Abstract
Aims
Using a pragmatic approach, the LIRA‐PRIME trial aims to address a knowledge gap by comparing efficacy in controlling glycaemia with glucagon‐like peptide‐1 analog liraglutide vs oral antidiabetic drugs (OADs) in patients with type 2 diabetes (T2D) uncontrolled with metformin monotherapy in primary care practice. We report the study design and patient baseline characteristics.
Materials and methods
This 104‐week, two‐arm, open‐label, active‐controlled trial is active in 219 primary care practices across nine countries. At screening, eligible patients with T2D were at least 18 years of age, had been using a stable daily dose of metformin ≥1500 mg or the maximum tolerated dose for ≥60 days, and had a glycated haemoglobin (HbA1c) of 7.5% to 9.0%, measured ≤90 days before screening. Patients were randomized (1:1) to liraglutide or OAD, both in addition to pre‐trial metformin. Individual OADs were chosen by the treating physician based on local guidelines. The primary endpoint is time to inadequate glycaemic control, defined as HbA1c above 7.0% at two scheduled consecutive visits after the first 26 weeks of treatment.
Results
The trial randomized 1997 patients with a mean (standard deviation) age of 56.9 (10.8) years, T2D duration of 7.2 (5.9) years (range, <1‐47 years), and HbA1c of 8.2%. One‐fifth of patients had a history of diabetes complications, and most were overweight (24.8%) or had obesity (65.3%).
Conclusions
This pragmatically designed, large‐scale, multinational, randomized clinical trial will help guide treatment decisions for patients with T2D who are inadequately controlled with metformin monotherapy and treated in primary care.
Keywords: GLP‐1, liraglutide, type 2 diabetes
1. INTRODUCTION
Because of the progressive nature of type 2 diabetes (T2D), treatment intensification with oral antidiabetic drugs (OADs) and injectable therapies is often needed to reach and maintain treatment targets.1 Early and continued glycaemic control reduces the risk of development and progression of diabetes complications.2 Despite this, a substantial proportion of patients using oral monotherapy remain in poor glycaemic control for several years before treatment is intensified,3 in part because of fears associated with treatment‐associated weight gain and hypoglycaemia, and also because patients may perceive more advanced treatment regimens to be too complex or burdensome.4 Furthermore, delay may be the result of clinical inertia.
Although the burden of care of patients with T2D generally falls within the realm of primary care,5 there remains a lack of evidence from randomized trials to guide clinical decision‐making in this setting. Randomized clinical trials conducted in specialist settings are often characterized by narrow inclusion criteria and strictly controlled interventions that require high compliance with study protocol. Translating results from these trials into general clinical practice can be challenging. Pragmatic trials in a primary care setting are associated with broader inclusion criteria and more loosely defined interventions, thereby providing clinical evidence that is more generalizable to a daily clinical care setting.6, 7, 8
Glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs), such as liraglutide, are recommended as a second‐line treatment option when metformin monotherapy is considered insufficent.1 Randomized controlled trials have demonstrated clinically significant reductions in glycated haemoglobin (HbA1c) with liraglutide, along with weight loss and low risk of hypoglycaemia, in patients with T2D as compared with other antidiabetic treatment regimens.9, 10, 11, 12 Additionally, a large cardiovascular (CV) outcomes trial, LEADER, reported a significant reduction in the risk of major CV events, all‐cause mortality and renal outcomes with liraglutide vs placebo, both in addition to standard of care, in patients with T2D who are at high CV risk.13, 14 However, no dedicated randomized pragmatic trial with liraglutide has been conducted in the primary care setting, and the efficacy of liraglutide in maintaining glycaemic control in patients inadequately controlled with metformin in primary care practice, as compared with other available OADs, is unknown. A durable, dual glucose‐lowering treatment regimen that keeps pace with disease progression without necessitating frequent regimen changes in this setting would be beneficial to both patients and healthcare providers.
Using a pragmatic approach, the LIRA‐PRIME trial aims to add valuable evidence to bridge this knowledge gap by comparing efficacy in controlling glycaemia with liraglutide vs OADs in patients with T2D who are uncontrolled with metformin monotherapy in primary care practice. This manuscript describes the study design and operational aspects, and provides baseline data for the trial population.
