Abstract
The aim of this investigation was to develop receiver and extraction fluids, and subsequently validate an analytical method to quantify the permeation and penetration of flurbiprofen into human pharynx tissue using a Franz diffusion cell. The solubility and stability of flurbiprofen in a suitable receiver fluid, and a suitable extraction method and fluid to recover and quantitate flurbiprofen from human pharynx tissue, were investigated using high‐performance liquid chromatography (HPLC). The potential interference of human pharynx tissue in the receiver fluid was also investigated. The HPLC analytical method was successfully validated according to current guidelines. The final receiver fluid demonstrated sufficient solubility and stability, and the extraction method and fluid resulted in >95% recovery of flurbiprofen following exposure to human pharynx tissue. The lower limit of quantitation of flurbiprofen was 0.045 μg/mL in both the receiver and extraction fluids. There was no interference of the human pharynx tissue with the HPLC method. This investigation validated an analytical method for quantitating flurbiprofen, and determined a suitable receiver fluid and extraction method and fluid, which can be used to investigate the permeation and penetration of flurbiprofen through human pharynx tissue using the Franz diffusion cell method.
Keywords: flurbiprofen, Franz cell, HPLC, pharyngitis, pharynx
1. INTRODUCTION
Flurbiprofen is a nonsteroidal anti‐inflammatory drug which, when delivered as a spray or lozenge, provides rapid relief from pharyngitis (sore throat) (de Looze et al., 2016; Radkova, Burova, Bychkova, & DeVito, 2017; Schachtel et al., 2014; Schachtel, Shephard, et al., 2018; Schachtel, Aspley, et al., 2018). This suggests that flurbiprofen works locally, rather than requiring absorption into the systemic circulation. Previous studies have shown that flurbiprofen is absorbed across the buccal mucosa (Barsuhn, Olanoff, Gleason, Adkins, & Ho, 1988; Gonzalez‐Younes, Wagner, Gaines, Ferry, & Hageman, 1991); however, the extent of penetration into the pharynx tissue is yet to be confirmed. Movement of flurbiprofen deeper into the layers of pharynx tissue would suggest that it acts locally on peripheral nerves located deep within the tissue and would be commensurate with its rapid onset of action (de Looze et al., 2016; Radkova et al., 2017; Schachtel et al., 2014; Schachtel, Aspley, et al., 2018; Schachtel, Shephard, et al., 2018).
To determine the penetration and permeation of drugs through tissues, the Franz diffusion cell method is often used to mimic in vivo conditions (Azzi et al., 2006; Franz, 1975; Ingram, Bartlett, Brown, Marriott, & Whitfield, 2003; Ng, Rouse, Sanderson, Meidan, & Eccleston, 2010) and has been used to determine the permeation rate of flurbiprofen through human skin (Takeuchi et al., 2011). High‐performance liquid chromatography (HPLC) has been used previously to assess the penetration of drugs into tissues (Azzi et al., 2006; Dhiman, Dhiman, & Sawant, 2009; Casey et al., 2017; Frosini, Bond, Loeffler, & Larner, 2017; Souza & Maia Campos, 2017), including the quantitation of flurbiprofen (Locatelli, Ferrone, Cifelli, Barbacane, & Carlucci, 2014; Yilmaz & Erdem, 2015).
The aim of this investigation was to develop receiver and extraction fluids, and subsequently validate an analytical method to quantify the permeation and penetration of flurbiprofen into human pharynx tissue using a Franz diffusion cell (Soham Scientific, Ely, UK).
2. EXPERIMENTAL
This investigation was conducted by MedPharm Ltd (Guildford, UK) in accordance with the International Conference on Harmonisation Pharmaceutical Quality System Q10, 2008.
2.1. HPLC for the quantitation of flurbiprofen
The HPLC methodology was developed using the LC2030C HPLC system (Shimadzu UK Ltd, Milton Keynes, UK) and Empower 3 Data Processing Software (Waters UK, Elstree, UK). Initial implementation of the HPLC method was conducted with flurbiprofen in a generic sample diluent (methanol–water) to ensure that the method was suitable for preliminary sample analysis before validation, and to provide information that the method was likely to be suitable for validation. The initial calibration standards were analyzed three times and QC standards were analyzed six times.
