Abstract
Aims
The aim of this study was to describe the risks of cardiovascular (CV) events and severe hypoglycaemia with insulin degludec (degludec) vs insulin glargine 100 units/mL (glargine U100) in patients with type 2 diabetes (T2D) aged 65 years or older.
Materials and methods
A total of 7637 patients in the DEVOTE trial, a treat‐to‐target, randomized, double‐blind trial evaluating the CV safety of degludec vs glargine U100, were divided into three age groups (50‐64 years, n = 3682; 65‐74 years, n = 3136; ≥75 years, n = 819). Outcomes by overall age group and randomized treatment differences were analysed for major adverse cardiovascular events (MACE), all‐cause mortality, severe hypoglycaemia and serious adverse events (SAEs).
Results
Patients with increasing age had higher risks of CV death, all‐cause mortality and SAEs, and there were non‐significant trends towards higher risks of MACE and severe hypoglycaemia. Treatment effects on the risk of MACE, all‐cause mortality, severe hypoglycaemia and SAEs were consistent across age groups, based on the non‐significant interactions between treatment and age with regard to these outcomes.
Conclusions
There were higher risks of CV death, all‐cause mortality and SAEs, and trends towards higher risks of MACE and severe hypoglycaemia with increasing age after adjusting for baseline differences. The effects across age groups of degludec vs glargine U100 on MACE, all‐cause mortality and severe hypoglycaemia were comparable, suggesting that the risk of MACE, as well as all‐cause mortality, is similar and the risk of severe hypoglycaemia is lower with degludec regardless of age. Evidence is conclusive only until 74 years of age.
Keywords: basal insulin, cardiovascular disease, hypoglycaemia, type 2 diabetes
1. INTRODUCTION
The global burden of type 2 diabetes (T2D) in patients aged 65 years or older is projected to increase substantially during the next few decades, as patients with diabetes live longer and the incidence of diabetes continues to rise.1 Thus, the growing problem of diabetes and its consequences in patients aged 65 years or older is an important public health concern.
The management of diabetes in patients aged 65 years or older presents unique challenges. Differences in drug metabolism as the result of deteriorating kidney and liver function and the challenges of polypharmacy related to the treatment of multiple co‐morbidities lead to a higher risk of drug‐drug interactions and adverse events in this population.2, 3 Cardiovascular disease (CVD), in particular, is a common complication and co‐morbidity among many older individuals with diabetes is the leading cause of mortality in this population.4 Further complicating treatment, patients aged 65 years or older are more susceptible to severe hypoglycaemic events than younger patients, in part because of a reduced ability to recognize and respond to symptoms.5, 6 Severe hypoglycaemia has been shown to be associated with a higher risk of adverse cardiovascular (CV) events and mortality.7, 8 In addition, severe hypoglycaemic episodes can increase utilization of healthcare resources.9 Several organizations have published guidelines for managing T2D in patients aged 65 years or older; however, most of these are based on expert opinion only, as there is a lack of high‐quality evidence from randomized clinical trials in this population.10, 11, 12, 13 Although most guidelines support the use of insulin as one of several treatment options, data suggest that it is under‐utilized in patients aged 65 years or older,14 and there are few long‐term studies in this population that demonstrate the efficacy and safety of basal insulins.15, 16, 17 Possible reasons for under‐utilization of insulin in this population include lack of clinical evidence, less stringent glycaemic targets suggested in recent recommendations, and the potential elevated risk of and concern about hypoglycaemia in these individuals.5, 11, 12, 13, 14, 17
CV safety and the lower risk of severe hypoglycaemia with insulin degludec (degludec) compared with insulin glargine 100 units/mL (glargine U100) was demonstrated in a double‐blind trial in patients with T2D who were at high risk of CV events (DEVOTE).16, 18 Given the mean age (65 years) of the study population,16 DEVOTE provides a unique opportunity to investigate CV safety and the risk of severe hypoglycaemia with degludec vs glargine U100 in patients with T2D aged 65 years or older who are at high risk of CV events.
2. METHODS
2.1. DEVOTE trial overview
DEVOTE was a multicentre, treat‐to‐target, randomized, double‐blind, active‐comparator trial that evaluated the CV safety of degludec vs glargine U100, in addition to standard of care, in patients with T2D who were at high risk of CV events. This trial was designed to continue until the occurrence of at least 633 MACEs, as confirmed by a central, blinded event adjudication committee (EAC).16, 18 The detailed trial design, trial protocol and primary results have been published previously.16, 18
DEVOTE (ClinicalTrials.gov NCT01959529) was conducted in accordance with the Declaration of Helsinki and ICH Good Clinical Practice Guideline.19, 20 The protocol was approved by independent ethics committees or institutional review boards for each centre. Written informed consent was obtained from each participant.
Patients were considered for the trial if they had been diagnosed with T2D and were undergoing treatment with at least one antihyperglycaemic agent, if they had an HbA1c value of at least 7.0% (53 mmol/mol) or were undergoing treatment with at least 20 units/day of basal insulin. Criteria for eligibility for the trial were: at least 50 years of age with a history of prior CVD or moderate chronic kidney disease (CKD); or were at least 60 years of age with one or more pre‐specified CV risk factors.16
Primary adjudicated outcome was the time from randomization to first occurrence of a three‐component MACE: CV death, non‐fatal myocardial infarction (MI) or non‐fatal stroke. Secondary confirmatory outcome was the number of EAC‐confirmed events of severe hypoglycaemia, defined according to American Diabetes Association guidelines as an episode requiring assistance of another person to actively administer carbohydrate or glucagon, or to take other corrective actions.8
2.2. Statistical analysis
In these secondary analyses, all randomized patients (n = 7637, full analysis set [FAS]) were categorized into three age groups: 50 to 64 years (n = 3682), 65 to 74 years (n = 3136) and ≥75 years (n = 819). These age groups and statistical analyses of all endpoints were pre‐specified, with the exception of analyses of serious adverse events (SAEs). Comparisons across age groups, treatment differences within each age group, and interaction between treatment and age groups were investigated for all endpoints. Analyses were based on FAS and followed previous analyses of the DEVOTE data,16 with the exception of adjustment for the following pre‐specified baseline variables: sex, region, diabetes duration, CV risk, insulin‐naïve, smoking status and kidney function. It was of particular importance to adjust for baseline CV risk, as patients between 50 and less than 60 years of age were required to have established CVD in order to be included in the trial, whereas those 60 years of age or older could have either CV risk factors or established CVD.
