As a result of our limited understanding of human pathophysiology, many drugs never become medicines. The use of quantitative systems pharmacology (QSP) has seen many advances with the goal of improving the probability of technical success. Many research and development (R&D) organizations have invested in QSP models; however, areas that require continuous efforts are best practices, training, education, and the clear understanding of regulatory expectations.
A Case for QSP
A recent publication highlighted four key areas as the future of pharmaceutical innovation1 and the core basis for many of the challenges as: “the number one obstacle to innovation is our lack of basic understanding of the disease.” To this end, pharmaceutical R&D has now become a race to collect and leverage human pathophysiology data. This obstacle is particularly concerning because our therapeutic arsenal includes traditional small‐molecule drugs, large‐molecule biologics, multitarget drugs, rational drug combinations, and gene and cellular therapies. For drug developers, the complexity in assessing how advances in cell and molecular biology and genetics can be used to design therapeutic strategies is a challenge. There is now clear realization that this multidimensional problem can only be wrestled with a fundamental “systems” and predictive approach.2 It is inconceivable that large and complex data will be converted into successful druggable targets without a quantitative systems approach. A path to industrialization needs our collective vision in which QSP modeling is a standard part of portfolio prioritization and a component of decision making within an R&D organization; and when an opportunity is presented, applied in regulatory decision making. For this to happen universally, we need to develop and leverage QSP models in core disease areas and project the probability of technical success.
Ideally, QSP modeling will provide a systematic process for target prioritization. The process starts by defining the present understanding of pathophysiology, assumptions, and supporting data. Next, the mechanism of action for novel targets are defined and incorporated into the model. Third, projected clinical outcomes are quantitatively visualized via simulations. Finally, the impact of biological uncertainty on projected outcomes are assessed using sensitivity analysis. Because human pathophysiology is expected to remain incompletely understood for some time, failure with trials and unexpected success will continue. Eventually, in a world of rational drug development, the company with the most knowledge will have the lowest failure rates.
At some point, the need for standard, precompetitive models of pathophysiology will be needed. The integration of a generation of scientists in discovery and development teams who have immersed themselves in understanding biology must be trained to turn data into knowledge. For this effort to succeed, these scientists must appreciate the need to not only build a foundation in quantitative skills but also develop a broad understanding of drug discovery and development.
Industrialization
There are many indicators of the industrialization of QSP. From workshops organized by the National Institutes of Health, white papers,3 systems and mathematical‐based training programs4 and the use of QSP by regulators5 there are several promising signals that the application of QSP in academia, industry, and regulatory agencies is widespread. However, because of the utility of the approach in drug discovery and development, the use of QSP in R&D is and has to be a driving force toward industrialization. Industry looks to construct fit‐for‐purpose models to generate mechanistic and testable biological hypotheses on therapeutic and safety profiles, with the following key issues in mind: (i) what the merits of are pursuing a pharmacological target, (ii) how to inform decisions around patient stratification and individualization, and (iii) how to rationalize selection and choice of combination treatments. Notably, neuroscience, oncology, and autoimmune disorders are the three disease areas with the most investment in QSP; neuroscience, especially in neurodegenerative disorders, is expected to have the most growth followed by continued efforts in oncology and autoimmune disorders.6
Regulatory Use
On September 12, 2014, the US Food and Drug Administration Endocrine and Metabolic Drugs Advisory Committee discussed the biologics license application for parathyroid hormone recombinant DNA or recombinant human parathyroid hormone (1‐84) for the long‐term treatment of hypoparathyroidism. The clinical pharmacology review included an assessment of the adequacy of the dosage regimen; this review used a published, publicly available model in the evaluation of alternate dosing regimens.7 Although there are many examples of using systems approaches in regulatory decisions, this example has served both to think of opportunities as well as calibrate our expectations for the use of QSP in a regulatory setting. This example highlights the need for a mechanistic approach, establishing the biological framework with external experts, steeped in clinical experience, and a clear, well‐formulated question. The availability of the QSP model in the public domain highlights the utility of open‐source platforms and how they enable decision making. Furthermore, we can anticipate that QSP may provide value in areas such as patient stratification and the need to rationalize dosing regimens especially in the development of drugs for diseases that are rare or drugs that are repurposed in untested populations. In these cases, a QSP approach may add to the evidence of effectiveness in the face of limited subject availability where the development and application of a QSP model is likely to be a high‐value proposition.8
Challenges
Although QSP is clearly being used in decision making at various stages of R&D, its varied use remains a challenge, and although computational capability is high, the universality of platforms9 and a common vocabulary are lacking. One major challenge has been the lack of transparency with QSP modeling. Historically, first‐generation models were large‐scale platform models that were never published. The size and complexity hindered a full understanding of the model components, but as the platforms and models evolve, the aim of the next generation of models must be transparency and acceptance within the scientific community it serves. A second challenge is the lack of standard practices for calibration and qualification. By the nature of what QSP is trying to accomplish, developing a model and assessing its fidelity is iterative. Thus, a realization that these models will evolve as new data are available will allow more realistic expectations on assessing predictive performance.
The distinction between those who use systems pharmacology and proponents of the pharmaco‐statistical approach has limited the broader development of QSP; pharmaco‐statistical models are a logical extension of system approaches and need to be appropriately used within the framework of R&D (Table 1). Another challenge has been the inclusion QSP modelers in research and development teams. There is no universal solution to this challenge other than to ensure that QSP modelers are engaged in strategic and scientific discussions to best understand key biology information gaps and questions. With a goal to transform drug discovery and development, both systems and pharmaco‐statistical models can complement one another to serve as key drivers in enabling a clinical trial (Figure 1).
