Abstract
Background
Hypertension is more prevalent in subjects with impaired glucose tolerance (IGT), but whether higher blood pressure per se or the mild hyperglycemia in combination with the hypertension enhanced the risk of cardiovascular disease (CVD) remains unclear.
Methods
Five hundred and sixty‐eight participants with IGT in the original Daqing diabetes prevention study, 297 with hypertension (HBP) and 271 without hypertension (NBP), were enrolled in 1986 and the intervention phase lasted for 6 years. In 2009, they were followed up to assess the outcomes of cardiovascular events (including stroke and myocardial infarction) and incidence of diabetes.
Results
Over 23 years, the incidence of diabetes was 93.9/1000 person‐years in HBP and 72.2/1000 person‐years in the NBP group, with an age‐ and sex‐adjusted hazard ratio of 1.26 (95% confidence interval [CI], 1.04‐1.54, P = 0.02). The yearly incidence of CVD events was 27.7/1000 person‐years and 16.6/1000 person‐years, indicating a 35% higher risk in HBP than in the NBP group (95% CI, 1.01‐1.81; P = 0.04). Cox proportional hazard analysis showed that a 10‐mm Hg increase of the baseline systolic blood pressure was associated with 9% increased risk of the development of diabetes (P = 0.02), together with a 7% higher risk of the CVD events (P = 0.02).
Conclusions
Hypertension predicted diabetes and enhances long‐term risk of CVD events in patients with IGT. An individualized strategy that targets hypertension as well as hyperglycemia is needed for diabetes and its cardiovascular complications.
Keywords: blood pressure, cardiovascular disease events, diabetes incidence, impaired glucose tolerance
摘要
背景
高血压在糖耐量受损(impaired glucose tolerance,IGT)人群中更为普遍。而它对心血管疾病(CVD)的影响是来自高血压本身还是轻度高血糖与血压的共同作用尚不清楚。
方法
1986年大庆糖尿病预防研究初始纳入IGT患者568例,分为IGT伴高血压组297例,IGT不伴高血压组271例。对IGT患者进行6年生活方式干预。随访至2009年评估两组的新发糖尿病和心血管事件(包括脑卒中和急性心肌梗死)发生率。
结果
随访23年中,IGT伴高血压组和不伴高血压组的2型糖尿病发生率分别为93.9/千人/年和72.2/千人/年。校正年龄和性别影响后,IGT伴高血压组的糖尿病发病风险为非高血压组1.26倍[95%可信区间(CI),1.04‐1.54,P = 0.02]。心血管事件的发生率在IGT伴高血压组为27.7/千人/年,不伴高血压组为16.6/千人/年,与不伴高血压组相比,IGT伴高血压组的发病风险增加35%(95% CI,1.01‐1.81,P = 0.04]。COX比例风险分析显示基线收缩压每升高10 mmHg,糖尿病的发生风险增加9.0%(P = 0.02),同时心血管事件的发生风险增加7%(P = 0.02)。
结论
在糖耐量受损人群中,高血压预测2型糖尿病的发生,并提高长期心血管事件的发生风险。个体化治疗策略应是降糖的同时还兼顾降压,以预防糖尿病及心血管并发症发生。
Keywords: 血压, 心血管事件, 糖尿病发病率, 糖耐量受损
1. INTRODUCTION
Over the past decade, an estimated 92‐113 million adults in China were diagnosed with diabetes, and approximately 148‐493 million others were found to be prediabetes, which placed them in a group with one of the prime causes of mortality and disease burden worldwide.1, 2
Cardiovascular (CV) events, including myocardial infarction and stroke, are the major causes of disability and mortality. An international study from 22 countries identified multiple stroke risk factors and found that hypertension and diabetes were the two important factors in addition to smoking status, abdominal obesity and history of cardiovascular disease. These factors account for 90% of the stroke risk.3 Moreover, patients with impaired glucose tolerance (IGT) frequently have similar vascular risk factors, such as obesity, hypertension and lipid metabolic abnormalities.4 Individuals with IGT comprise one‐third of patients experiencing a transient ischemic attack (TIA) or ischemic stroke5, 6 and have a 2‐fold greater risk of recurrent stroke.7
The 23‐year follow up of the Da Qing Diabetes Prevention Study (DQDPS) had reported that subjects with IGT had a 38% greater risk of cardiovascular diseases than people with normal glucose tolerance.8, 9 In the present post‐hoc analysis, we assessed the role of baseline blood pressure (BP) and plasma glucose on the risk of subsequent CVD events, including myocardial infarction and stroke, in relation to the progression of diabetes in people with IGT over the 23‐year follow up in the DQDPS.
