Abstract
Background
The prognosis of patients with pancreatic ductal adenocarcinoma (PDAC) is poor and selection of patients for surgery is challenging. This study examined the impact of a positive resection margin (R1) on locoregional recurrence (LRR) and overall survival (OS); and also aimed to identified tumour characteristics and/or technical factors associated with a positive resection margin in patients with PDAC.
Methods
Patients scheduled for pancreatic resection for PDAC between 2006 and 2016 were identified from an institutional database. The effect of resection margin status, patient characteristics and tumour characteristics on LRR, distant metastasis and OS was assessed.
Results
A total of 322 patients underwent pancreatectomy for PDAC. A positive resection (R1) margin was found in 129 patients (40·1 per cent); this was associated with decreased OS compared with that in patients with an R0 margin (median 15 (95 per cent c.i. 13 to 17) versus 22 months; P < 0·001). R1 status was associated with reduced time to LRR (median 16 versus 36 (not estimated, n.e.) months; P = 0·002). Disease recurrence patterns were similar in the R1 and R0 groups. Risk factors for early recurrence were tumour stage, positive lymph nodes (N1) and perineural invasion. Among 100 patients with N0 disease, R1 status was associated with shorter OS compared with R0 resection (median 17 (10 to 24) versus 45 (n.e.) months; P = 0·002), whereas R status was not related to OS in 222 patients with N1 disease (median 14 (12 to 16) versus 17 (15 to 19) months after R1 and R0 resection respectively; P = 0·068).
Conclusion
Although pancreatic resection with a positive margin was associated with poor survival and early recurrence, particularly in patients with N1 disease, disease recurrence patterns were similar between R1 and R0 groups.
Introduction
Surgical resection in combination with (neo)adjuvant chemotherapy is the only potentially curative treatment for patients with pancreatic ductal adenocarcinoma (PDAC). Overall survival (OS) among patients with PDAC is poor1. This may be related to incomplete resection of the tumour and a consequence of high recurrence rates2, 3, 4. Other factors affecting outcome include tumour size5, perineural and/or lymphangioinvasion6 and lymph node status6, 7, 8. Resection margin (R) status remains the most controversial4. Over two decades ago, Yeo and colleagues9 reported that patients who underwent radical pancreatoduodenectomy with tumour‐free resection margins (R0) had a 5‐year survival rate of 26 per cent, compared with only 8 per cent in those with positive margins (R1)9. Ghaneh and co‐workers10 reported a difference in median survival between R0 and R1 resection (24·9 versus 18·7 months respectively) in a large multicentre RCT.
Other studies11, 12, 13, 14 have reported that resection margin status is not an independent risk factor for survival. An explanation could be the lack of standardized pathological evaluation of the specimen, or definition and reporting of resection margin status15, 16. For example, in the USA, a resection margin is considered positive when tumour cells have reached the inked margin9, 17. In Europe, a positive resection margin is defined by the presence of tumour cells within 1 mm of the resection margin15, 18, 19. This discrepancy has led to a wide range of reported rates of resection margin involvement from less than 20 per cent to more than 75 per cent18, 20, 21, 22, 23, 24. Recently, Osipov and colleagues25 reported favourable disease‐free survival and OS after R0 resection, defined by the presence of tumour cells within 0·5 mm up to 2 mm from the resection margin. These results support the findings of Chang et al.26 and Gebauer and co‐workers27, who also recommended a resection margin of 1·5 or 2 mm.
An alternative hypothesis suggests that recurrence following R1 resection is not due to residual tumour cells, but reflects an aggressive tumour biology28, 29. This is based on the finding that isolated locoregional recurrence (LRR) without distant metastases is found in only 10–25 per cent of patients11, 30.
The aims of this study were to examine the impact of a positive resection margin on recurrence and survival; and to assess which tumour characteristics and/or technical factors are associated with R1 resection status in patients with PDAC.
Methods
This retrospective study was approved by the Institutional Medical Ethics Committee at Leiden University Medical Centre (LUMC), Leiden, the Netherlands. Patients with a histological diagnosis of PDAC who were scheduled for curative pancreatic resection between January 2006 and December 2016 were identified from an electronic institutional database. Follow‐up data were collected until October 2017. Patients with any other histopathological diagnosis were excluded, including those with malignant intraductal papillary mucinous neoplasms. Operative data and patient characteristics were collected in the database, including age at time of surgery, sex and type of surgery. Tumour characteristics recorded were: pTNM stage, grade, histopathological diagnosis, lymph node involvement, total number of resected lymph nodes, lymph node ratio, and lymphangioinvasion and/or perineural invasion.
After preoperative evaluation, including measurement of carbohydrate antigen 19.9 and carcinoembryonic antigen levels, contrast‐enhanced CT, and MRI and/or endoscopic ultrasonography where indicated, a multidisciplinary team of pancreatic surgeons, radiologists, gastroenterologists, pathologists and oncologists decided whether surgery with curative intent was feasible. The criteria for a non‐resectable tumour were: presence of distant metastases; obvious involvement of coeliac and/or para‐aortic nodes; and contact with the superior mesenteric artery, common hepatic artery, coeliac trunk of more than 90°, or encasement of the superior mesenteric or portal vein. All tumours were classified before surgery as resectable or borderline resectable according to Dutch Pancreatic Cancer Group (DPCG) 2012 criteria (PREOPANC trial)31.
