Abstract
Myocardial infarction is the most prevalent of cardiovascular diseases and pharmacological interventions do not lead to restoration of the lost cardiomyocytes. Despite extensive stem cell therapy studies, clinical trials using cardiac progenitor cells have shown moderate results. Furthermore, differentiation of endogenous progenitors to mature cardiomyocytes is rarely reported.
A metabolic switch from glucose to fatty acid oxidation occurs during cardiac development and cardiomyocyte maturation, however in vitro differentiation protocols do not consider the lack of fatty acids in cell culture media. The aim of this study was to assess the effect of this metabolic switch on control and differentiated adult cardiac progenitors, by fatty acid supplementation.
Addition of oleic acid stimulated the peroxisome proliferator-activated receptor alpha pathway and led to maturation of the cardiac progenitors, both before and after transforming growth factor-beta 1 differentiation. Addition of oleic acid following differentiation increased expression of myosin heavy chain 7 and connexin 43. Also, total glycolytic metabolism increased, as did mitochondrial membrane potential and glucose and fatty acid transporter expression.
This work provides new insights into the importance of fatty acids, and of peroxisome proliferator-activated receptor alpha, in cardiac progenitor differentiation. Harnessing the oxidative metabolic switch induced maturation of differentiated endogenous stem cells.
(200 words)
Keywords: Cardiac stem cells, Differentiation, Metabolism, Oleic acid OA, Fatty acid oxidation, PPARα
Abbreviations: A.A, Ascorbic Acid; BSA, Bovine Serum Albumin; CKIT, proto-oncogene Ckit / tyrosine-protein kinase Kit (CD117); CDM, Cardiac Differentiation Medium; CPC, Cardiac Progenitor Cell; CX43, Connexin 43; DIFF, Differentiation samples; DIFF + OA, Differentiation samples + Oleic Acid; DMSO, DiMethylSulfOxide; EB, Embryoid Body; FA, Fatty Acid; FBS, Fetal Bovine Serum; FLK1, Fetal Liver Kinase 1 / (KDR) (Type III Receptor Tyrosine Kinase); HF, Heart Failure; HRPT, Hypoxanthine-guanine phosphoribosyltransferase; GATA4, GATA binding protein 4; GLUT1, Glucose transporter type 1; GLUT4, Glucose transporter type 4; ISL-1, ISL1 transcription factor/ (Insulin gene enhancer protein – Islet1); mESC, mouse Embryonic Stem Cells; MEF2C, Myocyte Enhancer Factor 2C; MI, Myocardial Infarction; MYH7, myosin, heavy chain 7, cardiac muscle, beta; NKX2.5, NK2 homeobox 5; OA, Oleic Acid (unsaturated fatty acid); OCT-3/4, Octamer-binding Transcription factor 4 / (POU5F1); Px, Passage x (x = number); PDK4, Pyruvate Dehydrogenase Kinase-4; PGC-1Α, Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1; PFA, Paraformaldehyde; PPARΑ, Peroxisome Proliferator Activated Receptor Alpha; RXR, Retinoid X Receptor; SCA1, Stem cells antigen-1; SDHA, Succinate Dehydrogenase complex, subunit A; TERT, telomerase reverse transcriptase; TGF-β1, Transforming Growth Factor Beta 1; TNNT2, (Cardiac) Troponin T2
Graphical abstract
Highlights
-
•
Culture of CPCs with oleic acid (OA) upregulates glycolysis and glucose oxidation.
-
•
TGF-β differentiation of CPCs increases glycolytic flux.
-
•
OA increases glycolysis, glucose and fatty acid oxidations of differentiated CPCs.
-
•
The PPARα /PGC1α axis gets upregulated after oleic acid supplementation.
1. Introduction
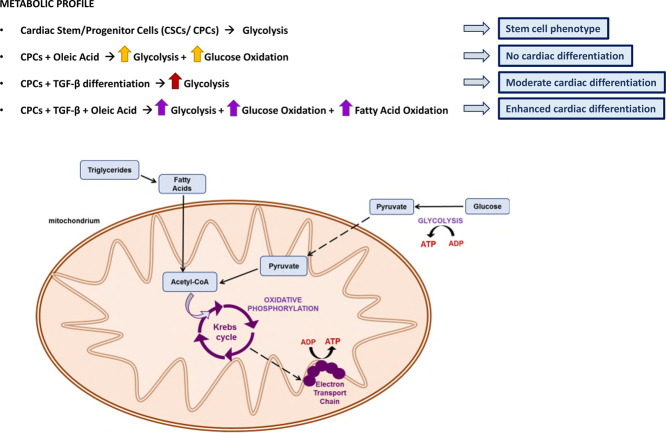
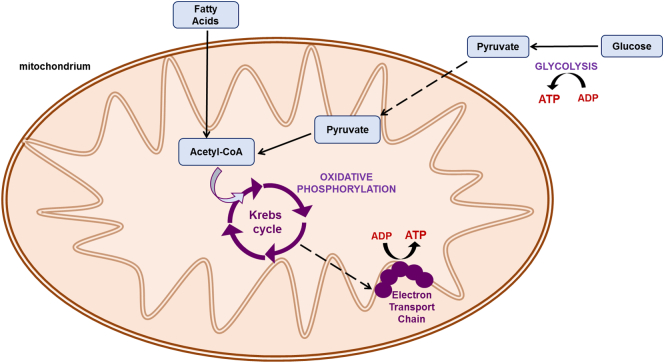
The heart uses a variety of substrates for energy production, while an array of metabolic networks guides adenosine triphosphate (ATP) production rates (Evans and Heather, 2016),(Taegtmeyer et al., 2016). ATP can be produced via glycolysis (non-oxidative breakdown of glucose) (DeBerardinis et al., 2007), alternatively, pyruvate can enter the mitochondria and be metabolised via oxidative phosphorylation (Madeira, 2012) (Fig. S1).
Fig. S1.
Schematic of basic cell metabolic pathways for energy production.
A well-studied metabolic switch characterises the transition from cardiac stem cells to cardiomyocytes (CMs), highlighted by a shift from anaerobic to oxidative metabolism (Kolwicz et al., 2013), which allows for the increased yield of ATP to sustain contraction (Schönfeld et al., 1996). Another metabolic shift is also experienced by cardiac progenitor cells (CPCs) during transplantation in vivo, since CPCs are transferred from the culture medium, which can contain between 5.5 mM – 25 mM glucose and no fatty acids (FAs), to substrates in plasma that vary substantially. In mice, glucose levels have been measured between ~3.4–9.6 mM (Wolfensohn and Lloyd, 2003), while FAs in the range of ~0.18–0.6 mM (Perbellini et al., 2014).
In addition, both oleic acid (OA) and Transforming Growth Factor-Beta 1 (TGF-β1) are naturally found in the body, so in the context of cell therapy it is necessary to understand their effect on the transplanted cells. FAs (including oleic, palmitic, linoleic, and arachidonic acid) are natural ligands for Peroxisome Proliferator-Activated Receptor α (PPARα) (Göttlicher et al., 1992). The PPARα signalling pathway comprise a group of nuclear receptor proteins (Nuclear Receptors Nomenclature, C, 1999), and their target genes are involved in fatty acid (Gulick et al., 1994) and glucose metabolism (Finck et al., 2002). Ding et al. showed that PPARα levels were upregulated during differentiation of Embryonic Stem Cells (ESCs) to beating CMs, and that PPARα inhibition prevented differentiation (Ding et al., 2007a). Furthermore, PPAR co-activator 1α (PGC-1α) (Sanderson et al., 2009), is involved in metabolic processes (Handschin and Spiegelman, 2006) including regulation of genes involved in FA oxidation (Vega et al., 2000). Interestingly, a study conducted in 2014 on the HepG2 hepatic cell line, showed that not all FAs have the same binding affinity to PPAR-alpha and therefore, do not necessarily result in its activation. While OA and palmitoleic acid increased PPRAα transactivation, palmitate and stearic acid inhibited it (Popeijus et al., 2014).
Various strategies have been applied to develop the optimal protocol for in vitro cardiac differentiation of stem cells (Malandraki-Miller et al., 2018). Goumans et al. used TGF-β1 for differentiation of human adult atrial stem cell antigen-1-positive (Sca1+) CPCs (Goumans et al., 2008; Smits et al., 2009).
Recently, there have been studies exploring metabolic manipulation and the subsequent effect on cell differentiation and maturation, in hPSC-CMs. In 2017, Correia et al. investigated how different substrates affect the functional maturation of hPSC-CMs (Correia et al., 2017). By testing different media compositions, they demonstrate that culture medium supplemented with fatty acids and galactose, lacking glucose, forced the cells to shift to oxidative phosphorylation. That resulted in a phenotype more similar to human adult CMs than previously reported. In addition, Hu et al. investigated the mechanism that links metabolism and the maturation of hPSC-CMs, showing that reliance on glucose and the HIF1α-LDHA axis are involved in the immature phenotype of hPSC-CMs (Hu et al., 2018). They afterwards proved that metabolic shift towards oxidative metabolism led to maturation of the hPSC-CMs. Hitherto, to the best of our knowledge, no cardiac differentiation protocol has investigated the effect of FA supplementation on adult CPCs' metabolism and differentiation.
On the basis of the link between metabolic state and cell phenotype, we suggest that manipulating substrate availability in vitro could trigger differentiation of CPCs. In addition, the different layers of regulation that PPARα and PGC-1α exert on differentiation and metabolism lead us to hypothesise that stimulating this axis could enhance cell differentiation. Here we reveal the effect of fatty acid (OA) availability, in culture, on the metabolic maturation of adult CPCs. We also elucidate the effect that OA has on control and differentiated CPCs.
2. Materials & methods
2.1. Animals
Male C57BL/6 mice (Harlan, Oxon, UK) were housed in a 12-h light–dark cycle, controlled temperature and humidity, with water and food ad libitum. All animal procedures were reviewed and approved by the University of Oxford Animal Welfare and Ethical Review Board and conforms to the Animals (Scientific Procedures) Act 1986 incorporating Directive 2010/63/EU of the European Parliament.
2.2. Isolation and expansion of mouse cardiac progenitor cells (CPCS)
Mouse atria were excised, washed with Dulbecco's phosphate buffered saline (DPBS) (Invitrogen, Fisher Scientific – UK Ltd) containing Penicillin / Streptomycin (P/S) and minced mechanically. The tissue-pieces were transferred in 0.5 ml of the digestion solution (comprising of 0.1% trypsin and 0.1% Collagenase II (Calbiochem, 286 U/ mg), incubated in a shaking water bath at 37 °C for 1 h. Every 10′ the digestion-mix was mechanically triturated, the supernatant was collected, neutralized with CPC medium and plated on fibronectin-coated 6-well plates. CPC medium comprised of Iscove's modified Dulbecco's medium (IMDM) (Invitrogen, Fisher Scientific – UK Ltd) with 20% foetal bovine serum (FBS) (Invitrogen, Fisher Scientific – UK Ltd), 1 U/ ml penicillin, 1 μg/ ml streptomycin and 0.2 mM l-glutamine (P/S/G), (Gibco, Life Technologies, Fisher Scientific – UK Ltd). Fresh digestion solution was added to the remaining tissue pieces as before, until 1 h was reached. This digestion produced surviving fibroblast-like cells, named “CPCs”, attaching after 2 days. The CPCs were passaged when they reached 90% confluency, for further experiments and analysis.
