Abstract
Background
General anaesthetics generate spatially defined brain oscillations in the EEG that relate fundamentally to neural-circuit architecture. Few studies detailing the neural-circuit activity of general anaesthesia in children have been described. The study aim was to identify age-related changes in EEG characteristics that mirror different stages of early human brain development during sevoflurane anaesthesia.
Methods
Multichannel EEG recordings were performed in 91 children aged 0–3 yr undergoing elective surgery. We mapped spatial power and coherence over the frontal, parietal, temporal, and occipital cortices during maintenance anaesthesia.
Results
During sevoflurane exposure: (i) slow–delta (0.1–4 Hz) oscillations were present in all ages, (ii) theta (4–8 Hz) and alpha (8–12 Hz) oscillations emerge by ∼4 months, (iii) alpha oscillations increased in power from 4 to 10 months, (iv) frontal alpha-oscillation predominance emerged at ∼6 months, (v) frontal slow oscillations were coherent from birth until 6 months, and (vi) frontal alpha oscillations became coherent ∼10 months and persisted in older ages.
Conclusions
Key developmental milestones in the maturation of the thalamo-cortical circuitry likely generate changes in EEG patterns in infants undergoing sevoflurane general anaesthesia. Characterisation of anaesthesia-induced EEG oscillations in children demonstrates the importance of developing age-dependent strategies to monitor properly the brain states of children receiving general anaesthesia. These data have the potential to guide future studies investigating neurodevelopmental pathologies involving altered excitatory–inhibitory balance, such as epilepsy or Rett syndrome.
Keywords: anaesthesia, general, child, development
Editor's key points.
-
•
Multichannel EEG recordings from 91 children aged 0–3 yr were used to map spatial power and coherence during sevoflurane anaesthesia.
-
•
Age-dependent changes detected in brain oscillations might reflect cortical maturation and integration.
-
•
These findings demonstrate the importance of age-dependent strategies for monitoring the brain states of children receiving general anaesthesia.
Each year, millions of children receive general anaesthesia for surgery. Sevoflurane is one of the most commonly used vapour anaesthetics in children because of its rapid induction, emergence, and recovery profile. Clinical studies using non-invasive brain monitoring show general anaesthetics and hypnotic agents produce EEG oscillations with specific spatial organisation that relate fundamentally to neural-circuit architecture and function.1, 2 For example, in adults under propofol anaesthesia, specific EEG patterns are observed, consisting of incoherent slow oscillations distributed across the entire head and strongly coherent alpha oscillations located across only the front of the head.3, 4 The association of unconsciousness with frontal alpha oscillations is a fairly consistent finding, although there are counterexamples.5
Prior studies suggest that neurophysiological response to sevoflurane changes as a function of age.6, 7, 8, 9, 10 However, these studies lack the detailed characterisation of anaesthesia-associated neural-circuit activity particularly at specific developmental ages associated with critical periods of heightened brain plasticity because of (i) low numbers of patients enrolled, (ii) grouped analysis over a broad age range that involves key developmental changes, (iii) limited electrodes and lack of spatial mapping, and (iv) use of EEG-derived algorithms that can lose valuable information.
The study aim was to investigate whether there are systematic changes in sevoflurane-induced brain oscillations that might mirror different stages of human brain development. We used multichannel EEG recordings in children up to 3 yr of age undergoing sevoflurane general anaesthesia for elective surgery. We mapped spatial power and coherence over the frontal, central, parietal, temporal, and occipital cortices. Our results suggest key developmental milestones in the assembly and maintenance of sevoflurane-induced circuit activity in the human brain.
Methods
Ethics approval
This study was approved by the Boston Children's Hospital Institutional Review Board (Protocol Number: P000003544) and classified as ‘no more than minimal risk’. Informed written consent was obtained from the parents/legal guardians. The study conformed to the Declaration of Helsinki.
Patient population
Children who were scheduled for an elective surgical procedure were recruited from the preoperative clinic at the Boston Children's Hospital from December 2011 to August 2016. The inclusion criteria consisted of 0–3 yr old and requiring surgery below the neck. All children were clinically stable on the day of study and ASA Physical Status I or II. The exclusion criteria were (i) born with congenital malformations or other genetic conditions thought to influence brain development, (ii) diagnosed with a neurological or cardiovascular disorder, or (iii) born at <32 weeks postmenstrual age.
Study design
Multichannel EEGs were recorded during the administration of sevoflurane general anaesthesia. The end-tidal anaesthetic gas concentration was time locked to the EEG recording. The spatial properties of the EEG were evaluated during a sevoflurane-maintained surgical state of anaesthesia.
Anaesthetic management
Anaesthetic management was adjusted per the anaesthetist's impression of clinical need. Each patient received anaesthesia induced with sevoflurane alone, or a combination of sevoflurane and nitrous oxide in oxygen. Nitrous oxide was added at the discretion of the anaesthetist (n=66). Nitrous oxide was discontinued after placement of a tracheal tube or laryngeal mask. Tracheal tube (n=62), laryngeal mask (n=24), or face mask (n=5) were used. Propofol bolus was used to facilitate tracheal intubation or to suppress motor reflexes in 44 subjects [median dose: 2.4 mg kg−1 [inter-quartile range (IQR): 1.7–3.3 mg kg−1]. Seven subjects were prescribed midazolam premedication on the day of surgery.
Data acquisition
All subjects were in the supine position throughout the study. Each subject was studied once.
EEG recording
An EEG cap was used to record the EEG activity (waveguard EEG cap; Advanced NeuroTechnology, Enschede, Netherlands) with 33 or 41 recording electrodes positioned per the modified international 10/20 electrode placement system. For 33-channel recording, the electrodes were positioned at FPz, FP1, FP2, F3, F4, F7, F8, FC1, FC2, FC5, FC6, Cz, CPz, C3, C4, CP1, CP2, CP5, CP6, Pz, P3, P4, P7, P8, T7, T8, M1, M2, POz, Oz, O1, and O2. For 41-channel recordings, additional electrodes were positioned at AF7, AF8, PO7, PO8, FT7, FT8, TP7, and TP8. Reference and ground electrodes were located at Fz and AFz, respectively. Impedance of the electrode–skin interface was minimised using an EEG prepping gel (Nuprep gel; Weaver and Company, Aurora, CO, USA), and conductive EEG gel was used to optimise electrode contact (OneStep clear gel; H + H Medical Devices, Dülmen, Germany).
The EEG activity from 0 to 500 Hz was recorded (EMU40EX; Natus Medical Incorporated, Oakville, ON, Canada). Signals were digitised at a 1024 Hz sampling rate (or 256 Hz in five patients) and 16-bit resolution.
Clinical data collection
Clinical information was collected from the electronic medical records and from the in-house anaesthesia information management system. End-tidal sevoflurane, oxygen, and nitrous oxide concentrations were downloaded from the anaesthetic monitoring device (Dräger Apollo; Dräger Medical Inc., Telford, PA, USA) to a recording computer in real time using ixTrend (Ixellence, Wildau, Germany). Signals were recorded at a 1 Hz sampling rate.
EEG analysis
Data preprocessing
Preprocessing was carried out with Natus NeuroWorks (Natus Medical Incorporated) and MATLAB (MathWorks, Natick, MA, USA). Ear electrodes (M1 and M2) were excluded from the final analysis because of poor surface-to-skin contact for most subjects.
EEG signals were re-montaged to a nearest-neighbour Laplacian reference using distances along the scalp surface and weighting the neighboring electrode contributions. We applied an anti-aliasing filter of 80 Hz and down sampled the EEG data to 256 Hz.