After surgery, each patient was discussed by the multidisciplinary team to determine whether adjuvant therapy was indicated. In the Netherlands, the standard for adjuvant chemotherapy is six cycles of gemcitabine. Because patients who undergo surgical resection receive adjuvant treatment at a site other than the referral hospital, detailed information regarding the dose, frequency and completeness of this planned treatment was not available. Therefore, the study included only whether each patient was recommended for adjuvant therapy or not. As neoadjuvant treatment is not standard of care and administered only in the context of clinical trials, patients who received neoadjuvant treatment were excluded.
Follow‐up information was obtained from the electronic patient records at LUMC, the primary care physician or the oncologist. Follow‐up imaging (primarily CT) was performed when indicated clinically; no standard protocol was used.
Recurrence was diagnosed based on evidence of disease on imaging. LRR was defined as the presence of disease in the surgical bed or present in the mesentery, periaortic soft tissue, pancreatojejunal anastomosis or in the nodes around the vena cava, coeliac axis and/or retroperitoneum. Distant metastasis was defined as the presence of disease in the omentum, peritoneum, solid organs and/or pelvic lymph nodes. Early recurrence was defined as recurrence occurring within 6 months after surgery32, 33. Overall recurrence was defined as any form of recurrence (locoregional or distant) that occurred first during follow‐up.
Pathological assessment
Macroscopic and microscopic examination of the pancreatoduodenectomy specimen was done using standard methods in accordance with the guidelines established by DPCG, which followed the recommendations of Verbeke and colleagues15, 18. Before 2010, examination was performed by bivalving the specimen. After the pancreas had been resected, the surgeon attached coloured beads to the different resection margins. Subsequently, the pathologist used multicolour inking of the specimen to identify these margins. The following terms were used to define the resection margins: posterior, vascular (superior mesenteric vein or superior mesenteric artery), common bile duct, anterior, pancreatic neck, caudal and circumferential margin. For analysis of the association between resection margin and outcome, only patients who had a Whipple's resection or pylorus‐preserving pancreatoduodenectomy (PPPD) were included.
Histological findings were reviewed to confirm the diagnosis, tumour characteristics and R status of the margins. Staging was determined using the TNM cancer staging system, seventh edition34. R1 resection was defined in accordance with Dutch guidelines, which were in line with the guidelines of the British Royal College of Pathology (https://www.rcpath.org/profession/guidelines/cancer‐datasets‐and‐tissue‐pathways.html); a surgical margin with malignant cells identified 1 mm or less from the inked margin was considered positive. A random sample of histological specimens was re‐examined.
Statistical analysis
Student's t test was used for analysis of continuous data with a normal distribution and the Mann–Whitney U test for data with a skewed distribution; categorical data were compared using the χ2 test. OS was calculated as the interval between either the date of death (event) or last follow‐up (censored) and the date of surgery, and is reported as median with 95 per cent confidence intervals. Time to recurrence was calculated as the interval between the date of surgery and the date of diagnosis of LRR or distant metastasis. The overall time to recurrence was calculated as the interval between date of surgery and first recurrence, either LRR or distant metastasis. If a patient died without evidence of recurrence (censored), the date of last follow‐up imaging or last follow‐up without clinical signs of recurrent disease was used. Therefore, death was not a competing risk in the analyses. Kaplan–Meier analysis and the log rank test were used to evaluate differences in recurrence and survival between groups. Characteristics associated with recurrence or survival were included in a Cox proportional hazards regression analysis. Differences were considered statistically significant at P < 0·050. SPSS® Statistics for Windows® version 23.0 was used for statistical analysis (IBM, Armonk, New York, USA).
Results
Some 322 patients underwent pancreatic resection (275 Whipple's procedure or PPPD, 35 distal pancreatectomy, 12 total pancreatectomy). A further 148 patients (31·5 per cent) underwent laparotomy with or without palliative bypass surgery owing to distant metastases or non‐resectable disease. Patient characteristics according to resection margin status are shown in Table 1. Before adopting the so‐called Verbeke protocol for gross examination of pancreas specimens (from 2006 to 2009), the R1 resection rate was 32 per cent (22 of 68); from 2010 onwards, the R1 resection rate was 42·1 per cent (107 of 254) (P = 0·144). Some 45 specimens were re‐examined randomly, with no change in R status. Median survival following resection was 18 (95 per cent c.i. 16 to 20) months.
Table 1.
