Abstract
Background
Disturbances in adipose tissue glucose uptake may play a role in the pathogenesis of type 2 diabetes, yet its examination by 2‐deoxy‐2‐[18F]fluorodeoxyglucose ([18F]FDG) PET/CT is challenged by relatively low uptake kinetics. We tested the hypothesis that performing [18F]FDG PET/CT during a hypoglycaemic clamp would improve adipose tissue tracer uptake to allow specific comparison of adipose tissue glucose handling between people with or without type 2 diabetes.
Design
We enrolled participants with or without diabetes who were at least overweight, to undergo a hyperinsulinaemic hypoglycaemic clamp or a hyperinsulinaemic euglycaemic clamp (n = 5 per group). Tracer uptake was quantified using [18F]FDG PET/CT.
Results
Hypoglycaemic clamping increased [18F]FDG uptake in visceral adipose tissue of healthy participants (P = 0.002). During hypoglycaemia, glucose uptake in visceral adipose tissue of type 2 diabetic participants was lower as compared to healthy participants (P < 0.0005). No significant differences were observed in skeletal muscle, liver or pancreas.
Conclusions
The present findings indicate that [18F]FDG PET/CT during a hypoglycaemic clamp provides a promising new research tool to evaluate adipose tissue glucose metabolism. Using this method, we observed a specific impairment in visceral adipose tissue [18F]FDG uptake in type 2 diabetes, suggesting a previously underestimated role for adipose tissue glucose handling in type 2 diabetes.
Keywords: [18F]FDG PET, adipose tissue, glucose metabolism, obesity, type 2 diabetes mellitus
1. INTRODUCTION
The amount and distribution of adipose tissue (AT) are important contributing factors in the development of insulin resistance and type 2 diabetes.1 While hepatic2 and muscle3 insulin resistance are generally regarded as the key factors in disrupted glucose homeostasis in type 2 diabetes, AT glucose handling could also play an important role. AT has been shown to make a considerable contribution to insulin‐stimulated glucose uptake from the circulation.4, 5, 6 Furthermore, there is substantial mechanistic evidence for impaired insulin‐stimulated glucose uptake in AT in relation to obesity.7
Several previous studies have used [18F]FDG PET during a hyperinsulinaemic euglycaemic clamp to examine glucose uptake in different AT depots in individuals of different metabolic health, but results have not been completely consistent. Both Oliveira et al8 and Virtanen et al4 have shown an obesity‐associated decrease in [18F]FDG uptake in visceral AT. However, a similar effect in subcutaneous AT was only demonstrated by one study4 and not by the other.8 In both these studies, [18F]FDG uptake in AT was not significantly different between metabolically healthy obese participants and obese individuals with type 2 diabetes.4, 8
These studies have used different methods of [18F]FDG PET imaging which could in part explain the different results. However, using 18F‐FDG PET during a hyperinsulinaemic euglycaemic clamp to study tracer uptake in AT is challenging because of the low uptake values of [18F]FDG in AT as compared to skeletal muscle. Consequently, it is difficult to determine differences in tracer uptake between different AT depots and between individuals. The role of AT in glucose homeostasis could therefore be underestimated. An interesting case was reported in 2011 by Hofman et al in which an [18F]FDG PET/CT scan was performed in a healthy lean individual during unintentional insulin‐induced hypoglycaemia. Surprisingly, an altered biodistribution of the radiopharmaceutical [18F]FDG was observed, with a markedly increased uptake in AT, most prominently in visceral AT, as compared to the uptake in skeletal muscle.9
Performing [18F]FDG PET during a hyperinsulinaemic hypoglycaemic clamp could thus stimulate tracer uptake in AT and thereby represent a new method to better examine AT glucose handling. We hypothesized that this method could elucidate differences in AT metabolism associated with type 2 diabetes, which would remain undetected in previous studies. In the current study, we therefore performed [18F]FDG PET/CT during a hyperinsulinaemic hypoglycaemic clamp in overweight or obese people with type 2 diabetes and healthy participants matched for BMI, to compare [18F]FDG uptake in AT between these groups.