Following the receiver and extraction fluid development, the HPLC method was validated using both solutions as sample diluent based on the acceptance criteria of the European Medicines Agency (EMA) guideline on bioanalytical method validation (EMEA/CHMP/EWP/192217/2009 Rev. 1 Corr. 2) and US Bioanalytical Method Validation [Food and Drug Administration (FDA) Guidance for Industry, September 2013 and May 2001].
2.2. Test drug
A flurbiprofen laboratory working standard (Reckitt Benckiser, manufactured in Bangplee, Thailand) was used for this investigation.
2.3. Calibration/QC standards
Standards for initial implementation were 0.1–100 μg/mL (60:40 v/v methanol–water). Following selection of suitable receiver and extraction fluids, the final calibration and quality control (QC) standards for validation were prepared from separate flurbiprofen stock solutions (500 μg/mL). The calibration standards ranged from 0.045 to 100.0 μg/mL (100, 85, 60, 40, 6, 0.15, 0.045 μg/mL) in receiver fluid and extraction fluid and the QC standards ranged from 0.045 to 85.0 μg/mL (85, 60, 40, 0.15, 0.045 μg/mL) in receiver and extraction fluids.
2.4. Human pharynx tissue
Human pharynx tissue ethically sourced from cadavers was supplied by Ethical Tissue (University of Bradford, UK; Research Ethics Committee reference 220367) and was stored at −20°C prior to use. The tissue was cut to ~0.5–1 cm2 prior to use in the development of receiver and extraction fluids and extraction methods.
2.5. Development of receiver fluid for the Franz diffusion cell
A suitable receiver fluid was required to ensure the permeation of the flurbiprofen was not limited by the solubility of the drug in the receptor compartment. Therefore, the solubility of flurbiprofen (from the laboratory working standard; see Section 2.2) was assessed in phosphate‐buffered saline (PBS), 20% v/v ethanol in PBS and 0.2% w/v Brij 98 in PBS. The solubility of flurbiprofen was determined by adding 10 mg (± 1 mg) of flurbiprofen to 500 mg (± 5 mg) of receiver fluid. The solution was maintained at 25°C and stirred for 24 h. After incubation, any undissolved flurbiprofen was removed via centrifugation for 10 min at 13,000 rpm (ca. 16,000g) at 25°C (centrifuge, Eppendorf 5430, VWR, Lutterworth, UK). The solution was diluted 1:200 to ensure that the drug concentration was within the calibration range for the HPLC method. The saturated system (50 mg) was weighed into a 10 mL volumetric flask which was made up to volume with sample diluent (60:40 v/v methanol–water) and thoroughly stirred. An aliquot of the diluted sample was analyzed using the HPLC method. The stability of flurbiprofen in the potential receiver fluids was assessed at 2–8 and 37°C at 24 and 48 h, and 5 days, using the HPLC method.
The potential interference of human pharynx tissue in the receiver fluid with the flurbiprofen peak in the HPLC method was also investigated, to ensure that no issues would be encountered in the full experiment. Pharynx tissue cut into ~0.6 cm2 sections was added to 28 mL of receiver fluid and stored in a 37°C water bath for 24 h. From the receiver fluid, 20 mL was removed and added to 5 mg of flurbiprofen and mixed by inversion. The solution was then made up to 100 mL using receiver fluid and analyzed by HPLC.
2.6. Development of extraction fluid and extraction methods for the Franz diffusion cell
A suitable extraction fluid was investigated to recover and quantify the flurbiprofen from human pharynx tissue. The following extraction fluids (EF) were assessed: 90:10 v/v methanol–water (EF1); 90:10 v/v ethanol–water (EF2); and 90:10 v/v ethanol–water with 1% v/v formic acid (EF3). To develop the extraction method, flurbiprofen 10 mg (± 2 mg) was weighed into a 10 mL flask (made up to volume with ethanol), and 10 μL of the solution was added to three cotton buds in a glass vial or human pharynx tissue cut into 0.5–1 cm2 pieces (all n = 3). For each, the percentage recovery of flurbiprofen compared with a control (empty vial) was assessed. Following a 6 h incubation in a water bath at 37°C, 1 mL of each test extraction fluid was added to the human pharynx tissue and homogenised (tissue homogenizer, Precellys 24, Peqlab Ltd, Southampton, UK) at 5800 rpm for 40 s at ambient temperature. An additional 1 mL of each extraction fluid was added to the tissue samples, and 2 mL of each extraction fluid was added to the remaining samples. Each sample was shaken for 16–20 h and centrifuged at 13,000 rpm (~16,000g) for 10 min at 25°C. The supernatant was analyzed via HPLC to determine the most suitable extraction fluid for the Franz diffusion cell method. Owing to poor recovery of flurbiprofen from the human pharynx tissue, the extraction method was repeated for EF2 (following an overnight freeze and additional beads added to the homogenizer vial ([Homogeniser vials: Fisher Scientific UK Ltd, Loughborough, UK]) and for EF3 (additional beads added but without freezing). As for the receiving fluid, the potential interference of human pharynx tissue in the extractor fluid with the flurbiprofen peak in the HPLC method was also investigated. Pharynx tissue cut into ~0.6 cm2 sections was added to 28 mL of each extractor fluid and stored in a 37°C water bath for 24 h. From each extractor fluid, 20 mL was removed and added to 5 mg of flurbiprofen then mixed by inversion. The solution was then made up to 100 mL using receiver fluid and analyzed by HPLC to confirm that there were no interfering peaks from the tissue. Details of a small‐scale experimental procedure are outlined in section S1.