2. MATERIALS AND METHODS
2.1. Study design and participants
The LIRA‐PRIME trial (ClinicalTrials.govNCT02730377) is a 104‐week, multi‐centre, randomized, two‐arm, open‐label, active‐controlled clinical trial, conducted in the primary care setting. In this trial, the term “primary care” was adapted from the definition used by the American Academy of Family Physicians.15 The trial has a pragmatic design to reflect diabetes management in primary care: a low number of eligibility criteria allowing enrollment of a broad patient population, treatments prescribed according to local labels, quarterly patient visits, and trial products dispensed by retail pharmacies. The trial is scheduled for completion in August 2019. Inclusion and exclusion criteria are presented in Table 1.
Table 1.
LIRA‐PRIME inclusion and exclusion criteria
| Inclusion criteria |
|
| Exclusion criteria |
|
Abbreviations: HbA1c, glycated haemoglobin; OAD, oral antidiabetic drug; T2D, type 2 diabetes.
The LIRA‐PRIME trial is being conducted in compliance with Good Clinical Practice (International Conference on Harmonization) and applicable regulatory requirements including Institutional Review Board approval16 and in accordance with the Declaration of Helsinki.17 Informed consent was obtained from all patients before any trial‐related activity. All patients have the right to withdraw from the trial at any time, without giving a specific reason.
2.2. Randomization and masking
After an initial screening period of up to 2 weeks, during which patients continued usual metformin treatment, patients were randomly assigned (1:1) to either liraglutide or a single OAD, both in addition to metformin, for a maximum of 104 weeks, followed by a 1‐week follow‐up period after the end of treatment (Figure 1). Six different classes of OADs were permitted: alpha‐glucosidase inhibitors, dipeptidyl peptidase‐4 (DPP‐4) inhibitors, meglitinides, sodium‐glucose cotransporter‐2 inhibitors (SGLT‐2is), sulphonylureas and thiazolidinediones. Patients are to continue using the same antidiabetic drugs, pre‐trial metformin plus either liraglutide or one selected OAD, throughout the trial. All trial products are commercial products used according to local labels, and are dispensed by a retail pharmacy or similar dispensary in accordance with different national and/or local regulations and requirements. Trial products are reimbursed by the trial sponsor throughout the individual patient's trial participation. An interactive web response system was used for randomization. After randomization, the trial is open‐label, with the exception of key trial staff from the sponsor, who are blinded until the end of the trial.
Figure 1.
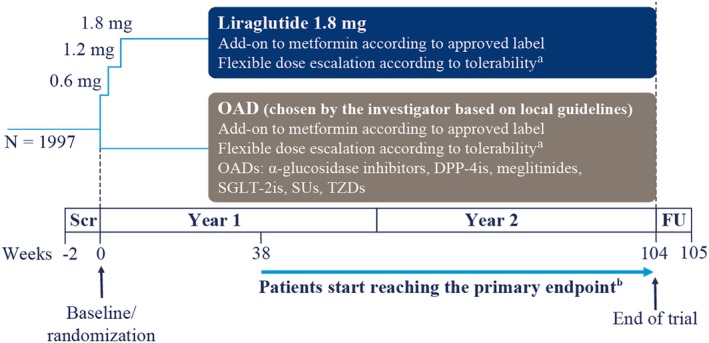
Study design. aPlease refer to text for further details. bPatients reaching the primary endpoint from Week 38 onwards will be withdrawn from the trial. Abbreviations: DPP‐4i, dipeptidyl peptidase‐4 inhibitor; FU, follow‐up; OAD, oral antidiabetic drug; Scr, screening; SGLT‐2is, sodium‐glucose cotransporter‐2 inhibitors; SUs, sulphonylureas; TZDs, thiazolidinediones
Patients randomized to liraglutide initiated treatment with 0.6 mg/d, with subsequent dose escalation up to 1.8 mg/d or maximum tolerated dose, according to patient needs and approved local label. Additional time for dose escalation was allowed at the discretion of the investigator. For both liraglutide and OADs, a maintenance dose below the maximum approved dose is acceptable if HbA1c is less than 7.0%; however, escalation to the maximum approved or maximum tolerated dose should be attempted, at the discretion of the investigator if HbA1c is 7.0% or greater. All patients will maintain the same pre‐trial dose of metformin during the entire trial, unless there is a safety concern.
Patient visits are scheduled at Weeks 2, 4, 16 and 26 after randomization, and on a quarterly basis thereafter (Table 2), to reflect the normal frequency of appointments in daily clinical routine.1 Unscheduled additional visits for medical reasons are allowed at the discretion of the investigator.
Table 2.
