Abstract
Aim
To systematically review and meta‐analyse studies of neurodevelopmental outcome of children born to mothers prescribed methadone in pregnancy.
Method
MEDLINE, Embase, and PsycINFO were searched for studies published from 1975 to 2017 reporting neurodevelopmental outcomes in children with prenatal methadone exposure.
Results
Forty‐one studies were identified (2283 participants). Eight studies were amenable to meta‐analysis: at 2 years the Mental Development Index weighted mean difference of children with prenatal methadone exposure compared with unexposed infants was −4.3 (95% confidence interval [CI] −7.24 to −1.63), and the Psychomotor Development Index weighted mean difference was −5.42 (95% CI −10.55 to −0.28). Seven studies reported behavioural scores and six found scores to be lower among methadone‐exposed children. Twelve studies reported visual outcomes: nystagmus and strabismus were common; five studies reported visual evoked potentials of which four described abnormalities. Factors that limited the quality of some studies, and introduced risk of bias, included absence of blinding, small sample size, high attrition, uncertainty about polydrug exposure, and lack of comparison group validity.
Interpretation
Children born to mothers prescribed methadone in pregnancy are at risk of neurodevelopmental problems but risk of bias limits inference about harm. Research into management of opioid use disorder in pregnancy should include evaluation of childhood neurodevelopmental outcome.
What this paper adds
Children born to opioid‐dependent mothers prescribed methadone are at risk of neurodevelopmental impairment.
Exposed infants have lower Mental Development Index and Psychomotor Development Index scores than unexposed children.
Atypical visual evoked potentials, strabismus, and nystagmus have increased prevalence.
Estimates of impairment may be biased by intermediate to poor quality evidence.
What this paper adds
Children born to opioid‐dependent mothers prescribed methadone are at risk of neurodevelopmental impairment.
Exposed infants have lower Mental Development Index and Psychomotor Development Index scores than unexposed children.
Atypical visual evoked potentials, strabismus, and nystagmus have increased prevalence.
Estimates of impairment may be biased by intermediate to poor quality evidence.
Editor's Choice
My Editor's Choice for the July 2019 issue is this systematic review. It highlights neurodevelopmental outcomes of children who had antenatal exposure to methadone given to their mother as maintenance treatment (as well as other, less documented risk factors). The surge in opiod dependence in the USA and other countries, particularly in young adults, raises specific concerns for the children's development.
This article is commented on by Skranes on page 738 of this issue.
This article's abstract has been translated into Spanish and Portuguese.
Follow the links from the abstract to view the translations.
Video Podcast: https://www.youtube.com/watch?v=Ixo12Fg0FJs
Resumen
Desarrollo neurológico infantil tras la prescripción de metadona de mantenimiento para el tratamiento de la dependencia de opioides durante el embarazo: revisión sistemática y meta‐análisis
Objetivo
Revisar sistemáticamente y realizar un meta‐análisis de estudios sobre el resultado del desarrollo neurológico de los niños nacidos de madres a quienes se les recetó metadona durante el embarazo.
Metodología
Se realizó una búsqueda en MEDLINE, Embase, y PsycINFO de estudios publicados desde el año 1975 al 2017 que informaran sobre el resultado del desarrollo neurológico de niños que hubieran tenido exposición prenatal a la metadona.
Resultados
Se identificaron 41 estudios (2283 participantes). Ocho estudios se pudieron someter al meta‐análisis: a los dos años de edad los niños con exposición prenatal a la metadona mostraron una diferencia de medias ponderada de ‐4,3 (95% intervalo de confianza [IC] −7,24 to −1,63) en el Índice de Desarrollo Mental (Mental Development Index) en comparación con los niños no expuestos. En el Índice de Desarrollo Psicomotor (Psychomotor Development Index) la diferencia de medias ponderada fue −5,42 (95% CI −10,55 to −0,28). 7 estudios mostraron las puntuaciones comportamentales y 6 de ellos encontraron puntuaciones más bajas entre los niños expuestos a la metadona. Doce estudios informaron sobre los resultados a nivel visual: el nistagmo y el estrabismo fueron comunes; 5 estudios informaron sobre los potenciales evocados visuales, de los cuáles cuatro describieron anormalidades. Los factores que limitaron la calidad de algunos estudios e introdujeron el riesgo de sesgo, incluyeron la ausencia de cegamiento, el pequeño tamaño de la muestra, el alto desgaste, la incertidumbre acerca de la exposición a varias drogas y la falta de validez del grupo de comparación.
Interpretación
Los niños nacidos de madres a quienes se les recetó metadona durante el embarazo se encuentran en riesgo de sufrir problemas de desarrollo neurológico, pero el riesgo de sesgo limita la inferencia sobre el daño. La investigación sobre el manejo del trastorno por uso de opioides en el embarazo debe incluir la evaluación del resultado del desarrollo neurológico infantil.
Resumo
Neurodesenvolvimento infantil após prescrição de metadona de manutenção para dependência de opióides na gestação: uma revisão sistemática e metanálise
Objetivo
Revisar sistematicamente e metanalisar os estudos com resultados do neurodesenvolvimento de crianças nascidas de mães que tiveram prescrição de metadona na gestação.
Método
MEDLINE, Embase, e PsycINFO foram pesquisadas por estudos publicados de 1974 a 2017 relatando resultados do neurodesenvolvimento em crianças expostas a metadona no período pré‐natal.
Resultados
Quarenta e um estudos foram identificados (2.283 participantes). Oito estudos foram possíveis de incluir na metanálise: aos 2 anos a diferença na média ponderada do Índice de Desenvolvimento Mental de crianças expostas a metadona pré‐natal comparadas com as não expostas foi −4,3 (intervalo de confiança [IC a 95%] −7,24 a −1,63), e a diferença na média ponderada do Índice de Desenvolvimento Psicomotor foi −5,42 (IC 95% −10,55 a −0,28). Sete estudos relataram escores comportamentais e seis encontraram escores menores entre crianças expostas a metadona. Doze estudos relataram resultados visuais: nistagmo e estrabismo foram comuns; cinco estudos reportaram potenciais evocados visuais, dos quais quatro descreveram anormalidades. Fatores que limitaram a qualidade de alguns estudos e introduziram risco de viéis incluíram falta de cegamento, reduzido tamanho amostral, desgaste alto, incerteza sobre exposição a outras drogas, e falta de validade por grupo de comparação.
Interpretação
Crianças nascidas de mães que receberam prescrição de metadona na gestação apresentam risco para problemas neurodesenvolvimentais, mas o risco de viés limita as inferências sobre o dano. Pesquisas sobre o manejo do uso de opióides na gestação devem incluir a avaliação do resultado do neurodesenvolvimento na infância.