2. METHODS
2.1. Research design
The details of the design of the DQDPS and the 20‐year follow‐up outcome studies have been mentioned previously.10, 11, 12 Briefly, in 1986, 2‐hours plasma glucose levels were measured in 110 660 subjects (87.3% of the eligible study population, 55 391 men and 55 269 women) from 33 care clinics after a standardized breakfast (100‐g steamed bread bun containing 80 g of carbohydrates). Non‐diabetes individuals with a 2‐hours plasma glucose level of 6.7 mmol/L or more received an oral glucose tolerance test (OGTT). Based on the results of these tests, 576 adults (312 men and 264 women) were identified as having IGT according to the 1985 World Health Organization (WHO) criteria.13 The participants were cluster randomized by clinics to one of three lifestyle interventions (diet, exercise, diet plus exercise) or a control group,12 and were re‐examined at 2‐year intervals over a 6‐year follow‐up period. We subsequently conducted a 23‐year follow‐up study to determine the incidence of diabetes and diabetes‐related complications. The study were approved by the institutional review boards at the WHO and Human Ethics Committee of the China‐Japan Friendship Hospital. All participants and their proxies for the deceased participants provided written informed consent for the follow‐up studies. Diabetes was defined according to the 1985 WHO criteria based on the results of OGTT performed every 2 years during the lifestyle intervention period (1986‐1992), and at the 20‐ and 23‐year re‐examinations, or by self‐reports of physician‐diagnosed diabetes or evidence of taking hypoglycemic medications in the patient's medical records.8, 9, 10, 11, 12 CVD events included stroke and myocardial infarction. Stroke was defined as the speedily developing clinical symptoms of local (or global) disturbance of cerebral function sustaining more than 24 hours or resulting in death with no other causes except cerebral vascular origin, while transient ischemic attacks were not covered in the study. Myocardial infarction was diagnosed with the clinical evidences, enzymology and X‐ray examinations. In the present analysis, 568 subjects with IGT were stratified into two groups based on whether they were hypertensive at entry. We measured sitting brachial BP twice with standard mercury sphygmomanometer, and used the mean value in the data analysis. Hypertension was defined as systolic BP (SBP) of 140 mm Hg or more and/or diastolic BP (DBP) of 90 mm Hg or more. There were 271 subjects in the non‐hypertensive group (NBP) and 297 subjects in the hypertension group (HBP). The hypertensive subjects received running antihypertensive therapy in local clinics according to the related guidelines throughout the long‐term follow‐up period.
2.2. Statistical analyses
The data are presented as the means (±SD) or counts (percentages). Descriptive statistics were compared between the HBP and NBP groups. Student's t test was used to compare normally distributed continuous variables, and the χ 2 test was used for categorical variables. CVD events, stroke, myocardial infarction and the diabetes incidence rates in the HBP and NBP groups were calculated according to the number of person‐years. One thousand person‐year incidence of different CVD events occurred before and after the progression to diabetes from IGT in different groups were also calculated. Survival time (in years) is reported as median unadjusted and Cox proportional hazards models were used to determine the different contribution adjusted for age and sex. In models investigating the independent association of BP and the onset of diabetes on subsequent risk of CVD events, the diabetes status was used as a time‐dependent variable in an extended Cox model,14, 15 controlling for baseline covariates, such as age, sex, body mass index (BMI), BP, smoking status, intervention and plasma cholesterol. Differences were considered significant if the two‐sided P‐values were 0.05 or less. Statistical analyses were conducted with SAS version 9.4 (SAS Institute, Cary, NC, USA).16
3. RESULTS
Five hundred and forty‐two participants (94%) had complete data for CVD events and 568 (99%) contributed diabetes data to the analysis (Figure S1). The clinical characteristics of the participants are shown in Table 1. At baseline, participants in the HBP were up to 5 years older and BMI and BP values were higher in the HBP than in the NBP group. The proportion of smokers was lower in the HBP (37.0%) than in the NBP (45.8%) group. No differences were found in sex and the plasma glucose concentrations between the HBP and NBP. At the end of the active intervention in 1992, participants in the HBP had higher BP (P < 0.0001) and fasting plasma glucose concentrations (P = 0.03) than those in the NBP group.