For each subject, a period of maintenance of general anaesthesia adequate for surgery and where end-tidal sevoflurane concentration was maintained at a steady concentration (±0.1 vol%) with air and oxygen was identified in the EEG. Within this steady-state segment, a 5 min epoch was selected from ‘artefact-free’ EEG, where motion or electrocautery artefacts were not present. Channels with noise or artefacts were excluded from the analysis by visual inspection. Epochs were analysed with a median time after the start of the surgical procedure of 15 min (IQR: 5–33 min). Two authors (L.C. and J.M.L.) visually inspected the EEG data and manually selected ‘artefact-free’ EEG segments for analysis.
Time–frequency analysis
The power spectral density quantifies the frequency distribution of power within a signal, where power is the 10 * log10 of the EEG signal amplitude squared. The spectrogram is a time-varying version of the power spectral density, estimated on consecutive windows of EEG data. In these spectrograms, frequencies are arranged along the y-axis and time along the x-axis, and power is indicated by colour on a decibel scale. Spectral analysis was performed with multi-taper methods using the Chronux toolbox (http://chronux.org/).11, 12 Multi-taper parameters were set using window lengths of T=2 s with a 1.9 s overlap, time–bandwidth product TW=2, and number of tapers K=3.
Subjects were divided into cohorts per postnatal age: (i) 0–3 months, (ii) 4–6 months, (iii) 7–9 months, (iv) 10–14 months, (v) 15–17 months, and (vi) 18–40 months. Age grouping was based on (i) results of our previous work in young infants,6 (ii) prior knowledge of age distribution of surgeries, and (iii) key stages of postnatal neurodevelopment.
First, group-averaged spectrograms were computed by taking the median power across the subjects at each time and frequency at the electrode of interest for each postnatal age cohort. Second, group-averaged spectra were computed by taking the median power across subjects at each frequency across the entire 5 min epoch, and then the median [95% confidence interval (CI)] power at each frequency was calculated for each postnatal age group. To identify age-varying changes in the frontal power spectra, we computed an age-varying spectrogram using an overlapping (1 month) moving window spanning a 3 month range. To obtain smoothed spectrograms, we applied a Kalman filter and a fixed-interval smoothing algorithm to the age-varying spectrogram.
To identify the topographic distribution for slow (0.1–1 Hz), delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta (12–25 Hz), and gamma (25–40 Hz) frequency bands, we first took the median power spectra of group-averaged spectrograms across the entire 5 min epoch for each electrode. Then, we averaged the median power spectra over each EEG frequency band of interest for each postnatal age group. Frequency-band power plots were performed using 3D interpolation of the electrode montage with the topoplot function in EEGLab.13
Coherence analysis
Coherence analysis was performed using custom-written MATLAB code (MathWorks Inc., Natick, MA).6 Coherence quantifies the degree of correlation between two signals at a given frequency. It is equivalent to a correlation coefficient indexed by frequency: a coherence of 1 indicates that two signals are perfectly correlated at that frequency, whilst a coherence of 0 indicates that the two signals are uncorrelated at that frequency. The coherence between signals i and j is given by
where Sij is the cross spectrum between signals xi(t) and xj(t), Si is the power spectrum of xi(t), and Sj is the power spectrum of xj(t).
For frontal-coherence analysis, we used raw EEG data recorded using a common reference (Fz) to avoid distortion by referencing. We estimated the coherence between the bipolar left frontal signal (F7–FP1) and bipolar right frontal signal (F8–FP2), using multi-taper methods with TW=4, K=7, and t=4 s with a 3 s overlap based on the analysis of adult EEG.14 A coherogram graphically illustrates coherence for a range of frequencies plotted across time. To compute the group-averaged coherogram, we took the median coherence across subjects at each time point and frequency. To identify age-varying changes in frontal coherence, we computed an age-varying coherogram using an overlapping (1 month) moving window spanning a 3 month range, and applied the Kalman and smoothing filter used in the spectral analysis.
For global-coherence analysis, we divided each segment into non-overlapping 2 s windows and computed the cross-spectral matrix. To remove the noise artefact, the median over 10 windows of the real and imaginary parts of each entry in the cross-spectral matrix was taken. Then, we performed an eigenvalue decomposition analysis of the cross-spectral matrix at each frequency. The cross-spectral matrix at each frequency can be factorised as
where UH is the complex conjugate transpose of U and a unitary matrix whose ith column is the eigenvector ui of S, and Λ is the diagonal matrix whose diagonal elements are the corresponding eigenvalues, Λii=λi. Global coherence is the ratio of the largest eigenvalue to the sum of eigenvalues3
When the largest eigenvalue is large compared with the remaining ones, the global coherence is close to 1. We computed the global coherence at each frequency using 5 min epochs for the maintenance of surgical anaesthesia. To determine the group-averaged global coherence, we took a median across subjects in each postnatal age group. We refer to the eigenvector umax corresponding to the largest eigenvalue λmax at a given frequency as the principal mode of oscillation for that frequency, and the coherence of electrode sites was obtained by the absolute square of the eigenvector, as previously applied in adult EEG studies.4 This means that the eigenvector described a coherent spatial distribution. Spatial coherence at each frequency band was computed by taking an average across the frequency range at each electrode. The group-averaged scalp coherence distribution was computed by taking the median across subjects and plotting with the topoplot function in EEGLab.
Statistical analysis
Analyses were performed using R version 3.4.3 with MedOr, moments, tableone, and tidyverse packages,15 and custom-written MATLAB code with polyfit and polyconf functions6 (MathWorks).
We used a paired-subject bootstrapping algorithm to compare spectral estimates between electrode locations (frontal vs occipital), as implemented in the Chronux toolbox.16 We drew bootstrap samples from the data set with replacement and estimated the best-fit regression for the bootstrap samples for each electrode location. Then, we took the difference between regression functions at specific electrode locations (using paired comparisons). We repeated this process 10 000 times and calculated the 95% CI for the difference between two regression functions to test for significant differences.
Use of previously published data sets
The EEG characteristics during general anaesthesia on selected subjects have been reported elsewhere: specifically, on spectral and coherence patterns at 0–6 months old (n=36),6 and on discontinuity (profoundly suppressed EEG activity) during deep levels of anaesthesia at 0–3 yr old (n=68).17
Code
Custom-written MATLAB code (with simulated data) for computing multi-taper spectra, global coherence, and bootstrap CIs is publicly available.6
Results
Continuous multichannel EEG recordings collected during maintenance of a surgical state of anaesthesia in 91 subjects from 0 to 3 yr old (0–40 months old) are reported. The subject summary characteristics are provided in Table 1 (age-group stratified) and Supplementary Table S1 (entire sample), and the study profile in Supplementary Figure S1.
Table 1.