3. RESULTS
3.1. HPLC
During assessment of the analytical method, the absorption spectra of the flurbiprofen peak and the lambda max were determined to be 247 nm. This detection wavelength was then set to ensure that the greatest level of sensitivity could be achieved.
Following implementation (Table 1) the HPLC analytical method was successfully validated (according to the relevant FDA/EMA guidelines) and the parameters were shown to be ‘fit for purpose’ for the detection and quantitation of flurbiprofen (Table 2; Figure 1). The limit of quantitation (LOQ) of flurbiprofen was 0.045 μg/mL (Table 2). The final HPLC system conditions are described in Table 3.
Table 1.
HPLC analytical method implementation results
| Method implementation element | Results | Specification | Comments |
|---|---|---|---|
| Linearity: correlation coefficient (R 2)a | R 2 = 1.0000 | R 2 ≥ 0.9990 | Meets specification |
| Accuracy | Low concentration 1 μg/mL = 98.86 ± 0.85% | 95–105% | Meets specification at three concentrations |
| Medium concentration 10 μg/mL = 100.69 ± 0.28% | |||
| High concentration 80 μg/mL = 99.55 ± 0.08% | |||
| Precision/repeatability | Low concentration 1 μg/mL = 0.86% | Relative SD < 2% | Meets specification at three concentrations |
| Medium concentration 10 μg/mL = 0.28% | |||
| High concentration 80 μg/mL = 0.08% | |||
| System suitability (1 μg/mL) | Capacity factor K = 9.61 | K > 2 | Meets specification |
| Tailing factor T = 1.01 | T ≤ 2 | Meets specification | |
| Theoretical plate number N = 11,502.09 | N > 2000 | Meets specification | |
| System suitability (10 μg/mL) | Capacity factor K = 9.87 | K > 2 | Meets specification |
| Tailing factor T = 1.13 | T ≤ 2 | Meets specification | |
| Theoretical plate number N = 7996.39 | N > 2000 | Meets specification | |
| System suitability (80 μg/mL) | Capacity factor K = 9.85 | K > 2 | Meets specification |
| Tailing factor T = 1.16 | T ≤ 2 | Meets specification | |
| Theoretical plate number N = 7707.72 | N > 2000 | Meets specification | |
| Quantitation limit for flurbiprofena | Theoretical LOQ = 0.045 μg/mL | Report results | LOQ established |
Data represented as mean ± standard deviation (SD).
n = 3, all other parameters n = 6.
HPLC, High‐performance liquid chromatography; LOQ, limit of quantitation.
Table 2.