Table 1.
Baseline characteristics in 1986 and characteristics at end of the intervention in 1992 in the IGT groups with and without hypertension
| Non‐hypertension group (n = 271) | Hypertension group (n = 297) | P‐value | |
|---|---|---|---|
| At baseline (in 1986) | |||
| Age (y) | 42.8 ± 8.0 | 47.4 ± 9.9 | <0.0001 |
| Sex | |||
| Men | 141 (52.0%) | 168 (56.6%) | 0.28 |
| Women | 130 (48.0%) | 129 (43.4%) | |
| Smoker (%) | 124 (45.8%) | 110 (37.0%) | 0.04 |
| Body mass index (kg/m2) | 24.9 ± 3.6 | 26.7 ± 3.8 | <0.0001 |
| FPG (mmol/L) | 5.6 ± 0.8 | 5.6 ± 0.8 | 0.58 |
| PG2h (mmol/L) | 8.9 ± 0.9 | 9.0 ± 0.9 | 0.17 |
| Cholesterol (mmol/L) | 5.0 ± 1.1 | 5.2 ± 1.5 | 0.06 |
| Blood pressure (mm Hg) | |||
| Systolic | 115.8 ± 10.8 | 148.3 ± 21.0 | <0.0001 |
| Diastolic | 76.7 ± 6.4 | 97.3 ± 10.4 | <0.0001 |
| At end (in 1992) | |||
| FPG (mmol/L) | 6.8 ± 2.2 | 7.4 ± 3.8 | 0.03 |
| PG2h (mmol/L) | 10.7 ± 4.3 | 11.4 ± 4.5 | 0.07 |
| Cholesterol (mmol/L) | 5.2 ± 1.2 | 5.2 ± 0.9 | 0.60 |
| Blood pressure (mm Hg) | |||
| Systolic | 122.5 ± 14.3 | 139.8 ± 21.6 | <0.0001 |
| Diastolic | 81.2 ± 11.0 | 88.9 ± 12.2 | <0.0001 |
Data are mean ± SD or percentage of participants in two groups. FPG, fasting plasma glucose; IGT, impaired glucose tolerance; PG2h, venous plasma glucose concentration 2 hours after 75‐g oral glucose load.
Table 2 shows that, during the 23‐year follow up, the cumulative incidence of diabetes was higher in the HBP group (80.8%, 240/297) than in the NBP (72.3%, 196/271, P = 0.02) group, with yearly incidence rates of 93.9/1000 person‐years in the HBP and 72.3/1000 person‐years in the NBP group. After adjusting for age and sex, the patients in the HBP group had a 26% (95% confidence interval [CI], 1.04‐1.54) increased risk of diabetes (Figure 1A). Median survival time in the HBP group was 8.2 years, while that in the NBP group was 10.4 years. However, the independent association between plasma glucose, BP at baseline and the development of diabetes was only found in the HBP group. After the adjustment of age, sex, smoking, BMI and intervention, it was showed that 1 mmol/L increase of 2‐hours plasma glucose at baseline was associated with 25% increased risk of the development of diabetes (hazard ratio [HR] = 1.25, 95% CI, 1.08‐1.44, P = 0.003) and a 10‐mm Hg increase of SBP was associated with 9% increased risk of development of diabetes. Of note, in the NBP group, that the increase of SBP was not associated with the development of diabetes but at the same model 1 mmol/L increase of 2‐hours plasma glucose at baseline was found to be associated with 36% increased risk of the development of diabetes (HR = 1.36, 95% CI, 1.16‐1.60, P = 0.0001), showed in Table 3.