Clinical characteristics of subjects studied.
| 0–3M (n=17) | 4–6M (n=23) | 7–9M (n=8) | 10–14M (n=17) | 15–17M (n=7) | 18M+ (n=19) | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age (months), median (IQR) | 2.9 (2.6–3.5) | 6 (5.4–6.1) | 8.4 (7.9–9.6) | 12.8 (11.3–13.9) | 17.5 (16.6–17.6) | 28.3 (20.3–33.9) |
| Weight at study (kg), median (IQR) | 5.7 (4.8–6.1) | 7.7 (6.8–8.2) | 8.1 (8–9) | 10.3 (9.2–11.4) | 11.1 (10.8–12) | 13.9 (12.4–15.2) |
| Sex (male), % (n) | 76.5 (13) | 91.3 (21) | 50 (4) | 88.2 (15) | 85.7 (6) | 78.9 (15) |
| Anaesthetic management | ||||||
| Duration of anaesthesia (min), median (IQR) | 103 (86–140) | 94 (77–170) | 148.5 (100.8–184.3) | 95 (86–132) | 133 (106.5–174.5) | 112 (67–169.5) |
| Propofol, % (n) | 64.7 (11) | 47.8 (11) | 37.5 (3) | 70.6 (12) | 0 (0) | 52.9 (9) |
| Local anaesthetic agents, % (n) | 82.3 (14) | 95.6 (22) | 87.5 (7) | 82.4 (14) | 57.1 (4) | 52.9 (9) |
| Opioid agents, % (n) | 82.3 (14) | 65.2 (15) | 87.5 (7) | 94.1 (16) | 85.7 (6) | 82.4 (14) |
| Neuromuscular blocking agents, % (n) | 70.6 (12) | 34.8 (8) | 37.5 (3) | 52.9 (9) | 42.9 (3) | 23.5 (4) |
| Maintenance epoch | ||||||
| End-tidal sevoflurane (%), mean (SD) | 2.3 (0.7) | 2.6 (0.6) | 2.3 (0.3) | 2.5 (0.6) | 2.7 (0.6) | 2.3 (0.4) |
IQR, interquartile range; SD, standard deviation.
Age-varying changes in power spectral properties
Slow and delta oscillations are present across all ages; theta and alpha emerge around 4 months old
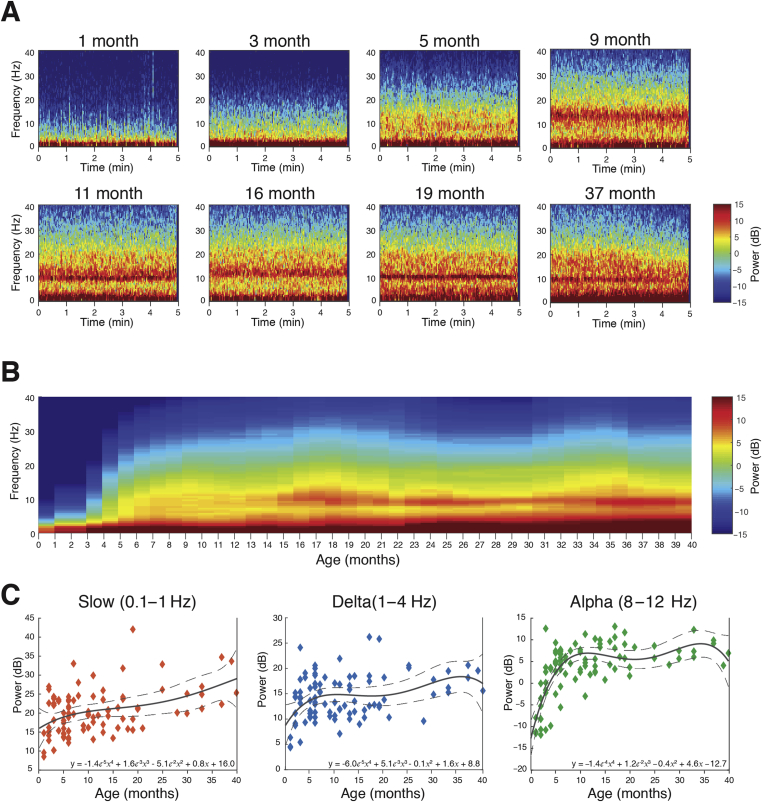
The frontal power spectral analysis in individual infants at electrode F7 shows slow (0.1–1 Hz) and delta power (1–4 Hz) were present at all ages (Fig. 1a). At the youngest ages, 1 and 3 months old, power in the 4–40 Hz frequency range was minimal. At 5 months old, power in the theta (4–8 Hz) and alpha (8–12 Hz) frequency ranges began to emerge. At 9, 11, and 16 months, higher frequencies in the low beta (13–17 Hz) ranges appear. At 19 and 37 months, the alpha frequency range began to increase in power compared with other frequencies above 4 Hz.
Fig 1.
Frontal spectrograms and band power during sevoflurane-maintained surgical anaesthesia in children from 0 to 3 yr. Frontal EEG spectral power for frequencies from 0.1 to 40 Hz during a 5 min period of surgical anaesthesia. (a) Frontal spectrogram for individual subjects; (b) age-varying spectral changes in frontal spectrogram; and (c) frontal peak band power in the slow (0.1–1 Hz), delta (1–4 Hz), and alpha (8–12 Hz) frequency bands. Delta oscillations exhibited a small, steady increase in power with age. Alpha oscillations increased in power during infancy, peaking at approximately 10 months, and remaining at a sustained level with age. The solid line represents a fourth-degree polynomial-regression model describing the relationship between age and EEG power, with an equation written in inset; the dashed lines represent the 95th confidence boundaries of the regression model. The F7 electrode is presented using nearest-neighbour Laplacian referencing (See Supplementary Fig. S2 for frontal EEG spectral properties across age groups.).
The age-varying frontal spectrogram (time-varying spectra) computed at F7 shows the distribution of slow–delta, theta, alpha, beta, and gamma (25–40 Hz) oscillations by age (Fig. 1b). Slow and delta were dominant frequencies across all ages. Theta and alpha oscillations were more prominent in subjects aged 4 months and older. Beta and gamma oscillations remained less prominent of all frequencies. The age-group analysis of frontal group-median power spectra shows the evolution of alpha oscillations with age (Supplementary Fig. S2).
Slow, delta, and alpha oscillations were the dominant frequency components observed across age. Frontal slow and frontal delta oscillations were present from birth, and steadily increased with age (Fig. 1c). Frontal alpha oscillations emerged at approximately 3–4 months, and steadily increased until plateauing at 10 months old (∼6 dB) (Fig. 1c).
Frontal alpha predominance emerges around 7–9 months old
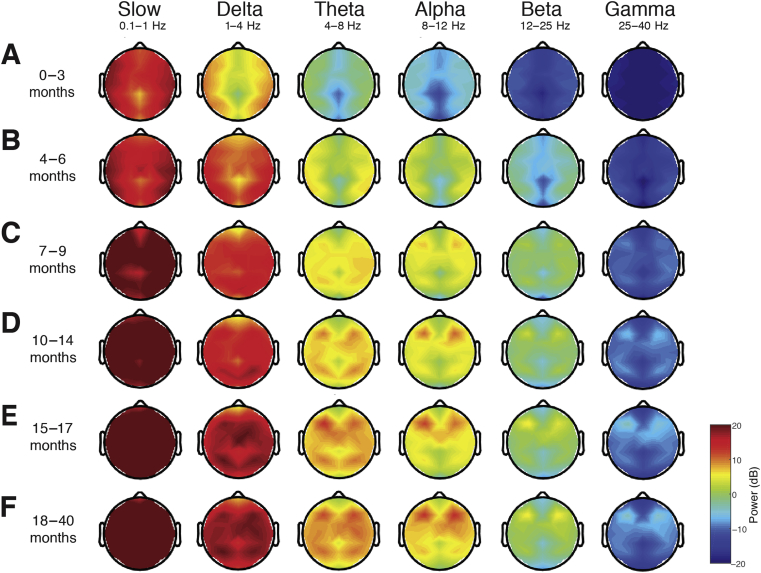
Topographic plots were computed for slow, delta, theta, alpha, beta, and gamma frequency bands in each age group (Fig. 2; individual infants are shown in Supplementary Fig. S3). For all age groups, slow, delta, and theta activities were generally widely distributed across the scalp. Alpha activity was present to a greater degree from around 4 months, and was largely regionalised to the frontal cortex from around 7–9 months old. Beta and gamma activities remained negligible across all ages.