Patient characteristics according to resection margin
|
All patients (n = 322) |
R0 (n = 193) |
R1 (n = 129) |
P § | |
|---|---|---|---|---|
| Age (years) * | 65(10) | 66(10) | 64(9) | 0·172¶ |
| Sex ratio (M : F) | 161: 161 | 95: 98 | 66: 63 | 0·733 |
| Death | 231 (71·7) | 130 (67·4) | 101 (78·3) | 0·033 |
| Survival (months) † | 18 (16, 20) | 22 (17, 27) | 15 (13, 17) | < 0·001# |
| Tumour size (mm) * | 29(15) | 26(15) | 33(15) | < 0·001¶ |
| Adjuvant therapy | 172 (53·4) | 95 (49·2) | 77 (59·7) | 0·065 |
| Tumour differentiation | 0·564 | |||
| Well | 63 (19·6) | 40 (20·7) | 23 (17·8) | |
| Moderate | 133 (41·3) | 80 (41·5) | 53 (41·1) | |
| Poor | 124 (38·5) | 71 (36·8) | 53 (41·1) | |
| Undifferentiated | 2 (0·6) | 2 (1·0) | 0 (0·0) | |
| No. of resected lymph nodes ‡ | 15 (11–20) | 15 (11–19) | 15 (12–21) | 0·318** |
| Lymph node‐positive | 222 (68·9) | 122 (63·2) | 100 (77·5) | 0·007 |
| No. of positive lymph nodes ‡ | 2 (0–4) | 1 (0–3) | 3 (1–5) | 0·001** |
| Lymph node ratio ‡ | 0·1 (0–0·28) | 0·08 (0–0·25) | 0·17 (0·04–0·31) | 0·003** |
| Tumour stage | < 0·001 | |||
| IA | 27 (8·4) | 23 (11·9) | 4 (3·1) | |
| IB | 11 (3·4) | 9 (4·7) | 2 (1·6) | |
| IIA | 59 (18·3) | 40 (20·7) | 19 (14·7) | |
| IIB | 207 (64·3) | 118 (61·1) | 89 (69·0) | |
| III | 15 (4·7) | 2 (1·0) | 13 (10·1) | |
| IV | 3 (0·9) | 1 (0·5) | 2 (1·6) | |
| Perineural invasion | 201 (62·4) | 104 (53·9) | 97 (75·2) | < 0·001 |
| Lymphangioinvasion | 70 (21·7) | 35 (18·1) | 35 (27·1) | 0·055 |
Values in parentheses are percentages unless indicated otherwise; values are
mean(s.d.),
median (95 per cent c.i.) and
median (i.q.r.). R0, resection margin free from tumour cells; R1, tumour cells within 1 mm of resection margin.
χ2 test, except
Student's t test,
log rank test and
Mann–Whitney U test.
Impact of R1 status on tumour recurrence
Of patients who underwent pancreatectomy, 196 (60·9 per cent) developed a recurrence: 45 (14·0 per cent) had LRR, 91 (28·3 per cent) distant metastasis, and 60 (18·6 per cent) developed both LRR and distant metastasis. Fifty‐five of the 196 patients (28·1 per cent) whose disease relapsed had an early recurrence within 6 months after surgery. Patients with early recurrence had a non‐significantly higher tumour stage, more tumour‐positive lymph nodes and a higher prevalence of perineural invasion than patients without early recurrence (Table 2). In patients who developed distant metastasis, liver metastases were more prevalent in the early recurrence group (39 of 55 (71 per cent) versus 60 of 267 (22·5 per cent); P < 0·001).
Table 2.
Patient characteristics according to time until recurrence
|
Recurrence within 6 months (n = 55) |
All other patients (n = 267) |
P ‡ | |
|---|---|---|---|
| Age (years) * | 64(10) | 66(10) | 0·150§ |
| Sex ratio (M : F) | 29: 26 | 132: 135 | 0·657 |
| Death | 53 (96) | 178 (66·7) | < 0·001 |
| Survival (months) † | 8 | 23 | < 0·001¶ |
| Tumour size (mm) * | 32(14) | 28(16) | 0·083§ |
| Adjuvant therapy | 23 (42) | 149 (55·8) | 0·058 |
| Tumour differentiation | 0·078 | ||
| Well | 6 (11) | 57 (21·3) | |
| Moderate | 20 (36) | 113 (42·3) | |
| Poor | 29 (53) | 95 (35·6) | |
| Undifferentiated | 0 (0) | 2 (0·7) | |
| Lymph node‐positive | 43 (78) | 179 (67·0) | 0·104 |
| No. of positive lymph nodes ‡ | 2 (0–4) | 2 (1–5) | 0·125# |
| Tumour stage | 0·192 | ||
| IA | 2 (4) | 25 (9·4) | |
| IB | 1 (2) | 10 (3·7) | |
| IIA | 9 (16) | 50 (18·7) | |
| IIB | 38 (69) | 169 (63·3) | |
| III | 3 (5) | 12 (4·5) | |
| IV | 2 (4) | 1 (0·4) | |
| Perineural invasion | 40 (73) | 161 (60·3) | 0·083 |
| Lymphangioinvasion | 15 (27) | 55 (20·6) | 0·275 |
| Resection margin | 0·071 | ||
| R0 | 27 (49) | 166 (62·2) | |
| R1 | 28 (51) | 101 (37·8) |
Values in parentheses are percentages unless indicated otherwise; values are
mean(s.d.),
median (95 per cent c.i.) and ‡median (i.q.r.). R0, resection margin free from tumour cells; R1, tumour cells within 1 mm of resection margin.
χ2 test, except
Student's t test,
log rank test and
Mann–Whitney U test.
The median time until LRR was 16 (95 per cent c.i. 12 to 20) months in the R1 group compared with 36 (not estimated, n.e.) months in the R0 group (P = 0·002) (Fig. 1 a). The LRR rate was similar in the R1 (7 patients) and R0 (12) groups (approximately 8 per cent) in the first 6 months after resection. Time to diagnosis of distant metastasis was also significantly shorter after R1 resection (15 (11 to 19) versus 20 (13 to 27) months; P = 0·036) (Fig. 1 b). Finally, time until any recurrence was shorter in the R1 group (13 (10 to 16) versus 15 (12 to 18) months; P < 0·001) (Fig. 1 c). LRR was associated significantly with perineural invasion (P = 0·031); the only significant predictor of distant metastasis was lymph node status (P = 0·001) (Table S1, supporting information).
Figure 1.
