Summary
Pregnancies complicated by diabetes have largely increased in number over the last 50 years. Pregnancy is characterized by a physiologic increase in insulin resistance, which, associated with increased oxidative stress and inflammations, could induce alterations of glucose metabolism and diabetes. If not optimally controlled, these conditions have a negative impact on maternal and foetal outcomes. To date, one can resort only to diet and lifestyle to treat obesity and insulin resistance during pregnancy, and insulin remains the only therapeutic option to manage diabetes during pregnancy. However, in the last years, in a variety of experimental models, inositol and antioxidants supplementation have shown insulin‐sensitizing, anti‐inflammatory, and antioxidant properties, which could be mediated by some possible complementary mechanism of action. Different isomers and multiple combinations of these compounds are presently available: Aim of the present review article is to examine the existing evidence in order to clarify and/or define the effects of different inositol‐ and antioxidant‐based supplements during pregnancy complicated by insulin resistance and/or by diabetes. This could help the clinician's evaluation and choice of the appropriate supplementation regimen.
Keywords: antioxidants, diabetes, glucose metabolism, inositol, pregnancy, safety
1. INTRODUCTION
Prevalence of obesity, type 2 diabetes, and gestational diabetes mellitus (GDM) have dramatically increased over the last 50 years in women in fertile age.1 A growing number of pregnant women are at risk for these conditions, all of which are characterized by insulin resistance, hyperinsulinaemia, inflammation, and increased oxidative stress.2 During pregnancy, a physiologic increase in insulin resistance occurs because of the release of placental hormones. These hormones aim to promote nutrients utilization by the foetus, especially in the second and third trimester.3 On the other hand, insulin resistance associated with pregnancy is the main pathogenic mechanism leading to the development of GDM.4
GDM does induce chronic maternal oxidative stress and inflammation.5 During pregnancy complicated by diabetes and/or obesity, an increase in the plasma concentration of circulating pro‐inflammatory cytokines such as tumour necrosis factor‐α (TNF‐α) and Interleukin 6 (IL‐6) is associated with a reduction of plasma levels of anti‐inflammatory molecules such as adiponectin and Interleukin 10 (IL‐10).6, 7 Furthermore, inflammatory mediators overexpression, together with an increase in reactive oxygen species (ROS), could lead to metabolic alterations and vascular disease.8 Given this, GDM could be viewed as a sort of short‐lived metabolic syndrome with oxidative stress‐related hyperglycaemia and inflammation playing a key role in the development of GDM. These alterations may induce modifications in signalling pathways responsible for several intracellular processes.9 Some of the processes are related to the inhibition of the insulin signalling pathway, resulting in insulin resistance,10 reduced insulin gene expression, and consequently, reduced β‐cells insulin secretion.11
In most countries, none of the oral antihyperglycaemic drugs are approved for use during pregnancy. For this reason, one can resort only to a diet and a certain lifestyle for treating obesity and insulin resistance during pregnancy, and insulin remains the only therapeutic option to manage diabetes during pregnancy.
In the last years, inositol (Myo‐ and d‐chiro‐inositol [DCI]) and antioxidants (eg, lipoic acid, resveratrol, and epigallocatechin‐3‐gallate [EGCG]) have shown anti‐inflammatory and insulin‐sensitizing properties in a variety of experimental models, with several data suggesting an important role for these molecules in modulating the pathogenesis of inflammation, oxidative stress, and insulin resistance.12
Inositol acts as a mediator of the action of insulin, and it is necessary to activate key enzymes in the metabolism of glucose. Antioxidants exert anti‐inflammatory activities and ameliorate cellular glucose metabolism by reducing the levels of oxidative stress.12
Inositol‐ and antioxidant‐based supplements could then represent a valid therapeutic approach in pregnancy complicated by diabetes and by insulin resistance in order to improve glucose metabolism and delay or avoid insulin therapy when needed.
However, each compound could target different mechanisms involved in the pathophysiology of insulin resistance and diabetes with useful and possible complementary effects. The selection of the inositol isomers or antioxidant molecules and their association should be made according to the type of alteration/disease presented. Moreover, scant information is available regarding the dosage and timing of these supplements administered, useful to obtain a significant improvement in metabolic homeostasis.
The aim of the present study is to review the available evidence (gathered through a PubMed literature search, from February 2018 to January 2019, including the terms “Inositol,” “Myo‐Inositol,” “d‐Chiro‐Inositol,” “Lipoic acid,” “resveratrol,” “diabetes,” “gestational diabetes,” “obesity,” “pregnancy,” “insulin resistance,” “oxidative stress,” “inflammation,” “glucose homeostasis,” and “safety”) on the effects of inositol‐ and antioxidant‐based supplement administration during pregnancy complicated by insulin resistance and/or by diabetes (Table 1). This could help to guide the clinician's evaluation and choice of the appropriate supplementation regimen.
Table 1.
Basic characteristics of discussed studies
| Study | Subjects | Daily Dosage | Duration of Treatment |
|---|---|---|---|
| Myo‐inositol | |||
| Corrado et al13 | 84, diet treated women with GDM | Myo 4 g plus folic acid 400 μg | 8 weeks |
| D'Anna et al14 | 220, pregnant women with a parent with type 2 diabetes at 12‐13 weeks of gestation | Myo 4 g plus folic acid 400 μg | From first trimester to delivery |
| D'Anna et al15 | 220, pregnant obese women | Myo 4 g plus folic acid 400 μg | From first trimester to delivery |
| Santamaria et al16 | 220, pregnant overweight women | Myo 4 g plus folic acid 400 μg | From first trimester to delivery |
| Matarrelli et al17 | 75, non‐obese singleton pregnant women with an elevated fasting glucose in the first or early second trimester | Myo 4 g plus folic acid 400 μg | From first trimester to delivery |
| d‐chiro‐inositol | |||
| Di Biase et al18 | 137, women with GDM | DCI 1 g | From first trimester to delivery |
| Comparison between Myo‐inositol, d‐chiro‐inositol, and Myo‐inositol plus d‐chiroinositol formulations | |||
| Malvasi et al19 | 65, healthy pregnant women between the 13th and 24th week of gestation | Myo 2 g, DCI 400 mg, folic acid 400 μg, plus manganese 10 mg | 8 weeks |
| Fraticelli et al20 | 80, GDM women | Myo 4 g plus folic acid 400 μg DCI 500 mg plus folic acid 400 μg Myo/DCI 1,1 g/27,6 mg plus folic acid 400 μg folic acid 400 μg (control treatment | 8 weeks |
| Dell'Edera et al21 | 83 women | 250 mg/day d‐chiro‐inositol, 1.75 g/day d‐myo‐inositol, 12.5 mg/day zinc, 10 mg/day methylsulfonylmethane, 400 μg/day 5‐methyltetrahydrofolic acid 400 μg/day folic acid (control group) | From first trimester to delivery |
| Farren et al22 | 240, women between 10 and 16 weeks ‘gestation | Myo/DCI 1.1/27.6 mg, folic acid 400 μg | From first trimester to delivery |
| Celentano et al23 | 157 pregnant women with fasting glucose≥92 mg/dl and ≤ 126 mg/dl at first trimester blood exams | Myo 4 g plus folic acid 400 μg DCI 500 mg plus folic acid 400 μg Myo/DCI 1,1 g/27,6 mg plus folic acid 400 μg folic acid 400 μg (control treatment | From first trimester to delivery |
| Comparison between Myo‐imnositol plus d‐chiro‐inositol and Myo‐inositol plus d‐chiro‐inositol plus Revifast formulation | |||
| Malvasi et al24 | 104, pregnant women | Revifast 80 mg plus Myo/DCI 200 mg/500 mg Myo 138 mg/DCI 500 mg placebo | From 24th and 28th weeks' gestation for 60 days |
| Epigallocatechin 3‐gallate | |||
| Zhang et al25 | 472 GDM women during third trimester | EGCG 500 mg | From third trimester to delivery |
Abbreviations: DCI, d‐chiro inositol; EGCG, epigallocatechin 3‐gallate; GDM, gestational diabetes; Myo, myoinositol.
2. INOSITOL MOLECULAR AND METABOLIC ASPECTS
Inositol (cyclohexanehexol), a cyclic carbohydrate with six hydroxyl groups, was originally isolated in 1850 from muscle extracts by Scherer and named Myo‐inositol (Myo), from the Greek word meaning “muscle.”26 Myo can be obtained through biosynthetic mechanisms and from dietary sources. Although Myo was initially considered as an essential nutrient belonging to the vitamin B family,27 it has been observed that the liver and the kidneys are able to produce up to 4 g/day of inositol.