Fig 2.
Topographic EEG maps of spectral power for distinct frequency bands during sevoflurane-maintained surgical anaesthesia in children 0–3 yr. Topographic EEG maps detailing group-averaged power for each EEG frequency band in infants aged (a) 0–3 months, (b) 4–6 months, (c) 7–9 months, (d) 10–14 months, (e) toddlers 15–17 months, and (f) 18–40 months. The slow-wave, delta, and theta activities are distributed across the scalp in all age groups. Alpha activity is present to a greater degree from around 4 months, and is largely regionalised to the frontal cortex from around 7 months of age. Beta and gamma oscillations remain negligible across all ages (See Supplementary Fig. S3 for spatial distribution of EEG spectral power for frequencies from 0.1 to 40 Hz during sevoflurane-maintained surgical anaesthesia in individual subjects.).
For infants 0–3 months, the spectral power along the midline (specifically the central–parietal locations) was lower across all frequencies. For subjects older than 3 months, the spectral power at the central–parietal and occipital midline was lower in the theta and alpha ranges. In all ages, slow and delta oscillations were broadly distributed across the scalp. Theta oscillations were also broadly distributed across the scalp and became more pronounced at around 10–14 months old. At 4–6 months old, alpha oscillations were broadly distributed across the scalp with lower power in the central–parietal and occipital midline. At 6 months, the peak alpha power was located over the frontal regions and expanded to include the central area with increasing age.
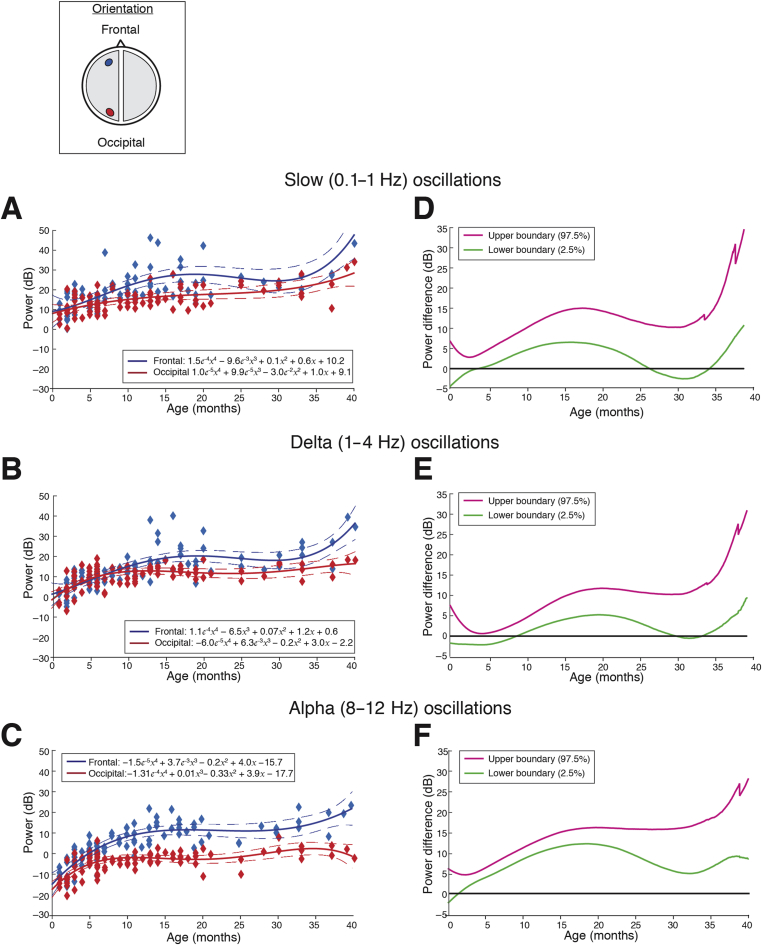
The frontal predominance of alpha power was evaluated by assessing the power spectral differences between the frontal (F3) and occipital (O1) electrodes across ages (Fig. 3). Frontal electrodes (F3 and F4) were identified as the locations with the greatest total power in the alpha frequency band from the topographic mapping, and were therefore used in this analysis. Frontal alpha oscillations were consistently greater in power compared with occipital oscillations from around 8 months and older. Slow and delta oscillations remained uniformly distributed over the frontal and occipital cortices. These data indicate that adultlike patterns of activity, anteriorisation of alpha oscillations, are detectable from late infancy onwards.
Fig 3.
Frontal and occipital power distribution at slow, delta, and alpha frequencies during sevoflurane-maintained surgical anaesthesia in children from 0 to 3 yr. Peak frontal and occipital EEG power in the (a) slow (0.1–1 Hz), (b) delta (1–4 Hz), and (c) alpha (8–12 Hz) frequency bands for each subject are plotted as a function of age. The solid line represents fourth-degree polynomial-regression model describing the relationship between age and EEG power (blue line, frontal power; red line, occipital power; dashed lines, 95th percentile confidence boundaries); the inset schematic indicates the location of the frontal and occipital channels. Differences in power between frontal and occipital channels in the (d) slow, (e) delta, and (f) alpha frequency bands are presented with 95% CI from bootstrap analysis (pink line, 97.5th percentile; green line, 2.5th percentile). Slow and delta oscillations exhibit a small and significant increase in frontal power compared with occipital power from mid-to late infancy until ∼26–30 months of age. Alpha oscillations emerge around 3–4 months of age and exhibit a significant and sustained frontal predominance of power that begins to emerge at 8 months of age and peaks at ∼15–20 months of age. These data indicate that adultlike patterns of activity are detectable from infancy. F3 and O1 electrodes are presented using nearest-neighbour Laplacian referencing. CI, confidence interval.
Age-varying changes in coherence properties
Frontal slow–delta coherence is present from birth to 3 months old; frontal alpha coherence emerges at 10 months
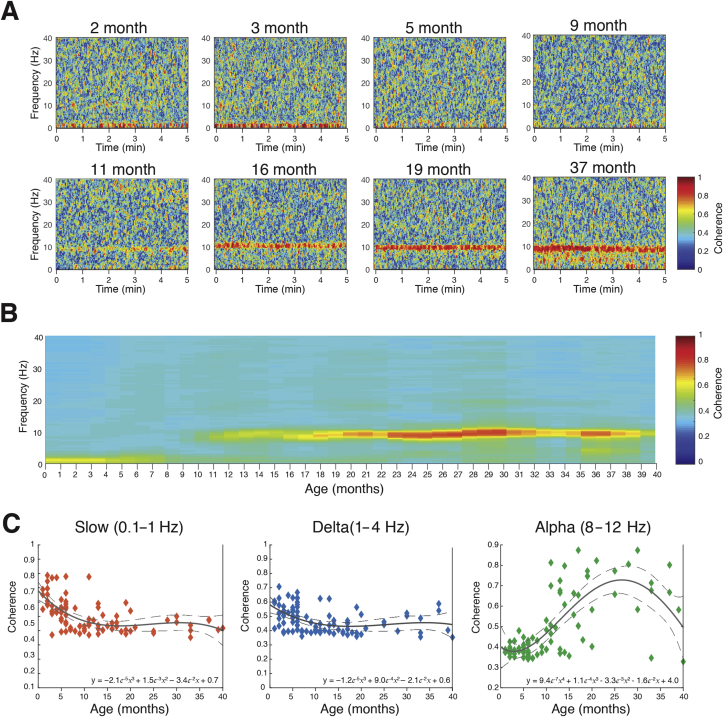
Frontal coherence—the level of local coordinated activity in the frontal regions of the scalp—was analysed by computing the coherogram (time-varying coherence) and coherence between the left (F7–FP1) and right (F8–FP2) electrodes. The frontal-coherence analysis in individual infants showed trends across age. At the youngest ages, 2 and 3 months old, slow and delta oscillations were highly coherent (>0.6). At 5 months old, slow and delta oscillations were less coherent, and at 9 months old were incoherent (uncoordinated). At 11 months old, alpha oscillations began to exhibit coherence. At 19 and 37 months old, slow and delta oscillations remained incoherent, and in contrast, alpha oscillations were highly coherent (Fig. 4a).