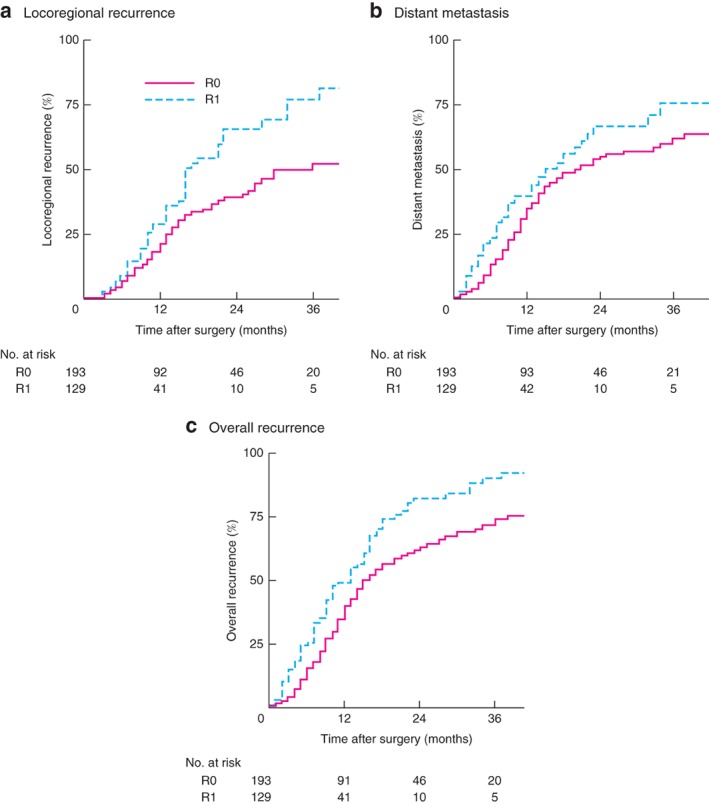
Locoregional recurrence, distant metastasis and overall recurrence according to resection margin status
a Locoregional recurrence (P = 0·002), b distant metastasis (P = 0·036) and c overall recurrence (P < 0·001) (log rank test).
Involvement of surgical margins and outcome
The vascular resection margin was involved with cancer cells in 56 of 107 patients (52·3 per cent) in the R1 group (Table S2, supporting information). The location of the R1 margin had no statistically significant association with surgical outcomes including OS and LRR (Fig. S1 a,c, supporting information).
Influence of margin status on long‐term distribution patterns of recurrence
There was no statistically significant difference in the distribution pattern of metastases between the R0 and R1 groups (Fig. 2). Some 41 of 277 patients (14·8 per cent) presented with distant metastasis within 5 months of surgery. Of 151 patients with distant metastasis, 38 (25·2 per cent) had liver metastases within 6 months after resection.
Figure 2.
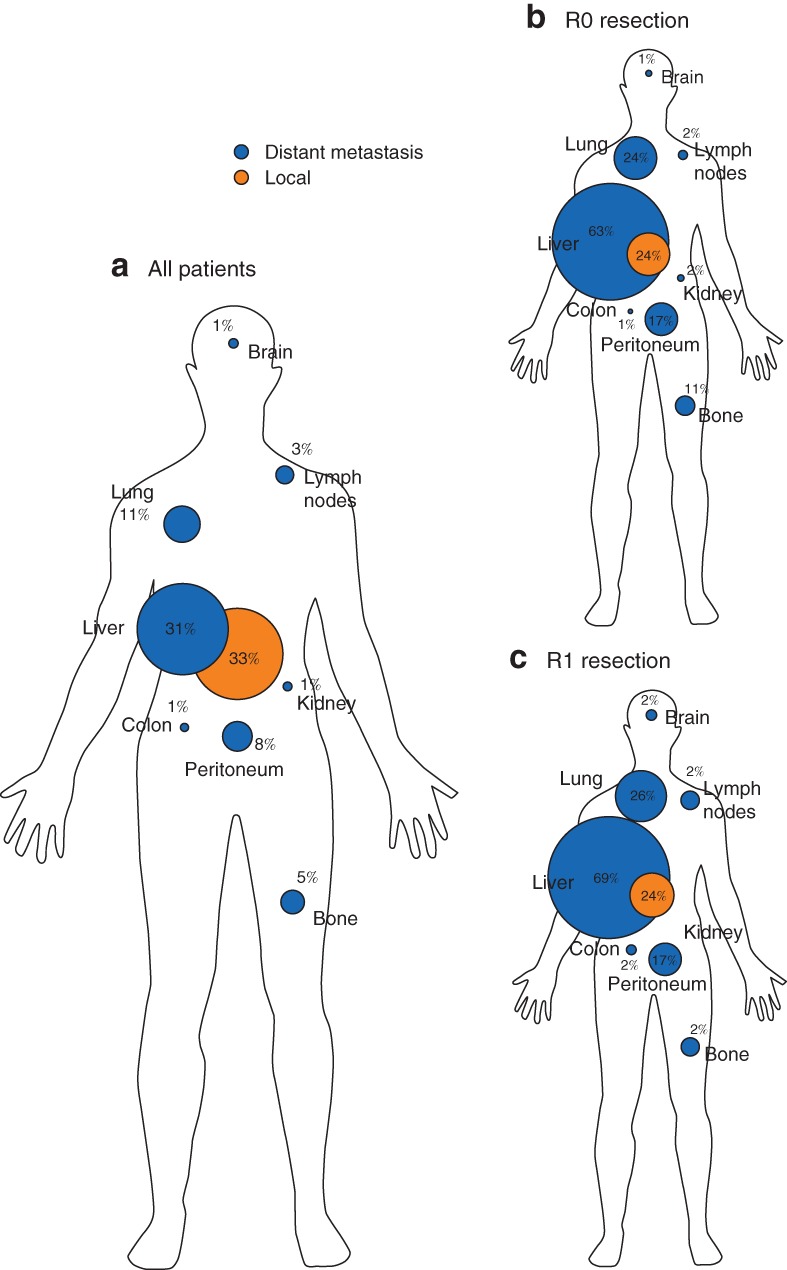
Spatial distribution of locoregional recurrence and distant metastasis following resection
a All patients, b after R0 resection, c after R1 resection. Percentages indicate the percentage of patients with recurrence at each site.
