Abstract
Trauma to the skull causing injury to the middle meningeal artery, middle meningeal vein, or dural venous sinuses is responsible for most cases of epidural hemorrhage (EDH). Spontaneous EDH is a rare entity in clinical practice. Common causes include sinusitis, coagulation abnormalities, dural metastasis, and Langerhans cell histiocytosis. Isolated nontraumatic EDH is an exceedingly rare complication of sickle cell disease (SCD). We report a case of spontaneous EDH in a patient with SCD and review the world literature regarding this rare entity. A 20-year-old African American female with sickle cell disease presented with vaso-occlusive crisis. About 24 hours after hospital admission, the patient had sudden deterioration of her mental status. An emergent CT scan of the head revealed a large right-sided frontoparietal epidural hematoma with midline shift, subfalcine, and uncal herniation. The patient underwent emergent hematoma evacuation but died 24 hours after surgery.
1. Introduction
Sickle cell disease (SCD) is caused by a single amino acid substitution on the beta globin chain resulting in the propensity of the hemoglobin molecule to polymerize in deoxygenated state. The abnormal polymerization is responsible for subsequent RBC injury, hemolysis, microvascular injury, and classical acute and chronic manifestations of the disease. Neurologic manifestations secondary to SCD are common and affect about 35% of patients [1]. Although reports vary [2], existing data suggest that 54% of these patients suffer from ischemic strokes and 34% from intracranial bleeding [1]. Intracerebral, subarachnoid, intraventricular, subdural, and epidural bleeds have been reported, with subarachnoid hemorrhage being the most common, especially in young adults [1–3]. Isolated nontraumatic spontaneous epidural hematoma (EDH) is an exceedingly rare complication of SCD. Other reported etiologies of spontaneous EDH are infectious [4–6], coagulation abnormalities associated with end stage renal disease and hemodialysis [7–9], dural metastasis [10–13], and Langerhans cell histiocytosis [14–16]. We present a case of spontaneous EDH in a sickle cell patient suffering from vaso-occlusive crisis and review the world literature regarding the rare entity.
2. Case Presentation
A 20-year-old African American female with a history of sickle cell disease (HbSS) and multiple previous admissions for vaso-occlusive crisis (VOC) presented to the hospital with severe generalized pain throughout her body. The patient was in severe distress. Her blood pressure was 155/101 mmHg, pulse 117 beats per minute, temperature 37.6 C, respiratory rate 25 breaths per minute, and oxygen saturation 98% on room air. Physical examination revealed poor bilateral air entry on lung auscultation due to splinting and an ejection systolic murmur over the aortic area. Mild-to-moderate tenderness was present over the extremities on palpation. Neurological examination was normal. Blood work showed leukocytosis, 12,800 with 53% neutrophil, 32% lymphocyte, and 1% band, hemoglobin 7.5 gm/dL, hematocrit 22.3%, platelet 181,000/dL, reticulocyte count 13%, lactate dehydrogenase 1144 IU/L, normal blood urea nitrogen, creatinine, and serum electrolyte studies. Liver function tests were normal except a total bilirubin level of 16.2 mg/dL. Chest X-ray was normal. The patient was started on IV hydration; analgesia was achieved by IV narcotics.
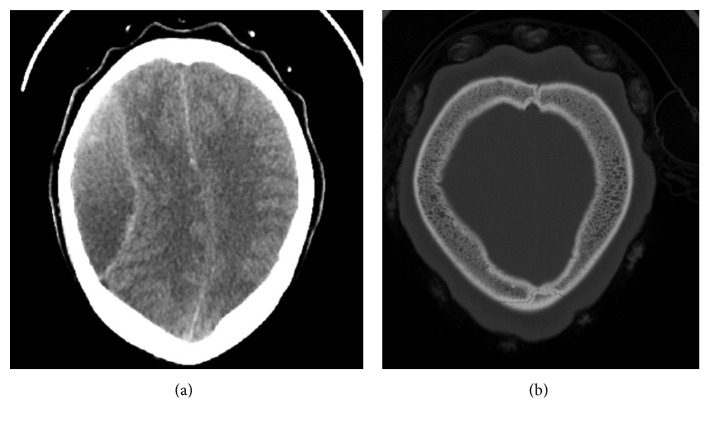
About 24 hours after hospital admission, the patient suddenly became unresponsive. Naloxone failed to improve her mental status. An emergent CT scan of the head revealed a large right-sided frontoparietal epidural hematoma with midline shift, subfalcine, and uncal herniation. No subgaleal or subperiosteal collection was noted. There was no noticeable bone infarction overlying the hematoma (Figure 1).
