Abstract
The aim of this study was to evaluate the efficacy and safety of pemafibrate in people with type 2 diabetes and hypertriglyceridaemia over a 52‐week period. Participants were randomly assigned to receive treatment with placebo or pemafibrate at a dose of 0.2 or 0.4 mg/d for 24 weeks (treatment period 1). The main results from treatment period 1 have been reported previously. The assigned treatment was continued up to week 52, except that the placebo was changed to pemafibrate 0.2 mg/d after week 24 (treatment period 2). The percentage changes in fasting serum triglyceride (TG) levels at week 52 (last observation carried forward) were −48.2%, −42.3%, and −46.4% in the placebo/pemafibrate 0.2 mg/d (n = 57), pemafibrate 0.2 mg/d (n = 54), and pemafibrate 0.4 mg/d (n = 55) groups, respectively. Levels of TG, non‐HDL cholesterol and total cholesterol stably decreased, whereas levels of HDL cholesterol increased with pemafibrate treatments over 52 weeks. Pemafibrate was well tolerated throughout the study period. The present study is the first to show that pemafibrate treatment substantially ameliorated lipid abnormalities and was well tolerated for 52 weeks in people with type 2 diabetes and hypertriglyceridaemia.
Keywords: clinical trial, dyslipidaemia, lipid‐lowering therapy, phase III study, randomized trial, type 2 diabetes
1. INTRODUCTION
Characteristic lipid abnormalities, such as high triglyceride (TG), low HDL cholesterol, and increased small dense LDL cholesterol levels, typically attributed to insulin resistance, are complications often noted in people with type 2 diabetes.1 Peroxisome proliferator‐activated receptor‐α (PPARα) agonists, such as fibrates, are highly effective in treating these lipid abnormalities.2 Pemafibrate, a novel selective PPARα modulator (SPPARMα), has potent TG‐lowering and HDL cholesterol‐increasing activities, almost no effect on serum creatinine levels, and an ameliorating effect on liver function test values.3 Furthermore, it has high efficacy at low doses and is safe for clinical use, similarly to placebo.4, 5, 6, 7
The aim of the present phase III trial was to evaluate the efficacy and safety of pemafibrate compared to placebo during the first 24‐week period (treatment period 1). After treatment period 1, treatment period 2 was initiated, from week 24 to week 52, with the placebo changed to 0.2 mg/d pemafibrate. The main results from treatment period 1 have been reported previously.6 This report summarizes the results including treatment period 2, which spanned 52 weeks. Furthermore, the efficacy and safety of pemafibrate in combination with and without statins were also investigated.
2. METHODS
The PROVIDE study (pemafibrate study to validate a 52‐week efficacy and safety in people with type 2 diabetes comorbid with elevated triglyceride levels) was a multicentre, placebo‐controlled, randomized, double‐blind, parallel‐group study. The study was conducted in accordance with the principles of the Declaration of Helsinki and the Ministerial Ordinance on Good Clinical Practice for Drugs issued by the Ministry of the Health and Welfare, Japan. The study protocol was approved by the institutional review boards of all the 34 sites. Written informed consent was obtained from all participants before their inclusion in the study.
The study methods have been reported previously.6 Briefly, patients with type 2 diabetes and hypertriglyceridemia (TG levels ≥150 mg/dL [1.7 mmol/L] and ≤1000 mg/dL [11.3 mmol/L]) were treated with placebo or pemafibrate at a dose of 0.2 or 0.4 mg/d (twice daily) for 24 weeks. After week 24, placebo was changed to pemafibrate 0.2 mg/d (placebo/pemafibrate 0.2 mg/d group), and treatment in the pemafibrate 0.2 mg/d and 0.4 mg/d groups was continued unchanged up to week 52 (Figure S1). A meal tolerance test was performed at weeks 0, 24 and 52 at facilities where the test was feasible.
The primary efficacy and safety endpoints were the percentage change in fasting serum TG levels and the incidence of adverse events (AEs) and adverse drug reactions (ADRs), respectively. Additional information can be found in Appendix S1.
3. RESULTS
Figure S2 shows the disposition of participants. Participant characteristics have been reported previously.6
The percentage changes in fasting serum TG levels at week 52 (last observation carried forward [LOCF]) were −48.2%, −42.3% and −46.4% in the placebo/pemafibrate 0.2 mg/d, pemafibrate 0.2 mg/d and pemafibrate 0.4 mg/d groups, respectively. TG, HDL cholesterol, non‐HDL cholesterol and total cholesterol levels stably improved in the pemafibrate groups over the 52‐week period (Figures 1 and S3). Similar findings were observed for remnant lipoprotein cholesterol and other apolipoprotein (Apo) levels (Table S1). Results of the meal tolerance test at week 52 were similar to those at week 24 (Figure S4).
Figure 1.
