Abstract
Objective
To provide a systematic review of economic evaluations that has been conducted for onchocerciasis interventions, to summarise current key knowledge and to identify research gaps.
Method
A systematic review of the literature was conducted on the 8th of August 2018 using the PubMed (MEDLINE) and ISI Web of Science electronic databases. No date or language stipulations were applied to the searches.
Results
We identified 14 primary studies reporting the results of economic evaluations of onchocerciasis interventions, seven of which were cost‐effectiveness analyses. The studies identified used a variety of different approaches to estimate the costs of the investigated interventions/programmes. Originally, the studies only quantified the benefits associated with preventing blindness. Gradually, methods improved and also captured onchocerciasis‐associated skin disease. Studies found that eliminating onchocerciasis would generate billions in economic benefits. The majority of the cost‐effectiveness analyses evaluated annual mass drug administration (MDA). The estimated cost per disability‐adjusted life year (DALY) averted of annual MDA varies between US$3 and US$30 (cost year variable).
Conclusions
The cost benefit and cost effectiveness of onchocerciasis interventions have consistently been found to be very favourable. This finding provides strong evidential support for the ongoing efforts to eliminate onchocerciasis from endemic areas. Although these results are very promising, there are several important research gaps that need to be addressed as we move towards the 2020 milestones and beyond.
Keywords: onchocerciasis, river blindness, economic evaluations, cost effectiveness, cost‐benefit analyses, cost, elimination, health economics
Abstract
Objectif
Fournir une analyse systématique des évaluations économiques réalisées pour les interventions contre l'onchocercose, résumer les principales connaissances actuelles et identifier les lacunes de la recherche.
Méthode
Une revue systématique de la littérature a été menée le 8 août 2018 en utilisant les bases de données électroniques PubMed (Medline) et ISI Web of Science. Aucune indication de date ou de langue n'a été appliquée aux recherches.
Résultats
Nous avons identifié 14 études principales rapportant sur les résultats d’évaluations économiques d'interventions contre l'onchocercose, dont 7 étaient des analyses coût‐efficacité. Les études identifiées ont utilisé diverses approches pour estimer les coûts des interventions/programmes étudiés. A l'origine, les études ne mesuraient que les avantages associés à la prévention de la cécité. Progressivement, les méthodes se sont améliorées et ont également capturé les maladies de la peau associées à l'onchocercose. Les études ont montré que l’élimination de l'onchocercose générerait des milliards de bénéfices économiques. La majorité des analyses coût‐efficacité ont évalué l'administration annuelle en masse de médicaments (AMD). Le coût estimé par année de vie ajustée par rapport à l'incapacité (DALY) corrigé pour l’AMD annuelle varie entre 3 et 30 USD (variable de l'année de coût).
ConclusionsLe rapport coût‐efficacité et la rentabilité des interventions contre l'onchocercose se sont toujours avérés très favorables. Cette constatation fournit un solide appui probant aux efforts en cours pour éliminer l'onchocercose des zones d'endémie. Bien que ces résultats soient très prometteurs, plusieurs lacunes importantes en matière de recherche doivent être comblées à mesure que nous nous approchons des étapes clés pour 2020 et au‐delà.
Keywords: onchocercose, cécité des rivières, évaluations économiques, rapport coût‐efficacité, analyses coût‐bénéfice, coût, élimination, économie de la santé
Introduction
Human onchocerciasis, also known as ‘river blindness’, is a parasitic infection caused by the filarial nematode Onchocerca volvulus. It is transmitted by the bites of Simulium blackflies. Of the 120 million people at risk, 99% live in sub‐Saharan Africa, although the disease has also been endemic in six countries of Latin America and is present in Yemen 1. Symptoms can include severe itching, disfiguring skin conditions, visual impairment, and permanent blindness 1. Onchocerciasis is the world's second leading infectious cause of blindness after trachoma 2. Onchocerciasis can also cause excess mortality 3, 4, 5 and is associated with epilepsy, nodding syndrome and hyposexual dwarfism (Nakalanga syndrome) 6, 7, 8.
During the last 40 years, there has been a remarkable expansion of onchocerciasis control programmes worldwide 9, 10, 11, 12, 13, 14, summarised in Box 1. These programmes have had a large impact on reducing onchocerciasis as a public health problem. Initially, control programmes (i.e. the Onchocerciasis Control Programme in West Africa (OCP)) only used large‐scale vector control as the drugs available at that time were too toxic for large‐scale use. This changed in 1987, when ivermectin (produced under the brand name Mectizan®), a drug suitable for mass treatment, was registered for human use against onchocerciasis, and large‐scale chemotherapeutic control programmes became feasible 11.
Box 1. Summary of the control programmes.
The Onchocerciasis Control Programme in West Africa (OCP, 1974–2002): The OCP was launched in 1974–1975, and originally covered a core area in seven countries of West Africa 11. However, by 1990 the OCP had expanded its operations to include larger zones and four additional countries following the southern and western extensions 10, 11. From 1974 to 1988 the OCP focused on a strategy of weekly aerial larviciding of blackfly breeding sites. In 1987 ivermectin was registered for human use against onchocerciasis, and due to the suitability of this drug for mass treatment, large‐scale chemotherapeutic control programmes became feasible 10. Large‐scale mass drug administration (MDA) of ivermectin began in the OCP regions in the late 1980s, initially administered by mobile teams of paid, local health professionals 10.
African Programme for Onchocerciasis Control (APOC, 1995–2015): The APOC was initiated in 1995 including 19 African countries 11, 149. The programme pioneered a community‐directed treatment approach, within which the local communities rather than health services directed the treatment process; the treatments were delivered by volunteer community‐directed distributors (CDDs) 149, 150, 151. APOC gradually expanded and by 2014 it had a network of over 699 656 volunteer CDDs 151. When the programme concluded at the end of 2015 it was supporting onchocerciasis control and elimination activities in 31 African countries (including the 19 original signatories of the Memorandum, South Sudan, and the 11 ex‐OCP participating countries) 149.
The Onchocerciasis Elimination Program for the Americas (OEPA, 2002–present): The OEPA started in 1992 in six countries of the Americas (across 13 discrete foci) 11, 12. The strategy is based on the biannual (twice a year) distribution of ivermectin, to all endemic communities (covering at least 85% of the eligible population) 12. As of December 2016, a total of four countries have successfully completed the World Health Organization process for verification of elimination 152.
In 1987, Merck & Co. committed to supply ivermectin to onchocerciasis endemic countries ‘as much as necessary for as long as necessary’ 15, 16. Over 7.8 billion ivermectin tablets have been donated for the treatment of onchocerciasis and lymphatic filariasis 17. This unprecedented donation allowed onchocerciasis control in endemic areas where vector control was not feasible or too expensive to sustain and ivermectin began to be distributed in the late 1980s. Initially, mobile teams of paid, local health professionals were used to distribute ivermectin; however, this was costly 18, and programmes mostly switched to using volunteer distributors and community‐directed approaches (Box 1).
Motivated by the successful elimination of the infection in some foci of Mali and Senegal 19, 20, there was a shift in onchocerciasis control policy in Africa, with the aim of programmes changing from elimination as a public health problem to elimination of transmission and ultimately infection. In 2012, the Joint Action Forum of the African Programme for Onchocerciasis Control (APOC), chaired by WHO and the ministers of health of endemic countries, set the target at elimination in 80% of African countries by 2025 21. WHO's roadmap on neglected tropical diseases (NTDs) also included goals for the elimination in several African countries by 2020 22.
The coverage of NTD mass drug administration (MDA) programmes has notably expanded over the last 10 years. In 2017, 1.762 billion treatments were delivered worldwide 23. NTD control programmes are becoming increasingly integrated, moving towards targeting multiple diseases within a multipronged programme 24, 25. In May 2016, the WHO launched the Expanded Special Project for Elimination of Neglected Tropical Diseases (ESPEN), a five‐year project to provide national NTD programmes with technical and fundraising support to help them accelerate the control and elimination of NTDs amenable to preventive chemotherapy, namely onchocerciasis, lymphatic filariasis, schistosomiasis, soil‐transmitted helminthiases and trachoma 26. The integrated and cross‐sectorial response to NTD control is relevant to the Sustainable Development Goals, including among others Universal Health Coverage, Water Sanitation and Hygiene (WASH) initiatives, global partnerships, alleviating poverty and hunger and improving education and economic growth 27.
The aim of this paper was to provide a systematic review of economic evaluations that have been conducted for onchocerciasis interventions, to summarise current key knowledge and to identify research gaps in this area.
Methods
Search strategy
A systematic review of the literature was conducted on the 8th of August 2018 using the PubMed (MEDLINE) and ISI Web of Science electronic databases. Variants of the following search terms were used to find relevant papers: river blindness, onchocerciasis, cost(s), cost‐benefit, cost‐effectiveness, economic(s), economic evaluation. No date or language stipulations were applied to the searches. A more detailed summary of the search terms and the PRISMA checklist are supplied in Appendix S1. The titles and abstracts of all the identified papers were examined initially for relevance and then the bibliographies of papers suitable for inclusion were scanned for studies not originally retrieved from the databases. The full selection process is outlined in Figure 1. Studies relating to cost recovery and willingness to pay were not explicitly included as an outcome of the systematic literature search (but are referenced within the review where relevant).
Figure 1.
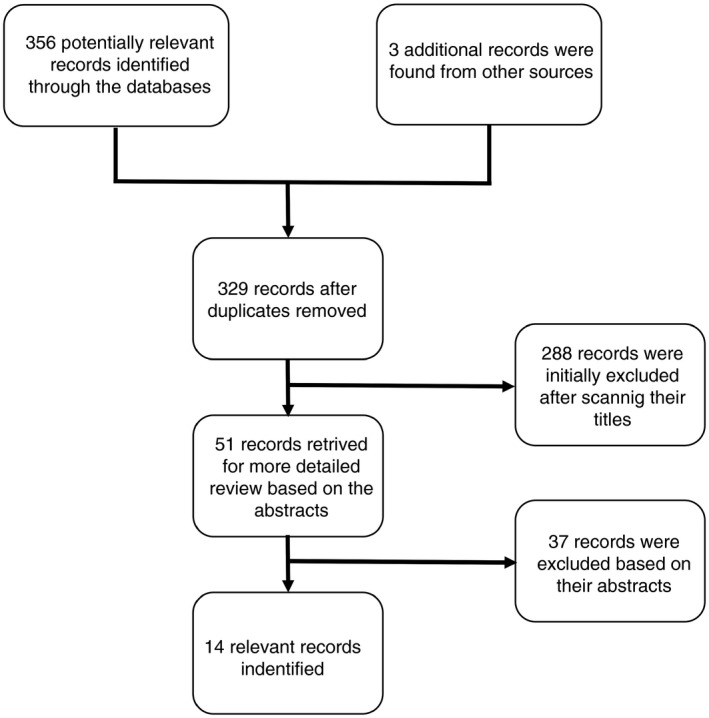
Decision tree outlining the inclusion and exclusion of the identified studies. Some studies reported both cost‐benefit and cost‐effectiveness estimates. A PRISMA checklist is provided in Appendix S1.
Results and discussion
We identified 14 primary studies reporting the results of economic evaluations of onchocerciasis interventions, which are described in Tables 1 and 2. It is important to note that both the OCP and APOC expanded over time (Box 1) and it was not always clear which specific countries/areas were being included in the different analyses.
Table 1.
