Abstract
Objective
The lateral hypothalamus (LH) is known for its role in feeding, and it also regulates other aspects of energy homeostasis. How genetically defined LH neuronal subpopulations mediate LH effects on energy homeostasis remains poorly understood. The behavioral effects of chemogenetically activating LH gamma‐aminobutyric acid (GABA) and the more selective population of LH GABA neurons that coexpress the leptin receptor (LepR) were compared.
Methods
LepR‐cre and VGAT‐cre mice were injected with AAV5‐hSyn‐DIO‐hM3DGq‐mCherry in the LH. The behavioral effects of LH GABA or LH LepR neuronal activation on feeding, locomotion, thermogenesis, and body weight were assessed.
Results
The activation of LH GABA neurons increased body temperature (P ≤ 0.008) and decreased body weight (P ≤ 0.01) despite decreased locomotor activity (P = 0.03) and transiently increased chow intake (P ≤ 0.009). Also, similar to other studies, this study found that activation of LH GABA neurons induced gnawing on both food and nonfood (P = 0.001) items. Activation of LH LepR neurons decreased body weight (P ≤ 0.01) and chow intake when presented on the cage floor (P ≤ 0.04) but not when presented in the cage top and increased locomotor activity (P = 0.002) and body temperature (P = 0.03).
Conclusions
LH LepR neurons are a subset of LH GABA neurons, and LH LepR activation more specifically regulates energy homeostasis to promote a negative energy balance.
Introduction
The lateral hypothalamus (LH) has emerged as the feeding center because lesions of the LH in rats and cats attenuate feeding and even result in starvation 1, 2, 3. The LH has also been associated with other aspects of body weight regulation, such as physical activity and thermogenesis 4, 5. Although the LH has gained attention for its role in body weight regulation, there is still a poor understanding of the role of specific LH neuronal subpopulations in this process.
The LH contains a heterogeneous assembly of cell populations, in which gamma‐aminobutyric acid (GABA)ergic neurons predominate 6, 7. LH GABA neurons are known to mediate multiple behaviors important for body weight regulation, such as promoting consumption of chow 8, 9, 10, 11, 12, 13 and palatable solutions 9, 10 and altering energy expenditure 10, 13. LH GABA neurons differ in expression of neurochemical markers, such as neurotensin, galanin, and the leptin receptor (LepR). How different neuronal subgroups contribute to LH GABA‐mediated behaviors remains largely unknown.
LH LepR neurons were shown to colocalize with LH GABA neurons 14. Leptin is a white adipose tissue‐derived hormone well known for decreasing food intake 15 and increasing energy expenditure 16, 17 via LepR signaling in the brain. In previous studies, intra‐LH leptin injections resulted in decreased feeding and body weight 14, leptin decreased the rewarding effects of LH stimulation 18, and deletion of LepR from specific LH neuronal subpopulations modulated energy expenditure and nutrient preference 19, 20.
We hypothesized that LH LepR neurons are a distinct group of LH GABA neurons and that these neurons more specifically affect body weight homeostasis and promote a negative energy balance compared with all LH GABA neurons. To test this hypothesis, we compared the effects of chemogenetically activating LH GABA and LH LepR neurons on multiple aspects of energy homeostasis: consumption of chow and palatable foods, locomotion, body temperature, and body weight.
Methods
All experiments were approved by the Animal Experimentation Committee of the University Utrecht and were carried out in agreement with Dutch law (Herziene Wet op Dierproeven, Art 10.a2, 2014) and European regulations (Guideline 2010/63/EU).
Animals
In‐house‐bred, adult, male, homozygote LepRb‐cre and VGAT‐cre transgenic mice (stock 008320 [B6.129‐Leprtm2(cre)Rck/J] and stock 028862 [B6J.129S6(Cg)‐Slc32a1tm2(cre)Lowl/MwarJ]; The Jackson Laboratory) were used for experiments. Animals were housed individually in plastic cages (Type II L, 365 × 207 × 140 mm, 530 cm2; Tecniplast) and maintained in a temperature (21°C ± 2°C)‐ and humidity (60%‐70%)‐controlled room on a 12‐hour reversed light/dark cycle (lights on at 9 pm) with ad libitum access to chow (3.1 kcal/g; Standard Diet Service) and water.
Surgeries
Mice were anesthetized with an intraperitoneal injection of ketamine (75 mg/kg, Narketan; Vetoquinol BV) and medetomidine (1 mg/kg, SedaStart; Ast Farma BV). Mice were given eye cream (CAF; CEVA Sante Animale BW) and placed in a stereotactic frame (Kopf Instruments). An incision was made along the midline of the skull, and additional analgesia was applied by spraying Xylocaine (lidocaine 100 mg/mL; AstraZeneca BV) on the skull. Microinjections of AAV5‐hSyn‐DIO‐hM3DGq‐mCherry (0.3 µL/side, 3.0 × 109 genomic copies/µL; UNC Vector Core) or AAV‐Ef1a‐DIO‐hChR2‐eYFP (0.3 µL/side, 3.0 × 109 genomic copies/µL; UNC Vector Core) were performed bilaterally in the LH (−1.2 mm anterior/posterior, + 2.0 mm medial/lateral, −5.4 mm dorsal/ventral, 10° angle) at a rate of 0.1 µL/min per side, followed by a 10‐minute waiting period before retracting the needles. Following surgery, mice were given carprofen (5 mg/kg, subcutaneous, Carporal; Ast Farma BV), atipamezole (2.5 mg/kg, intraperitoneal, SedaStop; AST Farma BV), and saline for rehydration. During the following 2 days, mice were given carprofen (5 mg/kg, subcutaneous) and were allowed to recover for at least 1 week. To ensure viral expression, testing commenced 3 weeks after virus injection.