Figure 1.
(a) Mixed density right frontoparietal epidural hematoma with midline shift. (b) Bony window showing massive expansion of the diploic bone from extramedullary hematopoiesis.
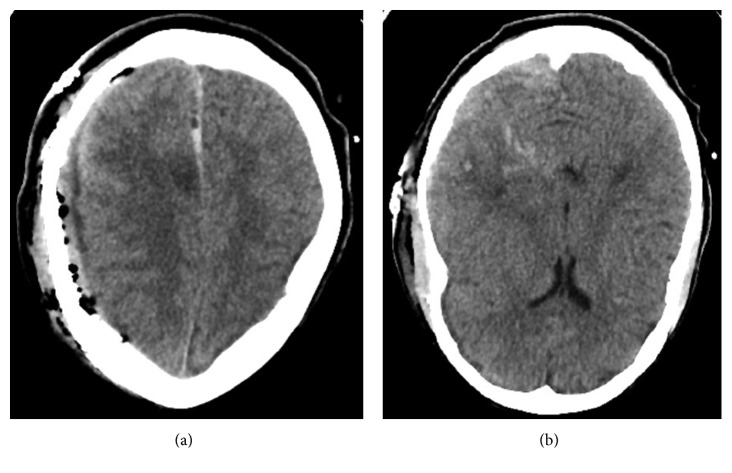
Laboratory data at this time demonstrated a platelet of 45,000/dL, prothrombin time 19.7 Seconds, INR 1.7, activated partial thromboplastin time 43 seconds, and a fibrinogen level of 96 mg/dL, consistent with a diagnosis of disseminated intravascular coagulation (DIC). The patient was emergently taken to the operating room for hematoma evacuation (Figure 2). Intraoperatively, no obvious bony abnormality was noted. Exchange transfusion was performed. The patient, however deteriorated, became hypotensive requiring multiple vasopressors and eventually died about 24 hour after surgical intervention from DIC.
Figure 2.
(a) Postoperative changes following hematoma evacuation with a small collection in the subdural space and significant improvement of midline shift (b) Punctate hemorrhage in the right temporal lobe postop.
Total 31 cases. R, right; L, left; N/S, not specified; B/L, bilateral; VOC, vaso-occlusive disease; FP, frontoparietal; PT, parietotemporal; DIC, disseminated intravascular coagulation; POA, present on admission; BS, bone scan; IO, intraoperatively.
3. Discussion
We have described a patient with SCD suffering from spontaneous EDH is the setting of VOC. Including the present case, we have identified 31 cases described in the literature, which are shown individually in Table 1. Since first reported about 3 decades ago, the reporting incidence has increased precipitously in the last decade possibly due to increased awareness, radiologic advancement, and more opportunities for publication rather than actual increase in incidence (Figure 3).
Table 1.
| Case report | Year | Age (years) | Sex | Haplotype | Presentation | Identification of EDH after hospitalization | Location of EDH | Subgaleal hemorrhage | Subperiosteal collection | Skull infarction | DIC present | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Current case | 2019 | 19 | Female | HbSS | VOC | 24 hours | R FP | No | No | No (CT/IO) | Yes | Died |
| Komarla et al. [17] | 2018 | 18 | Female | Unspecified | VOC | 24 hours | R parietal | No | No | Yes (MRI) | N/S | Survived |
| 17 | Male | Unspecified | Headache | POA | Bifrontal | Yes | Yes | Yes (MRI) | N/S | Survived | ||
| Banerjee et al. [18] | 2018 | Teenage | Male | HbSS | VOC | 6 hours | L frontal | No | No | No (MRI) | Yes | Died |
| Moyen et al. [19] | 2018 | 13 | Male | HbSS | Seizure | Imaging not performed till day 8 | R FP | No | N/S | No (MRI) | No | Died |
| Mishra et al. [20] | 2017 | 18 | Male | HbSS | VOC | 120 hours | R parietal | Yes | No | Yes (IO) | No | Survived |
| Gajjar and Gupta [21] | 2015 | 20 | Male | Unspecified | Headache | POA | L PT | No | No | No (CT) | No | Survived |