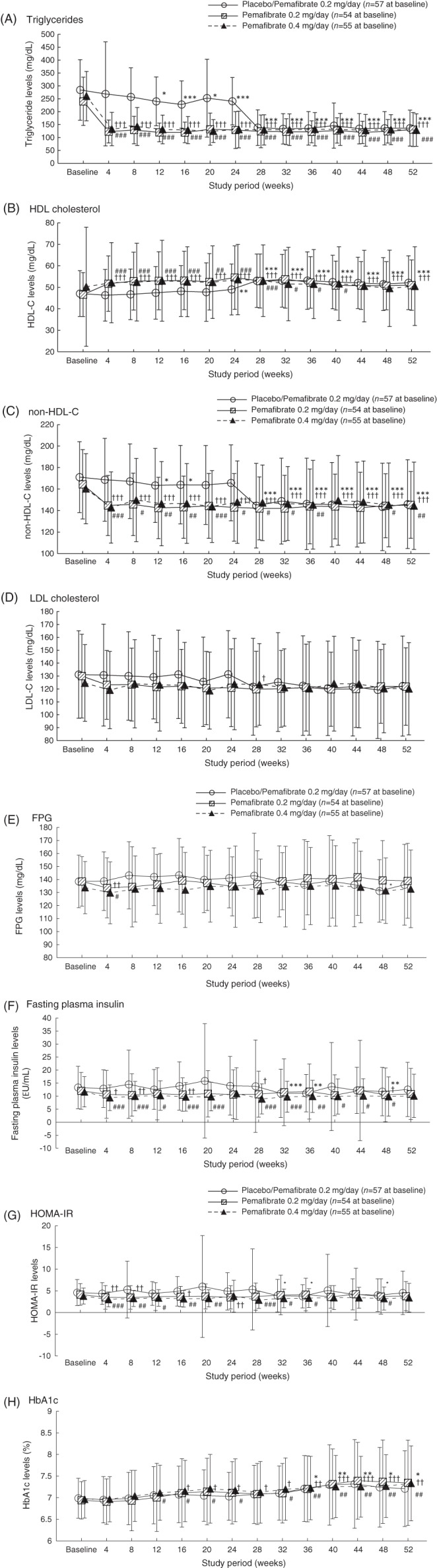
A–H, Lipids and levels of glucose‐related variables over 52 weeks. Values are presented as mean ± SD. *P <0.05, **P <0.01, ***P <0.001 vs. baseline for placebo/pemafibrate 0.2 mg/d (one‐sample t‐test). †P <0.05, ††P <0.01, †††P <0.001 vs. baseline for pemafibrate 0.2 mg/d (one‐sample t‐test). #P <0.05, ##P <0.01, ###P <0.001 vs. baseline for pemafibrate 0.4 mg/d (one‐sample t‐test). FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; HOMA‐IR, homeostasis model assessment of insulin resistance
The effects of pemafibrate on glucose metabolism were variable (Figures 1 and S3). Fasting insulin and homeostasis model assessment of insulin resistance (HOMA‐IR) decreased after switching from placebo to pemafibrate 0.2 mg/d at week 24. This trend was also observed at the start of pemafibrate administration in treatment period 1. Glycoalbumin levels tended to increase in treatment period 2, only in the pemafibrate 0.2 mg/d group. Glycated haemoglobin (HbA1c) levels increased over time in all groups. Pemafibrate did not have a major effect on the postprandial plasma glucose and insulin levels (Figure S4).
The incidence of AEs was 81.8%, 77.8% and 78.2% for the placebo/pemafibrate 0.2 mg/d (excluding the events during treatment period 1), pemafibrate 0.2 mg/d and pemafibrate 0.4 mg/d groups, respectively (Table 1). During the 52‐week period, no duration‐dependent treatment effect was noted on the incidence of AEs or ADRs (Table S2). Serious AEs occurred after pemafibrate treatment, with 21 episodes in 16 participants. A causal relationship with the study treatment was ruled out in all cases, apart from the possible development of a bile duct stone in one participant in the pemafibrate 0.2 mg/d group. There were 12 episodes of AEs that led to discontinuation of the study treatment in 12 participants. Causal relationships with the study treatment could not be ruled out for the eczema AE observed in the placebo/pemafibrate 0.2 mg/d group, bile duct stone in the pemafibrate 0.2 mg/d group, and acute renal failure and liver dysfunction in the pemafibrate 0.4 mg/d group.
Table 1.