Summary of the identified cost‐benefit analyses and estimates of the economic benefits of onchocerciasis interventions
| Source | Setting and time period of the intervention | Time horizon for the benefits | Discount rate | Cost year | Cost of the intervention | Total economic benefitb | Net present value | Internal rate of return |
|---|---|---|---|---|---|---|---|---|
| Benton & Skinner 67 | OCP (1974–2004) | 1974–2023 | 5–10% | 1985 US$ |
|
US$148 million (10% discount rate) to US$543 million (5% discount rate). | US$8 million (10% discount rate) to US$312 million (5% discount rate). | 11–13% |
| Kim & Benton 58 | OCP (1974–2002) | 1974–2012 | 3–10% | 1987 US$ |
|
Not stated. | US$485 million (10% discount rate) to US$3729 million (3% discount rate). | 20% |
| McFarland & Murray 124, a | OCP (10‐year project time period) | 1974–2023 | 5% | Not available |
|
Not available. | US$8 million. | ‐ |
| Benton 125 | APOC (1996–2007) | 1996–2017 | 10% | 1996 US$ |
|
Not stated. | US$53.7 million. | 17% |
| Haddix 69, a | APOC (1996–2007) | 1996–2017 | 3–10% | 1996 US$ |
|
Not available. | US$87.6 million (10% discount rate) to US$307.4 million (3% discount rate). | 24% |
| Kim et al. 60 | Potential benefits of achieving elimination scenarios in Africa | 2013–2045 | 3% | 2013 US$ | NA | Compared with the control scenario, the Elim I and II scenariosc would generate US$5.96 (2.53–7.28) billion and US$6.46 (2.83–8.09) billion in economic benefits respectively. | NA | NA |
| Redekop et al. 59 | Potential economic benefits of achieving the WHO 2020 targets | 2011–2030 | 3% | 2005 US$ | NA | US$3.3 (2.4–5.11) billion. | NA | NA |
| Turner et al. 95 | Potential impact of moxidectin on onchocerciasis elimination in African savannah settingsd | 50 years | 3% | 2012 US$ |
|
Annual moxidectin treatment would achieve similar reductions in programme duration as using biannual ivermectin treatment. If the moxidectin tablets were donated its use would lead to substantial in‐country cost savings. |
NA | NA |
APOC, African Programme for Onchocerciasis Control; NA, not applicable; OCP, Onchocerciasis Control Programme in West Africa; pOTTIS, provisional operational thresholds for treatment interruption followed by surveillance.
Information for this study was taken from Waters et al. 18.
The estimated economic benefits are outlined in further detail in Table 3.
The Control, Elim I and Elim II scenarios are described in Kim et al. 99.
Assumed that MDA would be stopped (determining the programme duration) once the pOTTIS would have been achieved (defined as the modelled microfilarial prevalence being <1.4%, measured just before the next treatment round).
Table 2.
Summary of the identified cost‐effectiveness analyses of onchocerciasis interventions
| Study | Setting and time period of the intervention | Time horizon for the benefits | Discount rate | Cost year | Cost of the intervention | Effectiveness | Cost‐effectiveness ratio |
|---|---|---|---|---|---|---|---|
| McFarland & Murray 124, a | OCP | ‐ | ‐ | Not available |
|
|
|
| Prescott et al. 126 and Prost & Prescott 127 | OCP ‐ Upper Volta (now Burkina Faso) (1975–1994) | 1975–1994 | 10% | 1977 US$ |
|
|
|
| Evans et al. 71 | OCP ‐ Burkina Faso (1974–1997) | 1974–1997 | 10% (but varied between 3–15%) | 1984 US$ |
|
|
|
| Benton 125 | APOC (1996–2007) | 1996–2017 | 3% | 1996 US$ |
|
|
|
| Coffeng et al. 30 | APOC (1995–2015) | 1995–2015 | 0% | Nominal values |
|
|
|
| Remme et al. 63 | APOC (over 15 years) | Over a 25‐year period | Unclear | Not stated |
|
|
|
| Turner et al. 73 | Annual MDA in an African savannah setting (up to 50 years)b , c | 50 years | 3% | 2012 US$ |
|
||
| Turner et al. 73 | Biannual MDA in an African savannah setting (up to 50 years)b , c | 50 years | 3% | 2012 US$ |
|
|
|
APOC, African Programme for Onchocerciasis Control; DALY, disability‐adjusted life years; MDA, mass drug administration; Nominal cost, values have not been adjusted for inflation; OCP, Onchocerciasis Control Programme in West Africa; pOTTIS, provisional operational thresholds for treatment interruption followed by surveillance.
Information for this study was taken from Waters et al. 18.
Assumed that MDA would be stopped (determining the programme duration and its total cost) once the pOTTIS would have been achieved (defined as the modelled microfilarial prevalence being <1.4%, measured just before the next treatment round).
Three different endemicity levels were explored (ranging between 40–80% microfilarial prevalence).
Estimated cost of onchocerciasis interventions
A key component of any cost‐benefit or cost‐effectiveness analysis (Box 2) of an intervention is its estimated cost. The studies identified used a variety of different approaches to estimate the costs of the investigated interventions/programmes. Many of the studies based their costs on programme budgets/reports and very few were based on comprehensive costing studies/approaches. There was variation in what types of costs were included within the analyses.
Box 2. Glossary.
Cost‐benefit analysis: A type of economic evaluation which compares the cost of an intervention to its monetary benefits. The results are typically expressed as an internal rate of return or net present value.
Cost‐effectiveness analysis: A type of economic evaluation in which the cost of an intervention is compared to the quantity of a single non‐monetary effectiveness measure (such as the number of deaths or cases averted). This avoids the issues associated with monetising the benefits of healthcare interventions. The results are expressed as a cost per unit of outcome (see cost‐effectiveness ratio).
Cost‐effectiveness ratio: A statistic used to summarise the cost‐effectiveness of an intervention. It is calculated by dividing the cost of an intervention by its effectiveness measure, such as a cost per disability‐adjusted life year (DALY) averted or healthy life year gained. An incremental cost‐effectiveness ratio is calculated by dividing the difference in costs by the difference in effectiveness outcomes of two alternative options (it summaries the ‘extra cost per additional unit of effect gained’).
Community‐directed distributors (CDDs): Also referred to as community drug distributors, these are volunteers selected by their communities to distribute treatment.
Disability‐adjusted life years (DALYs): A measure of disease burden that is calculated as the sum of the years of life lost due to premature mortality and the years of healthy life lost due to disability. The number of years of healthy life lost due to disability is calculated using a disability weight factor (between 0 and 1) that reflects the severity of the disease/disability. One DALY can be thought of as one year of ‘healthy’ life lost.
Economic costs (opportunity costs): These define the cost of a resource as its value in its next best alternative use (also known as an opportunity cost). This is a broader conceptualisation of a resource's value than its financial cost, as it recognises that using a resource makes it unavailable for productive use elsewhere. The rationale behind economic costs is that they are intended to represent the full value of all the resources used for an intervention, and they account for the fact that resources can have a value that is not (fully) captured by their financial costs (such as the ‘free’ use of building space provided by Ministries of Health, and the unpaid time devoted to mass drug administration by volunteer CDDs). This is particularly important when considering issues related to the sustainability and replicability of interventions.
Economies of scale: The reduction in the average cost per unit resulting from increased production/output; in this case, the reduction in the cost per treatment as a result of increasing the number of people treated.
Economies of scope: The reduction in the average cost per unit resulting when providing multiple goods/services jointly; in this case, the reduction in the cost per treatment when delivering more than one intervention at once (e.g. integrated control programmes or using the CDD platform to deliver more than a single intervention). Examples include administering treatment for both schistosomiasis and soil‐transmitted helminthiases within the same programme (instead of by separate vertical programmes).
Financial costs: The actual expenditure (i.e. the amount paid) for the goods, resources and services that are purchased.
Friction cost approach: The approach that takes the employer's perspective for valuing lost productivity, and therefore only counts as lost, the hours not worked by a sick employee before another employee takes over the work 65. It is based on the assumption that an ill individual will eventually be replaced by another healthy worker – therefore, the initial productivity levels are restored after this ‘friction period’.
Human capital approach: The approach that takes the patient's perspective for valuing lost productivity and therefore counts all the work they miss, as a productivity loss. With this approach, all potential production not performed by an individual because of morbidity or premature mortality is counted as a production loss 65.
Indirect costs (productivity costs): Indirect cost represents the value of productivity losses that result from illness, treatment, or premature death.
Internal rate of return (IRR): The discount rate applied to the monetised benefits and costs of an intervention, that makes its net present value equal to zero. Also known as the economic rate of return (ERR).
Mass drug administration (MDA): The large‐scale distribution of drugs to eligible people within populations at risk of infection, irrespective of current individual infection status, i.e. without the need for screening for or diagnosing infection prior to each treatment round.
Net present value (NPV): The difference between an intervention's monetised benefits and its cost. A positive NPV is an indicator of a successful investment.
Perspective: The viewpoint from which the intervention's costs and consequences are evaluated. When adopting the healthcare providers perspective, the costs falling outside the healthcare sector are ignored. In contrast, when adopting the societal perspective, all relevant cost categories should be included, including those incurred by the patients.
Time horizon: The time horizon for the analysis; the duration over which outcomes and costs are calculated.
The estimated intervention costs tended to be higher for the studies evaluating OCP activities. This is likely because the control measures used by the OCP (vector control and mass treatment delivered via mobile teams) were more expensive than the community‐directed treatment approach subsequently used by other programmes 28 (Box 1). The nominal financial cost (i.e. not adjusted for inflation or discounted) of the OCP (1974–2002) was just under US$1 billion 29 whereas the projected cost of the larger APOC (1995–2015) was US$478 million 30.
Often it was difficult to compare the reported costs from the different studies, as it was not always clear how costs were estimated, what activities were costed, and whether reported values were discounted or not. Some studies appeared only to adjust the costs for inflation and not discount them (Box 3).
Box 3. Discounting.
Healthcare interventions typically incur costs and generate health outcomes over a number of years. However, society does not place an equal value on costs or health outcomes that occur now compared to those that occur in the future. This is because there is an opportunity cost to spending money (as it could be invested to yield returns) and a desire to have benefits now rather than in the future. Economic evaluations therefore need to weight differently costs and health outcomes that occur in the future.
Discounting is the process used to convert costs or health outcomes occurring in the future into a present value 153, 154, 155. It makes costs and benefits occurring in the future worth less than those in the present. This allows the comparison of the costs and outcomes occurring over different time periods. The discount rate determines the strength of the time preference – the higher the discount rate the lower the value placed on future costs/outcomes. Note that adjusting for inflation (which accounts for the fact that the purchasing power of a currency changes over time) is not the same as discounting.
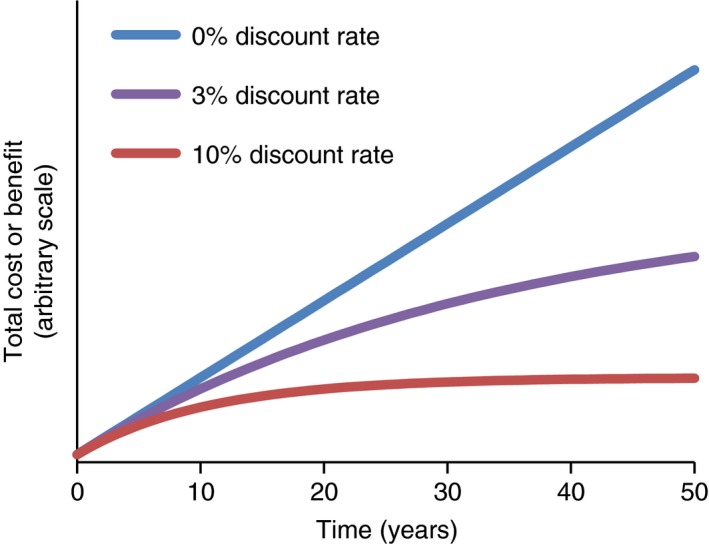
When different analyses have used different discount rates, it makes the results harder to compare them directly. The WHO's guide to cost‐effectiveness analysis recommends using a 3% discount rate for both costs and health outcomes (and testing the sensitivity of the results to using a 0% discount rate for health effects and a 6% discount rate for costs within the sensitivity analysis) 50. These recommendations have engendered greater consistency in the use of discount rates. However, although using the 3% discount rate has become the standard, there is still debate within the field, particularly on discounting of health effects 154, 155.
The studies evaluating programmes using community‐based mass treatment generally assumed delivery costs of around US$0.50 per treatment. This is consistent with the findings of a systematic review by Keating et al. 31, in which the average of identified onchocerciasis‐specific cost estimates was US$0.46 per treatment, as well as with recent MDA cost benchmarks estimated by the WHO 32, 33. However, it is important to note that the costs of MDA delivery vary across different settings. An important driver in this variation is the size of the targeted population 34, 35, 36. This is because MDA programmes generate economies of scale; as the number of people treated increases, the cost per treatment tends to decrease 37, 38. NTD control programmes have also become increasingly integrated and instead of using separate disease‐specific programmes they now often target multiple diseases within one programme. This can result in economies of scope, reducing the overall cost of the NTD interventions 31, 37, 38, 39, 40, 41, 42, 43. Most of the studies identified did not consider the potential impact of economies of scale or scope on the costs of the interventions within their analyses.