| Hettige et al. [22] | 2015 | 7 | Female | HbSS | Coma | POA | B/L parietal | No | No | No (CT/IO) | Yes | Died |
| Yogarajah at al. [23] | 2015 | 19 | Male | HbSC | VOC | 24 hours | R PT | No | No | No (CT/IO) | No | Survived |
| N'dri Oka et al. [24] | 2015 | 19 | Male | HbSC | Headache | POA | Occipital | Yes | No | No (CT) | No | Survived |
| Ilhan et al. [25] | 2014 | 15 | Male | HbSS | Headache | POA | Right frontal | Yes | No | Yes (MRI) | No | Survived |
| Serarslan et al. [26] | 2014 | 19 | Female | HbSS | Headache | POA | L FP | No | No | No (CT/IO) | No | Survived |
| Page et al. [27] | 2014 | 20 | Male | HbSS | VOC | 48 hours | L frontal | Yes | No | Yes (MRI) | No | Survived |
| 7 | Female | HbSS | Coma | POA | R temporal | No | Yes (MRI) | No | Survived | |||
| Babatola et al. [28] | 2012 | 18 | Male | HbSS | Headache | POA | R Frontal | No | No | No (CT/IO) | No | Survived |
| Bolke and Scherer [29] | 2012 | 19 | Male | Unspecified | VOC | 72 hours | L frontal | No | No | No (CT/IO) | No | Died |
| Patra et al. [30] | 2012 | 13 | Male | Unspecified | Headache | POA | B/L parietal | No | No | No (CT) | No | Survived |
| Arends et al. [31] | 2011 | 19 | Male | HbSC | Headache | POA | R parietal | No | No | Yes (MRI) | No | Survived |
| Sangle et al. [32] | 2011 | 15 | Male | Unspecified | Headache | 12 hours | Bifrontal | No | No | No (MRI) | No | Died |
| Azhar [33] | 2010 | 12 | Male | HbSD | VOC | 24 hours | L frontal | No | No | N/S (CT) | N/S | Survived |
| Dahdaleh et al. [34] | 2009 | 18 | Male | Unspecified | VOC | 12 hours | B/L FP | Yes | No | No (CT/IO) | No | Survived |
| Kotb et al. [35] | 2006 | 10 | Male | Unspecified | Headache | N/S | Bifrontal | Yes | No | Yes (MRI) | N/S | Survived |
| Kalala Okito et al. [36] | 2004 | 2 | Male | HbSS | Coma | POA | R FT | No | No | N/S (IO) | N/S | Died |
| 12 | Male | HbSS | VOC | N/S | L parietal | No | No | Yes (X-ray) | No | Survived | ||
| Ganesh et al. [37] | 2001 | 11 | Male | HbSS | Proptosis | POA | Bifrontal | Yes | No | Yes (BS) | N/S | Survived |
| Naran and Fontana [38] | 2001 | 16 | Male | Unspecified | Headache | ? POA | R Frontal | No | No | Yes (MRI/BS) | No | Survived |
| Cabon et al. [39] | 1997 | 14 | Female | HbSS | Unknown | Unknown | Bifrontal | Unknown | Unknown | Yes | Unknown | Unknown |
| Resar et al. [40] | 1996 | 14 | Male | HbSS | VOC | 48 hours | Left parietal and B/L frontal | Yes | Yes | Yes (MRI/BS) | No | Survived |
| Tony et al. [41] | 1995 | 35 | Male | Sickle- thalassemia | Proptosis | POA | Left frontal | No | No | Yes (BS) | No | Survived |
| Karacostas et al. [42] | 1991 | 19 | Male | HbSS | VOC | 48 hours | Left frontal | No | No | No (CT) | No | Survived |
| Mallouh et al. [43] | 1987 | 13 | Male | HbSS | Eye swelling | POA | Bifrontal | No | No | Yes | N/S | Survived |
Figure 3.