Abbreviations
- MAT
Medically assisted treatment
- MDI
Mental Development Index
- NAS
Neonatal abstinence syndrome
- PDI
Psychomotor Development Index
- VEP
Visual evoked potential
- WMD
Weighted mean difference
Opioid use, both prescribed and illicit, has been increasing globally since 2007. Past‐year prevalence of heroin use has almost doubled since 2007, and the rate of increase is higher among women compared with men. In the USA, the average rate of past‐year heroin use between 2013 and 2015 was 2.0 per 1000 women,1 and since 2000 there has been an almost fivefold increase in the prevalence of neonatal abstinence syndrome (NAS), a drug withdrawal syndrome commonly used as a proxy for opioid exposure during pregnancy.2 It is estimated that in the current opioid crisis up to 14.4% of pregnant women have opioid prescriptions dispensed during pregnancy.3
Pregnant women who use heroin are recommended medically assisted treatment (MAT) with an opioid substitute such as methadone as part of a comprehensive antenatal care plan because it is associated with improved use of antenatal services, reduced use of heroin during pregnancy, and reduced risk of preterm delivery, when compared with no treatment.4, 5, 6, 7, 8, 9 Fetal benefits of MAT include improved growth7, 10 and less risk of intrauterine death.8
Methadone is a synthetic long acting μ‐opioid agonist which freely crosses the placenta; despite the potential for methadone to affect the developing fetal brain, this treatment was introduced into practice without a randomized controlled study of childhood neurodevelopmental outcome. Preclinical studies suggest that exogenous opioids may exert pleiotropic harmful effects on the central nervous system,11, 12, 13 and diffusion magnetic resonance imaging studies show that the tract tissue microstructure of white matter (fractional anisotropy) is altered in neonates exposed prenatally to methadone.14, 15 Improved understanding of the neurodevelopmental outcome of children born to opioid‐dependent mothers and exposed prenatally to methadone is essential to inform management of their mothers during pregnancy.16, 17, 18 The issue is prescient because the optimal methadone dose regimen is uncertain,19 and alternative opioids such as buprenorphine may have a different risk profile for neonatal outcome,16, 17, 20 leading to equipoise about the optimal MAT strategy.
The aims of this study were to perform a systematic review of published literature on childhood neurodevelopmental outcomes after prescription of maintenance methadone in pregnancy, and to undertake a meta‐analysis of studies that used a common assessment tool.
Method
The study protocol was registered with the international prospective register of systematic reviews (PROSPERO), registration number CRD42017063987 (https://www.crd.york.ac.uk/prospero/). Methodology is reported according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement.21
We included all studies that reported neurodevelopmental outcome, including visual development, of children whose opioid‐dependent mothers were prescribed methadone during pregnancy. There was no language restriction. Exclusion criteria were prescription of alternative opioid substitutes during pregnancy and studies reporting only neonatal neurodevelopment.
Two reviewers (VJM, RH) independently searched MEDLINE, Embase, and PsycINFO for studies published between 1975 and 2017. Medical Subject Headings terms used were ‘methadone’ and ‘prenatal’ or ‘prenatal exposure’ or ‘prenatal drug exposure’ or ‘prenatal exposure delayed effects’ or ‘in utero’. Bibliographies of primary studies and review articles meeting the inclusion criteria were searched manually to identify further eligible studies.
Three reviewers (VJM, RH, HM) independently screened titles and abstracts to identify potentially eligible studies. Where necessary to determine eligibility, full text was retrieved and reviewed. Duplication was avoided if it was clear that the same cohort was reported in two publications; where more than one publication for a study was retrieved, only the report that contained the maximum data points was included.
Four reviewers (VJM, RH, HM, JPB) independently extracted data from included studies using a standardized template. Extracted information included study setting, design, population and participant demographics, details of methadone exposure if available, control conditions, recruitment and completion rates, age at outcome measurement, assessment tool, and outcome of assessment. Data were extracted from each study by two reviewers independently, and templates were combined to ensure complete data collection. Disagreements were resolved through discussion.
A quality assessment instrument was developed using the Grading of Recommendations Assessment Development and Evaluation Guidelines22, 23, 24 to provide a structured scoring system that aimed to describe quality and sources of bias in studies of neurodevelopment after prenatal drug exposure. It incorporated objective criteria about study design, sample size and characteristics, use of validated outcome measures, risk of bias (blinding, confounding, attrition), and data analysis. Each study was assessed independently by two reviewers (VJM, JPB) and scored as good (A, 6.5–8), intermediate (B, 3.5–6), or poor (C, 1–3) quality (Table SI, online supporting information).
Statistical analysis
Where studies used the same assessment tool for any outcome domain, quantitative data were pooled in random effects meta‐analysis using R software, version 3.2.2 (K Hornik; R Foundation for Statistical Computing, Vienna, Austria; https://cran.r-project.org/) with mean difference weighted by the inverse of the variance.25 Effect sizes were expressed as weighted mean differences (WMD) and their 95% confidence intervals (CI). For longitudinal studies, data for assessments at 6 months and at 2 years were analysed. Heterogeneity was assessed using the standard I 2 and τ statistics and graphically using forest plots. Where statistical pooling was not possible, data were collated in tables for outcomes across two domains (neurodevelopmental and visual development), and statements generated to represent the body of literature reviewed.
Results
Characteristics of included studies
Forty‐one eligible studies were identified, including a total of 1441 methadone‐exposed children and 842 unexposed children (Fig. S1, online supporting information). Twenty‐nine studies reported neurodevelopmental outcome (1247 methadone‐exposed vs 740 unexposed children), eight of which were amenable to meta‐analysis; 12 reported visual outcome (275 methadone‐exposed vs 128 unexposed). There were no randomized trials.
Only one study fulfilled criteria for good quality;26 26 (63%) studies were intermediate and 14 (34%) were poor quality (Table SI). Study deficiencies included lack of blinding, small sample size, high attrition rates, and lack of comparison group validity. Twenty‐four of 41 studies reported information about polydrug use during pregnancy, and 20 studies provided information about methadone dose exposure.
Thirty‐three of 41 studies reported infants receiving pharmacological treatment of NAS; in 19 of these the treatment regimen was described, with the most frequently used drugs being morphine, phenobarbital, benzodiazepines, or a combination. Sixteen studies stated that infants born preterm were included, while only seven explicitly excluded infants born before 36 weeks’ gestation. In 18 studies, it could not be determined whether infants born preterm were included.
Neurodevelopmental outcome
Of 29 studies reporting neurodevelopmental outcome,15 used the original Bayley Scales of Infant Development.27 Five of these 15 studies had no comparison individuals,28, 29, 30, 31, 32 one did not report a measure of variance,33 and one assessed children at 9 months only;34 leaving eight studies that were eligible for meta‐analysis of neurodevelopmental outcome based on the Bayley Scales of Infant Development (Table 1).
Table 1.