Method validation summary for flurbiprofen in receiver and extraction fluids over a concentration range of 0.045–100 μg/mL
| Method validation element | Specification | Experimental result for receiver fluid | Experimental result for extraction fluid |
|---|---|---|---|
| Specificity | Precision: CV ≤ 20% | Pass | Pass |
| Accuracy: 80–120% | Pass | Pass | |
| Percentage deviation: 4/6 replicates within 20% of the actual value | Pass | Pass | |
| Selectivity | S/N > 5 in 5/6 matrices | Pass | Pass |
| LLOQ | Peak area > 5× S/N ratio at the retention time of the analyte from a blank matrix | Pass | Pass |
| Intra‐assay validation | Precision of all QC standards: CV ≤ 15% | Pass | Pass |
| Accuracy of all QC standards: 85–115% | Pass | Pass | |
| Precision at the LLOQ: ≤ 20% | Pass | Pass | |
| Accuracy at the LLOQ: 80–120% | Pass | Pass | |
| Percentage deviation: 4/6 replicates within 15% (20% for LLOQ) of the actual values at each of the validation levels | Pass | Pass | |
| Inter‐assay validation | Precision of all QC standards: CV ≤ 15% | Pass | Pass |
| Accuracy of all QC standards: 85–115% | Pass | Pass | |
| Precision at the LLOQ: ≤20% | Pass | Pass | |
| Accuracy at the LLOQ: 80–120% | Pass | Pass | |
| Percentage deviation: 12/18 replicates within 15% of the actual values at each of the validation levels | Pass | Pass | |
| Coefficient of determination, intercept and slope: report result | Pass | Pass | |
| Short‐term stability | Precision: CV ≤ 15% at both low and high levels | Pass | Pass |
| Accuracy: 85–115% | Fail – freeze–thaw and 2‐week freezer stability only | Pass | |
| Percentage deviation: 4/6 replicates within 15% of the actual values at each of the validation levels | Pass | Pass | |
| Re‐injection reproducibility | Precision: CV ≤ 15% | Pass | Pass |
| Accuracy (percentage recovery): 85–115% | Pass | Pass | |
| Percentage deviation: within 15% of the actual values at each of the validation levels | Pass | Pass | |
| Stability of the analyte in solution | Precision: CV ≤ 15% | Pass | Pass |
| Accuracy (percentage recovery): 85–115% | Pass | Pass | |
| Percentage deviation: 4/6 stock dilutions within 15% of the freshly prepared stocks | Pass | Pass | |
| Carryover | <5% carryover | Pass | Pass |
CV, Coefficient of variation; LLOQ, lower limit of quantitation; QC, quality control; S/N, signal‐to‐noise ratio.
Figure 1.
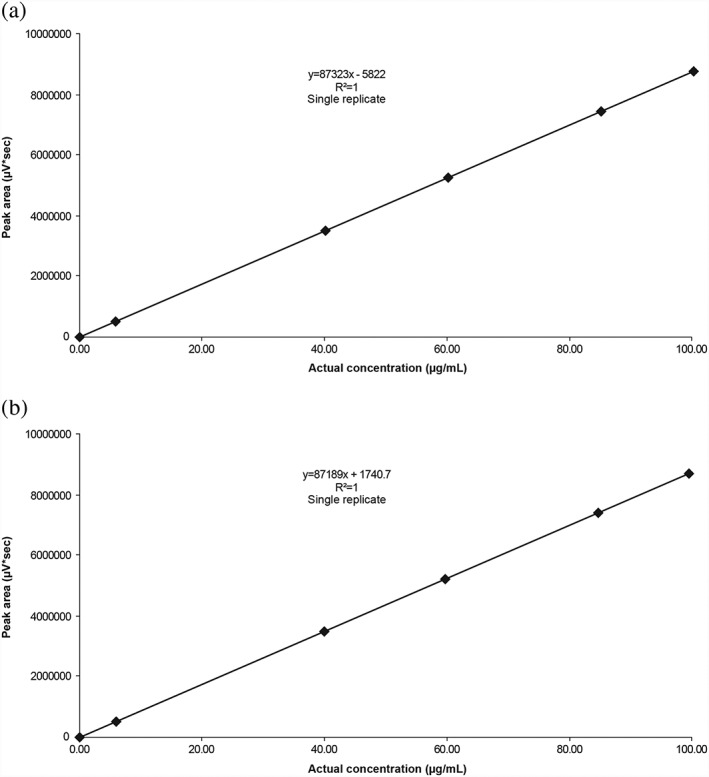
Representative flurbiprofen calibration curve in extraction fluid (a) and receiver fluid (b) over the validated range 0.045–100 μg/mL
Table 3.