Flow chart of patient visits
| Trial periods | Screeninga | Randomization | Treatment | End of treatment | Follow‐ up | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Visit type | S | S | P/S2b | S | S | S | S | S | S | S | S | S | P |
| Visit number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| Timing of visit, weeks | Up to − 2 | 0 | 2 | 4 | 16 | 26 | 38 | 52 | 65 | 78 | 91 | 104 | 105 |
| Visit window, days | ±7 | ±4 | ±10 | ±10 | ±10 | ±10 | ±10 | ±10 | ±10 | ±4 | +4 | ||
| Informed consent | X | ||||||||||||
| Inclusion/exclusion criteria | X | ||||||||||||
| Randomization | X | ||||||||||||
| IWRS session | X | X | |||||||||||
| Withdrawal criteriac | X | X | X | X | X | X | X | X | X | X | |||
| Medical history/concomitant illness | X | ||||||||||||
| Concomitant medication | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Demography | X | ||||||||||||
| Diagnosis of diabetes | X | ||||||||||||
| Diabetes complications | X | X | X | ||||||||||
| Diabetes treatment history | X | ||||||||||||
| Smoking status | X | ||||||||||||
| Pregnancy urine test | X | (X) | (X) | (X) | (X) | (X) | (X) | (X) | (X) | (X) | (X) | X | |
| Handout ID card | X | ||||||||||||
| Height | X | ||||||||||||
| Body weight | X | X | X | X | X | X | X | X | X | X | |||
| Blood sampling | X | X | X | X | X | X | X | X | X | X | |||
| ‐Fasting plasma glucose | X | X | X | X | X | ||||||||
| ‐HbA1cd | X | X | X | X | X | X | X | X | X | ||||
| ‐Lipids | X | X | X | ||||||||||
| ‐Biochemistry | X | X | X | ||||||||||
| ‐Haematology | X | X | X | ||||||||||
| ‐Attend visit fasting | X | X | X | X | X | ||||||||
| Systolic and diastolic blood pressure, sitting | X | X | X | X | X | X | X | X | X | X | |||
| Pulse, sitting | X | X | X | X | X | X | X | X | X | X | |||
| Handout diary and instruction in usee | X | X | X | X | X | X | X | X | X | ||||
| Handout blood glucose meter and instruction in use | X | ||||||||||||
| Prescription of trial product | X | X | X | X | X | X | X | X | X | ||||
| Information about trial product handling | X | (X)f | (X)f | (X)f | (X)f | (X)f | (X)f | (X)f | (X)f | (X)f | |||
| Drug accountability | X | X | X | X | X | X | X | X | X | ||||
| Adverse events | X | X | X | X | X | X | X | X | X | X | X | X | |
| Hypoglycaemic episodes | X | X | X | X | X | X | X | X | X | X | X | X | |
| Technical complaints | X | X | X | X | X | X | X | X | X | X | |||
| End of treatment | X | ||||||||||||
| End of trial | X | ||||||||||||
| Sign‐off casebook | X | ||||||||||||
Abbreviations: eCRF, electronic case report form; HbA1c, glycated haemoglobin; IWRS, Interactive Web Response System; NGSP, National Glycohemoglobin Standardization Program; P/S, phone contact/site visit.
Procedures and assessments relating to Visit 1 may be conducted at Visit 2 prior to randomization. Trial‐related procedures (including requesting the subject to fast) are not allowed before signing of informed consent.
Visit 3 can be performed as either a phone contact or site visit at the discretion of the investigator.
Participants withdrawing from the trial should attend end‐of‐treatment visit as soon as possible.
HbA1c was tested following the NGSP‐certified VARIANT II (HbA1c) method, developed by Bio‐Rad Laboratories.
Diaries should be reviewed at every visit. Relevant data should be recorded in the eCRF.
Information about and training in trial product handling to be given at the discretion of the investigator.
Patients were given diaries to record any new concomitant medication, dose changes to trial medications and adverse events. Patients were instructed to measure and record blood glucose when a hypoglycaemic episode18 is suspected. All plasma glucose values of 3.9 mmol/L (70 mg/dL) or less, or episodes with symptoms of hypoglycaemia with values greater than 3.9 mmol/L (70 mg/dL) are requested to be recorded for transcription into the electronic case report form by the healthcare provider. Patients are also required to report further information concerning hypoglycaemic episodes, including symptoms, physical activities or meals prior to the episode, and whether the patient was able to self‐treat, to allow for evaluation of the severity of the episode. In keeping with the real‐life design of the trial, regular self‐measured blood glucose profiles were not conducted, unless required as part of a treatment's local label.