Summary of the eight studies included in the meta‐analysis
| Study | Quality ratinga | Methadone‐exposed | Unexposed | Ageb | Drug informationc | Assessment tool | Main findingsd (results appear as methadone vs unexposed) | Commentse |
|---|---|---|---|---|---|---|---|---|
| Strauss et al.35,f | B | 25 | 26 | 3mo |
No dosing information No drug screening or polydrug information |
BSID (MDI, PDI) at all ages | 6mof: MDI 115.7 (16.8) vs 114.3 (20.9), PDI 109.4 (12.2) vs 111.7 (14.5) |
Original cohort 60 methadone‐exposed infants vs 53 unexposed infants (no information on matching); data reported only for infants who underwent BSID at all three time points. Attrition rate at 6mo was 58.4% vs 51%. One case of SIDS in the methadone‐exposed group. Gestational age at birth not stated. Assessor blinding not stated. No information about NAS or treatment. |
| 25 | 26 | 6mof | ||||||
| 25 | 26 | 1y | ||||||
| Kaltenbach et al.40,g | B | 26 | 27 | 1y |
Mean dose for 1y cohort: 30; mean dose for 2y cohort: 18 No drug screening or polydrug information |
BSID (MDI) at all ages | 2yg: MDI 90.88 (8.26) vs 94.62 (11.93) ns |
Original cohort 43 methadone‐exposed infants vs 51 unexposed (matched for maternal SES, ethnicity and medical conditions). Attrition rate at 2y 60.5% vs 53%. Assessors blinded to group. 62% 1y‐olds and 67% 2y‐olds had been treated for NAS in the neonatal period. No pharmacological agent stated |
| 17 | 24 | 2yg | ||||||
| Chasnoff et al.36,f,g | B | 31 | 34 | 3mo |
Mean dose 14.6±10.2 (5–40) Maternal interview and urine screening; 4 out of 31 used drugs in addition to heroin during pregnancy |
BSID (MDI, PDI) at all ages |
6mof: MDI 105.9 (12.4) vs 111.0 (12.3); PDI 103.9 (9.0) vs 107.6 (15.1) 2yg: MDI 97.5 (16.1) vs 96.2 (15.9); PDI 99.3 (16.9) vs 98.2 (8.9) No p‐values stated |
Original cohort 39 methadone exposed vs 34 unexposed (matched for maternal age, education, gravidity, and smoking). Attrition rate at 6mo was 66.7% vs 14.7%; at 2y attrition rate was 84.6% vs 58.8%. All infants were born at term. Assessor blinding not stated. No information about NAS or treatment. |
| 13 | 29 | 6mof | ||||||
| 11 | 27 | 1y | ||||||
| 6 | 14 | 2yg | ||||||
| Rosen and Johnson37,f,g | B | 41 | 23 | 6moa,f |
42 (mean of original cohort) Maternal urine screening; 56% of original cohort polydrug use (BDZ, opiates, cocaine, barbiturates, TCA) 15% reported ‘mod‐severe’ alcohol intake |
BSID (MDI, PDI) at 6mo, 12mo, 18mo, and 2y; M‐P at 3y |
6mof: MDI 95 (2.5) vs 100.7 (4.2), ns, PDI 101 (2.8) vs 105.1 (2.9), ns 2yg: MDI 90.4 (2.6) vs 96.9 (3.1) ns PDI 99.1 (2.7) vs 108 (2.7), p=0.05 All scores are mean (standard error)h |
Original cohort 61 methadone‐exposed infants vs 32 unexposed infants (matched for maternal ethnicity, SES, infant sex, birthweight, and gestational age). Attrition rate at 6mo was 32.8% vs 28.1%; at 2y was 44.3% vs 31.3%. Both groups included infants born preterm. Assessor blinding not stated. 75% methadone‐exposed had NAS; number treated pharmacologically not stated |
| 41 | 22 | 1y | ||||||
| 38 | 23 | 18mo | ||||||
| 34 | 22 | 2ya,g | ||||||
| 39 | 21 | 3y | ||||||
| Kaltenbach and Finnegan38,f,g | B | 27 | 17 | 6mof |
Mean dose 38.42 No drug screening or polydrug information |
BSID (MDI) at 6mo, 1y, and 2y; MSCA (GCI) at 3y 6mo–4y 6mo |
6mof: MDI 107.9 (12.23) vs 105.6 (7.31), no p‐value 2yg: MDI 100.9 (18.04) vs 103.9 (11.49) no p‐value |
No information about original cohort, therefore attrition rates unknown. Unexposed group were matched for maternal ethnicity and SES. Mean gestational age infants not stated. Blinding of assessors not stated. 92% were treated for NAS; pharmacological agent not stated. |
| 27 | 17 | 1y | ||||||
| 27 | 17 | 2yg | ||||||
| 27 | 17 | 3y 6mo– | ||||||
| 27 | 17 | 4y 6mo | ||||||
| Wilson41,g | B | 33 | 54 | 9mo |
No dosing information Maternal urine screening; 93% used psychoactive drugs |
BSID (MDI) at 9mo, 18mo, and 2yc | 2yg: MDI 88.8 (15.5) vs 90.2 (14.6) ns | Original cohort 39 methadone‐exposed vs 57 unexposed infants (matched for maternal age, ethnicity, SES, and marital status). Attrition rate at 2y was 18% vs 16%. Mean gestational age not stated for either group. Assessor blinding not stated. 87% original cohort treated for NAS, pharmacological agent not stated. |
| 29 | 42 | 18mo | ||||||
| 32 | 48 | 2yg | ||||||
| 26 | 41 | 3–5y | ||||||
| 12 | 12 | 6–11y | ||||||
| van Baar39, f,g | B | 21 | 37 | 6mof |
No dosing or screening information; Original cohort; six IV drug users; 94% used multiple drugs; 60% use cocaine |
BSID (MDI, PDI, NDI) at all ages WWPAf at 18mo (n=14), 2y (n=16), and 2y 6mo (n=15) |
6mof; MDI 103 (12) vs 107 (13), PDI 116 (18) vs 114 (21), NDI 105 (13) vs 109 (14) 2yg: MDI 86 (15) vs 98 (16) p<0.05 PDI 102 (16) vs 100 (18) ns, NDI 93 (16) vs 102 (22) |
Original cohort 35 methadone‐exposed vs 37 unexposed infants (not matched). Attrition rate at 6mo was 19.2% vs 0% and at 2y was 19.3% vs 8.1%. Assessor blinding not stated. 28 out of 35 (80%) were treated for NAS, pharmacological agent not stated. |
| 21 | 34 | 1y | ||||||
| 18 | 34 | 18mo | ||||||
| 21 | 34 | 2yg | ||||||
| 19 | 34 | 2y 6mo | ||||||
| Hans and Jeremy42, g | B | 33 | 45 | 4mo |
Mean <20 (range 3–40) Maternal interview and MUS; 13 out of 33 cocaine, 18 out of 33 cannabis, 11 out of 33 alcohol use |
BSID (MDI, PDI) at all ages | 2yg: MDI 92 (12.7) vs 96 (12.3); PDI 100 (14.2) vs 108 (14.9) |
Original cohort 47 methadone‐exposed vs 45 unexposed infants (matched for maternal age, SES, and IQ). Attrition rate 29.8% vs 0%. Assessors blinded to group. No information about NAS or treatment. |
| 33 | 45 | 8mo | ||||||
| 33 | 45 | 1y | ||||||
| 33 | 45 | 18mo | ||||||
| 33 | 45 | 2yg |
aQuality rating: A, good; B, intermediate; C, poor; based on modified Grading of Recommendations Assessment Development and Evaluation (GRADE) criteria (Table SI, online supporting information). bAge expressed in months (mo) or years (y). cDrug information includes mean daily methadone dose (in milligrams), maternal urine screening, and/or infant urine screening for drug exposure and information on maternal polydrug use (defined as methadone plus any other drug use during pregnancy, excluding tobacco), where these are reported. Unless otherwise stated, all information in this column refers to methadone‐exposed group only. dFull details from these studies is available in Table SII (online supporting information). Scores are presented as mean values (standard deviation) unless otherwise stated. eComments include information on attrition, matching, gestation, blinding, proportion of infants treated for NAS, and pharmacological treatment for NAS, where provided in the original study. fStudies included in meta‐analysis at 6mo. gStudies included in meta‐analysis at 2y. hStandard errors were converted to standard deviations for the meta‐analysis. BSID, Bayley Scales of Infant Development; MDI, Mental Developmental Index; PDI, Psychomotor developmental Index; SIDS, sudden infant death syndrome; NAS, neonatal abstinence syndrome; SES, socio‐economic status; BDZ, benzodiazepine; TCA, tricyclic antidepressant; M‐P, Merrill‐Palmer Scale; MSCA, McCarthy Scales of Childhood Abilities; GCI, General Cognitive Index; ns, not significant; IV, intravenous; NDI, non‐verbal developmental index; WWPA, Werry‐Weiss Peters Activity Scale; MUS, maternal urine screening.
Five studies reported Mental Development Index (MDI) at 6 months of age,35, 36, 37, 38, 39 and four of these reported Psychomotor Development Index (PDI)35, 36, 37, 39 (Fig. 1). Studies were all of intermediate quality with attrition rates ranging from 31% to 70%; three studies described maternal methadone doses, and gestational age was variably reported. For both MDI and PDI at 6 months, the difference in exposed versus non‐exposed infants was marginal and 95% CIs included the possibility of no difference: MDI, WMD of −1.56 (95% CI −4.98 to 1.87; Fig. 1a); PDI, WMD of −2.46 (95% CI −6.75 to 1.82; Fig. 1b).