Final HPLC chromatographic conditions for the assay of flurbiprofen
| HPLC system | Shimadzu LC2030C HPLC system Waters Empower 3 Data Processing Software |
|---|---|
| Column | Waters Symmetry C18 3.5 μm, 4.6 mm i.d. × 100 mm |
| Guard column | Waters Symmetry C18 guard column or equivalent |
| Mobile phase (MP) | 30:20:50 v/v/v MP A–MP B–MP C |
| MP A | 0.1% trifluoracetic acid in acetonitrile |
| MP B | 0.1% trifluoracetic acid in methanol |
| MP C | 0.1% trifluoracetic acid in water |
| Initial flow rate | 1 mL/min |
| Run time | 20 min |
| Wavelength | 247 nm |
| Column temperature | 35°C |
| Auto sampler temperature | Ambient laboratory temperature |
| Injection volume | 20 μL |
| Retention time of flurbiprofen | 14 min |
| Needle wash solvent | 100% ethanol |
| Sample and standard diluenta | 60:40 v/v methanol–water. Receiver fluid, PBS; extraction fluid, 90:10 v/v ethanol–water |
| Seal wash and line storage | 60:40 v/v methanol–water |
Initial assessment and implementation of the analytical method performed using 60:40 v/v methanol–water. Following selection of suitable receiver and extraction fluids samples the analytical method was validated and standards were prepared in the receiver or extraction fluid, as appropriate.
HPLC, High‐performance liquid chromatography; PBS, phosphate‐buffered saline.
3.2. Receiver fluid
All receiver fluids demonstrated sufficient solubility (at least 10‐fold greater than the maximum amount of flurbiprofen anticipated to permeate the pharynx tissue), with saturated solubility of the drug ranging from 0.12% w/w (PBS) to 0.14% w/w (0.2% w/v Brij 98 in PBS). Acceptable stability (95–105% compared with t = 0) of flurbiprofen from all three receiver fluids was observed across the 5 day stability period at both 2–8 and 37°C (Table 4). PBS was selected as the receiver fluid for further assessment. Human pharynx tissue in the receiver fluid demonstrated no interference when analyzing flurbiprofen with the HPLC analytical method.
Table 4.
Recovery of flurbiprofen from receiver fluid expressed as a percentage of t = 0
| Receiver fluid | t = 0 | 24 h | 48 h | 5 days | |||
|---|---|---|---|---|---|---|---|
| 2–8 °C | 37°C | 2–8 °C | 37°C | 2–8 °C | 37°C | ||
| PBS | 100.00 (99.64–100.21) | 100.50 (100.36–100.67) | 100.14 (99.96–100.24) | 99.32 (99.15–99.59) | 99.10 (98.96–99.27) | 99.49 (99.38–99.62) | 99.57 (99.34–99.73) |
| 20% v/v ethanol in PBS | 100.00 (98.80–100.24) | 100.55 (100.23–100.98) | 100.56 (100.55–100.57) | 99.33 (99.30–99.36) | 99.76 (99.56–99.94) | 99.64 (99.46–99.76) | 100.23 (100.18–100.28) |
| 0.2% w/v Brij 98 in PBS | 100.00 (99.80–100.19) | 100.44 (100.33–100.53) | 100.65 (100.58–100.71) | 99.52 (99.44–99.61) | 99.68 (99.55–99.78 | 99.79 (99.59–99.95) | 99.72 (99.51–99.85) |
Data presented as mean (range).
3.3. Extraction fluid and extraction method
Two of the extraction fluids (EF1, 90:10 v/v methanol–water, and EF2, 90:10 v/v ethanol–water) initially assessed had low recoveries (72.24–78.87% compared with the control vial) of flurbiprofen from human pharynx tissue. The protocol was developed further (freezing the tissue and adding additional beads in the tissue homogenizer vial), improving EF2 recovery to 96.63% compared with the control vial (Table 5). Using the third extraction fluid (EF3, 90:10 v/v ethanol–water with 1% formic acid) with additional beads but without freezing yielded a recovery of 95.50%. The recovery results were comparable between EF2 and EF3, and EF2 was selected for the Franz diffusion cell experiment (using the method with an overnight freeze step and additional beads added to the homogenizer vial). The recovery of flurbiprofen from cotton buds was 96.00% for EF2. The use of the different extraction fluids demonstrated no interference when analyzing flurbiprofen with the HPLC analytical method.
Table 5.
