Abstract
Background
Complex regional pain syndrome (CRPS) is much more prevalent in women than men but potential differences in clinical phenotype have not been thoroughly explored to date. Differences in the clinical presentation between sexes may point at new avenues for a more tailored management approach of CRPS. We therefore explored if in CRPS, the patient's sex is associated with differences in clinical and psychological characteristics.
Methods
In this cross‐sectional study of 698 CRPS patients (599 females) fulfilling the Budapest clinical or research criteria, CRPS signs and symptoms, CRPS severity, pain (average pain intensity in the previous week and McGill pain rating index), pain coping (Pain Coping Inventory), physical limitations (Radboud Skills Questionnaire (upper limb), Walking and Rising questionnaire (lower limb)), anxiety and depression (Hospital Anxiety and Depression scale) and kinesiophobia (Tampa scale for kinesiophobia) were evaluated.
Results
Male CRPS patients used more often extreme words to describe the affective qualities of pain, used more passive pain coping strategies, and were more likely to suffer from depression and kinesiophobia.
Conclusion
Sex‐related differences are present in CRPS, but the effect is generally small and mainly concerns psychological functioning. A greater awareness of sex‐specific factors in the management of CRPS may contribute to achieving better outcomes.
Significance
What is known? Nonsex‐specific clinical data of CRPS patients. What is new? Male CRPS patients used more often extreme words to describe the affective qualities of pain, used more passive pain coping strategies, and were more likely to suffer from depression and kinesiophobia.
1. INTRODUCTION
Complex regional pain syndrome (CRPS) patients suffer from intense pain with sensory, autonomic, motor and trophic changes of the affected limb, resulting in profound loss of quality of life (van Velzen et al., 2014). Typically, the syndrome is preceded by tissue damage of the affected limb (Marinus et al., 2011). Previous research efforts suggest that CRPS is a multifactorial disorder that is associated with an aberrant host response to tissue injury (Marinus et al., 2011). The various involvement of perturbed biological pathways underlying aberrant inflammation, vasomotor dysfunction and maladaptive neuroplasticity likely account for the clinical heterogeneity of CRPS (Marinus et al., 2011). Clinical heterogeneity is also encountered in studies directed at the development of therapeutic approaches for this condition, pointing to the existence of distinct subgroups that exhibit a varying response to treatment. Given the complex nature of CRPS, future treatment strategies likely will benefit from the identification of unique factors associated with treatment response in particular patients, enabling a more personalized approach. Although the syndrome is clearly much more common in females of all ages, affecting two to four times as many females as males, it is unclear whether sex is associated with differences in the clinical presentation of CRPS (De Mos et al., 2007). Findings in the general population indicate that in experimentally induced pain, women have lower pain thresholds and experience greater temporal summation of pain to brief, repeated or dynamic stimuli than men; however, women also show greater adaptation to sustained stimuli than men (Hashmi & Davis, 2014). In addition, women report higher prevalence and severity of pain in daily life, experience a higher severity of mood disturbance and seek more social support when suffering pain (Fillingim, 2000; Fillingim, King, Ribeiro‐Dasilva, Rahim‐Williams, & Riley, 2009; Greenspan et al., 2007; Pieretti et al., 2016; Rovner et al., 2017). Knowledge of sex‐related factors in the clinical presentation of CRPS patients could potentially reveal new avenues for a more tailored approach to management.
We therefore evaluated the clinical presentation of men and women with CRPS in a large cohort of almost 700 CRPS patients recruited in academic and regional hospitals in the Netherlands and used the term “sex” to denote the different groups. Specifically, we examined potential sex‐related differences in signs and symptoms, pain coping, self‐reported physical disability, anxiety, depression and kinesiophobia.
2. METHODS
2.1. Participants
Patients were recruited between January 2005 and December 2011 from five pain clinics and one department of neurology of academic and regional hospitals in the Netherlands participating in TREND (short for Trauma RElated Neuronal Dysfunction, a Dutch knowledge consortium on CRPS). The included patients were all 18 years or older and fulfilled generally accepted CRPS criteria, specifically the “Budapest clinical” (Bdp‐c) or the “Budapest research” (Bdp‐r) criteria (Figure 1). We excluded patients if they had other conditions that could account for the signs and symptoms encountered, dementia, cognitive impairment or any other condition that could affect the ability to understand and complete self‐assessment questionnaires.
Figure 1.
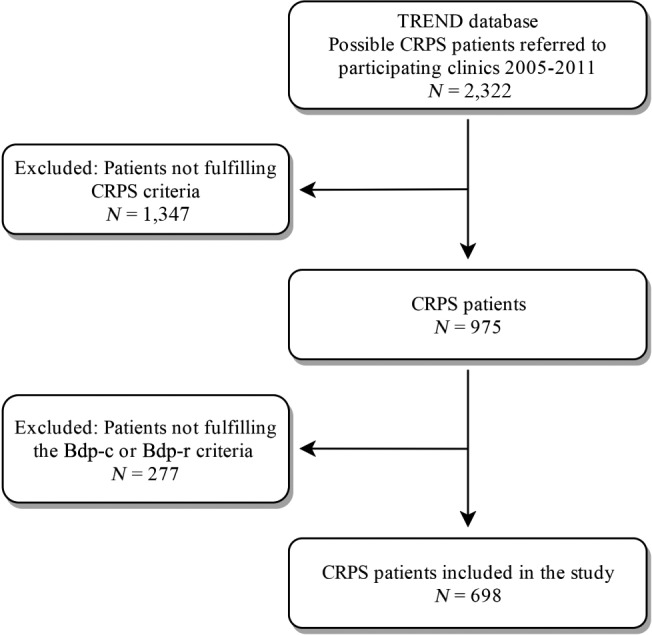
Flow‐chart of inclusion. Bdp‐c, Budapest clinical; Bdp‐r, Budapest research; CRPS, complex regional pain syndrome; TREND, Trauma RElated Neuronal Dysfunction
We use the term “sex” instead of “gender” to denote the different groups since we identify the patients based on their biological sexes, not “gender” which encompasses social and cultural values.
