/mp.2015.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Benedetti F, Poletti S, Hoogenboezem TA, Locatelli C, De HW, Wijkhuijs A, et al. Higher baseline proinflammatory cytokines mark poor antidepressant response in bipolar disorder. J Clin Psychiatry. 2017;78:e986–e993. doi: 10.4088/JCP.16m11310. [DOI] [PubMed] [Google Scholar]
- 74.Calati R, Crisafulli C, Balestri M, Serretti A, Spina E, Calabro M, et al. Evaluation of the role of MAPK1 and CREB1 polymorphisms on treatment resistance, response and remission in mood disorder patients. Prog Neuropsychopharmacol Biol Psychiatry. 2013;44:271–278. doi: 10.1016/j.pnpbp.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 75.Amare AT, Schubert KO, Hou L, Clark SR, Papiol S, Heilbronner U, et al. Association of polygenic score for schizophrenia and HLA antigen and inflammation genes with response to lithium in bipolar affective disorder: a genome-wide association study. JAMA Psychiatry. 2018;75:65–74. doi: 10.1001/jamapsychiatry.2017.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mechawar N, Savitz J. Neuropathology of mood disorders: do we see the stigmata of inflammation? Transl Psychiatry. 2016;6:e946. doi: 10.1038/tp.2016.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goldstein BI, Kemp DE, Soczynska JK, Mcintyre RS. Inflammation and the phenomenology, pathophysiology, comorbidity, and treatment of bipolar disorder: a systematic review of the literature. J Clin Psychiatry. 2009;70:1078–1090. doi: 10.4088/JCP.08r04505. [DOI] [PubMed] [Google Scholar]
- 78.Pandey GN. Inflammatory and innate immune markers of neuroprogression in depressed and teenage suicide brain. Mod Trends Pharmacopsychiatry. 2017;31:79–95. doi: 10.1159/000470809. [DOI] [PubMed] [Google Scholar]
- 79.Ghafelehbashi H, Pahlevan MK, Kular L, Moghbelinejad S, Ghafelehbashi SH. Decreased expression of IFNG-AS1, IFNG and IL-1B inflammatory genes in medicated Schizophrenia and Bipolar patients. Scand J Immunol. 2017;86:479–485. doi: 10.1111/sji.12620. [DOI] [PubMed] [Google Scholar]
- 80.Akcan U, Karabulut S, C İK, S Ç, Tüzün E. Bipolar disorder patients display reduced serum complement levels and elevated peripheral blood complement expression levels. Acta Neuropsychiatr 2018, 30: 70–78. [DOI] [PubMed]
- 81.Vogels RJ, Koenders MA, van Rossum EF, Spijker AT, Drexhage HA. T cell deficits and overexpression of hepatocyte growth factor in anti-inflammatory circulating monocytes of middle-aged patients with bipolar disorder characterized by a high prevalence of the metabolic syndrome. Front Psychiatry. 2017;8:34. doi: 10.3389/fpsyt.2017.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ferrari P, Parisi MM, Colombo R, Becker M, Fries G, Ascoli BM, et al. Depression and mania induce pro-inflammatory activation of macrophages following application of serum from individuals with bipolar disorder. Clin Psychopharmacol Neurosci. 2018;16:103–108. doi: 10.9758/cpn.2018.16.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Amare AT, Schubert KO, Hou L, Clark SR, Papiol S, Heilbronner U, et al. Association of polygenic score for schizophrenia and HLA antigen and inflammation genes with response to lithium in bipolar affective disorder: a genome-wide association study. JAMA Psychiatry. 2017;75:65–74. doi: 10.1001/jamapsychiatry.2017.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hayes JF, Khandaker GM, Anderson J, Mackay D, Zammit S, Lewis G, et al. Childhood interleukin-6, C-reactive protein and atopic disorders as risk factors for hypomanic symptoms in young adulthood: a longitudinal birth cohort study. Psychol Med. 2017;47:23–33. doi: 10.1017/S0033291716001574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zeni-Graiff M, Rizzo LB, Mansur RB, Maurya PK, Sethi S, Cunha GR, et al. Peripheral immuno-inflammatory abnormalities in ultra-high risk of developing psychosis. Schizophrenia Res. 2016;176:191–195. doi: 10.1016/j.schres.2016.06.031. [DOI] [PubMed] [Google Scholar]