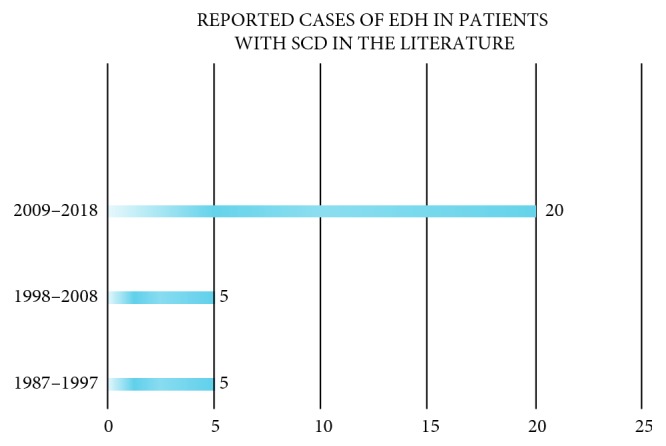
Reported cases of EDH in patients with SCD in the literature.
Most reported cases comprised males 25/31 (80%). The age ranged from 2–35 years (median 16.5) and 7–19 years (median 16), respectively, for males and females. 26/31 (84%) had SCD (17 patients with reported homozygosity and 9 known cases without specified haplotype). Other cases included 3 patients with HbSC disease, 1 with HbSD, and sickle thalassemia each.
Headache was present on admission in 37% (11/30), whereas symptoms consistent with VOC, primarily pain crisis, were the initial complaint in 40% (12/30). Other presenting symptoms were eye swelling, proptosis, seizure, and coma. In patients who presented with a headache, EDH was present on admission in 82% of the time. Patients who presented with VOC, progressed to have EDH within 6–120 hours, with a median of 24 hours after hospital admission.
Unilateral EDH was more common 20/31 (65%). Subgaleal hemorrhage was concurrently present in 9/30 (30%) of patients and associated with bone infarction in (7/9) 78% of cases. 48% of patients (15/31) with EDH showed evidence of overlying bony infarction. The overall mortality among the reported cases was 23% (7/30). Interestingly, patients with evidence of overlying bone infarction had a survival of 100% and patients with DIC had a mortality of 100%.
The most common cause of EDH is trauma causing injury to the middle meningeal artery, middle meningeal vein, the diploic vein, or the dural venous sinuses [44]. Spontaneous nontraumatic EDH is a rare manifestation of SCD and variant sickle cell syndromes. The pathophysiology of this rare occurrence is not completely understood.
Most reported cases in the literature are males. Whether any gender specific etiology is responsible for this discrepancy is not known. Low steady-state hemoglobin and high leukocyte count are known risk factors for hemorrhagic stroke in patients with SCD [1]. Whether this is also true for EDH is not clear. In the landmark study by Ohene-Frempong et al., hemorrhagic strokes were more prevalent in the adult SCD patients in the age range of 20–30 years [1]. However, EDH appears to be more prevalent in the adolescent age group.
Three pathophysiologic explanations have been proposed over the years for the causation of this rare entity: (1) Vaso-occlusion of the haematopoietically active calvarial diploic bone resulting in bone infarction and subsequent leaking of blood and proteinaceous material in the subperiosteal, epidural, or subgaleal space [38–41, 43, 45]. (2) Acute rapid expansion of hematopoiesis with resultant microfracture of already thinned inner cortex and extravasation of blood and hematopoietic tissue [24, 28, 36]. (3) Sludging of sickle cells in the diploic veins hampering venous drainage and oozing of blood due to vascular injury and elevated back pressure. A combination of different mechanisms could also be responsible. Presence of coagulopathy or platelet dysfunction worsens hematoma expansion and portends a dire clinical outcome.
Patients admitted with VOC developed EDH, did so with a median of 24 hours, suggesting early deterioration is expected in this patient population. Patients who suffered from concurrent bony infarction or subgaleal hemorrhage had a survival rate of 100%. This may indicate that vaso-occlusive etiology of the EDH carries better prognosis. This would also explain presence of EDH with contralateral subgaleal hemorrhage as skull bone infarction could be diffused [40]. The identification of bone infarction can be challenging. MRI appears to be the most sensitive tool. CT scan has low yield especially in the acute phase of the disease [18]. Infarcted bone can appear normal intraoperatively although thinning of the inner cortex is frequently seen. In 3 out of 7 patients who succumbed to their disease, an MRI ruled out any bony infarction. In the other 4 cases, no bone damage was noted by CT scan or on intraoperative inspection. We propose that patients without any obvious bony disruption and infarction, most likely suffer from microfracture of the inner table and extrusion of blood and hematopoietic cells in the epidural space due to rapid expansion of hematopoiesis. Presence of coagulopathy as in DIC makes the bleeding significantly worse. Patients who suffered from spontaneous EDH and suffered from DIC had a 100% mortality.