2. SUBJECTS AND METHODS
2.1. Enrolment
We enrolled five participants with type 2 diabetes who were at least overweight (BMI > 25 kg/m2) and five BMI‐matched individuals without diabetes to undergo a hyperinsulinaemic hypoglycaemic clamp. In addition, five nondiabetic participants, again matched for BMI, underwent a hyperinsulinaemic euglycaemic clamp. All people with type 2 diabetes met the following inclusion criteria: clinically overt type 2 diabetes for at least 2 years, treated by diet or oral glucose‐lowering medication alone (no previous insulin use), free from micro‐ and macrovascular complications except for background retinopathy and haemoglobin A1c (HbA1c) levels below 75 mmol/mol (9.0%). Healthy obese participants met the following inclusion criteria: fasting glucose < 6.1 mmol/L, HbA1c < 42 mmol/mol (6%) and a normal glucose tolerance test (plasma glucose levels below 6.5 mmol/L 2 hours after a 75 mg glucose challenge). The study was approved by the Radboud University Medical Center institutional review board and has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. All participants provided written consent.
2.2. Hyperinsulinaemic glucose clamps
All participants presented in the morning at 8.00 AM after an overnight fast (at least 8 hours). Participants with type 2 diabetes omitted their morning oral glucose‐lowering medication (if applicable). Upon arrival, two catheters were inserted intravenously. One catheter was placed into the antecubital vein for frequent blood sampling and was positioned in a heated box (55‐60ºC) to obtain arterialized venous blood. The second catheter was inserted in the antecubital vein of the contralateral arm for infusion of insulin (Insulin Aspart; Novo Nordisk) and glucose 20% w/w (Baxter). Subsequently, infusion of insulin was initiated at a rate of 120 mU per m2 per min after a bolus of 1 U (referred to as time point 0). Plasma glucose levels were brought to predetermined levels using a variable infusion of glucose 20%, based on plasma glucose measured at 5‐minutes intervals (Biosen C‐line; EKF Diagnostics), which was sustained during infusion of [18F]FDG, the incubation time and the scan. At the start of the clamping procedure, when plasma glucose levels were within the euglycaemic range, and just prior to positioning the patient in the scanner, when plasma glucose levels were within the predetermined range, blood was drawn to determine levels of growth hormone, cortisol, epinephrine and norepinephrine.
2.3. PET imaging with [18F]FDG
PET imaging was performed using a Siemens Biograph mCT‐40 time‐of‐flight PET/CT scanner. When stable plasma glucose levels were reached (targeting at 3.0 and 5.0 mmol/L for hypoglycaemia and euglycaemia, respectively), 1.6 MBq/kg [18F]FDG was infused. PET/CT images were obtained approximately 1 hour after injection from the base of the skull until the knees at 4 minutes per bed position. A low‐dose CT acquisition (40 mAs and 130 kV) of the same area as that covered by the PET scan was used for PET attenuation correction and as an anatomic reference. The size of the CT transaxial matrix was 512 × 512 (0.98 × 0.98 mm), and the CT slice width was 3 mm. High definition reconstruction of the images was performed with three iterations, 21 subsets and a post‐reconstruction Gaussian filter of 3 mm in full width at half maximum. The transaxial PET matrix size was 256 × 256, and pixel size was 3.18 × 3.18 × 3.
2.4. PET image tissue uptake quantification
PET/CT images were reviewed using Inveon Research Workplace software (version 4.1; Siemens Healthcare). CT images were smoothed with a Gaussian filter of 1 mm. Subsequently, regions of interest (ROIs) were drawn delineating different tissues on the CT image. AT was delineated by thresholding at −110 to −70 HU.6 The resulting ROI was then manually divided between visceral AT, abdominal subcutaneous AT and gluteofemoral AT. Skeletal muscle was delineated by manually drawing ROIs around the upper legs, lower back and right shoulder and performing thresholding at −21 to 104 HU within these ROIs to delineate skeletal muscle. ROIs around the liver and pancreas were drawn manually. All ROIs were drawn by a blinded observer. Tracer activity within the ROIs was recorded and expressed as standard uptake value (SUV), defined as activity per mL of tissue divided by the injected dose in MBq/g bodyweight.