Figure 1.
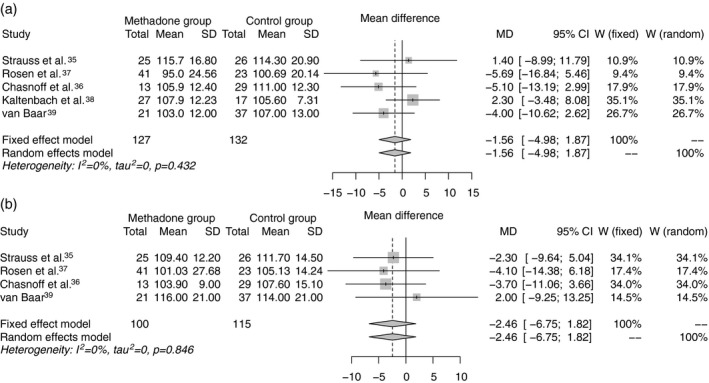
(a) Weighted mean difference in Mental Developmental Index and (b) Psychomotor Developmental Index of the Bayley Scales of Infant Development at age 6 months between methadone‐exposed and unexposed infants.
Seven studies reported MDI at 2 years of age,36, 37, 38, 39, 40, 41, 42 four of which reported PDI36, 37, 39, 42 (Table 1). All seven studies were rated intermediate quality, with attrition rates ranging from 18% to 84%; maternal methadone dose was variably reported; and where polydrug use was reported (five of seven studies), this ranged from 56% to more than 90% of mothers. The gestational age of participants was not stated in four studies.38, 40, 41, 42 Five studies reported rates of NAS between 67% and 92%; and no study described treatment for NAS. Compared with non‐exposed children, methadone exposure was associated with lower MDI, WMD of −4.43 (95% CI −7.24 to −1.63; Fig. 2a), and lower PDI, WMD of PDI −5.42 (95% CI −10.55 to −0.28; Fig. 2b).
Figure 2.
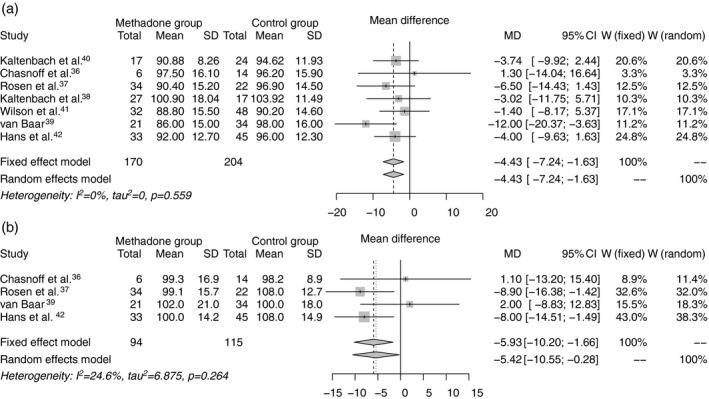
(a) Weighted mean difference in Mental Developmental Index and (b) Psychomotor Developmental Index of the Bayley Scales of Infant Development at age 18 to 24 months between methadone‐exposed and unexposed infants.
Of the remaining 21 neurodevelopmental studies, 13 were rated as having intermediate quality and eight as poor quality. Infant Behavior Record was reported in two studies: Marcus et al. reported poorer motor performance at 4 months in 15 methadone‐exposed infants compared with 23 unexposed infants;43 and Wilson et al.34 matched 33 methadone‐exposed infants with 55 unexposed infants for maternal age, ethnicity, socio‐economic status, and marital status, and reported poorer fine motor coordination, less attentiveness, and lower motor scores on the Bayley Scales of Infant Development at 9 months of age, but no difference in cognitive scores. Schneider and Hans reported no difference in focused attention during free play at 24 months between 30 exposed and 44 unexposed toddlers.44
Suffet et al.30 reported MDI and PDI in the normal range at 1 year, with females performing better than males (MDI mean 108.8 vs 102.7, p<0.05; PDI mean 102.3 vs 95.7, p<0.05). This association persisted for cognition up to 2 years of age (MDI 99.2 vs 82.0, p<0.01). Bier et al.45 reported MDI scores in the normal range at 4 months of age, in a cohort of 165 methadone‐exposed infants, with no difference between those infants exposed prenatally to either low dose (<100mg/d) or high dose (≥100mg/d) methadone.
At 6 months of age, using the Griffiths Scales of Mental Development, McGlone et al.46 noted reduced median scores across all domains which persisted after adjustment for prenatal alcohol exposure and maternal smoking. This study included 81 methadone‐exposed infants and 26 non‐drug‐exposed infants, matched for gestation and socio‐economic status. Scores were also lower for infants who had been treated for NAS (median general quotient 95 vs 99, p<0.008). Bunikowski et al.47 reported reductions in quotients for two subscales (hearing and speech; intellectual performance) at 1 year of age in a case series of 18 prenatally exposed infants compared with 42 unexposed children.
Twelve studies evaluated children older than 2 years using a range of assessment tools (Table 2). Participants included 323 methadone‐exposed children and 321 unexposed children.
Table 2.
Summary of 12 studies reporting neurodevelopmental outcomes in children beyond age 2y
| Study | Quality ratinga | Methadone‐exposed | Unexposed | Ageb | Drug informationc | Assessment tool | Main findingsd (results appear as methadone vs unexposed) |
|---|---|---|---|---|---|---|---|
| Strauss et al.48 | B | 31 | 27 | 5y | No information | MSCA | 86.8 (13.3) vs 86.2 (16.2), ns |
| Lifschitz et al.49 | B | 26 | 41 | 3y 5mo | 95% taking heroin or psychoactive drugs | MSCA | 90.4 (13) vs 89.4 (10.8), ns |
| Rosen and Johnson37 | B | 39 | 21 | 3y | 42 (mean of original cohort) | M‐P | 44.6 (2.1) vs 46.3 (2.3) ns |
| Davis and Templer53 | C | 12 | 28 | 8y 6mo | No information | WISC‐R | 89.58 (10.32) vs 96.32 (8.72) no p‐value |
| Wilson41 | B | 26 | 41 | 3–5y | No information |
MSCA (GCI) Survey and school reports IQ testing |
GCI: 90.4 (13.0) vs 89.4 (10.8) ns IQ 1–2 SD below norm 8% vs 5%, language disability 8% vs 5%, special education needs 16% vs 19%, behavioural problems 75% vs 48%, psychiatric referral 16% vs 5% |
| 12 | 12 | 6–11y | |||||
| Kaltenbach and Finnegan38 | B | 27 | 17 | 3y 6mo–4y 6mo | Mean dose 38.42 | MSCA (GCI) | GCI: 106.5 (12.96) vs 106.05 (13.10), t=0.11 |
| Sandberg et al.54 | B | 30 | 16 | 5–8y |
39.5 (males), 38.7 (females) Original cohort, 68% polydrug use; 15% moderate to heavy alcohol intake |
CGPQe
CBAQe (males only) |
Methadone‐exposed males showed more feminine game play than comparison males (p<0.04) |
| van Baar39 | B | 19 | 34 | 2y 6mo | No information |
BSID (MDI, PDI, NDI) WWPAe |
MDI 86 (15) vs 98 (16) p<0.05 PDI 102 (16) vs 100 (18) ns NDI 93 (16) vs 102 (22) WWPA 1.62 (1.03–2.66) vs 1.64 (1.28–2.52) ns |
| de Cubas and Field50 | B | 20 | 20 | 8y 6mo |
No drug information. ‘Moderate alcohol use’ |
SBIS KABC‐A RATC CBCLe |
SBIS: 97.6 vs 98.1, ns KABC‐A: 98.8 vs 102.4, no p‐value RATC: methadone‐exposed scored higher on anxiety, aggression, rejection, maladaptive outcome, p<0.01 for all CBCL: more behaviour problems p<0.05 |
| van Baar and de Graaff52 | B | 23 | 32 | 3y 6mo | Polydrug use; 16 out of 35 heroin and cocaine, only 2 out of 35 solely methadone |
SON‐IQ at 3y 6mo; RDLSC and RDLSE at 4y; RAKIT at 4y 6mo and 5y 6mo; IBR at 3y 6mo (n=22), 4y 6mo (n=23), 5y 6mo (n=22) |
SON‐IQ: 99 (9) vs 109 (11), p<0.01 RDLSC: 46 (6) vs 52 (6), p<0.01 RDLSE: 46 (9) vs 50 (6), p<0.05 RAKIT 4y 6mo: 85 (11) vs 103 (15), p<0.01 RAKIT 5y 6mo: 90 (12) vs 102 (17), p<0.05 IBR: results median (range): 3y 6mo: free of fear: 9 (4–9) vs 6.5 (2–9), p<0.05; activity level: 6 (3–9) vs 5 (2–9), p<0.05; attention: 5 (1–7) vs 5.5 (1–9) p<0.05; fine motor: 3 (1–5) vs 3 (1–5), p<0.05 4y 6mo: cooperation: 6 (2–9) vs 7 (3–9), p<0.01; endurance: 4 (2–9) vs 6 (1–9), p<0.01; attention: 8 (2–9) vs 5 (2–8), ns 5y 6mo: cooperation: 6 (1–9) vs 8 (4–9), p<0.01; free of fear: 8 (2–9) vs 9 (5–9), ns; attention: 5 (2–8) vs 5 (3–9), ns |
| 26 | 32 | 4y | |||||
| 23 | 31 | 4y | |||||
| 22 | 30 |
6mo 5y 6mo |
|||||