4. Conclusion
The understanding regarding this clinical entity is rapidly evolving in the era of advanced technology and improved awareness among healthcare providers. We believe this review will shed more light on the clinical as well as the pathophysiological aspect of the disease process and help in rapid identification of patients at risk of deterioration and formulate management plan accordingly.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Ohene-Frempong K., Weiner S. J., Sleeper L. A., et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91(1):288–294. [PubMed] [Google Scholar]
- 2.Gueguen A., Mahevas M., Nzouakou R., et al. Sickle-cell disease stroke throughout life: a retrospective study in an adult referral center. American Journal of Hematology. 2014;89(3):267–272. doi: 10.1002/ajh.23625. [DOI] [PubMed] [Google Scholar]
- 3.Strouse J. J., Hulbert M. L., DeBaun M. R., Jordan L. C., Casella J. F. Primary hemorrhagic stroke in children with sickle cell disease is associated with recent transfusion and use of corticosteroids. Pediatrics. 2006;118(5):1916–1924. doi: 10.1542/peds.2006-1241. [DOI] [PubMed] [Google Scholar]
- 4.Papadopoulos M. C., Dyer A., Hardwidge C. Spontaneous extradural haematoma with sinusitis. Journal of the Royal Society of Medicine. 2001;94(11):588–589. doi: 10.1177/014107680109401112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moonis G., Granados A., Simon S. L. Epidural hematoma as a complication of sphenoid sinusitis and epidural abscess. Clinical Imaging. 2002;26(6):382–385. doi: 10.1016/s0899-7071(02)00454-0. [DOI] [PubMed] [Google Scholar]
- 6.Chaiyasate S., Halewyck S., Van Rompaey K., Clement P. Spontaneous extradural hematoma as a presentation of sinusitis: case report and literature review. International Journal of Pediatric Otorhinolaryngology. 2007;71(5):827–830. doi: 10.1016/j.ijporl.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Shahlaie K., Fox A., Butani L., Boggan J. E. Spontaneous epidural hemorrhage in chronic renal failure. a case report and review. Pediatric Nephrology. 2004;19(10):1168–1172. doi: 10.1007/s00467-004-1551-8. [DOI] [PubMed] [Google Scholar]
- 8.Shimokawa S., Hayashi T., Anegawa S., et al. Spontaneous epidural hematoma in a patient undergoing hemodialysis: a case report. No to Shinkei = Brain and Nerve. 2003;55(2):163–166. [PubMed] [Google Scholar]
- 9.Zheng F., Chao Y. Spontaneous intracranial extradural hematoma: case report and literature review. Neurology India. 2009;57(3):324–326. doi: 10.4103/0028-3886.53288. [DOI] [PubMed] [Google Scholar]
- 10.Anegawa S., Hirohata S., Tokutomi T., Kuramoto S. Spontaneous epidural hematoma secondary to dural metastasis from an ovarian carcinoma. Neurologia Medico-Chirurgica. 1989;29(9):854–856. doi: 10.2176/nmc.29.854. [DOI] [PubMed] [Google Scholar]
- 11.McIver J. I., Scheithauer B. W., Rydberg C. H., Atkinson J. L. D. Metastatic hepatocellular carcinoma presenting as epidural hematoma: case report. Neurosurgery. 2001;49(2):447–449. doi: 10.1097/00006123-200108000-00034. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi K., Matsuo T., Kurihara M., Daikoku M., Kitange G., Shibata S. Skull metastasis of hepatocellular carcinoma associated with acute epidural hematoma: a case report. Surgical Neurology. 2000;53(4):379–382. doi: 10.1016/s0090-3019(00)00208-1. [DOI] [PubMed] [Google Scholar]
- 13.Shamim M. S., Bari M. E., Enam S. A. Dural metastases presenting as an extradural hematoma: a rare presentation. JPMA Journal of Pakistan Medical Association. 2005;55(11):509–510. [PubMed] [Google Scholar]
- 14.Cho D.-Y., Liau W.-R., Chiang I.-P. Eosinophilic granuloma with acute epidural hematoma. Pediatric Neurosurgery. 2001;35(5):266–269. doi: 10.1159/000050434. [DOI] [PubMed] [Google Scholar]
