Abstract
The differentiation of subtypes of primary progressive aphasia (PPA) remains challenging. We aimed to identify optimum neuropsychological measures for characterizing PPA, to examine the relationship between behavioural change and subtypes of PPA and to determine whether characteristic profiles of language, working memory, and behavioural changes occur in PPA. Forty‐seven patients with PPA and multi‐domain Alzheimer's disease (AD) together with 19 age‐matched controls underwent a large battery of working memory and language tests. We found that simple tasks of sentence ordering, narrative production, and buccofacial praxis were particularly useful in differentiating non‐fluent/agrammatic variant PPA (nfvPPA) from other PPA subtypes, whereas a test of single word comprehension was useful in detecting semantic dementia (SD). No individual tests were discriminating for logopenic variant PPA (lvPPA) relative to nfvPPA. LvPPA and multidomain AD exhibited similar language profiles. A principal components analysis revealed that characteristic PPA profiles extended beyond the realms of language, in particular, the presence of apraxia in nfvPPA, behavioural changes in SD, and working memory deficits in lvPPA. These findings suggest that not all tests are equally discriminatory for PPA and highlight the importance of a test profile in differentiating PPA. These results also support the view that lvPPA is a focal form of AD and emphasize the difficulties classifying lvPPA.
Keywords: Alzheimer's disease, frontotemporal lobar degeneration, logopenic variant primary progressive aphasia, non‐fluent/agrammatic variant primary progressive aphasia, primary progressive aphasia, semantic dementia
Background
Current diagnostic recommendations for primary progressive aphasia (PPA) include three main subtypes: non‐fluent/agrammatic (nfvPPA), characterized by agrammatism and/or apraxia of speech (AOS); logopenic (lvPPA), characterized by impaired repetition and word finding difficulties; and semantic (svPPA), characterized by anomia, impaired word comprehension, and impaired object recognition (Gorno‐Tempini et al., 2011). The terms nfvPPA and svPPA are commonly used interchangeably with the earlier designations progressive non‐fluent aphasia (PNFA) and semantic dementia (SD; Neary et al., 1998), although they are not precisely equivalent. In particular, the term SD acknowledges the multimodal nature of patients’ semantic loss and the fact that the earliest presenting symptom may be in the visual rather than verbal domain. The three PPA subtypes are associated with different distributions of atrophy: nfvPPA with left posterior fronto‐insular atrophy, lvPPA with left posterior perisylvian or parietal atrophy, and svPPA with anterior temporal lobe atrophy (Gorno‐Tempini et al., 2011). Most patients with nfvPPA and svPPA/SD have frontotemporal lobar degeneration (FTLD) spectrum pathologies and most lvPPA patients have Alzheimer's disease (AD) pathology, although this is not invariably the case (see Harris & Jones, 2014 for a review).
Neuropsychological studies suggest that current classifications do not encapsulate the full range of PPA syndromes observed, and patients may fulfil the criteria for more than one PPA variant (Botha et al., 2015; Harris et al., 2013). Furthermore, while each PPA subtype is associated with characteristic deficits, a degree of overlap exists between the subtypes. Indeed, the core features of lvPPA also occur frequently in nfvPPA (Sajjadi, Patterson, Arnold, Watson, & Nestor, 2012; Wicklund et al., 2014). These findings suggest that the characterization of PPA requires further refinement.
A potential source of variability in findings may lie in the choice of tests employed by clinicians and researchers. Thus, for example, in current classifications (Gorno‐Tempini et al., 2011) agrammatism is identified through tasks involving sentence production, yet different tasks may not have equal sensitivity or specificity. Indeed a recent review suggests that evaluation of agrammatism should include the assessment of comprehension and production of grammatical morphology, functional categories, verbs, and complex syntactic structures (Thompson & Mack, 2014). It would be important to determine optimum measures for detecting characteristic language deficits prospectively in a cohort of PPA patients.
Refinement of diagnosis may depend not only on identification of optimal language measures but also on the recognition of associated deficits. One such area worthy of consideration is praxis. AOS is a core feature of nfvPPA (Gorno‐Tempini et al., 2011), yet deficits may extend beyond the realm of speech to orofacial and/or limb apraxia (Joshi, Roy, Black, & Barbour, 2003; Rohrer, Rossor, & Warren, 2010; Tyrrell, Kartsounis, Frackowiak, Findley, & Rossor, 1991). Gestural apraxia might occur secondary to a central disorder of communication (Duffy & Duffy, 1981; Glosser, Wiener, & Kaplan, 1986). Apraxia might be predicted to be a more integral component of nfvPPA than of other forms of PPA.
Another domain of potential diagnostic importance is working memory. Phonological working memory has been suggested to play a pivotal role in the logopenic syndrome (Gorno‐Tempini et al., 2008; Meyer, Snider, Campbell, & Friedman, 2015), leading to patients’ difficulty in sentence repetition. Yet, the degree to which standard tests of working memory are able to differentiate lvPPA from nfvPPA is unclear. NfvPPA patients might be expected to perform poorly on verbal working memory tasks because of their marked speech production problems, and indeed there is compelling evidence of phonological processing impairments in nfvPPA to suggest problems in verbal/phonological working memory (Libon et al., 2007; Nestor et al., 2003). On the other hand, they might reasonably do well on working memory tasks that do not make phonological demands. The examination of performance on a variety of verbal and visual working memory tasks would help to identify optimal measures to aid differentiation.
In addition to the associated cognitive deficits, accompanying behavioural changes might be of diagnostic relevance. NfvPPA and svPPA/SD are pathologically linked to behavioural variant frontotemporal dementia (bvFTD) which is the commonest clinical disorder associated with FTLD spectrum pathology and is characterized by changes in behaviour and personality. Language and behavioural symptoms can co‐occur in disorders caused by FTLD pathology (Harris et al., 2016). Patients with SD have been reported to exhibit behavioural change, particularly stereotyped and repetitive behaviours (Rosen et al., 2006; Snowden et al., 2001), and behavioural change can also occur in nfvPPA (Rohrer & Warren, 2010). Increased apathy, anxiety, agitation, and depression have been described in lvPPA (Rohrer & Warren, 2010; Rosen et al., 2006). However, since lvPPA is typically not associated with FTLD but rather with AD pathology, these patients might be expected to have differing behavioural change when compared to patients with nfvPPA and svPPA/SD. The presence of certain behavioural characteristics might therefore aid differentiation of PPA subtypes and warrants further investigation.
The link between lvPPA and AD pathology raises the question whether the language characteristics of lvPPA mirror those seen in early‐onset AD, in which language problems typically constitute one component of a multi‐domain disorder that includes also deficits in episodic memory, working memory, visual perception, and spatial function, (Smits et al., 2012; Snowden et al., 2007). Reports have thus far been mixed with some authors describing logopenic‐type symptoms in non‐focal AD patients (Harris et al., 2015) and other authors describing a differing language profile in patients with AD (Ahmed, de Jager, Haigh, & Garrard, 2012).
In AD, long‐term and working memory may breakdown separately (Stopford, Snowden, Thompson, & Neary, 2007). Working memory has been associated with language functioning (Caza & Belleville, 2008; Stopford, Snowden, Thompson, & Neary, 2008; Stopford et al., 2007) but spatial span may also be impaired (Grossi, Becker, Smith, & Trojano, 1993; Trojano, Chiacchio, De Luca, & Grossi, 1994). A comparative study of lvPPA and AD showed poorer performance on tests of verbal working memory in lvPPA but similar performance in the two groups on tests of visuospatial working memory (Foxe, Irish, Hodges, & Piguet, 2013; Meyer et al., 2015). Whether this finding generalizes to other lvPPA and AD cohorts, and whether working memory profile distinguishes lvPPA from nfvPPA remains to be established.
This study had several aims. First, we aimed to identify optimum language measures for differentiating SD, nfvPPA, and lvPPA, focussing on tests of naming, single word comprehension, sentence processing, narrative production, reading, spelling, and repetition that tap core features of PPA conditions. Second, we examined the potential diagnostic contribution of non‐language measures, specifically in the domains of praxis, working memory, and behaviour. We included as a comparison group, patients with multidomain AD. We expected greater commonalities in language and non‐language performance with lvPPA than other forms of PPA, but it was an open question whether distinct characteristics would be identified between lvPPA and AD. Third, we aimed to determine, through principal component analysis of performance measures, the degree to which core factors can be identified that have predictive value in distinguishing forms of PPA and to delineate optimal combinations of discriminating features.
Methods
Participants
Participants comprised 47 consecutive patients with a clinical diagnosis of nfvPPA (12), lvPPA (13), SD (8), and multi‐domain AD (14) who attended a specialist early‐onset dementia clinic and agreed to take part in the study. These participants included new referrals and follow‐up patients. Most patients were seen within a year of first presenting to the clinic (mean 1.15 years, standard deviation 1.05 years). Participants with a history of alcohol abuse or head injury were excluded. Patients were diagnosed by experienced neurologists based on detailed clinical history, neuroimaging results, and neurological examination, using guidelines published by Snowden et al. (2011), which have been shown, in clinico‐pathological correlation studies, to yield high levels of diagnostic accuracy. Patients with nfvPPA and lvPPA met contemporary criteria (Gorno‐Tempini et al., 2011). All SD patients showed the language characteristics of svPPA (Gorno‐Tempini et al., 2011). However, as three patients showed early face and object recognition problems, in addition to their language disorder, and had greater right than left temporal lobe atrophy, the term SD (Neary et al., 1998) is used to designate the group. Diagnoses were supported by neuropsychological examination using the Manchester Neuropsychological Profile (Snowden et al., 2007, 2011; Thompson, Stopford, Snowden, & Neary, 2005). Nineteen age‐matched controls were also recruited.
Demographic data are shown in Table 1. There was a significant association between gender and group (Fisher's p = .026). The lvPPA group included a higher proportion of males than the control group (Fisher's p = .012) and the AD group (Fisher's p = .006). There was a significant group difference in age, F(4,61) = 7.357, p < .001. The AD group was significantly younger than the other groups (vs. nfvPPA p < .001, vs. lvPPA p < .001, vs. SD p = .011, and vs. controls p = .011). The youthful age of the AD patients, in part, reflects a referral bias of younger patients to the neurology clinic but also an inherent bias towards early onset in AD presenting with multi‐domain impairment.