2.2. Assessment methods and measurement instruments
The protocol was approved by the medical ethical committees of all participating centres in accordance with the Declaration of Helsinki. All participants gave written informed consent. We standardized methods of examination across centres and recorded signs and symptoms on a standard score sheet. All data were stored in a NEN‐7511 certified, central web‐based data management system (ProMISe©).
2.2.1. CRPS signs and symptoms
We examined 22 distinctive CRPS signs (observed during examination) and symptoms (reported by patients); specifically: allodynia (pain to normally innocuous stimuli) to light touch, to deep joint pressure and to movements; hyper‐ and hypoesthesia; hyper‐ and hypoalgesia; skin colour changes; temperature asymmetry; oedema; hyper‐ and hypohydrosis; trophic changes of hairs, nails and skin; muscle atrophy; decreased range of motion; paresis; abnormal postures; tremors; myoclonic jerks and bradykinesia.
2.2.2. CRPS severity score
We calculated the CRPS severity score (CCS) (Harden, Bruehl, & Perez, 2010), a measure designed to reflect the presence and severity of CRPS. The CCS is based on the presence or absence of nine signs (hyperpathia/hyperalgesia to pinprick; hyperpathia/hyperalgesia to light touch [brush], cold, warm, vibration, or deep manual joint pressure; temperature asymmetry; skin colour changes; oedema; sweating asymmetry; trophic/dystrophic changes; motor changes; and decreased active range of motion) and eight symptoms (hyperpathia/allodynia [all types]; bilateral temperature asymmetry; skin colour changes; oedema; sweating asymmetry; trophic/dystrophic changes (hair, nails, or skin); motor changes (e.g. weakness, tremor, dystonia); and decreased active range of motion. Range 0–17.
2.2.3. Pain
We used two measurement instruments to evaluate pain. First, the Numeric Rating Scale (NRS) which is the average pain intensity of the previous week on a scale from 0 to 10, with 10 reflecting the worst pain imaginable. Second, the McGill pain rating index to quantify pain (range 0–63; higher scores reflect more pain) (Melzack, 1975; Vanderiet, Adriaensen, Carton, & Vertommen, 1987). The Pain Rating Index is a sum score calculated over ranked words that express three qualities of pain, namely sensory qualities (such as temporal, spatial, pressure and thermal qualities), affective qualities (tension, fear and autonomic changes) and evaluative qualities (subjective intensity of pain).
2.2.4. Pain coping
Patients completed the Pain Coping Inventory (PCI) questionnaire to assess pain coping strategies (Jensen, Turner, Romano, & Strom, 1995; Kraaimaat, Bakker, & Evers, 1997). The questionnaire comprises six pain coping dimensions, grouped into “active” (Pain transformation, Distraction, Reducing demands; range 12–48) and “passive” domains (Retreating, Worrying, Resting; range 21–84); higher scores in these dimensions indicate more use of the corresponding strategy.
2.2.5. Self‐reported physical disability
To assess physical limitations in daily life, patients completed the Radboud Skills Questionnaire (RASQ) (Oerlemans; Oerlemans & Cup, 2000) if arms were affected and the Walking and Rising questionnaire (WRQ) (Roorda; Roorda et al., 2005) if legs were affected. The primary outcome of the RASQ is a summary score of six domains: personal care (e.g. personal hygiene), domestic activities (e.g. housekeeping), recreational activities (e.g. sports), social activities (e.g. going on outings), work (i.e. performing occupation) and other (e.g. using personal computer). Mean domain and total scores are calculated and ranged from 1 to 5, with higher scores reflecting worse functioning. For the WRQ, we used the summary score of the following three domains: walking inside, walking outside and rising; because of the different number of items for these three domains, subscale scores were first standardized to a 0–10 scale before adding up (total range 0–30; higher scores indicating worse walking ability).
For regression analyses (see Section 3), we used the physical health sum score (PHS) of the MOS 36‐Item Short‐Form Health Survey (SF36) (Aaronson & Muller, 1998; Ware & Sherbourne, 1994), which measures limitations in physical function. We used this scale because it addresses physical disability of the whole body in contrast to the RASQ and WRQ, which only measure limitations in upper and lower extremity function.
2.2.6. Anxiety and depression
To measure anxiety and depression, we used the Anxiety and Depression subscales of the Hospital Anxiety and Depression scale (HADS‐A and HADS‐D, respectively) (Bjelland, Dahl, Haug, & Neckelmann, 2002; Spinhoven et al., 1997).
2.2.7. Kinesiophobia
Kinesiophobia was measured using the Dutch version of the Tampa Scale for Kinesiophobia (TSK) (Vlaeyen, Kole‐Snijders, Boeren, & van Eek, 1995), a questionnaire consisting of 17 questions addressing patient's belief that activities that increase pain cause further harm (range 17–68, higher scores indicating more kinesiophobia).