- 15.Chen H. C., Shen W. C., Chou D. Y., Chiang I. P. Langerhans cell histiocytosis of the skull complicated with an epidural hematoma. AJNR American Journal of Neuroradiology. 2002;23(3):493–495. [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez-Lage J. F., Bermudez M., Martinez-Barba E., Fuster J. L., Poza M. Epidural hematoma from a cranial eosinophilic granuloma. Child’s Nervous System: ChNS: Official Journal of the International Society for Pediatric Neurosurgery. 2002;18(1-2):74–76. doi: 10.1007/s003810100491. [DOI] [PubMed] [Google Scholar]
- 17.Komarla R., Soares B. P., Chern J. J., Milla S. S. Spontaneous epidural hematoma secondary to bone infarction in sickle cell anemia: case report. Journal of Neurosurgery: Pediatrics PED. 2018;22(1):18–21. doi: 10.3171/2018.1.peds17407. [DOI] [PubMed] [Google Scholar]
- 18.Banerjee C., Yowtak J., Fridlyand D., Alleyne C., Jr. Acute spontaneous intracranial epidural haematoma and disseminated intravascular coagulation in a paediatric sickle cell patient. BMJ Case Reports. 2018;2018 doi: 10.1136/bcr-2018-224504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moyen E., Kambourou J., Ekouelé-Mbaki H. B., et al. Spontaneous epidural hematoma in a child with sickle cell disease. Internal Medicine Review. 2018;4(1):1–7. [Google Scholar]
- 20.Mishra S. S., Senapati S. B., Gouda A. K., Behera S. K., Patnaik A. Spontaneous extradural and subgaleal hematoma: a rare neurosurgical crisis of sickle cell disease. Asian Journal of Neurosurgery. 2017;12(1):47–50. doi: 10.4103/1793-5482.144177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gajjar R., Gupta P. Spontaneous extradural hematoma: a rare neurological crisis in sickle cell disease. IOSR Journal of Dental and Medical Sciences. 2015;14(12):94–96. [Google Scholar]
- 22.Hettige S., Sofela A., Bassi S., Chandler C. A review of spontaneous intracranial extradural hematoma in sickle-cell disease. Acta Neurochirurgica. 2015;157(11):2025–2029. doi: 10.1007/s00701-015-2582-6. [DOI] [PubMed] [Google Scholar]
- 23.Yogarajah M., Agu C. C., Sivasambu B., Mittler M. A. HbSC disease and spontaneous epidural hematoma with kernohan’s notch phenomena. Case Reports in Hematology. 2015;2015:3. doi: 10.1155/2015/470873.470873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.N’Dri Oka D., Tokpa A., Bah A., Derou L. Spontaneous intracranial extradural hematoma in sickle cell disease. Journal of Neurological Surgery Reports. 2015;76(1):e97–e99. doi: 10.1055/s-0035-1544953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ilhan N., Acipayam C., Aydogan F., et al. Orbital compression syndrome complicated by epidural hematoma and wide cephalohematoma in a patient with sickle cell disease. Journal of American Association for Pediatric Ophthalmology and Strabismus. 2014;18(2):189–191. doi: 10.1016/j.jaapos.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Serarslan Y., Aras M., Altaş M., Kaya H., Urfalı B. Non-traumatic spontaneous acute epidural hematoma in a patient with sickle cell disease. Neurocirugía. 2014;25(3):128–131. doi: 10.1016/j.neucir.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Page C., Gardner K., Height S., Rees D. C., Hampton T., Thein S. L. Nontraumatic extradural hematoma in sickle cell anemia: a rare neurological complication not to be missed. American Journal of Hematology. 2014;89(2):225–227. doi: 10.1002/ajh.23579. [DOI] [PubMed] [Google Scholar]