Inositol deficiency may arise through a plethora of different mechanisms, including reduced intake, increased catabolism and excretion, decreased biosynthesis, and an inhibition of intestinal and cellular uptake. Inositol deficiency can significantly affect several human pathological conditions.27 Myo is a precursor for many inositol‐containing compounds, playing critical and different roles in signal transduction, in membrane biogenesis, in vesicle trafficking, and in chromatin remodelling.28
Myo is only one of the nine possible structural isomers of inositol, as other naturally occurring stereoisomers have been found: scyllo‐, muco‐, epi‐, neo‐, allo‐, cis‐, d‐chiro‐, and l‐chiro‐inositol.26 In vivo Myo can be converted into DCI in tissues expressing the specific epimerase.29 The epimerase activity is strictly dependent on insulin and shows a tissue‐dependent modulation.30 It has been observed that the epimerase activity is dependent also on time, on pH, and on cofactors availability.31 In diabetic rats' insulin sensitive tissues (liver, fat, and muscle), the conversion from Myo to DCI is reduced (20%‐30% vs 5%, respectively), and the epimerase activity is significantly impaired.32 A lower activity of the enzyme epimerase seems to explain the decreased urine and tissue DCI content (and increased Myo to DCI ratios) in conditions characterized by insulin resistance.31 The action of inositol epimerase also determines the physiological tissue distribution of Myo and DCI, which influences their distinct physiological functions.33 Myo is associated with glucose transporters translocation and glucose utilization, while DCI is majorly involved in the synthesis of glycogen in the liver, in fat, and in muscle tissue.33 As a matter of fact, Myo and DCI seem to be both implicated in glucose homeostasis, since abnormalities in the metabolism of both has been associated to insulin resistance and diabetes.34 Conversely, glucose can interfere with Myo metabolism at different levels, both increasing Myo degradation and inhibiting its biosynthesis and absorption, thus contributing to the Myo depletion observed in diabetes.27
Several abnormalities in the Myo pathway have been associated with abnormalities of the glucose metabolism pathway.35 Inositol is actively transported into the cell through two different mechanisms, primarily involving sodium ion‐coupled (SMIT1 and SMIT2) and proton‐coupled transporters (HMIT1).36 SMIT1/2 are influenced in different ways by glucose and by glucose‐dependent metabolic pathways.27 For instance, inhibitors of the sodium/glucose transporter 1/2 prevent both inositol and glucose uptake in hepatocytes, probably because this transporter system is common to these molecules.37 Cellular inositol uptake is also decreased in the presence of high glucose concentrations. A competitive inhibition of sodium ion‐coupled transporters during hyperglycaemia leads to a Myo depletion in nervous tissues.38 Accordingly, in retinal epithelial cell cultures, inositol uptake could be significantly inhibited by high (20mM) glucose concentration via a competitive mechanism.39
As glucose interferes with inositol metabolism, inositol seems to exert a remarkable effect on glucose metabolism. In fact, it has been observed that inositol may directly act as a second messenger in the insulin signalling pathway having an important role in glucose metabolism as shown in Figure 1. Insulin can activate key enzymes involved in the oxidative and non‐oxidative glucose metabolism, which requires mediators such as inositol phosphoglycan (containing DCI).34 In this scenario, DCI is a mediator of insulin actions necessary to activate key enzymes in glucose metabolism, and in turn, it is involved in the pathogenesis of insulin resistance.40 Insulin stimulates glycosyl‐phosphatidylinositol hydrolysis through the direct activation of phospholipase C (PLC) and PLD, with the production of soluble inositol phosphoglycans. Upon insulin receptor activation, inositol phosphoglycans are released outside the cell and reimported by SMIT1/2 or HMIT1, activating cytosolic phosphoprotein phosphatase 2C‐α (PP2Cα) and mitochondrial PDH. This enhances oxidative glucose metabolism along the tricarboxylic acid cycle. The activated PP2Cα stimulates glycogen synthase by both direct and indirect mechanisms, through the phosphatidylinositide 3‐kinase (PI3K)/Akt pathway. Akt activation increases GLUT‐4 translocation and glucose uptake (Figure 1). Thus, intracellular inositol depletion can lead to insulin resistance, while restoring inositols cellular levels is expected to exert positive action on glucose metabolism.29
Figure 1.
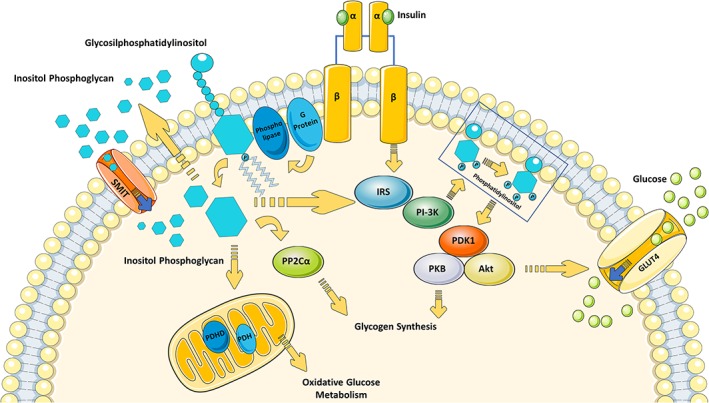
Role of inositol in insulin signalling pathway (inspired on Larner and Brautigan34). Upon binding with its receptor (IR), insulin induces IRS‐1 recruitment and activation. One of the principal IR/IRS target, PI3K, then generates Phosphatidilinositol to activate PDK1 and subsequently PKB/Akt. These actions are involved in GLUT4 translocation and in glycogen synthesis. According to the theory of Larner and Brautigan,34 IR activation might also be coupled to a heterotrimeric G protein, which in turn activates a phospholipase responsible for Glycosilphosphatidylinositol hydrolysis with the production of soluble inositol phosphoglycans. Upon insulin receptor activation, inositol phosphoglycans are released outside the cell and subsequently reimported by SMIT. Inositol phosphoglycans may act as a putative second insulin messenger. In the cytoplasm, inositol phosphoglycan binds to and allosterically activates PP2Cα and/or IRS, with the consequent direct stimulation of glycogen synthase. In the mitochondria, activated PDHP stimulates PDH and consequently glucose oxidative metabolism. Abbreviations: GLUT‐4 glucose transporter 4; GSK3, glycogen synthase kinase 3; IR, insulin receptor; IRS insulin receptor substrates; PDH, pyruvate dehydrogenase; PDHP, pyruvate dehydrogenase phosphatase; PDK‐1 phosphoinositide‐dependent kinase 1; PI3K, phosphoinositide 3 kinase; PKB/Akt, protein kinase B/Akt; SMIT, sodium/myo‐inositol transporter; PP2Cα, phosphoprotein phosphatase 2C alpha
A generalized deficiency of DCI bioactivity and content was observed in hemodialysate and in the muscle of type 2 diabetes patients compared with healthy subjects.41 Moreover, as compared with healthy controls, urinary DCI excretion was found significantly reduced in impaired glucose tolerance, in first‐degree type 2 diabetes subjects' relatives, and in type 2 diabetes subjects, whereas the Myo‐inositol excretion was elevated.34 In addition, urinary excretion of the DCI progressively decreases from subjects with normal glucose tolerance to subjects with impaired glucose tolerance and subjects with diabetes.42, 43 In contrast, Ostlund et al44 observed that DCI clearance was selectively elevated in both non–insulin‐dependent and insulin‐dependent diabetic subjects when compared with clearance of Myo. In addition, Baillargeon et al45 observed a direct relation between the degree of obesity or insulin resistance and DCI renal clearance. In these patients, however, the Myo/DCI ratio was still elevated, indicating that Myo excretion remains greater than DCI excretion.44
Insulin resistance is a physiologic phenomenon characterizing normal pregnancy, pathologically exacerbated in GDM. Scioscia et al46 observed that, as compared with healthy non‐pregnant women, in normal pregnancy, there is a higher urinary concentration of inositolphosphoglycan P‐type, which contains DCI; an even higher urinary concentration of DCI was measured in GDM compared with control pregnant women.47 A possible interpretation of these data is that during pregnancy, DCI production increases in response to maternal hyperinsulinaemia in order to improve glucose disposal. The same authors observed a positive association between DCI urinary excretion and poor glycaemic control in the second trimester of pregnancy.48 On the contrary, they observed that a poorer glycaemic control resulted in a reduction on Myo urinary content.49 The authors argue that increased DCI production/excretion, as well as the reduced Myo urine content, could represent a compensatory reaction to the hyperinsulinaemic state, rather than a primary effect mediated by insulin resistance. Thus, alterations in Myo/DCI metabolism could be among the mechanisms responsible for some diabetes‐related negative outcomes, such as macrosomia or impaired intrauterine growth.46
DCI deficiency has been observed in different conditions sharing insulin resistance as one of the etiopathogenic mechanisms, such as polycystic ovary syndrome (PCOS) or preeclampsia (a condition in which an insulin resistance state has been observed). Scioscia et al50 observed that, following insulin stimulation, the placenta membranes of a woman with preeclampsia released less DCI as compared with the placenta membrane of healthy women. These data further emphasize the correlation between impaired plasma DCI and insulin resistance and hints that the imbalance between DCI and Myo might be related more to insulin resistance rather than to type 2 diabetes.34
Few but interesting preclinical data exist regarding the role of inositols in the prevention and treatment of endothelial alterations. Nascimento et al51 observed that inositols improved the endothelial function in diabetic rat and rabbit vessels. A synthetic derivative of DCI (dibutyryl DCI) was effective to acutely reduce the ROS in endothelial cells and to preserve nitric oxide signalling in a dose‐dependent manner.51 Moreover, DCI reduces vascular damage progression by modulating the protein kinase C (PKC) activation, the hexosamine pathway activity, and advanced glycation end product formation.51 According to these findings, inositol could play a pivotal role in insulin signalling impairment, in metabolic disorders, and in endothelial dysfunction. Several clinical findings are emerging with regards to the impact of inositol supplementation in human pregnancy.
3. CLINICAL EFFECTS OF MYO‐INOSITOL SUPPLEMENTATION
In humans, it has been demonstrated that, as compared with normal pregnancy, patients with GDM exhibit significantly greater urinary inositol excretion in the first trimester, which is consistent with an alteration of insulin's effects on inositol metabolism in this group.52 According to these findings, a randomized, controlled, open‐label study was carried out in 84 pregnant women diagnosed for GDM between 24 and 28 weeks of gestation to assess the effect of Myo supplementation on insulin resistance parameters.13 Insulin resistance (HOMA) and adiponectin circulating levels were assessed. As compared with the control group, a significantly greater reduction in HOMA, fasting plasma glucose, and insulin levels (P = .0001), associated with a greater increase in circulating adiponectin levels (P = .009) were observed after 8 weeks of treatment with 4 g Myo/die.13
Fraticelli et al20 recently conducted in pregnant women affected by GDM an open‐label, parallel, randomized, controlled trial to compare the effects of 8 weeks of treatment with different dosages and combinations of inositol stereoisomers on insulin resistance (assessed by HOMA‐IR) and several maternal‐foetal outcomes. Women affected by GDM treated with Myo (4000 mg) showed a significant amelioration in insulin resistance and a lower variation in average weight gain during pregnancy as compared with DCI (500 mg) and to Myo/DCI (1100 mg/27.6 mg). Moreover, women exposed to Myo and Myo plus DCI required a significantly lower necessity of an intensified insulin treatment.20
Myo, at dosage of 2 to 4 g/day, significantly improved metabolic parameters in several conditions characterized by insulin resistance (menopause, metabolic syndrome, and PCOS).31
Given the evidence supporting the notion that Myo supplementation could improve insulin resistance, several randomized and controlled studies have been carried out in recent years to evaluate whether Myo supplementation may delay or prevent the GDM onset in pregnant women with risk factors for GDM.
The incidence of GDM was investigated in three randomized, controlled trials after 4‐g/die Myo supplementation in pregnant women (after the first trimester) with different risk factors: familiar history of type 2 diabetes,14 obesity,15 and overweight.16
As compared with placebo, the incidence of GDM was significantly reduced in women supplemented with Myo in all treatment groups. In particular, in women with a family history of type 2 diabetes, the incidence of GDM was of 6% (vs 15.3% in the placebo group, P = .04); in obese women, it was 14% (vs 33.6% in the placebo group, P = .001), and in overweight women, it was 11.6% (vs 27.4% in the placebo group, P = .004).