Table 2.
Cumulative incidence of diabetes and CVD events in IGT subjects with and without hypertension over 23 years
| Non‐hypertension group | Hypertension group | |
|---|---|---|
| Diabetes incidence | ||
| n (%) | 196 (72.3%) | 240 (80.8%) |
| Person‐years | 2713 | 2557 |
| Cases per 1000 person‐years (95% CI) | 72.2 (62.1‐82.4) | 93.9 (82.0‐105.7) |
| HR, P‐value† | HR = 1.26 (1.04‐1.54), P = 0.02 | |
| CVD events (including stroke and myocardial infarction) | ||
| n (%) | 80 (31.5%) | 133 (46.2%) |
| Person‐years | 4878 | 4799 |
| Cases per 1000 person‐years (95% CI) | 16.6 (13.0‐20.2) | 27.7 (23.0‐32.4) |
| HR, P‐value† | HR = 1.35 (1.01‐1.81), P = 0.04 | |
| Stroke | ||
| n (%) | 64 (25.2%) | 109 (37.9%) |
| Person‐years | 4949 | 4864 |
| Cases per 1000 person‐years (95% CI) | 12.9 (9.8‐16.1) | 22.4 (18.2‐26.6) |
| HR, P‐value† | HR = 1.43 (1.03‐1.98), P = 0.03 | |
| Myocardial infarction | ||
| n (%) | 16 (6.3%) | 24 (8.3%) |
| Person‐years | 5243 | 5503 |
| Cases per 1000 person‐years (95% CI) | 3.1 (1.6‐4.6) | 4.4 (2.6‐6.1) |
| HR, P‐value† | HR = 1.07 (0.55‐2.08), P = 0.84 | |
Compared with non‐hypertension group, age and sex adjusted.
Abbreviations: CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio; IGT, impaired glucose tolerance.
Figure 1.
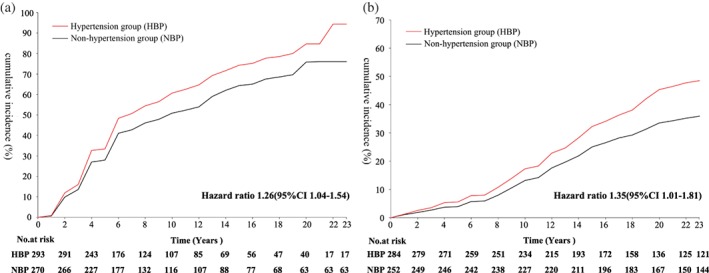
A, Cumulative incidence of diabetes after adjusted for age and sex in people with impaired glucose tolerance (IGT) with and without hypertension at baseline over the 23‐year follow up. Median survival time for the patients with IGT with hypertension was 8.18 years and median survival time for those without hypertension was 10.35 years. B, Cumulative incidence of CVD event after adjusted for age and sex in people with IGT with and without hypertension at baseline over the 23‐year follow up. CI, confidence interval
Table 3.
Influence of blood pressure and 2‐hours plasma glucose at baseline on the development of diabetes in IGT subjects with and without hypertension
| Non‐hypertension group | Hypertension group | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Hazard ratio | 95% CI | P | Hazard ratio | 95% CI | P | ||
| Age (y) | 1.01 | 0.99 | 1.03 | 0.22 | 0.99 | 0.98 | 1.01 | 0.21 |
| Sex (male = 1) | 0.89 | 0.64 | 1.25 | 0.499 | 0.98 | 0.74 | 1.31 | 0.91 |
| Smoker (yes = 1) | 1.16 | 0.83 | 1.63 | 0.38 | 0.98 | 0.74 | 1.30 | 0.88 |
| Intervention (yes = 1) | 0.54 | 0.39 | 0.75 | 0.0002 | 0.64 | 0.48 | 0.85 | 0.002 |
| BMI (kg/m2) | 1.09 | 1.05 | 1.14 | <0.0001 | 1.07 | 1.03 | 1.10 | 0.0002 |
| 2‐h plasma glucose (mmol/L) | 1.36 | 1.16 | 1.60 | 0.0001 | 1.25 | 1.08 | 1.44 | 0.003 |
| SBP (10 mm Hg) | 0.93 | 0.81 | 1.07 | 0.31 | 1.09 | 1.02 | 1.16 | 0.02 |
Abbreviations: BMI, body mass index; CI, confidence interval; IGT, impaired glucose tolerance; SBP, systolic blood pressure.