2.3. Statistics
We analysed all data using IBM® SPSS® statistics software version 23. First, we calculated group (sex) differences in all measures. In categorical data (dichotomous variables CRPS signs and symptoms; HADS‐A and D), sex differences were calculated using chi‐square tests with exact significance values in conjunction with odds ratio as a measure of effect size. In continuous data, t tests were used in conjunction with Hedges’ g as a measure of effect size (Hedges’ g due to unequal sample sizes of males and females; 0.20 = small, 0.50 = medium, 0.80 = large effect) (Ellis, 2010). In addition, if the previous analyses resulted in differences in continuous measures, the analysis was followed up with a multiple regression analysis to control for the potential influence of confounders. Independent variables were added to the model using a simultaneous forced entry method (“ENTER” method), which is used when a hierarchical order of the independent variables is not a priori known or considered relevant. We selected the following independent variables based on previous literature or on our assumption of possible interaction of the concerned variable with sex: “sex”; “age” at time of inclusion; “disease duration”; “McGill pain rating index”; “CRPS severity score”; “affected limbs” (upper limb(s), lower limb(s) or a combination of upper and lower limbs, imputed as two dummy variables in linear regression); and the “sum score physical health SF36 (PHS).” Missing values in the independent variables of the regression analysis were replaced by means if <5% of the independent variables were missing. Data were considered statistically significant if p‐values were <0.05. To control for false discovery rate, we used the Benjamini–Hochberg procedure (Thissen, Steinberg, & Kuang, 2002) with an alpha of <0.05 within the following different domains: CRPS signs and symptoms, pain scores (NRS and McGill pain rating index), CCS, self‐reported disability (WRQ, RASQ) and psychological variables (active and passive PCI, HADS‐A and D and kinesiophobia). Data in the text are presented as mean scores ± standard deviations.
3. RESULTS
Six hundred and ninety‐eight patients (age: 46.1 ± 14.2 years; 599 (86%) female) were included in the analysis, of which 267 (38.3%) with an affected upper limb, 278 (39.8%) with an affected lower limb and 153 (21.9%) with more than one limb affected. Mean disease duration at time of inclusion in male patients was 4.7 ± 6.9 and 5.2 ± 7.1 years in female patients. All 698 patients fulfilled the Bdp‐c criteria, of which 448 (64.2%) also the Bdp‐r criteria. Six hundred and eighty‐six patients (589 female) completed the McGill pain questionnaire, 565 patients (481 female) the NRS, 385 patient (335 female) the RASQ, 401 patients (349 female) the WRQ, 609 (522 female) the PCI, 684 patients (587 female) the HADS and 679 patients (582 female) the TSK. In the regression analyses, missing independent variables were replaced by means, which occurred in no more than 2% (disease duration 99%, CCS 99%, McGill pain rating index 98% complete data). Baseline results are listed in Table 1.
Table 1.
Patient characteristics
| Demographic and clinical variables | Male | Female | |
|---|---|---|---|
| Number of included patients (%) | 698 | 99 (14.2) | 599 (85.8) |
| Mean (SD) age, years | 46.1 (14.2) | 49.0 (12.9) | 45.7 (14.4) |
| Mean (SD) age at onset, years | 41.1 (15.4) | 44.0 (13.5) | 40.6 (15.7) |
| Median (IQR) disease duration, years | 2.0 (0.5–7.1) | 1.6 (0.4–6.1) | 2.1 (0.5–7.2) |
| N 1 arm/1 leg/>1 limbs | 267/278/153 | 40/42/17 | 227/236/136 |
| Fulfilling Bdp‐r criteria (%) | 448 (64.2) | 60 (60.1) | 388 (64.8) |
| CRPS Severity Score, median (IQR) | 12 (10–14) | 11.5 (3.0) | 11.8 (2.7) |
| McGill Pain Rating Index, mean (SD) | 27.2 (11.6) | 29.4 (12.0) | 26.9 (11.4) |
| Sensory qualities | 15.6 (7.0) | 16.2 (7.3) | 15.5 (6.9) |
| Affective qualities | 4.8 (3.4) | 5.9 (3.6) | 4.6 (3.4) |
| Evaluative qualities | 6.9 (2.9) | 7.3 (2.9) | 6.8 (2.9) |
| Numeric rating scale | 6.5 (1.8) | 6.3 (2.0) | 6.5 (1.8) |
| Pain Coping Inventory, active, mean (SD) | 28.5 (5.2) | 27.7 (5.6) | 28.7 (5.1) |
| Pain Coping Inventory, passive, mean (SD) | 42.1 (9.1) | 44.2 (10.4) | 41.7 (8.9) |
| Radboud Skills Questionnaire, mean (SD) | 3.2 (0.9) | 3.1 (0.9) | 3.3 (0.9) |
| Walking and Rising Questionnaire, mean (SD) | 19.9 (7.4) | 18.9 (7.5) | 20.0 (7.4) |
| Hospital Anxiety and Depression Scale‐depression subscale, mean (SD) | 5.1 (3.9) | 6.1 (4.3) | 4.9 (3.8) |
| Dichotomous (cut‐off ≥8) (%) | 164 (23.5) | 32 (32.3) | 132 (22.0) |
| Hospital Anxiety and Depression Scale‐ anxiety subscale, mean (SD) | 6.3 (3.8) | 6.7 (4.0) | 6.2 (3.7) |
| Dichotomous (cut‐off ≥8) (%) | 212 (30.4) | 32 (32.3) | 184 (30.7) |
| Tampa Scale of Kinesiophobia, mean (SD) | 38.1 (8.2) | 40.6 (7.8) | 37.7 (8.2) |
| MOS 36‐Item Short‐Form Health Survey, Physical health Sum Score, mean (SD) | 32.3 (16.3) | 33.9 (15.9) | 32.1 (16.4) |
Bdp‐c, Budapest clinical criteria; Bdp‐r, Budapest research criteria; CRPS, complex regional pain syndrome; IQR, interquartile range; SD, standard deviation.
