Abstract
Regeneration of lost cells in the central nervous system, especially the brain, is present to varying degrees in different species. In mammals, neuronal cell death often leads to glial cell hypertrophy, restricted proliferation, and formation of a gliotic scar, which prevents neuronal regeneration. Conversely, amphibians such as frogs and salamanders and teleost fish possess the astonishing capacity to regenerate lost cells in several regions of their brains. While frogs lose their regenerative abilities after metamorphosis, teleost fish and salamanders are known to possess regenerative competence even throughout adulthood. In the last decades, substantial progress has been made in our understanding of the cellular and molecular mechanisms of brain regeneration in amphibians and fish. But how similar are the means of brain regeneration in these different species? In this review, we provide an overview of common and distinct aspects of brain regeneration in frog, salamander, and teleost fish species: from the origin of regenerated cells to the functional recovery of behaviors.
Keywords: regeneration, brain, amphibian, teleost fish
Introduction
Both amphibians and teleost fish are excellent regenerative model systems and possess the ability to replace missing cells, appendages, and organs (Poss et al., 2002; Eguchi et al., 2011; Singh et al., 2012), including substantial parts of the central nervous system (Becker et al., 1997; Yoshino and Tochinai, 2004; Bernardos et al., 2007; Berg et al., 2010; Kroehne et al., 2011; Maden et al., 2013; Langhe et al., 2017). While common concepts certainly underlie regeneration among these different animals, recent research has also uncovered some surprising variations that provide insight into the diversity of ways in which regeneration can be achieved or inhibited. For example, the red spotted newt (Notophthalmus viridescens) and the axolotl (Ambystoma mexicanum), two salamander species separated by over 100 million years of evolution, use distinct mechanisms to regenerate muscle—namely via dedifferentiation or activation of satellite stem cells, respectively (Sandoval‐Guzmán et al., 2014). Heart and retina regeneration, which is present in zebrafish (Danio rerio) (Poss et al., 2002; Bernardos et al., 2007), is absent or impaired in another teleost fish species, the Japanese medaka (Oryzias latipes) (Lai et al., 2017; Lust and Wittbrodt, 2018). Such examples indicate the importance of studying numerous species to comprehend different strategies that have evolved and could eventually be used to induce regeneration in non‐regenerative species.
Teleost fish and amphibians each present advantages for studying brain regeneration. Teleost fish, especially the zebrafish, are established genetic models that have already contributed immense foundational knowledge on stem and progenitor cells involved in brain regeneration. The repertoire of mutants, genome editing tools as well as the availability of transgenic lines (Howe et al., 2013; Hwang et al., 2013; Kettleborough et al., 2013) make the zebrafish an excellent model for studying the molecular requirements of regeneration. Amphibian brain regeneration studies have a rich history; however, amphibians have been less studied in recent years. Anuran amphibians, such as the African clawed frog Xenopus laevis, are an interesting study model as they lose their regenerative abilities after metamorphosis, thus offering the opportunity to study the loss of regeneration within a single animal model. Conversely, urodele amphibians, such as newts and axolotls, are able to regenerate throughout their lifetime. Newts and axolotls might be particularly advantageous systems for studying telencephalon regeneration. The overall telencephalic neuroanatomies of newts and axolotls show greater similarities to those of mammals than the inverted anatomy in teleost fish (see Figure 1). Furthermore, the three currently most widely used species, the red spotted newt, the Iberian ribbed newt (Pleurodeles waltl), and the axolotl, exhibit clear differences in the proliferation pattern of brain ventricular cells. The proliferation of these cells ranges from complete quiescence to continuous proliferation, which offers an attractive base for comparative studies (Berg et al., 2010; Maden et al., 2013; Joven et al., 2018). Recent efforts in sequencing the genomes of Xenopus laevis (Session et al., 2016), axolotl (Nowoshilow et al., 2018), and the Iberian ribbed newt (Elewa et al., 2017) as well as the establishment of genetic tools, such as lineage tracing (Khattak et al., 2013; Joven et al., 2018) and CRISPR/Cas9‐mediated genome editing (Blitz et al., 2013; Fei et al., 2014, 2016, 2017; Aslan et al., 2017; Elewa et al., 2017) provide new opportunities to study brain regeneration in exquisite detail in these animals (Table 1).
Figure 1.
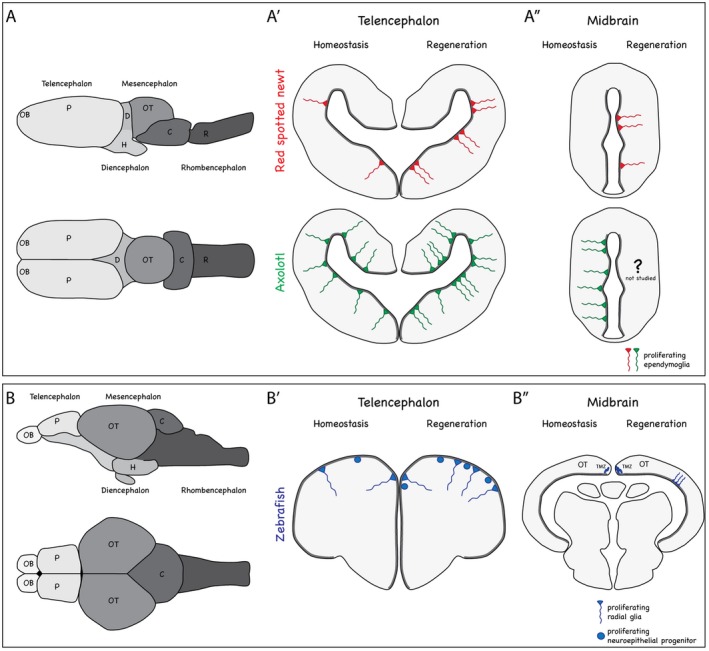
Brain structure and neurogenic niches during homeostasis and regeneration in salamanders and teleost fish. (A and B) Schematic illustrations of the lateral and dorsal views of salamander (A) and zebrafish (B) brains. OB = olfactory bulb, T = telencephalon, OT = optic tectum, D = diencephalon, H = hypothalamus, C = cerebellum, R = rhombencephalon. (A′ – B″) Cross sections through telencephalon and midbrain of red spotted newt, axolotl (A′, A″), and zebrafish (B′, B″). The left hemisphere depicts the proliferative behavior of cells during homeostasis, while the right hemisphere depicts the proliferative behavior of cells following injury. Thick grey lines indicate the ventricular zones, harboring radial glia or ependymoglia. Note the inverted organization of the teleost fish telencephalon, in which radial glia and non‐epithelial progenitors line the outside and neurons are located on the inside. (A′) In red spotted newts, ependymoglia (red) proliferate during homeostasis in confined hot spots, while in axolotl ependymoglia proliferation (green) is observed along the entire ventricular zone. In red spotted newts, ependymoglia cells at hot spots increase their proliferation rate in response to injury and additional hot spots of proliferation are generated. In axolotl, ependymoglia proliferation is increased. (A″) The midbrain of red spotted newts is quiescent during homeostasis, while axolotl midbrain ependymoglia are proliferative. Upon injury in red spotted newts, ependymoglia re‐enter the cell cycle locally where neurons were lost. Midbrain regeneration in axolotl has not been studied. (B′) In zebrafish, radial glia as well as non‐epithelial progenitors (blue) actively divide during homeostasis. Upon injury, additional radial glia and non‐epithelial progenitors are activated to proliferate. (B″) During homeostasis in the optic tectum, radial glia lining the roof of the tectal ventricle are quiescent, while neuroepithelial‐like progenitors located at the tectal marginal zone (TMZ) are proliferative. Upon injury, neuroepithelial‐like progenitors increase their proliferation and radial glia enter the cell cycle
Table 1.