| Hunt et al.51 | B | 67 | 44 | 3y | No information |
SBIS; VSMS; MSCA; RDLSC and RDLSE |
SBIS: 99.9 (15.1) vs 107.5 (13.4), p<0.01 VSMSL: 38.4 (8.1) vs 46.1 (7.7), p<0.05 MSCA: 49.5 (8.7) vs 53.9 (8.3), p<0.05 RDLSC: 42.4 (11.6) vs 49.2 (11.4), p<0.05 RDLSE: 35.5 (7.9) vs 42.8 (12.8), p<0.05 |
| Konijnenberg et al.55 | B | 24 | 0 | 4y |
Mean 85.96f; polydrug use in 40% (illegal drug use), 25% alcohol |
CBCLe | Scores >55 on aggressive behaviour and withdrawn behaviour |
aQuality rating: A, good; B, intermediate; C, poor; based on modified Grading of Recommendations Assessment Development and Evaluation criteria (Table SI, online supporting information). bAge expressed in months (mo) or years (y). cDrug information includes mean daily methadone dose (in milligrams) and information on maternal polydrug use (defined as methadone plus any other drug use during pregnancy, excluding tobacco), where these are reported. Unless otherwise stated, all information in this column refers to methadone‐exposed group only. dScores are presented as mean values (standard deviation) unless otherwise stated. eQuestionnaire completed by parent or caregiver. fMean methadone dose excludes outlier daily dose of 660mg methadone. MSCA, McCarthy Scales of Childhood Abilities; ns, not significant; M‐P, Merril‐Palmer Scale; WISC‐R, Wechsler Intelligence Scale for Children–Revised; GCI, general cognitive index (used in the MSCA); SD, standard deviation; CGPQ, Child Game Participation Questionnaire; CBAQ, Child Behavior Attitude Questionnaire; BSID, Bayley Scales of Infant Development (original version 1969); MDI, mean developmental index (cognitive score); PDI, psychomotor developmental index (motor score); NDI, non‐verbal developmental index; WWPA, Werry‐Weiss Peters Activity Scale; SBIS, Stanford‐Binet Intellectual Scale; KABC‐A, Kaufman Assessment Battery for Children, achievement component (tests the acquired knowledge of fact); RATC, Robert's Apperception Test for Children (tests the child's perception of common interpersonal situations); CBCL, Child Behavior Checklist; SON‐IQ, Snijders‐Oomen Nonverbal Intelligence Test; RDLSC, Reynell Developmental Language Scales (Comprehensive); RDLSE, Reynell Developmental Language Scales (Expressive); RAKIT, Revision of the Amsterdam Children's Intelligence Test; IBR, Infant behaviour record; VSMS, Vineland Social Maturity Scale.
Six out of 10 studies measuring cognitive outcomes reported no difference between methadone‐exposed and unexposed children,37, 38, 41, 48, 49, 50 and four studies reported lower cognitive performance in methadone‐exposed children at 2 years 6 months,39 3 years,51 3 years 6 months,52 4 years 6 months,52 5 years 6 months,52 and 8 years 6 months53 respectively. The Reynell Developmental Language Scales were used in two studies (93 methadone‐exposed vs 76 unexposed children) at 3 years51 and at 4 years;52 both reported reduced performance in expressive and comprehensive language. Of the seven studies assessing behaviour, six reported more behavioural problems in methadone‐exposed children than in unexposed children.41, 50, 51, 52, 54, 55 Details of all studies reporting childhood neurodevelopmental outcome after prenatal methadone exposure are summarized in Table SII (online supporting information).
Visual development and function
Twelve studies reported visual outcomes, five of which measured visual evoked potentials (VEPs; Table SIII, online supporting information). The VEP studies consisted of a total of 143 methadone‐exposed and 103 unexposed children, with one rated poor quality, three rated intermediate, and one rated good quality.
In two different cohorts, flash VEPs at 1 day to 4 days after birth were more frequently absent or immature and were smaller on average in methadone‐exposed infants compared with non‐exposed newborns.56, 57 At 4 months58 and at 6 months of age,26 pattern‐reversal and pattern‐onset VEP abnormalities persisted in methadone‐exposed infants. One follow‐up study of 10 3‐year‐old children previously tested at 4 months found no group difference in pattern‐reversal VEP peak times.59
A further six case series have described abnormal visual outcomes in a total of 108 methadone‐exposed children60, 61, 62, 63, 64, 65 (Table SIV, online supporting information). All six studies were rated as having poor quality evidence because they did not have comparison groups and did not correct for confounders owing to their observational design. However, collectively they describe common visual abnormalities, nystagmus (50 out of 108) and strabismus (51 out of 108), which may occur together (22 out of 108 cases). Nystagmus in all described cases was horizontal and either jerk or pendular in waveform.
In a case–control study of 100 methadone‐exposed infants, 81 of whom were followed up at 6 months of age, abnormal visual outcomes were present in 40% of methadone‐exposed children (nystagmus nine out of 81 cases, strabismus 20 out of 81 cases; both five out of 81) compared with two out of 26 non‐drug exposed infants matched for gestation and socio‐economic status.26 One intermediate quality study of methadone‐exposed 4‐year‐old children reported reduced visual selective attention in methadone‐exposed children compared with unexposed children.66
Discussion
This systematic review of neurodevelopmental and visual outcomes of children born to opioid‐dependent mothers prescribed methadone in pregnancy has synthesized data from 41 studies (1441 children whose mothers were prescribed methadone and 842 children whose mothers were not prescribed methadone during pregnancy). In the meta‐analysis, we found that point estimates of MDI and PDI in children exposed to prenatal methadone compared with children whose mothers were not prescribed methadone are reduced at 6 months of age, and by 2 years the 95% CIs of these estimates make the possibility of no group difference in MDI and PDI unlikely. The emergence of difficulties as children grow older is well‐recognized after complications during the perinatal period and is likely to reflect the ontogeny of higher‐order functions through childhood. The finding of behavioural problems in six out of seven studies that measured this domain, and lower cognitive performance in four out of 10 studies that reported outcome after 2 years, suggests that children of opioid‐dependent mothers prescribed methadone may be at increased risk of longer‐term problems.