3.1. CRPS signs and symptoms
No sex difference was found in CRPS signs and symptoms (see Supporting Information for the results of the uncorrected data).
3.2. CRPS severity score
The CCS was not significantly different between sexes (females: 11.8 ± 2.7, males: 11.5 ± 3.0): t(686) = −1.112, p = 0.266.
3.3. Pain
The average pain in the previous week as measured by the NRS was similar for female and male patients (females: 6.5 ± 1.8, males: 6.3 ± 2.0), t(−0.563) = −0.816, p = 0.390. In contrast, the McGill pain rating index was slightly higher for male CRPS patients than for female CRPS patients (females: 27.0 ± 11.5, males: 29.4 ± 12.0), t(684) = 2.011, p = 0.045, g Hedges = 0.22 (uncorrected results). This result which was entirely driven by the difference in affective quality of pain: male patients more often used extreme words to describe the affective qualities of pain (females 4.6 ± 3.4, males 5.9 ± 3.6, t[684] = 3.56, p < 0.001). Sensory qualities (females 15.4 ± 7.0, males 16.2 ± 7.3, t[684] = 0.99, p = 0.32) and evaluative qualities (females 7.3 ± 2.9, males 6.8 ± 2.9, t[684] = 1.45, p = 0.15) were not significantly different.
3.4. Pain coping
Male patients reported a higher use of passive pain coping mechanisms than female patients (females: 41.7 ± 8.9, males: 44.2 ± 10.4), t[607] = 2.37, p = 0.018, g Hedges = 0.15. Controlling for the potential effects of the confounders age, disease duration, CRPS severity score, pain, physical health and affected limb, the contribution of “sex” to the model remained significant, albeit small; β st‐sex = −0.077, p = 0.024 (Table 2).
Table 2.
Linear regression model Pain Coping Inventory
| Variables | B (SE) | β st | Sig. |
|---|---|---|---|
| Constant | 48.488 (2.298) | ||
| Sex | −1.882 (0.832) | −0.077 | 0.024 |
| Age | −0.002 (0.021) | −0.004 | 0.907 |
| Disease duration | 0.000 (0.046) | −0.000 | 0.997 |
| CRPS severity score | −0.079 (0.106) | −0.025 | 0.457 |
| McGill Pain rating index | 0.141 (0.029) | 0.190 | 0.000 |
| Physical health sum score | −0.195 (0.020) | −0.371 | 0.000 |
| Affected limbs A | −1.553 (0.652) | −0.090 | 0.017 |
| Affect limbs B | −3.804 (0.945) | −0.167 | 0.000 |
R 2 (variance explained by the model) = 0.226; sex: male > female; Sig. = significance (p < 0.05); affected limbs A = dummy variable affected lower limb(s) versus affected upper limb(s); affected limbs B = dummy variable affected lower limb(s) and upper limb(s) versus affected upper limb(s).
β st = standardized β; CRPS, complex regional pain syndrome; SE = standard error.
No difference in active pain coping mechanisms was found (females: 28.7 ± 5.1, males: 27.7 ± 5.6) t(607) = −1.66, p = 0.097.
3.5. Self‐reported physical disability
We found no group differences in the RASQ (females: 3.3 ± 0.9, males: 3.1 ± 0.9) t(383) = −0.999, p = 0.318 or WRQ (females: 20.0 ± 7.4, males: 18.9 ± 7.5) t(399) = −1.001, p = 0.317.
3.6. Anxiety and depression
No difference in anxiety scores (HADS‐A) was found between the groups: (females 6.1 ± 3.1, males 6.6 ± 4.1) t(680) = −0.294, p = 0.769.
Male CRPS patients had higher depression scores (HADS‐D) than female patients: (females 4.9 ± 3.8, males 6.1 ± 4.3) t(682) = 2.677, p = 0.008. The adjusted effect of sex in the logistic regression model remained significant (β st‐sex = −0.078, p = 0.024) (Table 3).
Table 3.
Regression model HADS‐D
| Variables | B (SE) | β st | Sig. |
|---|---|---|---|
| Constant | 9.139 (1.061) | ||
| Sex | −1.054 (0.384) | −0.096 | 0.006 |
| Age | 0.001 (0.01) | −0.005 | 0.881 |
| Disease duration | −0.021 (0.021) | −0.037 | 0.332 |
| CRPS severity score | −0.040 (0.049) | −0.029 | 0.410 |
| McGill Pain rating index | 0.033 (0.013) | 0.098 | 0.015 |
| Physical health sum score | −0.091 (0.009) | −0.384 | 0.000 |
| Affected limbs A | −0.800 (0.301) | −0.103 | 0.008 |
| Affect limbs B | −1.459 (0.436) | −0.142 | 0.001 |
R 2 (variance explained by the model) = 0.186; sex: male > female; Sig. = significance (p < 0.05); affected limbs A = dummy variable affected lower limb(s) versus affected upper limb(s); affected limbs B = dummy variable affected lower limb(s) and upper limb(s) versus affected upper limb(s).