The cumulative incidence rate of CVD events was significantly higher in the HBP patients (46.2%, 133/288) than in the NBP patients (31.5%, 80/254). Stroke was the most common CVD event in both groups, accounting for 82.0% of the CVD events in the HBP and 79.0% in the NBP group. The majority of strokes were ischemic (149/173) rather than hemorrhagic (24/173) stroke. No difference was found in myocardial infarction between the participants in the HBP and NBP groups. The age‐ and sex‐adjusted risk of CVD events and stroke remained higher in the HBP than in the NBP group (Table 2, Figure 1B).
Approximately two‐thirds of the CVD events in both NBP and HBP groups occurred after the progression to diabetes from IGT. Table S1 shows that the sex and updated‐age adjusted incidence of CVD events was higher after the onset of diabetes than before in the NBP (23.2% vs 8.7%) and in the HBP (30.6% vs 15.6%) group, respectively. The yearly incidence rate of CVD events was 24.5 vs 9.5 per 1000 person‐years with a HR of 2.09 (95% CI, 1.22‐3.59) after the onset of diabetes than before the development of diabetes in the NBP group. The corresponding incidence rate of events in the HBP group was 37.1 vs 20.1 per 1000 person‐years with a HR of 1.77 (95% CI, 1.21‐2.58). However, the incidence of stroke was significantly higher after the onset of diabetes (24.7% vs 13.2%) than that before the development of diabetes in the HBP group (HR = 1.77, 95% CI, 1.16‐2.68) but not in the NBP group (HR = 1.59, 95% CI, 0.88‐2.86). The difference in the incidence of myocardial infarction after and before the onset of diabetes was seen only in the HBP group (5.5% vs 0.8%) with a HR of 6.31 (95% CI, 1.34‐29.65) (Table S1).
A group of multivariable analyses showed, after the adjustment of some potential confounders such as age, sex, BMI, smoking status, plasma cholesterol and the influence of lifestyle intervention, that the baseline BP and progression to diabetes from IGT over the follow‐up period were independently associated with the development of first CVD events and stroke. A 10‐mm Hg increase in SBP was associated with a 7% (HR = 1.07, 95% CI, 1.011.12) increased risk of CVD events and an 8% (HR = 1.08, 95% CI, 1.01‐1.14) increased risk of stroke. Simultaneously, the progression to diabetes from IGT was associated with a 97% (HR = 1.97, 95% CI, 1.38‐2.80) higher risk of CVD events and a 91% (HR = 1.91, 95% CI, 1.29‐2.81) higher risk of stroke (Table 4). As for myocardial infarction, perhaps due to the small number, the above‐mentioned association was not significant.
Table 4.