Drugs
Clozapine N‐oxide (CNO 99%; AK Scientific, Inc.) was dissolved in 0.9% saline and injected intraperitoneally with a dose of 1.0 mg/kg. For all experiments, each animal received saline and CNO injections in a Latin square design.
Experimental procedures
Feeding experiments
To simplify the search for food pieces during measurements, all animals were habituated to a second cage (Type II L, 365 × 207 × 140 mm, 530 cm2; Tecniplast), in which no bedding but only three tissues (to retain comfort and environmental enrichment in the form of nest building) and a water bottle were present, henceforth referred to as the “feeding cage.” All feeding tests were done in these cages, were initiated 30 minutes prior to onset of the dark phase, and lasted 7 hours. Tests were done with standard rodent chow (3.1 kcal/g; Standard Diet Service), sugar cubes (4 kcal/g, Van Gilse), lard (9.1 kcal/g; Ossewit/Blanc de Boeuf), or a combination. Mice were habituated to novel foods overnight at least 3 days prior to testing to prevent neophobia. On test days, directly after saline/CNO injections, mice were placed in the feeding cage with a preweighed amount of food present and a water bottle. Chow or sugar cubes were present on the cage floor, and lard was presented on a suspended spoon (Walking Dinner Amuse Spoon, Yong). Food intake was measured 1, 2, 3, 5, and 7 hours after injections. If food was wet, food was replaced and wet food was dried before weighing.
Intake assessment of 20% sucrose
Mice were habituated to a bottle with 20% (weight per volume) granulated sugar solution (4 kcal/g; Jumbo) prior to testing. Tests were commenced 3 hours into the dark phase and lasted 2 hours, during which animals were placed in the feeding cages with only the sucrose bottle present.
Wood block exposure
During feeding experiments, we observed gnawing behavior after LH GABA stimulation. To test whether this was nonspecific (nonfood directed) behavior, we determined the extent of gnawing on a nonfood item, a wood block. Mice were habituated to a wood block prior to testing. Tests started 4 hours into the dark phase. Mice were placed in their feeding cage with a preweighed block of wood (~ 20 g) for 2 hours, after which wood blocks were dried and weighed. We also simultaneously presented mice with chow and a wood block, during which we more accurately separated intake from spilling by weighing pieces of chow and chow dust separately, making sure to remove all pieces of wood and feces.
Locomotion assessment
All animals were habituated to an empty cage (Type III H, 425 × 266 × 185 mm, 800 cm2; Tecniplast) for 1 hour on 2 separate days. Locomotion tests were performed 3 to 4 hours into the dark phase and lasted 1 hour. Thirty minutes prior to testing, animals were injected with either saline or CNO and placed in the behavioral testing room. Mice were placed in their own locomotion cage, and horizontal movement was tracked using a camera placed above the cages that was coupled to a computer running EthoVision 7 (Noldus).
Temperature measurements
Temperature measurements started 4 hours into the dark phase. Prior to baseline temperature measurements, food, water, and tissues were removed from the home cage. To measure temperature, mice were placed in a smaller cage, and a movie was made using a thermal camera (Flir T420; FLIR Systems, Inc.) and the accompanying software program ResearchIR (version 4.40.6.24, 64 bit; FLIR Systems). Fifteen and thirty minutes after removal, baseline temperature was recorded. Mice were then injected with either saline or CNO, and temperature was recorded 30, 60, and 90 minutes after injections. In between movies, mice were placed back in their home cage. ResearchIR was used to determine eye temperature by directing a 3 × 3‐sized pixel toward the region of the eyes with the least standard deviation with mice standing on their hind legs and looking upward. Temperature is displayed as a change in temperature from baseline (temperature − average of baseline temperatures).
Repeated injections
Mice were injected with saline or CNO at 8:30 am and at 3:30 pm for three consecutive days. Directly after injections, the mice, chow in the cage top, and water bottles were weighed. On the fourth day, no injections were given, but mice, food, and water were weighed at 8:30 am. Before repeating this task, animals were allowed to recover for at least 1 week.