In the three studies, the odds ratios (ORs) for occurrence of GDM in the Myo treated were 0.35 (95% CI, 0.13‐0.96); 0.34 (95% CI, 0.17‐0.68); and 0.33 (95% CI, 0.15‐0.70), respectively.
On the other hand, the evaluation of additional secondary outcomes (incidence of foetal macrosomia (birth weight > 4000 g), gestational hypertension, preterm delivery, caesarean section, shoulder dystocia, neonatal hypoglycaemia, and neonatal transfer to an intensive care unit) did not reveal appreciable differences between the Myo‐ and the placebo‐exposed groups. This could be explained by the low rate of occurrence of such events, limiting the statistical power of the studies. Since the three aforementioned studies were carried out on homogeneous populations and were performed with superimposable protocol and methodology, it was possible to conduct a pooled analysis relative to the rate of adverse foetal and maternal clinical outcomes.53 Myo treatment was associated with a reduction in the incidence of GDM (11.0% vs 25.3%, P < .001), in the risk of preterm birth (3.4% vs 7.6%, P = .03), in the prevalence of macrosomia (2.1% vs 5.3%, P = .04), in the percentage of large‐for‐gestational‐age (LGA) babies (4.8% vs 8.9%, P = .04). Only a trend towards a reduction was observed for gestational hypertension (1.4% vs 3.9%, P = .07).53
A recent meta‐analysis conducted on the same trials confirmed these results.54 It should be kept in mind that these are retrospective analyses based on open‐label studies still suffering from a lack of statistical power related to the relatively low occurrence of the outcome considered. Furthermore, the randomized controlled trials included in the analyses, having enrolled mostly Caucasian women, still remains unclear as to whether these results can be extended to women of different ethnicities.53, 54
Matarrelli et al17 also observed a lower incidence of GDM in a pilot, randomized, double‐blind, placebo‐controlled trial conducted with 4 g of Myo per day in 75 non‐obese pregnant women with elevated fasting glucose (blood glucose ≥92 mg/dl and ≤126 mg/dl) in the first or early second trimester. The GDM prevalence was 71% in the placebo group vs 6% in the Myo group, relative risk 0.127; 95% CI, 0.032‐0.502; P = .001, with an absolute risk decrease of more than 60%. Moreover, Myo supplementation was associated with less weight gain as compared with placebo (body mass index [BMI] increase 2.3 ± 1.1 vs 3.8 ± 2.5 kg/m2, respectively; 95% CI, 0.732‐2.5; P = .001). Women randomized to Myo also delivered at a longer gestational age, and less episodes of neonatal hypoglycaemia occurred in their offsprings. This was confirmed by a larger randomized controlled trial by Celentano et al23 who observed that Myo supplementation (2 g twice a day) in women at high risk for GDM reduced the incidence of diabetes as compared with DCI alone (500 mg) and also as compared with the association of Myo/DCI (1100 mg/27.6 mg).
Myo supplementation in GDM treatment was also the object of a Cochrane review in 2016.55 The authors concluded that it remained uncertain as to whether Myo supplementation could affect the need for insulin therapy during pregnancy. They also concluded that the evidence in support of a clinically significant Myo supplementation effect on fasting blood glucose and adverse pregnancy and/or neonatal outcomes is still to date insufficient.
All of the abovementioned clinical results on Myo supplementation need to be considered in light of the fact that to date, little is known about the bioavailability and optimal dosage of Myo. The allowed maximum daily inositol intake is presently up to 4 g. On the basis of the few available data, it seems that splitting the 4‐g dose into two daily administrations results in a full‐day coverage.56 It still remains to be ascertained as to whether some pharmaceutical forms are better than others and which they eventually could be.
4. CLINCAL EFFECTS OF D‐CHIRO‐INOSITOL SUPPLEMENTATION
Positive effects on metabolism have also been demonstrated for DCI.57, 58
To date, three studies reported the effects on metabolic parameters of supplementation with DCI alone during pregnancy. Di Biase et al18 observed the effects of a supplementation with 1 g of DCI on 137 pregnant women diagnosed with GDM after the 24th week of gestation. Women were randomized to receive either DCI 500 mg twice a day or no supplement. After 9 weeks of treatment, self‐assessed pre‐prandial capillary glucose values were lower in the DCI supplemented group. Maternal insulin requirement, maternal weight gain, newborn abdominal circumference, and head circumference also resulted significantly lower in the DCI group.
Opposite data were obtained in a study comparing different inositol stereoisomers in GDM women where insulin sensitivity was not affected by treatment with 250 mg of DCI plus 200 μg FA, twice a day.20 In addition, in a randomized controlled trial, Celentano et al23 used different inositol stereoisomers in order to investigate their ability to prevent GDM in high‐risk women and obtained the largest benefit with Myo supplementation, as compared with supplementation with DCI or with Myo/DCI.
5. CLINICAL EFFECTS OF MYO/DCI ASSOCIATION SUPPLEMENTATION
Although a much larger experience exists with Myo rather than with DCI particularly in GDM women, clinical data are available regarding the combination of the use of DCI and Myo/DCI.
In a pilot study involving 20 patients with type 2 diabetes mellitus, a combination of Myo 550 mg and DCI 13.8 mg added twice a day to glucose‐lowering drugs was associated with a significant reduction in blood glucose and HbA1c levels after a 3‐month follow‐up.59 Sixty days of treatment with a fixed combination of Myo/DCI/Folic Acid (2000 mg/400 mg/400 μg, respectively) plus 10 mg of manganese was shown to significantly improve plasma lipids and blood glucose in a study carried out in 48 healthy pregnant women, enrolled between 13 and 24 weeks of gestation.19
As mentioned above, supplementation with DCI alone (250 mg of DCI plus 200 μg FA, twice a day) or associated with Myo (Myo 550 mg/DCI 13.8 mg/FA 200 μg twice a day) in women with GDM did not produce a significant effect on insulin sensitivity.20 However, in women requiring insulin therapy, supplementation with both Myo or Myo/DCI was associated with less need to up‐titrate insulin.20
When pregnant women with fasting blood glucose less than 92 mg/dl in the first trimester were randomized in the study by Dell'Edera et al21 to intake either only folic acid (400 mg/die) or a fixed‐dosage combination of Myo/DCI (1750 mg/250 mg/die plus zinc, methylsulfonylmethane, and 5‐methyltetrahydrofolic acid), the incidence of GDM at 24 weeks of pregnancy was significantly greater only in the FA group (relative risk, RR = 3.35; 95% confidence interval, CI, 1.37‐8.17; P = .0028). Moreover, birthweight was significantly lower in the Myo/DCI group (RR = 5.12; 95% CI, 1.21‐21.68; P = .0099).
On the contrary, in the study by Farren et al,22 in 240 pregnant women with a family history of diabetes, recruited between the 10th and 16th weeks of gestation, a lower fixed‐dosage combination of Myo/DCI/FA (1100 mg/27.6 mg/400 μg, respectively) did not result in any reduction in the incidence of GDM as compared with the treatment with FA only. As already mentioned, Celentano et al23 recently demonstrated the greatest benefit of Myo (4000 mg) compared with DCI alone (500 mg) and Myo/DCI association (1100 mg/27.6 mg) in the prevention of GDM in high‐risk pregnant women. These results, at odds with the previous study by Dell'Edera et al,21 could be due to either the use of inadequate doses of Myo/DCI or to late intervention.22
6. INOSITOL SAFETY
Inositol supplementation during childbearing age and during pregnancy has risen greatly in recent years, and it is therefore appropriate to explore not only the efficacy but also the safety of these molecules.
Both animal model studies and several clinical trials have been conducted in order to evaluate the safety of inositol supplementation.60 Preclinical data indicate no toxic effects in terms of kidney and cognitive functions or carcinogenesis.61, 62, 63
In mouse models, the preimplantation embryo exposure to Myo (10mM) resulted in absence of early toxic effects, as suggested by normal prenatal and short‐term postnatal development, and in a significant increase in the overall rate of the live births obtained, as compared with embryos cultured in absence of Myo.64
The safety of inositol supplementation was also demonstrated in humans.60
Myo has been used in preterm infants (<29 weeks of gestational age) with respiratory distress syndrome in order to assess safety and pharmacokinetics of different daily inositol doses (10, 40, or 80 mg/kg/d). In this setting, treatment with Myo 80 mg/Kg/die for more than 10 weeks did not result in increased incidence of any adverse event as compared with control babies.65
In clinical trials in which inositol dosages ranged from 4 to 60 g/day and the exposure time ranged from 1 to 12 months, the only adverse events reported were mild gastrointestinal symptoms (nausea, flatus, and diarrhoea) but only at doses greater of 12 g per day. It is worth noting that the severity of the adverse event did not worsen with increasing dosage from 12 up to 30 g.60 However, these dosages are not currently allowed for supplementation. Furthermore, no side effects were observed in patients receiving intervention with DCI (27.5 up 500 mg/day) in the clinical studies available.18, 19, 20, 21, 22
According to the aforementioned data, inositol is defined as generally recognized as safe (GRAS) by the FDA, which allows its use also in infants.66
Regarding foetal safety, the transplacental passage of Myo to the foetus seems not to be clinically relevant.67 Further studies are required to establish long‐term maternal and foetal safety, involving a larger number of patients from different ethnicities and with different risk factors for GDM.
7. PRE‐CLINICAL AND CLINICAL EFFECTS OF INOSITOL PLUS α‐LIPOIC ACID AND OTHER ANTIOXIDANT MOLECULES SUPPLEMENTATION
Most of the MI/DCI fixed dosage combinations available for inositol supplementation also contain molecules with alleged antioxidant properties. For some of these molecules, evidence is available regarding their efficacy and safety reviewed below.
7.1. α‐Lipoic acid
α‐Lipoic acid (LA) is a potent biological antioxidant and a naturally occurring cofactor of mitochondrial dehydrogenase complexes.68 It is believed to directly scavenge ROS and reactive nitrogen species (RNS), both in vitro and in vivo.69, 70 LA regenerates essential antioxidant molecules, ie, coenzyme Q10, vitamin C, vitamin E, etc and chelates several heavy metals involved in oxidative processes.71, 72, 73 In addition, LA can also repair oxidative stress‐damaged proteins, lipids, and DNA.74
Besides its antioxidant properties, LA can positively modulate insulin signalling pathways associated with an increase in oxidative stress such as those activated by inflammation and/or hyperglycaemia, putatively involved in both type 2 diabetes and in vessel damage pathophysiology.70, 75
For this reason, the use of LA as a therapeutic agent has recently been proposed in diabetes and obesity.