Table 1.
Demographics
| Patient group | Gender (M:F) | Age at onset (years); mean (SD) | Age at testing (years); mean (SD) | Duration at testing | Family history of a similar condition in a first‐degree relative; N (%) |
|---|---|---|---|---|---|
| NfvPPA (12) | 7:5 | 66.71 (7.99) | 70.67 (7.23) | 4.00 (2.04) | 3/11 (27.3) |
| LvPPA (13) | 11:2 | 66.56 (6.95) | 70.35 (6.39) | 3.82 (2.20) | 3/13 (23.1) |
| SD (8) | 5:3 | 63.24 (3.77) | 66.83 (2.88) | 3.64 (1.16) | 4/8 (50.0) |
| Multi‐domain AD (14) | 4:10 | 57.16 (5.84) | 60.15 (5.75) | 3.03 (1.44) | 3/14 (21.4) |
| Controls (19) | 7:12 | n/a | 65.51 (5.20) | n/a | n/a |
AD, Alzheimer's disease; F, female; LvPPA, logopenic variant primary progressive aphasias; M, male; nfvPPA, non‐fluent variant primary progressive aphasia, SD, semantic dementia.
All participants provided written informed consent to take part in the study. The study was approved by North West NRES committee (REC Refs: 12/NW/0883).
Neuropsychology
Participants underwent a neuropsychological test battery with a particular focus on language and working memory. For performance measures, see Table A1 in Appendix S1.
Language assessment included tests of naming, single word comprehension, sentence processing, narrative production, reading, and spelling.
Naming
Single word comprehension
Manchester comprehension
This involves matching a printed word with one of four pictures (the same 40 target items are used as in the Manchester naming test).
Sentence processing tasks
Modified PALPA 55 subtest Auditory sentence comprehension test (Kay, Lesser, & Coltheart, 1992) and PALPA subtest 58 Auditory comprehension of locative relations (Kay et al., 1992).
Manchester sentence ordering
A locally constructed task, which requires participants to order five individually printed words to make a sentence.
Manchester tense production
In this locally constructed task, participants are shown action pictures (e.g., a boy kicking a ball) accompanied by the written word ‘yesterday’ or ‘tomorrow’. Participants are asked to generate a descriptive sentence beginning with the word provided (e.g., Yesterday, the boy kicked the ball).
Measures of narrative production
Cookie theft picture
Participants were recorded describing the picture (Goodglass & Kaplan, 1983). The number of phonetic errors, phonemic errors, fluency disruptions, length of utterance, number of dependent clauses per utterance, and percentage of well‐formed sentences were calculated. More details are provided online (Table A1 in Appendix S1).
Reading tests
Manchester reading. This test involves reading aloud the printed words used in the Manchester word‐picture matching test.
Modified PALPA subtest 36 Non‐word reading.
Modified PALPA test 31 Imageability/frequency reading.
Spelling/sound regularity reading (Glushko, 1979).
Spelling
Written spelling
Participants are requested to write 20 dictated words, drawn from the Manchester naming test.
Other tests evaluated working memory, praxis, calculation, and behaviour.
Working memory
The tasks addressed both verbal (phonological) and visuospatial working memory. Verbal tasks encompassed those with written as well as spoken presentation and tasks requiring pointing as well as spoken responses.
-
Verbal (phonological) tasks
Digits: Forwards and reverse Digit span (Wechsler, 1981).
Single words: Manchester word repetition. This test involves repetition of items from the Manchester naming/reading/comprehension tests.
Word Sequences: Manchester immediate and delayed word repetition. This is a locally constructed test, which involves repeating one or two words with variable delays of zero and five seconds.
Sentences: PALPA test 12 Sentence repetition (modified/shortened; Kay et al., 1992).
PALPA test 60 Pointing span for noun‐verb sequences (Kay et al., 1992).
Modified Brown–Peterson task of verbal working memory (see Stopford, Thompson, Neary, Richardson, & Snowden, 2010). In this test, participants are presented visually with three words. They are asked to repeat the words either immediately, following a five second delay or following a five second delay during which the participant reads aloud numbers.
-
Visuospatial tasks
Visual patterns span (Della Sala, Gray, Baddeley, & Wilson, 1997), involving copying from memory grids of black and white squares to blank grids.
Visual array comparison test constructed and modified from (Cowan et al., 2005; Luck & Vogel, 1997) and programmed in E‐Prime 2.0 (Psychology software tools inc, 2012). There are two subtests: colour and location. In both conditions, participants are briefly presented with one square and asked whether a second square is the same colour or in the same location as the first square. The arrays increase in size to a maximum of 10 squares.
Praxis
Orofacial and limb praxis were evaluated with the Manchester praxis screen, a locally constructed instrument taken from the Manchester neuropsychological profile, which is described previously (Snowden et al., 2011). Participants are asked to carry out the actions described below. If participants are unable to carry out the action to command, they are shown the action to copy.
Orofacial praxis tasks consist of five actions, including tongue protrusion and coughing, three speech sounds (alternating repetitive vowel sounds, consonant sounds, and maintaining an extended phoneme), three emotional gestures (surprise, anger, and happiness), and three pantomimes (sniffing, sucking, and blowing).
Upper limb praxis tasks included four gestures and four pantomimes. Each action is performed with both left and right upper limbs.
Calculation
The Manchester calculation test involves 12 calculations; 6 administered verbally and 6 in writing.
Behaviour
A locally constructed behavioural questionnaire was given to relatives/carers to complete. The questionnaire includes questions aiming to evaluate changes in areas of behaviour, which are known to be affected in bvFTD and are described below. Each question is rated on the basis of three options: increase, decrease, or no change. The behavioural domains have been found sensitive in distinguishing bvFTD from other forms of dementia (Bathgate, Snowden, Varma, Blackshaw, & Neary, 2001) and within subtypes of FTD (Snowden et al., 2001) and are incorporated within contemporary diagnostic criteria for bvFTD (Rascovsky et al., 2011):
Social behaviour and interactions with others: interest and enjoyment being around others, social greetings and courtesies, amount of eye contact and frequency of personal questions, and comments of a complimentary or critical nature.
Social emotions: happiness, sadness, fear, anger, guilt, surprise, disgust, embarrassment, empathy, laughter, and sense of humour.
Response to environment: awareness of danger and response to painful and neutral stimuli.
Eating and drinking: quantity and range of food eaten.
Self‐care: frequency of washing/bathing, changing clothes, and care about appearance.
Hobbies, order, and routine: frequency of doing puzzles, TV quizzes, clockwatching, carrying out simple or complex repetitive behaviour, and hoarding.
Statistical analysis
Statistical analyses were conducted with SPSS version 22. A p value of <.05 was adopted.
Group comparisons of demographic data were carried out with ANOVAs and non‐parametric equivalents as appropriate. Group comparisons on neuropsychological measures were analysed with Kruskal–Wallis tests. Due to the variation in ages and duration of symptoms across the cohort, group comparisons among the patient groups were also carried out with Quade's non‐parametric rank analysis of covariance (ANCOVA), with age and duration of illness entered as nuisance covariates. Post‐hoc Fisher's LSD tests were performed. A p value of <.05 was adopted.
Multiple post‐hoc comparisons were corrected for using Bonferroni corrections (i.e., for comparisons of all groups, p < .005 was adopted and for comparisons of the patient groups p < .0083 was adopted).
Sensitivity, specificity, positive and negative predictive values of individual tests were calculated. The tests and groups were chosen on the basis of group comparisons. Any test where a patient group scored significantly worse relative to the other patient groups was included in the sensitivity and specificity analyses. Receiver operating characteristics curves were fitted to the data and Louden's index was used to determine cut‐offs.
In order to delineate core factors underlying language, working memory, and behavioural deficits in these conditions, a principal components analysis (PCA) was carried out. A participant to variable ratio of 1.2 has yielded good results for PCA (Barrett & Kline, 1981). In order to have a reasonable participant to variable ratio, only scores from tests with both sensitivities and specificities greater than 50% were included in the analysis (see Table 4). For variables in which both ‘total’ and ‘subtests’ reached the threshold, for example, buccofacial praxis, only subtest variables were entered into the PCA. In total 25 variables were included in the PCA. Once missing data are taken into account, this study gives a 1.32 ratio. Factors above the inflection point on a scree plot were retained. In order to interpret factor loadings, a variance maximizing (varimax) rotation was performed. Factor loadings were used to gain individual participants’ factor scores, which provide information about the participants’ performance on the cognitive tests that cluster on said factor.
Individual participants’ factor scores were used for a stepwise linear canonical discriminant analysis (LDA) to determine the strongest neuropsychological discriminators between the four groups (nfvPPA, lvPPA, SD, and multi‐domain AD).
Results
Group comparisons
As expected, scores on many of the measures of working memory, speech and language, and praxis differed significantly in lvPPA and nfvPPA groups relative to healthy controls (data presented in Table A3 in Appendix S1). Only scores on tests of naming, comprehension, accuracy in reading aloud, and the percentage of nouns produced in narrative production and spelling differed between the SD group and healthy controls, in keeping with their semantic disorder. Scores on tests of naming, auditory sentence comprehension, spelling, several tests of repetition and auditory‐visual working memory differed between AD and controls. Direct comparisons between patient groups are presented in Tables 2 and 3 and highlighted in the sections below. These data are corrected for age and duration of illness. Sensitivity, specificity, and positive and negative predictive values of individual tests are presented in Table 4.
Table 2.