There are very few studies that have investigated the costs of alternative onchocerciasis interventions to annual community‐directed MDA (with ivermectin) 32. Turner et al. 44 found that the yearly cost of a community‐directed ivermectin treatment programme increases by 50–60% when increasing the treatment frequency from once to twice a year.
Several studies also considered the potential for cost‐recovery/cost‐sharing, where the communities contribute financially to the cost of the programme 28, 45, 46, 47 and the participants’ willingness to pay for ivermectin treatment 48, 49.
Economic costs vs. financial costs
The majority of the studies considered only the financial costs of the intervention. However, when performing economic evaluations of healthcare interventions, it is typically recommended to use ‘economic costs’ 50, 51 (Box 2). These conceptualise costs in a broader way than do financial costs and represent the full value of all resources used for an intervention, including the value (opportunity cost) of donated resources. Economic costs are important as they reflect the sustainability and replicability of interventions.
Ndyomugyenyi et al. 34 found that when accounting for the salaries of governmental personnel and the opportunity cost incurred by the volunteer community‐directed distributors (CDDs – Box 2), community‐directed treatment with ivermectin in Uganda cost US$0.78 per treatment (2004 prices). However, if these costs were excluded, the cost fell to just US$0.17 per treatment (2004 prices). The difference between the financial and economic costs was smaller (US$0.39 vs. US$0.45 (2011 prices)) in a study in Ghana 44.
A key economic cost for many MDA programmes is the value of the unpaid contribution of the volunteer CDDs. Turner et al. 52 found that the average economic costs relating to the volunteer CDDs unpaid time can be significant, varying between US$0.05–0.16 per treatment (cost year variable). They estimated that the time donated by volunteer CDDs to APOC (1997–2015) would be valued between US$60–90 million 52.
Economic value of ivermectin
Some of the identified studies considered the economic value of the donated ivermectin within their cost‐effectiveness analysis. Most used the quoted commercial value from the Mectizan Donation Program, i.e. US$1.50 (and US$0.0018 in shipping costs) per tablet. Assuming that, on average, a treatment requires 2.8 tablets, this results in an estimated average economic value of donated ivermectin of US$4.21 per treatment 30. Kim et al. 53 assumed a different economic value of US$1.5054 per treatment. Over the past 30 years, Merck & Co. has donated over 2.7 billion treatments of ivermectin for onchocerciasis and lymphatic filariasis 16. Based on these different assumed valuations of ivermectin this donation would be valued between US$4–11 billion.
In the context of these economic evaluations, it should be noted that it is difficult to estimate the true economic value of a donated drug 54. This is because the manufacturing costs of drugs are proprietary information, and the donating company may potentially mitigate some of the cost through charitable tax write‐offs as well as intangible benefits, such as enhanced public image among shareholders and employees 55. It is also argued that if ivermectin were not donated, it could potentially be procured from other sources at less than the proprietary cost. For example, Hernando et al. 55 estimate the price of an annual 9 mg ivermectin treatment with generic drugs on the global market 56 to be approximately US$0.78. Based on this, they estimated the direct cost for the tablets donated during 2005–2011 to be US$600 million, compared with the stated value of US$3.8 billion 55, and with potential tax write‐offs their net cost could be around US$180 million 55.
These arguments need to be interpreted with caution. Firstly, there is the issue of drug quality, which is a major challenge in low‐income countries, and especially with anthelmintics and similar popular drugs which commonly attract counterfeit manufacturers. Secondly, the donations themselves have a chilling effect on the markets, driving generic prices downwards. There is in fact, no established way to estimate the ‘real’ market price of these drugs, and it is notable that even the lowest estimates of the value of the donations are very substantial, measured in hundreds of millions of dollars per year. What is clear is that the ivermectin donation was the first substantial donation of its type, and its success led to the concept of the NTDs as solvable diseases of poverty, and to the massive donation of some 1.5 billion treatments for a range of NTDs by some 14 other pharmaceutical companies during the London Declaration 57.
The foundation of onchocerciasis control programmes is based on the commitment of ivermectin donation from Merck & Co. for as long as needed 17, 57. Therefore, it is debatable when the value of ivermectin should be included within an economic evaluation.
Estimated economic benefits and cost‐benefit analyses of onchocerciasis interventions
We identified several cost‐benefit analyses of onchocerciasis interventions (Table 1). The estimated internal rate of return (IRR ‐ Box 2) ranged from between 11–20% for the OCP and 17–24% for APOC (for comparison, an IRR above 10% is considered by the World Bank as the standard for a successful public health programme 58) (Table 1). The estimated net present values (NPV) were between US$8 million to US$3.7 billion for OCP and US$54–307 million for APOC (cost years variable). However, there was variation between the different studies regarding the time frame considered and the discount rate used (Box 3), making it difficult to compare the results directly. None of the cost‐benefit analyses identified included the economic value of the donated ivermectin tablets in their base case analysis (Table 1). Waters et al. 18 highlighted that this value could outweigh the estimated economic benefits from both the OCP and APOC programmes.
More recently, Redekop et al. 59 and Kim et al. 60 quantified the economic benefits associated with onchocerciasis elimination. Both those studies found that eliminating onchocerciasis would generate billions in economic benefits (Tables 1 and 3).
Table 3.
Summary of the estimated economic benefits of onchocerciasis interventions relating to productivity gains and land gains
| Source | Setting and time period of the intervention | Time horizon for the benefits | Cost year | Value of the productivity gainsb | Value of land gains |
|---|---|---|---|---|---|
| Benton & Skinner 67 | OCP (1974–2004) | 1974–2023 | 1985 US$ |
|
|
| Kim & Benton 58 | OCP (1974–2002) | 1974–2012 | 1987 US$ |
|
|
| McFarland & Murray 124, a | OCP (10‐year project time period) | 1974–2023 | Not available |
|
|
| Benton 125 | APOC (1996–2007) | 1996–2017 | 1996 US$ |
|
‐ |
| Haddix 69, a | APOC (1996–2007) | 1996–2017 | 1996 US$ |
|
|
| Kim et al. 60, d , e | Potential benefits of achieving elimination scenarios in Africa | 2013–2045 | 2013 US$ |
|
‐ |
| Redekop et al. 59 | Potential economic benefits of achieving the WHO 2020 targets | 2011–2030 | 2005 US$ |
|
‐ |
APOC, African Programme for Onchocerciasis Control; GDP, gross domestic product; OCP, Onchocerciasis Control Programme in West Africa.
Information for this study was taken from Waters et al. 18.
Further detail regarding how the productivity gains were calculated are provided in Table 5.
Assumption varied in the sensitivity analysis.
Also quantified the savings to the health systems and households (out‐of‐pocket payments) resulting from decreased usage of outpatient health services (Elim I: US$60.6 (30–80.7) million, Elim II: US$64.6 (31.8–86.4) million ‐ compared with the control scenario).
The Control, Elim I and Elim II scenarios are described in Kim et al. 99.
The identified studies quantified three different types of economic benefits of onchocerciasis interventions (namely, productivity gains, land use gains and reductions in outpatient services and out‐of‐pocket expenditures), but there was variation across the studies regarding how and which economic benefits were quantified (Table 3). Many of the broader benefits of onchocerciasis in terms of the Millennium Development Goals are highlighted by Dunn et al. 61.
Productivity gains
Onchocerciasis‐associated morbidity can affect an individual's economic productivity. Lenk et al. 62 recently conducted a systematic review of the productivity losses related to the NTDs eligible for preventive chemotherapy and a summary of the studies they identified for onchocerciasis is presented in Table 4. Unsurprisingly, the degree of the productivity loss was dependent on the type of onchocerciasis morbidity. Productivity losses were highest for blindness and lowest for skin disease.
Table 4.
Description of studies investigating the productivity losses associated with onchocerciasis‐associated morbidity (adapted from 62)
| Study | Country | Year | Study design | Population | Sample size | Sequela | Definition of productivity loss | Results |
|---|---|---|---|---|---|---|---|---|
| Evans 128 | Guinea | 1995 | Observational (survey) | Household members in a highly endemic area. | 319 | a) Visual impairment | Self‐reported ‘inactive’ occupational status. | a) 38% |
| b) Blindness | b) 79% | |||||||
| Kim et al. 129 | Ethiopia | 1997 | Case‐control | Coffee plantation workers. | 235 | a) OSD (intermediate) | a) Daily wages (individuals infected with OSD (intermediate) vs. those without). | a) 10% |
| b) OSD (severe) | b) Daily wages (individuals infected with OSD (severe) vs. those without). | b) 15% | ||||||
| Okeibunor et al. 130 | Cameroon, DRC, Nigeria, Uganda | 2011 | Observational (cross‐sectional) | Primarily residents from villages where ivermectin distribution was ongoing. | 1600 | General onchocerciasis | a) Increase in productivity from ivermectin treatment. | a) 76% |
| b) Percentage of respondents that referred ability to work better after ivermectin treatment. | b) 75.6% | |||||||
| Oladepo et al. 131 | Nigeria | 1993 | Case‐control | Male farmers. | 102 | OSD | Farm size that a man can keep satisfactorily weeded (workers with vs. without OSD). | 9117 vs. 13 850 m2 (34% loss) |
| Thomson 132 | Cameroon | 1971 | Case‐control | Estate workers in an onchocerciasis endemic area. | 420 | Unspecified (general) | Working days (workers with vs. without onchocerciasis). | 20% |
| Wogu & Okaka 133 | Nigeria | 2008 | Observational (survey) | Rural farming community in a mesoendemic area. | 200 | a) OSD (itching) | a) Percentage of respondents that reported a reduction in strength and concentration at work. | a) 13.5% |
| b) OSD (nodules) | b) Percentage of respondents that reported a decline in sales in business/trading. | b) 11% | ||||||
| c) Visual impairment (ocular lesions) | c) Percentage of respondents that reported giving up jobs (Productivity loss not specified). | c) 14% | ||||||
| Workneh et al. 134 | Ethiopia | 1993 | Case‐control | Male permanent coffee plantation workers. | 196 | OSD | Absenteeism/sick leave and net monthly pay (workers with vs. without OSD). | 25% |
| WHO & World Bank 70 | Nigeria, Ethiopia, Sudan | 1997 | Case‐control | Households in hyperendemic communities. | 824 | OSD | Time spent on productive activities (individuals with vs. without OSD signs and symptoms). | Not significant |
DRC, Democratic Republic of the Congo; OSD, onchocerciasis‐associated skin disease.
A key component of estimating the economic benefits of onchocerciasis interventions is the productivity gains that result from preventing onchocerciasis morbidity. The cost‐benefit analyses identified appeared to estimate only the productivity gains associated with preventing onchocerciasis‐associated blindness. However, the productivity losses associated with onchocerciasis‐associated skin disease and visual impairment are also significant (Tables 4 and 5). Consequently, only quantifying the productivity gains associated with prevented cases of blindness underestimates the economic benefits of onchocerciasis interventions 63.
Table 5.
Summary of the assumed productivity losses and disability weights used for onchocerciasis‐associated morbidity
| Study | Low vision | Blindness | Skin disease/troublesome itch | Source |
|---|---|---|---|---|
| Assumed productivity loss | ||||
| Benton & Skinner 67 | ‐ | 100% | ‐ | |
| Kim & Benton 58 | ‐ | 100% | ‐ | |
| McFarland & Murray 124 | Not available | Not available | Not available | |
| Benton 125 | ‐ | Unclear | ‐ | |
| Haddix 69 | ‐ | Not available | ‐ | |
| Kim et al. 60 | Patient: 38% Caregiver: 5% | Patient: 79% Caregiver: 10% | Severe itching: 19% | 128, 129, 134, 135, 136, 137 |
| Redekop et al. 59 | 38% | 79% | Moderate: 10% Mild: 0% | 128, 129 |
| Healthy life year weightsa | ||||
| Prescott et al. 126, 127 | ‐ | 1.0 | ‐ | |
| Evans et al. 71 | ‐ | 0.5 | ‐ | |
| Benton 125 | ‐ | 1.0 | ‐ | |
| DALY weightsa | ||||
| GBD 1990 | 0.245 | 0.488 (treated) 0.600 (untreated) | 0.068 | GBD 1990 138 |
| GBD 2000 138 | 0.224 (treated) 0.282 (untreated) | 0.60 | 0.068 | GBD 2000 138 |
| McFarland & Murray 124 | Not available | Not available | Not available | |
| Turner et al. 73 | 0.170 | 0.594 | 0.068 | GBD 2004 139 |
| Coffeng et al. 30 | 0.282 | 0.594 | 0.068 | GBD 2004 139 |
| Coffeng et al. 78 | 0.033 | 0.195 | 0.108b | GBD 2010 140 |
| de Vlas et al. 141 | 0.101c | 0.101c | 0.079b | GBD 2010 140 |
DALYs, disability‐adjusted life years; GBD, Global Burden of Disease Study.