Immunohistochemistry
Animals were anesthetized with an overdose of sodium pentobarbital (Euthanimal; Alfasan BV) and transcardially perfused with 1× phosphate‐buffered saline (PBS) followed by 4% paraformaldehyde in 1×PBS. Brains were dissected and kept in 4% paraformaldehyde for 24 hours at 4°C and then in 30% sucrose in 1×PBS for at least 48 hours at 4°C. Brain slices (40 μm) were washed 3 × 10 minutes in 1×PBS and then blocked for 1 hour in 1×PBS containing 10% normal goat serum (NGS) and 0.25% Triton‐X100. Slices were then placed in 1×PBS containing primary antibody (rabbit anti‐dsRed 1:500; number 632496; Clontech, Takara Bio USA Inc) and 2% NGS overnight at 4°C. At room temperature, slices were washed 3 × 10 minutes in 1×PBS and then placed in 1×PBS containing secondary antibody (goat anti‐rabbit 569, 1:500; number ab175471; Abcam Plc) and 2% NGS for 2 hours. Finally, slices were washed in 1×PBS, mounted onto glass slides, and covered using FluorSave (EMD Millipore Corp.). For overlays of viral expression, microscopic images of the LH at bregma −1.3 mm were analyzed using “analyze particles” in Fiji 21. Next, images were overlaid using Sum Slices in the Z Project option.
Data analysis and statistics
Behavioral data were analyzed using Microsoft Excel, Graphpad Prism (version 7.05, Graphpad Software Inc.) and SPSS Statistics software version 23 (IBM Corp.). Animals that ate < 1 g of chow over 7 hours after injections were removed from analyses (average chow intake after saline injections: 2.2 ± 0.1 g). One animal from the LH LepR group was removed because of a lack of viral expression. Paired t tests and two‐way repeated‐measures ANOVA tests were used where applicable with Bonferroni adjusted post hoc tests. On cumulative consumption and locomotion data, main effects of time are not reported because time will, by definition, increase when analyzing cumulative data. A significance criterion of P < 0.05, two tailed, was adopted in all statistical analyses.
Results
We injected VGAT‐cre and LepR‐cre mice with AAV5‐hSyn‐DIO‐hM3Dq‐mCherry targeted at the LH region. All LH VGAT (n = 6) and LH LepR (n = 8) mice had prominent viral expression in the LH (Figure 1A and Figure 2A). Because of viral spread, expression of hM3Dq‐mCherry extended to a region near the LH, the zona incerta (ZI). As expected, expression in LH VGAT mice was clearly higher than in LH LepR mice, reflecting lower numbers of LepR neurons.
Figure 1.
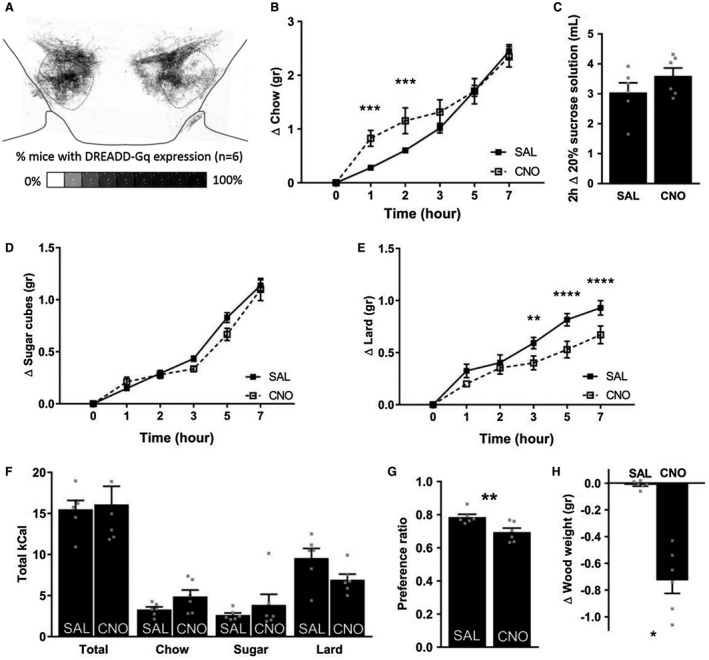
Feeding upon chemogenetic activation of LH GABA neurons. (A) Overlay of VGAT‐cre mice (n = 6) injected with AAV5‐hSyn‐DIO‐hM3DGq‐mCherry targeted at the lateral hypothalamus. Compared with saline injections, CNO (1.0 mg/kg) injections in VGAT‐cre mice (B) altered the feeding pattern of chow intake, (C) had no effect on sucrose solution consumption or (D) sugar cube intake, (E) decreased lard intake, (F) had no effect on total caloric intake or intake of separate foods when given free choice between chow, lard, and sugar (free‐choice high‐fat high‐sucrose), (G) decreased preference for lard and sugar, and (H) decreased the weight of a wood block. Mean ± SEM. Individual points are plotted as grey squares. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Figure 2.

Feeding upon chemogenetic activation of LH LepR neurons. (A) Overlay of LepR‐cre mice (n = 8) injected with AAV5‐hSyn‐DIO‐hM3DGq‐mCherry targeted at the lateral hypothalamus. Compared with saline injections, CNO (1.0 mg/kg) injections in LepR‐cre mice (B) decreased chow intake but did not affect (C) sucrose solution consumption, (D) sugar cube intake, (E) lard intake, (F) total caloric intake or intake of separate foods when given free choice between chow, lard, and sugar (free‐choice high‐fat high‐sucrose), (G) preference for lard and sugar, or (H) weight of a wood block. Mean ± SEM. Individual points are plotted as grey squares. *P < 0.05, **P < 0.01, ****P < 0.0001.
