Editor—Gaskell and colleagues1 conducted a secondary analysis of a heterogeneous, multicentre database to study the relationship between the presence of frontal alpha-delta EEG patterns and volitional responses assessed after anaesthesia induction using an isolated forearm technique. The authors conclude that that neither the presence of the frontal alpha-delta EEG patterns, nor any other EEG measure that they evaluated, reliably correlated with the volitional responses. Based on the data the authors present, this statement is not correct.
Phase-amplitude modulation discriminates responsiveness from unresponsiveness
The alpha and slow-delta oscillations that appear after the administration of a GABAergic anaesthetic demarcate several states. There is a state of no modulation of the alpha oscillations by the slow-delta oscillations; a trough-max state during which the maximum amplitude of the alpha oscillation occurs at the nadir of the slow oscillations; the peak-max state during which the maximum amplitude of the alpha oscillations occurs at the peak of the slow oscillations; and the transition state, which marks the evolution of the peak-max modulation into burst suppression.2 Purdon and colleagues2 showed that during induction and emergence from propofol-induced unconsciousness, the presence of the trough-max pattern predicted a non-zero probability of response to auditory stimuli in each of 10 study subjects. During the peak-max state, all of the subjects were unresponsive.
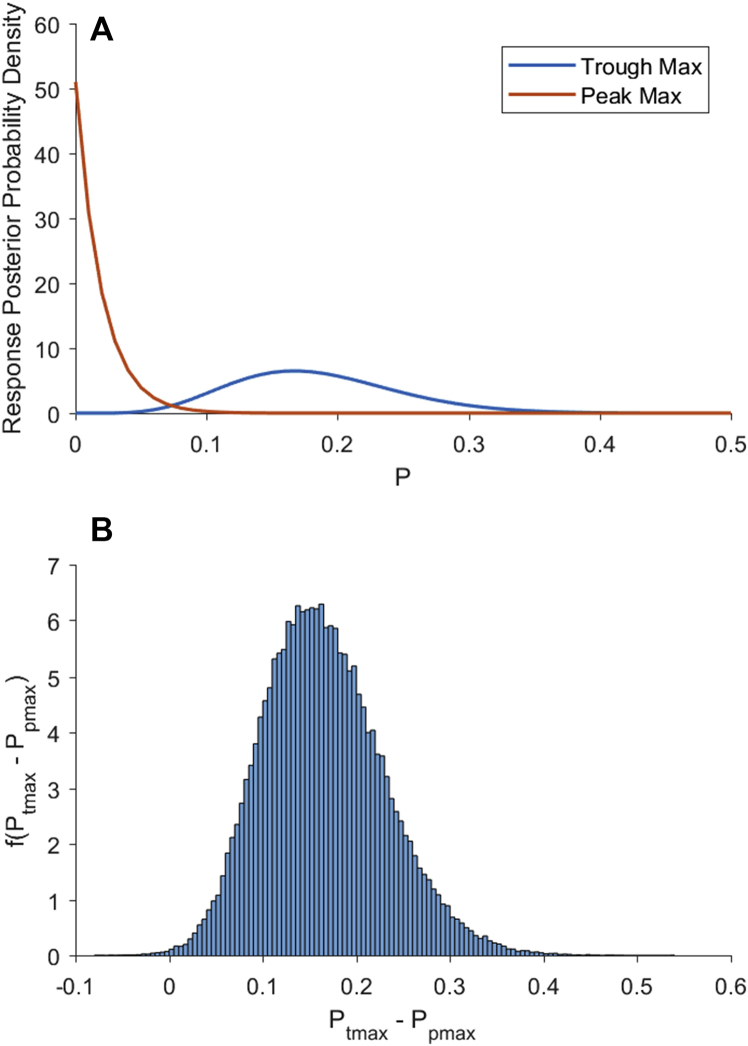
Gaskell and colleagues1 analysed EEG data from 86 patients. They reported that, of the 36 patients who showed either no modulation of the alpha oscillations by the slow-delta oscillations or the trough-max EEG pattern, six had volitional responses. Hence, in the presence of the trough-max pattern or no modulation of the alpha oscillations by the slow-delta oscillations, the volitional response probability was 0.17=6/36 (0.08, 0.32).3 The bracketed numbers are the 95% Bayesian credibility intervals based on a beta-binomial model (Fig 1A, blue curve). This observation is completely consistent with the non-zero probability of response reported by Purdon and colleagues2 in the presence of trough-max dynamics.
Figure 1.
Bayesian analysis of the probability of responding as a function of EEG signatures using a beta-binomial model.3 (A) Blue curve is f(ptmax), the posterior probability density of responding during the isolated forearm test given a trough-max EEG pattern. Orange curve is f(ptmax), the posterior probability density of responding during the isolated forearm test given a peak-max/burst suppression EEG pattern. (B) Probability density of the difference between the trough-max and the peak-max probability of responding computed by Monte Carlo convolution.3 The probability density f(ptmax − ppmax), gives the probability that the trough-max probability of response is greater than the peak-max probability of response. The probability that the trough max response probability is greater than the peak-max probability is 0.9984 (area under the curve to the right of zero). Hence, the trough-max and peak-max groups can be distinguished with near certainty.