Group comparisons of measures of language function; median (range)
| Measure | M (n) | SD (n = 8) | NfvPPA (n = 12) | LvPPA (n = 13) | AD (n = 14) | Quade's ANCOVA F (df = 3) | Controls (n = 19) |
|---|---|---|---|---|---|---|---|
| Naming | |||||||
| Object and action naming: objects/50 | 0 | 25.5 (14–48) | 47.0 (0–50) | 44.0 (22–50) | 45.5 (4–50) | 2.303 | 50.0 (48–50) |
| Object and action naming: actions/50 | 0 | 30.0 (20–49) | 42.0 (0–49) | 45.0 (17–49) | 42.5 (5–50) | 0.681 | 50.0 (48–50) |
| Proportion action to object naming | 1 | 1.2 (1.0–1.7)a , b , c | 0.9 (0.1–1.0)d | 1.0 (0.8–1.2)d | 1.0 (0.7–1.3)d | 11.101*** | 1.0 (1.0–1.0) |
| Manchester naming/40 | 0 | 21.0 (11–37) | 36.5 (0–40) | 36.0 (16–39) | 36.5 (4–40) | 2.518 | 40.0 (39–40) |
| Single word comprehension | |||||||
| Manchester comprehension/40 | 0 | 38.5 (27–40)a , b , c | 40.0 (40–40)d | 40.0 (40–40)d | 40.0 (38–40)d | 9.286*** | 40.0 (40–40) |
| Sentence processing | |||||||
| Auditory sentence comprehension/24 | 0 | 23.0 (11–24) | 20.5 (10–24) | 22.0 (15–24) | 18.0 (7–23) | 1.077 | 24.0 (23–24) |
| Auditory comprehension of locative relations /24 | 2 | 21.5 (5–24) | 15.0 (7–24) | 19.0 (7–24) | 11.0 (6–24) | 1.402 | 24.0 (19–24) |
| Manchester sentence ordering | |||||||
| Order/10 | 2 | 8.0 (3–10) | 1.0 (0–10)b | 9.5 (4–10)a | 9.0 (0–10) | 4.492** | 10.0 (7–10) |
| Reading/10 | 3 | 10.0 (10–10)a | 3.5 (0–10)b , c , d | 10.0 (6–10)a | 10.0 (0–10)a | 11.118*** | 10.0 (9–10) |
| To dictation/10 | 3 | 10.0 (7–10)a | 3.5 (0–10)b , c , d | 10.0 (8–10)a | 10.0 (0–10)a | 6.262*** | 10.0 (10–10) |
| Time (s) | 4 | 13.0 (8–28)a | 29.1 (10–48)d | 30.35 (11–55) | 28.2 (14–75) | 3.177* | 6.2 (2–17) |
| Manchester tense production | |||||||
| Uncued/20 | 7 | 17.0 (1–20) | 18.0 (2–20) | 19.0 (12–20) | 19.0 (0–20) | 0.845 | 20.0 (19–20) |
| Cued/20 | 7 | 18.0 (5–20) | 18.0 (2–20) | 20.0 (13–20) | 19.0 (0–20) | 1.356 | 20.0 (19–20) |
| Complete/20 | 2 | 20.0 (15–20) | 13.0 (10–20) | 20.0 (10–20) | 20.0 | 1.934 | 20.0 (20–20) |
| Measures of narrative production: cookie theft description | |||||||
| Words per minute | 1 | 159.5 (89–217)a , c | 47.8 (4–80)b , c , d | 122.1 (38–152)a | 116.9 (57–176)a , d | 14.415*** | 145.1 (120–198) |
| Phonetic errors per 100 words | 1 | 0.0 (0–0) | 0.0 (0–2.5) | 0.0 (0–0) | 0.0 (0–0) | 2.135 | 0.0 (0–0) |
| Phonemic errors per 100 words | 1 | 0.0 (0–0)a | 3.0 (0–67)b , c , d | 0.0 (0–5)a | 0.0 (0–5)a | 6.393** | 0.0 (0–0) |
| Fluency disruptions per 100 words | 1 | 4.6 (1–22)a | 31.3 (0–65)d | 16.1 (3–44) | 11.8 (0–64) | 4.648** | 6.8 (0–9) |
| Mean length of utterance (words) | 1 | 10.9 (7–13)a | 6.0 (0.0–9.6)b , c , d | 10.0 (5.3–21.4)a | 10.1 (5.7–17.7)a | 5.454** | 13.8 (9.4–22.7) |
| Number of dependent clauses per utterance | 2 | 0.1 (0–0.5) | 0.0 (0–0.1)b | 0.3 (0–1.3)a | 0.1 (0–0.7) | 6.046** | 0.5 (0.2–1.3) |
| Percent nouns | 2 | 9.9 (8–19)a | 19.9 (9–64)d | 14.6 (9–26) | 14.4 (6–23) | 3.281* | 19.6 (13–25) |
| Percent verbs | 2 | 29.4 (22–36) | 25.0 (14–38) | 26.8 (16–32) | 24.5 (17–32) | 1.390 | 24.7 (19.9–30.7) |
| Proportion nouns to verbs | 2 | 0.3 (0.2–0.9)a | 0.7 (0.4–4.5)d | 0.6 (0.4–1.1) | 0.6 (0.3–1.0) | 3.550* | 0.8 (0.5–1.2) |
| Percentage well‐formed sentences | 1 | 100.0 (86–100)a | 60.0 (0–100)d | 71.4 (43–100) | 80.0 (20–100) | 4.481** | 100.0 (85–100) |
| Reading | |||||||
| Manchester reading/40 | 2 | 40.0 (31–40) | 40.0 (33–40)b | 40.0 (40–40)a | 40.0 (31–40) | 2.954* | 40.0 (40–40) |
| Imageability/frequency reading/40 | 0 | 38.0 (20–40) | 37.0 (0–40) | 39.0 (31–40) | 39.0 (13–40) | 1.834 | 40.0 (39–40) |
| Difference high/low frequency | 0 | 1.5 (0–4) | 1.0 (0–5) | 0.0 (−3 to 2) | 0.5 (−2 to 3) | 2.422 | 0.0 (0–1) |
| Difference high/low imageability | 0 | 2.0 (0–10) | 1.0 (−1 to 5) | 0.0 (0–7) | 1.0 (0–9) | 2.531 | 0.0 (0–1) |
| Spelling/sound regularity reading/20 | 0 | 19.0 (9–20) | 18.0 (0–20) | 19.0 (16–20) | 19.0 (8–20) | 0.581 | 20.0 (19–20) |
| Difference regular/exception | 0 | 0.0 (−3 to 1) | 0.0 (−2 to 4) | 0.0 (−1 to 2) | 0.0 (−3 to 2) | 1.223 | 0.0 (−1 to 1) |
| Non‐word reading/10 | 0 | 10.0 (3–10) | 8.0 (0–10) | 10.0 (6–10) | 10.0 (1–10) | 2.333 | 10.0 (5–10) |
| Spelling | |||||||
| Manchester writing/20 | 5 | 13.0 (7–20) | 12.0 (2–20) | 19.0 (4–20) | 14.5 (0–20) | 0.550 | 20.0 (14–20) |
df, degrees of freedom; lvPPA, logopenic variant primary progressive aphasia; m, missing; nfvPPA, non‐fluent/agrammatic variant primary progressive aphasia; s, seconds; SD, semantic dementia.
*p < .05; **p < .01; ***p < .0001.
aSignificant versus non‐fluent/agrammatic patients.
bSignificant versus logopenic patients.
cSignificant versus AD patients.
dSignificant versus SD patients. N.B. For post‐hoc comparisons, significance is set at p < .0083.
Table 3.
Group comparisons of working memory, praxis, and calculation; median (range)
| M (n) | SD (n = 8) | NfvPPA (n = 12) | lvPPA (n = 13) | AD (n = 14) | Quade's ANCOVA F (df = 3) | Controls (n = 19) | |
|---|---|---|---|---|---|---|---|
| Working memory | |||||||
| Digit span forwards | 0 | 5.5 (3–8)a | 4.0 (0–6)b | 5.0 (2–6) | 4.5 (2–8) | 2.772 | 8.0 (5–9) |
| Digit span reverse | 0 | 4.0 (2–6)a , c | 2.0 (0–4)b | 3.0 (1–4) | 2.5 (1–4)b | 6.514** | 4.0 (3–8) |
| Manchester word repetition/40 | 1 | 40.0 (40–40) | 40.0 (0–40) | 40.0 (33–40) | 40.0 (35–40) | 3.100* | 40.0 (40–40) |
| Manchester immediate and delayed word repetition/36 | 0 | 35.5 (21–36) | 34.0 (0–36) | 33.0 (6–36) | 34.0 (5–36) | 1.325 | 36.0 (35–36) |
| Immediate/18 | 0 | 18.0 (14–18) | 17.0 (0–18) | 17.0 (6–18) | 18.0 (5–18) | 1.424 | 18.0 (17–18) |
| Delayed/18 | 0 | 17.5 (7–18) | 16.5 (0–18) | 16.0 (0–18) | 16.0 (0–18) | 0.857 | 18.0 (17–18) |
| Sentence repetition/24 | 1 | 24.0 (23–24)a | 14.0 (0–24)b | 23.0 (0–24) | 23.0 (0–24) | 3.865* | 24.0 (24–24) |
| Pointing span | 1 | 4.5 (4–9) | 5.0 (0–6) | 4.0 (0–7) | 3.0 (0–7) | 1.536 | 8.0 (5–11) |
| Modified Brown–Peterson total/54 | 2 | 50.5 (35–54)d | 44.5 (0–54) | 36.0 (5–46)b | 40.0 (0–54) | 4.047* | 53.5 (46–54) |
| Immediate/18 | 2 | 18.0 (16–18)c , d | 16.0 (0–18) | 15.0 (5–17)b | 15.0 (0–18)b | 5.100** | 18.0 (17–18) |
| 5‐s delay/18 | 2 | 17.0 (13–18)d | 15.0 (0–18) | 8.0 (0–17)b | 14.0 (0–18) | 3.293* | 18.0 (16–18) |
| Distraction & delay/18 | 2 | 16.5 (6–18)d | 13.0 (0–18) | 9.0 (0–16)b | 11.0 (0–18) | 2.960* | 18.0 (12–18) |
| Visual patterns test | 2 | 5.5 (3–9)c | 5.5 (0–9) | 5.0 (1–8) | 3.5 (0–7)b | 3.403* | 6.0 (4–9) |
| Visual array comparison test: colour/10 | 4 | 4.0 (1–7) | 4.0 (0–8) | 3.5 (0–10) | 1.5 (0–6) | 1.829 | 6.0 (1–10) |
| Visual array comparison test: location/10 | 4 | 7.0 (0–10) | 6.5 (0–10) | 8.0 (0–10) | 1.5 (0–5) | 2.287 | 8.0 (2–10) |
| Praxis: Manchester praxis screen | |||||||
| Orofacial/25 | 0 | 25.0 (22–25)a | 20.0 (4–23)b , c , d | 25.0 (21–25)a | 25.0 (22–25)a | 12.903*** | 25.0 (25–25) |
| Actions/10 | 10.0 (9–10)a | 8.0 (4–10)b , c , d | 10.0 (10–10)a | 10.0 (9–10)a | 8.069** | 10.0 (10–10) | |
| Sounds/speech praxis/3 | 3.0 (3–3)a | 1.0 (0–3)b , c , d | 3.0 (1–3)a | 3.0 (2–3)a | 23.325*** | 3.0 (3–3) | |
| Emotions/6 | 6.0 (3–6) | 5.0 (0–6)d | 6.0 (3–6)a | 6.0 (4–6) | 4.351** | 6.0 (6–6) | |
| Pantomime/6 | 6.0 (6–6)a | 6.0 (0–6)b | 6.0 (5–6) | 6.0 (4–6) | 3.571* | 6.0 (6–6) | |
| Upper limb/32 | 0 | 29.0 (24–32) | 26.5 (5–32) | 32.0 (22–32) | 28.0 (1–32) | 1.110 | 32.0 (32–32) |
| Right total/16 | 14.5 (12–16) | 13.5 (3–16) | 16.0 (12–16) | 14.5 (1–16) | 1.003 | 16.0 (16–16) | |
| Left total/16 | 14.5 (11–16) | 13.5 (0–16) | 16.0 (10–16) | 13.5 (0–16) | 1.027 | 16.0 (16–16) | |
| Gesture total/16 | 15.0 (10–16) | 14.5 (5–16) | 16.0 (12–16) | 15.5 (1–16) | 1.191 | 16.0 (16–16) | |
| Pantomime total/16 | 14.0 (12–16) | 13.0 (0–16) | 16.0 (9–16) | 13.5 (0–16) | 0.924 | 16.0 (16–16) | |
| Calculation | |||||||
| Manchester calculation test/12 | 12.0 (8–12)a , d | 4.0 (1–12)b | 6.0 (2–12)b , c | 3.0 (0–10)b , d | 11.035*** | 11.0 (7–12) | |
df, degrees of freedom; lvPPA, logopenic variant primary progressive aphasia; m, missing; nfvPPA, non‐fluent/agrammatic variant primary progressive aphasia; s, seconds; SD, semantic dementia.