High‐fat diet–fed rats exhibited less weight gain and a better plasma lipid profile as compared with the control group when supplemented with LA.76 A few studies suggest that the LA supplementation ability in mitigating insulin resistance could be related to the modulation of visceral adipose inflammation. In white adipose tissues, the LA is able to stimulate expression and synthesis of protective factors such as AMPK and adiponectin and attenuate the release of cytokines such as monocyte chemokine protein 1 (MCP‐1) and TNF‐α.77, 78
The LA could have direct actions on the insulin‐controlled metabolic pathways, such as glucose uptake and glycogen synthesis. In vitro studies showed that LA is able to increase the GLUT1 and GLU4 translocation on adipocytes plasma membranes,79 probably by increasing the activity insulin receptor substrate‐1 (IRS‐1), phosphatidylinositol PI‐3K, and serine/threonine kinase Akt1, key enzymes involved in the insulin signalling pathways.80 Consistent results were obtained in animal models of insulin resistance. LA restored skeletal muscle insulin‐stimulated glucose uptake in obese Zucker rats81, 82, 83 and reduced plasma glucose levels while enhancing skeletal muscle insulin‐stimulated glucose uptake in streptozotocin‐induced diabetic rats.84
LA supplementation could have beneficial role on pregnancy as supported by preclinical observations indicating a protective effect of this molecule against diabetic embryopathy, foetal losses, and ultrastructural alteration of diabetic placentas.85, 86
Treatment with LA reduces markers of oxidative stress in patients with diabetes.87 In obese subjects with or without glucose intolerance, LA doses ranging from 1000 to 1800 mg for up 20 weeks resulted in a 3% body weight loss (about 3 kg).88, 89, 90 In an additional study, a significant reduction in plasma levels of inflammation markers (CRP, TNF‐α, and IL‐6) was also observed in obese subjects after LA supplementation.91
When administrated intravenously in obese patients with glucose intolerance, 600 mg of LA for 2 weeks resulted in an improvement in insulin resistance and lipid profile and in decreased pro‐inflammatory cytokine (TNF‐α and IL‐6) plasma levels.92 Three hundred–milligram LA supplementation for 2 months in type 2 diabetic subjects significantly decreased fasting plasma glucose, postprandial glucose, and insulin resistance.93, 94
On the other hand, in a study by Xiao et al,95 a short‐term (2 weeks) oral LA supplementation did not result in any improvement in insulin resistance in obese or overweight subjects.
The described data suggest a potential active role for LA in modulating glucose metabolism, thus allowing to consider a possible beneficial role of LA supplementation in pregnancies complicated by diabetes and/or obesity. Indeed, LA administration was associated with a lower incidence of miscarriages,96 spontaneous contractions,71 and preterm premature rupture of human foetal membranes. This effect could be mediated by the inhibition of TNF‐α induced remodelling and weakening of human foetal membranes.97
To our knowledge, however, to date, no clinical trials have been conducted exploring the effects of LA supplementation in pregnant women with GDM. However, the evidence gathered so far in non‐pregnant subjects, in our opinion, should encourage the carrying out of these studies. They could confirm a positive and safe role of the use of LA acid in pregnancies, complicated by diabetes.
7.2. α‐Lipoic acid safety
LA supplementation is safe for humans. This molecule is commonly introduced with diet (vegetables, brown rice, and red meat are, among others, food rich in LA). LA intake with food and/or as a dietary supplement did not induce any side effects even at high‐dosage intake, and so far, no upper limit for LA intake has been established by the Italian Ministry of Health.98
In a large number of research studies exploring the efficacy of LA supplementation in different settings (mainly in relation to diabetic neuropathic treatment), LA resulted definitely safe even at high dosages (as high as 1200‐1800 mg/day for 5 years).89, 99, 100 This is not surprising, considering that this molecule is contained in a wide range of aliments and thus it is continuously taken with the diet, even during pregnancy.101
A German postmarketing surveillance programme showed a low rate of adverse reaction, in line with what emerged from controlled clinical trials.102
Several studies highlighted LA positive effects in ameliorating the strength of human foetal membranes.97, 103 Furthermore, animal studies have shown that LA has a protective effect on the foetus in mothers who are diabetic, alcoholic, or exposed to toxic pollutants such as dioxin.104, 105 Two human studies specifically exploring the use of LA in pregnancy did not evidence any adverse events both in mothers and foetuses, providing a reassuring picture regarding the safety of this molecule.71, 106
7.3. Resveratrol
Resveratrol, a polyphenol found in a number of plant‐based foods, for instance, red wine, has received a great deal of attention because of its several potential effects on health.107
In diabetic rats, resveratrol supplementation was associated with an improvement in metabolic parameters, the prevention of embryonal malformations,108 and the reduction in glucose excursion, likely as result of histone deacetylase Sirtuin 1 activation.109 Human studies have shown that resveratrol is readily absorbed by the intestinal tract and is then rapidly metabolized and excreted, accounting for its low bioavailability,110 which could however be improved by the use of more potent resveratrol isoforms or by enhanced delivery methods.111
The only data available on the use of resveratrol in GDM pregnancy were obtained with the use of trans resveratrol derived from Polygonumcuspidatum/magnesium hydroxide complex (Revifast). The effects of Revifast plus Myo/DCI were investigated in a sample of 104 overweight pregnant overweight women randomized to either Revifast plus Myo/DCI or MI/DCI alone or placebo in a 1:1:1 design. A significant improvement in lipids and blood glucose after 30 and 60 days of treatment was observed in women treated with Myo/DCI vs placebo (P < .02). As compared with Myo/DCI alone, Revifast plus Myo/DCI was also associated with a significant reduction in triglycerides and total cholesterol (P < .03). None of the enrolled women developed GDM, making it impossible to determine whether any of the explored treatments had an effect on the occurrence of GDM.24
7.4. Resveratrol safety
The European Food Safety Authority (EFSA), on the basis of the available data on trans‐resveratrol (taking into account 23 articles on human studies and a review that covered 14 additional human studies), concluded that the molecule is safe and is not nutritionally disadvantageous.
Genotoxicity, carcinotoxicity, and chronic toxicity studies did not demonstrate any harmful effects at a dosage of up to 150 mg/day. Considering the weight of the evidence, the panel concluded that the intake level of 150 mg/day for adults does not raise safety concerns.112
7.5. Epigallocatechin 3‐gallate
EGCG is the most abundant polyphenol in green tea leaves. Green tea has been associated with positive effects in the treatment of obesity and other associated comorbidities such as type 2 diabetes. These benefits are thought to be related to the anti‐inflammatory and antioxidant effects of green tea and to the reduction in body fat percentage exhibited by its bioactive compounds.
EGCG seems to be associated with positive effects when used in the treatment of obesity and type 2 diabetes. The observed benefits are thought to be related to potential anti‐inflammatory and antioxidant properties of EGCG and to the reduction in the percentage of body fat observed in subjects exposed to the molecule.113 However, available information on the EGCG role in metabolic disease are to date contradictory.114, 115
It has been observed that EGCG improves insulin‐stimulated glucose uptake in cells from mice skeletal muscle,116 and its use was associated with a reduction in body weight and albuminuria in diabetic nephropathic patients.115, 117
Zhang et al25 formally investigated, in a randomized, placebo controlled, double‐blind, clinical trial whether EGCG supplementation (500 mg/die) was able to improve maternal metabolic parameters and neonatal outcomes in 404 pregnant women affected by GDM. The EGCG intervention resulted in a significant reduction in fasting plasma glucose and insulin levels with a significant improvement in insulin sensitivity. Moreover, neonatal hypoglycaemic episodes, respiratory distress syndrome, and macrosomia were significantly lower in EGCG‐treated GDM patients as compared with the placebo group.
Although obtained so far only in one study, these data nevertheless support a likely beneficial effect of the use of EGCG supplementation in GDM patients.25
7.6. Epigallocatechin 3‐gallate safety
On March 2018, the EFSA Panel was asked to provide a scientific opinion on the safety of preparations containing EGCG. The Panel examined published scientific literature, including interventional studies, monographs, and reports by national and international authorities. From the analysis, what emerged is that food supplements containing green tea catechins provide a daily dose of EGCG in the range of 5 to 1000 mg/day for adult population in Europe. On the basis of the data available, the Panel concluded that catechins from green tea extracts are generally considered safe according to the presumption of the safety approach.
Rare cases of liver injury have been reported in interventional clinical trials at an intake of doses equal or above 800 mg EGCG/day, most probably due to an idiosyncratic reaction.
None of the intervention studies addressed pregnant women, breastfed infants, or children. However, a report evaluating the reproductive and developmental toxicity of EGCG showed that feeding pregnant rats with diets supplemented with 100, 300, and 1000 mg EGCG/kg/day during organogenesis was nontoxic to dams or foetuses.118
8. CONCLUSIONS
Both observational studies and clinical trials so far conducted indicate that supplementation with inositol and antioxidants (LA, resveratrol, ECGC) is safe and well tolerated in the general population.
A strong body of evidences suggests that, if used early in the course of pregnancy in women at high risk for GDM, inositols are able to significantly reduce the incidence of glucose metabolism alterations during pregnancy.14, 15, 16, 17, 20, 23 The use of these molecules in GDM is associated with improved glucose metabolism and reduced adverse foetal/neonatal outcomes. Among inositols supplementation, 2 g Myo twice a day seems to be the best therapeutic approach to improve metabolic alterations.
A recent consensus by the Italian Diabetes National Societies (SID) states that, although further studies are necessary to confirm efficacy and safety, Myo at a daily dose of 4000 mg can be considered in the prevention of GDM in Caucasian women (level I, strength B). Myo (4000 mg) can be used in Caucasian women in the treatment of GDM in aid of Nutritional Medical Therapy and lifestyle interventions (level II, strength B).119
In light of the fact that in diabetic rats and in GDM women, Myo epimerase activity is low and thus Myo‐DCI conversion is reduced,31 the combination Myo/DCI could theoretically be a better choice in pregnant diabetic women or in women affected by GDM. However, the evidence gathered so far, probably in light of the low dose of DCI used in the clinical trials, falls short from proving that this is definitely true.