Gaskell and colleagues1 reported that no volitional response was elicited from any of the 50 patients who had either a peak-max or burst suppression pattern.1 This gives a volitional response probability of 0.0=0/50 (0, 0.06) (Fig 1A orange curve). The probability that the response probability in the no modulation-trough-max group is greater than the response probability in the peak-max-burst suppression group is 0.9984, as computed by Monte Carlo convolution of the posterior densities from the two beta-binomial models (Fig 1B).3
This reanalysis shows that, contrary to what the authors conclude, observing alpha and slow-delta oscillations and then assessing the state of phase-amplitude modulation (trough-max, peak-max, or no modulation) discriminates with high probability (effective certainty) between the unconscious and the conscious state in patients receiving a GABAergic induction agent or inhaled ether.2 Source localisation has helped identify the brain regions responsible for these modulation patterns.4, 5 Mukamel and colleagues4 localised the trough-max pattern to brain regions believed to mediate internal consciousness,6 and arousal from unconsciousness.7 The peak-max pattern appears broadly across the cerebral cortex,4 consistent with pronounced cortical OFF states in which neuronal firing is silent, and intracortical communications are likely fragmented.5 Despite reporting six patients with volitional responses, no patient had recall of intraoperative events after surgery.1
Some respondents had a neutral modulation pattern which we interpret to mean that there was no apparent phase-amplitude modulation either with the trough-max or the peak-max pattern. We presume that these subjects were patients D, E or F (Gaskell and colleagues1 Fig 1d) or the patient in Figure 3 (Gaskell and colleagues1). The lack of any modulation suggests that there was not a significant production of slow-wave oscillations despite what was perceived to be an appropriate induction dose of propofol. Absence of slow-wave modulation, a marker of decreased excitatory inputs from the brainstem to the thalamus and cortex and cortical OFF-states, makes it more likely that these patients could be conscious. Administered doses of anaesthetics can be insufficient to induce unconsciousness despite apparent patient unresponsiveness. The presence of beta oscillations, absence of alpha and slow-delta oscillations, and absence of burst suppression strongly suggest this state.
Ketamine alters the dynamics of alpha oscillations
Formation of alpha oscillations is impeded by administration of ketamine. This is because alpha oscillation production requires enhanced inhibitory activity among GABAergic inhibitory interneurons in the thalamus and cortex.8, 9 The GABAergic anaesthetics enhance the activity of these interneurons, whereas low-dose ketamine inhibits the activity of inhibitory neurones with NMDA receptors.10, 11 As a consequence, patients receiving ketamine will not form reliable alpha oscillations. Akeju and colleagues12 demonstrated this by showing that initiation and maintenance of a ketamine infusion during sevoflurane and oxygen anaesthesia shifted alpha oscillations to beta oscillations (13–25 Hz). Mashour and Avidan13 cite this observation in their commentary. Figure 1d in the Gaskell paper shows that when the three patients who received ketamine (Patients D–F) were purportedly unconscious, each had peak power in the beta band and not in the alpha band. Furthermore, each of these three patients had diminished slow-delta power relative to other patients in their cohort. These EEG features suggest that principled approaches are needed to monitor unconsciousness in patients receiving a combination of ketamine and a GABAergic anaesthetic.
Volitional assessments were made with patients in a dynamic anaesthetic state
The authors state that anaesthetic drugs were administered to ‘induce anaesthesia’ without defining specific behavioural or neurophysiological endpoints, or fixed observational periods. The anaesthesiologists proceeded with tracheal intubation after making their clinical assessments, explicitly concluding that the anaesthetic state was adequate. Despite their clinical assessments, they found that the anaesthetic state was dynamic as six patients had volitional responses. That there could be a non-zero probability of response immediately after intubation is expected, because intubation is a potent nociceptive stimulus. In this study, there is the added observational confound of the nociceptive stimulus attributable to the isolated forearm test. Hence, the findings from this reanalysis shows that the clinical assessment of the anaesthetic state based solely on a patient's appearance and vital signs is likely to be inaccurate immediately after intubation. Moreover, it points to a need to view anaesthesia-induced unconsciousness as a dynamic state that can be modulated by external stimuli.
Anaesthetic state monitoring should combine clinical assessment with EEG monitoring and knowledge of the anaesthetic neurophysiology
For a patient anesthetised with a GABAergic anaesthetic alone, or with a GABAergic anaesthetic combined with an opioid and a muscle relaxant, the presence of a slow-delta and alpha oscillation pattern offers a basis for assessing the level of unconsciousness. The presence of phase-amplitude modulation in the peak- or trough-max states helps significantly to refine that assessment. In the operating room, we assess the spectral content of the EEG and the degree of phase-amplitude modulation by monitoring the spectrogram (density spectral array) and the raw EEG signal simultaneously.14
The findings of Gaskell and colleagues1 support the use of alpha and slow-delta oscillations to assess the level of unconsciousness in anaesthetised patients. Their study highlights the risks of drawing definitive conclusions from secondary investigations that lack prospective, systematic data collection, well-defined behavioural and neurophysiological endpoints, and appropriate statistical analyses. Their work further highlights the importance of using spectral analysis coupled with more refined EEG measures and an understanding of the neurophysiology underlying anaesthetic-induced oscillations to evaluate accurately the arousal levels of patients receiving general anaesthesia.10, 11, 14, 15
Declaration of interest
E.N.B. and P.L.P. have received speaker's honoraria from Masimo Corporation, and are inventors on pending patents on EEG monitoring that are assigned to Massachusetts General Hospital, some of which were licensed to Masimo Corporation. E.N.B. and P.L.P. have received institutionally distributed royalties for these licensed patents. O.A. and J.A. have no conflicts to declare.