As details of the analyses have been described previously,7, 16 only a brief summary is provided here. Time‐to‐first event were analysed using Cox proportional hazard regression models. The numbers of severe and nocturnal severe (00:01 am‐05:59 am, both inclusive) hypoglycaemic events and SAEs were analysed using a negative binomial‐regression model. Associations between severe hypoglycaemia and subsequent accidents and injuries (within one day, defined according to standardized Medical Dictionary for Regulatory Activities query), time to first MACE and time to all‐cause mortality (any time after a severe hypoglycaemic event) were analysed using a Cox regression model with treatment and previous severe hypoglycaemia (Yes/No) as time‐varying covariates, and were adjusted for the baseline covariates listed above, similar to those in DEVOTE 3.7
Interactions with age group, pooled across treatments for insulin dose (U/kg), HbA1c and fasting plasma glucose (FPG), were analysed using a mixed model for repeated measures (MMRM) within patients using an unstructured residual covariance matrix among visits. P values were not multiplicity adjusted and a P value less than 0.05 was considered significant.
3. RESULTS
3.1. Baseline characteristics
The lowest proportion of patients with established CVD/CKD, compared with the other age groups (50‐64 years or ≥75 years) was in the 65 to 74 years age group (Table 1), in part the result of the inclusion criteria. Compared with the 50 to 64 years age group, the 65 to 74 and ≥75 years age groups had a longer duration of diabetes and had lower body weight, body mass index (BMI), pulse, HbA1c, FPG, estimated glomerular filtration rate (eGFR), total cholesterol and triglycerides, and a lower proportion of patients in these groups were identified as smokers. Differences in the above baseline characteristics and demographic variables between age groups were significant (all P < 0.001).
Table 1.
Baseline characteristics by age groups
| Characteristic | 50‐64 years | 65‐74 years | ≥75 years |
|---|---|---|---|
| n = 3682 | n = 3136 | n = 819 | |
| Age (years) | 58.9 ± 4.0 | 68.8 ± 2.8 | 78.2 ± 3.1 |
| Male | 2273 (61.7) | 2008 (64.0) | 497 (60.7) |
| Ethnicity | |||
| Hispanic or Latino | 591 (16.1) | 438 (14.0) | 108 (13.2) |
| Race | |||
| White | 2596 (70.5) | 2510 (80.0) | 669 (81.7) |
| Asian | 452 (12.3) | 283 (9.0) | 41 (5.0) |
| Black | 500 (13.6) | 253 (8.1) | 79 (9.6) |
| Other | 134 (3.5) | 90 (2.8) | 30 (3.7) |
| Established CVD/CKD | 3169 (86.1) | 2620 (83.5) | 720 (87.9) |
| Smoker (yes) | 557 (15.1) | 262 (8.4) | 33 (4.0) |
| Diabetes duration (years) | 14.5 ± 7.9 | 17.8 ± 9.1 | 19.8 ± 10.2 |
| Body weight (kg) | 97.9 ± 24.0 | 95.5 ± 22.2 | 90.4 ± 19.0 |
| BMI (kg/m2) | 34.1 ± 7.2 | 33.4 ± 6.6 | 32.1 ± 5.8 |
| Blood pressure | |||
| Systolic (mmHg) | 135.1 ± 18.0 | 135.9 ± 18.1 | 136.0 ± 17.8 |
| Diastolic (mmHg) | 78.6 ± 10.0 | 74.4 ± 10.1 | 72.1 ± 10.2 |
| Heart rate (beats/min) | 74.8 ± 11.2 | 71.8 ± 11.3 | 70.4 ± 10.9 |
| HbA1c (%) | 8.7 ± 1.8 | 8.2 ± 1.5 | 8.0 ± 1.4 |
| [mmol/mol] | [71.8 ± 19.6] | [66.0 ± 16.1] | [64.1 ± 14.7] |
| FPG (mmol/L) | 10.0 ± 4.2 | 9.1 ± 3.6 | 8.9 ± 3.5 |
| [mg/dL] | [180.7 ± 76.2] | [164.2 ± 63.9] | [159.5 ± 62.2] |
| eGFR (mL/min/1.73m2) based on CKD‐EPI | 74.9 ± 21.9 | 63.2 ± 19.2 | 54.9 ± 16.9 |
| Total cholesterol (mmol/L) [mg/dL] | 4.4 ± 1.3 [170.8 ± 50.0] | 4.2 ± 1.2 [160.5 ± 44.2] | 4.1 ± 1.1 [157.1 ± 40.7] |
| LDL‐C (mmol/L) [mg/dL] | 2.3 ± 1.0 [89.4 ± 38.5] | 2.1 ± 0.9 [82.3 ± 34.5] | 2.1 ± 0.8 [79.5 ± 32.4] |
| HDL‐C (mmol/L) [mg/dL] | 1.1 ± 0.3 [43.8 ± 12.7] | 1.2 ± 0.3 [44.6 ± 12.9] | 1.2 ± 0.3 [46.5 ± 13.3] |