Correlation between margin status, tumour characteristics and outcome
Patients who underwent R1 resection had a median OS of 15 (95 per cent c.i. 13 to 17) months compared with 22 (17 to 27) months among those who had R0 resection (P < 0·001) (Fig. 3). Multivariable analyses revealed that perineural invasion, tumour‐positive lymph node status (N1) and R1 resection were significant predictors of OS, with hazard ratios (HRs) of 1·44 (P = 0·016), 2·15 (P < 0·001) and 1·37 (P = 0·031) respectively. Adjuvant chemotherapy had a protective effect on OS (HR 0·70; P = 0·012) (Table S3, supporting information).
Figure 3.
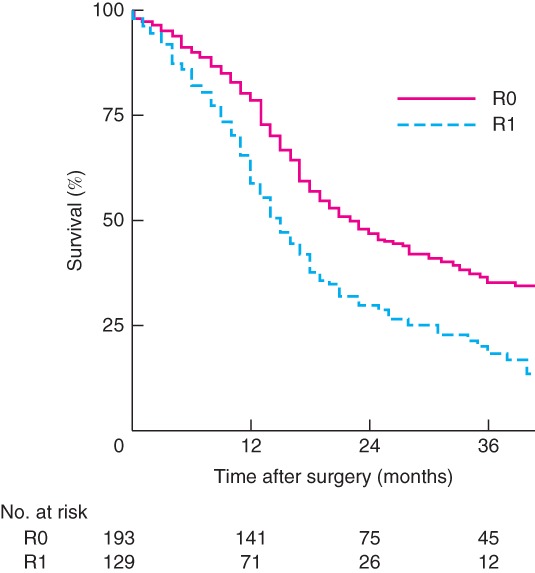
Overall survival according to resection margin status
P < 0·001 (log rank test).
Impact of surgical factors in patients with node‐negative disease
A separate analysis was done in which patients were grouped into those with lymph node‐negative (R0 N0, 71; R1 N0, 29) or node‐positive (R0 N1, 122; R1 N1, 100) disease. LRR occurred significantly earlier in patients with R1 N0 (median 17 (95 per cent c.i. 9 to 25) months) and R1 N1 status (16 (12 to 20) months) than in the R0 groups (Fig. 4 a). Patients with R0 N0 status had a significantly longer interval until the development of distant metastasis (67 (n.e.) months) than the other patients (Fig. 4 b).
Figure 4.
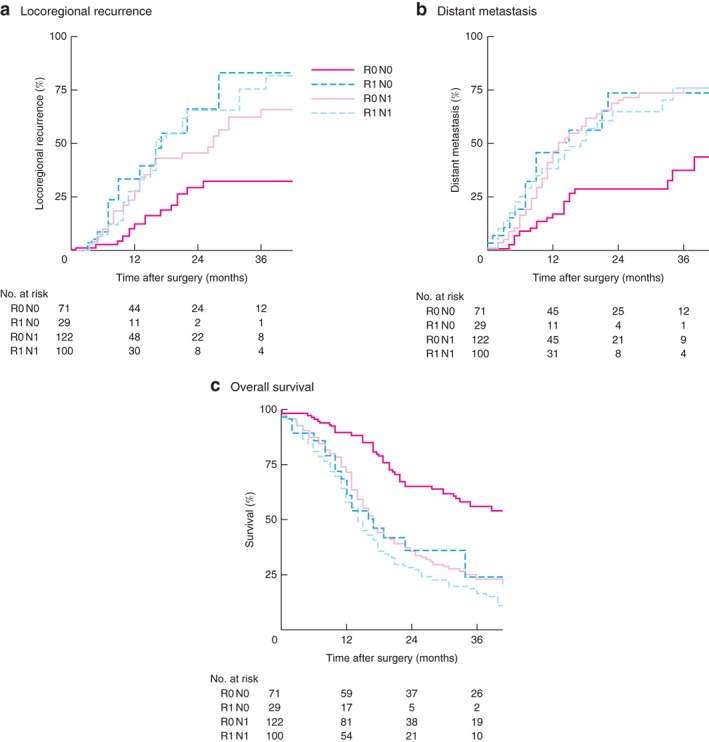
Subgroup analysis of effect of margin status (R0 or R1) and lymph node status (N0 or N1) on locoregional recurrence, distant metastasis and overall survival
a Locoregional recurrence (P < 0·001), b distant metastases (P < 0·001) and c overall recurrence (P < 0·001) (log rank test).