Overview of brain regeneration studies across different amphibian and teleost fish species
| Brain region | Organism | Regeneration | Injury type | Cells of origin | Molecular pathways | Functional recovery | |
|---|---|---|---|---|---|---|---|
| Telencephalon | Amphibians | African clawed frog (Xenopus laevis) | + | Removal of anterior half (Yoshino and Tochinai, 2004, 2006) | Musashi1+ progenitors (Yoshino and Tochinai, 2004) | − | Response to food odor (Yoshino and Tochinai, 2006) |
| only in larval stages 47–54 | |||||||
| Axolotl (Ambystoma mexicanum) | + | Partial removal of dorsal telencephalon (Kirsche and Kirsche, 1964a; Maden et al., 2013; Amamoto et al., 2016) | Ependymoglia (Maden et al., 2013) | − | Electrophysiological features (Amamoto et al., 2016) | ||
| Red spotted newt (Notophthalmus viridescens) | + | Ablation of cholinergic neurons (Berg et al., 2011; Kirkham et al., 2014) | Ependymoglia (Kirkham et al., 2014) | Notch (Kirkham et al., 2014) | − | ||
| Teleost Fish | Zebrafish (Danio rerio) | + | Stab lesion (via skull or nostril) (Kroehne et al., 2011; März et al., 2011; Baumgart et al., 2012; Kishimoto et al., 2012) | Radial glia and non‐glial progenitors (Kroehne et al., 2011) | Cysteinyl leukotriene receptor 1 (Kyritsis et al., 2012) | − | |
| Fibroblast growth factor (Kizil et al., 2012) | |||||||
| Gata3 (Kizil et al., 2012) | |||||||
| Id1(Rodriguez Viales et al., 2015) | |||||||
| Notch (Kishimoto et al., 2012) | |||||||
| Goldfish (Carassius auratus) | + | Removal (Bernstein, 1967; Bernstein and Sadlack, 1969) | − | − | Locomotor function (Venables et al., 2018) | ||
| Tyrosine hydroxylase‐positive neuron ablation (Venables et al., 2018) | |||||||
| Guppy (Lebistes reticulatus) | + | Complete removal (Maron, 1963) | − | − | − | ||
| Stickleback (Gasterosteus aculeatus) | + | Bilateral removal (area dorsalis pars lateralis) (Seegar, 1961) | Starts in ependymal layer (Seegar, 1961) | − | Parental and sexual behavior (Seegar, 1961) | ||
| Optic Tectum | Amphibians | African clawed frog (Xenopus laevis) | + | Removal of optic lobe (McKeown et al., 2013) | Sox2+ progenitors (McKeown et al., 2013) | − | Visual avoidance behavior (McKeown et al., 2013) |
| only in larval stages 47–54 | |||||||
| Italian crested newt (Triturus cristatus carnifex) | + | Partial removal (Minelli and Del Grande, 1974a, 1974b; Minelli et al., 1987, 1990) | − | − | − | ||
| Japanese fire belly newt (Cynops pyrrhogaster) | + | Unilateral removal (Okamoto et al., 2007) | − | − | − | ||
| Teleost Fish | Zebrafish (Danio rerio) | + | Stab lesion (Lindsey et al., 2018) | Neuroepithelial‐like progenitors and radial glia (Lindsey et al., 2018) | − | − | |
| Goldfish (Carassius auratus) | + | Unilateral removal (Davis and Schlumpf, 1984) | − | − | Reappearance of vision (Davis and Schlumpf, 1984) | ||
| Crucian carp (Carassius carassius) | + | Unilateral removal (Kirsche and Kirsche, 1961) | − | − | Locomotor function (Kirsche and Kirsche, 1961) | ||
| Mesencephalon | Amphibians | Red spotted newt (Notophthalmus viridescens) | + | Ablation of dopaminergic neurons (Parish et al., 2007; Berg et al., 2010, 2011) | Ependymoglia (Parish et al., 2007; Berg et al., 2010, 2011 | Dopamine (Berg et al., 2011) | Motor behavior (Parish et al., 2007) |
| Ablation of cholinergic neurons (Berg et al., 2011) | Reactive oxygen species (Hameed et al., 2015) | ||||||
| Sonic hedgehog (Berg et al., 2010) | |||||||
| Iberian ribbed newt (Pleurodeles walt) | + | Ablation of dopaminergic neurons (Joven et al., 2018) | Ependymoglia (Joven et al., 2018) | − | − | ||
| Cerebellum | Teleost Fish | Zebrafish (Danio rerio) | + | Unilateral ablation (Kaslin et al., 2017) | Neuroepithelial‐like stem cells (Kaslin et al., 2017) | − | Swimming behavior (Kaslin et al., 2017) |
| Brown ghost knifefish (Apteronotus leptorhynchus) | + | Stab lesion (Zupanc et al., 1998; Clint and Zupanc, 2001; Zupanc and Clint, 2001; Zupanc and Zupanc, 2006) | − | − | − | ||
Other anuaran amphibians such as Rana esculenta and Rana fusca do not exhibit brain regeneration.
+ Indicates that regeneration is present.
− Indicates that no studies have been performed.
Brain Regeneration in Anuran Amphibians
A key point of interest in anuran amphibians is the decline of regenerative abilities during, and after metamorphosis. The first report on brain regeneration in frogs dates back to 1890, in which removing the cerebral hemispheres from adult Xenopus laevis led to the formation of a cerebral mass, which was thought to contain newly born nerve cells (Danielewsky, 1890). Later studies revealed that Xenopus laevis can regenerate both telencephalon and optic tectum during larval stages (Srebro, 1957; Yoshino and Tochinai, 2004) (Filoni and Gibertini, 1969), whereas regeneration and wound closure are absent in adults (Srebro, 1965). Alongside this decline in regenerative abilities, the number of undifferentiated, proliferating cells decreases in the brains of larval and metamorphosing animals (Yoshino and Tochinai, 2004).
In Xenopus larvae, the source of regenerated cells lies within the ventricular zones of the respective brain region, similar to salamanders and teleost fish. In both the telencephalon and optic tectum, injury induces an increase in the proliferation of Musashi1‐positive tectal progenitors as well as Sox2‐expressing neural progenitors, which ultimately differentiate into N‐β‐tubulin‐positive neurons (Yoshino and Tochinai, 2004; McKeown et al., 2013). It has not been addressed if Musashi1‐positive and Sox2‐positive cells are the same cell population and harbor the same regenerative potentials.