| Triglycerides (mmol/L) [mg/dL] | 2.3 ± 2.2 [200.7 ± 191.0] | 2.0 ± 1.4 [173.2 ± 126.7] | 1.8 ± 1.2 [160.0 ± 106.9] |
| Antihyperglycaemic medication at baseline | |||
| Insulins | |||
| Long acting | 2101 (57.1) | 1973 (62.9) | 523 (63.9) |
| Intermediate actinga | 602 (16.3) | 384 (12.2) | 88 (10.7) |
| Short acting | 1281 (34.8) | 1244 (39.7) | 306 (37.4) |
| Premix | 413 (11.2) | 294 (9.4) | 75 (9.2) |
| Other antihyperglycaemic treatment (excluding insulins) | |||
| Metformin | 2406 (65.3) | 1800 (57.4) | 358 (43.7) |
| Sulfonylurea | 1050 (28.5) | 921 (29.4) | 258 (31.5) |
| Alpha glucosidase inhibitor | 73 (2.0) | 49 (1.6) | 11 (1.3) |
| Thiazolidinedione | 112 (3.0) | 128 (4.1) | 28 (3.4) |
| DPP‐4i | 438 (11.9) | 379 (12.1) | 126 (15.4) |
| GLP‐1RA | 292 (7.9) | 271 (8.6) | 41 (5.0) |
| SGLT‐2i | 83 (2.3) | 67 (2.1) | 18 (2.2) |
| Others | 34 (0.9) | 58 (1.8) | 26 (3.2) |
| CV medication at baseline | |||
| Antihypertensive therapy | 3389 (92.0) | 2948 (94.0) | 772 (94.3) |
| Diuretics | 1753 (47.6) | 1630 (52.0) | 433 (52.9) |
| Lipid‐lowering drugs | 2961 (80.4) | 2623 (83.6) | 690 (84.2) |
| Platelet aggregation inhibitors | 2631 (71.5) | 2267 (72.3) | 592 (72.3) |
| Anti‐thrombotic medication | 185 (5.0) | 296 (9.4) | 116 (14.2) |
Note. Full analysis set; data listed are number (proportion [%]) for discrete variables and mean ± SD for continuous variables.
Abbreviations: BMI, body mass index; CKD, chronic kidney disease; CV, cardiovascular; CVD, cardiovascular disease; DPP‐4i, dipeptidyl peptidase‐4 inhibitors; eGFR, estimated glomerular filtration rate; EPI, epidemiology collaboration formula; FPG, fasting plasma glucose; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; NPH, neutral protamine Hagedorn; SGLT‐2i, sodium‐glucose co‐transporter‐2 inhibitor.
Intermediate‐acting insulins include human insulins, neutral protamine Hagedorn and unknown types of insulins.
3.2. Cardiovascular and mortality outcomes
There was a trend towards higher risks of MACE and non‐fatal stroke across the age groups and a significantly higher risk of CV death in the ≥75 years age group, as compared with the 50 to 64 years age group (Figure 1). Compared with the 50 to 64 years and the 65 to 74 years age groups, the risk of all‐cause mortality was significantly higher in the ≥75 years age group (Figure 1). There was no evidence of heterogeneity of the effects of degludec vs glargine U100 on MACE, on all‐cause mortality (Figures 2 and S1) or on individual MACE components (CV death, non‐fatal MI and non‐fatal stroke) (Figures 2 and S2) among age groups (Figure 2).
Figure 1.
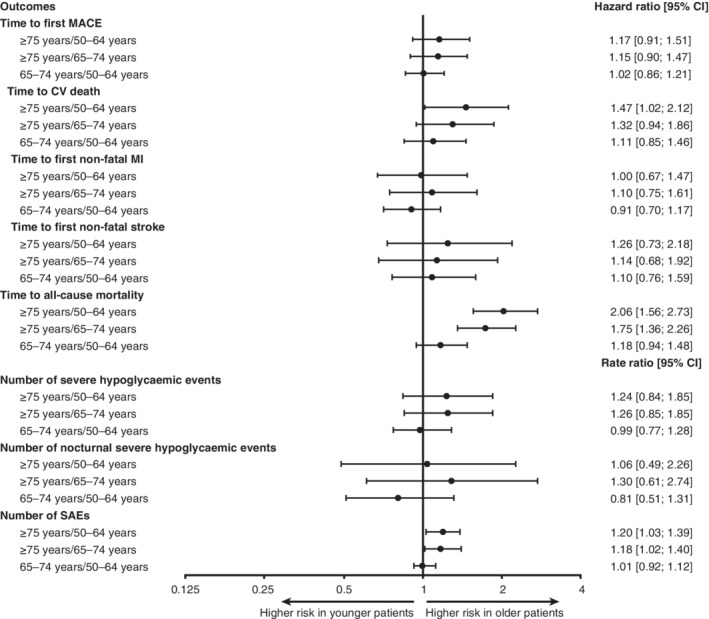
Age group comparisons concerning time to first MACE and its components (CV death, non‐fatal MI and non‐fatal stroke), time to all‐cause mortality, number of severe and nocturnal severe hypoglycaemic events and SAEs (pooled treatments; adjusted for baseline covariates). All comparisons accounted for age group, treatment, interactions between age group and treatment, sex, region, diabetes duration, CV risk, insulin‐naïve status, smoking status and kidney function at baseline. Severe hypoglycaemia was defined, according to the American Diabetes Association, as an episode requiring the assistance of another person to actively administer carbohydrate or glucagon, or to take other corrective actions.8 Nocturnal severe hypoglycaemia was defined as an episode with an investigator‐reported onset between 00:01 am and 5:59 am. Abbreviations: CI, confidence interval; CV, cardiovascular; MACE, major adverse cardiovascular event; MI, myocardial infarction; SAE, serious adverse event
Figure 2.