Assessment of HbA1c, fasting plasma glucose (FPG), lipids and serum biochemistry is conducted in centralized laboratories that are approved by local authorities according to International Federation of Clinical Chemistry and Laboratory Medicine standards.19 Treatment adherence is not measured because of the pragmatic design. As trial products are dispensed by local retail pharmacies, drug accountability is based on the prescribed trial product and empty packaging material returned to the investigator at clinic visits.
2.3. Outcomes
The primary objective of this study is to compare glycaemic control with liraglutide vs that with OADs, both as add‐on to metformin therapy, after up to 104 weeks of treatment. The primary endpoint is time to inadequate glycaemic control, defined as HbA1c greater than 7.0% at two scheduled consecutive visits after the first 26 weeks of treatment and up to 104 weeks. Patients meeting the primary endpoint after Week 26 and before Week 104 will be withdrawn from the trial and will be considered premature treatment discontinuations as a result of meeting the primary endpoint. The first possible discontinuation for this criterion (two consecutive HbA1c measurements greater than 7.0%) is at Week 38.
Key secondary endpoints include time to premature treatment discontinuation for any reason and change from baseline to Week 104 in HbA1c, FPG, body weight, body mass index (BMI), and systolic and diastolic blood pressure (BP). Also being evaluated is the number of patients who, at the end of the trial (Week 104) or at premature treatment discontinuation, achieve: (a) HbA1c ≤6.5%; (b) HbA1c ≤7.0% without weight gain; (c) HbA1c ≤7.0% without treatment‐emergent severe hypoglycaemic episodes or blood glucose‐confirmed symptomatic hypoglycaemic episodes18; and/or (d) HbA1c ≤7.0% without weight gain and no treatment‐emergent severe hypoglycaemic episodes or blood glucose‐confirmed symptomatic hypoglycaemic episodes.
Safety endpoints include the incidence of hypoglycaemic episodes, serious adverse events, and adverse events leading to permanent discontinuation of the investigational product. Diabetes complications recorded at baseline were reported by the investigator during the screening visit.
2.4. Statistical analyses
The sample size was determined to detect a difference in the time to inadequate glycaemic control (HbA1c >7.0%) between the liraglutide and OAD groups with 90% power. Based on previous clinical trials,12, 20 the proportion of patients expected to discontinue treatment prematurely without inadequate glycaemic control is 20%. Of the remaining patients, it is expected that 66% in the liraglutide group and 76% in the OAD group will have met the criteria for inadequate glycaemic control by Week 104. However, it is assumed that the treatment difference will not appear until after the visit at Week 65. With these assumptions, we calculated that 1994 randomized patients are required to ensure sufficient statistical power.
The time to inadequate glycaemic control will be compared between the liraglutide and OAD treatment groups using a two‐sided non‐parametric log rank test at a 5% significance level. The analysis will not be based on any model assumptions and it will not be adjusted for any covariates; this conservative approach means that any potential bias of baseline characteristics will be towards no treatment effect. The full analysis set includes all randomized patients and will be used in the analysis of the primary endpoint and other efficacy endpoints. Statistical evaluation of the full analysis set will follow the intention‐to‐treat principle, with patients contributing to the evaluation as randomized. After 26 weeks, if a patient discontinues treatment before the next planned visit, the last observation will be carried forward and the patient will be considered to have had an event of inadequate glycaemic control at the next planned visit. Otherwise, patients discontinuing treatment before 104 weeks, without inadequate glycaemic control, will be censored immediately after the last visit during treatment.
Summaries of patient demographics and clinical characteristics included in this paper are based on the data available at baseline for all randomized patients. Categorical data are presented as number and proportion of patients in each category. Continuous data are presented as arithmetic mean, standard deviation (SD) and range. Data are preliminary and may be subject to change before the database is locked at the end of the trial.
3. RESULTS
Between 28 March 2016 and 7 August 2017, the LIRA‐PRIME trial enrolled 1997 patients from 219 sites across nine countries (Figure 2). Baseline patient demographics and clinical characteristics are summarized in Table 3.
Figure 2.