β st = standardized β; CRPS, complex regional pain syndrome; SE = standard error.
3.7. Kinesiophobia
Male patients had higher scores of kinesiophobia than female patients (female 37.7 ± 8.2, males 40.6 ± 7.8) t(677) = 2.94, p = 0.001, g Hedges = 0.36).
The effect of sex in the regression model controlling for the potential influence of confounders remained significant (β st‐sex = −0.113, p = 0.002) (Table 4).
Table 4.
Linear regression model Tampa Scale of Kinesiophobia
| Variables | B (SE) | β st | Sig. |
|---|---|---|---|
| Constant | 41.692 (2.324) | ||
| Sex | −2.621 (0.842) | −0.113 | 0.002 |
| Age | 0.048 (0.022) | 0.084 | 0.026 |
| Disease duration | −0.004 (0.046) | −0.003 | 0.933 |
| CRPS severity score | 0.024 (0.107) | 0.008 | 0.825 |
| McGill Pain rating index | 0.066 (0.029) | 0.094 | 0.024 |
| Physical health sum score | −0.140 (0.020) | −0.283 | 0.000 |
| Affected limbs A | −1.441 (0.659) | −0.088 | 0.029 |
| Affect limbs B | −2.846 (0.956) | −0.132 | 0.003 |
R 2 (variance explained by the model) = 0.120; Sig. = significance (p < 0.05); sex: male > female; affected limbs A = dummy variable affected lower limb(s) versus affected upper limb(s); affected limbs B = dummy variable affected lower limb(s) and upper limb(s) versus affected upper limb(s).
β st, standardized β; CRPS, complex regional pain syndrome; SE, standard error.
4. DISCUSSION
We studied sex differences in 698 CRPS patients and found that male patients used more often extreme words to describe the affective qualities of pain, used slightly more often passive pain coping strategies, and were more likely to suffer from depression and kinesiophobia.
The NRS, which depicts the average pain in the previous week, was similar for female and male patients. In contrast, pain evaluated with the McGill pain rating index was somewhat higher in male patients. Although surprising considering the overwhelming evidence for the opposite in the general and pain population, the result appeared mainly driven by the questions concerning the affective qualities of pain; male patients more often used extreme words to describe the affective qualities of pain, whereas sensory and evaluative qualities of pain were not significantly different between the groups. Therefore, although pain intensity was not significantly higher, male CRPS patients might have suffered more from the pain than female patients, an effect that is potentially mediated by the higher levels of passive pain coping, depression and kinesiophobia found in male CRPS patients.
Male CRPS patients reported more passive pain coping strategies than female patients. Passive pain coping strategies are associated with decreased physical functioning and increased psychological distress (Kraaimaat & Evers, 2003). Indeed, in our sample, passive pain coping was negatively correlated with physical health (depicted by the SF‐36 physical health sum score, Table 1) and positively correlated with the RASQ and WRQ (Pearson's r = −0.39, r = 0.3.0 and 2.5, respectively; all p < 0.001). In CRPS patients with an affected lower limb, “resting” as a passive pain coping mechanism had the largest effect on difficulties in rising and walking (Marinus, Perez, & Eijs, 2013). This effect was even larger in comparison with pain or CRPS severity (Marinus et al., 2013). In addition, CRPS patients using active, instead of passive pain coping strategies do better in overall functioning, physical functioning, mood and the ability to cope with pain and pain flare‐ups (Mccormick, Gagnon, Caldwell, Patel, & Kornfeld, 2015). Female pain patients generally use a wider range of coping mechanisms than male patients, seek more social support and are more prone to pain‐related catastrophizing (Keogh & Denford, 2009). In contrast, male patients use less coping strategies, more avoidance, seek less social support, are more likely to use alcohol and more passive coping strategies when they perceive their pain as threatening (Keogh, 2015). In addition, male patients show lower levels of daily activities than female patients reporting the same pain severity (Rovner et al., 2017). Physicians may therefore consider assessing a patients’ resilience by inquiring about social ties and community support, use of sedatives and avoidance behaviour, especially when managing male patients.
Of note is that we found no difference in anxiety scores between the sexes. In the general population, females report higher anxiety scores and are at greater risk of anxiety disorders than men. Furthermore, in chronic (musculoskeletal) pain patients, anxiety has been found associated with pain in male, but not in female patients (Fillingim et al., 2009). In contrast, in our sample, an equally weak, positive correlation between pain and anxiety was found in both groups (Pearson's r = 0.21; with p = 0.04 in males and p < 0.001 in females). The presence of anxiety in CRPS patients is conceivable considering its influence on quality of life (van Velzen et al., 2014), physical health and clinical signs such as higher levels of pain, allodynia, motor disturbances, oedema, skin colour and temperature changes, and independent of the sex. Possibly, the comparatively high overall level of anxiety in this condition (>30% were classified as “anxious”), while mean group levels are close to the applied cut‐off value) outweighs the potential contribution of sex on these features.