Extensive evidences exist in support of LA antioxidant properties and of its role as a fine modulator of many pivotal anti‐inflammatory and metabolic pathways. This dietary supplement appears to be useful in reducing the incidence of spontaneous contractions and preterm birth. The best results, in the general population, were obtained with doses of up to 1800 mg per day, and these were not associated with any adverse effects.89 In pregnancy, a daily dose of 600 mg was not associated with any adverse effects in both mothers and infants.71, 106 However, the efficacy of using this molecule in pregnancy to ameliorate maternal/foetal metabolic outcomes is still far from being proven.
According to the mechanisms of actions described, we can speculate that inositols and LA might exert complementary effects on glucose metabolism. Inositols directly facilitate insulin signalling transduction pathways34 while LA might reduce cellular ROS formation and thus mitigate inflammation, which in turn has a positive impact on glucose metabolism.71
Resveratrol and EGCG potential beneficial effects in pregnancy have not been clearly demonstrated at the moment. Very few studies have been conducted in pregnancy, and a much larger body of evidence is needed to eventually confirm the usefulness of supplementation with these molecules.24, 25
On the basis of the evidence reviewed in this article, we definitely believe that Myo exerts a positive action on the metabolic alterations common in the pregnant state (Tables 1 and 2). As to supplementation with LA, although only a small number of clinical studies have been conducted in pregnancy, a large number of clinical trials conducted on the general population supports a positive impact on the glucose metabolism modulation also for this molecule.87, 88, 89, 90, 91, 92, 93, 94
Table 2.
Supplements effect on maternal and fetal outcomes
| Maternal Outcomes | Foetal Outcomes | ||||
|---|---|---|---|---|---|
| GDM Diagnosis | Metabolic Outcomes | Birth Weight | Hypoglycaemic Episodes | Respiratory Distress Syndrome | |
| Myo‐inositol (4000 mg/die)a | ↓ | Improved | ↓ | ↓ | No data available |
| d‐chiro inositol (>500 mg/die)a | ‐ | Improved | ‐ | ‐ | No data available |
| α‐Lipoic acid | No data available | Improved | No data available | No data available | No data available |
| Resveratrol | No data available | ‐ | No data available | No data available | No data available |
| Epigallocatechin 3‐gallate | No data available | Improved | ↓ | ↓ | ↓ |
Note. Cells with “‐” represent inconsistent data.
Period of treatment more than 8 weeks.
However, in order to widely recommend use of inositols and or LA supplementation in pregnancy, controlled clinical trials on large populations of women with different phenotypes and ethnicity are still needed. Should the results of these trials confirm the benefit, use of these compounds during pregnancy at risk of GDM or complicated by diabetes should become highly recommended (if not mandated) in order to (a) reduce the incidence of GDM, (b) delay the need for insulin treatment, and (c) reduce insulin doses. These additional trials should also allow to better identify the optimal supplement doses and formulations in relation to peculiar patient characteristics and metabolic alterations.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
AUTHOR CONTRIBUTIONS
G.F., M.P.A.B, F.G., and M.A.C. researched the data, made substantial contributions to the discussion of the content, wrote the first draft, and reviewed/edited the manuscript. A.C. and I.B. made substantial contributions to the discussion of the content and reviewed/edited the manuscript.
FUNDING
This article has not been funded.
Formoso G, Baldassarre MPA, Ginestra F, Carlucci MA, Bucci I, Consoli A. Inositol and antioxidant supplementation: Safety and efficacy in pregnancy. Diabetes Metab Res Rev. 2019;35:e3154 10.1002/dmrr.3154
REFERENCES
- 1. Simmons D. Diabetes and obesity in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25(1):25‐36. 10.1016/j.bpobgyn.2010.10.006 [DOI] [PubMed] [Google Scholar]
- 2. Pitocco D, Tesauro M, Alessandro R, Ghirlanda G, Cardillo C. Oxidative stress in diabetes: implications for vascular and other complications. Int J Mol Sci. 2013;14(11):21525‐21550. 10.3390/ijms141121525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lain KY, Catalano PM. Metabolic changes in pregnancy. Clin Obstet Gynecol. 2007;50(4):938‐948. 10.1097/GRF.0b013e31815a5494 [DOI] [PubMed] [Google Scholar]
- 4. American Diabetes Association . Gestational diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S103‐S105. http://www.ncbi.nlm.nih.gov/pubmed/12502631. Accessed August 2, 2018 [DOI] [PubMed] [Google Scholar]
- 5. Cosentino F, Hishikawa K, Katusic ZS, Lüscher TF. High glucose increases nitric oxide synthase expression and superoxide anion generation in human aortic endothelial cells. Circulation. 1997;96(1):25‐28. http://www.ncbi.nlm.nih.gov/pubmed/9236411. Accessed August 2, 2018 [DOI] [PubMed] [Google Scholar]
- 6. Sobrevia L, Yudilevich DL, Mann GE. Elevated d‐glucose induces insulin insensitivity in human umbilical endothelial cells isolated from gestational diabetic pregnancies. J Physiol. 1998;506(Pt 1):219‐230. http://www.ncbi.nlm.nih.gov/pubmed/9481683. Accessed August 2, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ceriello A. New insights on oxidative stress and diabetic complications may lead to a “causal” antioxidant therapy. Diabetes Care. 2003;26(5):1589‐1596. http://www.ncbi.nlm.nih.gov/pubmed/12716823. Accessed August 2, 2018 [DOI] [PubMed] [Google Scholar]
- 8. Giri H, Chandel S, Dwarakanath LS, Sreekumar S, Dixit M. Increased endothelial inflammation, sTie‐2 and arginase activity in umbilical cords obtained from gestational diabetic mothers. Mohanraj R, ed. PLoS ONE. 2013;8(12):e84546 10.1371/journal.pone.0084546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813‐820. 10.1038/414813a [DOI] [PubMed] [Google Scholar]
- 10. Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress‐activated signaling pathways mediators of insulin resistance and beta‐cell dysfunction? Diabetes. 2003;52(1):1‐8. http://www.ncbi.nlm.nih.gov/pubmed/12502486. Accessed August 2, 2018 [DOI] [PubMed] [Google Scholar]
- 11. Chang Y‐C, Chuang L‐M. The role of oxidative stress in the pathogenesis of type 2 diabetes: from molecular mechanism to clinical implication. Am J Transl Res. 2010;2(3):316‐331. http://www.ncbi.nlm.nih.gov/pubmed/20589170. Accessed August 2, 2018 [PMC free article] [PubMed] [Google Scholar]
- 12. Abdali D, Samson SE, Grover AK. How effective are antioxidant supplements in obesity and diabetes? Med Princ Pract. 2015;24(3):201‐215. 10.1159/000375305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Corrado F, D'Anna R, di Vieste G, et al. The effect of myoinositol supplementation on insulin resistance in patients with gestational diabetes. Diabet Med. 2011;28(8):972‐975. 10.1111/j.1464-5491.2011.03284.x [DOI] [PubMed] [Google Scholar]
- 14. D'Anna R, Scilipoti A, Giordano D, et al. Myo‐inositol supplementation and onset of gestational diabetes mellitus in pregnant women with a family history of type 2 diabetes: a prospective, randomized, placebo‐controlled study. Diabetes Care. 2013;36(4):854‐857. 10.2337/dc12-1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. D'Anna R, Di Benedetto A, Scilipoti A, et al. Myo‐inositol supplementation for prevention of gestational diabetes in obese pregnant women: a randomized controlled trial. Obstet Gynecol. 2015;126(2):310‐315. 10.1097/AOG.0000000000000958 [DOI] [PubMed] [Google Scholar]
- 16. Santamaria A, Di Benedetto A, Petrella E, et al. Myo‐inositol may prevent gestational diabetes onset in overweight women: a randomized, controlled trial. J Matern Neonatal Med. 2015;29(19):1‐4. 10.3109/14767058.2015.1121478 [DOI] [PubMed] [Google Scholar]
- 17. Matarrelli B, Vitacolonna E, D'angelo M, et al. Effect of dietary myo‐inositol supplementation in pregnancy on the incidence of maternal gestational diabetes mellitus and fetal outcomes: a randomized controlled trial. J Matern Neonatal Med. 2013;26(10):967‐972. 10.3109/14767058.2013.766691 [DOI] [PubMed] [Google Scholar]
- 18. Di Biase N, Martinelli M, Florio V, Meldolesi C, Bonito M. The effectiveness of d‐chiro inositol treatment in gestational diabetes. Diab Case Rep. 2017;2(131):2 10.4172/2572-5629.1000131 [DOI] [Google Scholar]
- 19. Malvasi A, Casciaro F, Minervini MM, et al. Myo‐inositol, d‐chiro‐inositol, folic acid and manganese in second trimester of pregnancy: a preliminary investigation. 2014;18(2):270‐274. [PubMed] [Google Scholar]
- 20. Fraticelli F, Celentano C, Zecca IAL, et al. Effect of inositol stereoisomers at different dosages in gestational diabetes: an open‐label, parallel, randomized controlled trial. Acta Diabetol. May 2018. 10.1007/s00592-018-1157-4;55(8):805‐812. [DOI] [PubMed] [Google Scholar]