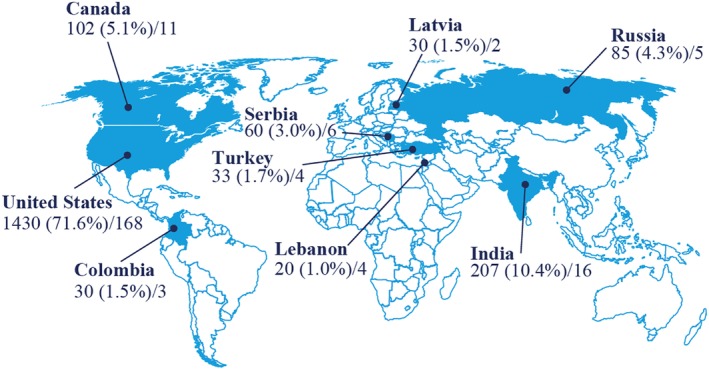
Number (%) of patients recruited and number of sites per country
Table 3.
Baseline demographics and clinical characteristics
| Baseline characteristic | Total population (N = 1997) |
|---|---|
| Female, n (%) | 949 (47.5%) |
| Diabetes duration, years | 7.2 (5.9) [0.2–47.3] |
| Age, years | 56.9 (10.8) [19.0–89.0] |
| Age group, n (%) | |
| 18 to < 45 | 255 (12.8%) |
| 45 to < 65 | 1242 (62.2%) |
| 65 to < 75 | 412 (20.6%) |
| ≥75 | 88 (4.4%) |
| Race, n (%) | |
| White | 1443 (72.3%) |
| Black or African American | 209 (10.5%) |
| Asian | 290 (14.5%) |
| Other | 55 (2.8%) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 366 (18.3%) |
| Not Hispanic or Latino | 1631 (81.7%) |
| Smoking status, n (%) | |
| Current smoker | 324 (16.2%) |
| Previous smoker | 463 (23.2%) |
| Never smoked | 1210 (60.6%) |
| HbA1c, % | 8.2 (1.0) [5.3–14.9] |
| HbA1c group, %, n (%) | |
| <7.0 | 113 (5.7) |
| 7.0 to < 7.5 | 285 (14.3) |
| 7.5 to < 8.0 | 496 (24.8) |
| 8.0 to < 8.5 | 429 (21.5) |
| 8.5 to < 9.0 | 317 (15.9) |
| 9.0 to < 9.5 | 182 (9.1) |
| ≥9.5 | 144 (7.3) |
| Fasting plasma glucose, mmol/L | 9.5 (2.8) [2.4–26.3] |
| Body weight, kg | 94.8 (24.4) [40.3–212.1] |
| BMI, kg/m2 | 33.5 (7.4) [17.2–68.7] |
| BMI group, kg/m2, n (%) | |
| <25 | 193 (9.7) |
| 25 to < 30 | 495 (24.8) |
| 30 to < 35 | 585 (29.3) |
| 35 to < 40 | 391 (19.6) |
| ≥40 | 327 (16.4) |
| Systolic blood pressure, mmHg | 131.3 (14.7) [70.0–202.0] |
| Diastolic blood pressure, mmHg | 79.8 (9.2) [42.0–123.0] |
| Pulse, beats/min | 75.3 (10.3) [45.0–119.0] |
| eGFR, mL/min/1.73 m2 a, n (%) | |
| G 1 (normal) ≥90 | 1109 (55.5) |
| G 2 (mildly decreased) 60–89 | 733 (36.7) |
| G 3 (moderately decreased) 30–59 | 126 (6.3) |
| G 4 (severely decreased) 15–29 | 2 (0.1) |
| Total cholesterol, mg/dL | 176.9 (41.9) [53.3–414.3] |
| LDL cholesterol, mg/dL | 98.1 (35.6) [6.6–291.9] |
| HDL cholesterol, mg/dL | 44.1 (10.9) [18.5–131.3] |
| Triglycerides, mg/dL | 187.2 (128.5) [31.2–2136] |
| Diabetes complications, n (%) [years, range] | 416 (20.8) |
| Diabetic nephropathy | 86 (4.3) [2.7, 0.0–20.1] |
| Diabetic neuropathy | 300 (15.0) [3.8; 0.0–27.5] |
| Diabetic retinopathy | 63 (3.2) [3.0, 0.0–13.5] |
| Macro‐angiopathy | 62 (3.1) [5.2; 0.0–43.3] |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; G, GFR category; HbA1c, glycated haemoglobin; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; n, number of patients; SD, standard deviation.