Summary of adverse events and adverse drug reactions occurring throughout the study period
| Placebo/Pemafibrate 0.2 mg/d | Pemafibrate 0.2 mg/d | Pemafibrate 0.4 mg/d | |
|---|---|---|---|
| Treatment period 2a | Treatment period 1 + 2 | Treatment period 1 + 2 | |
| (n = 55) | (n = 54) | (n = 55) | |
| Total AEs | 45 (81.8) | 42 (77.8) | 43 (78.2) |
| Serious AEs | 6 (10.9) | 6 (11.1) | 4 (7.3) |
| AEs leading to discontinuation | 4 (7.3) | 3 (5.6) | 5 (9.1) |
| Total ADRs | 12 (21.8) | 11 (20.4) | 15 (27.3) |
| Serious ADRs | 0 | 1 (1.9) | 0 |
| ADRs leading to discontinuation | 1 (1.8) | 1 (1.9) | 2 (3.6) |
| Laboratory tests | |||
| AST >3 × ULN | 0 | 2 (3.7) | 0 |
| AST >5 × ULN | 0 | 1 (1.9) | 0 |
| ALT >3 × ULN | 1 (1.8) | 0 | 0 |
| ALT >5 × ULN | 0 | 0 | 0 |
| GGT >3 × ULN | 2 (3.6) | 3 (5.6) | 0 |
| GGT >5 × ULN | 0 | 1 (1.9) | 0 |
| Creatine kinase >4 × ULN | 1 (1.8) | 1 (1.9) | 0 |
| Creatine kinase >5 × ULN | 1 (1.8) | 1 (1.9) | 0 |
| Creatine kinase >10 × ULN | 0 | 0 | 0 |
| Serum creatinine >1.5 mg/dL (132.6 μmol/L) | 2 (3.6) | 2 (3.7) | 2 (3.6) |
| Serum creatinine >2.0 mg/dL (176.8 μmol/L) | 1 (1.8) | 1 (1.9) | 1 (1.8) |
| Serum creatinine >1.5 × baseline | 2 (3.6) | 1 (1.9) | 1 (1.8) |
| Serum creatinine >2.0 × baseline | 0 | 0 | 0 |
Abbreviations: ADR, adverse drug reaction; AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma‐glutamyl transferase; ULN, upper limit of normal.
Data are presented as number of participants (%).
Excluding the events occurring in the placebo group during treatment period 1.
Alanine aminotransferase (ALT) and gamma‐glutamyl transferase (GGT) levels decreased after pemafibrate treatment for 52 weeks. Serum creatinine level increased and estimated glomerular filtration rate decreased slightly (Figure S5). No noteworthy findings were found with respect to increased levels above the cut‐off values of aspartate aminotransferase (AST), ALT, GGT, creatine kinase and serum creatinine, and no participant in the pemafibrate 0.4 mg/d group had increased levels of these, excluding serum creatinine (Table 1).
The incidence of AEs in the pemafibrate groups showed no significant difference from that in the placebo group, in participants with and without statin therapy (Table S3). Regardless of concomitant statin therapy, significant decreases in TG, remnant lipoprotein cholesterol, ApoB48, ApoCII, ApoCIII, ApoCIII/ApoCII and ApoE, and significant increases in ApoAII were observed in the pemafibrate groups compared with the placebo group at week 24 (LOCF; Figures S6 and S7). The effects of pemafibrate on glycaemic and safety variables were also similar between statin and no statin treatment (Figures S8 and S9).
4. DISCUSSION
This is the first study to evaluate the efficacy and safety of pemafibrate in people with type 2 diabetes and hypertriglyceridaemia for a 52‐week period.
Pemafibrate significantly improved lipid abnormalities accompanying type 2 diabetes, including high TG‐rich lipoprotein and low HDL cholesterol levels for a long‐term period of up to 52 weeks. Moreover, the effect of pemafibrate on lipid abnormalities was similar for statin and for no statin treatment. The efficacy of pemafibrate on lipid profiles seems to be similar for both 0.2 mg/d and 0.4 mg/d; however, in the pooled analyses of six placebo‐controlled trials with pemafibrate, pemafibrate 0.4 mg/d lowered TG‐rich lipoproteins, ApoCIII, fasting plasma glucose, insulin, GGT and ALP to a greater extent than pemafibrate 0.2 mg/d.8, 9 Cholesterol content in small‐sized HDL, which is thought to be more anti‐atherogenic, increased to a greater degree when treated with pemafibrate 0.4 mg/d than with pemafibrate 0.2 mg/d.6 Based on the above results, pemafibrate 0.4 mg/d seems to be more efficient in reducing atherosclerotic cardiovascular disease than pemafibrate 0.2 mg/d. This appropriate higher dose of pemafibrate (0.4 mg/d) has been used in the PROMINENT (Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes) study, evaluating the effects of pemafibrate on the prevention of cardiovascular diseases in combination with statin.10
Changes in serum creatinine levels with placebo, pemafibrate 0.2 mg/d, and pemafibrate 0.4 mg/d after 24 weeks were −0.013 mg/dL (−1.15 μmol/L), 0.026 mg/dL (2.30 μmol/L), and 0.031 mg/dL (2.74 μmol/L), respectively. Changes in serum creatinine levels with pemafibrate treatment were smaller than with fenofibrate treatment in the ACCORD lipid study11 (a ∼0.2‐mg/dL increase in the fenofibrate group and ~0.02‐mg/dL increase in the placebo group at month 12 from 0.9 ± 0.2 mg/dL at baseline). The FIRST study12 showed that, in the fenofibric acid group during the 104‐week treatment period, 2.7% of participants had a serum creatinine level >2.0 mg/dL and 20.7% and 2.1% of participants had a 1.5‐fold and 2‐fold increase from baseline, respectively, with these percentages being higher than those in the placebo group. Previous studies comparing the effects of pemafibrate with those of fenofibrate showed that pemafibrate had a smaller effect than fenofibrate on serum creatinine levels,4, 7, 13 which was also confirmed during our long‐term treatment of 52 weeks in people with type 2 diabetes (Table 1).