Patients with R0 N0 disease had significantly longer OS (median 45 (n.e.) months) than those with R0 N1 (17 (95 per cent c.i. 15 to 19) months; P < 0·001), R1 N0 (17 (10 to 24) months; P < 0·001) or R1 N1 (14 (12 to 16) months; P < 0·001) status (Fig. 4 c). Among patients with N0 tumours, OS was significantly worse after R1 compared with R0 resection (P = 0·002), although tumour characteristics were similar (Table 3). For patients with N1 disease, OS was similar after R1 and R0 resection (P = 0·068).
Table 3.
Effect of tumour biology
| Lymph node‐negative (N0) | Lymph node‐positive (N1) | |||||
|---|---|---|---|---|---|---|
|
R0 N0
(n = 71) |
R1 N0
(n = 29) |
P † |
R0 N1
(n = 122) |
R1 N1
(n = 100) |
P † | |
| Perineural invasion | 34 (48) | 16 (55) | 0·509 | 70 (57·4) | 81 (81·0) | < 0·001 |
| Lymphangioinvasion | 8 (11) | 6 (21) | 0·218 | 27 (22·1) | 29 (29·0) | 0·241 |
| Tumour differentiation | 0·802 | 0·741 | ||||
| Well | 16 (23) | 6 (21) | 24 (19·7) | 17 (17·0) | ||
| Moderate | 25 (35) | 10 (34) | 55 (45·1) | 43 (43·0) | ||
| Poor | 28 (39) | 13 (45) | 43 (35·2) | 40 (40·0) | ||
| Undifferentiated | 2 (3) | 0 (0) | 0 (0) | 0 (0) | ||
| Tumour size (mm) * | 27(20) | 27(18) | 0·933‡ | 26(12) | 34(13) | < 0·001‡ |
Values in parentheses are percentages unless indicated otherwise; values are
mean(s.d). R0, resection margin free from tumour cells; R1, tumour cells within 1 mm of resection margin.
χ2 test, except
Student's t test.
Discussion
This study showed a clear difference in OS and overall recurrence between patients who underwent pancreatectomy with negative resection margins and those with involved resection margins. A high recurrence rate within 6 months after surgery was found in both groups. This indicates the limitations of the current staging modalities used to exclude micrometastases and to select patients for curative surgery. This finding is clinically relevant as median OS among patients with early recurrence was only 8 months, compared with 11 months in patients who received palliative chemotherapy for non‐resectable or metastatic disease35. In pancreatic cancer surgery, the focus should be on improving the detection of distant disease and local resectability of the tumour as this could improve outcome following resection.
FOLFIRINOX (folinic acid, 5‐fluorouracil, irinotecan and oxaliplatin) chemotherapy for pancreatic cancer has an increasing role in downstaging the tumour and improving resection rates as well as representing a potent regimen for metastatic disease. The association between resection margin status and lymphangioinvasion and lymph node status suggests that the prognosis of tumours with aggressive biological characteristics may be improved by adding (neo)adjuvant therapy. Previous studies28, 29 reported that R1 status is probably not only a proxy for surgical quality but can also reflect the tumour's biological behaviour. In the present study, a difference was found in perineural invasion, tumour‐positive lymph nodes and tumour stage between the R0 and R1 groups. These results are similar to those of Kimbrough and colleagues36, who reported a significantly higher lymph node ratio and more microvascular invasion in the R1 group. This may also explain the present difference in OS between the R1 and R0 patient groups.
In a subgroup analysis, outcome in patients with node‐negative pancreatic cancer was significantly influenced by resection margin status. Therefore, in patients with clinical N0 disease, the role of the surgeon in achieving a radical resection is of great importance. Involvement of the large vessels should be evaluated carefully during surgery, as the vascular margin is the margin at greatest risk of tumour involvement. New molecular imaging modalities, such as fluorescence or photoacoustic imaging, could be used to improve the detection of tumour‐positive lymph nodes and may facilitate radical resection, especially at difficult margins including the posterior one37, 38, 39. These imaging techniques, with use of an exogenous agent directed against the tumour, allow the surgeon to image the lesion and may provide important additional information during the operation. In addition, molecular imaging may be beneficial in terms of disease staging. Contrast‐enhanced ultrasound imaging and/or tumour‐specific PET could be useful for preoperative detection of distant metastases and local staging40.
It is difficult to achieve a radical resection at the posterior surgical margin during pancreatoduodenectomy4, 25, 41. Vascular resection is an option to obtain a clear resection margin in locally advanced tumours. Therefore, an accurate method for detecting extension of the tumour before or during surgery is essential. Osipov and colleagues25 included the posterior surface, vascular groove and uncinate margins in the definition of posterior margin, in accordance with criteria established by the AJCC. Their data are consistent with the present finding that the vascular margin was associated with clinical outcomes, albeit not significantly. In a recent study10 of 1151 patients, a positive direct posterior resection margin was associated with reduced OS and recurrence‐free survival, whereas a positive direct superior mesenteric margin was associated with a higher local recurrence rate. For both margins, however, a R1 margin smaller than 1 mm did not affect clinically relevant outcomes. On the other hand, another study42 reported that only the transection margin of the pancreatic neck and the superior mesenteric artery‐facing margin were associated with prognosis. At present, it is unclear why involvement of a specific margin would affect prognosis more than other margins; the answer may lie in differences in the density of blood vessels, nerves and/or lymphatic vessels surrounding the pancreas.