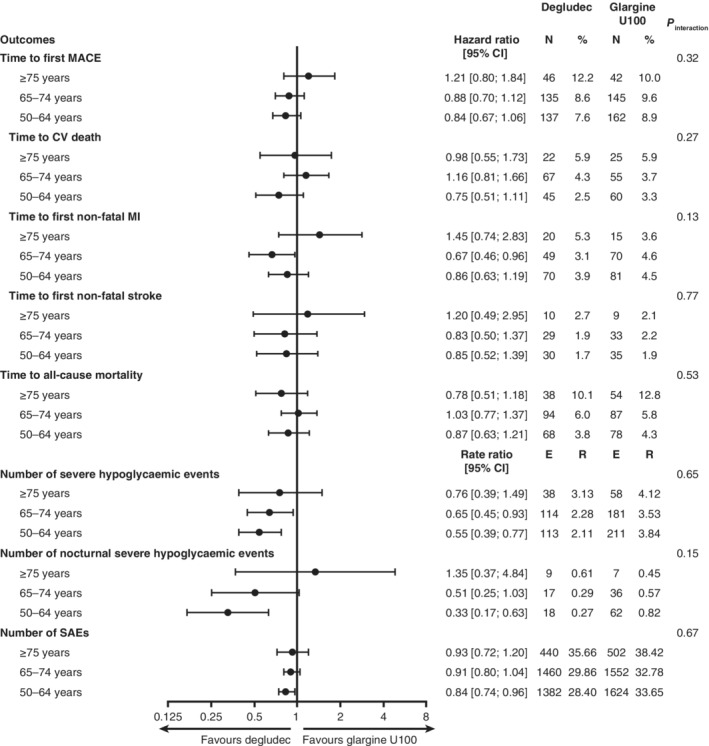
Treatment group comparisons (degludec vs glargine U100) concerning time to first MACE and its components (CV death, non‐fatal MI and non‐fatal stroke), time to all‐cause mortality, number of severe and nocturnal severe hypoglycaemic events and SAEs adjusted for baseline covariates. All comparisons accounted for age group, treatment, interactions between age group and treatment, sex, region, diabetes duration, CV risk, insulin‐naïve status, smoking status and kidney function at baseline. Severe hypoglycaemia was defined, according to the American Diabetes Association, as an episode requiring the assistance of another person to actively administer carbohydrate or glucagon, or to take other corrective actions.8 Nocturnal severe hypoglycaemia was defined as an episode with an investigator‐reported onset between 00:01 am and 5:59 am. Abbreviations: %, proportion of patients experiencing events; CI, confidence interval; CV, cardiovascular; E, number of events; glargine U100, insulin glargine 100 units/mL; MACE, major adverse cardiovascular event; MI, myocardial infarction; N, number of patients experiencing events; R, number of events per 100 patient‐years of observation; SAE, serious adverse event
3.3. Severe and nocturnal severe hypoglycaemia
The risks of severe and nocturnal severe hypoglycaemia in patients aged ≥75 years, compared with those aged 50 to 64 and 65 to 74 years (Figure 1) were numerically higher. There was a lower risk of severe hypoglycaemia with degludec vs glargine U100 across age groups, although a non‐significant trend was demonstrated in patients aged ≥75 years (Figures 2 and S3). This lower risk with degludec vs glargine U100 was also observed for nocturnal severe hypoglycaemia in patients aged 50 to 64 and 65 to 74 years but was not evident in the 16 events observed among patients aged ≥75 years (Figure 2). There was no evidence of interaction between randomized treatment and age group for severe or nocturnal severe hypoglycaemia (Figure 2).
3.4. Association between severe hypoglycaemia and time to first MACE and time to all‐cause mortality by age group
The risk of MACE after a severe hypoglycaemic event in the two older age groups was higher compared with before an event; this was significant in the 65 to 74 years age group (hazard ratio [HR], 1.69; 95% CI, 1.03‐2.77) and not significant in the ≥75 years age group (HR, 1.58; 95% CI, 0.71‐3.51) (Figure S4). Concerning time to all‐cause mortality, there was a significantly higher risk following a severe hypoglycaemic event in all age groups (≥75 years: HR, 2.20; 95% CI, 1.11‐4.33; 65‐74 years: HR, 2.36; 95% CI, 1.42‐3.92; 50‐64 years: HR, 1.95; 95% CI, 1.01‐3.75) (Figure S4).
3.5. Glycaemic control
Both total and basal insulin dose (U/kg) were significantly lower in the two older age groups after 24 months of treatment, compared with the younger age group (Figures S5 and S6A). However, there was no evidence of an association between randomized treatment and age group for total, basal and bolus insulin doses (P interaction = 0.63, 0.41 and 0.38, respectively) (Figure S6B).
Concerning change in HbA1c from baseline to Month 24, there was no significant difference between age groups, with the exception of the 65 to 74 years age group, which had a significantly greater reduction in HbA1c compared with the 50 to 64 years age group (Figure S7). Similarly, concerning change in FPG from baseline to Month 24, there was no significant difference across age groups; degludec achieved a significantly greater reduction in FPG during the same period compared with glargine U100 in all age groups (Figure S8). There was no evidence of an association between randomized treatment and age group for HbA1c and FPG (HbA1c: P interaction = 0.62; FPG: P interaction = 0.58).
Across the three age groups, there were no significant differences in day‐to‐day fasting self‐measured blood glucose (SMBG) variability (pooled treatments). In addition, there was a consistently lower day‐to‐day fasting SMBG variability with degludec compared with glargine U100 across age groups [P < 0.05].