2.3. RNA extraction, reverse transcription &qPCR reaction
Total RNA was extracted from frozen cell pellets, using the RNeasy Mini Kit (QIAGEN Ltd. – Manchester UK), following the manufacturer's protocol. Complementary DNA was synthesized, using the High Capacity cDNA Reverse Transcription kit (Life Technologies, Fisher Scientific – UK Ltd), following the manufacturer's protocol. cDNA aliquots were kept at −20 °C for further use. qPCR was performed using the StepOnePlus Real-Time PCR System (Applied Biosystems, Life Technologies, Fisher Scientific – UK Ltd). Relative mRNA levels were normalised to Sdha and Hrpt housekeeping genes. As detection system for the reaction SYBR green fluorescent intercalating dye was used (SYBR Green PCR mastermix, Life Technologies, Fisher Scientific – UK Ltd). For primer information see Supplementary material. Data was analysed using the published ΔΔCt method Livak method (Livak and Schmittgen, 2001), plotting the data as 2−ΔΔCt±SE.
2.4. Immunocytochemistry
CPCs P4 were seeded in 24-well plates, on fibronectin-coated cover slips (2 × 104 cells / well) and were fixed with 4% paraformaldehyde for 30′ at 4 °C. For intracellular proteins, cells were permeabilised with 0.2% Triton X for 10′. Then cells were blocked with a 2% FBS and 2% bovine serum albumin (BSA, Sigma-Aldrich Ltd. – Dorset, UK) solution for 30′. Primary antibody staining followed overnight at 4 °C. After washing with PBS, when required, cells were labelled with secondary antibody (30′, room temperature). Afterwards samples were co-stained with 0,1% 4′,6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich Ltd. – Dorset, UK), and mounted on glass slides using a 50% PBS and 50% Glycerol (Fisher Scientific – UK Ltd) solution. Slides were kept at 4 °C protected from light, until imaging. Immunostaining was assessed using light confocal microscopy (Inverted Olympus Fluoview FV1000 Confocal system). For image analysis FIJI software (“Fiji Is Just ImageJ”) was used. Positive staining was assessed compared to a negative control; either cells stained only with 4′,6-diamidino-2-phenylindole (DAPI) or with just the secondary antibody and DAPI. For antibody information see Supplemental material.
2.5. Mitochondrial staining
For mitochondrial imaging, the MitoTracker® Red CMXRos (Fisher Scientific – UK Ltd) intracellular dye was used, following manufacturer's protocol. CPCs at P4 were incubated for 40’with 10 nM MitoTracker in non-FBS medium. After washing with DPBS, they were fixed with 4% paraformaldehyde and imaged using light confocal microscopy. The average fluorescence intensity was analysed using the FIIJI image analysis software.
2.6. Mouse-esc differentiation – Embryoid bodies
The differentiated mouse ESCs, which were used as a positive control for comparison with differentiated CPCs in RT-PCR experiments, were donated by Dr. Richard Tyser (Prof. Paul Riley group, DPAG, Oxford). The samples were collected either at day 4, day 7,or day 14 of differentiation. Differentiation was induced using the hanging drop culture – embryoid body formation method (Boheler et al., 2002) (EBs d4, EBs d7, EBs d14).
2.7. TGF-β1 differentiation
Cardiac differentiation was induced using TGF-β1 (Smits et al., 2009). CPCs were seeded in CPC medium on gelatine-coated flasks 0,1% (Sigma-Aldrich Ltd. – Dorset, UK); at a density of 25 × 104 cells per 25 cm2 flask or at 2 × 104 cells per well in a 24-well plate. The next day, the medium was replaced with Cardiac Differentiation Medium (CDM) (47% IMDM, 47% Ham's F12 – GlutaMAX-I, 2% Horse serum, 1% MEM non-essential amino acids, 1% Insulin-Transferrin-Selenium, 2% Pen/Strep, containing 5 μM 5-Azacytidine. 5-Azacytidine was also added at the next 2 days, and then the medium was refreshed. From day 6 forward, 1 mM Ascorbic Acid (AA) was added every 2 days and 1 ng/ ml TGF-β1 twice weekly, while medium was refreshed every 2 days. The cells were collected after 25 days of differentiation for analysis. Undifferentiated, but over-confluent, cells were used as a control.
2.8. Oleic acid supplementation
CPCs P4 were seeded in 6-well plated for either 48 h, 1 week or 1 month, with the CDM basal medium (without any drug addition) supplemented with 75 μM, 150 μM and 300 μM OA conjugated to BSA. As a control, BSA supplemented CDM was used. The media was replenished every 2 days in all conditions.
Following the 25-day TGF-β1 differentiation, as described above, the differentiating cells were cultured in OA 300 μM-supplemented CDM, for either 1 week or 1 month.
2.9. 14C-glucose oxidation measurement
Glucose oxidation was measured using the method of the Collins et al. (Wolfensohn and Lloyd, 2003) with some modifications. CPCs were seeded on gelatine-coated 24-well plates (20 × 104 cells/ well) and were grown in basal CDM medium as untreated “control” condition (CNTRL), differentiated or treated with OA for 48 h or 1 week. Subsequently, they were incubated for 4 h in no-glucose DMEM (A14430, Gibco, Life Technologies, Fisher Scientific – UK Ltd) supplemented with 12 mM glucose containing 0.185 MBq D-U-14C-glucose (0.5 ml/ well). DMEM-alone was used as a negative CNTRL and wells without cells containing DMEM with 14C-glucose were used to measure background radioactivity. A 24-well plate formed the lid of the apparatus with a perforated rubber gasket, with holes corresponding to each well, separating the two plates. The 14CO2 produced by the glucose oxidation was trapped on KOH-soaked filter papers in the upper wells. To trigger the release of the 14CO2, perchloric acid was added to the well, at each desired time-point, to kill the cells. Subsequently, the samples were kept for 1 h to allow for the release of dissolved 14CO2. Filter papers containing trapped 14CO2 were analysed using a scintillation counter, and the radiation counts per minute (CPM) were measured.
2.10. 3H palmitate oxidation
CPCs were seeded in gelatine-coated 24-well plates (20 × 104 cells/ well) and were grown in basal CDM medium, as untreated CNTRL, differentiated or treated with OA for 48 h and 1 week. Subsequently the cells were incubated in no-glucose DMEM medium that was supplemented with 12 mM glucose, 300 μM palmitate, conjugated to 20 μM albumin and 0.022 MBq 3H-palmitate for 6 h. Palmitate oxidation rates were determined by the production of 3H2O from the mitochondria (Barr and Lopaschuk, 1997; Belke et al., 1999). Perfusate aliquots contained both 3H2O and 3H-palmitate, so the 3H2O was separated via Folch extraction. 1.88 ml of chloroform:methanol (1:2 v/v) solution, 625 μl chloroform and 625 μl KCl-HCl solution (2 M KCl, 0.4 M HCl) were added to 0.5 ml of the perfusate sample. The solution was rotated on a laboratory Stuart rotator SB3 at 40 rpm for 1 h before removing the top aqueous layer, while the organic layer was discarded. A solution of 1 ml choloform, 1 ml methanol and 0.9 ml KCl-HCl was added to the extracted aqueous solution, before further rotation for 1 h at 40 rpm. The top aqueous layer was once again removed and retained, and radioactivity was counted in counts per minute (CPM) using a scintillation counter. 0.5 ml medium at time 0 was used as a control to determine the specific activity of the buffer. For each aliquot sample, palmitate oxidation rates were calculated (Barr and Lopaschuk, 1997).
2.11. 3H glucose measurement of glycolytic flux
CPCs were seeded in gelatine-coated 24-well plates (20 × 104 cells/ well) and were grown in in basal CDM medium, as an untreated CNTRL, differentiated or treated with OA for 48 h and 1 week. Subsequently, they were incubated for 6 h in no-glucose basal DMEM supplemented with 12 mM glucose containing 0.118 MBq 3H-glucose. Glycolytic rates were determined through the conversion of 3H -glucose to 3H2O via enolase which converts 2-phosphoglycerate to phosphoenolpyruvate and releases H2O as a by-product. The samples contained both 3H2O and 3H-glucose. Therefore, the 3H2O was separated from the 3H-glucose using a Dowex 1 × 4 chloride form 100–200 mesh (Sigma, UK) anion exchange column. 250 g of Dowex resin was added to a 1.25 M NaOH and 1.61 M boric acid solution, and washed with distilled H2O until pH < 7.5. Dowex resin was added to glass Pasteur pipettes plugged with glass wool and the columns were washed with distilled H2O and allowed to drain. Then 200 μl of sample was added to each column allowing for the 3H -glucose to bind to the column for 15′, and 3H2O to be eluted into scintillation vials. Distilled H2O (2 ml) was added into the column to wash down any residual samples. Sample radioactivity was counted in counts per minute (CPM) using a scintillation counter.
2.12. Cell viability
To assess cell viability after Oleic Acid supplementation in the cell medium, treated CPCs were washed with PBS, trypsinised, and then counted using trypan blue stain, using both the standard haemocytometer approach and a Countess Automated Cell Counter (Invitrogen, Fisher Scientific – UK Ltd).
2.13. Statistical analysis
For all experiments (except for the case of mESCs) the ‘n’ number refers to biological replicates, that represent cells originating from different mice, and each tested once. The ‘n’ number for the EB mESCs represents technical replicates, of the donated cell line samples. Results are presented as means ±SE for qPCR and metabolic flux analyses, and means ±SD for other analyses. Differences were considered significant at p < .05, determined using analysis of variance with a Student's t-test. Experiments with >2 test groups were assessed by a one-way analysis of variance (ANOVA) with a Tukey post-hoc test (GraphPad Prism 7).
3. Results
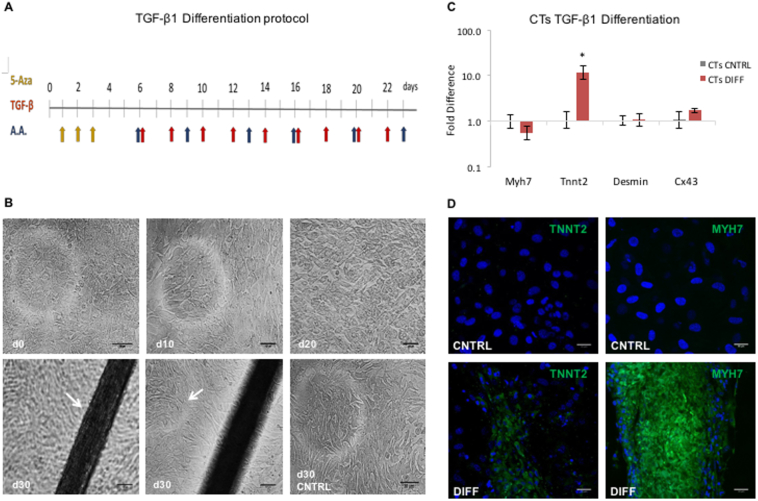
3.1. TGF-β1 differentiation increases expression of TNNT2 in mouse adult CPCs
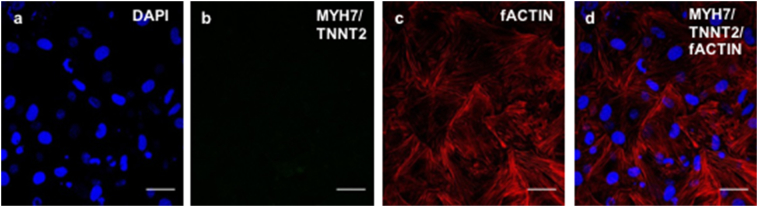
To assess the differentiation potential of the CPCs (for CPC characterisation profile see Supplementary Material and Fig. S2), the TGF-β1/5-Azacytidine/Ascorbic Acid (A.A.) protocol was used (Fig. 1A). The differentiating CPCs (“DIFF”) were monitored throughout, and at day 30 formed tubular structures (Fig. 1B). Gene expression analysis via qPCR showed a significant increase in cardiac troponin-T 2 (TnnT2) levels (Fig. 1C). The presence of TNNT2 and myosin heavy chain β (MYH7) was confirmed by immunocytochemistry, with the latter being confined to the organoids (Fig. 1D).