This study has shown that patients with tumour‐free resection margins have better survival than patients with involved resection margins after pancreatectomy. When the resection margin is involved, the vascular margin is most often the site of irradicality. Furthermore, because the overall distribution patterns of recurrence are similar after R0 and R1 resections, all patients may benefit from (neo)adjuvant treatment strategies. Moreover, achieving a radical resection can significantly change the outcome in patients with lymph node‐negative disease. Patients with suspected N0 disease should be identified, for example by improved imaging strategies; in these patients every attempt to achieve an R0 resection (for example by vascular resection) seems justified to achieve maximum local control.

Supporting information
Table S1 Factors associated with locoregional recurrence and distant metastases
Table S2 Percentage of involved margins in patients with pancreatic cancer
Fig. S1 Effect of vascular margin on (A) overall survival and (B) local recurrence in R0 and R1 patients. (C) Summary of the effect of resection margin on local recurrence and the time until recurrence in R0 and R1 patients
Table S3 Summary of factors that affect overall survival in patients with pancreatic cancer
Acknowledgements
This work was supported by a Bas Mulder Award (grant number UL2015‐7665) from the Dutch Cancer Society. W.S.T. was supported in part by the Michaël‐van Vloten Funds, the Lisa Waller Hayes Foundation, the Ketel 1 Study Funds Foundation, and the Stichting de Drie Lichten.
Disclosure: The authors declare no conflict of interest.
References
- 1. Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet 2011; 378: 607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010; 60: 277–300. [DOI] [PubMed] [Google Scholar]
- 3. Alexakis N, Halloran C, Raraty M, Ghaneh P, Sutton R, Neoptolemos JP. Current standards of surgery for pancreatic cancer. Br J Surg 2004; 91: 1410–1427. [DOI] [PubMed] [Google Scholar]
- 4. Ethun CG, Kooby DA. The importance of surgical margins in pancreatic cancer. J Surg Oncol 2016; 113: 283–288. [DOI] [PubMed] [Google Scholar]
- 5. Ansari D, Bauden M, Bergström S, Rylance R, Marko‐Varga G, Andersson R. Relationship between tumour size and outcome in pancreatic ductal adenocarcinoma. Br J Surg 2017; 104: 600–607. [DOI] [PubMed] [Google Scholar]
- 6. Bilici A. Prognostic factors related with survival in patients with pancreatic adenocarcinoma. World J Gastroenterol 2014; 20: 10802–10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baldwin S, Kukar M, Gabriel E, Attwood K, Wilkinson N, Hochwald SN et al Pancreatic cancer metastatic to a limited number of lymph nodes has no impact on outcome. HPB (Oxford) 2016; 18: 523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Elshaer M, Gravante G, Kosmin M, Riaz A, Al‐Bahrani A. A systematic review of the prognostic value of lymph node ratio, number of positive nodes and total nodes examined in pancreatic ductal adenocarcinoma. Ann R Coll Surg Engl 2017; 99: 101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yeo CJ, Cameron JL, Lillemoe KD, Sitzmann JV, Hruban RH, Goodman SN et al Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg 1995; 221: 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ghaneh P, Kleeff J, Halloran CM, Raraty M, Jackson R, Melling J et al; European Study Group for Pancreatic Cancer. The impact of positive resection margins on survival and recurrence following resection and adjuvant chemotherapy for pancreatic ductal adenocarcinoma. Ann Surg 2017; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 11. Raut CP, Tseng JF, Sun CC, Wang H, Wolff RA, Crane CH et al Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg 2007; 246: 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D et al; European Study Group for Pancreatic Cancer. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA 2010; 304: 1073–1081. [DOI] [PubMed] [Google Scholar]
- 13. Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H et al; European Study Group for Pancreatic Cancer. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004; 350: 1200–1210. [DOI] [PubMed] [Google Scholar]
- 14. Kato K, Yamada S, Sugimoto H, Kanazumi N, Nomoto S, Takeda S et al Prognostic factors for survival after extended pancreatectomy for pancreatic head cancer: influence of resection margin status on survival. Pancreas 2009; 38: 605–612. [DOI] [PubMed] [Google Scholar]
- 15. Verbeke CS. Resection margins and R1 rates in pancreatic cancer – are we there yet? Histopathology 2008; 52: 787–796. [DOI] [PubMed] [Google Scholar]
- 16. Khalifa MA, Maksymov V, Rowsell C. Retroperitoneal margin of the pancreaticoduodenectomy specimen: anatomic mapping for the surgical pathologist. Virchows Arch 2009; 454: 125–131. [DOI] [PubMed] [Google Scholar]
- 17. Khalifa MA. Intraoperative assessment of the Whipple resection specimen. J Clin Pathol 2007; 60: 975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Verbeke CS, Leitch D, Menon KV, McMahon MJ, Guillou PJ, Anthoney A. Redefining the R1 resection in pancreatic cancer. Br J Surg 2006; 93: 1232–1237. [DOI] [PubMed] [Google Scholar]
- 19. Esposito I, Kleeff J, Bergmann F, Reiser C, Herpel E, Friess H et al Most pancreatic cancer resections are R1 resections. Ann Surg Oncol 2008; 15: 1651–1660. [DOI] [PubMed] [Google Scholar]