Impact of hypertension and progression to diabetes from IGT on the risk of stroke, myocardial infarction and CVD events over 23 years
| Stroke | Myocardial infarction | CVD events (stroke and myocardial infarction) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Hazard ratio | 95% CI | P | Hazard ratio | 95% CI | P | Hazard ratio | 95% CI | P | |||
| Age (y) | 1.05 | 1.03 | 1.07 | <0.0001 | 1.08 | 1.04 | 1.12 | 0.0002 | 1.06 | 1.04 | 1.08 | <0.0001 |
| Sex (male = 1) | 1.33 | 0.92 | 1.90 | 0.13 | 1.17 | 0.57 | 2.38 | 0.67 | 1.35 | 0.98 | 1.86 | 0.07 |
| BMI (kg/m2) | 1.00 | 0.96 | 1.05 | 0.85 | 1.08 | 0.99 | 1.18 | 0.08 | 1.02 | 0.99 | 1.06 | 0.23 |
| Smoker (yes = 1) | 1.27 | 0.91 | 1.79 | 0.17 | 0.57 | 0.27 | 1.20 | 0.14 | 1.08 | 0.80 | 1.47 | 0.62 |
| Cholesterol† (mmol/L) | 1.31 | 0.62 | 2.80 | 0.48 | 0.28 | 0.06 | 1.10 | 0.07 | 0.93 | 0.47 | 1.83 | 0.84 |
| Intervention (yes = 1) | 1.00 | 0.71 | 1.40 | 0.98 | 0.76 | 0.39 | 1.49 | 0.43 | 0.98 | 0.72 | 1.32 | 0.87 |
| SBP (10 mm Hg) | 1.08 | 1.01 | 1.14 | 0.02 | 1.002 | 0.99 | 1.02 | 0.78 | 1.07 | 1.01 | 1.12 | 0.02 |
| Diabetes status (yes = 1)‡ | 1.91 | 1.29 | 2.81 | 0.001 | 1.82 | 0.77 | 4.34 | 0.17 | 1.97 | 1.38 | 2.80 | 0.0002 |
Log‐transformed.
Diabetes status as time‐dependent variable.
Abbreviations: BMI, body mass index; CI, confidence interval; CVD, cardiovascular disease; IGT, impaired glucose tolerance; SBP, systolic blood pressure.
4. DISCUSSION
A number of studies reported that the postprandial glucose level plays a significant role in the process leading to CVD events.17, 18, 19 Patients with IGT had a 20% increased risk of stroke even after multivariate adjustment (1.20,1.00‐1.45).20 A meta‐analysis of prospective studies also showed that IGT was an independent risk factor for stroke.20 However, in the IGT population, whether CVD is the result of the long‐standing mild hyperglycemia or the subsequent development of diabetes or its coexisting risk factors, such as hypertension, remains unclear.
Hypertension occurs in 50‐60% of subjects with T2DM21 and is 2‐ to 3‐fold more prevalent in patients with IGT than in the normal population.22 In the present study, the IGT subgroup with hypertension had significantly higher incidence of diabetes over the 23‐year follow‐up period. The age‐ and sex‐adjusted diabetes incidence rate among IGT subjects with hypertension was 26% higher than that in those IGT subjects without hypertension. Does the hypertension, which coexisted with mild hyperglycemia, contribute to the progression to diabetes among the participants with IGT? Our results showed that the baseline BP predicted subsequent risk of diabetes in the HBP but not in the NBP group independent of the contribution of baseline plasma glucose level, probably suggesting that hypertension in IGT patients played an accelerating role in the deterioration of glucose homeostasis at the presence of mild hyperglycemia. Hence, the coexistence of mild hyperglycemia and hypertension at baseline led to a higher rate of development of diabetes.
Previous studies have shown that hypertension is the most significant risk factor for all stroke subtypes in the general population.3 The risk of stroke increases continuously with increased BP. However, few previous articles have reported the risk of CVD events related to IGT in association with hypertension. In our study, we found that a high proportion in the IGT with HBP group experienced CVD events and stroke. After the age and sex adjustment, CVD events rates were approximately 35% greater than the rates in the NBP group and most of the CVD events in this IGT cohort occurred after the development of diabetes. Further analysis showed that the development of diabetes was associated with a double risk of CVD events even after full adjustment for BP and other confounders. These results may imply that hypertension in the IGT population increased the risk of diabetes, and the progression to diabetes from IGT further increased the CVD risk in our IGT cohort. Of note, the present data showed that a 10‐mm Hg increase in baseline SBP was associated with a 7.0% increased risk of CVD events independent of the progression to diabetes from IGT. This finding indicated that hypertension per se not only led to a higher incidence of cardio‐cerebral‐vascular disease, but also further increased the risk of CVD events by accelerating the development of diabetes among subjects with IGT. Thus, the results showed that hypertension had double effects in IGT patients, which was not clarified clearly before.
There is growing recognition that individuals with IGT represent a high‐risk population, and should be treated more aggressively to prevent CV events.23 Findings in our study suggested that preventing the onset of DM and decreasing the BP should be considered key strategies for reducing the macro‐complications of diabetes. Lifestyle interventions were as effective as other treatments in improving blood glucose11, 12 and BP,24 and even reducing the incidence of CVD‐related mortality in the DQDPS.9 Therefore, lifestyle interventions can be recommended as a fundamental policy in IGT patients with hypertension.