Data may be subject to minor changes at trial completion after database cleaning. Data are given as arithmetic mean (SD) [min; max] unless otherwise stated.
eGFR description and range adapted from Kidney Disease: Improving Global Outcomes (KDIGO) 2012. Kidney Int Suppl. 2013;3:1–150.25
Patients presented with a wide range of diabetes duration, from less than 1 year to 47 years, mean HbA1c of 8.2%, mean (SD) systolic BP of 131.3 (14.7) mmHg, and mean diastolic BP of 79.8 (9.2) mmHg. Most patients were overweight (24.8%) or had obesity (65.3%), and most (92.2%) had normal (estimated glomerular filtration rate [eGFR] ≥90 mL/min/1.73 m2) or mildly decreased (eGFR: 60‐89 mL/min/1.73 m2) renal function. Diabetic complications included neuropathy (15.0%), nephropathy (4.3%), retinopathy (3.2%) and macro‐angiopathy (3.1%).
4. DISCUSSION
Although improved glycaemic control with liraglutide in patients with T2D has been extensively assessed in specialist care settings,21 little is known about the treatment effect in the setting of routine primary care. With a pragmatic design, a more heterogeneous population at baseline than most randomized clinical trials in specialist care, and a number of treatment options compared in this study, the LIRA‐PRIME trial should provide further guidance for optimal treatment of patients with T2D who are managed in primary care practice. The wide ranges of baseline data and clinical characteristics collected reflect the primary care setting.22
While pragmatic trials conducted in diverse primary care settings around the world contribute to addressing the lack of data concerning primary care, they also present certain challenges. One challenge is to define endpoints and outcomes that are relevant to patients. These should be simple and should be assessed by procedures/methods widely available in clinical practice, without compromising quality and consistency. The primary endpoint in the LIRA‐PRIME trial is relevant to patients' daily care and reflects the questions concerning choice of the treatment that is most likely to be able to keep pace with disease progression. This concern is not likely to be addressed in randomized trials of short duration, for example, 6 months.
Another challenge in the LIRA‐PRIME trial derives from the simple inclusion criteria for HbA1c. Patients were included in the trial based on HbA1c values between 7.5% and 9.0%, measured within 90 days prior to screening at a local laboratory and documented in the medical records; however, one quarter of the patients at randomization had HbA1c values below 7.5% or above 9.0% as measured by the central laboratory. Discrepancies between the screening HbA1c level and the first centralized measurement were expected, but the observed extent of this discrepancy was greater than anticipated. The most likely explanations could be a change in HbA1c between the pre‐screening visit and randomization, with a maximum of 90 days between these two visits, and/or discrepancies in the methodology for HbA1c assessment between local and central laboratories. The presence of patients with HbA1c values greater than 9.5% may have an impact on the results generated from this trial, as lowering HbA1c to less than 7.0% will be more difficult in patients with HbA1c above 9.5% than in those with HbA1c below 9.0%. This could potentially affect the number and timings of premature withdrawals due to inadequate glycaemic control. However, given that randomization should have resulted in a fairly even distribution of participants with baseline HbA1c values outside the permitted range between the liraglutide and OAD groups, this effect should be similar in both arms.
Advertising for trial participants is a standard method used in most clinical trials to ensure that recruitment timelines are met. Because of the pragmatic approach of this trial and the primary care setting, patients were informed about the trial only at the level of the local clinic, through posters and direct conversation with physicians; however, recruiting a sufficient number of participants without a broader advertising campaign proved more difficult than anticipated. Thus, the recruitment period was extended from 12 to 18 months to allow sufficient time for physicians to recruit from their own patient populations. An important element of the pragmatic trial design was to have patients obtain trial products directly from a local retail pharmacy, but this requires different methods of reimbursement in each participating country, to conform with local regulations.
To obtain valid results, a certain extent of intervention was needed in the LIRA‐PRIME trial to achieve sufficient patient adherence to the protocol. Although the intent of the trial design was for patients to receive the maximum approved or tolerated dose as early as possible, the pragmatic design gave investigators the option of escalating the dose at their discretion to minimize side effects (eg, gastrointestinal adverse events). Although this discretion could introduce some clinical inertia within the trial, it may be balanced by increased adherence as the result of fewer side effects and regular follow‐up physician visits.23
Globally, different healthcare systems and facilities are available in different countries. One operational issue involved the use of commercial trial products, which revealed the need for country‐specific organization to conform with different regulations and requirements concerning trial products, such as labelling. Finally, investigators in the primary care setting are often less experienced in performing trials, for example, in handling products, and have limited resources as compared to investigators in specialist settings who are more experienced in conducting clinical trials. As choice of treatment in the comparator arm was at the discretion of the physician, we presume that unfamiliarity with drug options would not be an issue, but primary care physicians may have less experience with liraglutide, if typically prescribed in a specialist care setting in their country.