Feeding experiments
To test for effects of chemogenetically activating LH GABA and LH LepR neurons on feeding, we measured intake of regular chow and palatable foods over 7 hours. We pilot tested the effect of three doses of CNO (0.3 mg/kg, 1.0 mg/kg, and 3.0 mg/kg) on chow intake. Based on these experiments, we decided to continue using CNO 1.0 mg/kg because CNO 1.0 mg/kg gave the largest behavioral effect, and there were no nonspecific effects in control mice (data not shown).
Chemogenetically activating LH GABA neurons increased chow intake at 1 hour and 2 hours, but not at other time points (Figure 1B; interaction of Time × Injection: F 5,25 = 5.651, P = 0.0013; 1 hour: P = 0.00092, 2 hours: P = 0.00086). Because previous studies have reported increased consumption of palatable liquids 9, 10, we also presented mice with 20% sucrose solution injections, which revealed that five out of six mice increased the weight change of sucrose solution; however, this did not reach statistical significance (Figure 1C; t(5) = −1.714, P = 0.15). Next, we tested intake of nonliquid palatable foods: sugar cubes and lard. LH VGAT mice did not alter intake of sugar cubes after CNO injections (Figure 1D). Lard intake was decreased at 3, 5, and 7 hours upon CNO compared with vehicle injections (Figure 1E; interaction of Time × Injection: F 5,25 = 5.722, P = 0.0012; 3 hours: P = 0.0031, 5 hours: P = 0.000017, 7 hours: P = 0.000077). Finally, we presented mice with a combination of chow, sugar cubes, and lard. CNO‐treated VGAT animals did not consume more calories over 7 hours (“Total” in Figure 1F). When taking the separate foods into account, paired t tests revealed increased chow (t[5] = −2.661, P = 0.045) and decreased lard (t[5] = 3.100, P = 0.027) intake, but when corrected for multiple testing, these values did not reach statistical significance. Preference for palatable foods (calculated as preference ratio: caloric intake of sugar cubes + lard ÷ total caloric intake) was decreased in LH VGAT animals after CNO injections compared with vehicle injections (Figure 1G; t[5] = 5.248, P = 0.003).
Throughout the feeding experiments, we anecdotally observed torn tissues, wet cages, chow dust, and bite marks on plastic petri dishes by CNO‐ but not saline‐injected VGAT mice. We tested whether this urge to chew was nonspecific (nonfood directed) gnawing behavior. Therefore, we presented the animals with a block of wood similar to Navarro et al. 10. Indeed, CNO‐injected LH VGAT mice shredded the wood and decreased the wood weight (t[5] = 6.651, P = 0.001; Figure 1H). To further differentiate feeding from nonspecific gnawing, we simultaneously presented a wood block and chow. This revealed that gnawing on the wood block was increased irrespective of whether chow was presented or not (Supporting Information Figure S1A; main effect of Injection: F 1,5 = 96.18, P = 0.00019). We also more accurately assessed the consumption of chow by measuring both the total weight change of chow pieces and the weight of chow dust formed on the cage floor. This revealed that total weight change of chow was not affected by CNO (Supporting Information Figure S1B). However, the amount of chow dust was increased upon CNO injections (Supporting Information Figure S1C), but this did not result in a decrease in the amount of chow actually consumed (Supporting Information Figure S1D).
We performed the same feeding experiments in LH LepR mice. Activating LH LepR neurons decreased intake of chow at all time points measured (Figure 2B; interaction of Time × Injection: F 5,30 = 7.875, P = 0.000078; 1 hour: P = 0.041, 2 hours: P = 0.0018, 3 hours: P = 0.0000036, 5 hours: P = 0.000013, 7 hours: P = 0.000000053). Injecting CNO in LH LepR mice had no effect on the consumption of 20% sucrose solution, sugar cubes, or lard (Figure 2C‐2E). Finally, when LH LepR mice were given free choice of chow, sugar cubes, or lard, there was no effect of CNO on total caloric intake or on total caloric intake of the separate foods (Figure 2F). The preference for palatable foods over chow was not affected by CNO (Figure 2G).
We also assessed whether the more specific group of LH GABA‐LepR neurons contribute to LH GABA‐activation‐induced gnawing. We did not notice any urge to chew in CNO‐ or vehicle‐treated LH LepR mice. Similarly, LH LepR mice did not gnaw on wood after either vehicle or CNO injections (Figure 2H).
Locomotion and body temperature
To test two aspects of energy expenditure, we assessed both horizontal locomotor activity and body temperature. As a proxy for body temperature, we used the temperature of the eye, which we found to be the highest temperature spot detectable on images produced by a thermosensitive camera. Moreover, temperature within the eye is more stable across different voxels compared with other regions used to detect temperature, such as brown adipose tissue.