While the understanding of the molecular mechanisms underlying brain regeneration in anurans is currently sparse, the system has been important in demonstrating the requirement of nerves for brain regeneration. Input from the olfactory placode and the olfactory nerve is required for telencephalon regeneration in Xenopus and, similarly, optic tectum regeneration is dependent on optic nerve input (Yoshino and Tochinai, 2006). Interestingly, nerve dependency has long been studied in the context of limb regeneration in salamanders (Stocum, 2011) and Xenopus tadpoles (Filoni and Paglialunga, 1990) as well as fin regeneration in fish (Simões et al., 2014) where the nerves contribute factors that induce the proliferation of undifferentiated progenitor cells in the blastema. It remains to be addressed whether the nerve dependency of brain regeneration and limb regeneration are a result of common underlying mechanisms.
Another unanswered question is whether cells in the adult frog brain are intrinsically incompetent to perform a regenerative response or whether extrinsic signals create a regeneration‐deficient environment. To address this, larval or froglet telencephalon cell suspensions were transplanted into the space created in froglet brains by partially removing the telencephalon (Yoshino and Tochinai, 2004). Surprisingly, endogenous and transplanted cells were able to close the wound and generate a deformed telencephalon‐like structure that connected with the olfactory nerves. This led to the proposal that adult frogs do not lack the neural stem cells necessary for brain regeneration but that instead the failure of adult ependymal neural cells to migrate and close the wound might result in failed regeneration. These results raise an interesting question that could be addressed in the future: Would adult frogs be able to regenerate smaller injuries, or selective ablation of cell types, that do not require massive wound closure?
Brain regeneration in urodele amphibians
In contrast to anuran amphibians, urodeles such as newts and axolotls maintain their regenerative abilities throughout adulthood. The first studies on brain regeneration in salamanders date back to 1916 when Harold Burr found that forebrain regeneration occurs in axolotl larvae (Burr, 1916). Adult axolotls are also able to fully regenerate when a large portion of their telencephalon is removed (Kirsche and Kirsche, 1964a) and can even recover from complete lobectomy (Richter, 1968). Recent studies have revealed that the original neuronal diversity, even at the level of subpopulations, is regenerated after such injuries (Amamoto et al., 2016). Importantly however, resection of the whole telencephalic hemisphere does not lead to regeneration (Kirsche and Kirsche, 1964a). A detailed analysis of the proliferative dynamics in the axolotl brain using thymidine analogue incorporation has uncovered that ependymoglia cells display neurogenic potential across all brain regions under homeostatic conditions, as well as after injury to the telencephalon (Maden et al., 2013). As in Xenopus, the olfactory nerve has been shown to play a crucial role during telencephalic regeneration (Kirsche and Kirsche, 1964b; Maden et al., 2013). Removal of the olfactory nerve blocks regeneration until it is reconnected to the remaining part of the telencephalon (Maden et al., 2013). While some studies have speculated that the olfactory nerve is a source of cells that migrate into the injured telencephalon to initiate regeneration (Kirsche and Kirsche, 1964b; Winkelmann and Winkelmann, 1970; Richter, 1968), it is more probable that the nerve provides cues to stimulate the proliferation of ependymal cells in the telencephalon (Maden et al., 2013). The molecular nature of these signals, and how they reach the ependymoglia cells to initiate the regenerative program, remains unknown. It is also not known if nerve dependency in the brain reflects a need for extracellular signaling factors secreted by nerve cells, or for olfactory or visual experience (or a combination of both).
Several newt species have also been used to understand regeneration in different brain regions. Mechanical removal of large portions of the optic tectum leads to regeneration in the Italian crested newt (Triturus cristatus carnifex) (Minelli and Del Grande, 1974a, 1974b; Minelli et al., 1987, 1990). Optic tectum regeneration as well as retinotectal projection recovery can be observed in Japanese fire belly newts (Cynops pyrrhogaster) (Okamoto et al., 2007). The red spotted newt has been established as a great model to study cell type‐specific regeneration, for example of dopaminergic in the midbrain or cholinergic neurons in the telencephalon (Berg et al., 2010; Kirkham et al., 2014). In contrast to the axolotl, homeostatic proliferation in the red spotted newt is restricted to the forebrain, but quiescent ependymoglia cells can also proliferate in response to injury and regenerate lost cell types in other brain regions such as the midbrain (Berg et al., 2010). Ependymoglia have been identified as the source of brain regeneration in both the red spotted and Iberian newts, through thymidine analogue incorporation and lineage tracing by electroporation of reporter constructs (Berg et al., 2010; Kirkham et al., 2014; Joven et al., 2018)., Two types of ependymoglia have been identified in the telencephalon of the red spotted newt, and these are distributed unevenly along the ventricle (Kirkham et al., 2014). Type‐1 ependymoglia are dispersed along the ventricle and create new neurogenic regions after injury. They express both glutamine synthetase and glial fibrillary acidic protein and are considered to be slowly dividing stem cells as they retain the thymidine analogue BrdU for long periods of time in pulse‐chase experiments. Type‐2 ependymoglia are located in proliferative hot spots. They express glial fibrillary acidic protein and do not retain BrdU for long periods of time, leading to their classification as transit‐amplifying cells. Upon ablation of cholinergic neurons to de novo neurogenic niches, proliferation of type‐1 cells as well as appearance of type‐2 cells is observed. It will be important to determine the heterogeneities in ependymoglia cell types, exact lineage relationships, and their potencies during growth and regeneration in both the axolotl and newts to understand how similar or different they are to cells in other species.
The signaling pathways controlling brain regeneration have been studied in the red spotted newt using several experimental paradigms. Hameed and colleagues investigated how environmental factors may put species under a selective pressure to manifest regenerative abilities. The authors simulated experimentally the low oxygen conditions that newts experience in the wild during winter and examined the response of the brain to these conditions (Hameed et al., 2015). Reactive oxygen species (ROS) accumulated in ependymoglia and induced cell cycle re‐entry, ultimately leading to neurogenesis. Modulation of the oxygen levels also led to the activation of microglia, although these cells have no role in promoting the proliferation of ependymoglia. It was shown that regeneration under normoxia is also ROS‐dependent, indicating that this mechanism might have been co‐opted during evolution to allow regeneration.
Regeneration not only requires the activation of stem or progenitor cell proliferation but also the appropriate termination to avoid tumor formation. One mechanism to ensure this is through negative feedback from differentiated cells. In the newt midbrain, dopaminergic neurons exert a proliferation block on ependymoglia cells via dopamine signaling (Berg et al., 2011). Loss of dopamine production after targeted ablation of dopaminergic neurons lifts this block, allowing ependymoglia to enter the regenerative program until the block is reinstated via the regeneration of dopaminergic neurons. In addition, regeneration of dopaminergic neurons is also dependent on Sonic hedgehog signaling (Berg et al., 2010), which has also been studied in other models such as tail in axolotl (Schnapp et al., 2005) and lens regeneration in newts (Tsonis et al., 2004). Whether or not the same mechanisms are used to elicit regeneration in other parts of the brain and in other salamander species will be an interesting future direction to pursue. Indeed, it is a promising time to study salamander brain regeneration, as gene editing tools, transgenic Cre‐driver strains, as well as genomic resources have been recently developed for both the axolotl (Khattak et al., 2013; Fei et al., 2014, 2016, 2017; Nowoshilow et al., 2018) and the Iberian ribbed newt (Elewa et al., 2017).