Pemafibrate is a SPPARMα, which has been developed from the concept that PPARα agonists could trigger different biological responses via the same receptor and that their benefits could be separated from their unwanted side effects.3 The expression of several PPARα target genes differed under treatment with pemafibrate and fenofibrate.14 Although further investigation is needed to justify the difference in the clinical profile of pemafibrate from that of other fibrates, pemafibrate is reported to have a good benefit–risk balance. Pemafibrate had a TG‐lowering effect equal to or greater than that of fenofibrate, but the rates of AEs in the pemafibrate group were lower than those in the fenofibrate group.7, 13 Furthermore, pemafibrate, but not fenofibrate, decreased ALT and GGT levels. A phase II trial of pemafibrate in patients with non‐alcoholic fatty liver disease is ongoing (ClinicalTrials.gov Identifier: NCT03350165).
Throughout the study period of the present study, the dosage regimen of antidiabetic agents was changed in 22 participants. The effect of pemafibrate on glucose metabolism was not affected after excluding these 22 participants (Figures S10 and S11). Pemafibrate did not affect body weight during the 52‐week treatment period, although body weight significantly decreased at several timepoints. There were no specific trends in seasonal variability in glucose metabolism under pemafibrate treatment (data not shown).
The reason for the significant increase in HbA1c after 12 weeks of treatment is unclear, considering no remarkable changes in fasting plasma glucose, postprandial glucose or glycoalbumin levels were observed. The increase in HbA1c could be attributable to time‐dependent changes observed in people with diabetes or possibly a false increase. Fibrates improved red blood cell deformability by modifying erythrocyte membrane lipids,15 which might affect erythrocyte dynamics and lifespans, as well as HbA1c levels. Pemafibrate treatment led to a decrease in fasting plasma glucose, fasting insulin levels and HOMA‐IR during treatment period 1 in the previous study,6 and showed a significant increase in splanchnic glucose uptake rates, evaluated using the hyperinsulinaemic‐euglycaemic clamp study,16 suggesting that pemafibrate improves glucose metabolism, although the effects were limited.
The present study has several limitations. First, it included people with type 2 diabetes of relatively short duration, who were administered fewer combined antidiabetic agents. Further evidence and clinical experience in real‐world settings are therefore necessary to establish the efficacy and safety of pemafibrate. Second, the study participants were all Japanese, therefore, the generalizability of the results of this study to other ethnicities is unclear. Third, a placebo control was not used from week 24 to week 52, therefore, the efficacy and safety of pemafibrate during the 52‐week treatment should be assessed carefully.
In conclusion, the efficacy and safety of pemafibrate in people with type 2 diabetes with hypertriglyceridaemia noted during the previous 24‐week placebo‐controlled period were sustained over 52 weeks. Pemafibrate rarely had unfavourable effects on liver and renal function test results, even in participants with type 2 diabetes.