LH VGAT mice decreased 1‐hour locomotor activity after CNO injections (Figure 3A; t[5] = 3.031, P = 0.029). Furthermore, CNO injections increased eye temperature at all time points measured (Figure 3C; interaction of Time × Injection: F 3,15 = 9.043, P = 0.0012; 0‐30 minutes: P = 0.0057, 30‐60 minutes: P = 0.000009, 60‐90 minutes: P = 0.0078; Figure 3D; t[5] = 4.397, P = 0.0070).
Figure 3.

Energy expenditure upon chemogenetic activation of LH GABA neurons. (A) CNO (1.0 mg/kg) injections in VGAT‐cre mice (n = 6) decreased locomotor activity. (B) Examples of thermal images from a thermosensitive camera in which CNO‐injected LH VGAT mouse is standing on hind legs with eyes facing upward. (C) CNO increased eye temperature compared with saline injections. (D) Area under the curve of panel C. Mean ± SEM. Individual points are plotted as grey squares. *P < 0.05, **P < 0.01, ****P < 0.0001. [Colour figure can be viewed at wileyonlinelibrary.com]
Activation of LH LepR neurons increased both aspects of energy expenditure: LH LepR mice increased locomotor activity (Figure 4A; t[7] = −4.820, P = 0.002) and increased eye temperature after CNO compared with vehicle injections (Figure 4C; main effect of Injection F 1,7 = 7.495, P = 0.029; Figure 4D; t[7] = 2.505, P = 0.041).
Figure 4.

Energy expenditure upon chemogenetic activation of LH LepR neurons. (A) CNO (1.0 mg/kg) injections in LepR‐cre mice (n = 8) increased locomotor activity. (B) Examples of thermal images from a thermosensitive camera in which CNO‐injected LH VGAT mouse is standing on hind legs with eyes facing upward. (C) CNO increased eye temperature compared with saline injections. (D) Area under the curve of panel C. Mean ± SEM. Individual points are plotted as grey squares. *P < 0.05, **P < 0.01. [Colour figure can be viewed at wileyonlinelibrary.com]
Repeated CNO injections
To test whether the behavioral effects on feeding and energy expenditure affected body weight, mice were subjected to two daily injections of CNO or saline during the dark phase for three consecutive days. Throughout these days, we recorded body weight and changes in weight of the water bottle and cage top chow.
Repeated CNO injections in LH VGAT mice resulted in a larger decrease in body weight compared with vehicle injections (Figure 5A; interaction effect of Injection: F 3,15 = 5.353, P = 0.0104; day 1: P = 0.0032, day 2: P = 0.0199, day 3: P = 0.00029). Intake of chow was not affected (Figure 5B), but repeated CNO administration increased the weight change of water compared with vehicle (Figure 5C; interaction of Time × Injection: F 6,30 = 7.097, P = 0.000089; day 0.5: P = 0.029, day 1: P = 0.00053, day 1.5: P = 0.000029, day 2: P = 0.0000071, day 2.5: P = 0.00000051, day 3: P = 0.000000069).
Figure 5.

Body weight regulation upon repeated chemogenetic activation of LH GABA neurons. CNO (1.0 mg/kg) injections compared with saline injections in VGAT‐cre animals (n = 6) (A) increased body weight loss, (B) had no effect on food intake, and (C) increased water intake during a 3‐day twice‐daily injection scheme. Mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Similar to LH VGAT mice, repeated CNO injections in LH LepR mice decreased body weight to a greater extent than vehicle injections (Figure 6A; interaction of Time × Injection: F 3,21 = 3.74, P = 0.0269; day 2: P = 0.0013; day 3: P = 0.0087). In LH LepR mice, repeated CNO injections had no effect on either chow (Figure 6B) or water consumption (Figure 6C).
Figure 6.

Body weight regulation upon repeated chemogenetic activation of LH LepR neurons. CNO (1.0 mg/kg) injections compared with saline injections in LepR‐cre animals (n = 8) (A) increased body weight loss and (B) had no effect on food intake or (C) water intake during a 3‐day twice‐daily injection scheme. Mean ± SEM. *P < 0.05, **P < 0.01.
To control for nonspecific effects of CNO itself and of its reverse‐metabolized parent compound clozapine 22, we injected LepR‐cre and VGAT‐cre mice with a control virus that induces expression of channelrhodopsin, which does not respond to CNO or clozapine. Because we detected no differences in behavioral outcome between the mice, we combined them to form one control group. CNO injections in control mice had no effect on any parameter tested (Supporting Information Figure S2; n = 4). Therefore, we conclude that behavioral effects observed in hM3Dq‐injected mice are the result of enhanced neuronal activation in LH VGAT and LH LepR mice.
Discussion
Activating LH GABA neurons acutely and transiently increased feeding and decreased locomotion. In contrast, activating LH LepR neurons decreased feeding and increased locomotion. Both LH GABA and LH LepR activation increased body temperature, and repeated stimulation led to body weight loss. Our results showed that all behavioral effects mediated by LH LepR neurons promoted a negative energy balance, which was not the case for LH GABA neurons. Therefore, our data suggest that LH LepR neurons more specifically regulated parameters of energy balance compared with LH GABA neurons.