Brain Regeneration in Teleost Fish
Various teleost fish species have been studied in brain regeneration research, such as goldfish (Carassius auratus) (Bernstein and Sadlack, 1969; Davis and Schlumpf, 1984; Pflugfelder, 1965), adult sticklebacks (Gasterosteus aculeatus) (Seegar, 1961, 1962, 1965), guppies (Poecilia reticulata) (Maron, 1963), caruscian carps (Carassius carassius) (Kirsche and Kirsche, 1961), and the brown ghost knifefish (Apteronotus leptorhynchus) (Zupanc et al., 1998; Zupanc and Ott, 1999; Clint and Zupanc, 2001; Zupanc and Clint, 2001; Zupanc and Zupanc, 2006). However, in the last decades, the zebrafish has become the favored model to investigate regeneration of the telencephalon, optic tectum, and cerebellum.
The identification of the cells responsible for brain regeneration has been limited to thymidine analogue incorporations in most teleost fish (stickleback and goldfish). In contrast, the zebrafish has served as a great model to understand stem and progenitor cell dynamics during brain regeneration due to its compatibility with Cre‐loxP‐mediated lineage tracing. The teleost telencephalon harbors neurogenic radial glial cells that act as self‐renewing and multipotent progenitors at the single cell level, behaving as bona fide neural stem cells (Rothenaigner et al., 2011). In addition, non‐glial cycling neuroblasts, possible equivalents of mammalian transit‐amplifying progenitors, are dispersed along the telencephalic ventricle (März et al., 2010). Altogether four types of ventricular cells have been identified in the telencephalon: PCNA‐negative radial glia (“type I”), PCNA‐positive radial glia (“type II”), which are highly similar to the ependymoglia types found in the newt telencephalon, and dividing non‐glial progenitors (“type IIIa and IIIb”) (März et al., 2010). Upon injury, the proliferation rate of both radial glia and non‐glial progenitors is increased, ultimately leading to replacement of the lost cells (März et al., 2011; Kroehne et al., 2011; Baumgart et al., 2012; Kishimoto et al., 2012). Noninvasive in vivo imaging and lineage‐tracing of individual cells have been used to understand the dynamic behaviors of radial glia during homeostasis and regeneration (Barbosa et al., 2015; Dray et al., 2015). Surprisingly, it was found that mechanical lesion leads to increased symmetric, neurogenic divisions of radial glia, which results in the depletion of stem cells (Barbosa et al., 2015). While the authors showed that the zebrafish brain undergoes a decline in proliferative cells with age, it remains to be determined whether the ventricular zone undergoes reductions in size and cell number, as would be predicted. Moreover, these results raise the question of whether repeated injury to the telencephalon would eventually result in failed regeneration. Additionally, it could be of great interest to compare the injury‐induced behaviors of radial glia in different telencephalic regions, which could relate to the diverse behaviors observed during homeostasis (Dray et al., 2015).
Recent studies in other areas of the zebrafish brain have uncovered important differences in the source of regeneration, even in the same species. Whereas the telencephalon relies on both radial glia and non‐glial progenitors during homeostasis and regeneration, a clear separation between these two cell types can be determined in the optic tectum (Lindsey et al., 2018). Neuroepithelial‐like progenitors, which are restricted to the edge of the optic tectum, proliferate during homeostasis and increase their proliferation rate following stab lesion injury (Lindsey et al., 2018). Radial glia, which line the ventricle, are quiescent during homeostasis, but become activated to proliferate following injury (Lindsey et al., 2018). Conversely, during regeneration of the adult zebrafish cerebellum, radial glia cells play only minor roles. Here, neuroepithelial‐like stem cells are the source of regeneration, although they fail to regenerate all cell types (Kaslin et al., 2017).
How the presence of an injury reaches the cells responsible for regeneration, and the molecular pathways involved in regulating the proliferation and differentiation of these cells, has been extensively studied in zebrafish. Activation of the immune system is one of the first responses detected after injury and is necessary and sufficient to enhance the proliferation of radial glia (Kyritsis et al., 2012). These observations stand in contrast to results from red spotted newts, in which prolonged microglia responses are detrimental for the generation of dopaminergic neurons (Kirkham et al., 2011). In zebrafish, Cysteinyl leukotriene receptor 1 signaling is a necessary and sufficient component of this response. Overexpression of Cysteinyl leukotriene receptor 1 in the uninjured brain leads to increased proliferation, as well as expression of the zinc‐finger transcription factor gata3. Gata3 expression itself is induced only after injury in both radial glia and newborn neurons and is necessary, but not sufficient, for proliferation, neurogenesis, and distribution of newborn neurons in the tissue (Kizil et al., 2012). Interestingly, Gata3 has similar functions during regeneration of the heart and fins. Its expression is directly regulated by Fgf signaling in an injury‐dependent context in both brain and fin and is likely involved in the regenerative response of additional tissues, as its expression is also induced after injury to the cerebellum, optic tectum, spinal cord, and liver.
It will be exciting to investigate if the signaling pathways that have been studied in the context of telencephalon regeneration also play a conserved functional role in regeneration of the optic tectum and cerebellum, where different progenitors are induced to proliferate upon injury.
How can We Integrate and Compare Knowledge from Different Brain Injuries and Different Species?
When comparing our current knowledge on brain regeneration in aquatic species, it becomes clear that there are both similarities and differences. For example, both the newt and zebrafish telencephalon harbor similar glial cell populations: quiescent type I radial glia/ependymoglia as well as proliferating type II radial glia/ependymoglia. These two glia types are comparatively similar with respect to marker gene expression and proliferative behaviors. However, in addition to these glial cell types, each species contains unique cell types. Neuroepithelial‐like progenitors, like the type III cells in zebrafish, have not been detected in any salamander telencephalon studied to date. Conversely, glutamine synthetase‐negative, glial marker‐positive cells are not present in the zebrafish telencephalon.
One might additionally wonder how different types of injury affect the outcome of regeneration. Common, comparable injury models exist for other regenerative organs such as limbs in urodeles and anurans (Dent, 1962; Iten and Bryant, 1973). A diversity of injury paradigms have been used for the brain, which has complicated comparison, as some striking differences exist in the requirements for regeneration in each case. Selective ablation of specific neuronal cell types using drugs does not injure ependymoglia or radial glia and solely requires the regeneration of the ablated cell lineage. Large injuries, generated by the removal of brain tissue, lead to injury of the ventricular zone, likely death of ependymoglia or radial glia and multiple different cell types that need to be regenerated subsequently. It remains to be addressed whether the same cell populations are responsible for regeneration in both injury models, and whether they use comparable or differential signaling pathways.
