Editor—We recently wrote an editorial in the British Journal of Anaesthesia titled ‘Is consciousness fragile?’,1 and we now extend this discussion by reporting two cases informing us ‘Is consciousness frontal?’ To date, monitoring the frontal EEG under anaesthesia has not been shown to accurately detect intraoperative connected consciousness,2 although it may guide titration of anaesthesia to induce amnesia.3, 4, 5, 6, 7, 8, 9 Recent debate and experiment has proposed that posterior, not prefrontal, cortical brain regions represent the minimum neural correlates of consciousness.10, 11, 12 If proved, this would suggest that anaesthesia monitoring may be best targeted to other brain regions, such as the parietal cortex.13,14 Here we report two cases that further illustrate the potential limitations of monitoring the frontal cortex under anaesthesia.
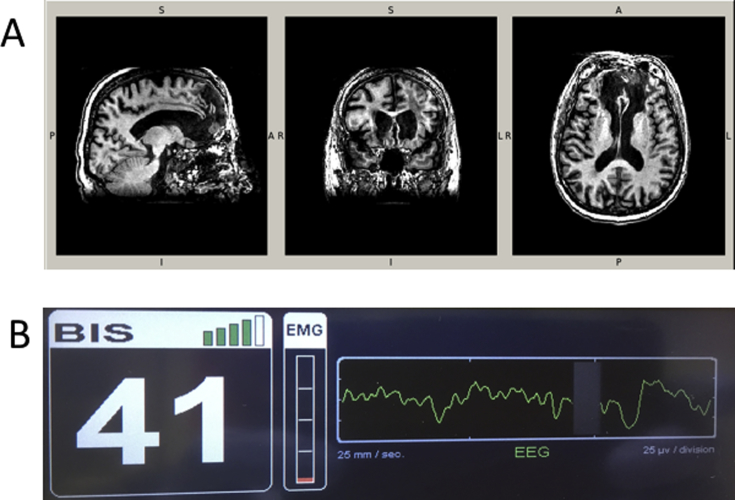
The first is of a subject who sustained severe prefrontal cortical injury as an adolescent, requiring major neurosurgery, who came to the clinic for elective endovascular aneurysm repair at age 78 yr. Preoperative T1 weighted magnetic resonance imaging showed clear evidence of bilateral injury to the prefrontal cortex (Fig. 1a). While he showed some evidence of cognitive impairment, including memory issues, he was obviously conscious when he presented for the procedure. He was anaesthetised with standard doses: i.v. induction with propofol 130 mg, fentanyl 50 μg, and lidocaine 40 mg, and maintenance with inhaled sevoflurane 1.6–1.9%. Such cases clearly indicate that subjects can be conscious while lacking significant portions of the prefrontal cortex. It appears likely that in this case, attempting to monitor the depth of anaesthesia by sampling prefrontal activity would have been uninformative for assessing consciousness.
Fig 1.
Consciousness is possible with damage to the prefrontal cortex and prefrontal slow wave activity. (a) Sagittal, coronal, and transverse planes of a preoperative magnetic resonance image collected as part of an ongoing cohort study (NCT03124303). The patient had some cognitive impairment but was conscious. (b) Screen photograph of the EEG monitor at the time that a different patient was opening eyes to command, showing the clear delta and theta waves on the raw EEG signal.
The majority of the data challenging the use of frontal EEG monitoring to identify intraoperative connected consciousness comes from studies of the isolated forearm technique (IFT).3, 4, 5, 6, 7, 8 However, there are concerns about the use of the IFT as a monitor of consciousness. For example, hand movements could be considered ambiguous to interpret (e.g. are they really purposeful and therefore linked to consciousness?) especially in the case of noxious stimuli (e.g. could they be reflex responses?). Therefore, while we maintain that the hand movements are an important form of behavioural report, we should strive to obtain multiple streams of evidence to assess the presence or absence of consciousness under anaesthesia.
Next, we report a case of an unparalysed patient who followed verbal command to open her eyes despite frontal slow wave activity in the EEG. This case is important as it parallels the IFT, but occurred in a non-paralysed patient who could produce an alternative, perhaps more standard, behavioural response of ‘eyes open’. The patient was a 72-yr-old slender (∼65 kg) female having a complex open repair of a fractured ankle, which required prone positioning. She was in general good health with hypertension and oesophageal reflux, and on treatment with verapamil and omeprazole. No other substance use was noted. A popliteal fossa block was performed under conscious sedation with 1 mg of midazolam. Before commencing induction with i.v. propofol, she had a total of midazolam 3 mg and fentanyl 100 μg. At this stage, she was drowsy but still awake. On induction with propofol, it was noticed that the bispectral index (BIS) had decreased to 54, but she was still responsive to verbal command and opened her eyes on request without alteration in the BIS wave pattern. Additional propofol was therefore given and she became unresponsive to command when the BIS reading reached <40. Propofol titration continued until the BIS reading decreased to 35. The total amount of propofol used to achieve this stage was 100 mg. At this point, vecuronium 6 mg was administered and her trachea was intubated. Anaesthesia was maintained using sevoflurane (1.7–2.3%) and infusions of propofol (200 mg h−1) and remifentanil (400 μg h−1). The maintenance dose was titrated to BIS readings of ∼33 (28–34), which was 10 below the last response to verbal command. Remifentanil was increased to 600 μg h−1 at ∼50 min into surgery for a tourniquet related increase in blood pressure. She also received clonidine (60 μg) and morphine (7 mg) titrated to tourniquet related response. No further relaxants were given. At the conclusion of the operation (∼3 h) she was turned supine and the sevoflurane and propofol stopped. The remifentanil was stopped when the BIS reading started to increase. When the BIS increased to between 40 and 50 she would open her eyes to repeated command and nod on request. She was comfortable and had no distress from the presence of the tracheal tube. Figure 1b shows that, at this point, she had a predominant slow wave pattern in her frontal EEG and low EMG tone. At a postoperative visit, she had no subsequent recollection of any of these events, and gave verbal consent for the details to be presented or published.