- 21. Dell'Edera D, Sarlo F, Allegretti A, Simone F, Lupo MG, Epifania AA. The influence of d‐chiro‐inositol and d‐myo‐inositol in pregnant women with glucose intolerance. Biomed Reports. 2017;7(2):169‐172. 10.3892/br.2017.939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Farren M, Daly N, McKeating A, Kinsley B, Turner MJ, Daly S. The prevention of gestational diabetes mellitus with antenatal oral inositol supplementation: a randomized controlled trial. Diabetes Care. 2017;40(6):759‐763. 10.2337/dc16-2449 [DOI] [PubMed] [Google Scholar]
- 23. Celentano C, Matarrelli B, Pavone G, et al. The influence of different inositol stereoisomers supplementation in pregnancy on maternal gestational diabetes mellitus and fetal outcomes in high‐risk patients: a randomized controlled trial. J Matern Neonatal Med. 2018;1‐9. 10.1080/14767058.2018.1500545 [DOI] [PubMed] [Google Scholar]
- 24. Malvasi A, Kosmas I, Mynbaev OA, et al. Can trans resveratrol plus d‐chiro‐inositol and myo‐inositol improve maternal metabolic profile in overweight pregnant patients? Clin Ter. 2017;168(4):e240‐e247. 10.7417/T.2017.2013 [DOI] [PubMed] [Google Scholar]
- 25. Zhang H, Su S, Yu X, Li Y. Dietary epigallocatechin 3‐gallate supplement improves maternal and neonatal treatment outcome of gestational diabetes mellitus: a double‐blind randomised controlled trial. J Hum Nutr Diet. 2017;30(6):753‐758. 10.1111/jhn.12470 [DOI] [PubMed] [Google Scholar]
- 26. Bizzarri M, Fuso A, Dinicola S, Cucina A, Bevilacqua A. Pharmacodynamics and pharmacokinetics of inositol(s) in health and disease. Expert Opin Drug Metab Toxicol. 2016;12(10):1181‐1196. 10.1080/17425255.2016.1206887 [DOI] [PubMed] [Google Scholar]
- 27. Dinicola S, Minini M, Unfer V, Verna R, Cucina A, Bizzarri M. Nutritional and acquired deficiencies in inositol bioavailability. Correlations with metabolic disorders. Int J Mol Sci. 2017;18(10):2187 10.3390/ijms18102187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carlomagno G, De Grazia S, Unfer V, Manna F. Myo‐inositol in a new pharmaceutical form: a step forward to a broader clinical use. Expert Opin Drug Deliv. 2012;9(3):267‐271. 10.1517/17425247.2012.662953 [DOI] [PubMed] [Google Scholar]
- 29. Bevilacqua A, Bizzarri M. Inositols in insulin signaling and glucose metabolism. Hindawi Int J Endocrinol. 2018;2018:1‐8. 10.1155/2018/1968450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun T, Heimark DB, Nguygen T, Nadler JL, Larner J. Both myo‐inositol to chiro‐inositol epimerase activities and chiro‐inositol to myo‐inositol ratios are decreased in tissues of GK type 2 diabetic rats compared to Wistar controls. Biochem Biophys Res Commun. 2002;293(3):1092‐1098. 10.1016/S0006-291X(02)00313-3 [DOI] [PubMed] [Google Scholar]
- 31. Croze ML, Soulage CO. Potential role and therapeutic interests of myo‐inositol in metabolic diseases. Biochimie. 2013;95(10):1811‐1827. 10.1016/j.biochi.2013.05.011 [DOI] [PubMed] [Google Scholar]
- 32. Pak Y, Hong Y, Kim S, Piccariello T, Farese RV, Larner J. In vivo chiro‐inositol metabolism in the rat: a defect in chiro‐inositol synthesis from myo‐inositol and an increased incorporation of chiro‐[3H]inositol into phospholipid in the Goto‐Kakizaki (G.K) rat. Mol Cells. 1998;8(3):301‐309. http://www.ncbi.nlm.nih.gov/pubmed/9666467. Accessed July 26, 2018 [PubMed] [Google Scholar]
- 33. Heimark D, McAllister J, Larner J. Decreased myo‐inositol to chiro‐inositol (M/C) ratios and increased M/C epimerase activity in PCOS theca cells demonstrate increased insulin sensitivity compared to controls. Endocr J. 2014;61(2):111‐117. 10.1507/endocrj.EJ13-0423 [DOI] [PubMed] [Google Scholar]
- 34. Larner J, Brautigan D. d‐chiro‐inositol glycans in insulin signaling and insulin resistance. Mol Med 2010;16(11‐12):543‐552. 10.2119/molmed.2010.00107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Croze ML, Géloën A, Soulage CO. Abnormalities in myo‐inositol metabolism associated with type 2 diabetes in mice fed a high‐fat diet: benefits of a dietary myo‐inositol supplementation. Br J Nutr. 2015;113(12):1862‐1875. 10.1017/S000711451500121X [DOI] [PubMed] [Google Scholar]
- 36. Schneider S. Inositol transport proteins. FEBS Lett. 2015;589(10):1049‐1058. 10.1016/j.febslet.2015.03.012 [DOI] [PubMed] [Google Scholar]
- 37. Kollros PE, Goldstein GW, Betz AL. Myo‐inositol transport into endothelial cells derived from nervous system microvessels. Brain Res. 1990;511(2):259‐264. http://www.ncbi.nlm.nih.gov/pubmed/2159359. Accessed February 1, 2019 [DOI] [PubMed] [Google Scholar]
- 38. Greene DA, Lattimer SA. Sodium‐ and energy‐dependent uptake of myo‐inositol by rabbit peripheral nerve. Competitive inhibition by glucose and lack of an insulin effect. J Clin Invest. 1982;70(5):1009‐1018. http://www.ncbi.nlm.nih.gov/pubmed/6813354. Accessed February 1, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thomas TP, Feldman EL, Nakamura J, et al. Ambient glucose and aldose reductase‐induced myo‐inositol depletion modulate basal and carbachol‐stimulated inositol phospholipid metabolism and diacylglycerol accumulation in human retinal pigment epithelial cells in culture. Proc Natl Acad Sci U S A. 1993;90(20):9712‐9716. http://www.ncbi.nlm.nih.gov/pubmed/8415767. Accessed February 1, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang LC, Heimark D, Linko J, Nolan R, Larner J. A model phosphatase 2C phosphatase 1 activation cascade via dual control of inhibitor‐1 (INH‐1) and DARPP‐32 dephosphorylation by two inositol glycan putative insulin mediators from beef liver. Biochem Biophys Res Commun. 1999;255(1):150‐156. 10.1006/bbrc.1999.0111 [DOI] [PubMed] [Google Scholar]
- 41. Asplin I, Galasko G, Larner J. chiro‐Inositol deficiency and insulin resistance: a comparison of the chiro‐inositol‐and the myo‐inositol‐containing insulin mediators isolated from urine, hemodialysate, and muscle of control and type II diabetic subjects. Med Sci. 1993;90:5924‐5928. https://doc‐0o‐6o‐apps‐viewer.googleusercontent.com/viewer/secure/pdf/a2vmvjnffuk5ijkjc8nkvda13sdhdepe/8fij2p7cplck08e0einffu4sfnfc3hqd/1522758375000/gmail/06233342817100067226/ACFrOgBz7UYXGoRF9m_F20‐5dGPLWjgkhc9_Nf7jiPpJ0cSZUtTkuidZJVh1J85lDIGmxroF8p7q‐Y. Accessed April 3, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Suzuki S, Kawasaki H, Satoh Y, et al. Urinary chiro‐inositol excretion is an index marker of insulin sensitivity in Japanese type II diabetes. Diabetes Care. 1994;17(12):1465‐1468. http://www.ncbi.nlm.nih.gov/pubmed/7882818. Accessed July 19, 2018 [DOI] [PubMed] [Google Scholar]
- 43. Jung T‐S, Hahm J‐R, Kim J‐J, et al. Determination of urinary Myo‐/chiro‐inositol ratios from Korean diabetes patients. Yonsei Med J. 2005;46(4):532‐538. 10.3349/ymj.2005.46.4.532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ostlund RE, McGill JB, Herskowitz I, et al. d‐chiro‐inositol metabolism in diabetes mellitus. Proc Natl Acad Sci U S A. 1993;90(21):9988‐9992. http://www.ncbi.nlm.nih.gov/pubmed/8234346. Accessed December 7, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Baillargeon J‐P, Diamanti‐Kandarakis E, Ostlund RE, Apridonidze T, Iuorno MJ, Nestler JE. Altered d‐chiro‐inositol urinary clearance in women with polycystic ovary syndrome. Diabetes Care. 2006;29(2):300‐305. 10.2337/DIACARE.29.02.06.DC05-1070 [DOI] [PubMed] [Google Scholar]
- 46. Scioscia M, Vimercati A, Selvaggi LE, Rodeck CH, Rademacher TW. Inositol phosphoglycan putative insulin mediator in human amniotic fluid. J Matern Neonatal Med. 2006;19(1):9‐12. 10.1080/14767050500362321 [DOI] [PubMed] [Google Scholar]
- 47. Scioscia M, Kunjara S, Gumaa K, McLean P, Rodeck CH, Rademacher TW. Urinary excretion of inositol phosphoglycan P‐type in gestational diabetes mellitus. Diabet Med. 2007;24(11):1300‐1304. 10.1111/j.1464-5491.2007.02267.x [DOI] [PubMed] [Google Scholar]
- 48. Scioscia M, Gumaa K, Selvaggi LE, Rodeck CH, Rademacher TW. Increased inositol phosphoglycan P‐type in the second trimester in pregnant women with type 2 and gestational diabetes mellitus. J Perinat Med. 2009;37(5):469‐471. 10.1515/JPM.2009.082 [DOI] [PubMed] [Google Scholar]
- 49. Scioscia M, Kunjara S, Gumaa K, et al. Altered urinary release of inositol phosphoglycan A‐type in women with gestational diabetes mellitus. Gynecol Obstet Investig. 2007;64(4):217‐223. 10.1159/000106494 [DOI] [PubMed] [Google Scholar]
- 50. Scioscia M, Nigro M, Montagnani M. The putative metabolic role of d‐chiro inositol phosphoglycan in human pregnancy and preeclampsia. J Reprod Immunol. 2014;101‐102(1):140‐147. 10.1016/j.jri.2013.05.006 [DOI] [PubMed] [Google Scholar]
- 51. Nascimento NRF, Lessa LMA, Kerntopf MR, et al. Inositols prevent and reverse endothelial dysfunction in diabetic rat and rabbit vasculature metabolically and by scavenging superoxide. Proc Natl Acad Sci U S A. 2006;103(1):218‐223. 10.1073/pnas.0509779103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Murphy A, Shamshirsaz A, Markovic D, Ostlund R, Koos B. Urinary excretion of Myo‐inositol and d‐chiro‐inositol in early pregnancy is enhanced in gravidas with gestational diabetes mellitus. Reprod Sci. 2016;23(3):365‐371. 10.1177/1933719115602767 [DOI] [PubMed] [Google Scholar]