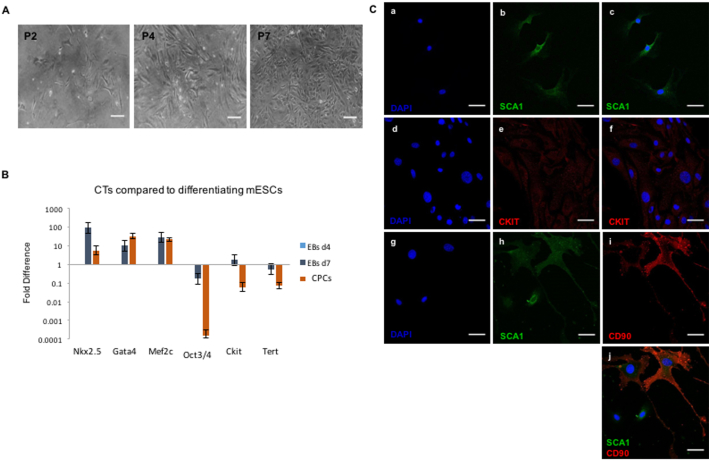
Fig. S2.
Characterisation of CPC cells. A) CPCs under phase contrast microscope, as observed at (a) p2, (b) p4, and (c) p7. (scale bars 100 μm). B) Gene expression levels of stemness and cardiac lineage markers in CPCs at P4, compared to mESCs differentiating to CMs at d4 and d7 of differentiation (n = 3). C) Immunocytochemistry on CPCs, (a-c) SCA1 (d-f), CKIT, (g-i) CD90/SCA1 double staining, with DAPI (a, d, g), (k) secondary antibodies for negative control. Scale bars: 50 μm.
Fig. 1.
Differentiation of CPCs: (A) Schematic representation of the timeline of the TGF-β1 differentiation protocol; yellow arrows mark the days when 5-Aza was added, blue is for Ascorbic Acid and red for TGF-β1. (B) Cell morphology of differentiating CPCs, at day 0, 10, 20 and 30, under light microscope. Arrow at d30 left image: indicating the tubular structure, at d30 right image: indicating the cell layer underneath the tubular structure (scale bars: 100 μm). (C) qPCR of differentiating CPCs normalised to the respective undifferentiated control samples (n = 4, *p < .002, error bars: standard error). (D) Differentiated CPCs, expression of MYH7 and TNNT2 shown in green; a,b: control samples, c,d: differentiated samples. (blue: DAPI, scale bars: 30 μm). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Stages of mESC differentiation VIA embryoid body formation
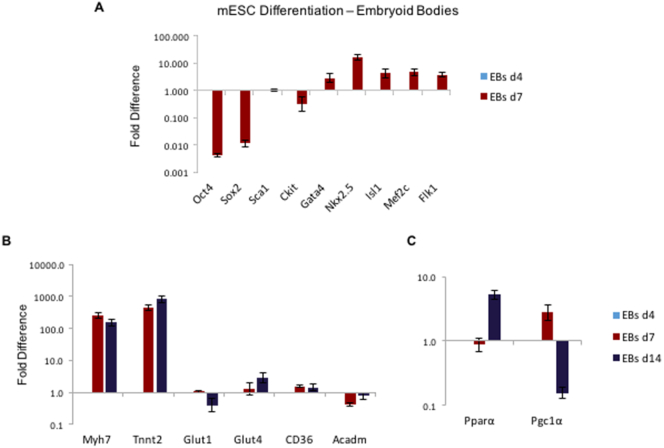
To characterise the metabolic changes we should expect to see during in vitro differentiation, a differentiating mESC line was used as a positive control. Agreeing with the anticipated genetic changes, the stemness genes (Oct4, Sox2, Sca1, Ckit) showed reduced expression as mESCs progressed from day 4 (EBs d4) to day 7 (EBs d7) of differentiation, while the early-onset cardiac markers (Gata4, Mef2c, Nkx2.5, Isl1) increased, as did the endothelial marker Flk1 (Fig. 2A). The gene expression of late-onset cardiac markers (Tnnt2 and Myh7) increased from day 4, through day 7 to day 14 (EBs d14) of differentiation. Expression of the insulin-sensitive glucose transporter Glut4 and the fatty acid transporter CD36 increased, while that of the glucose transporter Glut1, predominant at the fetal stage, was reduced (Fig. 2B). PPARα expression was unchanged from day 4 to day 7 of embryoid body differentiation and then showed an 8-fold increase. Pgc-1α was upregulated on day 7 and then downregulated when the cells were terminally differentiated (Fig. 2C).
Fig. 2.
Gene expression in differentiating ESCs: (A) Gene expression levels of stemness markers (Oct4, Sox2, Sca1, Ckit), early-onset cardiac (Gata4, Mef2c, Nkx2.5, Isl1) and endothelial (Flk1) markers in mESCs at d4 and d7 of differentiation of ESCs to CMs, normalised to EBs d4. (B) Gene expression levels of late-onset cardiac markers (TnnT2 and Myh7) and Glut1, Glut4 in mESCs at d4, d7 and d14 of differentiation to CMs, normalised to EBs d4. (C) Gene expression levels of PPARα and Pgc1α in mESCs at d4, d7 and d14 of differentiation to CMs, normalised to EBs d4 (n = 3).
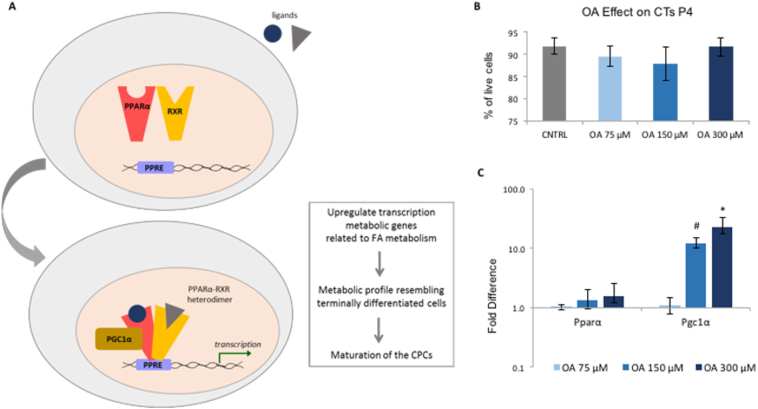
3.3. Oleic acid supplementation stimulates the PPARα pathway in mouse adult CPCs
To investigate the effect of FAs as a cell culture substrate, the CPC culture medium was supplemented with OA. The effect of 75 μM, 150 μM or 300 μM of OA on cell viability was checked and was not found to be toxic (Fig. 3B). 300 μM was selected as the highest dose because high levels of FA have been shown to be cytotoxic (De Sousa Andrade et al., 2005; Haeiwa et al., 2014). PGC-1α expression was significantly upregulated as the concentration of OA increased, but PPARα expression did not change (Fig. 3C). Since the OA supplement was pre-conjugated with BSA, the gene expression of PPARα and PGC-1α was also compared to the untreated controls which had been supplemented with BSA, at the respective concentrations. Both PPARα and PGC-1α expression increased >5-fold with 300 μM of OA, compared to the BSA controls (Supplementary Fig. S3).
Fig. 3.
OA stimulation of the PPARα pathway: (A) Schematic representation of the PPARα/PGC-1α axis: PPARα binds to RXR, the heterodimer is activated by ligands and PGC-1α acts as a co-activator for PPARα. The heterotrimer binds to the PPARα response element (PPRE), in the promoter of target genes. (B) The effect of 75 μM, 150 μM or 300 μM of OA on cell viability after 1 week of incubation. (C) Effect of 48 h supplementation with OA at increased concentrations. Effect of OA 75 μM, 150 μM, 300 μM on the expression of PPARα, PGC-1α in P4 CPCs, normalised to OA 75 μM. (n = 4, *p < .02, #p < .01, indicating difference to OA 75 μΜ; error bars: standard error).
Fig. S3.
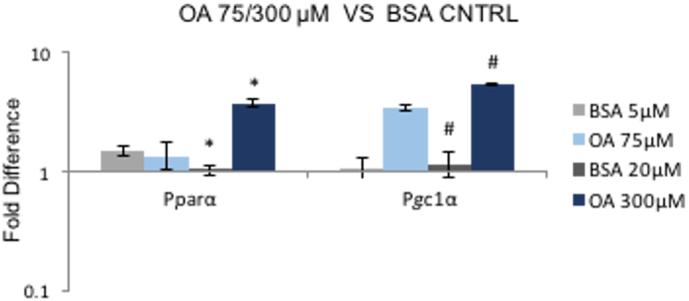
OA effect on PPARα / PGC-1α compared to the respective BSA control. Effect of 48 h supplementation with OA 75 μM and 300 μM on the expression of PPARα, Pgc1α, on CPCs P4, normalised to 48 h BSA supplementation with 5 μM and 20 μM, respectively. (n = 4; error bars: standard error, *p < .04, #p < .03, indicating difference to the respective BSA CNTRLs). Table showing the corresponding BSA concentrations for eack OA concentration, respectively.
3.4. Oleic acid supplementation metabolically matures mouse adult CPCs
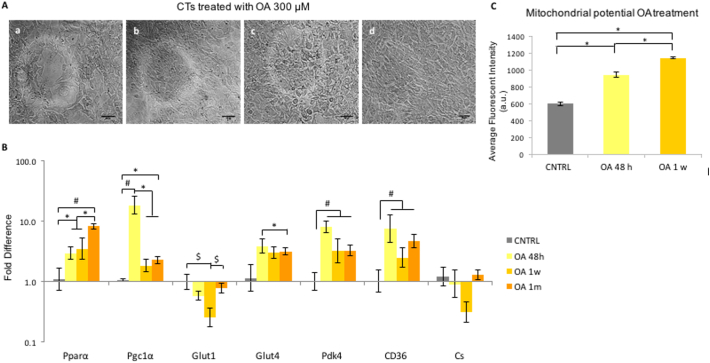
To test the long-term effect of OA treatment on the metabolism of CPCs, gene expression levels were investigated after treatment of CPCs with OA 300 μM for 48 h, 1 week and 1 month. The CPC morphology was observed for whole period, during which cells kept proliferating and no cell death was observed (Fig. 4A).