Reflect the severity of the disease sequelae with 0 representing perfect health and 1 representing death.
Used a weight representing an overall average for skin disease across more finely disaggregated strata/severity levels.
Used a weight representing an overall average for visual morbidity (i.e. the weight was not stratified by ‘low vision’ and ‘blindness’).
Where relevant additional studies that were not performing economic evaluations were included for comparison.
In contrast, both Kim et al. 60 and Redekop et al. 59 included the productivity gains associated with preventing onchocerciasis‐associated skin disease when quantifying the economic benefits of onchocerciasis interventions. Kim et al. 60 assumed that severe itching was associated with a 19% productivity loss and this made up 65% of their projected income/productivity gains. In Redekop et al.'s 59 study, the benefits associated with preventing skin disease represented 22% of the total projected economic benefit. This assumed that ‘moderate’ skin disease is associated with a 10% productivity loss and ‘mild’ skin disease is associated with no productivity loss (Table 5).
For these types of estimates, it is important to consider which method is used to value the productivity gains. Estimating the income of individuals affected by NTDs is challenging, as many of them are in informal employment (such as subsistence farmers). The analyses identified used a variety of different methods and sources to approximate the typical income of someone with onchocerciasis‐associated morbidity (such as the per capita gross domestic product (GDP), subsistence wage, the GDP per capita of the lowest income quintile). In some cases, the income source was not clearly stated. It is important to note that different income sources can give very different estimates of an individual's typical income, even when they relate to the same type of profession/socioeconomic status 40.
Kim et al. 60 also considered the potential income losses of the informal caregivers of patients with low vision and blindness (Table 5). Currently, there are very few primary data quantifying this. Ibe et al. 64 found that within their survey of onchocerciasis patients in Nigeria, the average productivity cost among the informal caregivers was US$3.50 per month (cost year unavailable).
In all studies productivity gains were estimated using the human capital approach, which takes the patient's perspective for valuing lost productivity. It should be noted that if the friction cost approach was used (which takes the employer's perspective, i.e. only counts as lost, the hours not worked before another employee takes over the patient's work 65), the estimated productivity gains would have been lower 66. There is continued debate within the field regarding which approach is most appropriate 65. In the context of studies for NTDs, it should be highlighted that the friction cost approach is hard to apply to populations that are predominately in informal employment. Kim et al. 60 and Kim & Benton 58 made adjustments for employment rates/labour force participation within their estimates.
Land use gains
Onchocerciasis caused many people to abandon the fertile river valleys within the countries covered by the OCP 2. It has been estimated that as a result of OCP interventions, 25 million hectares of abandoned arable land was able to be reclaimed for settlement and cultivation 58, 67 (capable of feeding 17 million people annually 67, 68). Several of the cost‐benefit analyses of the OCP included the projected economic benefits resulting from the increased agricultural output from the reclaimed land. These estimates varied between US$57–205 million (cost years variable) and were sensitive to the assumed time horizon and the discount rate (Table 3). The estimate from Kim & Benton 58 was even higher but the specific value was not stated (the NPVs (1974–2012) relating only to land use gains ranged between US$3154 million (using a 3% discount rate) and US$380 million (using a 10% discount rate)). It should be highlighted that such calculations are based on various assumptions surrounding rates of agricultural land use and repopulation of the reclaimed areas (Table 3). It is also important to consider that onchocerciasis may not have been the sole cause of depopulation of these areas 9. Because the same level of abandonment of river valleys was rarely seen in non‐OCP countries 63, the analyses of the countries covered by APOC generally did not include the economic benefits related to land‐use gains. One exception is the report by Haddix 69, which is reported to have re‐evaluated APOC interventions including the estimated economic benefit resulting from increased agricultural output (Table 3).
Outpatient services and out‐of‐pocket expenditure
Individuals with onchocerciasis‐associated morbidity may seek care from their local health services. Kim et al. 60 projected the economic benefits of onchocerciasis elimination resulting from the decreased use of outpatient health services. They estimated that between 2013 and 2045, when compared with a control scenario, the elimination of onchocerciasis in Africa would save the health systems US$35.9–38.6 million in outpatient service costs, and save the patients/households US$24.7–26.0 million in out‐of‐pocket payments (2013 prices) 60. Primary data on these costs or on how often individuals with onchocerciasis seek outpatient care or use local health services are scarce. An exception is the study by Ibe et al. 64, which found that the average direct cost incurred by onchocerciasis patient's per outpatient visit was US$14.00 (cost year unavailable), with the majority of this being for medications.
Interestingly, a study conducted by the World Bank and WHO reported that on average, people suffering from the manifestations of onchocerciasis‐associated skin disease were found to spend an additional US$8.10 (cost year unavailable) on health‐related expenditures over a six‐month period compared with those from the same community without these manifestations 70.
The cost‐effectiveness analyses of onchocerciasis interventions
We identified seven studies performing cost‐effectiveness analyses of onchocerciasis interventions. The majority of the studies evaluated annual MDA (Table 2). As with the cost‐benefit analyses, variation in the time frames and discount rates used (Box 3) make it difficult to compare directly some of the results.
Many older studies have used ‘healthy life years averted’ as their effectiveness metric (which was based on reductions in the number of onchocerciasis‐related blindness cases). These studies generally assumed that blindness results in complete disability, and that each year lived with blindness is equal to one full healthy life year lost, i.e. they assumed a disability weight of 1, which is equivalent to death (Table 5). One exception to this was Evans et al. 71, who used a lower disability weight for blindness of 0.5 (based on empirical evidence that blindness does not result in complete disability and that blind people are active both socially and economically). These studies did not appear to quantify the averted burden of other types of onchocerciasis morbidity (such as skin disease) (Table 5).
More recent studies have used disability‐adjusted life years (DALYs) averted, a standardised and more comprehensive effectiveness metric. When excluding the economic value of the donated ivermectin, the estimated cost per DALY averted of annual MDA varies between US$3 and US$30 (cost year variable). In comparison the cost per DALY averted for the MDA delivered within the Global Programme to Eliminate Lymphatic Filariasis was estimated to be US$24 when using financial costs and US$64 when using economic costs including the value of the donated drugs (2014 prices) 54. The cost‐effectiveness of onchocerciasis related MDA is also very favourable compared to other interventions conducted in low‐ and middle‐income countries (a comprehensive list of cost‐effectiveness estimates for a range of health interventions in these settings is provided within Horton et al. 72).
The most favourable cost‐effectiveness estimates relate to interventions in savannah settings 73. It is important to note that these estimates are not directly generalisable to onchocerciasis interventions in forest areas, where ocular pathology and morbidity is considered to be rarer (Figure 2) (but see 74). According to this assumption, intervention cost‐effectiveness in the latter areas would be lower. It should be noted that this assumption is based on limited data from forest settings (Figure 2).
Figure 2.
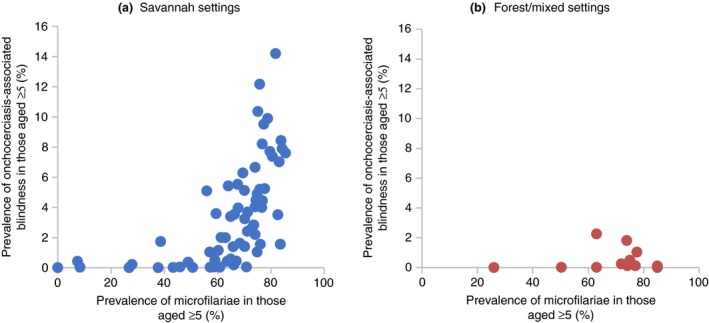
The relationship between the prevalence of onchocerciasis‐associated blindness and the prevalence of skin microfilariae in savannah (a) and forest/mixed forest‐savannah settings (b). The figures were adapted from Figures S3 and S4 in Coffeng et al. 30. The data were originally taken from 142, 143, 144, 145, 146, 147, 148. [Colour figure can be viewed at wileyonlinelibrary.com]
The estimated cost‐effectiveness ratio was also dependent on the assumed pre‐control endemicity level, with the cost per DALY averted being lower in higher endemicity settings. In the long term, this range is narrower than might be expected as, although fewer DALYs are averted in lower endemicity settings, the total cost of the intervention is also lower (as fewer treatment rounds will be needed to achieve a similar degree of control or to reach elimination) (Table 2).
The majority of studies have taken the healthcare providers perspective, which does not consider the costs falling on those outside of the healthcare sector. All the cost‐effectiveness analyses appeared to consider only the cost of the intervention, i.e. none of them included potential savings to outpatient services, prevented out‐of‐pocket costs, or productivity gains that result from prevented morbidity. Only a few identified studies considered the economic value of the donated ivermectin. Including this significantly increases interventions estimated cost and, therefore, decreases the estimated cost‐effectiveness. For example, Turner et al. 54 found that the estimated cost per DALY averted increased from US$3–15 to US$29–133 (2012 prices) when including the economic value of donated ivermectin (which would still be classed as cost effective). It is also debatable when the value of ivermectin should be included within an economic evaluation, particularly under the healthcare providers perspective.
Numerous approaches have been used to quantify the effectiveness of onchocerciasis interventions. Many older studies based the effectiveness on reductions in the incidence of blindness on limited empirical data and projected similar putative reductions with continuing intervention. More recent studies have used mathematical transmission models to project the ongoing future effectiveness of interventions more accurately, explicitly modelling disease dynamics through time 75. The two main models used for this purpose (EPIONCHO and ONCHOSIM) are described in Basáñez et al. 76. An important advantage of this approach is the capacity to account for the indirect benefits/herd effects of interventions (the indirect benefit afforded to individuals not directly targeted by an intervention that arises from the population‐wide reduction in transmission) 75. These models can also account for longer time horizons, accounting for the continued benefits of interventions even after the control programme has stopped.
DALY calculations
DALYs are calculated as the sum of two components; the years of healthy life lost due to disability, and the years of life lost due to premature mortality 77. In a DALY calculation, the years of healthy life lost due to disability are calculated using standardised disability weights, ranging between 0 and 1. This reflects the severity of the different disease sequelae, with 0 representing perfect health and 1 representing death. The disability weights used for onchocerciasis DALY calculations have changed over time (Table 5).
For the 2010 GBD study, the disability weights for vision loss were notably decreased compared to previous studies, whereas the weights for skin disease were increased (Table 5). Consequently, Coffeng et al. 78 found that when using the newer weights, the estimated number of DALYs averted by APOC (2000–2015) increased by 9% compared to when using the GBD 2004 weights. Moreover, skin disease, instead of eye disease, became the most important contributor to the burden of onchocerciasis. Since the GBD 2010 study, onchocerciasis‐associated skin disease has not been assigned a specific single disability weight, and more general disfigurement health states (stratified by three severity levels and whether or not the disfigurement is associated with itch or pain) are used. When using these updated GBD disability weights, studies have typically estimated an overall average skin disease weight (Table 5). Subsequent GBD studies have made additional changes to the DALY calculation for onchocerciasis, particularly relating to the weights attributed to the different types of skin disease and the types of skin disease included 79. A more detailed overview of the changes to the GBD study methodology used to calculate DALYs is presented in 80, 81, 82.
Several studies have accounted for the excess mortality associated with onchocerciasis visual morbidity 3 within their DALY calculations. However, it has generally not been considered that irrespective of visual morbidity, there is an increased risk of mortality associated with increasing microfilarial load, particularly in children and young adults aged below 20 years 4, 5. Estimates of onchocerciasis burden (and the cost‐effectiveness of its control) would generally increase if this onchocerciasis‐associated excess mortality were taken into account. For example, Turner et al. 83 found that, depending on the pre‐control endemicity level, excess mortality accounted for 29–43% of the estimated pre‐control DALY burden of onchocerciasis. If this had not been included, the estimated cost‐effectiveness would have been reduced substantively.