Recently, a study reported that LH GABA stimulation induced eating by low‐frequency optogenetic stimulation but induced gnawing at higher frequencies that did not elicit feeding 8. These results imply that LH GABA neurons modulate feeding and gnawing depending on the extent of stimulation and that we and others who have reported gnawing behavior did not examine true feeding effects. We observed that solid chow and water bottle sippers robustly induced gnawing behavior upon LH GABA activation, but porous (sugar cubes) or soft (lard) foods did not. The extent of the weight change of chow was almost identical to previous studies that have assessed the effect of LH GABA activation on chow intake in the dark phase 9, 10. Gnawing increased the weight change of food or water, which suggests that consumption of chow and water was increased. However, we observed that gnawing also resulted in spillage of water and food. Even more, when we directly measured the amount of chow actually consumed, we found that the amount consumed remained the same, even though gnawing of chow and wood occurred. These results suggest that the increased weight change in our original chow experiments did not represent an increase in consumption per se but was most likely the result of gnawing on the chow. Therefore, we suspect that previous reports on ingestive behaviors (reporting intake of chow and palatable solution) upon activation of LH GABA neurons may be similarly overestimated because of spillage as a result of gnawing.
We observed decreased consumption of chow when activating LH LepR neurons, which is in line with previous intra‐LH leptin injections 14. However, the LH LepR stimulation decrease in feeding was observed only when chow was presented on the floor and not during repeated CNO injections, when chow was in the cage top. Interestingly, after vehicle injections, mice ate more when chow was more easily accessible on the cage floor (2.0 ± 0.1 g) compared with chow in the cage top, which requires that they stand up or climb to consume chow (1.5 ± 0.1 g). Thus, activation of LH LepR neurons decreased consumption of chow only when food was easily accessible.
To our knowledge, we have provided the first evidence that stimulating LH VGAT and LH LepR neurons increases body temperature. In LH LepR mice, increased locomotor activity may have contributed to the increase in body temperature. However, LH VGAT mice decreased locomotion, indicating an effect of LH GABA activation on thermogenesis independent of locomotor activity. LH lesions have been shown to decrease body temperature 23, 24, 25, and disinhibition of neurons in the LH have been shown to increase thermogenesis 4. Furthermore, the periaqueductal grey (PAG) was shown to be involved in thermogenesis 26, and both LH LepR and GABAergic neurons are known to substantially project to the PAG 14, 27. Our data implicate both LH GABA and the more specific LH GABA‐LepR neurons in LH‐mediated thermogenesis, perhaps via projections to the PAG.
Deep brain stimulation of the LH in humans and rats has been shown to promote weight loss 21, 29, 30, 31. Our data reveal that repeated stimulation of both LH GABA and LH LepR neurons enhanced body weight loss and suggest that LH‐induced weight loss by deep brain stimulation was likely mediated by LH GABA neurons. Weight loss in LH LepR mice may have been the consequence of both increased locomotion and thermogenesis, whereas weight loss in LH VGAT mice seemed to be primarily driven by thermogenesis because locomotion was decreased.
LH LepR neurons have been shown to mainly coexpress neurotensin (Nts) and galanin (Gal) 32, 33. Our results that LH LepR neurons increased locomotor activity, but did not affect acute feeding, are in line with behavioral effects of LH Gal or LH Nts activation 13, 34, 35, 36. Furthermore, body weight loss was also observed upon repeated LH Nts neuronal activation, which was similarly mainly the result of increased energy expenditure 36. LH Nts neurons, however, increased consumption of liquids, such as water and sucrose solution, but also quinine solution 34, 36, whereas LH Gal neurons had no effect on ensure intake 13. Perhaps the simultaneous activation of LH Gal neurons suppressed the increase in intake of liquid induced by LH Nts neurons.
Several limitations to this study exist. First, expression of hM3Dq was not exclusive to the LH. In LH VGAT mice, there was copious expression of the DREADDGq in the ZI, which is similar to other LH GABA‐targeted experiments 9, 10, 13. ZI expression was also observed in LH LepR animals but to a far lesser degree. Activation of ZI GABA neurons was shown to increase body weight and feeding, including of palatable foods, and to not affect locomotion 37. In our study, activation of LH GABA neurons increased feeding but decreased lard intake, locomotion, and body weight. This suggests that it is unlikely that activation of ZI GABA neurons had a major effect on the parameters that we assessed here.
Secondly, leptin was shown to depolarize 33% and hyperpolarize 22% of LH LepR neurons 14. Changes in chow intake by LH LepR were observed after intra‐LH leptin injections 14, but not after LH LepR deletion, LepR deletion in neuronal subpopulations of the LH 19, 20, 38, or when we chemogenetically activated the majority of LH LepR neurons. This suggests that leptin‐induced feeding in the LH was regulated by the intricate activation and inhibition of LH LepR neurons, and perhaps effects on feeding were masked when increasing the activity of all LH LepR neurons.