*p < .05; **p < .01; ***p < .0001.
aSignificant versus non‐fluent/agrammatic patients.
bSignificant versus SD patients.
cSignificant versus AD patients.
dSignificant versus logopenic patients.
Table 4.
Sensitivity, specificity, positive and negative predictive values of cognitive measures
| Test (cut‐off) | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
|---|---|---|---|---|
| Semantic dementia versus other groups | ||||
| Manchester word picture comprehension test (<40/40) | 62.5 | 92.3 | 62.5 | 92.3 |
| Proportion action to object naming (>1.1) | 87.5 | 86.8 | 58.3 | 97.1 |
| Narrative: percent nouns (<11.5) | 75.0 | 84.6 | 50.0 | 94.3 |
| Narrative: proportion nouns to verbs (<0.4) | 62.5 | 89.7 | 55.6 | 92.1 |
| Behaviour: social behaviour (>5/9) | 75.0 | 75.9 | 30.0 | 95.7 |
| Behaviour: response to environment (>1/3) | 25.0 | 93.1 | 33.3 | 90.0 |
| Behaviour: hobbies, order and routines (>2/8) | 75.0 | 72.4 | 27.3 | 95.5 |
| Calculation (>8/12) | 87.5 | 76.3 | 43.7 | 96.7 |
| Non‐fluent/agrammatic variant primary progressive aphasia versus other groups | ||||
| Manchester reading (<40/40) | 40.0 | 91.4 | 57.1 | 84.2 |
| Manchester sentence ordering: order (<4/10) | 66.7 | 87.9 | 66.7 | 87.9 |
| Manchester sentence ordering: reading (<10/10) | 91.7 | 78.1 | 61.1 | 96.2 |
| Manchester sentence ordering: to dictation (<6/10) | 66.7 | 96.9 | 88.9 | 88.9 |
| Narrative: phonemic errors per 100 words (>0.9) | 81.8 | 80.0 | 56.3 | 93.3 |
| Narrative: words per minute (<82.5) | 100.0 | 85.7 | 68.8 | 100.0 |
| Narrative: percentage well‐formed sentences (<35.7%) | 62.5 | 97.1 | 83.3 | 91.9 |
| Narrative: mean length of utterance (<7.2 words) | 81.8 | 82.9 | 60.0 | 93.5 |
| Narrative: number of dependent clauses per utterance (<0.12) | 100.0 | 62.9 | 40.9 | 100.0 |
| Narrative: number of fluency disruptions per 100 words (>28.4) | 63.6 | 88.6 | 63.6 | 88.6 |
| Orofacial praxis total (<24/25) | 100.0 | 77.1 | 60.0 | 100.0 |
| Orofacial praxis actions (<9/10) | 58.3 | 100.0 | 100.0 | 87.5 |
| Orofacial praxis sounds (<3/3) | 91.7 | 91.4 | 78.6 | 97.0 |
| Orofacial praxis emotions (<6/6) | 75.0 | 80.0 | 56.3 | 90.3 |
| Orofacial praxis pantomimes (<4/6) | 41.7 | 100.0 | 100.0 | 83.3 |
| Logopenic variant primary progressive aphasia versus other groups | ||||
| Manchester immediate and delayed word repetition (<35/36) | 76.9 | 52.9 | 38.5 | 85.7 |
| Manchester immediate and delayed word repetition: immediate (<17/18) | 38.4 | 76.5 | 38.5 | 76.5 |
| Manchester immediate and delayed word repetition: delayed (<18/18) | 76.9 | 38.2 | 32.3 | 81.3 |
| Sentence repetition (<22/24) | 46.2 | 69.7 | 37.5 | 76.7 |
| Digit span forwards (<7) | 100.0 | 14.7 | 31.0 | 100.0 |
| Digit span reverse (<5) | 100.0 | 5.9 | 28.9 | 100.0 |
| Visual patterns span (<6) | 84.6 | 34.4 | 34.4 | 84.6 |
| Visual array comparison test: location (<1) | 25.0 | 77.4 | 30.0 | 72.7 |
| Visual array comparison test: colour (<7) | 91.7 | 9.7 | 28.2 | 75.0 |
| Pointing span (<5) | 69.2 | 42.4 | 32.1 | 77.8 |
| Brown–Peterson: total (<47/54) | 100.0 | 50.0 | 39.3 | 100.0 |
| Brown–Peterson: immediate (<18/18) | 100.0 | 38.2 | 34.4 | 100.0 |
| Brown–Peterson: delayed (<9/18) | 54.5 | 79.4 | 46.2 | 84.4 |
| Brown–Peterson: distraction (<17/18) | 100.0 | 29.4 | 31.4 | 100.0 |
| Logopenic variant primary progressive aphasia versus semantic dementia and non‐fluent variant PPA | ||||
| Manchester immediate and delayed word repetition (<35/36) | 76.9 | 60.0 | 55.6 | 80.0 |
| Manchester immediate and delayed word repetition: immediate (<17/18) | 38.5 | 75.0 | 50.0 | 65.2 |
| Manchester immediate and delayed word repetition: delayed (<17/18) | 61.5 | 60.0 | 50.0 | 70.6 |
| Sentence repetition (<22/24) | 46.2 | 68.4 | 50.0 | 65.0 |
| Digit span forwards (<6) | 84.6 | 30.0 | 44.0 | 75.0 |
| Digit span reverse (<4) | 76.9 | 35.0 | 43.5 | 70.0 |
| Visual patterns span (<6) | 84.6 | 50.0 | 55.0 | 81.8 |
| Visual array comparison test: location (<1) | 25.0 | 88.2 | 60.0 | 62.5 |
| Visual array comparison test: colour (<7) | 91.7 | 17.6 | 44.0 | 75.0 |
| Pointing span (<5) | 69.2 | 52.6 | 50.0 | 71.4 |
| Brown–Peterson: total (<47/54) | 100.0 | 65.0 | 61.1 | 100.0 |
| Brown–Peterson: immediate (<18/18) | 100.0 | 45.0 | 50.0 | 100.0 |
| Brown–Peterson: delayed (<16/18) | 81.8 | 60.0 | 52.9 | 85.7 |
| Brown–Peterson: distraction (<17/18) | 100.0 | 40.0 | 47.8 | 100.0 |
Patients in all groups showed problems in naming and naming scores alone did not differentiate the groups. However, there were qualitative differences. SD patients named a higher proportion of actions relative to objects than all other patient groups (Table 2) and they made more semantic errors than nfvPPA patients (and Table A4 in Appendix S1). Indeed, the disproportionately poor naming of nouns was reasonably sensitive (87.5%) and specific (86.8%) to SD (Table 4). NfvPPA made more phonological errors on naming tests than SD patients (Table A4 in Appendix S1).
Agrammatism is a core feature nfvPPA; yet, scores on only a subset of sentence processing tasks were significantly worse in nfvPPA compared to the other patient groups. The Manchester sentence ordering test yielded the highest level of significance. Notably, however, not all conditions of the test were equally discriminating. Ordering five words according to a dictated sentence (dictation condition), and reading aloud of an ordered sentence (reading condition), were poorer in nfvPPA compared to all other patient groups. By contrast, on the standard, ‘order’ condition, in which patients were required to arrange sets of five words to form a sentence, nfvPPA scores were poorer than in lvPPA but not SD or AD. Indeed the ‘order’ condition had lower specificity (87.9%; Table 4) for detecting nvPPA, than the dictation condition (specificity 96.9%, sensitivity 66.7%). The reading condition had the highest sensitivity (91.7%) but the lowest specificity (78.1%).
NfvPPA patients performed more poorly than other groups on several measures of speech derived from narrative production (Table 2). These include a shorter mean length of utterance and more phonemic errors compared to all other groups, fewer dependent clauses per utterance compared to lvPPA and a lower percentage of well‐formed sentences than SD (Table 2). Scores on a test of comprehension of complex syntax did not differ significantly between the patient groups.