CONFLICT OF INTEREST
E.A. reports personal fees from Kowa, during the conduct of the study, grants from Astellas Pharma, Ono Pharmaceutical, Nippon Boehringer Ingelheim, Taisho Toyama Pharmaceutical, Daiichi Sankyo, MSD, Sanofi, Takeda Pharmaceutical, Mitsubishi Tanabe Pharma, Novartis Pharma, Novo Nordisk Pharma and Eli Lilly Japan, and personal fees from Astellas Pharma, MSD, Sanofi, Ono Pharmaceutical and Novo Nordisk Pharma, outside the submitted work. S.Y. reports personal fees from Kowa, during the conduct of the study, grants from MSD, Bayer Yakuhin, Nippon Boehringer Ingelheim, Takeda Pharmaceutical, Ono Pharmaceutical, Astellas Pharma, Mitsubishi Tanabe Pharma, Kyowa Medex and Rohto Pharmaceutical, and personal fees from Kowa, MSD, Amgen Astellas BioPharma and Sanofi, outside the submitted work. H.A. reports personal fees from Kowa, during the conduct of the study, grants from Daiichi Sankyo, and personal fees from Daiichi Sankyo, Astellas Pharma, Sanofi, Kowa, MSD, Amgen Astellas BioPharma and Abbott Japan, outside the submitted work. K.Y. reports personal fees from Kowa, during the conduct of the study, grants from Astellas Pharma, MSD, Ono Pharmaceutical, Kowa Pharmaceutical, Kyowa Hakko Kirin, Shionogi, Daiichi Sankyo, Taisho Toyama Pharmaceutical, Sumitomo Dainippon Pharma, Takeda Pharmaceutical, Mitsubishi Tanabe Pharma, Teijin Pharma, Eli Lilly Japan, Nippon Boehringer Ingelheim, Pfizer Japan, Novo Nordisk Pharma and Mochida Pharmaceutical, personal fees from Astellas Pharma, Amgen Astellas BioPharma, AstraZeneca, Bayer, MSD, Ono Pharmaceutical, Sanwa Kagaku Kenkyusho, Kissei Pharmaceutical, Kyowa Hakko Kirin, Kaken Pharmaceutical, Kowa, Kowa Pharmaceutical, Sanofi, Shionogi, Daiichi Sankyo, Taisho Toyama Pharmaceutical, Sumitomo Dainippon Pharma, Takeda Pharmaceutical, Mitsubishi Tanabe Pharma, Eli Lilly Japan, Nippon Boehringer Ingelheim, Novartis Pharma, Novo Nordisk Pharma, Pfizer Japan, Mochida Pharmaceutical and Teijin Pharma, outside the submitted work. J.S. reports personal fees from Kowa, during the conduct of the study, personal fees from Astellas Pharma, AstraZeneca, Nippon Boehringer Ingelheim, Eli Lilly Japan, Kyowa Hakko Kirin, Mitsubishi Tanabe Pharma, MSD, Novo Nordisk Pharma, Ono Pharmaceutical, Pfizer Japan, Sanofi, Sanwa Kagaku Kenkyusho, Sumitomo Dainippon Pharma, Taisho Toyama Pharmaceutical and Takeda Pharmaceutical, outside the submitted work. T.I. reports personal fees from Kowa, during the conduct of the study, grants from Nippon Boehringer Ingelheim, Mitsubishi Tanabe Pharma, Novo Nordisk Pharma, Sanofi, Astellas Pharma, Kowa Pharmaceutical, AstraZeneca, Ono Pharmaceutical, Eli Lilly Japan, Sumitomo Dainippon Pharma and MSD, and personal fees from Nippon Boehringer Ingelheim, Mitsubishi Tanabe Pharma, Novo Nordisk Pharma, Taisho Toyama Pharmaceutical, Sanofi, Astellas Pharma, Kowa Pharmaceutical, Novartis Pharma, AstraZeneca, Ono Pharmaceutical, Takeda Pharmaceutical, Eli Lilly Japan, Kissei Pharmaceutical, Sumitomo Dainippon Pharma and MSD, outside the submitted work. J.N. reports personal fees from Kowa, during the conduct of the study, grants from Kissei Pharmaceutical, Nippon Boehringer Ingelheim, Astellas Pharma, MSD, Sanofi, Taisho Toyama Pharmaceutical, Mitsubishi Tanabe Pharma, Eli Lilly Japan, Japan Tobacco, Novartis Pharma, Pfizer Japan, Ono Pharmaceutical, AstraZeneca, Kyowa Hakko Kirin, Daiichi Sankyo, Sumitomo Dainippon Pharma, Takeda Pharmaceutical and Novo Nordisk Pharma, and personal fees from Nippon Boehringer Ingelheim, Astellas Pharma, MSD, Sanofi, Mitsubishi Tanabe Pharma, Eli Lilly Japan, Novartis Pharma, Ono Pharmaceutical, AstraZeneca, Daiichi Sankyo, Takeda Pharmaceutical, Novo Nordisk Pharma, Kowa Pharmaceutical, and Terumo, outside the submitted work. H.M. reports personal fees from Kowa, during the conduct of the study, grants from Astellas Pharma, AstraZeneca, Ono Pharmaceutical and Nippon Boehringer Ingelheim, and personal fees from Astellas Pharma, AstraZeneca, Ono Pharmaceutical, Nippon Boehringer Ingelheim, Taisho Toyama Pharmaceutical, Novo Nordisk Pharma, Eli Lilly Japan, MSD, Daiichi Sankyo, Mitsubishi Tanabe Pharma, Sanofi, Kowa