3.6. Serious adverse events
The most frequent SAEs were cardiac disorders, which occurred in 15.0% of patients aged 50 to 64 years, in 15.5% of patients aged 65 to 74 years and in 19.0% of patients aged ≥75 years of age (Table S1). The oldest age group (≥75 years) had significantly higher rates of SAEs compared with the 50 to 64 years and 65 to 74 years age groups (Figure 1). The proportion of patients in the ≥75 years age group who had accidents and injuries was 6.2% (rate, 4.19/100 patient‐years of observation [PYO]), whereas, in the 50 to 64 years and 65 to 74 years age groups, the proportions were 3.7% (rate, 2.29/100 PYO) and 3.5% (rate, 2.53/100 PYO), respectively. Furthermore, there was a significantly higher risk of accidents and injuries following, within one day, a severe hypoglycaemic event, regardless of age (229 times higher risk in the 50 to 64 years age group [P < 0.0001], 60 times higher risk in the 65 to 74 years age group [P < 0.0001] and 619 times higher risk in the ≥75 years age group [P < 0.0001]). However, there were no significant differences between treatments in the risk of accidents and injuries. There were trends towards a lower risk of SAEs with degludec in the 65 to 74 years and ≥75 years age groups, and a significantly lower risk of SAEs in the 50 to 64 years age group as compared with glargine U100 (Figure 2). In addition, similar to what was observed with primary outcomes, concerning SAEs, there were no significant interactions between randomized treatment and age group (Figure 2).
4. DISCUSSION
Results from these secondary analyses demonstrated that there was a trend towards higher risks of MACE and severe hypoglycaemia, and significantly higher risks of CV death, all‐cause mortality and SAEs with increasing age. There was no evidence for heterogeneity of the effects of degludec vs glargine U100 with regard to the risk of MACE, all‐cause mortality and SAEs at similar levels of glycaemic control in patients with T2D who are aged 65 years or older compared with younger patients. The results suggest that the CV safety and lower severe hypoglycaemia of degludec versus glargine U100 in patients with T2D observed in the overall results of the DEVOTE trial16 were similar for participants below or above 65 years of age. However, while there was strong evidence for treatment effects until the age of 74 years, there was no conclusive evidence for the group of patients who were 75 years or older, as reflected by the wide confidence intervals.
At baseline, there was a higher proportion of patients 75 years or older with established CVD/CKD, as compared with the 65 to 74 years age group, an observation that was not surprising given that these are common T2D‐related complications in patients 65 years or older.1 The higher proportion of patients with established CVD/CKD in the 50 to 64 years age group as compared to the 65 to 74 years age group probably resulted from the pre‐specified inclusion criterion that required CVD/CKD in those between 50 and less than 60 years of age. The ≥75 years age group was also characterized by a longer duration of T2D, although not to the same degree as the difference in age. To minimize the effect of differences between baseline characteristics and demographic variables, the statistical analyses in this study were adjusted for a number of baseline covariates. However, as age is probably still confounded by other baseline variables, the results can provide guidance on treatment decisions, but cannot determine the effect of age on outcomes in individuals.
The Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS) demonstrated that patients with increasing age had significantly higher risks of MACE, all‐cause mortality, severe hypoglycaemia and bone fractures,21 while pre‐specified subgroup analyses of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study demonstrated that increased age was significantly associated with a higher risk of hypoglycaemia that required medical assistance.22 A similar trend was observed in our study; however, there was a lack of a significant association between MACE, severe hypoglycaemia and age. While there was a numerically greater risk of severe hypoglycaemia with increasing age groups (RR, 1.24 for ≥75 years vs 50‐64 years and RR, 1.26 for ≥75 years vs 65‐74 years), the trend was not statistically significant. This may be explained by the markedly smaller sample size of the oldest age group compared with the other two age groups, leading to a higher degree of imprecision in estimates of outcomes. Furthermore, the magnitude of the association may have been influenced by under‐reporting of severe hypoglycaemia in patients living alone.
In the present study, there were higher risks of MACE, all‐cause mortality and accidents/injuries following compared with before a severe hypoglycaemic event in patients aged 65 years or older; this was the case especially in the ≥75 years age group, in which there was a 619 times higher risk of accidents/injuries following a severe hypoglycaemic event. This observation is consistent with a post hoc analysis of data from the Veterans Affairs Diabetes Trial (VADT) (mean age, 60.5 years), in which severe hypoglycaemia within the previous three months was significantly associated with higher risks of serious CV events, CV death and total mortality.23 Given this evidence and the vulnerability of this patient population to severe hypoglycaemia,24, 25 it is particularly important to minimize the occurrence of severe hypoglycaemia.26
The reduced risk of severe hypoglycaemia with degludec vs glargine U100 (24%−45% reduction across the age groups) was consistent with that revealed by a secondary analysis of the SWITCH 2 trial population stratified by age (37% reduction in patients >65 years and 48% reduction in patients ≤65 years with degludec compared with glargine U100 during the maintenance period; not significant).27 A separate pre‐planned meta‐analysis of seven trials in patients with T2D ≥65 years demonstrated that degludec was associated with a significant reduction (27% for overall hypoglycaemia and 39% for nocturnal hypoglycaemia) in hypoglycaemic events during the maintenance period, compared with glargine U100.28 This clinical benefit possibly derives from the improved pharmacokinetic/pharmacodynamic profile of degludec,29 characterized by reduced day‐to‐day fasting SMBG variability with degludec compared with glargine U100 across age groups, as observed in the present study and also supported by the finding of a strong association between variability and severe hypoglycaemia in all three pooled age groups. This property may allow patients with diabetes, of all ages and all disease stages, to aim for lower glycaemic targets without increasing their risk of hypoglycaemia.30, 31 In addition, the flexibility in dosing time with degludec32 may be beneficial in this older population that may require assistance and may not be able to administer insulin at regular daily intervals.