Fig 4.
Frontal coherograms and coherence during sevoflurane-maintained surgical anaesthesia in children from 0 to 3 yr. Frontal EEG coherence power for frequencies from 0.1 to 40 Hz during a 5 min period of surgical anaesthesia. (a) Frontal coherograms for individual subjects at 2, 3, 5, 9, 11, 16, 19, and 37 months of age; (b) age-varying coherence changes in frontal coherogram; and (c) frontal coherence in the slow (0.1–1 Hz), delta (1–4 Hz), and alpha (8–12 Hz) frequency bands for each subject. Slow and delta coherent oscillations are present in subjects from birth until around 8 months old. Alpha coherence appears to emerge around 10 months of age and gradually increases in 20 months where it persists to 3 yr of age. The solid line represents a third- or fourth-degree polynomial-regression model describing the relationship between age and EEG power; the dashed lines represent the 95th percentile confidence boundaries of the regression model (See Supplementary Fig. S4 for frontal-coherence properties during sevoflurane-maintained surgical anaesthesia across age groups.).
The age-varying frontal coherogram shows the distribution of slow–delta, theta, alpha, beta, and gamma oscillations (Fig. 4b). Slow and delta were dominant coherent frequencies from birth until around 8 months old, with the peak coherence in the first three months (∼0.6–0.7). Slow and delta oscillations were incoherent in children from 9 months and older. Despite alpha oscillations being present in the spectrogram from 3 to 4 months old, alpha oscillations were incoherent at this age and only became coherent at around 10 months old (at very low levels and with variability across subjects). Gradually, alpha-oscillation coherence increased until 20 months where it persisted through to 3 yr old. These results suggest that infants in the first 8 months of life have coordinated local frontal slow and delta oscillations, whilst from 10 months old the fine-tuning of coordinated alpha oscillations begins to emerge and is similar to the adult EEG.14 The age-group analysis of frontal group-median coherence computed shows the maturation of alpha oscillations with age (Supplementary Fig. S4).
Slow, delta, and alpha oscillations evolved in the degree of frontal coherence across age. Frontal slow–delta oscillations were highly coherent from birth and steadily decreased with age until around 8 months old (Fig. 4c). Frontal alpha oscillations were incoherent from birth until 10 months old (after appearing in the spectrogram at 3–4 months), and gradually increased coherence with increasing age (Fig. 4c).
Global coherence is weak across all frequencies in the first 9 months of age; frontal alpha coherence emerges at around 10 months old
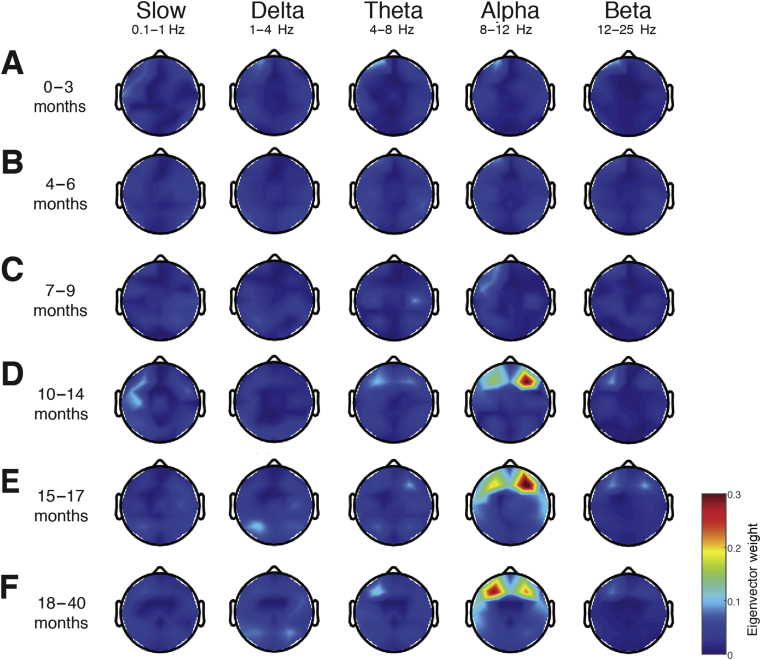
The global-coherence analysis characterises coordinated activity across multiple channels of EEG as a function of frequency. Despite frontally coherent slow–delta oscillations in infants from 0 to ∼8 months old, the global coherence was weak (Fig. 5a–c). In contrast, frontally coherent alpha oscillations were present in infants from 10 months old, with a global-coherence projection weight of around 0.3 (Fig. 5d). The global coherence in the slow and delta frequency ranges is consistent with the lack of slow oscillation coherence observed in adults.3, 4, 18 Furthermore, coherence in the alpha frequency range is similar to the highly coordinated, coherent frontal alpha activity observed in adults during propofol general anaesthesia.19
Fig 5.
Global coherence during sevoflurane-maintained surgical anaesthesia in children from 0 to 3 yr. Topographic EEG maps detailing group-averaged coherence for each EEG frequency band in children aged (a) 0–3 months, (b) 4–6 months, (c) 7–9 months, (d) 10–14 months, (e) 15–17 months, and (f) 18–40 months. Spatially coherent alpha oscillations that are concentrated in the frontal channels emerge at 10–14 months of age. Global coherence is low across all remaining frequencies and locations in all ages during surgical anaesthesia. This analysis suggests that global coherence becomes increasingly adultlike towards the end of the first year of life.