Fig. 4.
Long-term stimulation with OA: (A) P4 CPCs treated with OA for 48 h (4a), 1 week (4b) and 1 month (4c), as well as untreated CPCs grown for 1 month (scale bars: 100 μm). (B) Effect of 48 h, 1 week, 1 month supplementation of P4 CPCs with OA on the expression of metabolic genes, normalised to control untreated CPCs. (n = 3; error bars: standard error, *p < .05, #p < .02). (C) Effect of OA treatment for 48 h and 1 week, estimated as average fluorescent intensity, after staining with MitoTracker Red CMXRos red. *p < 0,00001 normalised to untreated CNTRL, and OA 48-h treatment, respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
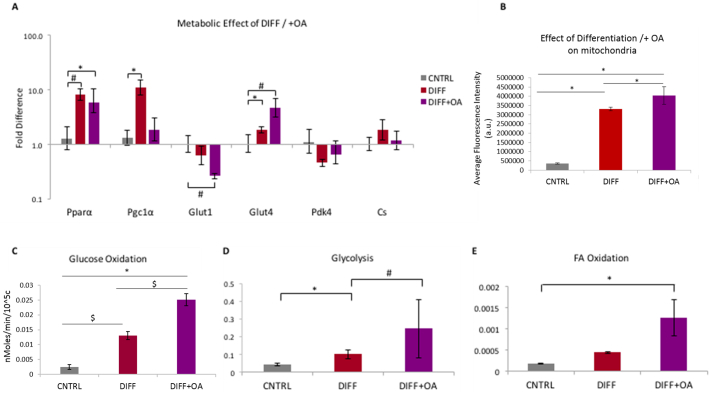
PGC-1α gene expression was significantly upregulated after 48 h and then decreased (Fig. 4B). PPARα and Glut4 were also significantly upregulated with OA supplementation (Fig. 4B). Pdk4 significantly increased with OA addition at all time-points, as did the fatty acid translocase receptor CD36, which functions as an OA receptor (Fig. 4B). No change in gene expression of citrate synthase (Cs) was observed (Fig. 4B).
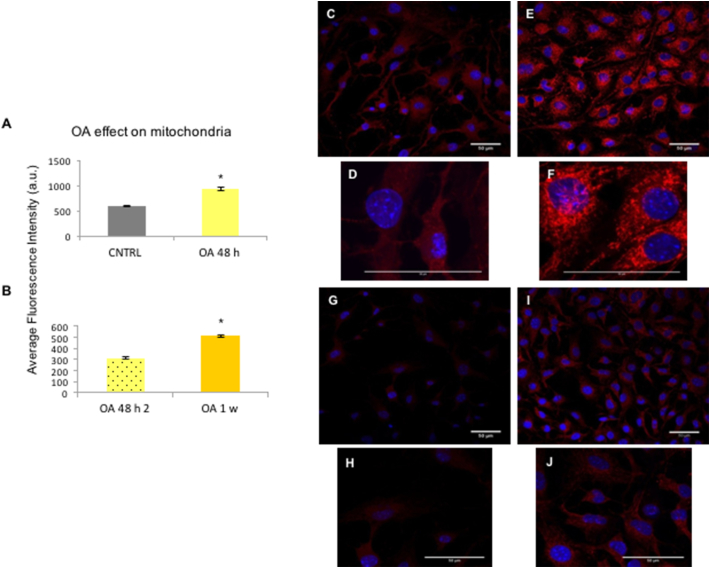
To investigate mitochondrial activity, CPCs were incubated with MitoTracker® Red CMXRos. CNTRL untreated CPCs were compared to 48 h of OA treatment, and 1 week of OA supplementation (Fig. 4C). A significant increase in fluorescence was observed following prolongation of the OA treatment (Fig. 4C, Supplemental Fig. S4), suggesting that the mitochondria were more active, since the CMXRos dye accumulates in live cells based on the mitochondrial membrane potential (Cottet-Rousselle et al., 2011).
Fig. S4.
Mitochondrial staining after OA treatment: Short-term effect of OA in cell mitochondria. Two separate experiments were conducted; average fluorescence intensity of untreated control CPCs P4, compared to CPCs P4 treated with OA 300 μΜ for 48 h (A), and compared to CPCs treated for 1 week (B). Raw data; untreated CNTRL (C,D) and treated with OA 300 μΜ for 48 h (E,F left), stained with MitoTracker® Red CMXRos. Images D, E are taken after 5% zoom of each respective group. (scale bars: 50 μm). Long-term effect of OA in cell mitochondria; CPCs P4; treated with OA 300 μΜ for 48 h (G,H) or 1 week (I,J), stained with MitoTracker® Red CMXRos. Images H,J are taken after 2.5% zoom of each respective group. (scale bars: 50 μm)
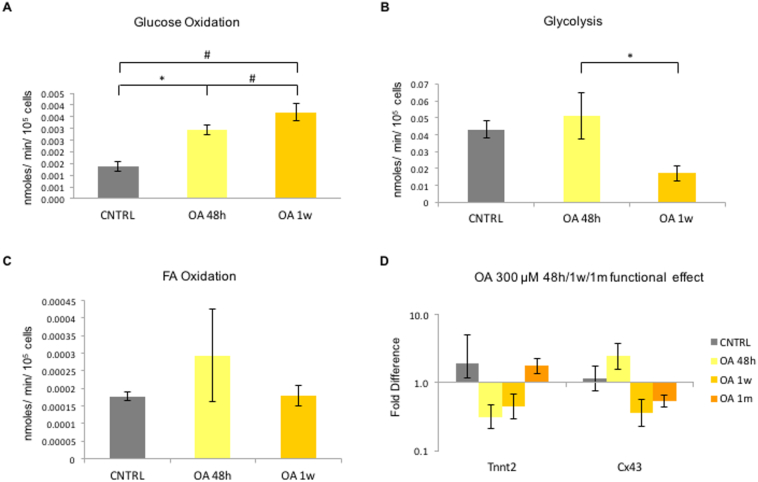
The level of substrate utilisation was assessed using radioactive tracer analysis. After supplementing the CPCs with OA for 48 h and 1 week, glucose oxidation was increased significantly (Fig. 5A), glycolytic flux was significantly reduced after 1 week of OA incubation (Fig. 5B), while FA oxidation remained unchanged (Fig. 5C). Despite these changes, qPCRanalysis showed no significant change in the gene expression of Tnnt2 or Cx43 after treatment with OA at any time point (Fig. 5D). Myh6 and Myh7 were not expressed under any of the conditions.
Fig. 5.
OA supplementation affects substrate metabolism: Effect of supplementation with OA on (A) glucose oxidation levels, (B) Glycolysis and (C) FA Oxidation levels, measured as nMoles/min/105 cells, compared to control untreated CPCs. (n = 4; *p < .04, #p < .01 indicating differences amongst the groups, error bars: standard error); and D) on the expression of differentiation genes in P4 CPCs normalised to control untreated CPCs. (n = 3; error bars: standard error).
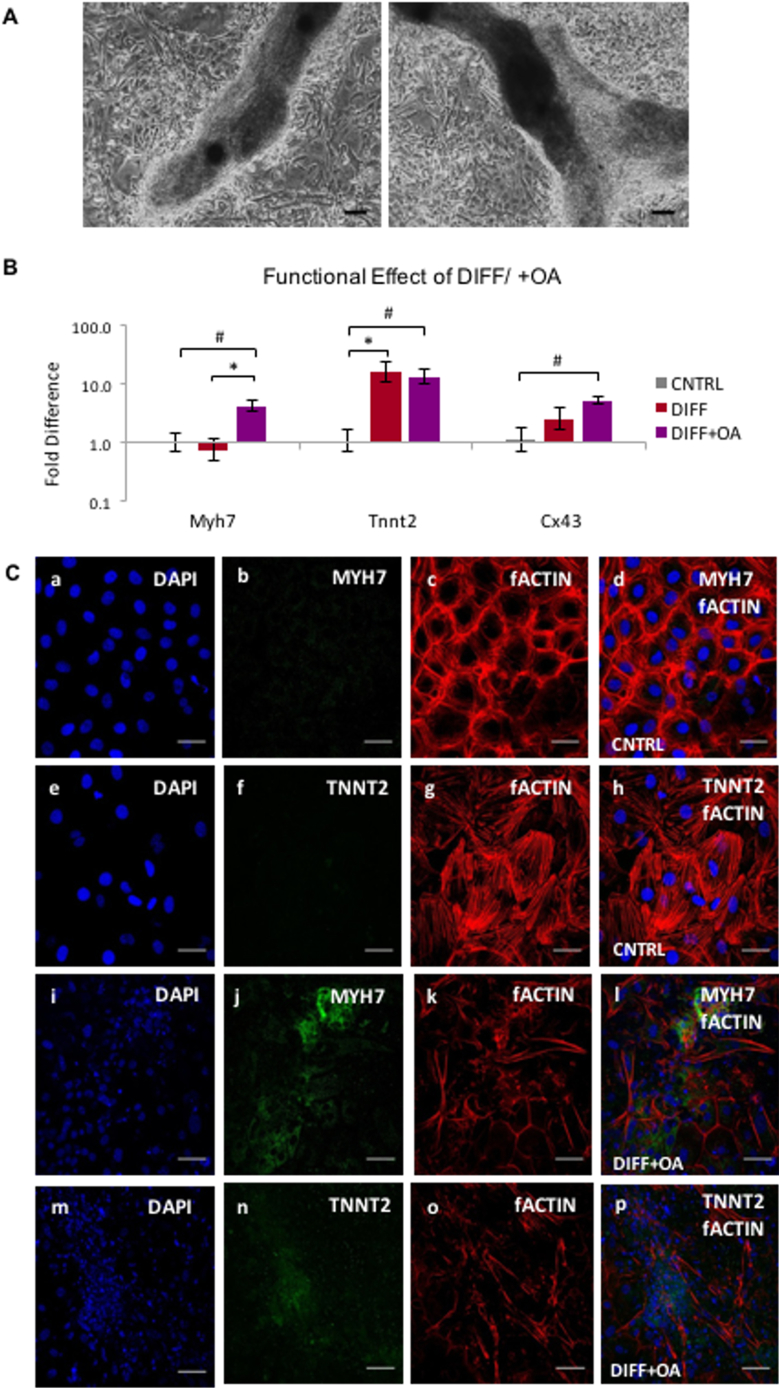
3.5. Oleic acid addition further matures differentiated CPCS functionally and metabolically
Based on the data indicating upregulation of the PPARα pathway after OA treatment of CPCs, OA was used after TGF-β1 differentiation (“DIFF+OA”) to assess whether differentiated cells could be matured metabolically. The differentiating CPCs formed cell clusters as before (Fig. 6A), but with the OA treatment the cells surrounding them were smaller than in the non-treated samples. qPCR analysis of mature cardiac genes (Myh7, Tnnt2, Cx43) showed that both Myh7 and Cx43, which were unchanged after differentiation (Fig. 1C), were significantly upregulated after OA treatment. Tnnt2 gene expression did not increase further (Fig. 6B). Immunocytochemistry confirmed the expression of MYH7 and TNNT2 in the differentiated CPCs treated with OA (Fig. 6C). Filamentous actin (fActin) staining, to assess the shape and structural components of the cells, did not indicate any CM-like striations (Fig. 6C).