Pharmaceutical and Takeda Pharmaceutical, outside the submitted work. N.Y. reports personal fees from Kowa, during the conduct of the study, and personal fees from Novo Nordisk Pharma, Sanofi and MSD, outside the submitted work. Y.T. reports personal fees from Kowa, during the conduct of the study, grants from Astellas Pharma, MSD, Ono Pharmaceutical, Taisho Toyama Pharmaceutical, Sumitomo Dainippon Pharma, Takeda Pharmaceutical, Nippon Boehringer Ingerheim, Kyowa Hakko Kirin, Sanwa Kagaku Kenkyusho, Shionogi, Daiichi Sankyo, Pfizer Japan, Bristol‐Myers Squibb and Eli Lilly Japan, and personal fees from Astellas Pharma, AstraZeneca, MSD, Ono Pharmaceutical, Kissei Pharmaceutical, Sanofi, Taisho Toyama Pharmaceutical, Sumitomo Dainippon Pharma, Takeda Pharmaceutical, Mitsubishi Tanabe Pharma, Nippon Boehringer Ingerheim and Novo Nordisc Pharma, outside the submitted work. H.W. reports personal fees from Kowa, during the conduct of the study, grants from Astellas Pharma, Sanofi, Mitsubishi Tanabe Pharma, Novo Nordisk Pharma, AstraZeneca, Takeda Pharmaceutical, Novartis Pharma, Nippon Boehringer Ingelheim, MSD, Sumitomo Dainippon Pharma, Eli Lilly Japan, Sanwa Kagaku Kenkyusho, Kissei Pharmaceutical, Kyowa Hakko Kirin, Daiichi Sankyo, Pfizer Japan, Nitto Boseki, Taisho Toyama Pharmaceutical, Johnson & Johnson and Teijin Pharma, and personal fees from Astellas Pharma, Sanofi, Mitsubishi Tanabe Pharma, Novo Nordisk Pharma, Kowa Pharmaceutical, AstraZeneca, Takeda Pharmaceutical, Nippon Boehringer Ingelheim, MSD, Eli Lilly Japan, Sanwa Kagaku Kenkyusho, Ono Pharmaceutical, Kissei Pharmaceutical, FUJIFILM Pharma, Daiichi Sankyo and Terumo, outside the submitted work. H.W. also belongs to the endowed chairs that receive the grants from Takeda Pharmaceutical, MSD, Mitsubishi Tanabe Pharma, Nippon Boehringer Ingelheim, Ono Pharmaceutical, Kowa and Sanwa Kagaku Kenkyusho, outside the submitted work. H.S. is an employee of Kowa. S.I. reports personal fees from Kowa, during the conduct of the study, grants from Eli Lilly Japan, Sanofi, Astellas Pharma, Nippon Boehringer Ingelheim, Mitsubishi Tanabe Pharma, Daiichi Sankyo, Takeda Pharmaceutical, Ono Pharmaceutical, Shionogi, Teijin Pharma and Kowa Pharmaceutical, and personal fees from MSD, Sanofi, Amgen Astellas BioPharma, Kowa Pharmaceutical, and Kowa, outside the submitted work.
AUTHOR CONTRIBUTIONS
E.A., S.Y., H.A., K.Y., J.S., T.I., J.N., H.M., N.Y., Y.T., H.W., H.S. and S.I. contributed to the concept, design and execution of the study, and to the interpretation of the data. E.A. substantially contributed to writing and critically review of the manuscript. S.Y., H.A., K.Y., J.S., T.I., J.N., H.M., N.Y., Y.T., H.W. and S.I. contributed to critically reviewing the manuscript. H.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Supporting information
Appendix S1Supplementary Appendix
Supplementary Table 1 Summary of lipid parameters change in week52(LOCF)
SupplementaryTable 2 The incidence of adverse events and adverse drug reactions every 12 weeks and after week 48
Supplementary Table 3 The incidence of adverse events during treatment period 1 by treatment group in subgroups with or without concomitant statin therapy
Supplementary Figure 1 Study design
Supplementary Figure 2 Disposition of patients.
Supplementary Figure 3 Total cholesterol and glycoalbuminlevels over 52 weeks.
Supplementary Figure 4 Postprandial TG, glucose, and insulin before and after treatment (week 24 [LOCF] and week 52 [LOCF]).
Supplementary Figure 5 Safety parameter levels over 52 weeks.
Supplementary Figure 6 Percent changes in lipids at week 24 (LOCF) in subgroups with or without concomitant statin therapy (full analysis set).
Supplementary Figure 7 Percent changes in apolipoproteins at week 24 (LOCF) in subgroups with or without concomitant statin therapy (full analysis set).
Supplementary Figure 8 Changes in glucose‐related parameters at week 24 (LOCF) in subgroups with or without concomitant statin therapy (full analysis set).
Supplementary Figure 9 Changes in safety laboratory parameters at week 24 in subgroups with or without concomitant statin therapy (safety analysis set).