Third, the group size of LH VGAT mice was low, and negative data should therefore be interpreted with caution. For instance, the weight change of sugar solution was increased in five out of six CNO‐injected LH VGAT mice but did not reach statistical significance. However, the findings that reached statistical significance were observed in all LH VGAT mice. Therefore, even though we used a low number of mice, the behavioral output was rather consistent between animals.
Fourth, to assess locomotor behavior, we tracked horizontal locomotion. LH VGAT activation decreased locomotion, which is line with findings reported by Navarro et al. 10, who also measured horizontal locomotion. However, this in contrast to findings by Qualls‐Creekmore et al. 13, who made use of beam breaks and subcutaneously implanted telemetric transmitters. A previous study showed that telemetry was more sensitive for detecting hyperactivity than a home cage monitoring system, let alone video tracking of horizontal movement 39. Considering smaller movements, such as when animals are gnawing on wood, the use of beam breaks or telemetry could increase the sensitivity of the locomotor assay and result in increased locomotion.
Conclusion
Our results reveal that LH LepR neurons more specifically regulate aspects of energy balance to promote a negative energy balance compared with LH GABA neurons. Furthermore, we have provided the first evidence that activation of LH GABA and LH LepR neurons modulates body temperature and, when stimulated repeatedly, enhances body weight loss. The latter is especially interesting in light of the obesity epidemic and may aid in the discovery of new treatment strategies to combat obesity.
Supporting information
Acknowledgments
We thank C. van der Meer and J. Brits for their practical assistance.
Funding agencies: This work was supported by the European Union Seventh Framework Programme (Grant Agreement Number 607310; Nudge‐It).
Disclosure: The authors declared no conflict of interest.
References
- 1. Anand B, Brobek J. Hypothalamic control of food intake in rats and cats. Yale J Biol Med 1951;24:123‐140. [PMC free article] [PubMed] [Google Scholar]
- 2. Harrell LE, Decastro JM, Balagura S. A critical evaluation of body weight loss following lateral hypothalamic lesions. Physiol Behav 1975;15:133‐136. [DOI] [PubMed] [Google Scholar]
- 3. Keesey R, Powley T. Self‐stimulation and body weight in rats with lateral hypothalamic lesions. Am J Physiol 1973;970‐978. [DOI] [PubMed] [Google Scholar]
- 4. Cerri M, Morrison SF. Activation of lateral hypothalamic neurons stimulates brown adipose tissue thermogenesis. Neuroscience 2005;135:627‐638. [DOI] [PubMed] [Google Scholar]
- 5. Inutsuka A, Inui A, Tabuchi S, Tsunematsu T, Lazarus M, Yamanaka A. Concurrent and robust regulation of feeding behaviors and metabolism by orexin neurons. Neuropharmacology 2014;85:451‐460. [DOI] [PubMed] [Google Scholar]
- 6. Kimura H, Kuriyama K. Distribution of gamma‐aminobutyric acid (GABA) in the rat hypothalamus: functional correlates of GABA with activities of appetite controlling mechanisms. J Neurochem 1975;24:903‐907. [DOI] [PubMed] [Google Scholar]
- 7. Vong L, Ye C, Yang Z, Choi B, Chua S, Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 2011;71:142‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barbano MF, Wang HL, Morales M, Wise RA. Feeding and reward are differentially induced by activating GABAergic lateral hypothalamic projections to VTA. J Neurosci 2016;36:2975‐2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jennings JH, Ung RL, Resendez SL, et al. Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell 2015;160:516‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Navarro M, Olney JJ, Burnham NW, et al. Lateral hypothalamus GABAergic neurons modulate GABAergic neurons modulate consummatory behaviors regardless of the caloric content or biological relevance of the consumed stimuli. Neuropsychopharmacology 2016;41:1505‐1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nieh EH, Matthews GA, Allsop SA, et al. Decoding neural circuits that control compulsive sucrose seeking. Cell 2015;160:528‐541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gigante ED, Benaliouad F, Zamora‐Olivencia V, Wise RA. Optogenetic activation of a lateral hypothalamic‐ventral tegmental drive‐reward pathway. PLoS One 2016;11:e0158885. doi: 10.1371/journal.pone.0158885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Qualls‐Creekmore E, Yu S, Francois M, et al. Galanin‐expressing GABA neurons in the lateral hypothalamus modulate food reward and noncompulsive locomotion. J Neurosci 2017;37:6053‐6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leinninger GM, Jo YH, Leshan RL, et al. Leptin acts via leptin receptor‐expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab 2009;10:89‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Halaas JL, Gajiwala KS, Maffei M, et al. Weight‐reducing effects of the plasma protein encoded by the obese gene. Science 1995;269:543‐546. [DOI] [PubMed] [Google Scholar]