An association between prenatal methadone exposure and atypical visual development has been described, with significant differences in VEPs in infancy and childhood, reflecting altered visual pathways.56, 58 McGlone et al.,26 in their cohort of 81 methadone‐exposed and 29 unexposed infants at 6 months of age, describe a methadone‐attributable risk of abnormal visual assessment of 80%, after correcting for excess prenatal alcohol exposure. The prevalence of childhood strabismus and nystagmus in the methadone‐exposed population is higher than expected, which suggests that disorders of childhood visual function, as well as altered electrophysiological measures, are associated with prenatal methadone exposure.
Our findings are consistent with a recent meta‐analysis of five studies of infants and preschool children exposed to chronic intrauterine illicit heroin and/or prescribed methadone, which reported neurobehavioural impairment in the opioid‐exposed group.67, 68 Our data provide additional information by focusing on studies of women prescribed methadone, analysis of studies that reported a wide range of outcomes including visual development, and inclusion criteria designed to achieve maximum representation of the target population. Specifically, because use of prescribed and non‐prescribed drugs and tobacco is common among pregnant women prescribed methadone (but ascertainment and reporting of polydrug exposure in studies is variable),69 and because our purpose was to determine outcomes of methadone‐exposed children rather than to investigate causation, we took a pragmatic approach and did not attempt to exclude on the basis of polydrug use.
The data are also consistent with the observation that fractional anisotropy is reduced throughout the white matter skeleton of neonates born to mothers who were prescribed methadone,15 because neonatal fractional anisotropy is associated with later neurodevelopmental impairment.70, 71 More broadly, these results contribute to an emerging literature suggesting that exposure of the brain to psychoactive drugs during the perinatal period may modify its development.72, 73
A strength of this work is its pragmatic and systematic approach to summarizing childhood neurodevelopmental outcome after prescribed prenatal methadone exposure. We excluded studies of neonatal neurodevelopment to prevent confounding by NAS, and we excluded studies of alternative opioid substitutes to derive maximum inference about methadone.
However, limitations of included studies mean that the risk of impairment in children whose mothers were prescribed methadone may be biased. In particular, comparison groups were often poorly described beyond the definition of ‘non‐opioid exposed’, with inadequate control for socio‐economic status, or environmental factors, making it difficult to know who the methadone‐exposed children were being compared with. Reporting of maternal methadone dosing and polydrug or alcohol use was variable and therefore it was difficult to obtain an accurate exposure profile of included children; only one study examined prenatal exposure in all infants in detail using extensive toxicology.57 Finally, only 15 of the 41 studies were published in the past decade, which might affect application of results to contemporary populations because patterns of drug misuse change over time and strategies for MAT of opioid use disorder in pregnancy have evolved. For example, the dose of methadone prescribed for MAT in current practice is typically higher than that reported in historical studies. Further study of contemporary populations is required to determine the neurodevelopmental and visual outcomes of children born to opioid‐dependent mothers.
Improved understanding of the effects of prenatal opioid use disorder and its treatment, including the use of alternative substitutes, has been identified as a research priority.74 Buprenorphine has been evaluated as an opioid substitute in pregnancy. Although less severe NAS, improved growth, shorter hospital stay, and longer gestation are all reported in buprenorphine‐exposed compared with methadone‐exposed infants,16, 75, 76, 77 a recent Cochrane review concluded that there are insufficient data to establish whether buprenorphine is equivalent for all maternal outcomes, including adherence to treatment.18 Furthermore, confounding by indication could explain improved neonatal outcomes in buprenorphine groups.17 Therefore, there remains clinical equipoise about the safest opioid substitute for mother and child.
The data presented highlight that being born to an opioid‐dependent mother who has been prescribed maintenance methadone in pregnancy is associated with adverse visual and neurodevelopmental outcomes in infancy and early childhood, but deficiencies in the existing literature limit causal inference about harm and factors other than methadone per se could account for these observations. Further research into optimal management of opioid‐dependent pregnant women is required; future studies should consider fetal brain development and long term neurodevelopmental and visual outcomes of the child.
Supporting information
Table SI: Quality assessment of 41 studies assessing childhood outcomes after prenatal methadone exposure
Table SII: Twenty‐nine studies reporting childhood neurodevelopmental outcomes after prenatal methadone exposure
Table SIII: Studies reporting childhood visual evoked potentials (VEPs) after prenatal methadone exposure
Table SIV: Studies reporting childhood visual outcomes after prenatal methadone exposure
Figure S1: Identification and selection. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flowchart showing process of inclusion and exclusion of studies.
Acknowledgements
We acknowledge Marshal Dozier, librarian at the University of Edinburgh, for her help and guidance with the original literature search and Oliver Koch for his help translating two articles written in German. This work was undertaken in the Medical Research Council Centre for Reproductive Health, which is funded by a Medical Research Council Centre grant (Medical Research Council G1002033). The authors have stated that they had no interests that might be perceived as posing a conflict or bias.
This article is commented on by Skranes.
References
- 1. United Nations Office on Drugs and Crime . World Drug Report 2017. United Nations publication E.17.XI.6: United Nations, 2017.