Orofacial praxis differentiated nfvPPA from other patient groups, with overall scores on the Manchester orofacial praxis screen showing good specificity (77.1%), and sensitivity (100.0%). nfvPPA patients’ deficits extended beyond the realms of AOS, although different orofacial tasks yielded different levels of sensitivity and specificity. Orofacial actions and pantomimes had the highest specificity (both 100.0%) but lower sensitivity (58.3% and 41.7% respectively). Perhaps unsurprisingly, production of repetitive and elongated speech sounds, that is, a measure of AOS, showed the best balance of specificity and sensitivity (91.7% and 91.4% respectively).
Patients with nfvPPA, lvPPA, and AD performed similarly on tests of working memory, whereas SD patients showed superior performance. NfvPPA patients performed worse than SD on digit span forwards and reverse conditions and on the sentence repetition test, whereas lvPPA patients performed worse than SD patients on all sections of the Brown–Peterson test. AD patients scored lower on the reverse digit span, immediate Brown–Peterson test, and the visual patterns test relative to SD patients. As working memory tests were expected to be useful in differentiating lvPPA from other groups, the sensitivity and specificity of these measures for lvPPA were calculated. The total score on the Brown–Peterson test showed the highest sensitivity for lvPPA (100.0%) but considerably lower specificity (50.0%). When multidomain AD patients were excluded from the analysis, the specificity increased to 65.0%. The only tests to reach over 70.0% specificity for lvPPA were the visual array comparison location test and the Manchester immediate word repetition test (77.4% and 76.5% respectively), but the sensitivities were very low (25.0% and 38.4%).
Behavioural data
Behavioural changes in the four patient groups are depicted in Figure 1 and Table A5 in Appendix S1. Behavioural data are absent from 7 patients. This was either because the patient attended the appointment without an informant or else carers/relatives were distressed and it was deemed inappropriate to approach them.
Figure 1.
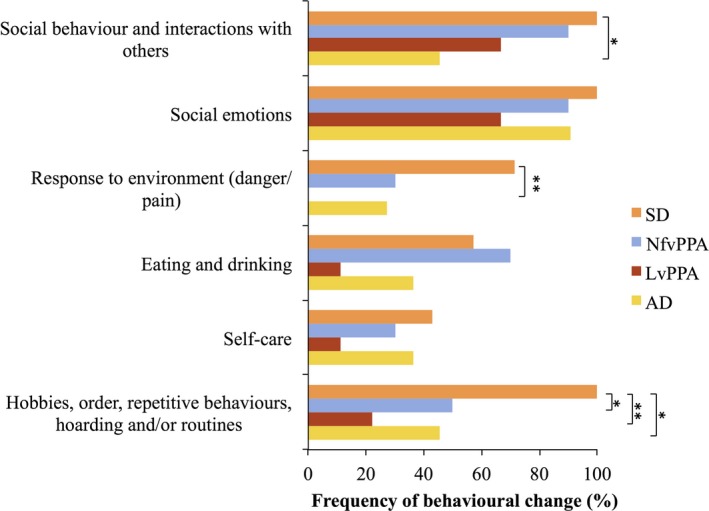
Behavioural change in the cohort. lvPPA, logopenic variant primary progressive aphasia; nfvPPA, non‐fluent/agrammatic variant primary progressive aphasia; SD, semantic dementia; AD, multi‐domain Alzheimer's disease. *p <. 05, **p < .01. [Colour figure can be viewed at wileyonlinelibrary.com]
Patients with SD exhibited more behavioural changes than the other patient groups, except in the domain of eating and drinking (Figure 1). There was no difference in frequency of behavioural changes in SD patients with predominantly right and predominantly left‐sided atrophy (Figure A1 in Appendix S1). Behavioural changes were not exclusive to SD and some patients in each group exhibited changes in each category of behaviour evaluated. The only exception was that no patient with lvPPA exhibited altered ‘response to environment’. Group differences reached significance for the following behavioural domains: ‘response to environment’ (Fisher's exact test, p = .018), ‘social behaviour’ (Fisher's exact test, p = .038) and ‘hobbies, order and routine’ (Fisher's exact test, p = .015). Changes in ‘hobbies, order and routine’ were more common in SD than nfvPPA, lvPPA, and AD (Fisher's exact test, p = .044, p = .003 and .038 respectively) and changes in patients’ ‘response to their environment’ were more common in SD than lvPPA (Fisher's exact test, p = .005). Changes in ‘social behaviour’ were more common in SD than AD (Fisher's exact test, p = .038). Indeed, changes in these behavioural domains had relatively high sensitivities and specificities for SD (Table 4).
The breakdown of specific behavioural changes is shown in (Table A5 in Appendix S1). A large proportion of patients with nfvPPA, lvPPA, and AD exhibited a withdrawal in social behaviours and interaction with others, in particular, a relatively high proportion of lvPPA (44.4%) and nfvPPA (40.0%) patients enjoyed being with others less. In contrast, a high proportion of SD patients enjoyed being with others more (42.9%). Changes in social emotions were more prevalent in nfvPPA and SD patients than in lvPPA or AD.
The majority of SD patients (57.1%) showed a reduction in the range of food they eat and an increase in the following features: reaction to painful stimuli (66.7%), clockwatching (57.1%), simple (71.4%) and complex (66.7%) repetitive behaviours, following a daily routine (57.1%), and enjoyment of puzzles (71.4%). In contrast, an increase in these features rarely occurred in the other patient groups. Nevertheless just under a third of nfvPPA patients liked TV quizzes and sweet food more than previously.
Principal components analysis
Fourteen patients had missing data on at least one of the 25 test variables due to anxiety/the magnitude of their difficulties and had to be excluded from the PCA. The remaining 33 patients comprised 9 nfvPPA, 7 lvPPA, 7 SD, and 10 multi‐domain AD. Evaluation of the sampling adequacy using the Kaiser–Meyer–Olkin revealed favourable results (0.504). The PCA yielded a 3‐factor solution, which accounted for 55.700% of the variance in performance (F1 = 26.032%, F2 = 15.130%, F3 = 14.538%). The rotated (varimax) factor loadings for the cognitive assessments are shown in Table 5.
Table 5.
Factor scores
| Factor | |||
|---|---|---|---|
| Grammaticality/speech production | Working memory | Semantic/behavioural | |
| Manchester immediate and delayed word repetition | 0.760 | ||
| Brown–Peterson test: immediate | 0.762 | ||
| Brown–Peterson test: delayed | 0.805 | ||
| Visual patterns span | |||
| Pointing span | 0.775 | ||
| Manchester word picture comprehension test | −0.843 | ||
| Proportion action to object naming | 0.579 | ||
| Narrative: percent nouns | |||
| Narrative: proportion nouns to verbs | |||
| Narrative: phonemic errors per 100 words | −0.715 | ||
| Calculation | |||
| Manchester sentence ordering: order | 0.768 | ||
| Manchester sentence ordering: reading | 0.851 | ||
| Manchester sentence ordering: dictation | 0.866 | ||
| Narrative: words per minute | 0.732 | ||
| Narrative: mean length of utterance (words) | |||
| Narrative: number of dependent clauses per utterance | |||
| Narrative: fluency disruptions per 100 words | −0.772 | ||
| Narrative: percentage well‐formed sentences | 0.661 | ||
| Orofacial praxis: actions | 0.710 | ||
| Orofacial praxis: sounds | 0.848 | ||
| Orofacial praxis: emotions | |||
| Behaviour: social Behaviour | 0.871 | ||
| Behaviour: response to environment | 0.782 | ||
| Behaviour: hobbies, order, and routine | 0.823 | ||
Tests of speech production, sentence processing, and buccofacial praxis loaded heavily onto factor one, together with one test of repetition. Tests of verbal/phonological working memory loaded heavily onto factor two. Single word comprehension, the proportion of actions versus objects named, and behavioural variables loaded heavily onto factor three.
Univariate analyses with Fisher's procedure employed for post‐hoc pairwise comparisons revealed that scores on the ‘speech production/grammaticality’ factor and the ‘semantic/behaviour’ factor differed significantly in the three groups, F(3,29) = 14.178, p < .001 and F(3,29) = 7.838, p = .001 respectively. Patients with nfvPPA scored significantly lower on factor one (the ‘speech production/grammaticality’ factor) than patients in the other groups (all p < .001). Patients with SD scored higher on factor three (the ‘semantic/behaviour’ factor) than patients with nfvPPA, lvPPA, or AD (p = .002, p < .001 and p < .001 respectively). Factor two scores did not yield group differences.
Linear discriminant analysis
Scores for factors 1–3 for the 33 patients were entered into a LDA to determine how well the factors derived from the PCA were able to differentiate the three diagnostic groups.
Three discriminant functions were obtained. Two of the three discriminant functions were statistically significant, Wilks’ λ = .127; χ2 (9) = 58.905; p < .001, Wilks’ λ = .486; χ2 (4) = 20.578; p < .001 and Wilks’ λ = .986; χ2 (1) = 0.403; p = .525 respectively.
The discriminant model correctly classified 72.7% of patients (88.9% of nfvPPA patients, 42.9% of lvPPA patients, 71.4% of SD, and 80.0% of multi‐domain AD patients). The model showed 88.9% sensitivity and 100.0% specificity for diagnosing nfvPPA. The sensitivity for diagnosis of SD was 71.4% with a specificity of 96.2%. The sensitivity for diagnosis of lvPPA was 42.9% with a specificity of 96.2%. The sensitivity for diagnosis of multi‐domain AD was 80.0% with a specificity of 69.6%.
Two patients with SD were predicted to be in the AD group. One patient with nfvPPA was predicted to be in the AD group. Four patients with lvPPA were predicted to be in the AD group. One patient with AD was predicted to be in the lvPPA group and a further patient with AD was predicted to be in the SD group.
While the model using the three factors was reasonably accurate at classifying nfvPPA and SD, it was unable to distinguish between the multi‐domain AD and lvPPA groups.