Surprisingly, male CRPS patients were more likely to suffer from depression. This contradicts the common notion that females, both in the general and chronic pain populations, are twice as likely to suffer from depression than males (Munce & Stewart, 2007). Moreover, in one much smaller study in CRPS patients (n = 24), female CRPS patients scored higher in depression (Geertzen, de Bruijn‐Kofman, de Bruijn, van de Wiel, & Dijkstra, 1998). For both sexes, the association between CRPS and depression has been documented before (for review see Lohnberg & Altmaier, 2013), and in one study, previous day pain was a significant predictor of next day's negative and depressed mood (Feldman, Downey, & Schaffer‐Neitz, 1999). However, there is evidence suggesting that in male patients with chronic pain, depression is associated with impairment of activity, and less so with pain (Haley, Turner, & Romano, 1985). Against this background, it is relevant to take into account that CRPS is strongly associated with reduced physical health (van Velzen et al., 2014), which may result in male patients being more depressed than female patients. Indeed, in our study we found a weaker negative correlation between physical health and depression in female as compared to male patients (Pearson's r = −0.25 and r = −0.37, respectively; both p < 0.001).
Male CRPS patients also had higher scores of kinesiophobia than female patients. This finding is in line with those of previous studies in chronic musculoskeletal pain patients (Bränström & Fahlström, 2008; Vlaeyen et al., 1995), although, to the best of our knowledge, a clear explanation for these findings is lacking. Kinesiophobia is common in CRPS and may contribute to functional limitations (Jong, Vlaeyen, Gelder, & Patijn, 2011), although this association was not found by others (Marinus et al., 2013). In addition, in patients with pain‐related fear, therapies that focussed on physical exposure instead of pain reduction resulted in better physical performance (den Hollander et al., 2016; Jong, Vlaeyen, & Onghena, 2005). This underlines the necessity of incorporating kinesiophobia assessment in the management of CRPS. Our data suggest that this might even be more important in male than female CRPS patients.
We found no significant differences in CRPS signs or symptoms. However, potential differences in signs and symptoms were much more difficult to detect, given that patients had to meet Budapest criteria to be included in the study. Concerning the results of the noncorrected data, subsequent research could focus on a potential differences in allodynia to deep joint pressure, since this sign was the most promising of all distinguishing female from male patients.
The strengths of this study are the large sample size and the use of—and regular training in—standardized assessments of CRPS signs and symptoms in the participating clinics. However, some main limitations need to be mentioned; one is the cross‐sectional study design which makes it impossible to draw conclusions on causality. The second limitation is that this study was executed in patients who were treated in specialized academic centres and referral bias can therefore not be ruled out, although it should be noted that, in particular at the time the data for this study were collected, most CRPS patients in the Netherlands were referred to specialized clinics such as those in which the present data were collected. It is further of note that we have little reason to assume that any potential referral bias would have affected the relationship between sex and the variables that were identified. Third, all patients were recruited only in the Netherlands. It may be worthwhile to explore if they also hold for other regions.
To summarize, male CRPS patients seem to experience a slightly higher psychological burden than female CRPS patients in the absence of significant differences in clinical presentation. Of note is that, except for depression, the effect sizes were generally small and that variables other than sex often accounted for more of the variance in the investigated outcomes. Although results of cross‐sectional studies cannot be causally interpreted, they may nevertheless provide clues that may be relevant to follow‐up. A greater awareness of sex‐specific factors in the management of CRPS may contribute to achieving better outcomes.
CONFLICTS OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
G.V., J.M. and J.H. contributed to the design and analysis of the article. F.H., M.K., F.E., J.M. and J.H. collected data. All authors contributed to the writing of the article. All authors revised the article and approved the final version.
Supporting information
ACKNOWLEDGEMENT
We commemorate Prof. Dr. Roberto Perez. A very enthusiastic, talented and kind colleague who passed away last year at the age of 49. Roberto was a true pioneer of CRPS research and a dedicated member of the TREND consortium. We here acknowledge Roberto's important contribution to the design and data collection of this study.
van Velzen GAJ, Huygen FJPM, van Kleef M, van Eijs FV, Marinus J, van Hilten JJ. Sex matters in complex regional pain syndrome. Eur J Pain. 2019;23:1108–1116. 10.1002/ejp.1375
Funding information
This study was performed within TREND and supported by a grant from the Netherlands’ Ministry of Economic Affairs (grant number BSIK03016).