- 53. Santamaria A, Alibrandi A, Di Benedetto A, et al. Clinical and metabolic outcomes in pregnant women at risk for gestational diabetes mellitus supplemented with myo‐inositol. A secondary analysis from 3 RCTs. Am J Obstet Gynecol. 2018;219(3):300‐e1. 10.1016/j.ajog.2018.05.018 [DOI] [PubMed] [Google Scholar]
- 54. Guo X, Guo S, Miao Z, Li Z, Zhang H. Myo‐inositol lowers the risk of developing gestational diabetic mellitus in pregnancies: a systematic review and meta‐analysis of randomized controlled trials with trial sequential analysis. J Diabetes Complicat. 2018;32(3):342‐348. 10.1016/j.jdiacomp.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 55. Brown J, Crawford TJ, Alsweiler J, Crowther CA. Dietary supplementation with myo‐inositol in women during pregnancy for treating gestational diabetes. Cochrane Database Syst Rev. 2016;9:CD012048 10.1002/14651858.CD012048.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Orrù B, Circo R, Logoteta P, Petousis S, Carlomagno G. Finding the best therapeutic approach for PCOS: the importance of inositol (s) bioavailability. Eur Rev Med Pharmacol Sci. 2017;21(2 Suppl):83‐88. [PubMed] [Google Scholar]
- 57. Nestler JE, Jakubowicz DJ, Reamer P, Gunn RD, Allan G. Ovulatory and metabolic effects of d‐ chiro ‐inositol in the polycystic ovary syndrome. N Engl J Med. 1999;340(17):1314‐1320. 10.1056/NEJM199904293401703 [DOI] [PubMed] [Google Scholar]
- 58. Iuorno MDMJ, Jakubowicz MDDJ, Baillargeon MDJ‐P, et al. Effects of d‐chiro‐inositol in lean women with the polycystic ovary syndrome. Endocr Pract. 2002;8(6):417‐423. 10.4158/EP.8.6.417 [DOI] [PubMed] [Google Scholar]
- 59. Pintaudi B, Di Vieste G, Bonomo M. The effectiveness of Myo‐inositol and d‐chiro inositol treatment in type 2 diabetes. Int J Endocrinol. 2016;2016:1‐5. 10.1155/2016/9132052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Carlomagno G, Unfer V. Inositol safety: clinical evidences. Eur Rev Med Pharmacol Sci. 2011;15(8):931‐936. http://www.ncbi.nlm.nih.gov/pubmed/21845803. Accessed February 5, 2019 [PubMed] [Google Scholar]
- 61. Pugliese G, Tilton RG, Speedy A, et al. Modulation of hemodynamic and vascular filtration changes in diabetic rats by dietary myo‐inositol. Diabetes. 1990;39(3):312‐322. http://www.ncbi.nlm.nih.gov/pubmed/2307293. Accessed February 5, 2019 [DOI] [PubMed] [Google Scholar]
- 62. Coppey LJ, Gellett JS, Davidson EP, Dunlap JA, Yorek MA. Effect of treating streptozotocin‐induced diabetic rats with sorbinil, myo‐inositol or aminoguanidine on endoneurial blood flow, motor nerve conduction velocity and vascular function of epineurial arterioles of the sciatic nerve. Int J Exp Diabetes Res. 2002;3(1):21‐36. http://www.ncbi.nlm.nih.gov/pubmed/11900277. Accessed February 5, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kassie F, Kalscheuer S, Matise I, et al. Inhibition of vinyl carbamate‐induced pulmonary adenocarcinoma by indole‐3‐carbinol and myo‐inositol in A/J mice. Carcinogenesis. 2010;31(2):239‐245. 10.1093/carcin/bgp174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kuşcu N, Bizzarri M, Bevilacqua A. Myo‐inositol safety in pregnancy: from preimplantation development to newborn animals. Int J Endocrinol. 2016;2016:1‐10. 10.1155/2016/2413857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Phelps DL, Ward RM, Williams RL. Safety and pharmacokinetics of multiple dose myo‐inositol in preterm infants. Pediatr Res. 2016;80(2):209‐217. 10.1038/pr.2016.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. FDA . Food And Drug Administration Department Of Health And Human Services Subchapter B‐‐Food For Human Consumption. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1370. Accessed February 5, 2019.
- 67. Staat BC, Galan HL, Harwood JEF, et al. Transplacental supply of mannose and inositol in uncomplicated pregnancies using stable isotopes. J Clin Endocrinol Metab. 2012;97(7):2497‐2502. 10.1210/jc.2011-1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Packer L, Witt EH, Tritschler HJ. Alpha‐lipoic acid as a biological antioxidant. Free Radic Biol Med. 1995;19(2):227‐250. http://www.ncbi.nlm.nih.gov/pubmed/7649494. Accessed August 1, 2018 [DOI] [PubMed] [Google Scholar]
- 69. Monastra G, De Grazia S, Cilaker Micili S, Goker A, Unfer V. Immunomodulatory activities of alpha lipoic acid with a special focus on its efficacy in preventing miscarriage. Expert Opin Drug Deliv. 2016;13(12):1695‐1708. 10.1080/17425247.2016.1200556 [DOI] [PubMed] [Google Scholar]
- 70. Di Tomo P, Di Silvestre S, Cordone VGP, et al. Centella asiatica and lipoic acid, or a combination thereof, inhibit monocyte adhesion to endothelial cells from umbilical cords of gestational diabetic women. Nutr Metab Cardiovasc Dis. 2015;25(7):659‐666. 10.1016/j.numecd.2015.04.002 [DOI] [PubMed] [Google Scholar]
- 71. Parente E, Colannino G, Picconi O, Monastra G. Safety of oral alpha‐lipoic acid treatment in pregnant women: a retrospective observational study. Eur Rev Med Pharmacol Sci. 2017;21(18):4219‐4227. [PubMed] [Google Scholar]
- 72. Suh JH, Shenvi SV, Dixon BM, et al. Decline in transcriptional activity of Nrf2 causes age‐related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci U S A. 2004;101(10):3381‐3386. 10.1073/pnas.0400282101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ou P, Tritschler HJ, Wolff SP. Thioctic (lipoic) acid: a therapeutic metal‐chelating antioxidant? Biochem Pharmacol. 1995;50(1):123‐126. http://www.ncbi.nlm.nih.gov/pubmed/7605337. Accessed August 1, 2018 [DOI] [PubMed] [Google Scholar]
- 74. Spector A, Huang RR, Yan GZ, Wang RR. Thioredoxin fragment 31‐36 is reduced by dihydrolipoamide and reduces oxidized protein. Biochem Biophys Res Commun. 1988;150(1):156‐162. http://www.ncbi.nlm.nih.gov/pubmed/3122752. Accessed August 1, 2018 [DOI] [PubMed] [Google Scholar]
- 75. Gomes M, Negrato C. Alpha‐lipoic acid as a pleiotropic compound with potential therapeutic use in diabetes and other chronic diseases. Diabetol Metab Syndr. 2014;6(1):80 10.1186/1758-5996-6-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cho K‐J, Moon H‐E, Moini H, Packer L, Yoon D‐Y, Chung A‐S. Alpha‐lipoic acid inhibits adipocyte differentiation by regulating pro‐adipogenic transcription factors via mitogen‐activated protein kinase pathways. J Biol Chem. 2003;278(37):34823‐34833. 10.1074/jbc.M210747200 [DOI] [PubMed] [Google Scholar]
- 77. Prieto‐Hontoria PL, Pérez‐Matute P, Fernández‐Galilea M, Alfredo Martínez J, Moreno‐Aliaga MJ. Effects of lipoic acid on AMPK and adiponectin in adipose tissue of low‐ and high‐fat–fed rats. Eur J Nutr. 2013;52(2):779‐787. 10.1007/s00394-012-0384-7 [DOI] [PubMed] [Google Scholar]
- 78. Yi X, Nickeleit V, James LR, Maeda N. α‐Lipoic acid protects diabetic apolipoprotein E‐deficient mice from nephropathy. J Diabetes Complicat. 2011;25(3):193‐201. 10.1016/j.jdiacomp.2010.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Estrada DE, Ewart HS, Tsakiridis T, et al. Stimulation of glucose uptake by the natural coenzyme alpha‐lipoic acid/thioctic acid: participation of elements of the insulin signaling pathway. Diabetes. 1996;45(12):1798‐1804. http://www.ncbi.nlm.nih.gov/pubmed/8922368. Accessed August 2, 2018 [DOI] [PubMed] [Google Scholar]
- 80. Yaworsky K, Somwar R, Ramlal T, Tritschler HJ, Klip A. Engagement of the insulin‐sensitive pathway in the stimulation of glucose transport by alpha‐lipoic acid in 3T3‐L1 adipocytes. Diabetologia. 2000;43(3):294‐303. http://www.ncbi.nlm.nih.gov/pubmed/10768090. Accessed August 2, 2018 [DOI] [PubMed] [Google Scholar]
- 81. Jacob S, Streeper RS, Fogt DL, et al. The antioxidant alpha‐lipoic acid enhances insulin‐stimulated glucose metabolism in insulin‐resistant rat skeletal muscle. Diabetes. 1996;45(8):1024‐1029. http://www.ncbi.nlm.nih.gov/pubmed/8690147. Accessed August 2, 2018 [DOI] [PubMed] [Google Scholar]
- 82. Streeper RS, Henriksen EJ, Jacob S, Hokama JY, Fogt DL, Tritschler HJ. Differential effects of lipoic acid stereoisomers on glucose metabolism in insulin‐resistant skeletal muscle. Am J Phys. 1997;273(1 Pt 1):E185‐E191. 10.1152/ajpendo.1997.273.1.E185 [DOI] [PubMed] [Google Scholar]
- 83. Peth JA, Kinnick TR, Youngblood EB, Tritschler HJ, Henriksen EJ. Effects of a unique conjugate of alpha‐lipoic acid and gamma‐linolenic acid on insulin action in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2000;278(2):R453‐R459. 10.1152/ajpregu.2000.278.2.R453 [DOI] [PubMed] [Google Scholar]
- 84. Khamaisi M, Potashnik R, Tirosh A, et al. Lipoic acid reduces glycemia and increases muscle GLUT4 content in streptozotocin‐diabetic rats. Metabolism. 1997;46(7):763‐768. http://www.ncbi.nlm.nih.gov/pubmed/9225829. Accessed August 2, 2018 [DOI] [PubMed] [Google Scholar]