This case confirms previous observations obtained with the IFT paradigm that prefrontal slow waves are not necessarily indicative of unconsciousness,3 but may reflect amnesia (although amnesia can also occur without slow waves3), lack of patient distress, or both.3 Hence, similar to Mashour and Avidan,9 we propose that prefrontal EEG monitoring of anaesthesia should not be abandoned. However, as our patients largely expect to be unconscious and not just amnestic during anaesthesia,15 we must strive to identify anaesthesia monitoring strategies that assess the level of consciousness during anaesthesia and surgery. Looking beyond monitoring of prefrontal regions appears a sensible course of action.
Authors' contributions
Wrote first draft of paper: R.D.S., N.M.
Contributed to writing paper: all authors.
Data collection: N.M., H.L.
Funding
R.D.S. is supported by National Institute of Health, National Institute on Aging K23 AG055700 and G.T. by NIH/NCCAM P01AT004952 and NIH/NIMH 5P20MH077967.
Declaration of interest
Dr. Tononi has consulted for Philips Respironics and has been involved in a research study in humans supported by Philips Respironics. The article submitted is not related to any of these relationships. GT Dr. Tononi is a consultant for the Allen Institute for Brain Research. The article submitted is not related to this relationship.
References
- 1.Sanders R.D., Raz A., Banks M.I., Boly M., Tononi G. Is consciousness fragile? Br J Anaesth. 2016;116:1–3. doi: 10.1093/bja/aev354. [DOI] [PubMed] [Google Scholar]
- 2.Sanders R.D., Tononi G., Laureys S., Sleigh J.W. Unresponsiveness ≠ unconsciousness. Anesthesiology. 2012;116:946–959. doi: 10.1097/ALN.0b013e318249d0a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaskell A.L., Hight D.F., Winders J. Frontal alpha-delta EEG does not preclude volitional response during anaesthesia: prospective cohort study of the isolated forearm technique. Br J Anaesth. 2017;119:664–673. doi: 10.1093/bja/aex170. [DOI] [PubMed] [Google Scholar]
- 4.Sanders R.D., Gaskell A., Raz A. Incidence of connected consciousness after tracheal intubation: a prospective, international, multicenter cohort study of the isolated forearm technique. Anesthesiology. 2017;126:214–222. doi: 10.1097/ALN.0000000000001479. [DOI] [PubMed] [Google Scholar]
- 5.Russell I.F. The Narcotrend 'depth of anaesthesia' monitor cannot reliably detect consciousness during general anaesthesia: an investigation using the isolated forearm technique. Br J Anaesth. 2006;96:346–352. doi: 10.1093/bja/ael017. [DOI] [PubMed] [Google Scholar]
- 6.Russell I.F. The ability of bispectral index to detect intra-operative wakefulness during isoflurane/air anaesthesia, compared with the isolated forearm technique. Anaesthesia. 2013;68:1010–1020. doi: 10.1111/anae.12357. [DOI] [PubMed] [Google Scholar]
- 7.Russell I.F., Sanders R.D. Monitoring consciousness under anaesthesia: the 21st century isolated forearm technique. Br J Anaesth. 2016;116:738–740. doi: 10.1093/bja/aew112. [DOI] [PubMed] [Google Scholar]
- 8.Linassi Br J Anaesth. 2018 [Google Scholar]
- 9.Mashour G.A., Avidan M.S. Black swans: challenging the relationship of anaesthetic-induced unconsciousness and electroencephalographic oscillations in the frontal cortex. Br J Anaesth. 2017;119:563–565. doi: 10.1093/bja/aex207. [DOI] [PubMed] [Google Scholar]
- 10.Koch C., Massimini M., Boly M., Tononi G. Neural correlates of consciousness: progress and problems. Nat Rev Neurosci. 2016;17:307–321. doi: 10.1038/nrn.2016.22. [DOI] [PubMed] [Google Scholar]
- 11.Boly M., Massimini M., Tsuchiya N., Postle B.R., Koch C., Tononi G. Are the neural correlates of consciousness in the front or in the back of the cerebral cortex? Clinical and neuroimaging evidence. J Neurosci. 2017;37:9603–9613. doi: 10.1523/JNEUROSCI.3218-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siclari F., Baird B., Perogamvros L. The neural correlates of dreaming. Nat Neurosci. 2017;20:872–878. doi: 10.1038/nn.4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee U., Muller M., Noh G.J., Choi B., Mashour G.A. Dissociable network properties of anesthetic state transitions. Anesthesiology. 2011;114:872–881. doi: 10.1097/ALN.0b013e31821102c9. [DOI] [PubMed] [Google Scholar]
- 14.Lee M., Sanders R.D., Yeom S.K. Network properties in transitions of consciousness during propofol-induced sedation. Sci Rep. 2017;7:16791. doi: 10.1038/s41598-017-15082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowley P., Boncyk C., Gaskell A. What do people expect of general anaesthesia? Br J Anaesth. 2017;118:486–488. doi: 10.1093/bja/aex040. [DOI] [PubMed] [Google Scholar]