Supplementary Figure 10 Glucose‐related parameter levels over 52 weeks (excluding 22 patients for whom antidiabetic agents were added or changed during the treatment period).
Supplementary Figure 11 Glucose‐related parameter levels over 52 weeks (for only 22 patients for whom antidiabetic agents were added or changed during the treatment period).
ACKNOWLEDGMENTS
We thank the investigators and patients who participated in this study. This study was conducted at NTT‐East Sapporo Hospital (So Nagai), Iwate Medical University Hospital (Noriko Takebe), Juntendo University Hospital (Fuki Ikeda), Aichi Medical University Hospital (Jiro Nakamura), Shiga University of Medical Science Hospital (Hiroshi Maegawa), Yamaguchi University Hospital (Yasuharu Ohta), Kyushu University Hospital (Toyoshi Inoguchi), Kumamoto University Hospital (Takeshi Matsumura), Chiba University Hospital (Minoru Takemoto), Sugiura Clinic (Toshiyuki Sugiura), Musashi Fujisawa Central Clinic (Seiki Wada), Nakayama Clinic (Mikihiro Nakayama), Goshi Hospital (Takeo Naito), Tao Internal Medicine Clinic (Tsuyoshi Tao), BOOCS Clinic Fukuoka (Kazuyuki Saito), Diabetes Centre, Shin‐Koga Hospital (Shoichi Akazawa and Eiji Kawasaki), Kurihara Diabetic Care Clinic (Yoshio Kurihara), Okuguchi Clinic of Internal Medicine (Fuminobu Okuguchi), Naka Kinen Clinic (Takeshi Osonoi), Tomonaga Clinic (Osamu Tomonaga), Kurashiki Central Hospital (Takashi Matsuoka), Kanauchi Medical Clinic (Rie Wada), Osaka Gyoumeikan Hospital (Shinya Makino), Osaka Ekisaikai Hospital (Haruyuki Taguchi), Tohoku Medical and Pharmaceutical University Wakabayashi Hospital, formerly NTT‐East Tohoku Hospital (Kazumi Yamato), Tokyo‐Eki Centre‐building Clinic (Arihiro Kiyosue), Sakakibara Sapia Tower Clinic (Makiko Abe), Chikamori Hospital (Yoshitaka Kumon), Ayame Medical Clinic (Hideo Ayame), Ehime Rosai Hospital (Kazuaki Nakai), Saiseikai Matsuyama Hospital (Hiroaki Miyaoka), Matsuyama Red Cross Hospital (Shiori Kondo), Matsuba Clinic (Ikuro Matsuba), and Manda Memorial Hospital (Shinji Taneda).
This work was supported by Kowa Company, Ltd.
Araki E, Yamashita S, Arai H, et al. Efficacy and safety of pemafibrate in people with type 2 diabetes and elevated triglyceride levels: 52‐week data from the PROVIDE study. Diabetes Obes Metab. 2019;21:1737–1744. 10.1111/dom.13686
Funding information This work was supported by Kowa Company, Ltd.
REFERENCES
- 1. Bell DS, Al Badarin F, O'Keefe JH Jr. Therapies for diabetic dyslipidaemia. Diabetes Obes Metab. 2011;13(4):313‐325. [DOI] [PubMed] [Google Scholar]
- 2. Okopien B, Buldak L, Boldys A. Fibrates in the management of atherogenic dyslipidemia. Expert Rev Cardiovasc Ther. 2017;15(12):913‐921. [DOI] [PubMed] [Google Scholar]
- 3. Fruchart JC. Selective peroxisome proliferator‐activated receptor alpha modulators (SPPARMalpha): the next generation of peroxisome proliferator‐activated receptor alpha‐agonists. Cardiovasc Diabetol. 2013;12:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ishibashi S, Yamashita S, Arai H, et al. Effects of K‐877, a novel selective PPARalpha modulator (SPPARMalpha), in dyslipidaemic patients: a randomized, double blind, active‐ and placebo‐controlled, phase 2 trial. Atherosclerosis. 2016;249:36‐43. [DOI] [PubMed] [Google Scholar]
- 5. Arai H, Yamashita S, Yokote K, Araki E, Suganami H, Ishibashi S. Efficacy and safety of K‐877, a novel selective peroxisome proliferator‐activated receptor alpha modulator (SPPARMalpha), in combination with statin treatment: two randomised, double‐blind, placebo‐controlled clinical trials in patients with dyslipidaemia. Atherosclerosis. 2017;261:144‐152. [DOI] [PubMed] [Google Scholar]
- 6. Araki E, Yamashita S, Arai H, et al. Effects of pemafibrate, a novel selective PPARalpha modulator, on lipid and glucose metabolism in patients with type 2 diabetes and hypertriglyceridemia: a randomized, double‐blind, placebo‐controlled, phase 3 trial. Diabetes Care. 2018;41(3):538‐546. [DOI] [PubMed] [Google Scholar]