- 86.Cheslack-Postava K, Cremers S, Bao Y, Shen L, Schaefer CA, Brown AS. Maternal serum cytokine levels and risk of bipolar disorder. Brain Behav Immun. 2017;63:108–114. doi: 10.1016/j.bbi.2016.07.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Park M, Newman LE, Gold PW, Luckenbaugh DA, Yuan P, Machado-Vieira R, et al. Change in cytokine levels is not associated with rapid antidepressant response to ketamine in treatment-resistant depression. J Psychiatr Res. 2017;84:113–118. doi: 10.1016/j.jpsychires.2016.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van den Ameele S, van Diermen L, Staels W, Coppens V, Dumont G, Sabbe B, et al. The effect of mood-stabilizing drugs on cytokine levels in bipolar disorder: A systematic review. J Affect Disord. 2016;203:364–373. doi: 10.1016/j.jad.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 89.Barbisan F, van Azzolin VF, Teixeira CF, Mastella MH, Ribeiro EE, do Prado-Lima PAS, et al. Xanthine-catechin mixture enhances lithium-induced anti-inflammatory response in activated macrophages in vitro. Biomed Res Int. 2017;2017:4151594. doi: 10.1155/2017/4151594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Valvassori SS, Resende WR, Dal-Pont G, Sangaletti-Pereira H, Gava FF, Peterle BR, et al. Lithium ameliorates sleep deprivation-induced mania-like behavior, hypothalamic-pituitary-adrenal (HPA) axis alterations, oxidative stress and elevations of cytokine concentrations in the brain and serum of mice. Bipolar Disord. 2017;19:246–258. doi: 10.1111/bdi.12503. [DOI] [PubMed] [Google Scholar]
- 91.Benedetti F, Poletti S, Radaelli D, Locatelli C, Pirovano A, Lorenzi C, et al. Lithium and GSK-3beta promoter gene variants influence cortical gray matter volumes in bipolar disorder. Psychopharmacology (Berl) 2015;232:1325–1336. doi: 10.1007/s00213-014-3770-4. [DOI] [PubMed] [Google Scholar]
- 92.Valvassori SS, Resende WR, Varela RB, Arent CO, Gava FF, Peterle BR, et al. The effects of histone deacetylase inhibition on the levels of cerebral cytokines in an animal model of mania induced by dextroamphetamine. Mol Neurobiol. 2018;55:1430–1439. doi: 10.1007/s12035-017-0384-y. [DOI] [PubMed] [Google Scholar]
- 93.Leu SJ, Yang YY, Liu HC, Cheng CY, Wu YC, Huang MC, et al. Valproic acid and lithium meditate anti-inflammatory effects by differentially modulating dendritic cell differentiation and function. J Cell Physiol. 2017;232:1176–1186. doi: 10.1002/jcp.25604. [DOI] [PubMed] [Google Scholar]
- 94.Bortolasci CC, Spolding B, Callaly E, Martin S, Panizzutti B, Kidnapillai S, et al. Mechanisms underpinning the polypharmacy effects of medications in psychiatry. Int J Neuropsychopharmacol 2018. [DOI] [PMC free article] [PubMed]
- 95.Miller AH, Haroon E, Raison CL, Felger JC. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety. 2013;30:297–306. doi: 10.1002/da.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rosenblat JD, Kakar R, Berk M, Kessing LV, Vinberg M, Baune BT, et al. Anti-inflammatory agents in the treatment of bipolar depression: a systematic review and meta-analysis. Bipolar Disord. 2016;18:89–101. doi: 10.1111/bdi.12373. [DOI] [PubMed] [Google Scholar]
- 97.Savitz JB, Teague TK, Misaki M, Macaluso M, Wurfel BE, Meyer M, et al. Treatment of bipolar depression with minocycline and/or aspirin: an adaptive, 2×2 double-blind, randomized, placebo-controlled, phase IIA clinical trial. Transl Psychiatry. 2018;8:27. doi: 10.1038/s41398-017-0073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Soczynska JK, Kennedy SH, Alsuwaidan M, Mansur RB, Li M, Mcandrews MP, et al. A pilot, open-label, 8-week study evaluating the efficacy, safety and tolerability of adjunctive minocycline for the treatment of bipolar I/II depression. Bipolar Disord. 2017;19:198–213. doi: 10.1111/bdi.12496. [DOI] [PubMed] [Google Scholar]
- 99.Arabzadeh S, Ameli N, Zeinoddini A, Rezaei F, Farokhnia M, Mohammadinejad P, et al. Celecoxib adjunctive therapy for acute bipolar mania: a randomized, double-blind, placebo-controlled trial. Bipolar Disord. 2015;17:606–614. doi: 10.1111/bdi.12324. [DOI] [PubMed] [Google Scholar]
- 100.Rosenblat JD, Brietzke E, Mansur RB, Maruschak NA, Lee Y, McIntyre RS. Inflammation as a neurobiological substrate of cognitive impairment in bipolar disorder: Evidence, pathophysiology and treatment implications. J Affect Disord. 2015;188:149–159. doi: 10.1016/j.jad.2015.08.058. [DOI] [PubMed] [Google Scholar]