Funding
This work was partially supported by National Institutes of Health (NIH) Transformative Research Award R01 GM104948, NIH Award P01 GM118629, by funds from Massachusetts General Hospital (MGH) and by funds from Massachusetts Institute of Technology (MIT), and by funds from the Picower Institute for Learning and Memory at MIT (to E.N.B); by NIH Award R01 AG053582 (to O.A.); by NIH Award R01AG056015 (to P.L.P.); and by the A∗STAR NSS MD-PhD Program and by the Institute for Medical Engineering and Science MIT at (to J.A).
References
- 1.Gaskell A.L., Hight D., Winders J. Frontal alpha-delta EEG does not preclude volitional response during anaesthesia: prospective cohort study of the isolated forearm technique. Br J Anaesth. 2017;119:664–673. doi: 10.1093/bja/aex170. [DOI] [PubMed] [Google Scholar]
- 2.Purdon P.L., Pierce E.T., Mukamel E.A. Electroencephalogram signatures of loss and recovery of consciousness from propofol. Proc Natl Acad Sci U S A. 2013;110:E1142–E1151. doi: 10.1073/pnas.1221180110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chemali J.J., Van Dort C.J., Brown E.N., Solt K. Active emergence from propofol general anesthesia is induced by methylphenidate. Anesthesiology. 2012;116:998–1005. doi: 10.1097/ALN.0b013e3182518bfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mukamel E.A., Pirondini E., Babadi B. A transition in brain state during propofol-induced unconsciousness. J Neurosci. 2014;34:839–845. doi: 10.1523/JNEUROSCI.5813-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis L.D., Weiner V.S., Mukamel E.A. Rapid fragmentation of neuronal networks at the onset of propofol-induced unconsciousness. Proc Natl Acad Sci U S A. 2012;109:E3377–E3386. doi: 10.1073/pnas.1210907109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanhaudenhuyse A., Demertzi A., Schabus M. Two distinct neuronal networks mediate the awareness of environment and of self. J Cogn Neurosci. 2011;23:570–578. doi: 10.1162/jocn.2010.21488. [DOI] [PubMed] [Google Scholar]
- 7.Langsjo J.W., Alkire M.T., Kaskinoro K. Returning from oblivion: imaging the neural core of consciousness. J Neurosci. 2012;32:4935–4943. doi: 10.1523/JNEUROSCI.4962-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ching S., Cimenser A., Purdon P.L., Brown E.N., Kopell N.J. Thalamocortical model for a propofol-induced alpha-rhythm associated with loss of consciousness. Proc Natl Acad Sci U S A. 2010;107:22665–22670. doi: 10.1073/pnas.1017069108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flores F.J., Hartnack K.E., Fath A.B. Thalamocortical synchronization during induction and emergence from propofol-induced unconsciousness. Proc Natl Acad Sci U S A. 2017;114:E6660–E6668. doi: 10.1073/pnas.1700148114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown E.N., Lydic R., Schiff N.D. General anesthesia, sleep, and coma. N Engl J Med. 2010;363:2638–2650. doi: 10.1056/NEJMra0808281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown E.N., Purdon P.L., Van Dort C.J. General anesthesia and altered states of arousal: a systems neuroscience analysis. Annu Rev Neurosci. 2011;34:601–628. doi: 10.1146/annurev-neuro-060909-153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akeju O., Hamilos A.E., Song A.H. GABAA circuit mechanisms are associated with ether anesthesia-induced unconsciousness. Clin Neurophysiol. 2016;127:2472–2481. doi: 10.1016/j.clinph.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mashour G.A., Avidan M.S. Black swans: challenging the relationship of anaesthetic-induced unconsciousness and electroencephalographic oscillations in the frontal cortex. Br J Anaesth. 2017;119:563–565. doi: 10.1093/bja/aex207. [DOI] [PubMed] [Google Scholar]
- 14.Purdon P.L., Sampson A., Pavone K.J., Brown E.N. Clinical electroencephalography for anesthesiologists: Part I: background and basic signatures. Anesthesiology. 2015;123:937–960. doi: 10.1097/ALN.0000000000000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown E.N., Purdon P.L. The aging brain and anesthesia. Curr Opin Anaesthesiol. 2013;26:414–419. doi: 10.1097/ACO.0b013e328362d183. [DOI] [PubMed] [Google Scholar]