- 2. Patrick SW, Davis MM, Lehmann CU, Cooper WO. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. J Perinatol 2015; 35: 650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bateman BT, Hernandez‐Diaz S, Rathmell JP, et al. Patterns of opioid utilization in pregnancy in a large cohort of commercial insurance beneficiaries in the United States. Anesthesiology 2014; 120: 1216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burns L, Mattick RP, Lim K, Wallace C. Methadone in pregnancy: treatment retention and neonatal outcomes. Addiction 2007; 102: 264–70. [DOI] [PubMed] [Google Scholar]
- 5. Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev 2009; 3: CD00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zelson C, Lee SJ, Casalino M. Neonatal narcotic addiction. Comparative effects of maternal intake of heroin and methadone. N Engl J Med 1973; 289: 1216–20. [DOI] [PubMed] [Google Scholar]
- 7. Kandall SR, Albin S, Lowinson J, Berle B, Eidelman AI, Gartner LM. Differential effects of maternal heroin and methadone use on birthweight. Pediatrics 1976; 58: 681–5. [PubMed] [Google Scholar]
- 8. Kandall SR, Albin S, Gartner LM, Lee KS, Eidelman A, Lowinson J. The narcotic‐dependent mother: fetal and neonatal consequences. Early Hum Dev 1977; 1: 159–69. [DOI] [PubMed] [Google Scholar]
- 9. Doberczak TM, Thornton JC, Bernstein J, Kandall SR. Impact of maternal drug dependency on birth weight and head circumference of offspring. Am J Dis Child 1987; 141: 1163–7. [DOI] [PubMed] [Google Scholar]
- 10. Hulse GK, Milne E, English DR, Holman CD. The relationship between maternal use of heroin and methadone and infant birth weight. Addiction 1997; 92: 1571–9. [PubMed] [Google Scholar]
- 11. Vestal‐Laborde AA, Eschenroeder AC, Bigbee JW, Robinson SE, Sato‐Bigbee C. The opioid system and brain development: effects of methadone on the oligodendrocyte lineage and the early stages of myelination. Dev Neurosci 2014; 36: 409–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thompson BL, Levitt P, Stanwood GD. Prenatal exposure to drugs: effects on brain development and implications for policy and education. Nat Rev Neurosci 2009; 10: 303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hutchinson MR, Shavit Y, Grace PM, Rice KC, Maier SF, Watkins LR. Exploring the neuroimmunopharmacology of opioids: an integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol Rev 2011; 63: 772–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Walhovd KB, Watts R, Amlien I, Woodward LJ. Neural tract development of infants born to methadone‐maintained mothers. Pediatric Neurol 2012; 47: 1–6. [DOI] [PubMed] [Google Scholar]
- 15. Monnelly VJ, Anblagan D, Quigley A, et al. Prenatal methadone exposure is associated with altered neonatal brain development. Neuroimage Clin 2017; 18: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jones HE, Kaltenbach K, Heil SH, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med 2010; 363: 2320–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brogly SB, Saia KA, Walley AY, Du HM, Sebastiani P. Prenatal buprenorphine versus methadone exposure and neonatal outcomes: systematic review and meta‐analysis. Am J Epidemiol 2014; 180: 673–86. [DOI] [PubMed] [Google Scholar]
- 18. Minozzi S, Amato L, Bellisario C, Ferri M, Davoli M. Maintenance agonist treatments for opiate‐dependent pregnant women. Cochrane Database Syst Rev 2013; 12: CD006318. [DOI] [PubMed] [Google Scholar]
- 19. McCarthy JJ, Leamon MH, Finnegan LP, Fassbender C. Opioid dependence and pregnancy: minimizing stress on the fetal brain. Am J Obstet Gynecol 2017; 216: 226–31. [DOI] [PubMed] [Google Scholar]
- 20. Zedler BK, Mann AL, Kim MM, et al. Buprenorphine compared with methadone to treat pregnant women with opioid use disorder: a systematic review and meta‐analysis of safety in the mother, fetus and child. Addiction 2016; 111: 2115–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011; 64: 383–94. [DOI] [PubMed] [Google Scholar]
- 23. Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence–study limitations (risk of bias). J Clin Epidemiol 2011; 64: 407–15. [DOI] [PubMed] [Google Scholar]
- 24. Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011; 64: 401–6. [DOI] [PubMed] [Google Scholar]
- 25. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986; 7: 177–88. [DOI] [PubMed] [Google Scholar]
- 26. McGlone L, Hamilton R, McCulloch DL, MacKinnon JR, Bradnam M, Mactier H. Visual outcome in infants born to drug‐misusing mothers prescribed methadone in pregnancy. Br J Ophthalmol 2014; 98: 238–45. [DOI] [PubMed] [Google Scholar]
- 27. Bayley N. Bayley Scales of Infant Development: Birth to Two Years. New York, NY: Psychological Corporation, 1969. [Google Scholar]
- 28. Ramer C, Lodge A. Neonatal addiction: a two‐year study. Part I. Clinical and developmental characteristics of infants of mothers on methadone maintenance. Addict Dis 1975; 2: 227–34. [PubMed] [Google Scholar]
- 29. Zarin‐Ackerman J. In: Beschner GM, Brotman R, Abuse NIo D. editors. Symposium on Comprehensive Health Care for Addicted Families and Their Children, 20th and 21st May 1976, New York, NY. National Institute on Drug Abuse, 1977.
- 30. Suffet F, Brotman R. A comprehensive care program for pregnant addicts: obstetrical, neonatal, and child development outcomes. Int J Addict 1984; 19: 199–219. [DOI] [PubMed] [Google Scholar]
- 31. Kaltenbach K, Finnegan LP. Neonatal abstinence syndrome, pharmacotherapy and developmental outcome. Neurobehav Toxicol Teratol 1986; 8: 353–5. [PubMed] [Google Scholar]
- 32. Doberczak TM, Shanzer S, Cutler R, Senie RT, Loucopoulos JA, Kandall SR. One‐year follow‐up of infants with abstinence‐associated seizures. Arch Neurol 1988; 45: 649–53. [DOI] [PubMed] [Google Scholar]
- 33. Kaltenbach K, Finnegan LP. Perinatal and developmental outcome of infants exposed to methadone in‐utero. Neurotoxicol Teratol 1987; 9: 311–3. [DOI] [PubMed] [Google Scholar]
- 34. Wilson GS, Desmond MM, Wait RB. Follow‐up of methadone‐treated and untreated narcotic‐dependent women and their infants: health, developmental, and social implications. J Pediatr 1981; 98: 716–22. [DOI] [PubMed] [Google Scholar]
- 35. Strauss ME, Starr RH, Ostrea EM, Chavez CJ, Stryker JC. Behavioral concomitants of prenatal addiction to narcotics. J Pediatrics 1976; 89: 842–6. [DOI] [PubMed] [Google Scholar]
- 36. Chasnoff IJ, Schnoll SH, Burns WJ, Burns K. Maternal nonnarcotic substance abuse during pregnancy: effects on infant development. Neurobehav Toxicol Teratol 1984; 6: 277–80. [PubMed] [Google Scholar]
- 37. Rosen TS, Johnson HL. Long‐term effects of prenatal methadone maintenance. NIDA Res Monogr 1985; 59: 73–83. [PubMed] [Google Scholar]
- 38. Kaltenbach K, Finnegan LP. Children exposed to methadone in utero: assessment of developmental and cognitive ability. Ann N Y Acad Sci 1989; 562: 360–2. [Google Scholar]
- 39. van Baar A. Development of infants of drug dependent mothers. J Child Psychol Psychiatry 1990; 31: 911–20. [DOI] [PubMed] [Google Scholar]
- 40. Kaltenbach K, Graziani LJ, Finnegan LP. Methadone exposure in utero: developmental status at one and two years of age. Pharmacol Biochem Behav 1979; 11: 15–7. [PubMed] [Google Scholar]
- 41. Wilson GS. Clinical studies of infants and children exposed prenatally to heroin. Ann N Y Acad Sci 1989; 562: 183–94. [DOI] [PubMed] [Google Scholar]
- 42. Hans SL, Jeremy RJ. Postneonatal mental and motor development of infants exposed in utero to opioid drugs. Infant Ment Health J 2001; 22: 300–15. [Google Scholar]
- 43. Marcus J, Hans SL, Jeremy RJ. Patterns of 1‐day and 4‐month motor functioning in infants of women on methadone. Neurobehav Toxicol Teratol 1982; 4: 473–6. [PubMed] [Google Scholar]