Discussion
This study demonstrates three key findings in relation to PPA, which extend established knowledge. Firstly, they show that not all test measures within a language domain are equally valuable or discriminating. Secondly, they highlight features outside the language domain that contribute to the differentiation between subtypes of PPA. A PCA elicited three distinct factors that combined pure linguistic and extra linguistic features, which were useful in discriminating the groups. Thirdly, the findings show that despite high sensitivity of several measures to lvPPA they showed relatively poor specificity in discriminating lvPPA both from nfvPPA and from multidomain AD, highlighting the challenge for the diagnosis and classification of lvPPA. These aspects are considered in more detail below.
As expected, SD patients showed severely impaired naming. Yet, raw scores on naming tests did not differentiate SD from the other patient groups. A more useful marker was the relative magnitude of the naming impairment for nouns compared to that for verbs, which was significantly greater in SD compared to other groups. The Manchester comprehension test (word‐picture matching), involving high frequency words, was highly discriminatory because SD patients alone showed reduced scores. A limitation is that the test is sufficiently easy that it elicited ceiling level scores in some SD patients, and so may be insensitive to mild comprehension impairments. This finding highlights the importance of including for diagnostic purposes a variety of tests that range in level of difficulty.
NfvPPA was the only group to perform worse than controls and other patient groups on tests of grammar. Interestingly, however, production of grammatical sentences in picture description, a conventional measure of grammaticality, as well as a sentence completion using the correct verb tense, were less discriminating than the simple Manchester sentence ordering task involving the ordering of five words to form a grammatically correct sentence. Different conditions of the Manchester sentence ordering task were not equally discriminatory. The core ‘order’ condition, in which patients have to order five words to make a sentence, was reasonably sensitive to nfvPPA (i.e., most patients with nfvPPA exhibited reduced scores on this test), yet it was not as specific as some of the other conditions (i.e., patients in other groups also had reduced scores in this domain). The likely explanation is that performance could be compromised for reasons other than agrammatism. For example, SD patients might have difficulty understanding the component words of a sentence, whereas lvPPA and AD patients might be overloaded by task demands due to reduced working memory capacity. The ‘dictation’ condition, in which participants are dictated a full sentence and asked to order the words accordingly, increased specificity. The dictated sentence improved performance in other patient groups, while some nfvPPA patients remained unable to order the sentences correctly.
NfvPPA patients predictably performed poorly on tests of orofacial praxis relative to the other patient groups. Impaired production of repetitive and elongated speech sounds (orofacial praxis: sounds), that is, indicators of AOS, had high sensitivity and specificity for nfvPPA. Problems in orofacial praxis in nfvPPA extended beyond the realm of AOS, with impairments being demonstrated also in production of emotional gesture and orofacial actions, highlighting the association between gestural and spoken communication (Duffy & Duffy, 1981; Glosser et al., 1986). Notably, on the PCA, tests of orofacial actions, sentence processing and narrative production loaded heavily onto the same factor (speech production and grammaticality), on which patients in the nfvPPA group had the lowest factor scores.
Behavioural changes, particularly changes in frequency of engagement in hobbies, increased preference for order and routines, repetitive behaviours, and hoarding, occurred more frequently in SD than the other patient groups. Indeed, an increase in repetitive behaviours and increased enjoyment of quizzes occurred in the majority of SD patients. Nevertheless, each core behavioural domain evaluated in this study was affected in some patients with nfvPPA. Most of the behavioural changes we observed are characteristic of bvFTD (Rascovsky et al., 2011). Consistent with previous findings (Harris et al., 2016), it is apparent that overlapping characteristics exist in these three syndromes predominantly predicated on FTLD pathology, that is, SD, nfvPPA, and bvFTD.
In this study, there were no significant differences in the behavioural profile of SD patients presenting with left and right predominant temporal lobe atrophy, and these behavioural features have been well documented (Bozeat, Gregory, Ralph, & Hodges, 2000; Green & Patterson, 2009; Lambon Ralph & Patterson, 2008; Liu et al., 2004; Rohrer & Warren, 2010; Rosen et al., 2006; Snowden et al., 2001). Furthermore, a ‘semantic and behavioural’ factor emerged from the principal components analysis on which SD patients’ scores differed from the other patient groups. The ubiquitous nature of behavioural changes in SD underscores the fact that this disorder extends beyond the realm of semantic memory and calls into question whether it should be considered primarily a disorder of language. Similarities between patients with left and right predominant temporal lobe atrophy also raises the question of the usefulness of the contemporary distinction between syndromes associated, respectively, with left and right temporal lobe degeneration.
Individual test scores failed to show a clear pattern of deficits in lvPPA relative to the nfvPPA group. Impaired repetition is a central feature of lvPPA (Gorno‐Tempini et al., 2011) and may be predicated on phonological working memory impairment (Gorno‐Tempini et al., 2008). In this study, we performed a detailed evaluation of working memory including several tests of repetition. Performance on working memory tests was undoubtedly impaired in lvPPA relative to controls, and the Brown–Peterson measures, in particular, discriminated lvPPA from SD. However, impairments on working memory tests were also apparent in the nfvPPA and AD groups and the specificity of these measures was generally low for lvPPA. Indeed another factor analytic study of PPA found that repetition clustered with agrammatism and AOS, core features of nfvPPA (Sajjadi et al., 2012). Given the marked speech production problems exhibited by patients with nfvPPA, it was perhaps unsurprising that they were impaired to a similar degree on tasks of verbal working memory as lvPPA patients. Our study supports the supposition that, in isolation, this feature has limited utility as a core diagnostic marker of lvPPA (Sajjadi et al., 2012). Interestingly, the PCA elicited a ‘working memory’ factor which, together with a ‘speech production and grammaticality’ factor and a ‘semantics and behaviour’ factor, was able to differentiate lvPPA patients from nfvPPA and SD patients relatively well when evaluated with a linear discriminant analysis. Composite measures of working memory may therefore prove useful diagnostically in the classification of PPA.
The PCA analysis did not distinguish lvPPA from multi‐domain AD. The findings suggest that common language and working memory deficits occur in lvPPA and multi‐domain AD. Our findings differ from those of some previous studies, which have found differences between AD and lvPPA patients in the realm of verbal working memory (Foxe et al., 2013; Meyer et al., 2015) and language (Ahmed et al., 2012). Differences in findings might reflect phenotypic variation in study cohorts, of which age is known to be one determinant (Snowden et al., 2007). Differences in assessment methods might also be relevant. The study by Ahmed et al., for example, involved analysis of speech samples rather than explicit measures of phonological short‐term memory. Stage of illness might also be relevant. The finding by Foxe et al. (2013) and Meyer et al. (2015) of poorer phonological short‐term memory in lvPPA were compared with AD, despite patients being matched for visual short‐term memory performance might represent a transient distinction. It is of interest in this regard that longitudinal studies of lvPPA have indicated a convergence over time with the clinical symptoms of AD (Leyton, Hsieh, Mioshi, & Hodges, 2013) and a spread of atrophy consistent with AD (Brambati et al., 2015)(Rohrer et al., 2013). It is possible that at an earlier stage of disease greater differences might have been elicited between the two groups. However, the average number of years since disease onset was relatively low in the lvPPA group (mean 3.82 years) and in line with that cited in other studies of lvPPA (Foxe et al., 2016; Krishnan et al., 2017; Whitwell et al., 2015; Win et al., 2017). Moreover, the majority of patients were assessed within a year of their medical referral, so were still at a relatively early stage of illness. Further studies involving incident cases and longitudinal investigation of working memory in a spectrum of different AD subtypes with varying ages of onset would be useful.
The ‘grammaticality and speech production’ factor was the most useful factor in classifying the three patient groups. It has been suggested that the absence of frank impairments in grammar and comprehension should be a core feature of lvPPA, rather than impaired repetition (Mesulam, Wieneke, Thompson, Rogalski, & Weintraub, 2012; Mesulam & Weintraub, 2014; Mesulam et al., 2014). Our findings support the notion that grammaticality (together with speech production) is more effective than impaired repetition in differentiation of PPA. However, supporting criteria for lvPPA are almost exclusively made up of features denoting preserved areas of speech and language. The only other core feature, impaired single word retrieval, is a notoriously common feature across dementia syndromes, in this study, proved non‐discriminatory. Thus, a change in the core criteria from ‘impaired phrase repetition’ to ‘preserved grammaticality and speech production’ would constitute denoting a disorder exclusively by absence of features, rather than the presence of characteristic features. The likely effect of this would be that any patient with PPA who does not have the defining features of the other two subtypes would be classified as lvPPA. While this would increase the sensitivity of the criteria for lvPPA, it would have a detrimental effect on the specificity, as mild cases of nfvPPA and svPPA and atypical PPA patients would probably fulfil criteria for lvPPA.
The study has several limitations. Firstly, inevitably there is some circularity when evaluating classification of patients on the same areas of cognition that are used to reach a diagnosis. Nevertheless, clinical diagnoses were made independently by clinical history and observation and neurological examination, with supportive neuropsychological evaluation. Furthermore, most of the tests employed as part of this study are not routinely used in the clinic and some measures were purely experimental. Indeed, all patients with lvPPA exhibited impairments in sentence repetition during clinical diagnostic testing; yet, some patients performed at ceiling on the relatively undemanding PALPA sentence repetition task. The choice of the sentence repetition task might be criticized for being too easy. On the other hand, it did elicit impairments in the nfvPPA group sufficient to distinguish nfvPPA from SD. Sentence repetition is therefore unlikely to prove a useful diagnostic marker for lvPPA, to allow it to be distinguished from nvfPPA, irrespective of the level of difficulty of the repetition task. In addition, the individual‐rotated factors elicited from the PCA included combinations of tests and behavioural measures that would not routinely be used in classification of patients. Secondly, by necessity of the prospective nature of the study, the sample size was relatively small (n = 47). The number of patients included in the PCA was smaller still (n = 33). However, as outlined in the methods, the ratio of participants to variables was acceptable (Barrett & Kline, 1981). Nevertheless, future confirmatory studies with larger numbers of participants would be useful. A third limitation is that, due to the relatively small sample, in order to have a reasonable participant to variable ratio for the PCA, the study did not involve an exhaustive assessment of non‐linguistic and linguistic factors. Cognitive domains were chosen owing to their theoretical importance in a study of progressive aphasia; yet, there are some notable omissions. One such factor is episodic memory, which has been found to be significantly impaired in AD relative to lvPPA (Win et al., 2017) and in lvPPA relative to nfvPPA (Piguet, Leyton, Gleeson, Hoon, & Hodges, 2015). In this study, standard episodic memory measures were omitted on the grounds that performance may be compromised secondarily by problems in language and working memory. However, further studies including carefully selected episodic memory variables would be interesting, as would the inclusion of other behavioural domains such as emotion processing and more detailed evaluation of connected speech, which may be useful in differential diagnosis of PPA (Piguet et al., 2015; Wilson et al., 2010). A final limitation relates to gender differences. The participants were consecutive referrals who met the selection criteria and agreed to participate. An unintended consequence was that the groups were not matched for gender. In future studies it would be important to consider the possible influence of gender on differential performance patterns.