Discussion
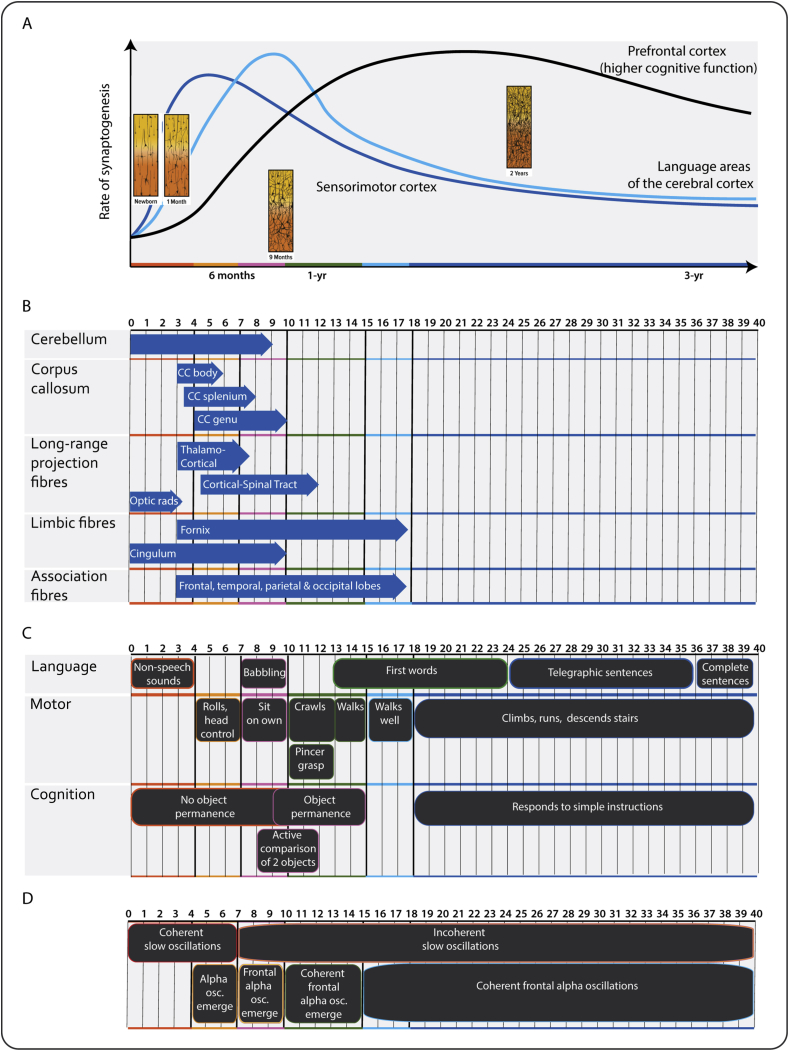
We found age-dependent changes in the spatial EEG during sevoflurane general anaesthesia. These included the following: (i) slow–delta oscillations were present in all ages; (ii) theta and alpha oscillations emerged around 4 months; (iii) alpha oscillations increased in power from 4 to 10 months, where they plateaued and remained at a sustained power level at older ages; (iv) frontal alpha-oscillation predominance emerged around 7 months; (v) frontal slow oscillations were coherent from birth until around 6 months; and (vi) frontal alpha oscillations became coherent around 10 months and persist in older ages. These age-related changes in the EEG likely relate to changes in neurodevelopmental processes that occur in early childhood, including synaptogenesis, axonal growth, neural pruning, myelination, and maturation of the thalamo-cortical and cortico-cortical functional connectivity20, 21, 22, 23 (Fig. 6). These unique data provide putative markers of postnatal maturation of functional activity in the human infant brain.
Fig 6.
Postnatal brain development in a human. (a) Rate of synaptogenesis in the prefrontal cortex (black), language areas of the cortex (light blue), and sensorimotor cortex (dark blue). Inset: schematic histology sections from the cortex illustrate a shift in synaptic and dendritic connections with age. (b) White-matter maturation (blue arrows indicate onset of mature myelination). Corpus callosum, thalamo-cortical, and associated fibres are the last regions to myelinate. (c) Neurodevelopmental milestones and (d) EEG features under sevoflurane maintenance anaesthesia. Thick solid black lines represent age grouping in the current study. Osc., oscillations. Adapted from Thompson and Nelson,21 Corel,22 Dubois and colleagues,23 and Frankenburg and colleagues.24
Studies of EEG dynamics in adults under general anaesthesia
Monitoring brain activity in response to general anaesthesia has been extensively characterised in adults. Specifically, the brain states induced by general anaesthetic drugs have been well studied through detailed characterisations of behaviour and consciousness at varying drug levels in adult human volunteers or in surgical patients.4, 5, 14, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 A dose-dependent effect on frontal predominance of alpha oscillations under deep sedation with sevoflurane occurs in healthy adult volunteers.25 More recently, a high-density EEG analysis demonstrated that the unconsciousness induced by sevoflurane or propofol is associated with a functional disconnection in the alpha oscillations between the anterior and posterior structures.3, 4, 30, 34 The anterior thalamo-cortical information processing is thought to play a major role in the generation of consciousness, and impairment of this processing might be an important neurophysiological mechanism of action of general anaesthetics.
Studies of EEG dynamics of the infant brain under general anaesthesia
We observed that anaesthesia-induced EEG oscillations change with brain development and become increasingly adultlike by the end of the first year of life. Age-related differences in EEG signatures have been shown in infants and toddlers. Davidson and colleagues7 monitored 8 leads of EEG in 17 infants (0–6 months), 19 toddlers (6 months–2 yr), and 21 children (2–10 yr) during emergence from anaesthesia. Power, limited to 2–20 Hz, was lower during anaesthesia in infants compared with older children, and power was similar during anaesthesia in toddlers and children. Hayashi and colleagues8 conducted a retrospective study on EEG data collected using a 2-lead EEG from 62 children ranging from 1 day to 2 yr old who received sevoflurane anaesthesia. The spectral edge frequency was low even under light anaesthesia, and changes with deeper anaesthesia were small in infants younger than 3 months old. Sury and colleagues9 studied 20 infants at 1 week to 10 months old during sevoflurane anaesthesia using a 4-lead EEG, and analysed the spectral power between 1 and 28 Hz. Infants 3 months and older had greater power in the 5–20 Hz range during maintenance compared with younger infants. Akeju and colleagues10 retrospectively characterised frontal EEG data power spectra and coherence using a 4-lead EEG montage (electrode positions: FP1, FP2, F7, and F8) in 54 subjects ranging in age from 0.4 to 28 yr (4 infants and 12 aged 1–6 yr old). Frontal slow oscillations were present from birth and frontal alpha oscillations were present after 1 yr. Slow frequencies were transiently coherent up to 1 yr old, whereas alpha oscillations were coherent around 1 yr and older.
Few studies have evaluated spatial changes in the EEG during anaesthesia administration in young children. Lo and colleagues35 characterised the spatial EEG patterns in 37 children, 22 days to 3.6 yr, who received sevoflurane or isoflurane anaesthesia using 128 leads. They found spatial differences in the power of EEG frequency bands—ranging from 1 to 50 Hz—over the frontal cortex compared with the occipital cortex at high concentrations of the two agents. However, none of the analyses were stratified by age.
We have previously reported spatial EEG changes in 36 infants, 0–6 months old, which indicate that slow oscillations were present and coherent in all infants, and alpha oscillations emerged around 3–4 months old and remained incoherent.6 The current report expanded on this age range to include an almost three-fold increase in patient numbers and developmental profile. The coherent frontal alpha oscillations seen in adults are thought to involve a gamma-aminobutyric acid (GABA)-associated thalamo-cortical oscillation.19 Therefore, we speculate that age-dependent changes in coherence might reflect functional changes in GABA-associated frontal thalamo-cortical circuits (Fig. 6).
Postnatal development of brain structure and function
The dramatic maturation of different cortical rhythms during the first year of life is likely caused by synaptogenesis, dendrite elaboration, myelination, white-matter tract development, and continued sub-plate growth. In this study, we observed that, at ages ≤3 months old, before the thalamo-cortical and cortico-thalamic connections are fully mature, slow oscillations were dominant. This is possibly caused by the decreased excitatory inputs from the thalamus and the brainstem to the cortex that result in an enhanced cortical pyramidal neurone hyperpolarisation.2 At 3–9 months, the thalamo-cortical and cortico-thalamic connections are weakly established with minimal to no coherence in the alpha range observed. At older ages, ≥10 months old, robust thalamo-cortical and cortico-thalamic connections are present and, consequently, highly coherent alpha oscillations are observed (Fig. 6).