Evidence that different injury models lead to divergent outcomes was found in the zebrafish telencephalon. Radial glia in zebrafish react to stab wound injuries through the skull by upregulation of glial fibrillary acidic protein and glial swelling (März et al., 2010; Kishimoto et al., 2012), while this reaction is not induced when injury is generated through the nostril (Kroehne et al., 2011; Baumgart et al., 2012). It is likely that the injury through the skull is more comparable to large ablations, as ependymoglia and the ventricle are injured and the cerebrospinal fluid can leak into the brain tissue. Whether this results in different injury signals being present and different ependymoglia being activated remains to be addressed.
Finally, one might also wonder whether different injury methods might reveal differential regenerative potentials. It is curious to note that the adult goldfish telencephalon does not undergo regeneration when a large brain mass is removed, while, in contrast, it can regenerate dopaminergic neurons after selective chemical ablation (Bernstein, 1967; Venables et al., 2018). In light of the recent findings that radial glia in the zebrafish telencephalon have a limited capacity for self‐renewal (Barbosa et al., 2015), one wonders whether regeneration of large injuries can be accomplished at all in zebrafish. Even though the removal of telencephalic regions has not been performed in zebrafish, it could be speculated that massive injuries would not be regenerated due to the lack of sufficient self‐renewing divisions. Newts and axolotl have the ability to regenerate large injuries, opening up the question of whether the self‐renewing capacities of salamander glial cells differ from those observed in zebrafish. It is possible that signaling pathways such as the planar cell polarity pathway, which has been shown to direct self‐renewing divisions in axolotl spinal cord regeneration (Albors et al., 2015) and in mouse brain development (Delaunay et al., 2014), are active in salamander glia cells but not in teleost fish. Additionally, these results raise the question of whether the lack of regeneration in adult Xenopus is to some extent caused by the lack of glial self‐renewal. It is tempting to speculate that low numbers of self‐renewing divisions, leading to a rapid depletion of glia cells, might impede the closure of large injuries. The fact that the transplantation of both larval and froglet brain cells into an injured froglet brain results in improved regeneration could also be explained by this hypothesis: increased numbers of ependymal cells, regardless of whether they are derived from larvae or froglets, could provide an increased cellular source for regeneration. With this in mind, it will be interesting to address whether adult frogs are able to regenerate neuronal cells in response to selective ablations.
Functional Recovery and The Perfection of Brain Regeneration
Limb regeneration, as well as spinal cord regeneration, in salamanders predominantly results in the reappearance of a perfect, functional copy of the original structure (Schnapp et al., 2005; Diogo et al., 2014). Is this also achieved after brain regeneration? As the brain is the central unit that orchestrates the execution of behaviors, it is extremely attractive to address the restoration of function.
One important requirement to understand whether an original copy can be regenerated faithfully is the ability to distinguish different cell types, such as neuronal subtypes and their localization across brain areas. Amamoto and colleagues have addressed this problem to great detail in the axolotl telencephalon, which regenerates all neuronal subtypes after removal of a large portion of the telencephalon (Amamoto et al., 2016). However, the regenerated structure is disorganized: the distribution of neurons is altered and neuronal subtypes are not arranged in distinct domains as seen before injury. The reason for this uncoupling of cell type diversity and spatial organization is not yet known, and the functional consequences of such a disorganized, regenerated brain area remain to be analyzed in the future. In the zebrafish retina, it has been shown that the specificity of neuronal connections is to largely maintained during regeneration (Yoshimatsu et al., 2016). H3 horizontal cells, which preferentially connect to ultraviolet cones, re‐establish their connections following ultraviolet cone ablation and regeneration. However, the cues determining synaptic specificity can only be maintained within a limited time period after injury. Upon regeneration, delay by repeated ablation of ultraviolet cones synaptic specificity is not restored. These results indicate that comparing large injuries, which require long time periods to be regenerated, to smaller, cell‐type specific ablations could be important when addressing the re‐emergence of synaptic connections during brain regeneration.
Studying functional recovery after brain regeneration requires the recording and quantification of stereotypic behaviors. Xenopus has proven to be an optimal model, as it performs highly stereotypic behaviors. A food‐induced response known as “forelimb sweeping” has been used to study functional telencephalic regeneration in larval Xenopus brains (Hutchison, 1964; Avila and Frye, 1977; Yoshino and Tochinai, 2006). A visual avoidance response to moving stimuli serves as a readout for functional optic tectum regeneration (McKeown et al., 2013). A commonly used assay to study functional recovery after brain injury in teleost fish and salamanders is the re‐occurrence of locomotor function. Such assays have been utilized after injury and regeneration of the cerebellum in zebrafish (Kaslin et al., 2017) as well as after targeted ablation and regeneration of dopaminergic neurons in the goldfish telencephalon (Venables et al., 2018). Likewise, in red spotted newts, locomotor recovery assays were used to study functional regeneration of dopaminergic and cholinergic neurons (Parish et al., 2007).
In the future, it will be exciting to determine if a regenerated brain is able to fulfill the same functions as the original, and whether plasticity in neuronal connections and a failure to re‐establish synaptic connections might be reasons why brain regeneration does not lead to perfect regeneration in some cases.
Conclusion
We have acquired substantial knowledge on the processes of injury‐induced proliferation and neurogenesis in the brains of different species. In the future, it will be interesting to use comparable injury models to assess the differences and similarities between species more rigorously. By doing so, we will be able to achieve a more comparative understanding of the processes involved in brain regeneration, which will help determine the strategies that could be used to induce brain repair in non‐regenerative species.
Conflict of Interest
The authors indicate no potential conflicts of interests.
Acknowledgements
We are grateful to Leo Otsuki for his helpful comments on the manuscript.
This work was supported by the Research Institute of Molecular Pathology (IMP).
K.L. is supported by a Long‐Term Fellowship from the Human Frontier Science Program LT000605/2018‐L.