REFERENCES
- Aaronson, N. , & Muller, M. (1998). Translation, validation, and norming of the Dutch language version of the SF‐36 Health Survey in community and chronic disease populations. Journal of Clinical Epidemiology, 51, 1055–1068. 10.1016/S0895-4356(98)00097-3 [DOI] [PubMed] [Google Scholar]
- Bjelland, I. , Dahl, A. A. , Haug, T. T. , & Neckelmann, D. (2002). The validity of the Hospital Anxiety and Depression Scale. An updated literature review. Journal of Psychosomatic Research, 52, 69–77. 10.1016/S0022-3999(01)00296-3 [DOI] [PubMed] [Google Scholar]
- Bränström, H. , & Fahlström, M. (2008). Kinesiophobia in patients with chronic musculoskeletal pain: Differences between men and women. Journal of Rehabilitation Medicine, 40, 375–380. 10.2340/16501977-0186 [DOI] [PubMed] [Google Scholar]
- De Mos, M. , de Bruijn, A. G. , Huygen, F. J. , Dieleman, J. P. , Stricker, B. H. , & Sturkenboom, M. C. (2007). The incidence of complex regional pain syndrome: A population‐based study. Pain, 129, 12–20. 10.1016/j.pain.2006.09.008 [DOI] [PubMed] [Google Scholar]
- Ellis, P . (2010). The essential guide to effect sizes: Statistical power, meta-analysis, and the interpretation of research results. Cambridge: Cambridge University Press. [Google Scholar]
- Feldman, S. I. , Downey, G. , & Schaffer‐Neitz, R. (1999). Pain, negative mood, and perceived support in chronic pain patients: A daily diary study of people with reflex sympathetic dystrophy syndrome. Journal of Consulting and Clinical Psychology, 67, 776–785. 10.1037/0022-006X.67.5.776 [DOI] [PubMed] [Google Scholar]
- Fillingim, R. B. (2000). Sex, gender, and pain: Women and men really are different. Current Review of Pain, 4, 24–30. 10.1007/s11916-000-0006-6 [DOI] [PubMed] [Google Scholar]
- Fillingim, R. B. , King, C. D. , Ribeiro‐Dasilva, M. C. , Rahim‐Williams, B. , & Riley, J. L. (2009). Sex, gender, and pain: A review of recent clinical and experimental findings. Journal of Pain, 10, 447–485. 10.1016/j.jpain.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geertzen, J. H. , de Bruijn‐Kofman, A. T. , de Bruijn, H. P. , van de Wiel, H. B. , & Dijkstra, P. U. (1998). Stressful life events and psychological dysfunction in Complex Regional Pain Syndrome type I. Clinical Journal of Pain, 14, 143–147. 10.1097/00002508-199806000-00009 [DOI] [PubMed] [Google Scholar]
- Greenspan, J. D. , Craft, R. M. , LeResche, L. , Arendt‐Nielsen, L. , Berkley, K. J. , Fillingim, R. B. , … Traub, R. J. (2007). Studying sex and gender differences in pain and analgesia: A consensus report. Pain, 132(Suppl 1), S26–S45. 10.1016/j.pain.2007.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley, W. E. , Turner, J. A. , & Romano, J. M. (1985). Depression in chronic pain patients: Relation to pain, activity, and sex differences. Pain, 23, 337–343. 10.1016/0304-3959(85)90003-X [DOI] [PubMed] [Google Scholar]
- Harden, R. , Bruehl, S. , & Perez, R. (2010). Development of a severity score for CRPS. Pain, 151, 870–876. 10.1016/j.pain.2010.09.031 [DOI] [PubMed] [Google Scholar]
- Hashmi, J. A. , & Davis, K. D. (2014). Deconstructing sex differences in pain sensitivity. Pain, 155, 10–13. 10.1016/j.pain.2013.07.039 [DOI] [PubMed] [Google Scholar]
- den Hollander, M. , Goossens, M. , de Jong, J. , Ruijgrok, J. , Oosterhof, J. , Onghena, P. , … Vlaeyen, J. W. S. (2016). Expose or protect? A randomized controlled trial of exposure in vivo vs pain‐contingent treatment as usual in patients with complex regional pain syndrome type 1 Pain, 157, 2318–2329. 10.1097/j.pain.0000000000000651 [DOI] [PubMed] [Google Scholar]
- Jensen, M. P. , Turner, J. A. , Romano, J. M. , & Strom, S. E. (1995). The chronic pain coping inventory: Development and preliminary validation. Pain, 60, 203–216. 10.1016/0304-3959(94)00118-X [DOI] [PubMed] [Google Scholar]
- Jong, J. de, Vlaeyen, J. , Gelder, J. de, & Patijn, J. (2011). Pain‐related fear, perceived harmfulness of activities, and functional limitations in complex regional pain syndrome type I. Journal of Pain, 12, 1209–1218. 10.1016/j.jpain.2011.06.010 [DOI] [PubMed] [Google Scholar]
- Jong, J. de, Vlaeyen, J. , & Onghena, P. (2005). Reduction of pain‐related fear in complex regional pain syndrome type I: The application of graded exposure in vivo. Pain, 116, 264–275. 10.1016/j.pain.2005.04.019 [DOI] [PubMed] [Google Scholar]
- Keogh, E. (2015). Men, masculinity, and pain. Pain, 156, 2408–2412. 10.1097/j.pain.0000000000000328 [DOI] [PubMed] [Google Scholar]
- Keogh, E. , & Denford, S. (2009). Sex differences in perceptions of pain coping strategy usage. European Journal of Pain, 13, 629–634. 10.1016/j.ejpain.2008.07.002 [DOI] [PubMed] [Google Scholar]
- Kraaimaat, F. W. , Bakker, A. , & Evers, A. W. M. (1997). Pijncoping‐strategieën bij chronische pijnpatiënten: De ontwikkeling van de Pijn‐Coping‐Inventarisatielijst (PCI). Gedragstherapie, 30, 185–201. [Google Scholar]