The study had some limitations. First, glucose tolerance tests were examined systematically only at defined intervals during the 6‐year intervention period but not continued throughout the follow‐up period, even though an examination was conducted at 20 and 23 years. The clinical diabetes diagnostic procedure may delay the assessment of the time to onset of diabetes compared with systemic OGTT and may lead to underestimation of the influence of new‐onset diabetes on the development of cardiovascular disease events such as stroke and myocardial infarction. Second, we did not collect data of medications used in the long‐term follow‐up period; thus, we cannot adjust its influence on CVD‐related events.
5. CONCLUSION
The present study found a long‐term increased risk in the development of diabetes among the IGT subjects with hypertension, and the coexistence of hypertension and mild hyperglycemia in this population led to a significantly higher risk of CVD events, especially the occurrence of stroke. It suggests that an individualized intervention targeted to control hypertension together with preventing the development of diabetes may favor the reduction of the diabetes‐related macro‐complications among the IGT population with hypertension.
CONFLICTS OF INTEREST
The authors have no conflict of interests to declare.
Supporting information
FIGURE S1 Trial profile. CVD, cardiovascular disease.
TABLE S1 Cumulative incidence of stroke, myocardial infarction and CVD events before and after progression to diabetes in IGT subjects with and without hypertension.
ACKNOWLEDGMENTS
The authors thank all the research subjects for their participation in the original Da Qing IGT and Diabetes Study, the DQDPS, and the Da Qing Diabetes Prevention Follow‐up Study. The authors thank Peter H. Bennett who gave advice on the manuscript. We especially thank the late Professor Xiaoren Pan, as this study would not have been possible without his leadership in the design and implementation of the original DQDPS.
Li X, Wang J, Shen X, et al. Higher blood pressure predicts diabetes and enhances long‐term risk of cardiovascular disease events in individuals with impaired glucose tolerance: Twenty‐three‐year follow‐up of the Daqing diabetes prevention study. Journal of Diabetes. 2019;11:593–598. 10.1111/1753-0407.12887
Our research began in 1986, when there was no registration requirement, so there is no registration.
Funding information National Center for Chronic Disease Prevention and Health Promotion through CDC/WHO Cooperative Agreement No. U58/CCU424123‐01‐02 and by the China‐Japan Friendship Hospital
REFERENCES
- 1. Wenying Yang MD, Juming Lu MD, Jianping Weng MD, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090‐1101. [DOI] [PubMed] [Google Scholar]
- 2. Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310(9):948‐959. [DOI] [PubMed] [Google Scholar]
- 3. O'Donnell MJ, Xavier D, Liu L, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case‐control study. Lancet. 2010;376(9735):112‐123. [DOI] [PubMed] [Google Scholar]
- 4. DeFronzo RA, Abdul‐Ghani M. Assessment and treatment of cardiovascular risk in prediabetes: impaired glucose tolerance and impaired fasting glucose. Am J Cardiol. 2011;108(suppl 3):3B‐24B. [DOI] [PubMed] [Google Scholar]
- 5. Dave JA, Engel ME, Freercks R, et al. Abnormal glucose metabolism in non‐diabetic patients presenting with an acute stroke: prospective study and systematic review. QJM. 2010;103(7):495‐503. [DOI] [PubMed] [Google Scholar]
- 6. Fonville S, Zandbergen AA, Vermeer SE, Dippel DW, Koudstaal PJ, den Hertog HM. Prevalence of prediabetes and newly diagnosed diabetes in patients with a transient ischemic attack or stroke. Cerebrovasc Dis. 2013;36(4):283‐289. [DOI] [PubMed] [Google Scholar]
- 7. Vermeer SE, Sandee W, Algra A. Impaired glucose tolerance increases stroke risk in nondiabetic patients with transient ischemic attack or minor ischemic stroke. Stroke. 2006;37(6):1413‐1417. [DOI] [PubMed] [Google Scholar]