Literature Cited
- Albors, A.R. , Tazaki, A. , Rost, F. , Nowoshilow, S. , Chara, O. and Tanaka, E.M. (2015) Planar cell polarity‐mediated induction of neural stem cell expansion during axolotl spinal cord regeneration. Elife, 4, e10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amamoto, R. , Gisselle, V. , Huerta, L. , Takahashi, E. , Dai, G. , Grant, A. , et al. (2016) Adult axolotls can regenerate original neuronal diversity in response to brain injury. Elife, 5, e13998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslan, Y. , Tadjuidje, E. , Zorn, A.M. and Cha, S.‐W. (2017) High efficiency non‐mosaic CRISPR mediated knock‐in and mutations in F0 Xenopus . Development, 144, 2852–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila, V.L. and Frye, P.G. (1977) Feeding behavior in the African clawed frog (Xenopus laevis Daudin). Herpetologica, 33, 152–161. [Google Scholar]
- Barbosa, J.S. , Sanchez‐Gonzalez, R. , Di Giaimo, R., Baumgart, E.V. , Theis, F.J. , Götz, M. , et al. (2015) Live imaging of adult neural stem cell behavior in the intact and injured zebrafish brain. Science, 348, 789–793. [DOI] [PubMed] [Google Scholar]
- Baumgart, E.V. , Barbosa, J.S. , Bally‐cuif, L. , Götz, M. and Ninkovic, J. (2012) Stab wound injury of the zebrafish telencephalon: a model for comparative analysis of reactive gliosis. Glia, 60, 343–357. [DOI] [PubMed] [Google Scholar]
- Becker, T. , Wullimann, M.F. , Becker, C.G. , Bernhardt, R.R. and Schachner, M. (1997) Axonal regrowth after spinal cord transection in adult zebrafish. The Journal of Comparative Neurology, 377, 577–595. [DOI] [PubMed] [Google Scholar]
- Berg, D.A. , Kirkham, M. , Beljajeva, A. , Knapp, D. , Habermann, B. , Ryge, J. , et al. (2010) Efficient regeneration by activation of neurogenesis in homeostatically quiescent regions of the adult vertebrate brain. Development, 138, 4127–4134. [DOI] [PubMed] [Google Scholar]
- Berg, D.A. , Kirkham, M. , Wang, H. , Frisén, J. and Simon, A. (2011) Dopamine controls neurogenesis in the adult salamander midbrain in homeostasis and during regeneration of dopamine neurons. Cell Stem Cell, 8, 426–433. [DOI] [PubMed] [Google Scholar]
- Bernardos, R.L. , Barthel, L.K. , Meyers, J.R. and Raymond, P.A. (2007) Late‐stage neuronal progenitors in the retina are radial Müller glia that function as retinal stem cells. Journal of Neuroscience, 27, 7028–7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein, J.J. (1967) The regenerative capacity of the telencephalon of the goldfish and rat. Experimental Neurology, 17, 44–56. [DOI] [PubMed] [Google Scholar]
- Bernstein, J. and Sadlack, F. (1969) The formation of new neurons following telencephalic lesions in goldfish. The Anatomical Record, 163. [Google Scholar]
- Blitz, I.L. , Biesinger, J. , Xie, X. and Cho, K.W.Y. (2013) Biallelic genome modification in F0 Xenopus tropicalis embryos using the CRISPR/Cas system. Genesis, 51, 827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr, H. (1916) Regeneration in the brain of Amblystoma Journal of Comparative Neurology, 26, 203–211. [Google Scholar]
- Clint, S.C. and Zupanc, G.K.H. (2001) Neuronal regeneration in the cerebellum of adult teleost fish, Apteronotus leptorhynchus: guidance of migrating young cells by radial glia. Developmental Brain Research, 130, 15–23. [DOI] [PubMed] [Google Scholar]
- Danielewsky, B. (1890) Über die Regeneration der Grosshirn Hemisphären beim Frosch. Verh X inter med Kongr Bd. 2.
- Davis, R. and Schlumpf, B. (1984) Visual recovery in goldfish following unilateral optic tectum ablation. Evidence of competition between optic axons for tectal targets. Behavioural Brain Research, 3, 287–291. [DOI] [PubMed] [Google Scholar]
- Delaunay, D. , Cortay, V. , Patti, D. , Knoblauch, K. and Dehay, C. (2014) Mitotic spindle asymmetry: a Wnt/PCP‐regulated mechanism generating asymmetrical division in cortical precursors. Cell Reports, 6, 400–414. [DOI] [PubMed] [Google Scholar]
- Dent, J.N. (1962) Limb regeneration in larvae and metamorphosing individuals of the South African clawed toad. Journal of Morphology, 110, 61–77. [DOI] [PubMed] [Google Scholar]
- Diogo, R. , Nacu, E. and Tanaka, E.M. (2014) Is salamander limb regeneration really perfect? Anatomical and morphogenetic analysis of forelimb muscle regeneration in GFP‐transgenic axolotls as a basis for regenerative, developmental, and evolutionary studies. Anatomical Record, 297, 1076–1089. [DOI] [PubMed] [Google Scholar]
- Dray, N. , Bedu, S. , Vuillemin, N. , Alunni, A. , Coolen, M. , Krecsmarik, M. , et al (2015) Large‐scale live imaging of adult neural stem cells in their endogenous niche. Development, 142, 3592–3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi, G. , Eguchi, Y. , Nakamura, K. , Yadav, M.C. , Millán, J.L. and Tsonis, P.A. (2011) Regenerative capacity in newts is not altered by repeated regeneration and ageing. Nature Communications, 2, 384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elewa, A. , Wang, H. , Talavera‐López, C. , Joven, A. , Brito, G. , Kumar, A. , et al. (2017) Reading and editing the Pleurodeles waltl genome reveals novel features of tetrapod regeneration. Nature Communications, 8, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei, J. , Schuez, M. , Tazaki, A. , Taniguchi, Y. , Roensch, K. and Tanaka, E.M. (2014) CRISPR‐mediated genomic deletion of Sox2 in the axolotl shows a requirement in spinal cord neural stem cell amplification during tail regeneration. Stem Cell Reports, 3, 444–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei, J. , Knapp, D. , Schuez, M. , Murawala, P. , Zou, Y. , Singh, S.P. , et al (2016) Tissue‐ and time‐directed electroporation of CAS9 protein – gRNA complexes in vivo yields efficient multigene knockout for studying gene function in regeneration. Regenerative Medicine, 1, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei, J.‐F. , Schuez, M. , Knapp, D. , Taniguchi, Y. , Drechsel, D.N. and Tanaka, E.M. (2017) Efficient gene knockin in axolotl and its use to test the role of satellite cells in limb regeneration. Proceedings of the National Academy of Sciences, 114, 12501–12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filoni, S. and Gibertini, G. (1969) A study of the regenerative capacity of the central nervous system of anuran amphibia in relation to their stage of development. I. Observations on the regeneration of the optic lobe of Xenopus laevis (Daudin) in the larval stages. Archives of Biological, 80, 369–411. [PubMed] [Google Scholar]
- Filoni, S. and Paglialunga, L. (1990) Effect of denervation on hindlimb regeneration in Xenopus laevis larvae. Differentiation, 43, 10–19. [DOI] [PubMed] [Google Scholar]