Recovery of flurbiprofen using different extraction procedure expressed as a percentage compared with the control
| Extraction fluid | Sample | Recovery of flurbiprofen compared with the control (%) |
|---|---|---|
| 90:10 v/v methanol–water (EF1) | Cotton bud | 92.43 (90.88–95.53) |
| Pharynx | 78.87 (68.98–88.52) | |
| Empty vial (control) | 100.00 (97.39–104.51) | |
| 90:10 v/v ethanol–water (EF2) | Cotton bud | 96.00 (95.94–96.07)a |
| Pharynx | 72.24 (65.15–80.86) | |
| Empty vial (control) | 100.00 (93.34–103.83) | |
| 90:10 v/v ethanol–water (EF2) additional development | Pharynx (frozen) | 96.63 (91.41–106.43) |
| Empty tissue homogenizer vial (control) | 105.51 (103.12–108.94) | |
| Empty vial (control) | 100.00 (92.65–105.24) | |
| 90:10 v/v ethanol–water with 1% v/v formic acid (EF3) | Pharynx | 95.50 (94.93–96.07)a |
| Empty tissue homogenizer vial (control) | 103.06 (100.92–104.55) | |
| Empty vial (control) | 100.00 (95.49–103.28) |
n = 2 replicates only. Data presented as mean (range).
EF, Extraction fluid.
4. DISCUSSION
This investigation developed a suitable receiver fluid, extraction method and fluid, and validated an analytical method for investigating and quantifying flurbiprofen permeation and penetration in human pharynx tissue in a Franz diffusion cell. To our knowledge, this is the first study of its kind. The final analytical method was validated according to all relevant FDA and EMA guidelines and was determined to be ‘fit for purpose’. All validation experiments have shown that the analytical method used is accurate and precise over the analytical range investigated, and offers advantages relative to previous analytical methods with respect to sensitivity to detect permeation and penetration of flurbiprofen into the target tissue (Akhlaq et al., 2011; Hussain, Al‐Ajmi, Amir, & Ali, 2016; Yilmaz & Erdem, 2015).
The selected receiver fluid (PBS) demonstrated sufficient solubility (saturated solubility of flurbiprofen was 0.12% w/w) and acceptable stability across 5 days at both 2–8 and 37°C. The extraction method and fluid resulted in >95% recovery of flurbiprofen following exposure to human pharynx tissue, indicating that flurbiprofen could be successfully recovered from the samples tested and is stable in the presence of the pharynx tissue for up to 6 h incubation at 37°C. Most importantly, there was no interference of the human pharynx tissue in the receiver fluid and extraction fluid with the HPLC method.
This investigation determined that the LOQ for flurbiprofen was 0.045 μg/mL. Compared with other studies using HPLC as the detection method, this method provides a more sensitive measure [other studies have reported LOQs of 0.10 μg/mL (Yilmaz & Erdem, 2015) and 0.578 μg/mL (Akhlaq et al., 2011)]; however, there are more sensitive methods available for determining flurbiprofen concentration, such as liquid chromatography–mass spectrometry (LC–MS) (Lee et al., 2014; Mano, Narui, Nikaido, & Goto, 2002). Therefore, potential further investigations using LC–MS could be conducted to further improve the sensitivity of the methodology.
5. CONCLUSION
This investigation validated an analytical method for quantitating flurbiprofen, and determined a suitable receiver fluid and extraction method and fluid, which can be used to investigate the permeation and penetration of flurbiprofen through human pharynx tissue using the Franz diffusion cell method. The final analytical method was validated according to all relevant FDA and EMA guidelines.
AUTHOR CONTRIBUTIONS
Rob Turner and Marc Brown (CSO of MedPharm) provided technical expertise for the validation and performance testing; Sean Robert Wevrett was the Study Director overseeing the project and Suzanne Edmunds was the Scientific Lead involved in decision‐making and data interpretation. All authors contributed to the conception and development of this manuscript, and all authors have approved the final draft and take full responsibility for the contents of the manuscript.
DECLARATION OF FINANCIAl/OTHER RELATIONSHIPS
Robert Atkinson and Tim Shea are employees of Reckitt Benckiser. Rob Turner, Sean Robert Wevrett, Suzanne Edmunds and Marc Brown are employees of MedPharm Ltd.
DATA SHARING STATEMENT
All data relating to this investigation are reported within the manuscript.
Supporting information
Data S1. 2.7 Protocol for small scale experiment. Receiver fluid (200 μL) was removed at the following time points t=0, 5, 15, 30, 45, 60, 90, 120 minutes and 4 hours. The surface of the human pharynx tissue was swabbed for residual flurbiprofen and the tissue was homogenised to extract the flurbiprofen from within the tissue. Recovery from the surface of the tissue, within the tissue and the receiver fluid was calculated, full mass balance was not performed, therefore the residual flurbiprofen present on the Franz cell apparatus was not quantified.