Cost‐effectiveness thresholds
In cost‐effectiveness analyses, the cost per DALY averted is compared to a willingness to pay threshold to determine whether an intervention is cost effective. However, the most appropriate cost‐effectiveness thresholds are debated and remain somewhat arbitrary 84, 85, 86. The cost‐effectiveness threshold set by the WHO‐CHOICE 87 (a cost per DALY averted < 3 times the country's GDP per capita) is now considered to be too high 84, 85, 86, 88, 89. Most analyses within the NTD field have not used it 40, 41, 90, many opting instead for the more conservative cost‐effectiveness threshold set by the World Bank 91 (≤ US$251 per DALY averted, when adjusted for inflation to 2016 prices 92). Interestingly, recent analyses have indicated that a cost‐effectiveness threshold closer to < ½ the country's per capita GDP would be more appropriate for low‐income countries 88, 93. For comparison, the Disease Control Priorities project (Third Edition) used a threshold of US$200 per DALY averted to identify priority interventions for consideration in low‐income countries 94. Despite this ambiguity on cost‐effectiveness thresholds, onchocerciasis interventions would remain classed as cost effective by any of the proposed measures.
Elimination and evaluation of alternative interventions
Traditional cost‐effectiveness analyses of new interventions evaluate their incremental effectiveness and incremental cost compared to the current practice (calculating incremental cost‐effectiveness ratios, Box 2). This framework has been widely and successfully used to evaluate the comparative cost‐effectiveness of new intervention strategies for disease control. However, this incremental cost‐effectiveness framework is less informative and somewhat ill‐suited for disease elimination or eradication programmes. For example, Turner et al. 73 found that that increasing the treatment frequency of ivermectin distribution from once to twice per year yielded very small incremental health gains (only a 3–4% increase in the number of DALYs averted) but could have a large influence on a programme's overall total cost and duration (Table 2). In such cases, where an intervention is aimed at accelerating and sustaining elimination, an incremental cost‐effectiveness ratio may not reflect its true value. Instead, as applied in Turner et al., the absolute cost of the intervention and the time it takes to achieve the desired elimination goal can be more informative. The same framework was applied to an economic evaluation of moxidectin 95 (Table 1), a newly registered treatment for onchocerciasis (https://www.medicinesdevelopment.com/news-180613.htm). Kastner et al. 96 have also highlighted that the number of DALYs averted may not fully capture the long‐term consequences and broader benefits of disease eradication programmes.
Another important aspect to consider when evaluating elimination programmes is the time horizon of the analysis 40. This is because the costs of elimination programmes are typically higher than would be needed for disease control 97. After elimination is certified, the estimated cost‐effectiveness of an elimination programme will steadily increase as the discounted benefits continue to accumulate but the costs have stopped (with the potential exception of ongoing surveillance). The benefits and potential future cost savings resulting from achieving elimination/eradication are not infinite 40, 73, as the costs/cost savings being considered must be restricted within a suitable time horizon and are typically discounted into the future (Box 3). In contrast, for disease control programmes, the intervention costs will typically be incurred for the full‐ time horizon. Because of this, elimination programmes can be cost‐saving in the long term. However, it can take time for the longer‐term benefits and cost savings associated with achieving elimination to outweigh the initial increases in costs associated with achieving elimination. Consequently, with short‐term time horizons, elimination programmes are unlikely to be more cost effective than disease control programmes.
Eradication investment cases have been developed for a number of NTDs 98, including onchocerciasis 53, 60, 99. These latter studies have compared onchocerciasis control and elimination/eradication scenarios and have quantified the duration of the programme, its financial and economic cost, the number of ivermectin treatments required, the workload of the community healthcare workers/volunteers, costs related to outpatient healthcare services, and the productivity gains resulting from preventing onchocerciasis‐associated morbidity. The overall conclusions of these studies are that eradication and elimination of onchocerciasis are both justifiable on both cost‐effectiveness and benefit‐cost analysis grounds, and that eradication tends to be more favoured over the long‐term time horizon.
Sensitivity analysis
The majority of identified studies either did not perform a sensitivity analysis or undertook only a univariate analysis, changing one parameter at a time to evaluate its impact independent of other parameters. The main exceptions to this were Redekop et al. 59 and Kim et al. 60.
Redekop et al. 59 performed probabilistic sensitivity analysis (PSA) in which the values of three input parameters (the estimates of disease prevalence in 2010, the percentage of productivity loss/the amount of out‐of‐pocket payments, and the patient's income) were allowed to vary simultaneously. Kim et al. 60 first conducted a one‐way deterministic sensitivity analysis to examine which parameters are key drivers. They then conducted PSA to assess the robustness of the results to the joint uncertainties around all selected parameters 60.
Limitations of this analysis
A potential source of bias of the search strategy is that it did not capture economic evaluations published outside of the searched electronic databases (i.e. grey literature such as policy documents/reports, and many non‐English language publications etc.). Efforts were made to minimise this bias by searching the bibliographies of selected studies. There could also be a degree of publication bias, with economic evaluations with negative or unfavourable results less likely to be published. It should be noted that the selection of studies was not performed independently by two researchers.
Areas of further research
The results of the systematic review highlight that the standard onchocerciasis control strategies are consistently found to be very cost effective. However, there are some important research gaps that need further research.
Data on intervention costs and the economic burden of onchocerciasis
The costs of MDA delivery have been shown to vary across different settings 31, 32, 37, 41, 100. This variation potentially affects the generalisability of any cost‐effectiveness/cost‐benefit analysis 75, and future studies need to quantify the impact of this in greater detail. It should be noted that the costs of conducting MDA in areas where onchocerciasis and loiasis are co‐endemic may be higher – due to the need for enhanced surveillance and community sensitisation.
In addition, further studies are needed to investigate how integrating NTD control programmes 31, 39 may influence the costs/cost‐effectiveness of implementing different control strategies 37, 40. There is notable variation in the methodological approaches used to quantify the economic costs incurred by CDDs. It would be beneficial if future studies adopted more consistent approaches (outlined in 52).
The cost of onchocerciasis control programmes will likely increase significantly as they approach the ‘last mile’ towards elimination 37. This is partly because of the increase in the costs resulting from expanding the programmes to target harder‐to‐reach areas/groups (diseconomies of scale) 37. This is an important issue for NTD programmes in general, and further costing studies are needed to quantify it.
Further studies are needed on quantifying the medical costs incurred by those with onchocerciasis seeking treatment. Such studies could yield more robust estimates of the economic benefits of onchocerciasis control 60. It would also be beneficial if future studies sought to quantify the productivity losses incurred by informal caregivers, not just those caring for the blind. Note also that with the rise in sophistication of health systems in the endemic countries, the health system costs and the contribution of out‐of‐pocket expenses are both likely to rise 101.
Our analysis also revealed that studies used cost data collected in different years, but it was not always clear if and how costs were adjusted for inflation. Future studies should report this more explicitly 102 and within a given study the costs would be standardised to a consistent year.
Economic evaluations of alternative interventions
In certain epidemiological and programmatic circumstances, alternative strategies to annual MDA will be required to achieve the current goals for onchocerciasis control/elimination 21, 22, 103. Such strategies include increased frequency of MDA (up to four times per year), localised low‐cost vector control and treatment strategies using moxidectin, anti‐Wolbachia therapies and new macrofilaricidal drugs 104, 105, 106. However, there currently are very few costing studies and economic evaluations relating to these alternative interventions 32, 107. Studies are needed to evaluate the cost and cost‐effectiveness of such strategies. When such studies are performed, it will be vital that the generalisability of the estimated cost across different programmatic settings is considered 37. It will also be important to consider the value of these alternative interventions not only in reducing the disease burden where they are implemented, but also in their capacity to help eliminate onchocerciasis more quickly. This will be particularly important in reducing the risk that ‘hot spots’ of sustained transmission seed and re‐establish transmission in areas where onchocerciasis has been eliminated.
This area of research is particularly important for interventions targeting onchocerciasis in Loa loa co‐endemic areas. It has been recently predicted that, at the 2025 horizon, two‐thirds of onchocerciasis remaining cases will be living in hypoendemic areas for onchocerciasis where the risk of Loa‐related post‐ivermectin severe adverse events (SAEs) has so far been considered to outweigh the benefits for the communities. The paradigm shift from control to elimination implies that hypoendemic areas start receiving community treatment with ivermectin. A test‐and‐not‐treat strategy for onchocerciasis has recently been successfully piloted in a health area of Central Cameroon where community‐directed treatment with ivermectin had to be permanently halted after a series of SAEs occurred in 1999 108. Costing studies of this approach are ongoing. Critical questions when addressing the cost‐effectiveness of this intervention include the counterfactual – what it would cost to leave all those populations untreated? – and cost‐effectiveness relative to other locally important health issues.
Future economic analyses of alternative interventions need to be tailored to the key policy questions from the different decision makers and stakeholders. These different questions may require different methodological approaches and perspectives for the analyses. This further emphasises the need for transparent reporting of methodology in economic evaluations.
Quantifying the health benefits of interventions
Many older studies only quantified the health and economic benefits resulting from the number of blindness cases averted. More advanced disease models have been developed that account for averted visual impairment, skin disease, and excess human mortality. However, further refinements are needed to better capture the relationship between infection and skin disease 109, and to account for related neurological disorders such as epilepsy and nodding syndrome 6, 7, 8.
When evaluating an intervention aimed at reducing morbidity, the number of DALYs averted is often the best effectiveness metric, as it allows cost‐effectiveness estimates to be directly compared to estimates relating to other interventions/diseases as well as to standardised cost‐effectiveness thresholds. This makes the results of economic evaluations easier to interpret by policymakers. However, DALYs do have limitations and there are controversies surrounding their calculation 110. For example:
The universal disability weights do not account for how the local context may influence the burden of a disease. Consequently, the potential that the burden of a disease or its sequelae may be worse for those that are living in poverty is not accounted for. It has been argued that this aspect of DALY calculations may significantly underestimate the burden of poverty‐related diseases 110, 111.
The DALY disability weights do not fully account for the psycho‐social implications of a disease or its sequelae 112 and its overall impact on quality of life. They also do not explicitly account for the impact of the disease on patients’ informal caregivers. In particular, the updated disability weight for blindness (decreasing from 0.60 to 0.19) has been controversial within the field 113, 114. A possible reason for this significant change is that within the updated GBD framework (post‐GBD 2010), the disability weights are intended to be solely measures of losses of ‘optimal health’ and are not intended to represent losses of well‐being/welfare 80, 115.
Interestingly, the same age group (those aged below 20 years) for which there is a statistically significant higher risk of mortality for a given microfilarial load in comparison to those aged 20 years and older 5, is the group with the onset and higher incidence of nodding syndrome, a type of epilepsy that is increasingly recognised as associated with onchocerciasis 116, 117. Preliminary studies of the disease burden of onchocerciasis‐associated epilepsy have been conducted 118. These studies should be followed by economic evaluations of both onchocerciasis‐associated epilepsy and the impact of onchocerciasis interventions.
Joint/auxiliary benefits
Ivermectin is a broad‐spectrum antiparasitic drug that also has an impact on other co‐endemic parasitic infections (such as soil‐transmitted helminthiases, lymphatic filariasis, loiasis and scabies). Krotneva et al. 119 assessed the auxiliary benefits of APOC and estimated that between 1995 and 2010, ivermectin mass treatment (in APOC regions) cumulatively averted approximately 500 000 DALYs from co‐endemic soil‐transmitted helminth infections, lymphatic filariasis, and scabies. This highlights that the overall cost‐effectiveness of onchocerciasis interventions may be even higher than previously reported. Further quantification of these auxiliary benefits would be useful to improve estimations of the impact of onchocerciasis interventions.
Modelling and elimination thresholds
Dynamic transmission models have an important role, particularly for the evaluation of novel interventions and how they compare to the standard strategy of MDA with ivermectin. These models (reviewed in Basáñez et al. 76) have undergone extensive refinement in recent years to better capture parasite population dynamics during interventions 120 and to produce more robust projections on the likelihood of elimination. These modelling efforts will be particularly useful in identifying epidemiological and programmatic circumstances in which alternative strategies will be required to reach elimination (for example in highly endemic settings, settings with suboptimal responses to ivermectin 121, 122, or where the initiation of programmes has previously been delayed 103). Moreover, transmission models (as opposed to so‐called ‘static’ models) capture explicitly our current understanding of the changing parasite dynamics during interventions 75.