Structural magnetic-resonance-imaging studies indicate that the corpus callosum, which serves to join the left and right hemispheres of the brain, is uniformly thin during the neonatal period, and undergoes rapid growth spurts from 3 months onwards until it begins to have an adultlike appearance by about 9 months old.36, 37 Myelin is already present during the first year in all associated regions, but maturation is ongoing—with the frontal cortex being one of the last to fully myelinate (Fig. 6).23
Clinical implications for brain monitoring under anaesthesia
Critical periods of brain development are strongly influenced by the maturation of GABAergic interneurones, and represent a major milestone in the development of neural circuits. Animal studies show these critical periods can be prematurely opened, kept open, or prematurely closed by exposure to GABA-modulating compounds, such as benzodiazepines.38 Considerably low alpha oscillatory power during general anaesthesia is reported in children with complex medical history or neurodevelopmental disorders, including preterm birth and autism spectrum disorder.39, 40 These spectral changes are likely caused by changes in thalamo-cortical circuits that drive alpha activity under anaesthesia.19
The results from animal studies suggest that sustained exposure to GABAA receptor agonists and N-methyl-d-aspartate receptor antagonists in the neonatal and infant periods accelerates neurodegenerative mechanisms, resulting in impaired learning and memory in later life.41 In human studies, the evidence is less clear and remains hotly debated, as accurate translation of animal behaviour to human outcomes is challenging.41 Some retrospective studies suggest that repeated exposure to general anaesthesia in early life (<2 yr) might increase the risk for adverse long-term neurodevelopment, whilst more recent prospective studies suggest otherwise.41
Our results encourage further examinations of large-scale functional brain network in ‘typically developing infants’ and in infants exposed to repeated anaesthesia or prolonged sedation, and in neurodevelopmental pathologies involving altered excitatory–inhibitory balance, such as epilepsy, Rett syndrome, or fragile X syndrome.
Study weaknesses
Anaesthetic management was administered according to the anaesthetist's discretion, rather than in a controlled, titrated fashion. The observed EEG features could be confounded by systematic age-related differences in drug administration. To prevent this, epochs were identified at a period of surgical anaesthesia and reflect a brain state consistent with anaesthesia-induced unconsciousness across the sample population. The spectral and coherence effects are unlikely to be the result of over-anaesthetising younger subjects, and are more likely age-related differences in underlying neurophysiology. Although challenging to pursue, there is a need for more detailed EEG studies featuring differing states of consciousness, controlled drug administration, and pharmacokinetic measurements in children.
Conclusions
The novelty of this study is in (i) enrolment of a large cohort of children (n=91) with high numbers at <1 yr old, (ii) multichannel EEG to analyse power and coherence properties over multiple brain regions (including the frontal, parietal, central, temporal, and occipital cortices), and (iii) application of advanced signal-processing approaches to reduce noise and enhance the resolution of the raw EEG. We show that general anaesthetic-induced brain oscillations might indicate cortical maturation and integration through neurophysiological signatures. The characterisation of functional brain networks in children requiring general anaesthesia demonstrates the importance of developing age-dependent strategies to monitor properly the brain states of children receiving anaesthesia. Furthermore, these data have the potential to guide the design of future studies investigating neurodevelopmental pathologies involving altered excitatory–inhibitory balance.
Authors' contributions
Study design: L.C., C.B.B.
Patient recruitment: L.C., J.M.L.
Data collection: L.C., J.M.L.
Computational analysis methodology: S.E.K., P.L.P., E.N.B.
Data analysis: S.E.K., L.C., J.M.L.
Writing of the original manuscript: L.C., P.L.P., C.B.B., S.E.K., E.N.B.
Equal contribution to this work: L.C., S.E.K.
Equal contribution to this work as senior authors: E.N.B., P.L.P., C.B.B.
Acknowledgements
The authors would like to thank the preoperative and operating-room staff at Boston Children's Hospital for their assistance, the families who took part in this study, and A.-M. Bergin of the Department of Neurology for reviewing all EEG recordings for potential incidental findings.
Handling editor: H.C. Hemmings Jr.
Editorial decision: January 30, 2018
Declarations of interest
E.N.B and P.L.P. have patents pending describing the use of EEG measures for brain monitoring during general anaesthesia and sedation, and a patent licensing agreement with Masimo Corporation (Switzerland). The application numbers are 2016–0331307, 2016-0324446, 2015-0080754, 2015-0011907, 2014-0323898, 2014-0323897, 2014-0316218, 2014-0316217, 2014-0187973, 2014-0180160, and 2008-0306397. All other authors declare that no competing interest exists.
Funding
The Boston Children's Hospital Anesthesia Research Distinguished Trailblazer Award to L.C.; the International Anesthesia Research Society Mentored Research Award to L.C.; Harvard Medical School, Scholars in Medicine Office and Harvard-MIT Health Sciences and Technology to J.M.L.; Massachusetts General Hospital to E.N.B. and P.L.P.; National Institutes of Health (R01-GM104948 and DP1-OD003646) to E.N.B. and (DP2-OD006454) to P.L.P.; the Sara Page Mayo Endowment for Pediatric Pain Research and Treatment to C.B.B.; and the National Research Foundation of Korea (NRF-2017R1C1B5017254) to S.K.
References
- 1.Brown E.N., Purdon P.L., Van Dort C.J. General anesthesia and altered states of arousal: a systems neuroscience analysis. Annu Rev Neurosci. 2011;34:601–628. doi: 10.1146/annurev-neuro-060909-153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Purdon P.L., Sampson A., Pavone K.J., Brown E.N. Clinical electroencephalography for anesthesiologists: part I: background and basic signatures. Anesthesiology. 2015;123:937–960. doi: 10.1097/ALN.0000000000000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cimenser A., Purdon P.L., Pierce E.T. Tracking brain states under general anesthesia by using global coherence analysis. Proc Natl Acad Sci USA. 2011;108:8832–8837. doi: 10.1073/pnas.1017041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Purdon P.L., Pierce E.T., Mukamel E.A. Electroencephalogram signatures of loss and recovery of consciousness from propofol. Proc Natl Acad Sci USA. 2013;110:E1142–E1151. doi: 10.1073/pnas.1221180110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaskell A.L., Hight D.F., Winders J. Frontal alpha-delta EEG does not preclude volitional response during anaesthesia: prospective cohort study of the isolated forearm technique. Br J Anaesth. 2017;119:664–673. doi: 10.1093/bja/aex170. [DOI] [PubMed] [Google Scholar]
- 6.Cornelissen L., Kim S.-E., Purdon P.L., Brown E.N., Berde C.B. Age-dependent electroencephalogram (EEG) patterns during sevoflurane general anesthesia in infants. eLife. 2015;4:e06513. doi: 10.7554/eLife.06513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson A.J., Sale S.M., Wong C. The electroencephalograph during anesthesia and emergence in infants and children. Paediatr Anaesth. 2008;18:60–70. doi: 10.1111/j.1460-9592.2007.02359.x. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi K., Shigemi K., Sawa T. Neonatal electroencephalography shows low sensitivity to anesthesia. Neurosci Lett. 2012;517:87–91. doi: 10.1016/j.neulet.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 9.Sury M.R.J., Worley A., Boyd S.G. Age-related changes in EEG power spectra in infants during sevoflurane wash-out. Br J Anaesth. 2014;112:686–694. doi: 10.1093/bja/aet409. [DOI] [PubMed] [Google Scholar]