- 44. Schneider JW, Hans SL. Effects of prenatal exposure to opioids on focused attention in toddlers during free play. J Dev Behav Pediatr 1996; 17: 240–7. [PubMed] [Google Scholar]
- 45. Bier JB, Finger AS, Bier BA, Johnson TA, Coyle MG. Growth and developmental outcome of infants with in‐utero exposure to methadone vs buprenorphine. J Perinatol 2015; 35: 656–9. [DOI] [PubMed] [Google Scholar]
- 46. McGlone L, Mactier H. Infants of opioid‐dependent mothers: neurodevelopment at six months. Early Hum Dev 2015; 91: 19–21. [DOI] [PubMed] [Google Scholar]
- 47. Bunikowski R, Grimmer I, Heiser A, Metze B, Schäfer A, Obladen M. Neurodevelopmental outcome after prenatal exposure to opiates. Eur J Pediatr 1998; 157: 724–30. [DOI] [PubMed] [Google Scholar]
- 48. Strauss ME, Lessen‐Firestone JK, Chavez CJ, Stryker JC. Children of metadone‐treated women at five years of age. Pharmacol Biochem Behav 1979; 11: 3–6. [PubMed] [Google Scholar]
- 49. Lifschitz MH, Wilson GS, O'Brian Smith E, Desmond MM. Factors affecting head growth and intellectual function in children of drug addicts. Pediatrics 1985; 75: 269–74. [PubMed] [Google Scholar]
- 50. de Cubas MM, Field T. Children of methadone‐dependent women: developmental outcomes. Am J Orthopsychiatry 1993; 63: 266–76. [DOI] [PubMed] [Google Scholar]
- 51. Hunt RW, Tzioumi D, Collins E, Jeffery H. Adverse neurodevelopmental outcome of infants exposed to opiate in‐utero. Early Hum Dev 2008; 84: 29–35. [DOI] [PubMed] [Google Scholar]
- 52. van Baar A, de Graaff BM. Cognitive development at preschool‐age of infants of drug‐dependent mothers. Dev Med Child Neurol 1994; 36: 1063–75. [DOI] [PubMed] [Google Scholar]
- 53. Davis DD, Templer DI. Neurobehavioral functioning in children exposed to narcotics in utero. Addict Behav 1988; 13: 275–83. [DOI] [PubMed] [Google Scholar]
- 54. Sandberg DE, Meyer‐Bahlburg HF, Rosen TS, Johnson HL. Effects of prenatal methadone exposure on sex‐dimorphic behavior in early school‐age children. Psychoneuroendocrinology 1990; 15: 77–82. [DOI] [PubMed] [Google Scholar]
- 55. Konijnenberg C, Lund IO, Melinder A. Behavioural outcomes of four‐year‐old children prenatally exposed to methadone or buprenorphine: a test of three risk models. Early Child Dev Care 2015; 185: 1641–57. [Google Scholar]
- 56. McGlone L, Mactier H, Hamilton R, et al. Visual evoked potentials in infants exposed to methadone in utero. Arch Dis Child 2008; 93: 784–6. [DOI] [PubMed] [Google Scholar]
- 57. McGlone L, Hamilton R, McCulloch DL, et al. Neonatal visual evoked potentials in infants born to mothers prescribed methadone. Pediatrics 2013; 131: e857–63. [DOI] [PubMed] [Google Scholar]
- 58. Whitham JN, Spurrier NJ, Sawyer MG, et al. The effects of prenatal exposure to buprenorphine or methadone on infant visual evoked potentials. Neurotoxicol Teratol 2010; 32: 280–8. [DOI] [PubMed] [Google Scholar]
- 59. Whitham JN, Spurrier NJ, Baghurst PA, Weston P, Sawyer MG. Visual evoked potential latencies of three‐year‐old children prenatally exposed to buprenorphine or methadone compared with non‐opioid exposed children: the results of a longitudinal study. Neurotoxicol Teratol 2015; 52: 17–24. [DOI] [PubMed] [Google Scholar]
- 60. Nelson LB, Ehrlich S, Calhoun JH. Occurrence of strabismus in infants born to drug‐dependent women. Am J Dis Child 1987; 141: 175–8. [DOI] [PubMed] [Google Scholar]
- 61. Gaillard MC, Borruat FX. New finding: transitory horizontal pendular nystagmus secondary to neonatal abstinence syndrome (In French). Klin Monbl Augenheilkd 2002; 219: 317–9. [DOI] [PubMed] [Google Scholar]
- 62. Hamilton R, McGlone L, MacKinnon JR, Russell HC, Bradnam MS, Mactier H. Ophthalmic, clinical and visual electrophysiological findings in children born to mothers prescribed substitute methadone in pregnancy. Br J Ophthalmol 2010; 94: 696–700. [DOI] [PubMed] [Google Scholar]
- 63. Gupta M, Mulvihill AO, Lascaratos G, Fleck BW, George ND. Nystagmus and reduced visual acuity secondary to drug exposure in utero: long‐term follow‐up. J Pediatr Ophthalmol Strabismus 2012; 49: 58–63. [DOI] [PubMed] [Google Scholar]
- 64. Tinelli F, Gamucci A, Battini R, Cioni G. Congenital nystagmus in two infants born from mothers exposed to methadone during pregnancy. Ital J Pediatr 2013; 39: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yoo SH, Jansson LM, Park HJ. Sensorimotor outcomes in children with prenatal exposure to methadone. J AAPOS 2017; 21: 316–21. [DOI] [PubMed] [Google Scholar]
- 66. Konijnenberg C, Melinder A. Visual selective attention is impaired in children prenatally exposed to opioid agonist medication. Eur Addict Res 2015; 21: 63–70. [DOI] [PubMed] [Google Scholar]
- 67. Baldacchino A, Arbuckle K, Petrie DJ, McCowan C. Erratum: neurobehavioral consequences of chronic intrauterine opioid exposure in infants and preschool children: a systematic review and meta‐analysis. BMC Psychiatry 2015; 15: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Baldacchino A, Arbuckle K, Petrie DJ, McCowan C. Neurobehavioral consequences of chronic intrauterine opioid exposure in infants and preschool children: a systematic review and meta‐analysis. BMC Psychiatry 2014; 14: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. McGlone L, Mactier H, Hassan H, Cooper G. In utero drug and alcohol exposure in infants born to mothers prescribed maintenance methadone. Arch Dis Child Fet Neonat Ed 2013; 98: F542–4. [DOI] [PubMed] [Google Scholar]
- 70. van Kooij BJ, de Vries LS, Ball G, et al. Neonatal tract‐based spatial statistics findings and outcome in preterm infants. AJNR Am J Neuroradiol 2012; 33: 188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bassi L, Ricci D, Volzone A, et al. Probabilistic diffusion tractography of the optic radiations and visual function in preterm infants at term equivalent age. Brain 2008; 131: 573–82. [DOI] [PubMed] [Google Scholar]
- 72. Duerden EG, Guo T, Dodbiba L, et al. Midazolam dose correlates with abnormal hippocampal growth and neurodevelopmental outcome in preterm infants. Ann Neurol 2016; 79: 548–59. [DOI] [PubMed] [Google Scholar]
- 73. Salzwedel AP, Grewen KM, Vachet C, Gerig G, Lin W, Gao W. Prenatal drug exposure affects neonatal brain functional connectivity. J Neurosci 2015; 35: 5860–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. World Health Organization . Guidelines for the Identification and Management of Substance Use and Substance Use Disorders in Pregnancy. Geneva: World Health Organization, 2014. [PubMed] [Google Scholar]
- 75. Metz V, Jagsch R, Ebner N, et al. Impact of treatment approach on maternal and neonatal outcome in pregnant opioid‐maintained women. Hum Psychopharmacol 2011; 26: 412–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pritham UA, Paul JA, Hayes MJ. Opioid dependency in pregnancy and length of stay for neonatal abstinence syndrome. J Obstet Gynecol Neonat Nurs 2012; 41: 180–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lacroix I, Berrebi A, Garipuy D, et al. Buprenorphine versus methadone in pregnant opioid‐dependent women: a prospective multicenter study. Eur J Clin Pharmacol 2011; 67: 1053–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI: Quality assessment of 41 studies assessing childhood outcomes after prenatal methadone exposure
Table SII: Twenty‐nine studies reporting childhood neurodevelopmental outcomes after prenatal methadone exposure
Table SIII: Studies reporting childhood visual evoked potentials (VEPs) after prenatal methadone exposure
Table SIV: Studies reporting childhood visual outcomes after prenatal methadone exposure
Figure S1: Identification and selection. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flowchart showing process of inclusion and exclusion of studies.