ACKNOWLEDGEMENTS
This study was funded by Reckitt Benckiser. MedPharm Ltd (Surrey Research Park, Guildford, UK) received funding from Reckitt Benckiser to conduct the study. Medical writing assistance was provided by Elements Communications Ltd (Westerham, UK) and was funded by Reckitt Benckiser Healthcare Ltd, UK. The authors gratefully acknowledge the contribution of John Farrah.
Turner R, Wevrett SR, Edmunds S, Brown M, Atkinson R, Shea T. Validation of an analytical method to quantify the permeation and penetration of flurbiprofen into human pharynx tissue. Biomedical Chromatography. 2019;33:e4499 10.1002/bmc.4499
REFERENCES
- Akhlaq, M. , Khan, G. M. , Wahab, A. , Khan, A. , Hussain, A. , Nawaz, A. , & Abdelkader, H. (2011). A simple high‐performance liquid chromatographic practical approach for determination of flurbiprofen. Journal of Advanced Pharmaceutical Technology & Research, 2(3), 151–155. 10.4103/2231-4040.85529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi, C. , Zhang, J. , Purdon, C. H. , Chapman, J. M. , Nitcheva, D. , Hebert, J. R. , & Smith, E. W. (2006). Permeation and reservoir formation of 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanone (nnk) and benzo[a]pyrene (b[a]p) across porcine esophageal tissue in the presence of ethanol and menthol. Carcinogenesis, 27(1), 137–145. 10.1093/carcin/bgi173 [DOI] [PubMed] [Google Scholar]
- Barsuhn, C. L. , Olanoff, L. S. , Gleason, D. D. , Adkins, E. L. , & Ho, N. F. (1988). Human buccal absorption of flurbiprofen. Clinical Pharmacology and Therapeutics, 44(2), 225–231. 10.1038/clpt.1988.141 [DOI] [PubMed] [Google Scholar]
- Casey, A. L. , Karpanen, T. J. , Conway, B. R. , Worthington, T. , Nightingale, P. , Waters, R. , & Elliott, T. S. J. (2017). Enhanced chlorhexidine skin penetration with 1,8‐cineole. BMC Infectious Diseases, 17(1), 350 10.1186/s12879-017-2451-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Looze, F. , Russo, M. , Bloch, M. , Montgomery, B. , Shephard, A. , Smith, G. , & Aspley, S. (2016). Efficacy of flurbiprofen 8.75 mg spray in patients with sore throat due to an upper respiratory tract infection: A randomised controlled trial. The European Journal of General Practice, 22(2), 111–118. 10.3109/13814788.2016.1145650 [DOI] [PubMed] [Google Scholar]
- Dhiman, M. K. , Dhiman, A. , & Sawant, K. K. (2009). Transbuccal delivery of 5‐fluorouracil: Permeation enhancement and pharmacokinetic study. AAPS PharmSciTech, 10(1), 258–265. 10.1208/s12249-009-9203-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz, T. J. (1975). Percutaneous absorption on the relevance of in vitro data. The Journal of Investigative Dermatology, 64(3), 190–195. 10.1111/1523-1747.ep12533356 [DOI] [PubMed] [Google Scholar]
- Frosini, S. M. , Bond, R. , Loeffler, A. , & Larner, J. (2017). Opportunities for topical antimicrobial therapy: Permeation of canine skin by fusidic acid. BMC Veterinary Research, 13(1), 345 10.1186/s12917-017-1270-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez‐Younes, I. , Wagner, J. G. , Gaines, D. A. , Ferry, J. J. , & Hageman, J. M. (1991). Absorption of flurbiprofen through human buccal mucosa. Journal of Pharmaceutical Sciences, 80(9), 820–823. 10.1002/jps.2600800903 [DOI] [PubMed] [Google Scholar]
- Hussain, A. , Al‐Ajmi, M. F. , Amir, S. , & Ali, I. (2016). Development and validation of SPMMTE HPLC method for analysis of profens from human plasma. Biomedical Chromatography, 30(8), 1263–1269. 10.1002/bmc.3676 [DOI] [PubMed] [Google Scholar]
- Ingram, R. J. , Bartlett, A. , Brown, M. B. , Marriott, C. , & Whitfield, P. J. (2003). Penetration of human skin by the cercariae of schistosoma mansoni: An investigation of the effect of multiple cercarial applications. Journal of Helminthology, 77(1), 27–31. 10.1079/joh2002157 [DOI] [PubMed] [Google Scholar]