- 20. Willett CG, Lewandrowski K, Warshaw AL, Efird J, Compton CC. Resection margins in carcinoma of the head of the pancreas. Implications for radiation therapy. Ann Surg 1993; 217: 144–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nitecki SS, Sarr MG, Colby TV, van Heerden JA. Long‐term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann Surg 1995; 221: 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sperti C, Pasquali C, Piccoli A, Pedrazzoli S. Survival after resection for ductal adenocarcinoma of the pancreas. Br J Surg 1996; 83: 625–631. [DOI] [PubMed] [Google Scholar]
- 23. Millikan KW, Deziel DJ, Silverstein JC, Kanjo TM, Christein JD, Doolas A et al Prognostic factors associated with resectable adenocarcinoma of the head of the pancreas. Am Surg 1999; 65: 618–623. [PubMed] [Google Scholar]
- 24. Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA et al Resected adenocarcinoma of the pancreas – 616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg 2000; 4: 567–579. [DOI] [PubMed] [Google Scholar]
- 25. Osipov A, Nissen N, Rutgers J, Dhall D, Naziri J, Chopra S et al Redefining the positive margin in pancreatic cancer: impact on patterns of failure, long‐term survival and adjuvant therapy. Ann Surg Oncol 2017; 24: 3674–3682. [DOI] [PubMed] [Google Scholar]
- 26. Chang DK, Johns AL, Merrett ND, Gill AJ, Colvin EK, Scarlett CJ et al Margin clearance and outcome in resected pancreatic cancer. J Clin Oncol 2009; 27: 2855–2862. [DOI] [PubMed] [Google Scholar]
- 27. Gebauer F, Tachezy M, Vashist YK, Marx AH, Yekebas E, Izbicki JR et al Resection margin clearance in pancreatic cancer after implementation of the Leeds Pathology Protocol (LEEPP): clinically relevant or just academic? World J Surg 2015; 39: 493–499. [DOI] [PubMed] [Google Scholar]
- 28. Hernandez J, Mullinax J, Clark W, Toomey P, Villadolid D, Morton C et al Survival after pancreaticoduodenectomy is not improved by extending resections to achieve negative margins. Ann Surg 2009; 250: 76–80. [DOI] [PubMed] [Google Scholar]
- 29. Vogel I, Kalthoff H, Henne‐Bruns D, Kremer B. Detection and prognostic impact of disseminated tumor cells in pancreatic carcinoma. Pancreatology 2002; 2: 79–88. [DOI] [PubMed] [Google Scholar]
- 30. Van den Broeck A, Sergeant G, Ectors N, Van Steenbergen W, Aerts R, Topal B. Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur J Surg Oncol 2009; 35: 600–604. [DOI] [PubMed] [Google Scholar]
- 31. Versteijne E, van Eijck CH, Punt CJ, Suker M, Zwinderman AH, Dohmen MA et al; Dutch Pancreatic Cancer Group (DPCG). Preoperative radiochemotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC trial): study protocol for a multicentre randomized controlled trial. Trials 2016; 17: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pahk E, Jesin R, Kluger Y, Epelbaum R, Lachter J. Predictors of early recurrence of adenocarcinoma of the head of the pancreas after curative resection. JOP 2015; 16: 597–600. [Google Scholar]
- 33. Fischer R, Breidert M, Keck T, Makowiec F, Lohrmann C, Harder J. Early recurrence of pancreatic cancer after resection and during adjuvant chemotherapy. Saudi J Gastroenterol 2012; 18: 118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010; 17: 1471–1474. [DOI] [PubMed] [Google Scholar]
- 35. Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y et al; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364: 1817–1825. [DOI] [PubMed] [Google Scholar]
- 36. Kimbrough CW, St Hill CR, Martin RC, McMasters KM, Scoggins CR. Tumor‐positive resection margins reflect an aggressive tumor biology in pancreatic cancer. J Surg Oncol 2013; 107: 602–607. [DOI] [PubMed] [Google Scholar]
- 37. Vahrmeijer AL, Hutteman M, van der Vorst JR, van de Velde CJ, Frangioni JV. Image‐guided cancer surgery using near‐infrared fluorescence. Nat Rev Clin Oncol 2013; 10: 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zackrisson S, van de Ven SMWY, Gambhir SS. Light in and sound out: emerging translational strategies for photoacoustic imaging. Cancer Res 2014; 74: 979–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Handgraaf HJ, Boonstra MC, Van Erkel AR, Bonsing BA, Putter H, Van De Velde CJ et al Current and future intraoperative imaging strategies to increase radical resection rates in pancreatic cancer surgery. Biomed Res Int 2014; 2014: 890230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tummers WS, Miller SE, Teraphongphom NT, Gomez A, Steinberg I, Huland DM et al Intraoperative pancreatic cancer detection using tumor‐specific multimodality molecular imaging. Ann Surg Oncol 2018; 25: 1880–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gnerlich JL, Luka SR, Deshpande AD, Dubray BJ, Weir JS, Carpenter DH et al Microscopic margins and patterns of treatment failure in resected pancreatic adenocarcinoma. Arch Surg 2012; 147: 753–760. [DOI] [PubMed] [Google Scholar]
- 42. Jamieson NB, Foulis AK, Oien KA, Going JJ, Glen P, Dickson EJ et al Positive mobilization margins alone do not influence survival following pancreatico‐duodenectomy for pancreatic ductal adenocarcinoma. Ann Surg 2010; 251: 1003–1010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Factors associated with locoregional recurrence and distant metastases
Table S2 Percentage of involved margins in patients with pancreatic cancer
Fig. S1 Effect of vascular margin on (A) overall survival and (B) local recurrence in R0 and R1 patients. (C) Summary of the effect of resection margin on local recurrence and the time until recurrence in R0 and R1 patients
Table S3 Summary of factors that affect overall survival in patients with pancreatic cancer