In conclusion, simple and brief tasks of sentence ordering, narrative production, buccofacial praxis, and single word comprehension are helpful in the discrimination of forms of PPA. Nevertheless, evaluating single tests in isolation has limitations. Namely, tests may lack sensitivity or specificity because they are too easy or because failure can arise for different underlying reasons. A test profile is important, as exemplified by the PCA. Characteristic profiles in PPA include non‐language features, in particular, the presence of apraxia in nfvPPA, which extends beyond AOS, behavioural changes in SD, and working memory deficits in lvPPA. Notably, while factors elicited by the PCA were effective at differentiating nfvPPA, SD, and lvPPA, these measures were unable to differentiate lvPPA and multi‐domain AD. The similarity in language profile in lvPPA and multidomain AD is important because it supports the contention that lvPPA is largely a focal, linguistic form of AD rather than FTLD. Nevertheless, lvPPA risks being a diagnosis of exclusion. The classification of forms of PPA that are distinct from nfvPPA and SD remains a challenge.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Supporting information
Appendix S1. Supplementary material.
Acknowledgements
The authors thank the neurologists who work at the Cerebral Function Unit and contributed to the original clinical evaluation of patients included in this study and the clinical colleagues from the Northwest Region of UK who referred patients.
References
- Ahmed, S. , de Jager, C. A. , Haigh, A. M. , & Garrard, P. (2012). Logopenic aphasia in Alzheimer's disease: Clinical variant or clinical feature? Journal of Neurology, Neurosurgery & Psychiatry, 83, 1056–1062. 10.1136/jnnp-2012-302798 [DOI] [PubMed] [Google Scholar]
- Barrett, P. T. , & Kline, P. (1981). The observation to variable ratio in factor analysis. Personality Study and Group Behaviour, 1, 23–33. [Google Scholar]
- Bathgate, D. , Snowden, J. , Varma, A. , Blackshaw, A. , & Neary, D. (2001). Behaviour in frontotemporal dementia, Alzheimer's disease and vascular dementia. Acta Neurologica Scandinavica, 103, 367–378. https://doi.org/ane236%5bpii%5d [DOI] [PubMed] [Google Scholar]
- Botha, H. , Duffy, J. R. , Whitwell, J. L. , Strand, E. A. , Machulda, M. M. , Schwarz, C. G. , … Josephs, K. A. (2015). Classification and clinicoradiologic features of primary progressive aphasia (PPA) and apraxia of speech. Cortex, 69, 220–236. 10.1016/j.cortex.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozeat, S. , Gregory, C. A. , Ralph, M. A. , & Hodges, J. R. (2000). Which neuropsychiatric and behavioural features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer's disease? Journal of Neurology, Neurosurgery and Psychiatry, 69(2), 178–186. 10.1136/jnnp.69.2.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambati, S. M. , Amici, S. , Racine, C. A. , Neuhaus, J. , Miller, Z. , Ogar, J. , … Gorno‐Tempini, M. L. (2015). Longitudinal gray matter contraction in three variants of primary progressive aphasia: A tenser‐based morphometry study. NeuroImage Clinical, 8, 345–355. 10.1016/j.nicl.2015.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caza, N. , & Belleville, S. (2008). Reduced short‐term memory capacity in Alzheimer's disease: The role of phonological, lexical, and semantic processing. Memory, 16, 341–350. 10.1080/09658210701881758 [DOI] [PubMed] [Google Scholar]
- Cowan, N. , Elliott, E. M. , Scott Saults, J. , Morey, C. C. , Mattox, S. , Hismjatullina, A. , & Conway, A. R. (2005). On the capacity of attention: Its estimation and its role in working memory and cognitive aptitudes. Cognitive Psychology, 51(1), 42–100. 10.1016/j.cogpsych.2004.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Sala, S. , Gray, C. , Baddeley, A. , & Wilson, L. (1997). Visual patterns test: A test of short term visual recall. Bury St Edmunds, UK: Thames Valley Test Company. [Google Scholar]
- Druks, J. , & Masterson, J. (2000). An object & action naming battery. Philadelphia, PA: Psychology Press. [Google Scholar]
- Duffy, R. J. , & Duffy, J. R. (1981). Three studies of deficits in pantomimic expression and pantomimic recognition in aphasia. Journal of Speech, Language, and Hearing Research, 24(1), 70–84. 10.1044/jshr.2401.70 [DOI] [PubMed] [Google Scholar]
- Foxe, D. G. , Irish, M. , Hodges, J. R. , & Piguet, O. (2013). Verbal and visuospatial span in logopenic progressive aphasia and Alzheimer's disease. Journal of the International Neuropsychological Society, 19(3), 247–253. 10.1017/S1355617712001269 [DOI] [PubMed] [Google Scholar]
- Foxe, D. , Leyton, C. E. , Hodges, J. R. , Burrell, J. R. , Irish, M. , & Piguet, O. (2016). The neural correlates of auditory and visuospatial span in logopenic progressive aphasia and Alzheimer's disease. Cortex, 83, 39–50. 10.1016/j.cortex.2016.07.003 [DOI] [PubMed] [Google Scholar]
- Glosser, G. , Wiener, M. , & Kaplan, E. (1986). Communicative gestures in aphasia. Brain and Language, 27, 345–359. 10.1016/0093-934X(86)90024-6 [DOI] [PubMed] [Google Scholar]
- Glushko, R. J. (1979). Organization and activation of orthographic knowledge in reading aloud. Journal of Experimental Psychology: Human Perception and Performance, 5, 674–691. 10.1037//0096-1523.5.4.674 [DOI] [Google Scholar]
- Goodglass, H. , & Kaplan, E. (1983). Boston diagnostic aphasia examination (BDAE). Philadelphia, PA: Lea and Febiger. [Google Scholar]
- Gorno‐Tempini, M. L. , Brambati, S. M. , Ginex, V. , Ogar, J. , Dronkers, N. F. , Marcone, A. , … Miller, B. L. (2008). The logopenic/phonological variant of primary progressive aphasia. Neurology, 71, 1227–1234. 10.1212/01.wnl.0000320506.79811.da [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno‐Tempini, M. L. , Hillis, A. E. , Weintraub, S. , Kertesz, A. , Mendez, M. , Cappa, S. F. , … Grossman, M. (2011). Classification of primary progressive aphasia and its variants. Neurology, 76, 1006–1014. 10.1212/wnl.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, H. A. C. , & Patterson, K. (2009). Jigsaws‐A preserved ability in semantic dementia. Neuropsychologia, 47, 569–576. 10.1016/j.neuropsychologia.2008.10.015 [DOI] [PubMed] [Google Scholar]
- Grossi, D. , Becker, J. T. , Smith, C. , & Trojano, L. (1993). Memory for visuospatial patterns in Alzheimer's disease. Psychological Medicine, 23(1), 65–70. 10.1017/S003329170003885X [DOI] [PubMed] [Google Scholar]
- Harris, J. M. , Gall, C. , Thompson, J. C. , Richardson, A. M. , Neary, D. , du Plessis, D. , … Jones, M. (2013). Classification and pathology of primary progressive aphasia. Neurology, 81, 1832–1839. 10.1212/01.wnl.0000436070.28137.7b [DOI] [PubMed] [Google Scholar]
- Harris, J. M. , & Jones, M. (2014). Pathology in primary progressive aphasia syndromes. Current Neurology and Neuroscience Reports, 14, 466 10.1007/s11910-014-0466-4 [DOI] [PubMed] [Google Scholar]
- Harris, J. M. , Jones, M. , Gall, C. , Richardson, A. M. T. , Neary, D. , du Plessis, D. , … Thompson, J. C. (2016). Co‐occurrence of language and behavioural change in frontotemporal lobar degeneration. Dementia and Geriatric Cognitive Disorders Extra, 6, 205–213. 10.1159/000444848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, J. M. , Thompson, J. C. , Gall, C. , Richardson, A. M. , Neary, D. , du Plessis, D. , … Jones, M. (2015). Do NIA‐AA criteria distinguish Alzheimer's disease from frontotemporal dementia? Alzheimer's & Dementia, 11(2), 207–215. 10.1016/j.jalz.2014.04.516 [DOI] [PubMed] [Google Scholar]
- Joshi, A. , Roy, E. A. , Black, S. E. , & Barbour, K. (2003). Patterns of limb apraxia in primary progressive aphasia. Brain and Cognition, 53, 403–407. 10.1016/S0278-2626(03)00116-7 [DOI] [PubMed] [Google Scholar]
- Kay, J. , Lesser, R. , & Coltheart, M. (1992). Psycholinguistic assessment of language processing in aphasia. Hove, UK: Psychology Press. [Google Scholar]
- Krishnan, K. , Machuda, M. M. , Whitwell, J. L. , Butts, A. M. , Duffy, J. R. , Strand, E. A. , … Josephs, K. A. (2017). Varying degrees of temporoparietal hypometabolism on FDG‐PET reveal amyloid‐positive logopenic primary progressive aphsia is not a homogeneneous clinical entity. Journal of Alzheimer's Disease, 55, 1019–1029. 10.3233/JAD-160614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph, M. A. , & Patterson, K. (2008). Generalization and differentiation in semantic memory: Insights from semantic dementia. Annals of the New York Academy of Sciences, 1124, 61–76. 10.1196/annals.1440.006 [DOI] [PubMed] [Google Scholar]
- Leyton, C. E. , Hsieh, S. , Mioshi, E. , & Hodges, J. R. (2013). Cognitive decline in logopenic aphasia: More than losing words. Neurology, 80(10), 897–903. 10.1212/WNL.0b013e318285c15b [DOI] [PubMed] [Google Scholar]