Fig. 6.
OA supplementation of differentiating CPCs: (A) Cell morphology of CPCs differentiated using the TGF-β1 protocol, followed by 1 week of OA 300 μM supplementation, under light microscope (scale bars: 100 μM). (B) Effect of OA treatment post-differentiation on CPCs, compared to non-treated differentiated CPCs and CNTRL undifferentiated P4 CPCs. (n = 4, *p < .02, $p < .01 compared to CNTRL, error bars: standard error). (C) Immunocytochemistry on control undifferentiated samples (top two rows) and differentiated samples with OA addition (DIFF+OA; bottom two rows). (MYH7 and TNNT2 shown in green, co-stained with DAPI in blue, scale bars: 50 μm). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
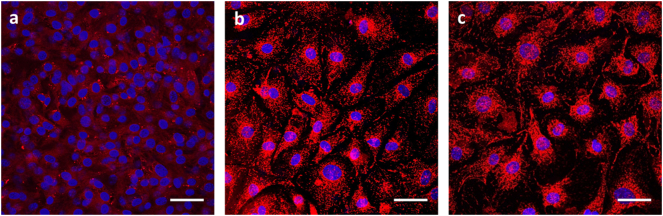
The TGF-β1 protocol increased gene expression of both PPARα and PGC-1α. Addition of OA for 1 week maintained the PPARα gene expression at the same level, but PGC-1α expression decreased (Fig. 7A). Glut1 gene expression was significantly downregulated after OA treatment, accompanied by a significant increase in Glut4 expression after TGF-β1 differentiation, which increased further following OA treatment. Pdk4 and Cs expression remained unchanged in both treatments (Fig. 7A). Assessment of the mitochondrial membrane potential, showed an increase in the fluorescence between the CNTRL undifferentiated CPCs, compared to the DIFF group and the DIFF+OA differentiated CPCs (Supplemental Fig. S5), (Fig. 7B).The glucose oxidation rates were increased after differentiation with TGF-β1, and afterwards further in differentiated cells treated with OA compared with rates in CNTRL undifferentiated cells (Fig. 7C). FA oxidation (Fig. 7E) increased in differentiated cells treated with OA, whereas rates of glycolytic flux which had been significantly increased by differentiation alone, were further elevated with OA treatment (Fig. 7D).
Fig. 7.
OA supplementation affects substrate metabolism in differentiated CPCs: (A) Metabolic effect of 1 week supplementation with OA post-differentiation on gene expression in CPCs, compared to non-treated differentiated CPCs and CNTRL undifferentiated CPCs. (n = 4, *p < 0,05, $p < 0,01 indicating difference to CNTRL, error bars: standard error). (B) Effect of differentiation, and differentiation followed by 1 week of OA 300 μM, on cell mitochondria, estimated as average fluorescent intensity, after staining with MitoTracker Red CMXRos red. (n = 12 cells, 3 replicates, *p < .001). (C) Effect of TGF-β1 differentiation, and differentiation followed by 1 week supplementation with OA, on the glucose oxidation levels, (D) Glycolysis, and E) FA Oxidation, measured as nMoles/ min/ 105 cells, normalised to control untreated CPCs. (n = 3; *p < .03, #p < .01, $p < .001 indicating differences amongst the groups, error bars: standard error). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. S5.
Mitochondrial staining after differentiation + OA: Effect of differentiation of CPCs P4, followed by 1 week of OA 300 μM, on cell mitochondria (c), compared with CNTRL undifferentiated CPCs (a) and differentiated CPCs without OA supplementation (b); all stained with MitoTracker® Red CMXRos. (scale bars: 50 μm)
4. Discussion
To our knowledge, this is the first report that investigates the effect of fatty acid supplementation on adult cardiac progenitors, using metabolic flux analyses, focusing on optimising differentiation efficiency. Our results shed light on the metabolic profile of differentiating CPCs and highlight the importance of substrate availability, and the PPARα pathway, in this process.
Different groups have used the TGF-β1 family (Smits et al., 2009; Goumans et al., 2008; Ye et al., 2012) and 5-Aza (Oh et al., 2003; Qian et al., 2012; Goumans et al., 2008) on various cardiac cell types to induce cardiac differentiation in vitro. The methods of assessment of the level of CPC differentiation vary, but entail changes in cell morphology, as well as expression of markers such as Tnnt2, Myh7 and Cx43 (Goumans et al., 2008; Qian et al., 2012; Linke et al., 2005). Smits et al. (Smits et al., 2009) observed that cells formed multiple layers with different alignments and confluency, after TGF-β1 differentiation of selected Sca1+ CPCs, as well as upregulation in Tnnt2, Cx43 and Myh7, which agrees with our observation of cell culture morphology and marker expression (Fig. 1B and C). The pattern of MYH7 expression was concentrated within the tubular structures (Fig. 1D), suggesting that there was still a fraction of undifferentiated cells growing around the differentiating ones, which may indicate that cells need to be highly confluent for efficient differentiation. The main cell culture issue that we faced, had to do with the fact that the cells were difficult to dissociate as they formed clumps after the differentiation of month. Subsequently, the cells were trypsinised, before pelleting for RNA extraction, which did not work well with the cell clusters in the differentiated samples, which may have biased the analysis of gene expression to the easily-dissociated monolayer cells (Fig. 1C).
The upregulation of cardiac transcription factors, shown in studies of hESC differentiation (Kehat et al., 2004; Xu, 2002) and mESC (Sachinidis et al., 2003; Fijnvandraat et al., 2003) into CMs, as well as MHC and Troponin (Fijnvandraat et al., 2003; Van Laake et al., 2005), matched those seen in the differentiating mESCs at d4, d7 and d14. (Fig. 2A). The downregulation of Glut1 and upregulation of Glut4 observed has been reported as an indication of the metabolic maturation of cells (Wang and Hu, 1991; Studelska et al., 1992; Postic et al., 1994) (Fig. 2B). The early increase in PGC-1α could indicate upregulation of the mitochondrial metabolism early in the differentiating SCs (Chung et al., 2010; Porter et al., 2011; Lehman et al., 2000), followed by an increase in PPARα, as seen previously in murine cardiac differentiation in vitro (Ding et al., 2007b; Vega et al., 2000) (Fig. 2C). These observations suggested that the mESCs differentiating to beating CMs in vitro recapitulate the metabolic shift towards oxidative metabolism and express mature cardiac cell markers, rendering them a good control for this study.
After identifying the metabolic changes characterising successful differentiation of mESCs to beating CMs, we proceeded to study the metabolism of differentiating mouse adult CPCs. The upregulation of PPARα and PGC-1α after OA supplementation (Fig. 2C &3C) agrees with OA, and FAs in general, being activators of the PPARα pathway (Popeijus et al., 2014; Göttlicher et al., 1992). Pdk4, which has been shown to be upregulated by PGC-1α (Wende et al., 2005) was upregulated at 48 h, while CD36, which mediates the uptake of fatty acids in a variety of cell types (Pepino et al., 2014); Coburn et al., 2001), was upregulated at all time-points (Fig. 3B). The shift from Glut1 to Glut4 was similar to that seen with mESC differentiation, consistent with cell maturation (Wang and Hu, 1991; Studelska et al., 1992; Postic et al., 1994) (Figs. 3B & 2B). Also, OA treatment increased mitochondrial membrane potential (Figs. 4C & Supplementary Figs. S4 & S5) after 48 h and 1 week of treatment. The effect was on mitochondrial activity, rather than the mitochondrial numbers, since CS was found unchanged – both of which are necessary for cell maturation (Porter et al., 2011).
Glucose oxidation was upregulated significantly, while glycolytic flux decreased, after 1 week of OA treatment, suggesting a shift towards a more mature metabolic phenotype, but FA oxidation rates were unchanged (Fig. 5A, B and C). This is the first study assessing the metabolism of differentiating adult CPCs in vitro, so there is no prior evidence of the expected metabolic profile. It may be that the OA-treated cells required a longer supplementation period in order to significantly upregulate FA oxidation, or that the undifferentiated cells obtained sufficient energy from glucose oxidation.
To investigate whether the metabolic effect that followed the OA treatment could assist the differentiation of adult CPCs, the cell culture medium was supplemented for 1 week following the TGF-β1 differentiation protocol. The gene expression changes of the OA-treated differentiated CPCs revealed a more structurally mature phenotype, with both Myh7 and Cx43 being significantly upregulated (Fig. 6B). In addition, TGF-β1 differentiation alone was sufficient to stimulate the PPARα – PGC-1α pathway, as well as a shift from Glut1 to Glut4 gene expression (Fig. 7A), as seen during the mESC differentiation previously. PPARα and PGC-1α are upregulated during differentiation (Ding et al., 2007b), and the expression of GLUT4 is higher in adult tissues (Wang and Hu, 1991; Studelska et al., 1992; Postic et al., 1994), suggesting that TGF-β1 differentiation induces a degree of metabolic maturation.
Addition of OA after differentiation retained the levels of PPARα, Glut1 and Glut4, but led to a reduction in PGC-1α (Fig. 7A), a decrease that was also seen at d14 of mESCs progressing to terminal differentiation (Fig. 2C), and after long-term exposure of CNTRL cells with OA (Fig. 4B). This could suggest that PGC-1α does not need to be constitutively upregulated after changes have been induced, although Birket et al. reported consistent activation of PGC-1α in ESCs during cardiomyocyte differentiation (Birket et al., 2013).
Glucose and FA oxidation, as well as glycolytic flux, were significantly upregulated in the TGF-β1 differentiated CPCs treated with OA (Fig. 7C, D and E), indicating a holistic metabolic maturation of the differentiated cells after OA supplementation. The TGF-β1 differentiated CPCs seem to acquire a more mature metabolic phenotype (because of the pharmacological agent), and respond differently to undifferentiated CPCs when OA is added, by upregulating FA oxidation. The fact that OA supplementation had a positive effect in the maturation of the differentiated CPCs came as no surprise, given the recent study from Correia et al. (Correia et al., 2017) that demonstrated the importance of the metabolic shift in the maturation of hPSC-CMs. The hPSCs that were supplemented with FAs had enhanced differentiated profile with increased oxidative metabolism and functional maturation, compared to those grown in the standard glucose media, a finding that agrees with our observations. In this study the FA treatment entailed combination of both oleic and palmitic acids, as well as galactose, whereas we only used oleic acid, but it would be interesting to explore a better combination of substrates that can enhance cardiac differentiation to mature CMs from adult cardiac progenitors.
5. Conclusions
This is the first study that uses radiolabelled substrate consumption for metabolic flux analyses of adult cardiac progenitors, after FA supplementation, with the aim to enhance their cardiac differentiation. In addition, we explore the role of the PPARα/PGC-1α axis during metabolic manipulation.
We have shown that OA supplementation matured both control and differentiated cells metabolically, while also increased the expression of cardiac markers in the latter. In addition we highlight that the PPARα pathway was stimulated by cardiac differentiation in both CPCs and mESCs. Our results allow for a better understanding of the effect of fatty acids, on cardiac differentiation of adult cardiac progenitors, complementing the metabolic manipulation studies of hPSC-CMs, and development of a protocol that would allow for more efficient differentiation to mature CMs.