We recognize that there are limitations to the trial design. For example, physician selection of OAD can be influenced by the knowledge that the efficacy of these agents is being compared with that of a GLP‐1RA, potentially leading physicians to select a more potent agent than usual, and influenced also by the external funding of treatment, which potentially removes cost considerations from treatment decisions. Both of these factors could lead to treatment practices that do not reflect those of the everyday clinical setting. Another limitation is that, to closely mimic real‐world management, treatment adherence was not monitored. It is possible that the hypoglycaemia and weight‐loss benefits may favour adherence to liraglutide,23 potentially biasing treatment outcomes; however, the injection regimen of liraglutide and its early gastrointestinal side effects,24 which are not present with other OADs used in this study, with the exception of DPP‐4is, might disfavour adherence in patients treated with liraglutide, thus biasing treatment outcomes in the opposite direction. Finally, a third limitation, which may be intrinsic to any trial, is that the updating of treatment guidelines and the emergence of new standards of care may render the study treatment(s) sub‐optimal in certain scenarios; however, because of the open‐label design of the LIRA‐PRIME trial, investigators are aware of the drug a patient is receiving and participants can be withdrawn from the study at any time, should there be any safety concerns.
In summary, the LIRA‐PRIME trial has successfully enrolled the desired number of patients (n = 1997) from 219 primary care practices across nine countries. The demographic and clinical profiles of these individuals suggest that they represent a heterogeneous group. Primary care physicians will soon have comparative data concerning the safety and efficacy of liraglutide as compared to OADs within a primary care setting for patients not reaching glycaemic targets with metformin alone. Results of the trial should be available in 2020.
CONFLICT OF INTEREST
J. U. is an advisory board member, consultant and speakers bureau member for, and has received research support from, Novo Nordisk A/S. D. C. A., D. L., K. L. and J. K. P. have received research support from Novo Nordisk A/S. M. K. and M. B. T. are employees of and shareholders in Novo Nordisk A/S. M. Z. has received research support from Novo Nordisk A/S and has presented lectures for Novo Nordisk A/S, AstraZeneca, MSD and Eli Lilly Ltd. M. C., G. M. and M. S. have nothing to declare.
Author contributions
J. U. conducted follow‐up assessments of patients and oversaw the study. D. C. A. conducted follow‐up assessments of patients. M. C. conducted follow‐up assessments of patients. K. L. conducted screening and follow‐up assessments of patients. D. L. conducted follow‐up assessments of patients. G. M. conducted follow‐up assessments of patients. J. K. P. contributed to study design and data management. M. S. conducted follow‐up assessments of patients. M. K. contributed to trial design, medical oversight during trial conduct, data cleaning and analysis and interpretation of the data. M. B. T. contributed to analysis and interpretation of the data. M. Z. conducted follow‐up assessments of patients. All authors interpreted the data and participated in writing the manuscript with the assistance of medical writing services provided by the trial sponsor. All authors read the manuscript critically and approved the submitted article prior to submission.
ACKNOWLEDGMENTS
The authors thank all trial personnel, investigators and patients who were involved in this study, and acknowledge Martin Linder and Hans Askelund Sævereid, Novo Nordisk, for review of and input to the statistical analyses.
Medical writing assistance and editorial and submission support were provided by Ugo Battaglia, PhD, and Izabel James, MBBS, both from Watermeadow Medical, an Ashfield Company, part of UDG Healthcare plc, funded by Novo Nordisk A/S.
Part of this study was presented as a poster at the American Diabetes Association 78th Scientific Sessions, Orlando, Florida, USA, June 22‐26, 2018.
Subject level analysis datasets for this publication are available from the corresponding author upon reasonable request.
Unger J, Allison DC, Carlton M, et al. Trial design and baseline data for LIRA‐PRIME: A randomized trial investigating the efficacy of liraglutide in controlling glycaemia in type 2 diabetes in a primary care setting. Diabetes Obes Metab. 2019;21:1543–1550. 10.1111/dom.13682
Funding information Novo Nordisk A/S; Funding for the LIRA‐PRIME trial is provided by the trial sponsor, Novo Nordisk A/S, Denmark (no grants were issued).
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.13682.