This study has a number of strengths, including the double‐blind, active‐control design, the high level of CV risk of the population, and the prospective capture and independent adjudication of CV events, mortality and severe hypoglycaemic events. The prospective design and multicentre, international nature of this trial and the high level of patient follow‐up further contributed to the generalizability and robustness of the analyses. In addition, this secondary analysis was pre‐specified, with the exception of statistical analyses of the SAEs. Although the DEVOTE trial was not powered to investigate confirmatory outcomes in the three age groups separately, as the only randomized, double‐blind cardiovascular outcome clinical trial directly comparing two basal insulins in such large numbers, it still provides valuable information concerning these important endpoints in a population aged 65 years or older. Nevertheless, the smaller sample size leads to a higher degree of imprecision in results concerning the ≥75 years age group. Finally, there was no correction for multiplicity of comparisons in these analyses.
In conclusion, there were higher risks of CV death, all‐cause mortality and SAEs, as well as a trend towards higher risks of MACE and severe hypoglycaemia, with increasing age in patients with T2D. The effects across age groups of degludec vs glargine U100 on MACE, all‐cause mortality and severe hypoglycaemia were consistent with those of the overall DEVOTE population, suggesting that, regardless of age, the risk of MACE and death in patients with T2D who are at high risk of CV are comparable, and the risk of severe hypoglycaemia is lower with degludec compared with glargine U100.
CONFLICT OF INTEREST
R. E. P. has received consultancy and speaker fees from AstraZeneca, Takeda and Novo Nordisk; has received consultancy fees from Boehringer Ingelheim, GlaxoSmithKline, Hanmi Pharmaceutical Co. Ltd., Janssen Scientific Affairs LLC, Ligand Pharmaceuticals, Inc., Eli Lilly, Merck, Pfizer and Eisai, Inc.; and has received research grants from Gilead Sciences, Lexicon Pharmaceuticals, Ligand Pharmaceuticals, Inc., Eli Lilly, Merck, Sanofi US LLC and Takeda, all of which were paid directly to Florida Hospital, a non‐profit organization.
S. S. E. has received personal fees related to Data Monitoring Committees from CTI BioPharma, Arena Pharmaceuticals, SFJ Pharmaceuticals, BioMarin, Medivation, Biom'up, Bristol‐Myers Squibb, Dynavax, Genentech, GlaxoSmithKline, Janssen Research, Novartis, Pfizer, Roche, Sarepta Therapeutics and Xoma; has received personal fees related to other statistical consulting from Amgen, AstraZeneca, Celltrion, Daiichi Sankyo, Nektar Pharmaceuticals, Novo Nordisk, Sage Therapeutics, Shire, Sprout Pharmaceuticals, Sanofi, Takeda Pharmaceutical Company, Collegium Pharmaceutical, Intercept, Coherus BioMedical and Emmaus Life Sciences; and has received research grant support from the National Heart, Lung, and Blood Institute (NHLBI).
E. F. has received personal fees related to advisory board activities from AstraZeneca, Boehringer Ingelheim, Merck Sharp & Dohme Corp. and Novo Nordisk; and has received speaker's fees from AstraZeneca, Bristol‐Myers Squibb, Boehringer Ingelheim, Eli Lily, Merck, Merck Sharp & Dohme Corp., Novo Nordisk and Servier.
M. P. G. has received personal fees as a consultant from Sanofi US and Novo Nordisk.
S. P. M. has received personal fees from Abbott Vascular, Novo Nordisk, University of Oxford and Bristol‐Myers Squibb; and has received research support from Novo Nordisk, the Medicines Company and Terumo Medical.
D. K. M. has received personal fees for clinical trial leadership from Boehringer Ingelheim, Janssen Research and Development LLC, Sanofi US, Merck Sharp and Dohme Corp., Pfizer, Lilly USA, Novo Nordisk, GlaxoSmithKline, AstraZeneca, Lexicon Pharmaceuticals, Eisai and Esperion; and has received fees for consultancy from AstraZeneca, Sanofi Aventis, Lilly US, Boehringer Ingelheim, Merck & Co, Pfizer, Novo Nordisk, Applied Therapeutics and Metavant.
T. R. P. has received research support from Novo Nordisk and AstraZeneca, paid directly to the Medical University of Graz; has received personal fees for consulting from AstraZeneca, Bristol‐Myers Squibb, Eli Lilly, Novo Nordisk and Roche Diabetes Care; and is the Chief Scientific Officer of CBmed (Center for Biomarker Research in Medicine), a public‐funded biomarker research company.
B. Z. has received grant support from Boehringer Ingelheim, AstraZeneca and Novo Nordisk; and has received consulting fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk and Sanofi.
C. T. H., M. V. H. and T. M. are full‐time employees of, and hold stock in, Novo Nordisk A/S.
A. C. M. was a full‐time employee of Novo Nordisk A/S at the time of the study and remains a consultant to Novo Nordisk, and holds stock in the company.
J. B. B. has received contracted consulting fees, paid to the University of North Carolina, from Adocia, AstraZeneca, Eli Lilly, MannKind, NovaTarg, Novo Nordisk, Senseonics, and vTv Therapeutics; has received grant support from Novo Nordisk, Sanofi and vTv Therapeutics; is a consultant to Neurimmune AG; is supported by a grant from the National Institutes of Health (UL1TR002489); and holds stock options in Mellitus Health, PhaseBio and Stability Health.