Conclusions
The cost benefit and cost effectiveness of onchocerciasis interventions have consistently been found to be very favourable. This finding provides strong evidential support for the ongoing efforts to eliminate onchocerciasis from endemic areas.
MDA against other NTDs has also been found to be cost effective 40, 54, 90 and, therefore, a logical next step would be to quantify the net cost‐effectiveness of more closely integrating these programmes, which are already often running side‐by‐side in co‐endemic areas. Indeed, the primary rationale for the aggregation of common infectious diseases of the poor under the denomination of ‘Neglected Tropical Diseases’ was the perception that treating many diseases using a single common delivery system would be inherently cost effective. It would be important to future policy making to explore the evidence base for that perception. Further systematic reviews of this type on other NTDs would also be useful.
We identify three main research gaps in this area. First, the need to be more inclusive in quantifying burden. Originally, studies only quantified the benefits of preventing blindness, and then also captured onchocerciasis‐associated skin disease. An improved understanding of other factors, such as onchocerciasis‐associated epilepsy 117, 118 would enhance the precision of the calculated benefits. Second, the evaluation of interventions targeting Loa loa co‐endemic areas which will become more important in the end‐game for elimination. Finally, the need to increase the comparability of economic analyses. Greater adherence to standardised guidelines for reporting the results of economic evaluations (such as CHEERS for cost‐effectiveness analysis 123) would be beneficial and increase the reliability and reproducibility of reported findings.
Programmes to eliminate onchocerciasis have always been in the vanguard of global efforts to eliminate diseases of poverty. Lessons learned here from the economic analysis of onchocerciasis programmes have direct relevance to the design of programmes addressing all the other NTDs.
Supporting information
Appendix S1. PRISMA checklist.
Acknowledgements
HCT is supported by the Wellcome Trust [089276/B/09/7]. MW's and MGB's research on NTDs is supported through grants from the Bill & Melinda Gates Foundation via the NTD Modelling Consortium, Wellcome and the European & Developing Countries Clinical Trials Partnership (EDCTP). MGB acknowledges joint Centre funding from the UK Medical Research Council and the Department for International Development (grant no. MR/R015600/1).
References
- 1. Duke BO. Human onchocerciasis–an overview of the disease. Acta Leiden 1990: 59: 9–24. [PubMed] [Google Scholar]
- 2. World Health Organization . Signs, symptoms and treatment of onchocerciasis. (Available from: http://www.who.int/onchocerciasis/symptoms/en/).
- 3. Kirkwood B, Smith P, Marshall T, Prost A. Relationships between mortality, visual acuity and microfilarial load in the area of the Onchocerciasis Control Programme. Trans R Soc Trop Med Hyg 1983: 77: 862–868. [DOI] [PubMed] [Google Scholar]
- 4. Little MP, Breitling LP, Basáñez MG, Alley ES, Boatin BA. Association between microfilarial load and excess mortality in onchocerciasis: an epidemiological study. Lancet 2004: 363: 1514–1521. [DOI] [PubMed] [Google Scholar]
- 5. Walker M, Little MP, Wagner KS, Soumbey‐Alley EW, Boatin BA, Basáñez MG. Density‐dependent mortality of the human host in onchocerciasis: relationships between microfilarial load and excess mortality. PLoS Negl Trop Dis 2012: 6: e1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Colebunders R, Hendy A, van Oijen M. Nodding Syndrome in Onchocerciasis Endemic Areas. Trends Parasitol 2016: 32: 581–583. [DOI] [PubMed] [Google Scholar]
- 7. Kaiser C, Pion SDS, Boussinesq M. Case‐control studies on the relationship between onchocerciasis and epilepsy: systematic review and meta‐analysis. PLoS Negl Trop Dis 2013: 7: e2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Colebunders R, Hendy A, Mokili JL et al Nodding syndrome and epilepsy in onchocerciasis endemic regions: comparing preliminary observations from South Sudan and the Democratic Republic of the Congo with data from Uganda. BMC Res Notes 2016: 9: 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Basáñez M‐G, Pion SDS, Churcher TS, Breitling LP, Little MP, Boussinesq M. River blindness: a success story under threat? PLoS Med 2006: 3: e371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boatin B. The Onchocerciasis Control Programme in West Africa (OCP). Ann Trop Med Parasitol 2008: 102(Suppl 1): 13–17. [DOI] [PubMed] [Google Scholar]
- 11. Richards FO Jr, Boatin B, Sauerbrey M, Seketeli A. Control of onchocerciasis today: status and challenges. Trends Parasitol 2001: 17: 558–563. [DOI] [PubMed] [Google Scholar]
- 12. Sauerbrey M. The Onchocerciasis Elimination Program for the Americas (OEPA). Ann Trop Med Parasitol 2008: 102(Suppl 1): 25–29. [DOI] [PubMed] [Google Scholar]
- 13. Roungou J‐B, Yameogo L, Mwikisa C, Boakye DA, Bundy DAP. 40 years of the APOC partnership. PLoS Negl Trop Dis 2015: 9: e0003562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lawrence J, Sodahlon YK. Onchocerciasis: the beginning of the end. Int Health 2018: 10(Suppl_1): i1–i2. [DOI] [PubMed] [Google Scholar]
- 15. Colatrella B. The Mectizan Donation Program: 20 years of successful collaboration ‐ a retrospective. Ann Trop Med Parasitol 2008: 102(Suppl 1): 7–11. [DOI] [PubMed] [Google Scholar]
- 16. Gustavsen KM, Colatrella BD, McCoy T. For as long as necessary: examining 30 years of msd's focus on achieving elimination of onchocerciasis and lymphatic filariasis. Int Health 2018: 10(Suppl_1): i3–i6. [DOI] [PubMed] [Google Scholar]
- 17. World Health Organization . Contribution of pharmaceutical companies to the control of neglected tropical diseases. (Available from: http://www.who.int/neglected_diseases/pharma_contribution/en/).
- 18. Waters HR, Rehwinkel JA, Burnham G. Economic evaluation of Mectizan distribution. Trop Med Int Health 2004: 9: A16–A25. [DOI] [PubMed] [Google Scholar]
- 19. Diawara L, Traoré MO, Badji A et al Feasibility of onchocerciasis elimination with ivermectin treatment in endemic foci in Africa: first evidence from studies in Mali and Senegal. PLoS Negl Trop Dis 2009: 3: e497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Traore MO, Sarr MD, Badji A et al Proof‐of‐principle of onchocerciasis elimination with ivermectin treatment in endemic foci in Africa: final results of a study in Mali and Senegal. PLoS Negl Trop Dis 2012: 6: e1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. African Programme for Onchocerciasis Control (APOC) . Final Communiqué, Eighteenth Session of the Joint Action Forum 11‐13 December 2012. 2012. (Available from: http://www.who.int/apoc/publications/recommendations/en/index.html).
- 22. World Health Organization . Accelerating work to overcome the global impact of neglected tropical diseases– A roadmap for implementation 2012. (Available from: http://www.who.int/neglected_diseases/NTD_RoadMap_2012_Fullversion.pdf).
- 23. World Health Organization . Update on the global status of implementation of preventive chemotherapy (PC). (Available from: https://www.who.int/neglected_diseases/preventive_chemotherapy/PC_Update.pdf).
- 24. Hanson C, Weaver A, Zoerhoff KL et al Integrated implementation of programs targeting neglected tropical diseases through preventive chemotherapy: identifying best practices to roll out programs at national scale. Am J Trop Med Hyg 2012: 86: 508–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Linehan M, Hanson C, Weaver A et al Integrated implementation of programs targeting neglected tropical diseases through preventive chemotherapy: proving the feasibility at national scale. Am J Trop Med Hyg 2011: 84: 5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. World Health Organization . Expanded Special Project for Elimination of Neglected Tropical Diseases. (Available from: https://www.afro.who.int/health-topics/expanded-special-project-elimination-neglected-tropical-disease).
- 27. Bangert M, Molyneux DH, Lindsay SW, Fitzpatrick C, Engels D. The cross‐cutting contribution of the end of neglected tropical diseases to the sustainable development goals. Infect Dis Poverty 2017: 6: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Amazigo U, Noma M, Boatin BA, Etya'ale DE, Seketeli A, Dadzie KY. Delivery systems and cost recovery in Mectizan treatment for onchocerciasis. Ann Trop Med Parasitol 1998: 92(Suppl 1): S23–S31. [PubMed] [Google Scholar]
- 29. Bundy DAP, Dhomun B, Daney X, Schultz LB, Tembon A. Investing in onchocerciasis control: financial management of the African Programme for Onchocerciasis Control (APOC). PLoS Negl Trop Dis 2015: 9: e0003508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Coffeng LE, Stolk WA, Zoure HG et al African Programme For Onchocerciasis Control 1995‐2015: model‐estimated health impact and cost. PLoS Negl Trop Dis 2013: 7: e2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Keating J, Yukich JO, Mollenkopf S, Tediosi F. Lymphatic filariasis and onchocerciasis prevention, treatment, and control costs across diverse settings: a systematic review. Acta Trop 2014: 135: 86–95. [DOI] [PubMed] [Google Scholar]
- 32. Fitzpatrick C, Madin‐Warburton M, Schneiderd T et al Benchmarks for the cost per person of mass treatment against neglected tropical diseases: a literature review and metaregression with web‐based software application. PLoS Negl Trop Dis 2016: 12: e0005037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fitzpatrick C, Nwankwo U, Lenk E, de Vlas SJ. An Investment Case for Ending Neglected Tropical Diseases. Disease Control Priorities (3rd edn), (Volume 6): Major Infectious Diseases. Washington, DC: World Bank, 2017; 411–431. [PubMed] [Google Scholar]
- 34. Ndyomugyenyi R, Lakwo T, Habomugisha P, Male B. Progress towards the elimination of onchocerciasis as a public‐health problem in Uganda: opportunities, challenges and the way forward. Ann Trop Med Parasitol 2007: 101: 323–333. [DOI] [PubMed] [Google Scholar]
- 35. Katabarwa M, Mutabazi D, Richards F Jr. The community‐directed, ivermectin‐treatment programme for onchocerciasis control in Uganda–an evaluative study (1993‐1997). Ann Trop Med Parasitol 1999: 93: 727–735. [DOI] [PubMed] [Google Scholar]
- 36. Katabarwa MN, Habomugisha P, Richards FO Jr. Implementing community‐directed treatment with ivermectin for the control of onchocerciasis in Uganda (1997‐2000): an evaluation. Ann Trop Med Parasitol 2002: 96: 61–73. [DOI] [PubMed] [Google Scholar]
- 37. Turner HC, Toor J, Hollingsworth TD, Anderson RM. Economic evaluations of mass drug administration: the importance of economies of scale and scope. Clin Infect Dis 2017: 66: 1298–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Conteh L, Engels T, Molyneux DH. Socioeconomic aspects of neglected tropical diseases. Lancet 2010: 375: 239–247. [DOI] [PubMed] [Google Scholar]
- 39. Brady MA, Hooper PJ, Ottesen EA. Projected benefits from integrating NTD programs in sub‐Saharan Africa. Trends Parasitol 2006: 22: 285–291. [DOI] [PubMed] [Google Scholar]
- 40. Gedge LM, Bettis AA, Bradley MH, Hollingsworth TD, Turner HC. Economic evaluations of lymphatic filariasis interventions: a systematic review and research needs. Parasit Vectors 2018: 11: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Turner HC, Truscott JE, Hollingsworth TD, Bettis AA, Brooker SJ, Anderson RM. Cost and cost‐effectiveness of soil‐transmitted helminth treatment programmes: systematic review and research needs. Parasit Vectors 2015: 8: 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Evans D, McFarland D, Adamani W et al Cost‐effectiveness of triple drug administration (TDA) with praziquantel, ivermectin and albendazole for the prevention of neglected tropical diseases in Nigeria. Ann Trop Med Parasitol 2011: 105: 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bundy DAP, Appleby LJ, Bradley M et al Mass Deworming Programs in Middle Childhood and Adolescence. Chapter 13 in Disease Control Priorities, 3rd edn (Volume 8): Child and Adolescent Health and Development. Washington, DC: World Bank, 2017; 165–182. [PubMed] [Google Scholar]
- 44. Turner HC, Osei‐Atweneboana MY, Walker M et al The cost of annual versus biannual community‐directed treatment of onchocerciasis with ivermectin: Ghana as a case study. PLoS Negl Trop Dis 2013: 7: e2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Godin C. Cameroon and Chad: cost recovery. Ann Trop Med Parasitol 1998: 92(Suppl 1): S163–S164. [PubMed] [Google Scholar]
- 46. Hopkins AD. Mectizan delivery systems and cost recovery in the Central African Republic. Ann Trop Med Parasitol 1998: 92(Suppl 1): S97–S100. [DOI] [PubMed] [Google Scholar]
- 47. Onwujekwe OE, Shu EN, Okonkwo PO. Community financing of local ivermectin distribution in Nigeria: potential payment and cost‐recovery outlook. Trop Doct 2000: 30: 91–94. [DOI] [PubMed] [Google Scholar]
- 48. Onwujekwe OE, Shu EN, Nwagbo D, Akpala CO, Okonkwo PO. Willingness to pay for community‐based ivermectin distribution: a study of three onchocerciasis‐endemic communities in Nigeria. Trop Med Int Health 1998: 3: 802–808. [DOI] [PubMed] [Google Scholar]
- 49. Onwujekwe OE, Shu EN, Okonkwo PO. Willingness to pay for the maintenance of equity in a local ivermectin distribution scheme in Toro, Northern Nigeria. Public Health 1999: 113: 193–194. [PubMed] [Google Scholar]
- 50. WHO‐CHOICE . Making choices in health: WHO guide to cost‐effectiveness analysis. Geneva: World Health Organization, 2003. (Available from: https://www.who.int/choice/publications/p_2003_generalised_cea.pdf).