- Hameed, L.S. , Berg, D.A. , Belnoue, L. , Jensen, L.D. , Cao, Y. and Simon, A. (2015) Environmental changes in oxygen tension reveal ROS‐dependent neurogenesis and regeneration in the adult newt brain. Elife, 4, e08422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe, K. , Clark, M.D. , Torroja, C.F. , Torrance, J. , Berthelot, C. , Muffato, M. , et al. (2013) The zebrafish reference genome sequence and its relationship to the human genome. Nature, 496, 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison, J.B. (1964) Investigations on the neural control of clasping and feeding in Xenopus laevis (Daudin). Behaviour, 24, 47–65. [DOI] [PubMed] [Google Scholar]
- Hwang, W.Y. , Fu, Y. , Reyon, D. , Maeder, M.L. , Tsai, S.Q. , Sander, J.D. , et al (2013) Efficient genome editing in zebrafish using a CRISPR‐Cas system. Nature Biotechnology, 31, 227–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iten, L.E. and Bryant, S.V. (1973) Forelimb regeneration from different levels of amputation in the newt, Notophthalmus viridescens: length, rate, and stages. Wilhelm Roux’ Archiv für Entwicklungsmechanik der Organismen, 173, 263–282. [DOI] [PubMed] [Google Scholar]
- Joven, A. , Wang, H. , Pinheiro, T. , Hameed, L.S. , Belnoue, L. and Simon, A. (2018) Cellular basis of brain maturation and acquisition of complex behaviors in salamanders. Development, 145, dev160051. [DOI] [PubMed] [Google Scholar]
- Kaslin, J. , Kroehne, V. , Ganz, J. , Hans, S. and Brand, M. (2017) Distinct roles of neuroepithelial‐like and radial glia‐like progenitor cells in cerebellar regeneration. Development, 144, 1462–1471. [DOI] [PubMed] [Google Scholar]
- Kettleborough, R.N.W. , Busch‐Nentwich, E.M. , Harvey, S.A. , Dooley, C.M. , De Bruijn, E. , Van Eeden, F. , et al. (2013) A systematic genome‐wide analysis of zebrafish protein‐coding gene function. Nature, 496, 494–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattak, S. , Schuez, M. , Richter, T. , Knapp, D. , Haigo, S.L. , Sandoval‐Guzmán, T. , et al (2013) Germline transgenic methods for tracking cells and testing gene function during regeneration in the axolotl. Stem Cell Reports, 1, 90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham, M. , Berg, D.A. and Simon, A. (2011) Microglia activation during neuroregeneration in the adult vertebrate brain. Neuroscience Letters, 497, 11–16. [DOI] [PubMed] [Google Scholar]
- Kirkham, M. , Hameed, L.S. , Berg, D.A. , Wang, H. and Simon, A. (2014) Progenitor cell dynamics in the newt telencephalon during homeostasis and neuronal regeneration. Stem Cell Reports, 2, 507–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsche, W. and Kirsche, K. (1961) Experimentelle Untersuchungen zur Frage der Regeneration und Funktion des Tectum Opticum von Carassius carassius . Zeitschrift fuer Mikroskopisch‐Anatomische Forschung, 67, 140–182. [PubMed] [Google Scholar]
- Kirsche, K. and Kirsche, W. (1964a) Regenerative processes in the telencephalon of Ambystoma mexicanum . Journal fur Hirnforschung 7, 421–436. [PubMed] [Google Scholar]
- Kirsche, K. and Kirsche, W. (1964b) Experimentelle Untersuchung über den Einfluß der Regeneration des Nervus olfactorius auf die Vorderhirnregeneration von Ambystoma mexicanum . Journal fur Hirnforschung, 7, 315–333. [PubMed] [Google Scholar]
- Kishimoto, N. , Shimizu, K. and Sawamoto, K. (2012) Neuronal regeneration in a zebrafish model of adult brain injury. Disease Models & Mechanisms, 5, 200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizil, C. , Kyritsis, N. , Dudczig, S. , Kroehne, V. , Freudenreich, D. , Kaslin, J. , et al (2012) Regenerative neurogenesis from neural progenitor cells requires injury‐induced expression of Gata3. Developmental Cell, 23, 1230–1237. [DOI] [PubMed] [Google Scholar]
- Kroehne, V. , Freudenreich, D. , Hans, S. , Kaslin, J. and Brand, M. (2011) Regeneration of the adult zebrafish brain from neurogenic radial glia‐type progenitors. Development, 138, 4831–4841. [DOI] [PubMed] [Google Scholar]
- Kyritsis, N. , Kizil, C. , Zocher, S. , Kroehne, V. , Kaslin, J. , Freudenreich, D. , et al (2012) Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science, 338, 1353–1356. [DOI] [PubMed] [Google Scholar]
- Lai, S. , Kuenne, C. , Kuan, J. , Lai, H. , Tsedeke, A.T. , Guenther, S. , et al (2017) Reciprocal analyses in zebrafish and medaka reveal that harnessing the immune response promotes cardiac regeneration. Elife, 6, e25605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhe, R. , Chesneau, A. , Colozza, G. , Hidalgo, M. , Ail, D. , Locker, M., et al (2017) Müller glial cell reactivation in Xenopus models of retinal degeneration. Glia, 65, 1333–1349. [DOI] [PubMed] [Google Scholar]
- Lindsey, B.W. , Aitken, G.E. , Tang, J.K. , Khabooshan, M. and Vandestadt, C. (2018) Tectal stem cells display diverse regenerative capacities. bioRxiv 268136, 10.1101/268136 [DOI] [PMC free article] [PubMed]
- Lust, K. and Wittbrodt, J. (2018) Activating the regenerative potential of Müller glia cells in a regeneration‐deficient retina. Elife, 7, e32319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden, M. , Manwell, L.A. and Ormerod, B.K. (2013) Proliferation zones in the axolotl brain and regeneration of the telencephalon. Neural Development, 8, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron, K. (1963) Endbrain regeneration in Lebistes reticulatus . Folia Biologica (Praha), 11, 1–10. [Google Scholar]
- März, M. , Chapouton, P. , Diotel, N. , Vaillant, C. , Hesl, B. , Takamiya, M. , et al (2010) Heterogeneity in progenitor cell subtypes in the ventricular zone of the zebrafish adult telencephalon. Glia, 58, 870–888. [DOI] [PubMed] [Google Scholar]
- März, M. , Schmidt, R. , Rastegar, S. and Strahle, U. (2011) Regenerative response following stab injury in the adult zebrafish telencephalon. Developmental Dynamics, 240, 2221–2231. [DOI] [PubMed] [Google Scholar]
- McKeown, C.R. , Sharma, P. , Sharipov, H.E. , Shen, W. and Cline, H.T. (2013) Neurogenesis is required for behavioral recovery after injury in the visual system of Xenopus laevis . The Journal of Comparative Neurology, 521, 2262–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minelli, G. and Del Grande, P. (1974a) Histoautoradiographic studies of encephalic reactions in regeneration of the optic tectum of Triturus cristatus carnifex . Archivio Italiano di Anatomia e di Embriologia, 79, 103–114. [PubMed] [Google Scholar]
- Minelli, G. and Del Grande, P. (1974b) Localization and quantitative analysis of the elements leading to the regeneration of the optic tectum in the adult Triturus cristatus carnifex . Zeitschrift für mikroskopisch‐anatomische Forschung, 88, 209–224. [PubMed] [Google Scholar]
- Minelli, G. , Franceschini, V. , Del Grande, P. and Ciani, F. (1987) Newly‐formed neurons in the regenerating optic tectum of Triturus cristatus carnifex . Basic and Applied Histochemistry, 31,43–52. [PubMed] [Google Scholar]