REFERENCES
- 1. American Diabetes Association . 8. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(suppl 1):S73‐S85. [DOI] [PubMed] [Google Scholar]
- 2. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577‐1589. [DOI] [PubMed] [Google Scholar]
- 3. Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care. 2013;36:3411‐3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Russell‐Jones D, Pouwer F, Khunti K. Identification of barriers to insulin therapy and approaches to overcoming them. Diabetes Obes Metab. 2018;20:488‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davidson JA. The increasing role of primary care physicians in caring for patients with type 2 diabetes mellitus. Mayo Clin Proc. 2010;85(suppl 12):S3‐S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chalkidou K, Tunis S, Whicher D, Fowler R, Zwarenstein M. The role for pragmatic randomized controlled trials (pRCTs) in comparative effectiveness research. Clin Trials. 2012;9:436‐446. [DOI] [PubMed] [Google Scholar]
- 7. EMA . Scientific guidance on post‐authorisation efficacy studies. 2016. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/12/WC500219040.pdf. Accessed May 2018.
- 8. Ford I, Norrie J. Pragmatic trials. N Engl J Med. 2016;375:454‐463. [DOI] [PubMed] [Google Scholar]
- 9. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD‐3 Mono): a randomised, 52‐week, phase III, double‐blind, parallel‐treatment trial. Lancet. 2009;373:473‐481. [DOI] [PubMed] [Google Scholar]
- 10. Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26‐week randomised, parallel‐group, multinational, open‐label trial (LEAD‐6). Lancet. 2009;374:39‐47. [DOI] [PubMed] [Google Scholar]
- 11. Zinman B, Gerich J, Buse JB, et al. Efficacy and safety of the human glucagon‐like peptide‐1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD‐4 met+TZD). Diabetes Care. 2009;32:1224‐1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pratley RE, Nauck M, Bailey T, et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26‐week, randomised, parallel‐group, open‐label trial. Lancet. 2010;375:1447‐1456. [DOI] [PubMed] [Google Scholar]
- 13. Marso SP, Gilbert H, Daniels MD, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mann JFE, Ørsted DD, Brown‐Frandsen K, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377:839‐848. [DOI] [PubMed] [Google Scholar]
- 15. American Academy of Family Physicians . Primary Care. 2016. Available at https://www.aafp.org/about/policies/all/primary-care.html#1. Accessed February 2018.
- 16. ICH Harmonised Tripartite Guideline . International Conference on Harmonisation. ICH Harmonised Tripartite Guideline. Guideline for Good Clinical Practice E6 (R1), Step 4. June 10, 1996. Available at https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. Accessed May 2018.
- 17. World Medical Association . Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. 64th WMA General Assembly, Fortaleza. 1 Oct 2013. Available at https://www.wma.net/policies‐post/wma‐declaration‐of‐helsinki‐ethical‐principles‐for‐medical‐research‐involving‐human‐subjects/. Accessed May 2018.
- 18. Seaquist ER, Andersons MD, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36:1384‐1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. International Federation of Clinical Chemistry and Laboratory Medicine, IFCC Scientific Division , Mosca A, Goodall I, et al. Global standardization of glycated hemoglobin measurement: the position of the IFCC Working Group. Clin Chem Lab Med. 2007;45:1077‐1080. [DOI] [PubMed] [Google Scholar]
- 20. Pratley RE, Nauck M, Bailey T, et al. One year of liraglutide treatment offers sustained and more effective glycaemic control and weight reduction compared with sitagliptin, both in combination with metformin, in patients with type 2 diabetes: a randomised, parallel‐group, open‐label trial. Int J Clin Pract. 2011;65:397‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scott LJ. Liraglutide: a review of its use in adult patients with type 2 diabetes mellitus. Drugs. 2014;74:2161‐2174. [DOI] [PubMed] [Google Scholar]
- 22. Spann SJ, Nuttling PA, Galliher JM, et al. Management of type 2 diabetes in the primary care setting: a practice‐based research network study. Ann Fam Med. 2006;4:23‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garcia‐Pérez LE, Alvarez M, Dilla T, Gil‐Guillén V, Orozco‐Beltrán D. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther. 2013;4:175‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jackson SH, Martin TS, Jones JD, Seal D, Emanuel F. Liraglutide (victoza): the first once‐daily incretin mimetic injection for type‐2 diabetes. P T. 2010;35:498‐529. [PMC free article] [PubMed] [Google Scholar]
- 25. KDIGO 2012 . Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1‐150. [DOI] [PubMed] [Google Scholar]