- 85. Sugimura Y, Murase T, Kobayashi K, et al. α‐Lipoic acid reduces congenital malformations in the offspring of diabetic mice. Diabetes Metab Res Rev. 2009;25(3):287‐294. 10.1002/dmrr.947 [DOI] [PubMed] [Google Scholar]
- 86. Wiznitzer A, Ayalon N, Hershkovitz R, et al. Lipoic acid prevention of neural tube defects in offspring of rats with streptozocin‐induced diabetes. Am J Obstet Gynecol. 1999;180(1 I):188‐193. 10.1016/S0002-9378(99)70173-0 [DOI] [PubMed] [Google Scholar]
- 87. Golbidi S, Badran M, Laher I. Diabetes and alpha lipoic acid. Front Pharmacol. 2011. 10.3389/fphar.2011.00069;2:‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. McNeilly AM, Davison GW, Murphy MH, et al. Effect of α‐lipoic acid and exercise training on cardiovascular disease risk in obesity with impaired glucose tolerance. Lipids Health Dis. 2011;10(1):217 10.1186/1476-511X-10-217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Koh EH, Lee WJ, Lee SA, et al. Effects of alpha‐lipoic acid on body weight in obese subjects. Am J Med. 2011;124(1):85.e1‐85.e8. 10.1016/j.amjmed.2010.08.005 [DOI] [PubMed] [Google Scholar]
- 90. Ratliff JC, Palmese LB, Reutenauer EL, Tek C. An open‐label pilot trial of alpha‐lipoic acid for weight loss in patients with schizophrenia without diabetes. Clin Schizophr Relat Psychoses. 2015;8(4):196‐200. 10.3371/CSRP.RAPA.030113 [DOI] [PubMed] [Google Scholar]
- 91. Carbonelli MG, Di Renzo L, Bigioni M, De Daniele N, De Lorenzo A, Fusco MA. Alpha‐lipoic acid supplementation: a tool for obesity therapy? Curr Pharm Des. 2010;16(7):840‐846. http://www.ncbi.nlm.nih.gov/pubmed/20388095. Accessed August 2, 2018 [DOI] [PubMed] [Google Scholar]
- 92. Zhang Y, Han P, Wu N, et al. Amelioration of lipid abnormalities by α‐lipoic acid through antioxidative and anti‐inflammatory effects. Obesity (Silver Spring). 2011;19(8):1647‐1653. 10.1038/oby.2011.121 [DOI] [PubMed] [Google Scholar]
- 93. Ansar H, Mazloom Z, Kazemi F, Hejazi N. Effect of alpha‐lipoic acid on blood glucose, insulin resistance and glutathione peroxidase of type 2 diabetic patients. Saudi Med J. 2011;32(6):584‐588. http://www.ncbi.nlm.nih.gov/pubmed/21666939. Accessed August 2, 2018 [PubMed] [Google Scholar]
- 94. Kamenova P. Improvement of insulin sensitivity in patients with type 2 diabetes mellitus after oral administration of alpha‐lipoic acid. Hormones (Athens). 2006;5(4):251‐258. 10.14310/horm.2002.11191 [DOI] [PubMed] [Google Scholar]
- 95. Xiao C, Giacca A, Lewis GF. Short‐term oral α‐lipoic acid does not prevent lipid‐induced dysregulation of glucose homeostasis in obese and overweight nondiabetic men. Am J Physiol Metab. 2011;301(4):E736‐E741. 10.1152/ajpendo.00183.2011 [DOI] [PubMed] [Google Scholar]
- 96. Porcaro G, Brillo E, Giardina I, Di Iorio R. Alpha lipoic acid (ALA) effects on subchorionic hematoma: preliminary clinical results. Eur Rev Med Pharmacol Sci. 2015;19(18):3426‐3432. http://www.ncbi.nlm.nih.gov/pubmed/26439038. Accessed August 2, 2018 [PubMed] [Google Scholar]
- 97. Moore RM, Novak JB, Kumar D, Mansour JM, Mercer BM, Moore JJ. Alpha‐lipoic acid inhibits tumor necrosis factor‐induced remodeling and weakening of human fetal membranes 1. Biol Reprod. 2009;80(4):781‐787. 10.1095/biolreprod.108.073205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Italian Ministry of Health . ALTRI NUTRIENTI E ALTRE SOSTANZE AD EFFETTO NUTRITIVO O FISIOLOGICO (Revisione Gennaio 2019) 2019. http://www.salute.gov.it/imgs/C_17_pagineAree_1268_listaFile_itemName_4_file.pdf. Accessed February 5, 2019.
- 99. Ruessmann H‐J. German society of out patient diabetes centres AND (Arbeitsgemeinschaft niedergelassener diabetologisch tätiger Arzte e.V.). Switching from pathogenetic treatment with alpha‐lipoic acid to gabapentin and other analgesics in painful diabetic neuropathy: a real‐world study in outpatients. J Diabetes Complicat. 2009;23(3):174‐177. 10.1016/j.jdiacomp.2008.02.002 [DOI] [PubMed] [Google Scholar]
- 100. Ziegler D, Low PA, Litchy WJ, et al. Efficacy and safety of antioxidant treatment with α‐lipoic acid over 4 years in diabetic polyneuropathy: the NATHAN 1 trial. Diabetes Care. 2011;34(9):2054‐2060. 10.2337/dc11-0503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Durrani AI, Schwartz H, Nagl M, Sontag G. Determination of free α‐lipoic acid in foodstuffs by HPLC coupled with CEAD and ESI‐MS. Food Chem. 2010;120(4):1143‐1148. 10.1016/J.FOODCHEM.2009.11.045 [DOI] [Google Scholar]
- 102. Rathmann W, Haastert B, Delling B, Gries FA, Giani G. Postmarketing surveillance of adverse drug reactions: a correlational study approach using multiple data sources. Pharmacoepidemiol Drug Saf. 1998;7(1):51‐57. [DOI] [PubMed] [Google Scholar]
- 103. Moore RM, Schatz F, Kumar D, et al. Alpha‐lipoic acid inhibits thrombin‐induced fetal membrane weakening in vitro. Placenta. 2010;31(10):886‐892. 10.1016/j.placenta.2010.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Al Ghafli MHM, Padmanabhan R, Kataya HH, Berg B. Effects of alpha‐lipoic acid supplementation on maternal diabetes‐induced growth retardation and congenital anomalies in rat fetuses. Mol Cell Biochem. 2004;261(1‐2):123‐135. http://www.ncbi.nlm.nih.gov/pubmed/15362495. Accessed February 5, 2019 [DOI] [PubMed] [Google Scholar]
- 105. Padmanabhan R, Mohamed S, Singh S. Beneficial effect of supplemental lipoic acid on diabetes‐induced pregnancy loss in the mouse. Ann N Y Acad Sci. 2006;1084(1):118‐131. 10.1196/annals.1372.015 [DOI] [PubMed] [Google Scholar]
- 106. Costantino M, Guaraldi C, Costantino D, De Grazia S, Unfer V. Peripheral neuropathy in obstetrics: efficacy and safety of α‐lipoic acid supplementation. Eur Rev Med Pharmacol Sci. 2014;18(18):2766‐2771. http://www.ncbi.nlm.nih.gov/pubmed/25317815. Accessed February 5, 2019 [PubMed] [Google Scholar]
- 107. Bitterman JL, Chung JH. Metabolic effects of resveratrol: addressing the controversies. Cell Mol Life Sci. 2015;72(8):1473‐1488. 10.1007/s00018-014-1808-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Singh CK, Kumar A, Hitchcock DB, et al. Resveratrol prevents embryonic oxidative stress and apoptosis associated with diabetic embryopathy and improves glucose and lipid profile of diabetic dam. Mol Nutr Food Res. 2011;55(8):1186‐1196. 10.1002/mnfr.201000457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Sharma S, Misra CS, Arumugam S, et al. Antidiabetic activity of resveratrol, a known SIRT1 activator in a genetic model for type‐2 diabetes. Phytother Res. 2011;25(1):67‐73. 10.1002/ptr.3221 [DOI] [PubMed] [Google Scholar]
- 110. Cottart C‐H, Nivet‐Antoine V, Laguillier‐Morizot C, Beaudeux J‐L. Resveratrol bioavailability and toxicity in humans. Mol Nutr Food Res. 2010;54(1):7‐16. 10.1002/mnfr.200900437 [DOI] [PubMed] [Google Scholar]
- 111. Singh CK, Kumar A, Lavoie HA, Dipette DJ, Singh US. Diabetic complications in pregnancy: is resveratrol a solution? Exp Biol Med (Maywood). 2013;238(5):482‐490. 10.1177/1535370212473704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) . Safety of synthetic trans‐resveratrol as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2016;14(1):4368 10.2903/j.efsa.2016.4368 [DOI] [Google Scholar]
- 113. Bogdanski P, Suliburska J, Szulinska M, Stepien M, Pupek‐Musialik D, Jablecka A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr Res. 2012;32(6):421‐427. 10.1016/j.nutres.2012.05.007 [DOI] [PubMed] [Google Scholar]
- 114. Chowdhury A, Sarkar J, Chakraborti T, Pramanik PK, Chakraborti S. Protective role of epigallocatechin‐3‐gallate in health and disease: a perspective. Biomed Pharmacother. 2016;78:50‐59. 10.1016/j.biopha.2015.12.013 [DOI] [PubMed] [Google Scholar]
- 115. Suzuki T, Pervin M, Goto S, Isemura M, Nakamura Y. Beneficial effects of tea and the green tea catechin epigallocatechin‐3‐gallate on obesity. Molecules. 2016;21(10):5‐8. 10.3390/molecules21101305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Zhang ZF, Li Q, Liang J, et al. Epigallocatechin‐3‐O‐gallate (EGCG) protects the insulin sensitivity in rat L6 muscle cells exposed to dexamethasone condition. Phytomedicine. 2010;17(1):14‐18. 10.1016/j.phymed.2009.09.007 [DOI] [PubMed] [Google Scholar]
- 117. Borges CM, Papadimitriou A, Duarte DA, Lopes De Faria JM, Lopes De Faria JB. The use of green tea polyphenols for treating residual albuminuria in diabetic nephropathy: a double‐blind randomised clinical trial. Sci Rep. 2016;6(May):1‐9. 10.1038/srep28282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Isbrucker RA, Edwards JA, Wolz E, Davidovich A, Bausch J. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 3: teratogenicity and reproductive toxicity studies in rats. Food Chem Toxicol. 2006;44(5):651‐661. 10.1016/j.fct.2005.11.002 [DOI] [PubMed] [Google Scholar]
- 119. 17/10/2018 ‐ POSITION STATEMENT AMD ‐ SID “Integratori vitaminici, inositolo e probiotici nelle donne con iperglicemia in gravidanza.” http://www.siditalia.it/news/2103‐17‐10‐2018‐position‐statement‐amd‐sid‐integratori‐vitaminici‐inositolo‐e‐probiotici‐nelle‐donne‐con‐iperglicemia‐in‐gravidanza. Accessed February 18, 2019.