AUTHOR CONTRIBUTIONS
All authors confirm that they meet the uniform requirements for authorship of the International Committee of Medical Journal Editors. Specifically, all authors made substantial contributions to the interpretation of data, drafted and critically revised the manuscript, provided final approval of the version to be published and agreed to be accountable for all aspects of the manuscript. Novo Nordisk contributed to the trial design, data collection, statistical analyses and the preparation of the clinical study report. All authors had full access to data and shared final responsibility for the content of the manuscript and the decision to submit for publication.
Subject level analysis data sets for the research presented in this publication are available from the corresponding author upon reasonable request.
Supporting information
Table S1. SAEs by age group
Figure S1 Kaplan‐Meier plots for time to first MACE in A, ≥75 years, B, 65 to 74 years, C, 50 to 64 years, and time to all‐cause mortality in D, ≥75 years, E, 65 to 74 years, F, 50 to 64 years, by treatment group
Figure S2 Kaplan‐Meier plots for time to CV death (A−C), time to first non‐fatal MI (D−F) and time to first non‐fatal stroke (G−I) by age and treatment groups
Figure S3 Mean cumulative function plots for number of severe hypoglycaemic episodes in A, ≥75 years, B, 65 to 74 years, C, 50 to 64 years by treatment group
Figure S4 Association between severe hypoglycaemia and time to first MACE and time to all‐cause mortality adjusted for baseline covariates
Figure S5 A, Total insulin dose; B, Basal insulin dose; C, Bolus insulin dose over time by age group
Figure S6 A, Age group comparisons (pooled data), and B, treatment group comparisons on total, basal and bolus insulin dose at month 24
Figure S7 HbA1c over time by age and treatment groups
Figure S8 FPG over time by age and treatment groups
ACKNOWLEDGMENTS
All authors thank the trial investigators, trial staff and trial participants for their participation, and they thank Jin Heppell and Beverly La Ferla of Watermeadow Medical, an Ashfield company (sponsored by Novo Nordisk) for providing medical writing and editorial support.
DEVOTE research activities were supported at numerous US centres by Clinical and Translational Science Awards from the National Institutes of Health's National Center for Advancing Translational Science.
Pratley RE, Emerson SS, Franek E, et al. Cardiovascular safety and lower severe hypoglycaemia of insulin degludec versus insulin glargine U100 in patients with type 2 diabetes aged 65 years or older: Results from DEVOTE (DEVOTE 7). Diabetes Obes Metab. 2019;21:1625–1633. 10.1111/dom.13699
Funding information This trial and the secondary analysis were funded by Novo Nordisk, with all analyses planned and conducted in collaboration with the Executive Steering Committee of the DEVOTE trial
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.13699.
REFERENCES
- 1. Corriere M, Rooparinesingh N, Kalyani RR. Epidemiology of diabetes and diabetes complications in the elderly: an emerging public health burden. Curr Diab Rep. 2013;13:805‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nobili A, Pasina L, Tettamanti M, et al. Potentially severe drug interactions in elderly outpatients: results of an observational study of an administrative prescription database. J Clin Pharm Ther. 2009;34:377‐386. [DOI] [PubMed] [Google Scholar]
- 3. Pratley R, Heller S, Miller M. Treatment of type 2 diabetes mellitus in the older adult: a review. Endocr Pract. 2014;20:722‐736. [DOI] [PubMed] [Google Scholar]
- 4. Regensteiner JG, Golden S, Huebschmann AG, et al. Sex differences in the cardiovascular consequences of diabetes mellitus. Circulation. 2015;132:2424‐2447. [DOI] [PubMed] [Google Scholar]
- 5. Huang ES, Laiteerapong N, Liu JY, John PM, Moffet HH, Karter AJ. Rates of complications and mortality in older diabetes patients: the diabetes and aging study. JAMA Intern Med. 2014;174:251‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pratley RE, Rosenstock J, Pi‐Sunyer FX, et al. Management of type 2 diabetes in treatment‐naive elderly patients. Diabetes Care. 2007;30:3017‐3022. [DOI] [PubMed] [Google Scholar]
- 7. Pieber TR, Marso SP, McGuire DK, et al. DEVOTE 3: temporal relationships between severe hypoglycaemia, cardiovascular outcomes and mortality. Diabetologia. 2018;61:58‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36:1384‐1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heller SR, Frier BM, Herslov ML, Gundgaard J, Gough SC. Severe hypoglycaemia in adults with insulin‐treated diabetes: impact on healthcare resources. Diabet Med. 2016;33:471‐477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650‐2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sinclair A, Morley JE, Rodriguez‐Mañas L, et al. Diabetes mellitus in older people: position statement on behalf of the International Association of Gerontology and Geriatrics (IAGG), the European Diabetes Working Party for Older People (EDWPOP), and the International Task Force of Experts in Diabetes. J Am Med Dir Assoc. 2012;13:497‐502. [DOI] [PubMed] [Google Scholar]
- 12. American Geriatrics Society Expert Panel on the Care of Older Adults with Diabetes Mellitus . Guidelines abstracted from the American Geriatrics Society Guidelines for improving the care of older adults with diabetes mellitus: 2013 update. J Am Geriatr Soc. 2013;61:2020‐2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. International Diabetes Federation . IDF Diabetes Atlas, 8th ed.; 2017. http://diabetesatlas.org/IDF_Diabetes_Atlas_8e_interactive_EN/. Accessed October 1, 2018.