- Minelli, G. , del Grande, P. , Franceschini, V. and Ciani, F. (1990) Proliferative response of the mesencephalic matrix areas in the reparation of the optic tectum of Triturus cristatus carnifex . Zeitschrift für mikroskopisch‐anatomische Forschung, 104, 17–25. [PubMed] [Google Scholar]
- Nowoshilow, S. , Schloissnig, S. , Fei, J.F. , Dahl, A. , Pang, A.W.C. , Pippel, M. , et al. (2018) The axolotl genome and the evolution of key tissue formation regulators. Nature, 554, 50–55. [DOI] [PubMed] [Google Scholar]
- Okamoto, M. , Ohsawa, H. , Hayashi, T. , Owaribe, K. and Tsonis, P.A. (2007) Regeneration of retinotectal projections after optic tectum removal in adult newts. Molecular Vision, 13, 2112–2118. [PubMed] [Google Scholar]
- Parish, C.L. , Beljajeva, A. , Arenas, E. and Simon, A. (2007) Midbrain dopaminergic neurogenesis and behavioural recovery in a salamander lesion‐induced regeneration model. Development, 134, 2881–2887. [DOI] [PubMed] [Google Scholar]
- Pflugfelder, O. (1965) Reparative und regenerative prozesse nach partieller zerstörung von fischgehirnen. Zool. Jb. Physiol, 71, 301–314. [Google Scholar]
- Poss, K.D. , Wilson, L.G. and Keating, M.T. (2002) Heart regeneration in zebrafish. Science, 298, 2188–2190. [DOI] [PubMed] [Google Scholar]
- Richter, W. (1968) Regenerative vorgange nach einseitiger enfernung des caudalen endhirnabschnittes einschliesslich des telo-diencephalen grenzbereiches bei ambystoma mexicanum. Journal für Hirnforschung, 10, 515–534. [PubMed] [Google Scholar]
- Rodriguez Viales, R. , Diotel, N. , Ferg, M. , Armant, O. , Eich, J. , Alunni, A. , März, M. , Bally-Cuif, L. , Rastegar, S. and Strähle, U. (2015) The helix-loop-helix protein Id1 controls stem cell proliferation during regenerative neurogenesis in the adult zebrafish telencephalon. Stem Cells, 33, 892–903. [DOI] [PubMed] [Google Scholar]
- Rothenaigner, I. , Krecsmarik, M. , Hayes, J.A. , Bahn, B. , Lepier, A. , Fortin, G. , et al (2011) Clonal analysis by distinct viral vectors identifies bona fide neural stem cells in the adult zebrafish telencephalon and characterizes their division properties and fate. Development, 138, 1459–1469. [DOI] [PubMed] [Google Scholar]
- Sandoval‐Guzmán, T. , Wang, H. , Khattak, S. , Schuez, M. , Roensch, K. , Nacu, E. , et al (2014) Fundamental differences in dedifferentiation and stem cell recruitment during skeletal muscle regeneration in two salamander species. Cell Stem Cell, 14, 174–187. [DOI] [PubMed] [Google Scholar]
- Schnapp, E. , Kragl, M. , Rubin, L. and Tanaka, E.M. (2005) Hedgehog signaling controls dorsoventral patterning, blastema cell proliferation and cartilage induction during axolotl tail regeneration. Development, 132, 3243–3253. [DOI] [PubMed] [Google Scholar]
- Seegar, J. (1961) Telencephalon and behaviour in Gasterosteus aculeatus . Behaviour, 18, 256–287. [Google Scholar]
- Seegar, J. (1962) Die Funktion des Vorderhirns in Bezug auf das angeborene Verhalten des dreidornigen Stichlingsmännchen – zugleich ein Beitrag über Neuronenregeneration im Fischgehirn. Acta Morph Neerl Scand, 5, 49–64. [Google Scholar]
- Seegar, J. (1965) Behavioural aspects of degeneration and regeneration in fish brain. Progress in Brain Research, 14, 143–231. [DOI] [PubMed] [Google Scholar]
- Session, A.M. , Uno, Y. , Kwon, T. , Chapman, J.A. , Toyoda, A. , Takahashi, S. , et al. (2016) Genome evolution in the allotetraploid frog Xenopus laevis . Nature, 538, 336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões, M.G. , Bensimon‐Brito, A. , Fonseca, M. , Farinho, A. , Valério, F. , Sousa, S. , et al (2014) Denervation impairs regeneration of amputated zebrafish fins. BMC Developmental Biology, 14, 10–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, S.P. , Holdway, J.E. and Poss, K.D. (2012) Regeneration of amputated zebrafish fin rays from de novo osteoblasts. Developmental Cell, 22, 879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srebro, Z. (1957) Regeneration of the endbrain in Xenopus laevis tadpoles. Folia Biologica (Krakow), 5, 211–232. [Google Scholar]
- Srebro, Z. (1965) Endbrain regeneration in adult Xenopus laevis . Folia Biologica, 13, 269–280. [PubMed] [Google Scholar]
- Stocum, D.L. (2011) The role of peripheral nerves in urodele limb regeneration. European Journal of Neuroscience, 34, 908–916. [DOI] [PubMed] [Google Scholar]
- Tsonis, P.A. , Vergara, M.N. , Spence, J.R. , Madhavan, M. , Kramer, E.L. , Call, M.K. , et al (2004) A novel role of the hedgehog pathway in lens regeneration. Developmental Biology, 267, 450–461. [DOI] [PubMed] [Google Scholar]
- Venables, M.J. , Xing, L. , Edington, C.C. and Trudeau, V.L. (2018) Neuronal regeneration in the goldfish telencephalon following 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine (MPTP) insult. FACETS, 3, 358–374. [Google Scholar]
- Winkelmann, E. and Winkelmann, A. (1970) Experimentelle untersuchungen zur regeneration des telencephalon von ambystoma mexicanum nach resektion beider hemispharen. Zeitschrift für Mikroskopisch-Anatomische Forschung, 82, 149–171. [PubMed] [Google Scholar]
- Yoshimatsu, T. , D’Orazi, F.D. , Gamlin, C.R. , Suzuki, S.C. , Suli, A. , Kimelman, D. , et al (2016) Presynaptic partner selection during retinal circuit reassembly varies with timing of neuronal regeneration in vivo. Nature Communications, 7, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino, J. and Tochinai, S. (2004) Successful reconstitution of the non‐regenerating adult telencephalon by cell transplantation in Xenopus laevis . Development, Growth and Differentiation, 46, 523–533. [DOI] [PubMed] [Google Scholar]
- Yoshino, J. and Tochinai, S. (2006) Functional regeneration of the olfactory bulb requires reconnection to the olfactory nerve in Xenopus larvae. Development, Growth and Differentiation, 48, 15–24. [DOI] [PubMed] [Google Scholar]
- Zupanc, G.K.H. and Clint, S.C. (2001) Radial glia‐mediated up‐regulation of somatostatin in the regenerating adult fish brain. Neuroscience Letters, 309, 149–152. [DOI] [PubMed] [Google Scholar]
- Zupanc, G.K.H. and Ott, R. (1999) Cell proliferation after lesions in the cerebellum of adult teleost fish: time course, origin, and type of new cells produced. Experimental Neurology, 160, 78–87. [DOI] [PubMed] [Google Scholar]
- Zupanc, M.M. and Zupanc, G.K.H. (2006) Upregulation of calbindin‐D28k expression during regeneration in the adult fish cerebellum. Brain Research, 1095, 26–34. [DOI] [PubMed] [Google Scholar]
- Zupanc, G.K.H. , Kompass, K.S. , Horschke, I. , Ott, R. and Schwarz, H. (1998) Apoptosis after injuries in the cerebellum of adult teleost fish. Experimental Neurology, 152, 221–230. [DOI] [PubMed] [Google Scholar]