- Libon, D. J. , Xie, S. X. , Moore, P. , Farmer, J. , Antani, S. , McCawley, G. , … Grossman, M. (2007). Patterns of neuropsychological impairment in frontotemporal dementia. Neurology, 68, 369–375. 10.1212/01.wnl.0000252820.81313.9b [DOI] [PubMed] [Google Scholar]
- Liu, W. , Miller, B. L. , Kramer, J. H. , Rankin, K. , Wyss‐Coray, C. , Gearhart, R. , … Rosen, H. J. (2004). Behavioral disorders in the frontal and temporal variants of frontotemporal dementia. Neurology, 62, 742–748. 10.1212/01.WNL.0000113729.77161.C9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck, S. J. , & Vogel, E. K. (1997). The capacity of visual working memory for features and conjunctions. Nature, 390, 279–281. 10.1038/36846 [DOI] [PubMed] [Google Scholar]
- Mesulam, M. M. , & Weintraub, S. (2014). Is it time to revisit the classification guidelines for primary progressive aphasia? Neurology, 82, 1108–1109. 10.1212/WNL.0000000000000272 [DOI] [PubMed] [Google Scholar]
- Mesulam, M. M. , Weintraub, S. , Rogalski, E. J. , Wieneke, C. , Geula, C. , & Bigio, E. H. (2014). Asymmetry and heterogeneity of Alzheimer's and frontotemporal pathology in primary progressive aphasia. Brain, 137(Pt 4), 1176–1192. 10.1093/brain/awu024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam, M. , Wieneke, C. , Thompson, C. , Rogalski, E. , & Weintraub, S. (2012). Quantitative classification of primary progressive aphasia at early and mild impairment stages. Brain, 135(Pt 5), 1537–1553. 10.1093/brain/aws080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, A. M. , Snider, S. F. , Campbell, R. E. , & Friedman, R. B. (2015). Phonological short‐term memory in logopenic variant primary progressive aphasia and mild Alzheimer's disease. Cortex, 71, 183–189. 10.1016/j.cortex.2015.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary, D. , Snowden, J. , Gustafson, L. , Passant, U. , Stuss, D. , Black, S. , … Benson, D. F. (1998). Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology, 51, 1546–1554. 10.1212/WNL.51.6.1546 [DOI] [PubMed] [Google Scholar]
- Nestor, P. J. , Graham, N. L. , Fryer, T. D. , Williams, G. B. , Patterson, K. , & Hodges, J. R. (2003). Progressive non‐fluent aphasia is associated with hypometabolism centred on the left anterior insula. Brain, 126(Pt 11), 2406–2418. 10.1093/brain/awg240 [DOI] [PubMed] [Google Scholar]
- Piguet, O. , Leyton, C. E. , Gleeson, L. D. , Hoon, C. , & Hodges, J. R. (2015). Memory and emotion processing performance contributes to the diagnosis of non‐semantic primary progressive aphasia syndromes. Journal of Alzheimer's Disease, 44, 541–547. 10.3233/JAD-141854 [DOI] [PubMed] [Google Scholar]
- Psychology software tools inc . (Producer). (2012). E‐Prime 2.0.
- Rascovsky, K. , Hodges, J. R. , Knopman, D. , Mendez, M. F. , Kramer, J. H. , Neuhaus, J. , … Miller, B. L. (2011). Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain, 134(Pt 9), 2456–2477. 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer, J. D. , Caso, F. , Mahoney, C. , Henry, M. , Rosen, H. J. , Rabinovici, G. , … Gorno‐Tempini, M. L. (2013). Patterns of longitudinal brain atrophy in the logopenic variant of primary progressive aphasia. Brain and Language, 127(2), 121–126. 10.1016/j.bandl.2012.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer, J. D. , Rossor, M. N. , & Warren, J. D. (2010). Apraxia in progressive nonfluent aphasia. Journal of Neurology, 257, 569–574. 10.1007/s00415-009-5371-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer, J. D. , & Warren, J. D. (2010). Phenomenology and anatomy of abnormal behaviours in primary progressive aphasia. Journal of the Neurological Sciences, 293(1–2), 35–38. 10.1016/j.jns.2010.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen, H. J. , Allison, S. C. , Ogar, J. M. , Amici, S. , Rose, K. , Dronkers, N. , … Gorno‐Tempini, M. L. (2006). Behavioral features in semantic dementia vs other forms of progressive aphasias. Neurology, 67, 1752–1756. 10.1212/01.wnl.0000247630.29222.34 [DOI] [PubMed] [Google Scholar]
- Sajjadi, S. A. , Patterson, K. , Arnold, R. J. , Watson, P. C. , & Nestor, P. J. (2012). Primary progressive aphasia: A tale of two syndromes and the rest. Neurology, 78, 1670–1677. 10.1212/WNL.0b013e3182574f79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits, L. L. , Pijnenburg, Y. A. , Koedam, E. L. , van der Vlies, A. E. , Reuling, I. E. , Koene, T. , … van der Flier, W. M. (2012). Early onset Alzheimer's disease is associated with a distinct neuropsychological profile. Journal of Alzheimer's Disease, 30(1), 101–108. 10.3233/jad-2012-111934 [DOI] [PubMed] [Google Scholar]
- Snodgrass, J. G. , & Vanderwart, M. (1980). A standardized set of 260 pictures: Norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology: Human Learning and Memory, 6(2), 174–215. 10.1037/0278-7393.6.2.174 [DOI] [PubMed] [Google Scholar]
- Snowden, J. S. , Bathgate, D. , Varma, A. , Blackshaw, A. , Gibbons, Z. C. , & Neary, D. (2001). Distinct behavioural profiles in frontotemporal dementia and semantic dementia. Journal of Neurology, Neurosurgery and Psychiatry, 70, 323–332. 10.1136/jnnp.70.3.323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden, J. S. , Stopford, C. L. , Julien, C. , Thompson, J. C. , Davidson, Y. , Gibbons, L. , … Mann, D. (2007). Cognitive phenotypes in Alzheimer's disease and genetic risk. Cortex, 43, 835–845. 10.1016/S0010-9452(08)70683-X [DOI] [PubMed] [Google Scholar]
- Snowden, J. S. , Thompson, J. C. , Stopford, C. L. , Richardson, A. M. , Gerhard, A. , Neary, D. , & Mann, D. M. (2011). The clinical diagnosis of early‐onset dementias: Diagnostic accuracy and clinicopathological relationships. Brain, 134(Pt 9), 2478–2492. 10.1093/brain/awr189 [DOI] [PubMed] [Google Scholar]
- Stopford, C. L. , Snowden, J. , Thompson, J. C. , & Neary, D. (2007). Distinct memory profiles in Alzheimer's disease. Cortex, 43, 846–857. 10.1016/S0010-9452(08)70684-1 [DOI] [PubMed] [Google Scholar]
- Stopford, C. L. , Snowden, J. , Thompson, J. C. , & Neary, D. (2008). Variability in cognitive presentation of Alzheimer's disease. Cortex, 44(2), 185–195. 10.1016/j.cortex.2005.11.002 [DOI] [PubMed] [Google Scholar]
- Stopford, C. L. , Thompson, J. C. , Neary, D. , Richardson, A. M. , & Snowden, J. (2010). Working memory, attention, and executive function in Alzheimer's disease and frontotemporal dementia. Cortex, 48, 429–446. 10.1016/j.cortex.2010.12.002 [DOI] [PubMed] [Google Scholar]
- Thompson, C. K. , & Mack, J. E. (2014). Grammatical impairments in PPA. Aphasiology, 28, 1018–1037. 10.1080/02687038.2014.912744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, J. C. , Stopford, C. L. , Snowden, J. , & Neary, D. (2005). Qualitative neuropsychological performance characteristics in frontotemporal dementia and Alzheimer's disease. Journal of Neurology, Neurosurgery and Psychiatry, 76, 920–927. 10.1136/jnnp.2003.033779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojano, L. , Chiacchio, L. , De Luca, G. , & Grossi, D. (1994). Exploring visuospatial short‐term memory defect in Alzheimer's disease. Journal of Clinical and Experimental Neuropsychology, 16, 911–915. 10.1080/01688639408402702 [DOI] [PubMed] [Google Scholar]
- Tyrrell, P. J. , Kartsounis, L. D. , Frackowiak, R. S. , Findley, L. J. , & Rossor, M. N. (1991). Progressive loss of speech output and orofacial dyspraxia associated with frontal lobe hypometabolism. Journal of Neurology, Neurosurgery and Psychiatry, 54, 351–357. 10.1136/jnnp.54.4.351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler, D. (1981). Wechsler adult intelligence scale‐revised. New York, NY: Psychological Corporation. [Google Scholar]
- Whitwell, J. L. , Jones, D. T. , Duffy, J. R. , Strand, E. A. , Machuda, M. M. , Przybelski, B. S. , … Josephs, K. A. (2015). Working memory and language network dysfunction in logopenic aphasia: A task‐free fMRI comparison to Alzheimer's dementia. Neurobiology of Aging, 36, 1245–1252. 10.1016/j.neurobiolaging.2014.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicklund, M. R. , Duffy, J. R. , Strand, E. A. , Machulda, M. M. , Whitwell, J. L. , & Josephs, K. A. (2014). Quantitative application of the primary progressive aphasia consensus criteria. Neurology, 82, 1119–1126. 10.1212/WNL.0000000000000261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, S. M. , Henry, M. L. , Besbris, M. , Ogar, J. M. , Dronkers, N. F. , Jarrold, W. , … Gorno‐Tempini, M. L. (2010). Connected speech production in three variants of primary progressive aphasia. Brain, 133(Pt 7), 2069–2088. 10.1093/brain/awq129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win, K. T. , Pluta, J. , Yushkevich, P. , Irwin, D. J. , McMillan, C. T. , Rascovsky, K. , … Grossman, M. (2017). Neural correlates of verbal episodic memory and lexical retrieval in Logopenic Variant Primary Progressive Aphasia. Frontiers in Neuroscience, 11, 330 10.3389/fnins.2017.00330 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supplementary material.