- 51. Drummond MF, Sculpher MJ, Torrance GW, O'Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programme, 3rd edn Oxford: Oxford University Press, 2005. [Google Scholar]
- 52. Turner HC, Toor J, Bettis AA et al Valuing the unpaid contribution of community health volunteers to mass drug administration programs. Clin Infect Dis 2018; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kim YE, Sicuri E, Tediosi F. Financial and Economic Costs of the Elimination and Eradication of Onchocerciasis (River Blindness) in Africa. PLoS Negl Trop Dis 2015: 9: e0004056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Turner HC, Bettis AA, Chu BK et al Investment success in public health: an analysis of the cost‐effectiveness and cost‐benefit of the Global Programme to Eliminate Lymphatic Filariasis. Clin Infect Dis 2017: 64: 728–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hernando Y, Colwell K, Wright BD. Doing well while fighting river blindness: the alignment of a corporate drug donation programme with responsibilities to shareholders. Trop Med Int Health 2016: 21: 1304–1310. [DOI] [PubMed] [Google Scholar]
- 56. Management Sciences for Health . The International Medical Products Price Guide. (Available from: http://mshpriceguide.org/en/home/).
- 57. Uniting to Combat NTDs . London Declaration on Neglected Tropical Diseases. (Available from: https://unitingtocombatntds.org/london-declaration-neglected-tropical-diseases/).
- 58. Kim A, Benton B. Cost‐benefit analysis of the onchocerciasis control program (OCP). The World Bank; 1995. (Available from: http://documents.worldbank.org/curated/en/205811468739219136/pdf/multi0page.pdf).
- 59. Redekop WK, Lenk EJ, Luyendijk M et al The Socioeconomic Benefit to Individuals of Achieving the 2020 Targets for Five Preventive Chemotherapy Neglected Tropical Diseases. PLoS Negl Trop Dis 2017: 11: e0005289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kim YE, Stolk WA, Tanner M, Tediosi F. Modelling the health and economic impacts of the elimination of river blindness (onchocerciasis) in Africa. BMJ Glob Health 2017: 2: e000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dunn C, Callahan K, Katabarwa M et al The Contributions of Onchocerciasis Control and Elimination Programs toward the Achievement of the Millennium Development Goals. PLoS Negl Trop Dis 2015: 9: e0003703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lenk EJ, Redekop WK, Luyendijk M, Rijnsburger AJ, Severens JL. Productivity loss related to neglected tropical diseases eligible for preventive chemotherapy: a systematic literature review. PLoS Negl Trop Dis 2016: 10: e0004397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Remme JHF, Feenstra P, Lever PR, Medici AC, Morel CM. Tropical diseases targeted for elimination: chagas disease, lymphatic dilariasis, onchocerciasis, and leprosy In: Dean T Jamison, Joel G Breman, Anthony R Measham, George Alleyne, Mariam Claeson, David B Evans, Prabhat Jha, Anne Mills, Philip Musgrove (eds). Disease Control Priorities in Developing Countries, 2nd edn New York: Oxford University Press, 2006; 433–449. [PubMed] [Google Scholar]
- 64. Ibe O, Onwujekwe O, Uzochukwu B, Ajuba M, Okonkwo P. Exploring Consumer Perceptions and Economic Burden of Onchocerciasis on Households in Enugu State, South‐East Nigeria. PLoS Negl Trop Dis 2015: 9: e0004231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Krol M, Brouwer W, Rutten F. Productivity costs in economic evaluations: past, present, future. Pharmacoeconomics 2013: 31: 537–549. [DOI] [PubMed] [Google Scholar]
- 66. van den Hout WB. The value of productivity: human‐capital versus friction‐cost method. Ann Rheum Dis 2010: 69(Suppl 1): i89–i91. [DOI] [PubMed] [Google Scholar]
- 67. Benton B, Skinner ED. Cost‐benefits of onchocerciasis control. Acta Leiden 1990: 59: 405–411. [PubMed] [Google Scholar]
- 68. World Health Organization . Prevention, control and elimination of onchocerciasis. (Available from: http://www.who.int/onchocerciasis/control/en/).
- 69. Haddix A. Economic Re‐evaluation of the African Programme for Onchocerciasis Control. School of Public Health of Emory University: Rollins Atlanta, 1997. [Google Scholar]
- 70. World Health Organization, World Bank . Economic Impact of Onchocercal Skin Disease (OSD). Report of a Multi‐country Study. TDR Applied Field Research Report World Health Organization, Geneva. 1997.
- 71. Evans TG, Murray CJL. A critical re‐examination of the economics of blindness prevention under the onchocerciasis control programme. Soc Sci Med 1987: 25: 241–249. [DOI] [PubMed] [Google Scholar]
- 72. Horton S, Gelband H, Jamison D, Levin C, Nugent R, Watkins D. Ranking 93 health interventions for low‐ and middle‐income countries by cost‐effectiveness. PLoS ONE 2017: 12: e0182951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Turner HC, Walker M, Churcher TS et al Reaching the London declaration on neglected tropical diseases goals for onchocerciasis: an economic evaluation of increasing the frequency of Ivermectin treatment in Africa. Clin Infect Dis 2014: 59: 923–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cheke RA, Garms R. Indices of onchocerciasis transmission by different members of the Simulium damnosum complex conflict with the paradigm of forest and savanna parasite strains. Acta Trop 2013: 125: 43–52. [DOI] [PubMed] [Google Scholar]
- 75. Turner HC, Walker M, French MD, Blake IM, Churcher TS, Basáñez MG. Neglected tools for neglected diseases: mathematical models in economic evaluations. Trends Parasitol 2014: 30: 562–570. [DOI] [PubMed] [Google Scholar]
- 76. Basáñez MG, Walker M, Turner HC, Coffeng LE, de Vlas SJ, Stolk WA. River blindness: mathematical models for control and elimination. Adv Parasitol 2016: 94: 247–341. [DOI] [PubMed] [Google Scholar]
- 77. Gold MR, Stevenson D, Fryback DG. HALYS and QALYS and DALYS, Oh My: similarities and differences in summary measures of population Health. Annu Rev Public Health 2002: 23: 115–134. [DOI] [PubMed] [Google Scholar]
- 78. Coffeng LE, Stolk WA, Zoure HG et al African programme for onchocerciasis control 1995‐2015: updated health impact estimates based on new disability weights. PLoS Negl Trop Dis 2014: 8: e2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Global Burden of Disease Study 2013 Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990‐2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015: 386: 743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. World Health Organization . WHO methods and data sources for global burden of disease estimates 2000‐2011. 2013. (Available from: https://www.who.int/healthinfo/statistics/GlobalDALYmethods_2000_2011.pdf).
- 81. Voigt K, King NB. Disability weights in the global burden of disease 2010 study: two steps forward, one step back? Bull World Health Organ 2014: 92: 226–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Murray CJ, Lopez AD. Measuring global health: motivation and evolution of the Global Burden of Disease Study. Lancet 2017: 390: 1460–1464. [DOI] [PubMed] [Google Scholar]
- 83. Turner HC, Walker M, Churcher TS, Basáñez M‐G. Modelling the impact of ivermectin on river blindness and its burden of morbidity and mortality in African Savannah: EpiOncho projections. Parasit Vectors 2014: 7: 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Newall AT, Jit M, Hutubessy R. Are current cost‐effectiveness thresholds for low‐ and middle‐income countries useful? Examples from the world of vaccines. Pharmacoeconomics 2014: 32: 525–531. [DOI] [PubMed] [Google Scholar]
- 85. Marseille E, Larson B, Kazi DS, Kahn JG, Rosen S. Thresholds for the cost–effectiveness of interventions: alternative approaches. Bull World Health Organ 2015: 93: 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Leech AA, Kim DD, Cohen JT, Neumann PJ. Use and Misuse of Cost‐Effectiveness Analysis Thresholds in Low‐ and Middle‐Income Countries: Trends in Cost‐per‐DALY Studies. Value in Health. 2018. [DOI] [PMC free article] [PubMed]
- 87. Hutubessy R, Chisholm D, Edejer TT‐T, Who C. Generalized cost‐effectiveness analysis for national‐level priority‐setting in the health sector. Cost Eff Resour Alloc 2003: 1: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Woods B, Revill P, Sculpher M, Claxton K. Country‐level cost‐effectiveness thresholds: initial estimates and the need for further research. Value Health 2016: 19: 929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Shillcutt SD, Walker DG, Goodman CA, Mills AJ. Cost‐effectiveness in low‐ and middle‐income countries: a review of the debates surrounding decision rules. Pharmacoeconomics 2009: 27: 903–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Fitzpatrick C, Nwankwo U, Lenk EJ, de Vlas SJ, Bundy DAP. An Investment Case for Ending Neglected Tropical Diseases. Disease Control Priorities, 3rd edn (Volume 6): World Bank: Washington, DC, 2017. [PubMed] [Google Scholar]
- 91. World Bank . World development report 1993: investing in health. New York: Oxford University Press; 1993. (Available from: http://documents.worldbank.org/curated/en/468831468340807129/World-development-report-1993-investing-in-health)
- 92. Holmstrom O. Point‐of‐care mobile digital microscopy and deep learning for the detection of soil‐transmitted helminths and Schistosoma haematobium. PLoS Negl Trop Dis 2017: 10: 1337325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ochalek J, Lomas J, Claxton K. Cost per DALY averted thresholds for low‐ and middle‐income countries: evidence from cross country data. University of York, Centre for Health Economics, Working Paper 122 2015(Available from: https://pure.york.ac.uk/portal/en/publications/cost-per-daly-averted-thresholds-for-low-and-middleincome-countries(12487fa5-e63f-4ac3-9fa4-03b2795065eb).html).