The following are the supplementary data related to this article.
Fig. S6.
Negative control staining for differentiation imaging: Negative control staining with secondary antibodies for immunocytochemistry of MHC and TnnT2 on differentiated CPCs with the TGF-β1 protocol (see Fig. 6C), co-stained with DAPI in blue (scale bars: 50 μm)
Supplementary material
Acknowledgments
Acknowledgements
We thank Dr. Richard Tyser and Professor Paul Riley for donation of the differentiating mESCs.
We would also like to thank Dr. Vicky Ball for her technical support and training she provided for the radioactive experiments.
Author contributions
Sophia Malandraki-Miller: Conception and design, Collection and assembly of data, Data analysis and interpretation, Manuscript writing, Manuscript revisions and final approval.
Colleen Lopez: Collection and assembly of data, Data analysis and interpretation.
Ujang Purnama: Collection and assembly of data for the revision corrections.
Rita Alonaizan: Collection and assembly of data for the revision corrections.
Filippo Perbellini: Provided support and consulting for experimental techniques.
Kathy Pakzad: Initial set up of the 14C-Glucose Oxidation Measurement technique.
Carolyn Carr: Conception and design, Financial support, Administrative support, Data analysis and interpretation, Manuscript writing, Final approval of manuscript.
Funding
This work was supported by the British Heart Foundation (PG/13/34/30216) and the Rosetrees Trust (M315).
Declarations of interest
None.
Contributor Information
Sophia Malandraki-Miller, Email: sophia.malandraki-miller@dpag.ox.ac.uk.
Colleen A. Lopez, Email: colleen.lopez@dpag.ox.ac.uk.
Rita Alonaizan, Email: rita.alonaizan@dpag.ox.ac.uk.
Ujang Purnama, Email: ujang.purnama@dpag.ox.ac.uk.
Filippo Perbellini, Email: f.perbellini@imperial.ac.uk.
Kathy Pakzad, Email: khadij.pakzad@icloud.com.
Carolyn A. Carr, Email: carolyn.carr@dpag.ox.ac.uk.
References
- Barr R.L., Lopaschuk G.D. Direct measurement of energy metabolism in the isolated working rat heart. J. Pharmacol. Toxicol. Methods. 1997;38:11–17. doi: 10.1016/s1056-8719(97)86574-4. [DOI] [PubMed] [Google Scholar]; Barr, R.L., Lopaschuk, G.D., 1997. Direct measurement of energy metabolism in the isolated working rat heart. J. Pharmacol. Toxicol. Methods 38, 11–17. https://doi.org/10.1016/S1056-8719(97)86574-4 [DOI] [PubMed]
- Belke D.D., Larsen T.S., Lopaschuk G.D., Severson D.L. Glucose and fatty acid metabolism in the isolated working mouse heart. Am. J. Phys. 1999;277:R1210–R1217. doi: 10.1152/ajpregu.1999.277.4.R1210. [DOI] [PubMed] [Google Scholar]; Belke, D.D., Larsen, T.S., Lopaschuk, G.D., Severson, D.L., 1999. Glucose and fatty acid metabolism in the isolated working mouse heart. Am. J. Physiol. 277, R1210-7. [DOI] [PubMed]
- Birket M.J., Casini S., Kosmidis G., Elliott D.A., Gerencser A.A., Baartscheer A., Schumacher C., Mastroberardino P.G., Elefanty A.G., Stanley E.G., Mummery C.L. PGC-1?? and reactive oxygen species regulate human embryonic stem cell-derived cardiomyocyte function. Stem Cell Rep. 2013;1:560–574. doi: 10.1016/j.stemcr.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; Birket, M.J., Casini, S., Kosmidis, G., Elliott, D.A., Gerencser, A.A., Baartscheer, A., Schumacher, C., Mastroberardino, P.G., Elefanty, A.G., Stanley, E.G., Mummery, C.L., 2013. PGC-1?? and reactive oxygen species regulate human embryonic stem cell-derived cardiomyocyte function. Stem Cell Reports 1, 560–574. https://doi.org/10.1016/j.stemcr.2013.11.008 [DOI] [PMC free article] [PubMed]
- Boheler K.R., Czyz J., Tweedie D., Yang H.T., Anisimov S.V., Wobus A.M. Differentiation of pluripotent embryonic stem cells into cardiomyocytes. Circ. Res. 2002 doi: 10.1161/01.res.0000027865.61704.32. [DOI] [PubMed] [Google Scholar]; Boheler, K.R., Czyz, J., Tweedie, D., Yang, H.T., Anisimov, S. V., Wobus, A.M., 2002. Differentiation of pluripotent embryonic stem cells into cardiomyocytes. Circ. Res. https://doi.org/10.1161/01.RES.0000027865.61704.32 [DOI] [PubMed]
- Chung S., Arrell D.K., Faustino R.S., Terzic A., Dzeja P.P. Glycolytic network restructuring integral to the energetics of embryonic stem cell cardiac differentiation. J. Mol. Cell. Cardiol. 2010;48:725–734. doi: 10.1016/j.yjmcc.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chung, S., Arrell, D.K., Faustino, R.S., Terzic, A., Dzeja, P.P., 2010. Glycolytic network restructuring integral to the energetics of embryonic stem cell cardiac differentiation. J. Mol. Cell. Cardiol. 48, 725–734. https://doi.org/10.1016/j.yjmcc.2009.12.014 [DOI] [PMC free article] [PubMed]
- Correia C., Koshkin A., Duarte P., Hu D., Teixeira A., Domian I., Serra M., Alves P.M. Distinct carbon sources affect structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Sci. Rep. 2017 doi: 10.1038/s41598-017-08713-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; Correia, C., Koshkin, A., Duarte, P., Hu, D., Teixeira, A., Domian, I., Serra, M., Alves, P.M., 2017. Distinct carbon sources affect structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Sci. Rep. https://doi.org/10.1038/s41598-017-08713-4 [DOI] [PMC free article] [PubMed]
- Cottet-Rousselle C., Ronot X., Leverve X., Mayol J.F. Cytometric assessment of mitochondria using fluorescent probes. Cytom. Part A. 2011 doi: 10.1002/cyto.a.21061. [DOI] [PubMed] [Google Scholar]; Cottet-Rousselle, C., Ronot, X., Leverve, X., Mayol, J.F., 2011. Cytometric assessment of mitochondria using fluorescent probes. Cytom. Part A. https://doi.org/10.1002/cyto.a.21061 [DOI] [PubMed]
- De Sousa Andrade L.N., De Lima T.M., Curi R., De Lauro Castrucci A.M. Toxicity of fatty acids on murine and human melanoma cell lines. Toxicol. in Vitro. 2005;19:553–560. doi: 10.1016/j.tiv.2005.02.002. [DOI] [PubMed] [Google Scholar]; De Sousa Andrade, L.N., De Lima, T.M., Curi, R., De Lauro Castrucci, A.M., 2005. Toxicity of fatty acids on murine and human melanoma cell lines. Toxicol. Vitr. 19, 553–560. https://doi.org/10.1016/j.tiv.2005.02.002 [DOI] [PubMed]
- DeBerardinis R.J., Mancuso A., Daikhin E., Nissim I., Yudkoff M., Wehrli S., Thompson C.B. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. U. S. A. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]; DeBerardinis, R.J., Mancuso, A., Daikhin, E., Nissim, I., Yudkoff, M., Wehrli, S., Thompson, C.B., 2007. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A 104, 19345–19350. https://doi.org/10.1073/pnas.0709747104 [DOI] [PMC free article] [PubMed]
- Ding L., Liang X.-G., Lou Y.-J. Time-dependence of cardiomyocyte differentiation disturbed by peroxisome proliferator-activated receptor alpha inhibitor GW6471 in murine embryonic stem cells in vitro. Acta Pharmacol. Sin. 2007;28:634–642. doi: 10.1111/j.1745-7254.2007.00558.x. [DOI] [PubMed] [Google Scholar]; Ding, L., Liang, X.-G., Lou, Y.-J., 2007a. Time-dependence of cardiomyocyte differentiation disturbed by peroxisome proliferator-activated receptor alpha inhibitor GW6471 in murine embryonic stem cells in vitro. Acta Pharmacol. Sin. 28, 634–42. https://doi.org/10.1111/j.1745-7254.2007.00558.x [DOI] [PubMed]
- Ding L., Liang X., Zhu D., Lou Y. Peroxisome proliferator-activated receptor alpha is involved in cardiomyocyte differentiation of murine embryonic stem cells in vitro. Cell Biol. Int. 2007;31:1002–1009. doi: 10.1016/j.cellbi.2007.03.013. [DOI] [PubMed] [Google Scholar]; Ding, L., Liang, X., Zhu, D., Lou, Y., 2007b. Peroxisome proliferator-activated receptor alpha is involved in cardiomyocyte differentiation of murine embryonic stem cells in vitro. Cell Biol. Int. 31, 1002–9. https://doi.org/10.1016/j.cellbi.2007.03.013 [DOI] [PubMed]
- Fijnvandraat A.C., Van Ginneken A.C.G., Schumacher C.A., Boheler K.R., Lekanne Deprez R.H., Christoffels V.M., Moorman A.F.M. Cardiomyocytes purified from differentiated embryonic stem cells exhibit characteristics of early chamber myocardium. J. Mol. Cell. Cardiol. 2003;35:1461–1472. doi: 10.1016/j.yjmcc.2003.09.011. [DOI] [PubMed] [Google Scholar]; Fijnvandraat, A.C., Van Ginneken, A.C.G., Schumacher, C.A., Boheler, K.R., Lekanne Deprez, R.H., Christoffels, V.M., Moorman, A.F.M., 2003. Cardiomyocytes purified from differentiated embryonic stem cells exhibit characteristics of early chamber myocardium. J. Mol. Cell. Cardiol. 35, 1461–1472. https://doi.org/10.1016/j.yjmcc.2003.09.011 [DOI] [PubMed]
- Finck B.N., Lehman J.J., Leone T.C., Welch M.J., Bennett M.J., Kovacs A., Han X., Gross R.W., Kozak R., Lopaschuk G.D., Kelly D.P. The cardiac phenotype induced by PPARa overexpression mimics that caused by diabetes mellitus. J. Clin. Invest. 2002;109:121–130. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]; Finck, B.N., Lehman, J.J., Leone, T.C., Welch, M.J., Bennett, M.J., Kovacs, A., Han, X., Gross, R.W., Kozak, R., Lopaschuk, G.D., Kelly, D.P., 2002. The cardiac phenotype induced by PPARa overexpression mimics that caused by diabetes mellitus. J. Clin. Invest. 109, 121–130. https://doi.org/10.1172/JCI200214080 [DOI] [PMC free article] [PubMed]