- 8. Chen Y, Wang J, An Y, et al. Cardiovascular events and mortality after lifestyle intervention for prediabetes in the Da Qing diabetes prevention study: a 23‐year follow‐up study. Chin J Intern Med. 2015;54(01):13‐17. [Google Scholar]
- 9. Li G, Zhang P, Wang J, et al. Cardiovascular mortality, all‐cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing diabetes prevention study: a 23‐year follow‐up study. Lancet Diabetes Endocrinol. 2014;2:474‐480. [DOI] [PubMed] [Google Scholar]
- 10. Gong Q, Gregg EW, Wang J, et al. Long term effects of a randomised trial of a 6‐year lifestyle intervention in impaired glucose tolerance on diabetes‐related microvascular complications: the China Da Qing diabetes prevention outcome study. Diabetologia. 2011;54:300‐307. [DOI] [PubMed] [Google Scholar]
- 11. Li G, Zhang P, Wang J, et al. The long‐term effect of lifestyle interventions to prevent diabetes in the China Da Qing diabetes prevention study: a 20‐year follow‐up study. Lancet. 2008;371:1783‐1789. [DOI] [PubMed] [Google Scholar]
- 12. Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and diabetes study. Diabetes Care. 1997;20:537‐544. [DOI] [PubMed] [Google Scholar]
- 13. World Health Organization Study Group . Diabetes Mellitus. Report of a WHO Study Group. Geneva: World Health Organization; 1985. (Tech. Rep.Ser., no.727). [PubMed] [Google Scholar]
- 14. Andersson TM, Dickman PW, Eloranta S, Lambert PC. Estimating and modelling cure in population‐based cancer studies within the framework of flexible parametric survival models. BMC Med Res Methodol. 2011;11:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fisher LD, Lin DY. Time‐dependent covariates in the cox proportional‐hazards regression model. Annu Rev Public Health. 1999;20:145‐157. [DOI] [PubMed] [Google Scholar]
- 16. SAS Institute Inc . SAS/STAT User's Guide, Version 9.4. Cary, NC: SAS Institute Inc; 2012. [Google Scholar]
- 17. The DECODE Study Group . Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. Lancet. 1999;354:617‐621. [PubMed] [Google Scholar]
- 18. De Vegt F, Dekker JM, Ruhe HG, et al. Hyperglycaemia is associated with all‐cause and cardiovascular mortality in the Hoorn population: the Hoorn study. Diabetologia. 1999;42:926‐931. [DOI] [PubMed] [Google Scholar]
- 19. Meigs JB, Nathan DM, D'Agostino RB Sr, Wilson PW. Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham offspring study. Diabetes Care. 2002;25:1845‐1850. [DOI] [PubMed] [Google Scholar]
- 20. Huang Y, Cai X, Mai W, et al. Association between prediabetes and risk of cardiovascular disease and all causes mortality: systematic review and meta‐analysis. BMJ. 2016;355:i5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Salomaa VV, Strandberg TE, Vanhanen H, Naukkarinen V, Sarna S, Miettinen TA. Glucose tolerance and blood pressure: long term follow up in middle aged men. BMJ. 1991;302:493‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Segura J, Ruilope LM. Treatment of prehypertension in diabetes and metabolic syndrome: what are the pros? Diabetes Care. 2009;32(suppl 2):S284‐S289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ford ES, Zhao G, Li C. Pre‐diabetes and the risk for cardiovascular disease: a systematic review of the evidence. J Am Coll Cardiol. 2010;55:1310‐1317. [DOI] [PubMed] [Google Scholar]
- 24. Campbell NR, Lackland DT, Niebylski ML, World Hypertension League Committee; International Society of Hypertension Executive Committee . High blood pressure: why prevention and control are urgent and important: a 2014 fact sheet from the World Hypertension League and the International Society of Hypertension. J Clin Hypertens (Greenwich). 2014;16:551‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Trial profile. CVD, cardiovascular disease.
TABLE S1 Cumulative incidence of stroke, myocardial infarction and CVD events before and after progression to diabetes in IGT subjects with and without hypertension.