- 94. Horton S. Cost‐Effectiveness Analysis in Disease Control Priorities, 3rd edn, Volume 9, Disease Control Priorities. Washington, DC: World Bank, 2017. [PubMed] [Google Scholar]
- 95. Turner HC, Walker M, Attah SK et al The potential impact of moxidectin on onchocerciasis elimination in Africa: an economic evaluation based on the Phase II clinical trial data. Parasit Vectors 2015: 8: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kastner RJ, Stone CM, Steinmann P, Tanner M, Tediosi F. Chapter Seven ‐ lessons learned from developing an eradication investment case for lymphatic filariasis In: Maria Gloria Basáñez, Roy M. Anderson (eds) Advances in Parasitology, Volume 94 London UK: Academic Press, 2016; 393–417. [DOI] [PubMed] [Google Scholar]
- 97. Sicuri E, Evans DB, Tediosi F. Can Economic Analysis Contribute to Disease Elimination and Eradication? A Systematic Review. PLoS ONE 2015: 10: e0130603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Tediosi F, Steinmann P, de Savigny D, Tanner M. Developing eradication investment cases for onchocerciasis, lymphatic filariasis, and human African trypanosomiasis: rationale and main challenges. PLoS Negl Trop Dis 2013: 7: e2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kim YE, Remme JH, Steinmann P, Stolk WA, Roungou JB, Tediosi F. Control, elimination, and eradication of river blindness: scenarios, timelines, and ivermectin treatment needs in Africa. PLoS Negl Trop Dis 2015: 9: e0003664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Goldman AS, Guisinger VH, Aikins M et al National mass drug administration costs for lymphatic filariasis elimination. PLoS Negl Trop Dis 2007: 1: e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Global Burden of Disease Health Financing Collaborator Network . Evolution and patterns of global health financing 1995‐2014: development assistance for health, and government, prepaid private, and out‐of‐pocket health spending in 184 countries. Lancet 2017: 389: 1981–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kumaranayake L. The real and the nominal? Making inflationary adjustments to cost and other economic data. Health Policy Plan 2000: 15: 230–234. [DOI] [PubMed] [Google Scholar]
- 103. Verver S, Walker M, Kim YE et al How can onchocerciasis elimination in Africa be accelerated? Modeling the impact of increased ivermectin treatment frequency and complementary vector control. Clin Infect Dis 2018: 66(suppl_4): S267–S274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Boussinesq M, Fobi G, Kuesel AC. Alternative treatment strategies to accelerate the elimination of onchocerciasis. Int Health 2018: 10(suppl_1): i40–i48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kuesel AC. Research for new drugs for elimination of onchocerciasis in Africa. Int J Parasitol Drugs Drug Resist 2016: 6: 272–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Jacob BG, Loum D, Lakwo TL et al Community‐directed vector control to supplement mass drug distribution for onchocerciasis elimination in the Madi mid‐North focus of Northern Uganda. PLoS Negl Trop Dis 2018: 12: e0006702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Turner HC, Osei‐Atweneboana MY, Walker M et al The cost of annual versus biannual community‐directed treatment with ivermectin: Ghana as a case study. PLoS Negl Trop Dis 2013: 7: e2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kamgno J, Pion SD, Chesnais CB et al A Test‐and‐Not‐Treat Strategy for Onchocerciasis in Loa loa‐Endemic Areas. N Engl J Med 2017: 377: 2044–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ozoh GA, Murdoch ME, Bissek AC et al The African programme for onchocerciasis control: impact on onchocercal skin disease. Trop Med Int Health 2011: 16: 875–883. [DOI] [PubMed] [Google Scholar]
- 110. King CH. Health metrics for helminth infections. Acta Trop 2015: 141: 150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. King CH, Bertino AM. Asymmetries of poverty: why global burden of disease valuations underestimate the burden of neglected tropical diseases. PLoS Negl Trop Dis 2008: 2: e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Alonso LM, Murdoch ME, Jofre‐Bonet M. Psycho‐social and economical evaluation of onchocerciasis: a literature review. Soc Med 2009: 4: 8–31. [Google Scholar]
- 113. Taylor HR, Jonas JB, Keeffe J et al Disability weights for vision disorders in Global Burden of Disease study. Lancet: 381: 23. [DOI] [PubMed] [Google Scholar]
- 114. Salomon JA, Vos T, Murray CJL. Disability weights for vision disorders in Global Burden of Disease study ‐ Authors’ reply. Lancet 381: 23–24. [DOI] [PubMed] [Google Scholar]
- 115. Nord E. Disability weights in the Global Burden of Disease 2010: unclear meaning and overstatement of international agreement. Health Policy 2013: 111: 99–104. [DOI] [PubMed] [Google Scholar]
- 116. Colebunders R, Titulaer MJ. Nodding syndrome: preventable and treatable. Sci Transl Med 2017: 9: eaam8532. [DOI] [PubMed] [Google Scholar]
- 117. Chesnais CB, Nana‐Djeunga HC, Njamnshi AK et al The temporal relationship between onchocerciasis and epilepsy: a population‐based cohort study. Lancet Infect Dis 2018: 18: 1278–1286. [DOI] [PubMed] [Google Scholar]
- 118. Vinkeles Melchers NVS, Mollenkopf S, Colebunders R et al Burden of onchocerciasis‐associated epilepsy: first estimates and research priorities. Infect Dis Poverty 2018: 7: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Krotneva SP, Coffeng LE, Noma M et al African Program for Onchocerciasis Control 1995–2010: impact of Annual Ivermectin Mass Treatment on Off‐Target Infectious Diseases. PLoS Negl Trop Dis 2015: 9: e0004051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Walker M, Stolk WA, Dixon MA et al Modelling the elimination of river blindness using long‐term epidemiological and programmatic data from Mali and Senegal. Epidemics 2017: 18: 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Frempong KK, Walker M, Cheke RA et al Does Increasing Treatment Frequency Address Suboptimal Responses to Ivermectin for the Control and Elimination of River Blindness? Clin Infect Dis 2016: 62: 1338–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Doyle SR, Bourguinat C, Nana‐Djeunga HC et al Genome‐wide analysis of ivermectin response by Onchocerca volvulus reveals that genetic drift and soft selective sweeps contribute to loss of drug sensitivity. PLoS Negl Trop Dis 2017: 11: e0005816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Husereau D, Drummond M, Petrou S et al Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013: 11: 346. [DOI] [PubMed] [Google Scholar]
- 124. McFarland D, Murray J. A review of the economic impact of treatment with ivermectin for Onchocerciasis. Atlanta: Emory University School of Public Health and the International Health Program Office at the Centers for Disease Control and Prevention, 1994.
- 125. Benton B. Economic impact of onchocerciasis control through the African Programme for Onchocerciasis Control: an overview. Ann Trop Med Parasitol 1998: 92(Suppl 1): S33–S39. [DOI] [PubMed] [Google Scholar]
- 126. Prescott N, Prost A, Le Berre R. The economics of blindness prevention in Upper Volta under the Onchocerciasis Control Program. Soc Sci Med 1984: 19: 1051–1055. [DOI] [PubMed] [Google Scholar]
- 127. Prost A, Prescott N. Cost‐effectiveness of blindness prevention by the Onchocerciasis Control Programme in Upper Volta. Bull World Health Organ 1984: 62: 795–802. [PMC free article] [PubMed] [Google Scholar]
- 128. Evans TG. Socioeconomic consequences of blinding onchocerciasis in west Africa. Bull World Health Organ 1995: 73: 495–506. [PMC free article] [PubMed] [Google Scholar]
- 129. Kim A, Tandon A, Hailu A. Health and Labor Productivity: The Economic Impact of Onchocercial Skin Disease. Policy, Research working paper; no WPS 1836 Washington, DC: World Bank. 1997 (Available from: http://documentsworldbankorg/curated/en/417751468767965429/Health-and-labor-productivity-the-economic-impact-of-onchocercal-skin-disease).
- 130. Okeibunor JC, Amuyunzu‐Nyamongo M, Onyeneho NG et al Where would I be without ivermectin? Capturing the benefits of community‐directed treatment with ivermectin in Africa. Trop Med Int Health 2011: 16: 608–621. [DOI] [PubMed] [Google Scholar]
- 131. Oladepo O, Brieger WR, Otusanya S, Kale OO, Offiong S, Titiloye M. Farm land size and onchocerciasis status of peasant farmers in south‐western Nigeria. Trop Med Int Health 1997: 2: 334–340. [DOI] [PubMed] [Google Scholar]
- 132. Thomson IG. Onchocerciasis in an oil palm estate. Trans R Soc Trop Med Hyg 1971: 65: 484–489. [DOI] [PubMed] [Google Scholar]
- 133. Wogu MD, Okaka CE. Prevalence and socio‐economic effects of onchocerciasis in Okpuje, Owan West Local Government Area, Edo State, Nigeria. Int J Biomed Health Sci 2008: 4: 113–119. [Google Scholar]
- 134. Workneh W, Fletcher M, Olwit G. Onchocerciasis in field workers at Baya Farm, Teppi Coffee Plantation Project, southwestern Ethiopia: prevalence and impact on productivity. Acta Trop 1993: 54: 89–97. [DOI] [PubMed] [Google Scholar]
- 135. Frick KD, Foster A, Bah M, Faal H. Analysis of costs and benefits of the Gambian Eye Care Program. Arch Ophthalmol 2005: 123: 239–243. [DOI] [PubMed] [Google Scholar]
- 136. Smith TS, Frick KD, Holden BA, Fricke TR, Naidoo KS. Potential lost productivity resulting from the global burden of uncorrected refractive error. Bull World Health Organ 2009: 87: 431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Shamanna BR, Dandona L, Rao GN. Economic burden of blindness in India. Indian J Ophthalmol 1998: 46: 169–172. [PubMed] [Google Scholar]
- 138. World Health Organization . Global burden of onchocerciasis in the year 2000: Summary of methods and data sources. 2006. (Available from:https://www.who.int/healthinfo/statistics/bod_onchocerciasis.pdf).
- 139. World Health Organization . Global Burden of Disease 2004 update: Disability weights for diseases and conditions. 2008. (Available from: https://www.who.int/healthinfo/global_burden_disease/GBD2004_DisabilityWeights.pdf).
- 140. Salomon JA, Vos T, Hogan DR et al Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet 2012: 380: 2129–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. de Vlas SJ, Stolk WA, le Rutte EA et al Concerted efforts to control or eliminate neglected tropical diseases: how much health will be gained? PLoS Negl Trop Dis 2016: 10: e0004386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Remme J, Dadzie KY, Rolland A, Thylefors B. Ocular onchocerciasis and intensity of infection in the community. I. West African savanna. Trop Med Parasitol 1989: 40: 340–347. [PubMed] [Google Scholar]
- 143. Remme JHF. The Global Burden of Onchocerciasis in 1990. Geneva: WHO, 2004. (Available from: https://www.who.int/healthinfo/global_burden_disease/Onchocerciasis%201990.pdf).
- 144. Brown R, Shannon R. Prevalence, intensity and ocular manifestations of Onchocerca volvulus infection in Dimbelenge, Zaire. Ann Soc Belg Med Trop 1989: 69: 137–142. [PubMed] [Google Scholar]
- 145. Henry MC, Meredith SE. The onchocerciasis focus at Kinsuka/Kinshasa (Republic of Zaire) in 1985. I. Entomological aspect. Ann Trop Med Parasitol 1990: 84: 369–379. [DOI] [PubMed] [Google Scholar]
- 146. Whitworth JAG, Morgan D, Gilbert CE, Mabey DM, Maude GH, Taylor DW. Effects of repeated doses of ivermectin on ocular onchocerciasis: community‐based trial in Sierra Leone. Lancet 1991: 338: 1100–1103. [DOI] [PubMed] [Google Scholar]
- 147. Whitworth JA, Gilbert CE, Mabey DM, Morgan D, Foster A. Visual loss in an onchocerciasis endemic community in Sierra Leone. Br J Ophthalmol 1993: 77: 30–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Kayembe DL, Kasonga DL, Kayembe PK, Mwanza JC, Boussinesq M. Profile of eye lesions and vision loss: a cross‐sectional study in Lusambo, a forest‐savanna area hyperendemic for onchocerciasis in the Democratic Republic of Congo. Trop Med Int Health 2003: 8: 83–89. [DOI] [PubMed] [Google Scholar]
- 149. Fobi G, Yameogo L, Noma M et al Managing the Fight against Onchocerciasis in Africa: APOC Experience. PLoS Negl Trop Dis 2015: 9: e0003542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Homeida M, Braide E, Elhassan E et al APOC's strategy of community‐directed treatment with ivermectin (CDTI) and its potential for providing additional health services to the poorest populations. African Programme for Onchocerciasis Control. Ann Trop Med Parasitol 2002: 96(Suppl 1): S93–S104. [DOI] [PubMed] [Google Scholar]
- 151. World Health Organization . African Programme for Onchocerciasis Control (APOC): About us. (Available from: http://www.who.int/apoc/about/en/).
- 152. World Health Organization . Progress towards eliminating onchocerciasis in the WHO Region of the Americas: verification of elimination of transmission in Guatemala. Wkly Epidemiol Rec 2016: 91: 501–505. [PubMed] [Google Scholar]
- 153. Torgerson DJ, Raftery J. Discounting. BMJ 1999: 319: 914–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Sheldon TA. Discounting in health care decision‐making: time for a change? J Public Health Med 1992: 14: 250–256. [PubMed] [Google Scholar]
- 155. Attema AE, Brouwer WBF, Claxton K. Discounting in economic evaluations. Pharmacoeconomics 2018: 36: 745–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. PRISMA checklist.