- 14. Du YF, Ou HY, Beverly EA, Chiu CJ. Achieving glycemic control in elderly patients with type 2 diabetes: a critical comparison of current options. Clin Interv Aging. 2014;18:1963‐1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ritzel RA‐O, Harris SB, Baron H, et al. A randomized controlled trial comparing efficacy and safety of insulin glargine 300 units/mL versus 100 units/mL in older people with type 2 diabetes: results from the SENIOR study. Diabetes Care. 2018;41:1672‐1680. [DOI] [PubMed] [Google Scholar]
- 16. Marso SP, McGuire DK, Zinman B, et al. Efficacy and safety of degludec versus glargine in type 2 diabetes. N Engl J Med. 2017;377:723‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pratley RE, Gilbert M. Clinical management of elderly patients with type 2 diabetes mellitus. Postgrad Med. 2012;124:133‐143. [DOI] [PubMed] [Google Scholar]
- 18. Marso SP, McGuire DK, Zinman B, et al. Design of DEVOTE (trial comparing cardiovascular safety of insulin degludec vs insulin glargine in patients with type 2 diabetes at high risk of cardiovascular events) ‐ DEVOTE 1. Am Heart J. 2016;179:175‐183. [DOI] [PubMed] [Google Scholar]
- 19. World Medical Association . Declaration of Helsinki (2013) Ethical principles for medical research involving human subjects. JAMA. 2013;310:2191‐2194. [DOI] [PubMed] [Google Scholar]
- 20. ICH harmonised tripartite guideline: guideline for good clinical practice. J Postgrad Med. 2001;47:199‐203. [PubMed] [Google Scholar]
- 21. Bethel MA, Engel SS, Green JB, et al. Assessing the safety of sitagliptin in older participants in the Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS). Diabetes Care. 2017;40:494‐501. [DOI] [PubMed] [Google Scholar]
- 22. Miller ME, Bonds DE, Gerstein HC, et al. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:b5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Davis SN, Duckworth W, Emanuele N, et al. Effects of severe hypoglycemia on cardiovascular outcomes and death in the Veterans Affairs Diabetes Trial. Diabetes Care. 2018;42:157‐163. 10.2337/dc18-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bode BW, Brett J, Falahati A, Pratley RE. Comparison of the efficacy and tolerability profile of liraglutide, a once‐daily human GLP‐1 analog, in patients with type 2 diabetes >/=65 and <65 years of age: a pooled analysis from phase III studies. Am J Geriatr Pharmacother. 2011;9:423‐433. [DOI] [PubMed] [Google Scholar]
- 25. Laiteerapong NH, Elbert S. Diabetes in older adults In: Cowie CC, Casagrande SS, Menke A, et al., eds. Diabetes in America. 3rd ed. Bethesda, MD: National Institutes of Health; , NIH Pub No. 17‐1468 2018:1‐26. Accessed April 3, 2019. https://www.niddk.nih.gov/‐/media/Files/Strategic‐Plans/Diabetes‐in‐America‐3rd‐Edition/DIA_Ch16_V2.pdf?la=en [Google Scholar]
- 26. Cha S‐A, Yun J‐S, Lim T‐S, et al. Severe hypoglycemia and cardiovascular or all‐cause mortality in patients with type 2 diabetes. Diabetes Metab J. 2016;40:202‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heller SR, Hans Devries J, Wysham CH, Hansen CT, Hansen MV, Frier BM. Insulin degludec has lower hypoglycemia risk than insulin glargine u100 in older people with type 2 diabetes (T2D). Diabetes. 2018;67:107‐OR. [Google Scholar]
- 28. Sorli C, Warren M, Oyer D, Mersebach H, Johansen T, Gough S. Elderly patients with diabetes experience a lower rate of nocturnal hypoglycaemia with insulin degludec than with insulin glargine: a meta‐analysis of phase IIIa trials. Drugs Aging. 2013;30:1009‐1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heise T, Hövelmann U, Nosek L, Hermanski L, Bøttcher SG, Haahr H. Comparison of the pharmacokinetic and pharmacodynamic profiles of insulin degludec and insulin glargine. Expert Opin Drug Metab Toxicol. 2015;11:1193‐1201. [DOI] [PubMed] [Google Scholar]
- 30. Heise T, Hermanski L, Nosek L, Feldman A, Rasmussen S, Haahr H. Insulin degludec: four times lower pharmacodynamic variability than insulin glargine under steady‐state conditions in type 1 diabetes. Diabetes Obes Metab. 2012;14:859‐864. [DOI] [PubMed] [Google Scholar]
- 31. Philis‐Tsimikas A, Lane W, Pedersen‐Bjergaard U, et al. Relationship between A1C and hypoglycemia risk in individual patients comparing insulin degludec with insulin glargine U100. Diabetes. 2018;67:300‐OR. [Google Scholar]
- 32. Meneghini L, Atkin SL, Gough SCL, et al. The efficacy and safety of insulin degludec given in variable once‐daily dosing intervals compared with insulin glargine and insulin degludec dosed at the same time daily. Diabetes Care. 2013;36:858‐864. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. SAEs by age group
Figure S1 Kaplan‐Meier plots for time to first MACE in A, ≥75 years, B, 65 to 74 years, C, 50 to 64 years, and time to all‐cause mortality in D, ≥75 years, E, 65 to 74 years, F, 50 to 64 years, by treatment group
Figure S2 Kaplan‐Meier plots for time to CV death (A−C), time to first non‐fatal MI (D−F) and time to first non‐fatal stroke (G−I) by age and treatment groups
Figure S3 Mean cumulative function plots for number of severe hypoglycaemic episodes in A, ≥75 years, B, 65 to 74 years, C, 50 to 64 years by treatment group
Figure S4 Association between severe hypoglycaemia and time to first MACE and time to all‐cause mortality adjusted for baseline covariates
Figure S5 A, Total insulin dose; B, Basal insulin dose; C, Bolus insulin dose over time by age group
Figure S6 A, Age group comparisons (pooled data), and B, treatment group comparisons on total, basal and bolus insulin dose at month 24
Figure S7 HbA1c over time by age and treatment groups
Figure S8 FPG over time by age and treatment groups