- 16. Hwa JJ, Fawzi AB, Graziano MP, et al. Leptin increases energy expenditure and selectively promotes fat metabolism in ob/ob mice. Am J Physiol 1997;272(4 Pt 2):R1204‐R1209. [DOI] [PubMed] [Google Scholar]
- 17. Ribeiro AC, Ceccarini G, Dupré C, Friedman JM, Pfaff DW, Mark AL. Contrasting effects of leptin on food anticipatory and total locomotor activity. PLoS One 2011;6:e23364. doi: 10.1371/journal.pone.0023364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fulton S, Woodside B, Shizgal P. Modulation of brain reward circuitry by leptin. Science 2000;287:125‐128. [DOI] [PubMed] [Google Scholar]
- 19. Laque A, Yu S, Qualls‐Creekmore E, et al. Leptin modulates nutrient reward via inhibitory galanin action on orexin neurons. Mol Metab 2015;4:706‐717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brown JA, Bugescu R, Mayer TA, et al. Loss of action via neurotensin‐leptin receptor neurons disrupts leptin and ghrelin‐mediated control of energy balance. Endocrinology 2017;158:1271‐1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schindelin J, Arganda‐Carreras I, Frise E, et al. Fiji: an open‐source platform for biological‐image analysis. Nat Methods 2012;9:676‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Manvich DF, Webster KA, Foster SL, et al. The DREADD agonist clozapine N‐oxide (CNO) is reverse‐metabolized to clozapine and produces clozapine‐like interoceptive stimulus effects in rats and mice. Sci Rep 2018;8:3840. doi: 10.1038/s41598-018-22116-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Refinetti R, Carlisle HJ. Effects of lateral hypothalamic lesions on thermoregulation in the rat. Physiol Behav 1986;38:219‐228. [DOI] [PubMed] [Google Scholar]
- 24. Refinetti R, Carlisle HJ. A reevaluation of the role of the lateral hypothalamus in behavioral temperature regulation. Physiol Behav 1987;40:189‐192. [DOI] [PubMed] [Google Scholar]
- 25. Clark GC, Magoun HW, Ranson SW. Hypothalamic regulation of body temperature. J Neurophysiol 1939;61‐80. [Google Scholar]
- 26. Chen X‐M, Nishi M, Taniguchi A, Nagashima K, Shibata M, Kanosue K. The caudal periaqueductal gray participates in the activation of brown adipose tissue in rats. Neurosci Lett 2002;331:17‐20. [DOI] [PubMed] [Google Scholar]
- 27. Venner A, Anaclet C, Broadhurst RY, Saper CB, Fuller PM. A novel population of wake‐promoting GABAergic neurons in the ventral lateral hypothalamus. Curr Biol 2016;26:2137‐2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Quaade F, Yaernet K, Larsson S. Stereotaxic stimulation and electrocoagulation of the lateral hypothalamus in obese humans. Acta Neurochir (Wien) 1974;30:111‐117. [DOI] [PubMed] [Google Scholar]
- 29. Sani S, Jobe K, Smith A, Kordower JH, Bakay RA. Deep brain stimulation for treatment of obesity in rats. J Neurosurg 2007;107:809‐813. [DOI] [PubMed] [Google Scholar]
- 30. Soto‐Montenegro ML, Pascau J, Desco M. Response to deep brain stimulation in the lateral hypothalamic area in a rat model of obesity: in vivo assessment of brain glucose metabolism. Mol Imaging Biol 2014;16:830‐837. [DOI] [PubMed] [Google Scholar]
- 31. Whiting DM, Tomycz ND, Bailes J, et al. Lateral hypothalamic area deep brain stimulation for refractory obesity: a pilot study with preliminary data on safety, body weight, and energy metabolism. J Neurosurg 2013;119:56‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Laque A, Zhang Y, Gettys S, et al. Leptin receptor neurons in the mouse hypothalamus are colocalized with the neuropeptide galanin and mediate anorexigenic leptin action. Am J Physiol Endocrinol Metab 2013;304:E999‐E1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leinninger GM, Opland DM, Jo Y‐H, et al. Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab 2011;14:313‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kurt G, Woodworth HL, Fowler S, Bugescu R, Leinninger GM. Activation of lateral hypothalamic area neurotensin‐expressing neurons promotes drinking [published online September 25, 2018]. Neuropharmacology 2018. doi: 10.1016/j.neuropharm.2018.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Patterson CM, Wong J‐MT, Leinninger GM, et al. Ventral tegmental area neurotensin signaling links the lateral hypothalamus to locomotor activity and striatal dopamine efflux in male mice. Endocrinology 2015;156:1692‐1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Woodworth HL, Beekly BG, Batchelor HM, et al. Lateral hypothalamic neurotensin neurons orchestrate dual weight loss behaviors via distinct mechanisms. Cell Rep 2017;21:3116‐3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang X, van den Pol AN. Rapid binge‐like eating and body weight gain driven by zona incerta GABA neuron activation. Science 2017;356:853‐859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Davis JF, Choi DL, Schurdak JD, et al. Leptin regulates energy balance and motivation through action at distinct neural circuits. Biol Psychiatry 2011;69:668‐674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Harkin A, O'Donnell JM, Kelly JP. A study of VitalView™ for behavioural and physiological monitoring in laboratory rats. Physiol Behav 2002;77:65‐77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