- 28.Babatola B. O., Salman Y. A., Abiola A. M., Okezie K. O., Oladele A. S. Spontaneous epidural haematoma in sickle cell anaemia: case report and literature review. Journal of Surgical Technique and Case Report. 2012;4(2):135–137. doi: 10.4103/2006-8808.110271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bolke E., Scherer A. Sickle cell disease. Canadian Medical Association Journal. 2012;184(3):p. E201. doi: 10.1503/cmaj.111475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patra S., Mishra S., Das S. A rare case of spontaneous bilateral extradural hematoma in a sickle cell disease child. Journal of Pediatric Neurosciences. 2012;7(1):77–78. doi: 10.4103/1817-1745.97636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arends S., Coebergh J. A., Kerkhoffs J. L., van Gils A., Koppen H. Severe unilateral headache caused by skull bone infarction with epidural haematoma in a patient with sickle cell disease. Cephalalgia. 2011;31(12):1325–1328. doi: 10.1177/0333102411414441. [DOI] [PubMed] [Google Scholar]
- 32.Sangle S., Lohiya R., Karne S., Chugh K. Spontaneous epidural hematoma: a rare complication of sickle cell anemia. Neurology India. 2011;59(2):301–302. doi: 10.4103/0028-3886.79156. [DOI] [PubMed] [Google Scholar]
- 33.Azhar M. J. Extradural hemorrhage: a rare complication and manifestation of stroke in sickle cell disease. Oman Medical Journal. 2010;25(4):p. e017. doi: 10.5001/omj.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dahdaleh N. S., Lindley T. E., Kirby P. A., Oya H., Howard M. A., 3rd A “neurosurgical crisis” of sickle cell disease. Journal of Neurosurgery: Pediatrics. 2009;4(6):532–535. doi: 10.3171/2009.7.peds09219. [DOI] [PubMed] [Google Scholar]
- 35.Kotb M. M., Tantawi W. H., Elsayed A. A., Damanhouri G. A., Malibary H. M. Brain MRI and CT findings in sickle cell disease patients from Western Saudi Arabia. Neurosciences (Riyadh, Saudi Arabia) 2006;11(1):28–36. [PubMed] [Google Scholar]
- 36.Kalala Okito J. P., Van Damme O., Calliauw L. Are spontaneous epidural haematoma in sickle cell disease a rare complication? A report of two new cases. Acta Neurochirurgica. 2004;146(4):407–410. doi: 10.1007/s00701-003-0214-z. [DOI] [PubMed] [Google Scholar]
- 37.Ganesh A., William R. R., Mitra S., et al. Orbital involvement in sickle cell disease: a report of five cases and review literature. Eye. 2001;15(6):774–780. doi: 10.1038/eye.2001.248. [DOI] [PubMed] [Google Scholar]
- 38.Naran A. D., Fontana L. Sickle cell disease with orbital infarction and epidural hematoma. Pediatric Radiology. 2001;31(4):257–259. doi: 10.1007/s002470000406. [DOI] [PubMed] [Google Scholar]
- 39.Cabon I., Hladky J. P., Lambilliotte A., Cotten A., Dhellemmes P. Uncommon etiology of extradural hematoma. Neurochirurgie. 1997;43(3):173–176. [PubMed] [Google Scholar]
- 40.Resar L. M. S., Oliva M. M., Casella J. F. Skull infarction and epidural hematomas in a patient with sickle cell anemia. Journal of Pediatric Hematology/Oncology. 1996;18(4):413–415. doi: 10.1097/00043426-199611000-00017. [DOI] [PubMed] [Google Scholar]
- 41.Tony J., Subramanya G., Kallur K. G., Chalapathy A. V., Sheshadri S., Lakhkar B. Proptosis, skull infarction and epidural haematoma in sickle thalassemia. Postgraduate Medical Journal. 1995;71(837):p. 445. doi: 10.1136/pgmj.71.837.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karacostas D., Artemis N., Papadopoulou M., Christakis J. Case report: epidural and bilateral retroorbital hematomas complicating sickle cell anemia. American Journal of the Medical Sciences. 1991;302(2):107–109. doi: 10.1097/00000441-199108000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Mallouh A. A., Young M., Hamdan J., Salamah M. M. Proptosis, skull infarction, and retro-orbital and epidural hematomas in a child with sickle cell disease. Clinical Pediatrics. 1987;26(10):536–538. doi: 10.1177/000992288702601009. [DOI] [PubMed] [Google Scholar]
- 44.Bullock M. R., Chesnut R., Ghajar J., et al. Surgical management of acute epidural hematomas. Neurosurgery. 2006;58(3):S7–15. doi: 10.1227/01.neu.0000210367.14043.0e. [DOI] [PubMed] [Google Scholar]
- 45.Watanabe M., Saito N., Nadgir R. N., et al. Craniofacial bone infarcts in sickle cell disease. Journal of Computer Assisted Tomography. 2013;37(1):91–97. doi: 10.1097/rct.0b013e3182752967. [DOI] [PubMed] [Google Scholar]