- Kraaimaat, F. W. , & Evers, A. W. M. (2003). Pain‐coping strategies in chronic pain patients: Psychometric characteristics of the pain‐coping inventory (PCI) TL – 10. International Journal of Behavioral Medicine, 10, 343–363. 10.1207/S15327558IJBM1004_5 [DOI] [PubMed] [Google Scholar]
- Lohnberg, J. A. , & Altmaier, E. M. (2013). A review of psychosocial factors in complex regional pain syndrome. Journal of Clinical Psychology in Medical Settings, 20, 247–254. 10.1007/s10880-012-9322-3 [DOI] [PubMed] [Google Scholar]
- Marinus, J. , Moseley, G. L. , Birklein, F. , Baron, R. , Maihöfner, C. , Kingery, W. S. , & van Hilten, J. J. (2011). Clinical features and pathophysiology of complex regional pain syndrome. Lancet Neurology, 10, 637–648. 10.1016/S1474-4422(11)70106-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinus, J. , Perez, R. , & Eijs, F. van (2013). The Role of Pain Coping and Kinesiophobia in Patients With Complex Regional Pain Syndrome Type 1 of the Legs. Clinical Journal of Pain, 29, 563–569. 10.1097/AJP.0b013e31826f9a8a [DOI] [PubMed] [Google Scholar]
- Mccormick, Z. L. , Gagnon, C. M. , Caldwell, M. , Patel, J. , & Kornfeld, S. (2015). Short‐term functional, emotional, and pain outcomes of patients with complex regional pain syndrome treated in a comprehensive interdisciplinary pain management program. Pain Medicine, 16, 2357–2367. 10.1111/pme.12817 [DOI] [PubMed] [Google Scholar]
- Melzack, R. (1975). The McGill Pain Questionnaire: Major properties and scoring methods. Pain, 1, 277–299. 10.1016/0304-3959(75)90044-5 [DOI] [PubMed] [Google Scholar]
- Munce, S. E. P. , & Stewart, D. E. (2007). Gender differences in depression and chronic pain conditions in a national epidemiologic survey. Psychosomatics, 48, 394–399. 10.1176/appi.psy.48.5.394 [DOI] [PubMed] [Google Scholar]
- Oerlemans, M. H. Vragenlijst Vaardigheden. Retrieved from https://meetinstrumentenzorg.blob.core.windows.net/test-documents/Instrument330/450_3_N.pdf
- Oerlemans, H. M. , & Cup, E. H. (2000). The Radboud skills questionnaire: Construction and reliability in patients with reflex sympathetic dystrophy of one upper extremity. Disability and Rehabilitation, 22, 233–245. 10.1080/096382800296809 [DOI] [PubMed] [Google Scholar]
- Pieretti, S. , Di Giannuario, A. , Di Giovannandrea, R. , Marzoli, F. , Piccaro, G. , Minosi, P. , & Aloisi, A. M. (2016). Gender differences in pain and its relief In Alleva E. (Ed.), Annali Dell'Istituto Superiore Di Sanita (pp. 184–189). Rome: Istituto superiore di sanità. [DOI] [PubMed] [Google Scholar]
- Roorda, L. D. Walking and Rising questionnaire (WRQ) Dutch. Retrieved from https://www.leo-d-roorda.nl/en/vragenlijst-lopen-vl35/
- Roorda, L. D. , Roebroeck, M. E. , Van Tilburg, T. , Molenaar, I. W. , Lankhorst, G. J. , & Bouter, L. M. (2005). Measuring activity limitations in walking: Development of a hierarchical scale for patients with lower‐extremity disorders who live at home. Archives of Physical Medicine and Rehabilitation, 86, 2277–2283. 10.1016/j.apmr.2005.06.014 [DOI] [PubMed] [Google Scholar]
- Rovner, G. S. , Sunnerhagen, K. S. , Björkdahl, A. , Gerdle, B. , Börsbo, B. , Johansson, F. , & Gillanders, D. (2017). Chronic pain and sex‐differences; Women accept and move, while men feel blue. PLoS ONE, 12, e0175737 10.1371/journal.pone.0175737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinhoven, P. , Ormel, J. , Sloekers, P. P. A. , Kempen, G. I. J. M. , Speckens, A. E. M. , & Van Hemert, A. M. (1997). A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychological Medicine, 27, 363–370. 10.1017/S0033291796004382 [DOI] [PubMed] [Google Scholar]
- Thissen, D. , Steinberg, L. , & Kuang, D. (2002). Quick and easy implementation of the Benjamini‐Hochberg procedure for controlling the false positive rate in multiple comparisons. Journal of Educational and Behavioral Statistics, 27, 77–83. 10.3102/10769986027001077 [DOI] [Google Scholar]
- van Velzen, G. A. J. , Perez, R. S. G. M. , van Gestel, M. A. , Huygen, F. J. P. M. , van Kleef, M. , van Eijs, F. , … Marinus, J. (2014). Health‐related quality of life in 975 patients with complex regional pain syndrome type 1. Pain, 155, 629–634. 10.1016/j.pain.2013.12.017 [DOI] [PubMed] [Google Scholar]
- Vanderiet, K. , Adriaensen, H. , Carton, H. , & Vertommen, H. (1987). The McGill Pain Questionnaire constructed for the Dutch language (MPQ‐DV). Preliminary data concerning reliability and validity. Pain, 30, 395–408. 10.1016/0304-3959(87)90027-3 [DOI] [PubMed] [Google Scholar]
- Vlaeyen, J. W. S. , Kole‐Snijders, A. M. J. , Boeren, R. G. B. , & van Eek, H. (1995). Fear of movement/(re)injury in chronic low back pain and its relation to behavioral performance. Pain, 62, 363–372. 10.1016/0304-3959(94)00279-N [DOI] [PubMed] [Google Scholar]
- Ware, J. , & Sherbourne, C. (1994). The MOS 36‐item short‐form health survey (SF‐36). Medical Care, 30, 473–483. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