- 10.Akeju O., Pavone K.J., Thum J.A. Age-dependency of sevoflurane-induced electroencephalogram dynamics in children. Br J Anaesth. 2015;115:i66–76. doi: 10.1093/bja/aev114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bokil H., Andrews P., Kulkarni J.E., Mehta S., Mitra P.P. Chronux: a platform for analyzing neural signals. J Neurosci Methods. 2010;192:146–151. doi: 10.1016/j.jneumeth.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim S.-E., Behr M.K., Ba D., Brown E.N. State-space multitaper time-frequency analysis. Proc Natl Acad Sci USA. 2018;115:E5–E14. doi: 10.1073/pnas.1702877115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delorme A., Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Akeju O., Westover M.B., Pavone K.J. Effects of sevoflurane and propofol on frontal electroencephalogram power and coherence. Anesthesiology. 2014;121:990–998. doi: 10.1097/ALN.0000000000000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2013. R: A language and environment for statistical computing. URL: http://www.R-project.org/ [Google Scholar]
- 16.Kirch C., Politis D.N. TFT-bootstrap: resampling time series in the frequency domain to obtain replicates in the time domain. Ann Stat. 2011;39:1427–1470. [Google Scholar]
- 17.Cornelissen L., Bergin A.M., Lobo K., Donado C., Soul J.S., Berde C.B. Electroencephalographic discontinuity during sevoflurane anesthesia in infants and children. Paediatr Anaesth. 2017;27:251–262. doi: 10.1111/pan.13061. [DOI] [PubMed] [Google Scholar]
- 18.Lewis L.D., Weiner V.S., Mukamel E.A. Rapid fragmentation of neuronal networks at the onset of propofol-induced unconsciousness. Proc Natl Acad Sci USA. 2012;109:E3377–E3386. doi: 10.1073/pnas.1210907109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ching S., Cimenser A., Purdon P.L., Brown E.N., Kopell N.J. Thalamocortical model for a propofol-induced alpha-rhythm associated with loss of consciousness. Proc Natl Acad Sci USA. 2010;107:22665–22670. doi: 10.1073/pnas.1017069108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verriotis M., Chang P., Fitzgerald M., Fabrizi L. The development of the nociceptive brain. Neuroscience. 2016;338:207–219. doi: 10.1016/j.neuroscience.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 21.Thompson R.A., Nelson C.A. Developmental science and the media. Early brain development. Am Psychol. 2001;56:5–15. doi: 10.1037/0003-066x.56.1.5. [DOI] [PubMed] [Google Scholar]
- 22.Corel J.L. Harvard University Press; Cambridge, MA: 1975. The Postnatal Development of the Human Cerebral cortex. [Google Scholar]
- 23.Dubois J., Dehaene-Lambertz G., Kulikova S., Poupon C., Hüppi P.S., Hertz-Pannier L. The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience. 2014;276C:48–71. doi: 10.1016/j.neuroscience.2013.12.044. [DOI] [PubMed] [Google Scholar]
- 24.Frankenburg W.K., Dodds J., Archer P., Shapiro H., Bresnick B. The denver II: a major revision and restandardization of the denver developmental screening test. Pediatrics. 1992;89:91–97. [PubMed] [Google Scholar]
- 25.Gugino L.D., Chabot R.J., Prichep L.S., John E.R., Formanek V., Aglio L.S. Quantitative EEG changes associated with loss and return of consciousness in healthy adult volunteers anaesthetized with propofol or sevoflurane. Br J Anaesth. 2001;87:421–428. doi: 10.1093/bja/87.3.421. [DOI] [PubMed] [Google Scholar]
- 26.Mhuircheartaigh R.N., Rosenorn-Lanng D., Wise R., Jbabdi S., Rogers R., Tracey I. Cortical and subcortical connectivity changes during decreasing levels of consciousness in humans: a functional magnetic resonance imaging study using propofol. J Neurosci. 2010;30:9095–9102. doi: 10.1523/JNEUROSCI.5516-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ku S.-W., Lee U., Noh G.-J., Jun I.-G., Mashour G.A. Preferential inhibition of frontal-to-parietal feedback connectivity is a neurophysiologic correlate of general anesthesia in surgical patients. PloS One. 2011;6:e25155. doi: 10.1371/journal.pone.0025155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mhuircheartaigh R.N., Warnaby C., Rogers R., Jbabdi S., Tracey I. Slow-wave activity saturation and thalamocortical isolation during propofol anesthesia in humans. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3006007. 208ra148. [DOI] [PubMed] [Google Scholar]
- 29.Barttfeld P., Bekinschtein T.A., Salles A. Factoring the brain signatures of anesthesia concentration and level of arousal across individuals. Neuroimage Clin. 2015;9:385–391. doi: 10.1016/j.nicl.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blain-Moraes S., Tarnal V., Vanini G. Neurophysiological correlates of sevoflurane-induced unconsciousness. Anesthesiology. 2015;122:307–316. doi: 10.1097/ALN.0000000000000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Purdon P.L., Pavone K.J., Akeju O. The ageing brain: age-dependent changes in the electroencephalogram during propofol and sevoflurane general anaesthesia. Br J Anaesth. 2015;115:i46–57. doi: 10.1093/bja/aev213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akeju O., Kim S.-E., Vazquez R. Spatiotemporal dynamics of dexmedetomidine-induced electroencephalogram oscillations. PloS One. 2016;11 doi: 10.1371/journal.pone.0163431. e0163431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hight D., Voss L.J., Garcia P.S., Sleigh J. Changes in alpha frequency and power of the electroencephalogram during volatile-based general anesthesia. Front Syst Neurosci. 2017;11:36. doi: 10.3389/fnsys.2017.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pavone K.J., Su L., Gao L. Lack of responsiveness during the onset and offset of sevoflurane anesthesia is associated with decreased awake-alpha oscillation power. Front Syst Neurosci. 2017;11:38. doi: 10.3389/fnsys.2017.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lo S.S., Sobol J.B., Mallavaram N. Anesthetic-specific electroencephalographic patterns during emergence from sevoflurane and isoflurane in infants and children. Paediatr Anaesth. 2009;19:1157–1165. doi: 10.1111/j.1460-9592.2009.03128.x. [DOI] [PubMed] [Google Scholar]
- 36.Barkovich A.J., Kjos B.O. Normal postnatal development of the corpus callosum as demonstrated by MR imaging. Am J Neuroradiol. 1988;9:487–491. [PMC free article] [PubMed] [Google Scholar]
- 37.Provenzale J.M., Isaacson J., Chen S. Progression of corpus callosum diffusion-tensor imaging values during a period of signal changes consistent with myelination. Am J Roentgenol. 2012;198:1403–1408. doi: 10.2214/AJR.11.7849. [DOI] [PubMed] [Google Scholar]
- 38.Hensch T.K. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 39.Poorun R., Hartley C., Goksan S. Electroencephalography during general anaesthesia differs between term-born and premature-born children. Clin Neurophysiol. 2016;127:1216–1222. doi: 10.1016/j.clinph.2015.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walsh E., Lee J., Terzakis K. 2016. Changes in the propofol-induced frontal electroencephalogram in children with autism spectrum disorder.http://www.asaabstracts.com/strands/asaabstracts/abstract.htm%20%20?year=2016&index=16&absnum=3810 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vutskits L., Xie Z. Lasting impact of general anaesthesia on the brain: mechanisms and relevance. Nat Rev Neurosci. 2016;17:705–717. doi: 10.1038/nrn.2016.128. [DOI] [PubMed] [Google Scholar]