Table 1.
Opportunities for application of model‐informed drug discovery–based approaches in clinical drug development
| Model | Components | Model evaluation |
|---|---|---|
| Systems pharmacology | Pathway parameters; simulation models | Belief, biological plausibility, experimental data. Sensitivity analysis/alternate hypothesis |
| Covariate–Pharmacokinetic‐Pharmacodynamic outcomes trial | Structure, parameters, variability (simulation and estimation models) | Belief, biological plausibility, experimental data, pharmaco‐statistical approaches |
Figure 1.
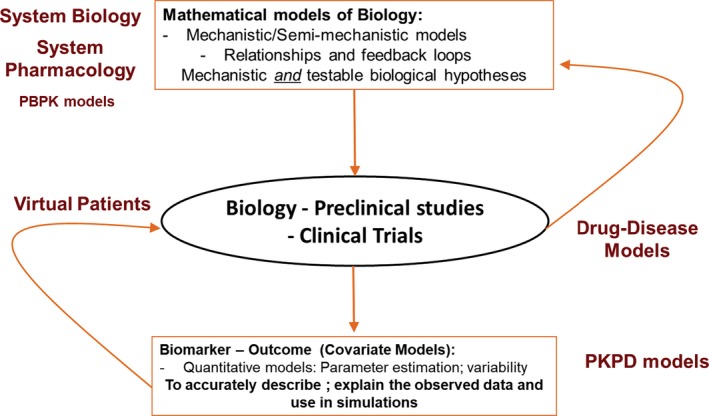
Knowledge cycles in drug discovery and development. PBPK, physiologically‐based pharmacokinetic models; PKPD, pharmacokinetic–pharmacodynamic.
The perceived need to establish regulatory impact often is a distraction from the real value proposition, which is to project efficacy based on our present understanding of human pathophysiology and provide a platform to test assumptions and alternative hypotheses. The lack of universal platforms and often uncertainty with decision makers will need some level of “qualification” for a core set of QSP models. Although a time‐consuming and strenuous process, the benefits of model qualification ensure scientific consensus and regulatory acceptance.10
There has also been a clear and unnecessary distinction of those who embrace a “systems” approach and those who do not. We are now realizing that QSP is part of a modeling continuum, and QSP modelers can learn much from more established paradigms such as the use of population approaches in understanding covariate effects on dosing and assessment of drug‐interactions using physiologically‐based pharmacokinetic models. Although there will be personal and organizational preferences to the role of QSP in R&D, discovery sciences, clinicians, and decision makers must drive both the need and application of QSP through which it may be fully embraced as indispensable in R&D decision making.
In summary, the challenges faced by QSP are no different than those faced by any emerging science. Most immediately, establishing best practices that can be universally adopted, reaching out to decision makers, and sharing examples of applications are needed for QSP to continue in its path toward industrialization.
Funding
Merck & Co. Inc. sponsored and funded the work in this article.
Conflict of Interest
M.E.T., B.T., and V.S. are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, and may own stock/stock options in the company. V.S. also reports stock/stock options, patents, and royalties from Eli Lilly and Co., and as an Associate Editor for CPT: Pharmacometrics & Systems Pharmacology, was not involved in the review or decision process for this article.
References
- 1. The Economist Intelligence Unit . The innovation imperative: the future of drug development 2018 <https://druginnovation.eiu.com/>.
- 2. Danhof, M. , Klein, K. , Stolk, P. , Aitken, M. & Leufkens, H. The future of drug development: the paradigm shift towards systems therapeutics. Drug Discov. Today 23, 1990–1995 (2018). [DOI] [PubMed] [Google Scholar]
- 3. Rogers, M. , Lyster, P. & Okita, R. NIH support for the emergence of quantitative and systems pharmacology. CPT Pharmacometrics Syst. Pharmacol. 2, 1–3 37 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Friedrich, C.M. A model qualification method for mechanistic physiological QSP models to support model-informed drug development. CPT Pharmacometrics Syst. Pharmacol. 5, 43–53 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khurana, M. et al Use of a systems pharmacology model based approach toward dose optimization of parathyroid hormone therapy in hypoparathyroidism. Clin. Pharmacol. Ther. 105, 710–718 (2019). [DOI] [PubMed] [Google Scholar]
- 6. Nijsen, M.J. et al Preclinical QSP modeling in the pharmaceutical industry: an IQ consortium survey examining the current landscape. CPT Pharmacometrics Syst. Pharmacol. 7, 135–146 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peterson, M.C. & Riggs, M.M. A physiologically based mathematical model of integrated calcium homeostasis and bone remodeling. Bone 46, 49–63 (2010). [DOI] [PubMed] [Google Scholar]
- 8. Kaddi, C.D. et al Quantitative systems pharmacology modeling of acid sphingomyelinase deficiency and the enzyme replacement therapy olipudase alfa is an innovative tool for linking pathophysiology and pharmacology. CPT Pharmacometrics Syst. Pharmacol. 7, 442–452 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Watkins, P.B. The DILI‐sim Initiative: insights into hepatotoxicity mechanisms and biomarker interpretation. Clin. Transl. Sci. 12, 122–129 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Romero, K. et al The future is now: model‐based clinical trial design for Alzheimer's disease. Clin. Pharmacol. Ther. 97, 210–214 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]