- Göttlicher M., Widmark E., Li Q., Gustafsson J.A. Fatty acids activate a chimera of the clofibric acid-activated receptor and the glucocorticoid receptor. Proc. Natl. Acad. Sci. U. S. A. 1992;89:4653–4657. doi: 10.1073/pnas.89.10.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]; Göttlicher, M., Widmark, E., Li, Q., Gustafsson, J.A., 1992. Fatty acids activate a chimera of the clofibric acid-activated receptor and the glucocorticoid receptor. Proc. Natl. Acad. Sci. U. S. A. 89, 4653–7. https://doi.org/10.1073/pnas.89.10.4653 [DOI] [PMC free article] [PubMed]
- Goumans M.-J., de Boer T.P., Smits A.M., van Laake L.W., van Vliet P., Metz C.H.G., Korfage T.H., Kats K.P., Hochstenbach R., Pasterkamp G., Verhaar M.C., van der Heyden M.A.G., de Kleijn D., Mummery C.L., van Veen T.A.B., Sluijter J.P.G., Doevendans P.A. TGF-β1 induces efficient differentiation of human cardiomyocyte progenitor cells into functional cardiomyocytes in vitro. Stem Cell Res. 2008;1:138–149. doi: 10.1016/j.scr.2008.02.003. [DOI] [PubMed] [Google Scholar]; Goumans, M.-J., de Boer, T.P., Smits, A.M., van Laake, L.W., van Vliet, P., Metz, C.H.G., Korfage, T.H., Kats, K.P., Hochstenbach, R., Pasterkamp, G., Verhaar, M.C., van der Heyden, M.A.G., de Kleijn, D., Mummery, C.L., van Veen, T.A.B., Sluijter, J.P.G., Doevendans, P.A., 2008. TGF-β1 induces efficient differentiation of human cardiomyocyte progenitor cells into functional cardiomyocytes in vitro. Stem Cell Res. 1, 138–149. https://doi.org/10.1016/j.scr.2008.02.003 [DOI] [PubMed]
- Gulick T., Cresci S., Caira T., Moore D.D., Kelly D.P. The peroxisome proliferator-activated receptor regulates mitochondrial fatty acid oxidative enzyme gene expression. Proc. Natl. Acad. Sci. U. S. A. 1994;91:11012–11016. doi: 10.1073/pnas.91.23.11012. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gulick, T., Cresci, S., Caira, T., Moore, D.D., Kelly, D.P., 1994. The peroxisome proliferator-activated receptor regulates mitochondrial fatty acid oxidative enzyme gene expression. Proc. Natl. Acad. Sci. U. S. A. 91, 11012–6. [DOI] [PMC free article] [PubMed]
- Haeiwa H., Fujita T., Saitoh Y., Miwa N. Oleic acid promotes adaptability against oxidative stress in 3T3-L1 cells through lipohormesis. Mol. Cell. Biochem. 2014;386:73–83. doi: 10.1007/s11010-013-1846-9. [DOI] [PubMed] [Google Scholar]; Haeiwa, H., Fujita, T., Saitoh, Y., Miwa, N., 2014. Oleic acid promotes adaptability against oxidative stress in 3T3-L1 cells through lipohormesis. Mol. Cell. Biochem. 386, 73–83. https://doi.org/10.1007/s11010-013-1846-9 [DOI] [PubMed]
- Handschin C., Spiegelman B.M. Peroxisome proliferator-activated receptor a coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr. Rev. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]; Handschin, C., Spiegelman, B.M., 2006. Peroxisome proliferator-activated receptor a coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr. Rev. 27, 728–735. https://doi.org/10.1210/er.2006-0037 [DOI] [PubMed]
- Hu D., Linders A., Yamak A., Correia C., Kijlstra J.D., Garakani A., Xiao L., Milan D.J., van der Meer P., Serra M., Alves P.M., Domian I.J. Metabolic maturation of human pluripotent stem cell-derived cardiomyocytes by inhibition of HIF1α and LDHA. Circ. Res. 2018 doi: 10.1161/CIRCRESAHA.118.313249. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hu, D., Linders, A., Yamak, A., Correia, C., Kijlstra, J.D., Garakani, A., Xiao, L., Milan, D.J., van der Meer, P., Serra, M., Alves, P.M., Domian, I.J., 2018. Metabolic Maturation of Human Pluripotent Stem Cell-Derived Cardiomyocytes by Inhibition of HIF1α and LDHA. Circ. Res. https://doi.org/10.1161/CIRCRESAHA.118.313249 [DOI] [PMC free article] [PubMed]
- Kehat I., Khimovich L., Caspi O., Gepstein A., Shofti R., Arbel G., Huber I., Satin J., Itskovitz-Eldor J., Gepstein L. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat. Biotechnol. 2004;22:1282–1289. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]; Kehat, I., Khimovich, L., Caspi, O., Gepstein, A., Shofti, R., Arbel, G., Huber, I., Satin, J., Itskovitz-Eldor, J., Gepstein, L., 2004. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat. Biotechnol. 22, 1282–9. https://doi.org/10.1038/nbt1014 [DOI] [PubMed]
- Kolwicz S.C., Purohit S., Tian R. Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. Circ. Res. 2013 doi: 10.1161/CIRCRESAHA.113.302095. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kolwicz, S.C., Purohit, S., Tian, R., 2013. Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. Circ. Res. https://doi.org/10.1161/CIRCRESAHA.113.302095 [DOI] [PMC free article] [PubMed]
- Lehman J.J., Barger P.M., Kovacs A., Saffitz J.E., Medeiros D.M., Kelly D.P. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J. Clin. Invest. 2000;106:847–856. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lehman, J.J., Barger, P.M., Kovacs, A., Saffitz, J.E., Medeiros, D.M., Kelly, D.P., 2000. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J. Clin. Invest. 106, 847–56. https://doi.org/10.1172/JCI10268 [DOI] [PMC free article] [PubMed]
- Linke A., Müller P., Nurzynska D., Casarsa C., Torella D., Nascimbene A., Castaldo C., Cascapera S., Böhm M., Quaini F., Urbanek K., Leri A., Hintze T.H., Kajstura J., Anversa P. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc. Natl. Acad. Sci. U. S. A. 2005;102:8966–8971. doi: 10.1073/pnas.0502678102. [DOI] [PMC free article] [PubMed] [Google Scholar]; Linke, A., Müller, P., Nurzynska, D., Casarsa, C., Torella, D., Nascimbene, A., Castaldo, C., Cascapera, S., Böhm, M., Quaini, F., Urbanek, K., Leri, A., Hintze, T.H., Kajstura, J., Anversa, P., 2005. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc. Natl. Acad. Sci. U. S. A. 102, 8966–71. https://doi.org/10.1073/pnas.0502678102 [DOI] [PMC free article] [PubMed]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2^- ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]; Livak, K.J., Schmittgen, T.D., 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2^- ΔΔCT Method. Methods 25, 402–8. https://doi.org/10.1006/meth.2001.1262 [DOI] [PubMed]
- Madeira V.M.C. Overview of mitochondrial bioenergetics. Methods Mol. Biol. 2012;810:1–6. doi: 10.1007/978-1-61779-382-0_1. [DOI] [PubMed] [Google Scholar]; Madeira, V.M.C., 2012. Overview of mitochondrial bioenergetics. Methods Mol. Biol. 810, 1–6. https://doi.org/10.1007/978-1-61779-382-0_1 [DOI] [PubMed]
- Malandraki-Miller S., Lopez C.A., Al-Siddiqi H., Carr C.A. Changing metabolism in differentiating cardiac progenitor cells-can stem cells become metabolically flexible cardiomyocytes? Front Cardiovasc Med. 2018;5:119. doi: 10.3389/fcvm.2018.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]; Malandraki-Miller S., Lopez C.A., Al-Siddiqi H., Carr C.A., Changing Metabolism in Differentiating Cardiac Progenitor Cells-Can Stem Cells Become Metabolically Flexible Cardiomyocytes?, Front Cardiovasc Med. 2018;5:119. doi: 10.3389/fcvm.2018.00119. [DOI] [PMC free article] [PubMed]
- Nuclear Receptors Nomenclature, C A unified nomenclature system for the nuclear receptor superfamily. Cell. 1999;97:161–163. doi: 10.1016/s0092-8674(00)80726-6. [DOI] [PubMed] [Google Scholar]; Nuclear Receptors Nomenclature, C., 1999. A unified nomenclature system for the nuclear receptor superfamily. Cell 97, 161–163. https://doi.org/10.1016/S0092-8674(00)80726-6 [DOI] [PubMed]
- Oh H., Bradfute S.B., Gallardo T.D., Nakamura T., Gaussin V., Mishina Y., Pocius J., Michael L.H., Behringer R.R., Garry D.J., Entman M.L., Schneider M.D. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc. Natl. Acad. Sci. U. S. A. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]; Oh, H., Bradfute, S.B., Gallardo, T.D., Nakamura, T., Gaussin, V., Mishina, Y., Pocius, J., Michael, L.H., Behringer, R.R., Garry, D.J., Entman, M.L., Schneider, M.D., 2003. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc. Natl. Acad. Sci. U. S. A. 100, 12313–8. https://doi.org/10.1073/pnas.2132126100 [DOI] [PMC free article] [PubMed]
- Pepino M.Y., Kuda O., Samovski D., Abumrad N. a. Structure-function of CD36 and importance of fatty acid signal transduction in fat metabolism. Annu. Rev. Nutr. 2014;34:281–303. doi: 10.1146/annurev-nutr-071812-161220. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pepino, M.Y., Kuda, O., Samovski, D., Abumrad, N. a, 2014. Structure-Function of CD36 and Importance of Fatty Acid Signal Transduction in Fat Metabolism. Annu. Rev. Nutr. 34, 281–303. https://doi.org/10.1146/annurev-nutr-071812-161220 [DOI] [PMC free article] [PubMed]
- Perbellini F., Gomes R.S.M., Vieira S., Buchanan D., Malandraki-Miller S., Bruyneel A. Chronic high-fat feeding affects the mesenchymal cell population expanded from adipose tissue but not cardiac atria. Stem Cells Transl. Med. 2015;4:1403–1414. doi: 10.5966/sctm.2015-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]; Perbellini, F., Buchanan, D.A., Gomes, R.S.M., Vieira, S., Clarke, K., Faggian, G., Carr, C.A., 2014. The effect of a high fat diet on cardiac and adipose mesenchymal cells . Cardiovasc. Res. .