- Lee, H. I. , Choi, C. I. , Byeon, J. Y. , Lee, J. E. , Park, S. Y. , Kim, Y. H. , & Lee, S. Y. (2014). Simultaneous determination of flurbiprofen and its hydroxy metabolite in human plasma by liquid chromatography–tandem mass spectrometry for clinical application. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences, 971, 58–63. 10.1016/j.jchromb.2014.09.007 [DOI] [PubMed] [Google Scholar]
- Locatelli, M. , Ferrone, V. , Cifelli, R. , Barbacane, R. C. , & Carlucci, G. (2014). Microextraction by packed sorbent and high performance liquid chromatography determination of seven non‐steroidal anti‐inflammatory drugs in human plasma and urine. Journal of Chromatography A, 1367, 1–8. 10.1016/j.chroma.2014.09.034 [DOI] [PubMed] [Google Scholar]
- Mano, N. , Narui, T. , Nikaido, A. , & Goto, J. (2002). Separation and determination of diastereomeric flurbiprofen acyl glucuronides in human urine by lc/esi‐ms with a simple column‐switching technique. Drug Metabolism and Pharmacokinetics, 17(2), 142–149. 10.2133/dmpk.17.142 [DOI] [PubMed] [Google Scholar]
- Ng, S. F. , Rouse, J. J. , Sanderson, F. D. , Meidan, V. , & Eccleston, G. M. (2010). Validation of a static franz diffusion cell system for in vitro permeation studies. AAPS PharmSciTech, 11(3), 1432–1441. 10.1208/s12249-010-9522-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radkova, E. , Burova, N. , Bychkova, V. , & DeVito, R. (2017). Efficacy of flurbiprofen 8.75 mg delivered as a spray or lozenge in patients with sore throat due to upper respiratory tract infection: A randomized, non‐inferiority trial in the Russian Federation. Journal of Pain Research, 10, 1591–1600. 10.2147/jpr.s135602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtel, B. , Aspley, S. , Shephard, A. , Schachtel, E. , Lorton, M. B. , & Shea, T. (2018). Onset of analgesia by a topically administered flurbiprofen lozenge: A randomised controlled trial using the double stopwatch method. British Journal of Pain (in press), 12, 208–216. 10.1177/2049463718756152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtel, B. , Aspley, S. , Shephard, A. , Shea, T. , Smith, G. , Sanner, K. , … Schachtel, E. (2014). Onset of action of a lozenge containing flurbiprofen 8.75 mg: A randomized, double‐blind, placebo‐controlled trial with a new method for measuring onset of analgesic activity. Pain, 155(2), 422–428. 10.1016/j.pain.2013.11.001 [DOI] [PubMed] [Google Scholar]
- Schachtel, B. , Shephard, A. , Schachtel, E. , Lorton, M. B. , Shea, T. , & Aspley, S. (2018). Qualities of sore throat index (quasti): Measuring descriptors of sore throat in a randomized, placebo‐controlled trial. Pain Manag, 8(2), 85–94. 10.2217/pmt-2017-0041 [DOI] [PubMed] [Google Scholar]
- Souza, C. , & Maia Campos, P. (2017). Development of a hplc method for determination of four UV filters in sunscreen and its application to skin penetration studies. Biomedical Chromatography, 31(12). 10.1002/bmc.4029 [DOI] [PubMed] [Google Scholar]
- Takeuchi, H. , Mano, Y. , Terasaka, S. , Sakurai, T. , Furuya, A. , Urano, H. , & Sugibayashi, K. (2011). Usefulness of rat skin as a substitute for human skin in the in vitro skin permeation study. Experimental Animals, 60(4), 373–384. 10.1538/expanim.60.373 [DOI] [PubMed] [Google Scholar]
- Yilmaz, B. , & Erdem, A. F. (2015). Determination of flurbiprofen in human plasma by high‐performance liquid chromatography. Journal of Chromatographic Science, 53(9), 1443–1448. 10.1093/chromsci/bmv032 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. 2.7 Protocol for small scale experiment. Receiver fluid (200 μL) was removed at the following time points t=0, 5, 15, 30, 45, 60, 90, 120 minutes and 4 hours. The surface of the human pharynx tissue was swabbed for residual flurbiprofen and the tissue was homogenised to extract the flurbiprofen from within the tissue. Recovery from the surface of the tissue, within the tissue and the receiver fluid was calculated, full mass balance was not performed, therefore the residual flurbiprofen present on the Franz cell apparatus was not quantified.