- 7. Arai H, Yamashita S, Yokote K, Araki E, Suganami H, Ishibashi S. Efficacy and safety of pemafibrate versus fenofibrate in patients with high triglyceride and low HDL cholesterol levels: a multicenter, placebo‐controlled, double‐blind, randomized trial. J Atheroscler Thromb. 2018;25(6):521‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ishibashi S, Arai H, Yokote K, Araki E, Suganami H, Yamashita S. Efficacy of pemafibrate on atherogenic dyslipidemia: results of a pooled analysis of pemafibrate phase II/III clinical trials compared with placebo. Atherosclerosis Suppl. 2018;32:25‐26. [Google Scholar]
- 9. Yokote K, Yamashita S, Arai H, Araki E, Suganami H, Ishibashi S. A pooled analysis of pemafibrate phase II/III clinical trials indicated significant improvement in glycemic and liver function‐related parameters. Atherosclerosis Suppl. 2018;32:154‐155. [Google Scholar]
- 10. Pradhan AD, Paynter NP, Everett BM, et al. Rationale and design of the pemafibrate to reduce cardiovascular outcomes by reducing triglycerides in patients with diabetes (PROMINENT) study. Am Heart J. 2018;206:80‐93. [DOI] [PubMed] [Google Scholar]
- 11. Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1563‐1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davidson MH, Rosenson RS, Maki KC, et al. Effects of fenofibric acid on carotid intima‐media thickness in patients with mixed dyslipidemia on atorvastatin therapy: randomized, placebo‐controlled study (FIRST). Arterioscler Thromb Vasc Biol. 2014;34(6):1298‐1306. [DOI] [PubMed] [Google Scholar]
- 13. Ishibashi S, Arai H, Yokote K, Araki E, Suganami H, Yamashita S. Efficacy and safety of pemafibrate (K‐877), a selective peroxisome proliferator‐activated receptor alpha modulator, in patients with dyslipidemia: results from a 24‐week, randomized, double blind, active‐controlled, phase 3 trial. J Clin Lipidol. 2018;12(1):173‐184. [DOI] [PubMed] [Google Scholar]
- 14. Raza‐Iqbal S, Tanaka T, Anai M, et al. Transcriptome analysis of K‐877 (a novel selective PPARalpha modulator (SPPARMalpha))‐regulated genes in primary human hepatocytes and the mouse liver. J Atheroscler Thromb. 2015;22(8):754‐772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Labios M, Martinez M, Vaya A, Gabriel F, Guiral V, Aznar J. Effect of a modified fibrate (Biniwas retard) on hemorheological alterations in hyperlipemic patients. Clin Hemorheol Microcirc. 1999;21(2):79‐85. [PubMed] [Google Scholar]
- 16. Matsuba I, Matsuba R, Ishibashi S, et al. Effects of a novel selective peroxisome proliferator‐activated receptor‐alpha modulator, pemafibrate, on hepatic and peripheral glucose uptake in patients with hypertriglyceridemia and insulin resistance. J Diabetes Investig. 2018;9(6):1323‐1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1Supplementary Appendix
Supplementary Table 1 Summary of lipid parameters change in week52(LOCF)
SupplementaryTable 2 The incidence of adverse events and adverse drug reactions every 12 weeks and after week 48
Supplementary Table 3 The incidence of adverse events during treatment period 1 by treatment group in subgroups with or without concomitant statin therapy
Supplementary Figure 1 Study design
Supplementary Figure 2 Disposition of patients.
Supplementary Figure 3 Total cholesterol and glycoalbuminlevels over 52 weeks.
Supplementary Figure 4 Postprandial TG, glucose, and insulin before and after treatment (week 24 [LOCF] and week 52 [LOCF]).
Supplementary Figure 5 Safety parameter levels over 52 weeks.
Supplementary Figure 6 Percent changes in lipids at week 24 (LOCF) in subgroups with or without concomitant statin therapy (full analysis set).
Supplementary Figure 7 Percent changes in apolipoproteins at week 24 (LOCF) in subgroups with or without concomitant statin therapy (full analysis set).
Supplementary Figure 8 Changes in glucose‐related parameters at week 24 (LOCF) in subgroups with or without concomitant statin therapy (full analysis set).
Supplementary Figure 9 Changes in safety laboratory parameters at week 24 in subgroups with or without concomitant statin therapy (safety analysis set).
Supplementary Figure 10 Glucose‐related parameter levels over 52 weeks (excluding 22 patients for whom antidiabetic agents were added or changed during the treatment period).
Supplementary Figure 11 Glucose‐related parameter levels over 52 weeks (for only 22 patients for whom antidiabetic agents were added or changed during the treatment period).