- Popeijus H.E., van Otterdijk S.D., van der Krieken S.E., Konings M., Serbonij K., Plat J., Mensink R.P. Fatty acid chain length and saturation influences PPARα transcriptional activation and repression in HepG2 cells. Mol. Nutr. Food Res. 2014 doi: 10.1002/mnfr.201400314. [DOI] [PubMed] [Google Scholar]; Popeijus, H.E., van Otterdijk, S.D., van der Krieken, S.E., Konings, M., Serbonij, K., Plat, J., Mensink, R.P., 2014. Fatty acid chain length and saturation influences PPARα transcriptional activation and repression in HepG2 cells. Mol. Nutr. Food Res. https://doi.org/10.1002/mnfr.201400314 [DOI] [PubMed]
- Porter G.A., Hom J.R., Hoffman D.L., Quintanilla R.A., Bentley K.L. de M., Sheu S.S. Bioenergetics, mitochondria, and cardiac myocyte differentiation. Prog. Pediatr. Cardiol. 2011;31:75–81. doi: 10.1016/j.ppedcard.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; Porter, G.A., Hom, J.R., Hoffman, D.L., Quintanilla, R.A., Bentley, K.L. de M., Sheu, S.S., 2011. Bioenergetics, mitochondria, and cardiac myocyte differentiation. Prog. Pediatr. Cardiol. 31, 75–81. https://doi.org/10.1016/j.ppedcard.2011.02.002 [DOI] [PMC free article] [PubMed]
- Postic C., Leturque A., Printz R.L., Maulard P., Loizeau M., Granner D.K., Girard J. Development and regulation of glucose transporter and hexokinase expression in rat. Am. J. Phys. 1994;266:E548–E559. doi: 10.1152/ajpendo.1994.266.4.E548. [DOI] [PubMed] [Google Scholar]; Postic, C., Leturque, A., Printz, R.L., Maulard, P., Loizeau, M., Granner, D.K., Girard, J., 1994. Development and regulation of glucose transporter and hexokinase expression in rat. Am J Physiol 266, E548-59. [DOI] [PubMed]
- Qian Q., Qian H., Zhang X., Zhu W., Yan Y., Ye S., Peng X., Li W., Xu Z., Sun L., Xu W. 5-Azacytidine induces cardiac differentiation of human umbilical cord-derived mesenchymal stem cells by activating extracellular regulated kinase. Stem Cells Dev. 2012;21:67–75. doi: 10.1089/scd.2010.0519. [DOI] [PMC free article] [PubMed] [Google Scholar]; Qian, Q., Qian, H., Zhang, X., Zhu, W., Yan, Y., Ye, S., Peng, X., Li, W., Xu, Z., Sun, L., Xu, W., 2012. 5-Azacytidine Induces Cardiac Differentiation of Human Umbilical Cord-Derived Mesenchymal Stem Cells by Activating Extracellular Regulated Kinase. Stem Cells Dev. 21, 67–75. https://doi.org/10.1089/scd.2010.0519 [DOI] [PMC free article] [PubMed]
- Sanderson L.M., Degenhardt T., Koppen A., Kalkhoven E., Desvergne B., Müller M., Kersten S. Peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta) but not PPARalpha serves as a plasma free fatty acid sensor in liver. Mol. Cell. Biol. 2009;29:6257–6267. doi: 10.1128/MCB.00370-09. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sanderson, L.M., Degenhardt, T., Koppen, A., Kalkhoven, E., Desvergne, B., Müller, M., Kersten, S., 2009. Peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta) but not PPARalpha serves as a plasma free fatty acid sensor in liver. Mol. Cell. Biol. 29, 6257–6267. https://doi.org/10.1128/MCB.00370-09 [DOI] [PMC free article] [PubMed]
- Smits A.M., van Vliet P., Metz C.H., Korfage T., Sluijter J.P., Doevendans P. a, Goumans M.-J. Human cardiomyocyte progenitor cells differentiate into functional mature cardiomyocytes: an in vitro model for studying human cardiac physiology and pathophysiology. Nat. Protoc. 2009;4:232–243. doi: 10.1038/nprot.2008.229. [DOI] [PubMed] [Google Scholar]; Smits, A.M., van Vliet, P., Metz, C.H., Korfage, T., Sluijter, J.P., Doevendans, P. a, Goumans, M.-J., 2009. Human cardiomyocyte progenitor cells differentiate into functional mature cardiomyocytes: an in vitro model for studying human cardiac physiology and pathophysiology. Nat. Protoc. 4, 232–243. https://doi.org/10.1038/nprot.2008.229 [DOI] [PubMed]
- Studelska D.R., Campbell C., Pang S., Rodnick K.J., James D.E. Developmental expression of insulin-regulatable glucose transporter GLUT-4. Am. J. Physiol. Endocrinol. Metab. 1992;263:E102–E106. doi: 10.1152/ajpendo.1992.263.1.E102. [DOI] [PubMed] [Google Scholar]; Studelska, D.R., Campbell, C., Pang, S., Rodnick, K.J., James, D.E., 1992. Developmental expression of insulin-regulatable glucose transporter GLUT-4. Am. J. Physiol. – Endocrinol. Metab. 263, E102–E106. [DOI] [PubMed]
- Taegtmeyer H., Young M.E., Lopaschuk G.D., Abel E.D., Brunengraber H., Darley-Usmar V., Des Rosiers C., Gerszten R., Glatz J.F., Griffin J.L., Gropler R.J., Holzhuetter H.-G., Kizer J.R., Lewandowski E.D., Malloy C.R., Neubauer S., Peterson L.R., Portman M.A., Recchia F.A., Van Eyk J.E., Wang T.J. Assessing cardiac metabolism a scientific statement from the American Heart Association. Circ. Res. 2016 doi: 10.1161/RES.0000000000000097. [DOI] [PMC free article] [PubMed] [Google Scholar]; Taegtmeyer, H., Young, M.E., Lopaschuk, G.D., Abel, E.D., Brunengraber, H., Darley-Usmar, V., Des Rosiers, C., Gerszten, R., Glatz, J.F., Griffin, J.L., Gropler, R.J., Holzhuetter, H.-G., Kizer, J.R., Lewandowski, E.D., Malloy, C.R., Neubauer, S., Peterson, L.R., Portman, M.A., Recchia, F.A., Van Eyk, J.E., Wang, T.J., 2016. Assessing Cardiac Metabolism A Scientific Statement From the American Heart Association, Circulation Research. https://doi.org/10.1161/RES.0000000000000097 [DOI] [PMC free article] [PubMed]
- Van Laake L.W., Van Hoof D., Mummery C.L. Cardiomyocytes derived from stem cells. Ann. Med. 2005;37:499–512. doi: 10.1080/07853890500327843. https://doi.org/N782Q30313536680 [pii]\r. [DOI] [PubMed] [Google Scholar]; Van Laake, L.W., Van Hoof, D., Mummery, C.L., 2005. Cardiomyocytes derived from stem cells. Ann Med 37, 499–512. https://doi.org/N782Q30313536680 [pii]\r10.1080/07853890500327843 [DOI] [PubMed]
- Coburn C.T., Hajri T., Ibrahimi A., Abumrad N.A. Role of CD36 in membrane transport and utilization of long-chain fatty acids by different tissues. J. Mol. Neurosci. 2001;16:117–121. doi: 10.1385/JMN:16:2-3:117. discussion 151–7. [DOI] [PubMed] [Google Scholar]; Coburn, C.T., Hajri, T., Ibrahimi, A., Abumrad, N.A., 2001. Role of CD36 in membrane transport and utilization of long-chain fatty acids by different tissues. J. Mol. Neurosci. 16, 117-21; discussion 151–7. https://doi.org/10.1385/JMN:16:2-3:117 [DOI] [PubMed]
- Evans R.D., Heather L.C. Metabolic pathways and abnormalities. Surg. 2016;1–7 [Google Scholar]; Evans, R.D., Heather, L.C., 2016. Metabolic pathways and abnormalities. Surg. 1–7. https://doi.org/10.1016/j.mpsur.2016.03.010
- Sachinidis A., Fleischmann B.K., Kolossov E., Wartenberg M., Sauer H., Hescheler J. Cardiac specific differentiation of mouse embryonic stem cells. Cardiovasc. Res. 2003 doi: 10.1016/s0008-6363(03)00248-7. [DOI] [PubMed] [Google Scholar]; Sachinidis, A., Fleischmann, B.K., Kolossov, E., Wartenberg, M., Sauer, H., Hescheler, J., 2003. Cardiac specific differentiation of mouse embryonic stem cells. Cardiovasc. Res. https://doi.org/10.1016/S0008-6363(03)00248-7 [DOI] [PubMed]
- Schönfeld P., Schild L., Bohnensack R. Expression of the ADP/ATP carrier and expansion of the mitochondrial (ATP + ADP) pool contribute to postnatal maturation of the rat heart. Eur. J. Biochem. 1996;241:895–900. doi: 10.1111/j.1432-1033.1996.00895.x. [DOI] [PubMed] [Google Scholar]; Schönfeld, P., Schild, L., Bohnensack, R., 1996. Expression of the ADP/ATP carrier and expansion of the mitochondrial (ATP + ADP) pool contribute to postnatal maturation of the rat heart. Eur. J. Biochem. [DOI] [PubMed]
- Vega R.B., Huss J.M., Kelly D.P. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol. Cell. Biol. 2000;20:1868–1876. doi: 10.1128/mcb.20.5.1868-1876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]; Vega, R.B., Huss, J.M., Kelly, D.P., 2000. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol. Cell. Biol. 20, 1868–76. https://doi.org/10.1128/MCB.20.5.1868-1876.2000 [DOI] [PMC free article] [PubMed]
- Wang C., Hu S.M. Developmental regulation in the expression of rat heart glucose transporters. Biochem. Biophys. Res. Commun. 1991;177:1095–1100. doi: 10.1016/0006-291x(91)90651-m. [DOI] [PubMed] [Google Scholar]; Wang, C., Hu, S.M., 1991. Developmental regulation in the expression of rat heart glucose transporters. Biochem. Biophys. Res. Commun. 177, 1095–100. [DOI] [PubMed]
- Wende A.R., Huss J.M., Schaeffer P.J., Giguère V., Kelly D.P. PGC-1α coactivates PDK4 gene expression via the orphan nuclear receptor ERRα: a mechanism for transcriptional control of muscle glucose metabolism. Mol. Cell. Biol. 2005;25:10684–10694. doi: 10.1128/MCB.25.24.10684-10694.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wende, A.R., Huss, J.M., Schaeffer, P.J., Giguère, V., Kelly, D.P., 2005. PGC-1α Coactivates PDK4 Gene Expression via the Orphan Nuclear Receptor ERRα: a Mechanism for Transcriptional Control of Muscle Glucose Metabolism. Mol. Cell. Biol. 25, 10684–10694. https://doi.org/10.1128/MCB.25.24.10684-10694.2005 [DOI] [PMC free article] [PubMed]
- Wolfensohn S., Lloyd M. 3rd ed. Blackwell Publishing Ltd.; 2003. Handbook of Laboratory Animal Management and Welfare. [Google Scholar]; Wolfensohn, S., Lloyd, M., 2003. Handbook of Laboratory Animal Management and Welfare, Third. ed. lackwell Publishing Ltd.
- Xu C. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ. Res. 2002;91:501–508. doi: 10.1161/01.res.0000035254.80718.91. [DOI] [PubMed] [Google Scholar]; Xu, C., 2002. Characterization and Enrichment of Cardiomyocytes Derived From Human Embryonic Stem Cells. Circ. Res. 91, 501–508. https://doi.org/10.1161/01.RES.0000035254.80718.91 [DOI] [PubMed]
- Ye J., Boyle A., Shih H., Sievers R.E., Zhang Y., Prasad M., Su H., Zhou Y., Grossman W., Bernstein H.S., Yeghiazarians Y. Sca-1 + cardiosphere-derived cells are enriched for isl1-expressing cardiac precursors and improve cardiac function after myocardial injury. PLoS One. 2012;7 doi: 10.1371/journal.pone.0030329. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ye, J., Boyle, A., Shih, H., Sievers, R.E., Zhang, Y., Prasad, M., Su, H., Zhou, Y., Grossman, W., Bernstein, H.S., Yeghiazarians, Y., 2012. Sca-1 + cardiosphere-derived cells are enriched for isl1-expressing cardiac precursors and improve cardiac function after myocardial injury. PLoS One 7. https://doi.org/10.1371/journal.pone.0030329 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material