2.5. Statistical analysis
Data analysis was performed using SPSS (version 22; SPSS) with P < 0.05 considered statistically significant. Results are expressed as mean ± SD. Glucose infusion rates during the procedure were compared between the groups using a two‐way ANOVA repeated‐measures analysis. The effects of type 2 diabetes and hypoglycaemia were tested using one‐way ANOVA. Post hoc analyses were performed using the Bonferroni‐Dunn test to reveal statistically significant differences between the groups. Levels of hormones before and during the procedure were compared using a Wilcoxon signed rank test.
3. RESULTS
3.1. Study participant characteristics
Table 1 shows the baseline characteristics of the participants. Patients with type 2 diabetes were generally well‐controlled and older than the two control groups, but well matched for BMI to the healthy obese participants.
Table 1.
Clinical characteristics of the study participants
| Nondiabetic | Type 2 diabetes | ||
|---|---|---|---|
| Euglycaemic clamp (n = 5) | Hypoglycaemic clamp (n = 5) | Hypoglycaemic clamp (n = 5) | |
| Age (y) | 30 ± 4 | 27 ± 6 | 60 ± 5 |
| Sex (female:male) | 2:3 | 4:1 | 0:5 |
| Bodyweight (kg) | 110 ± 8 | 89 ± 5 | 88 ± 4 |
| BMI (kg/m2) | 31.0 ± 1.1 | 30.0 ± 1.1 | 29.3 ± 0.4 |
| Duration of diabetes (y) | NA | NA | 11 ± 3 |
| Diet | 1 | ||
| Oral medication | |||
| Metformin | 3 | ||
| Sulfonylurea | 1 | ||
| Fasting glucose (mmol/L) | 4.86 ± 0.25 | 5.08 ± 0.12 | 7.86 ± 0.49 |
| HbA1c (%)/(mmol/mol) | 3.3 ± 0.1/ | 3.2 ± 0.1/ | 5.0 ± 0.2/ |
| 35.8 ± 0.9 | 34.8 ± 1.4 | 55.0 ± 2.1 | |
Data are shown as means ± SD or as number (%).
3.2. Plasma glucose levels during the clamps
During the clamping procedure, glucose levels were 5.0 ± 0.3 mmol/L for the euglycaemia group. In the hypoglycaemia groups, glucose levels were 3.0 ± 0.1 for the healthy obese participants and 3.0 ± 0.1 for the patients with type 2 diabetes (P = NS, Figure 1A). Mean glucose infusion rates during the procedure were 7.1 ± 1.9 mg/kg/min for the euglycaemia group. In the hypoglycaemia groups, glucose infusion rates were 4.0.0 ± 1.2 mg/kg/min for the healthy obese participants and 2.8 ± 1.0 mg/kg/min for the patients with type 2 diabetes (P = 0.023, Figure 1B).
Figure 1.
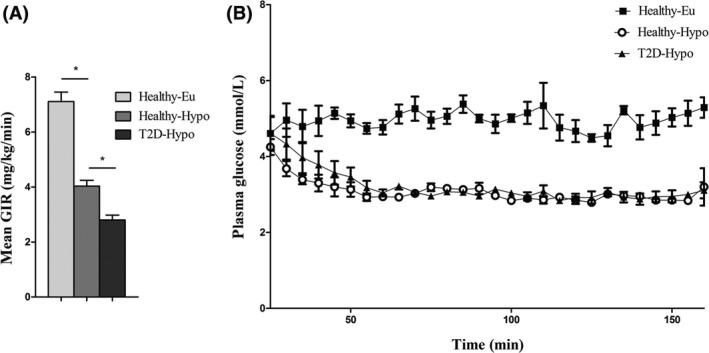
A, Time course of plasma glucose levels during the clamps. At time point 0, insulin infusion started. Black squares indicate values of the euglycaemia group. For hypoglycaemia: open circles, healthy participants; black triangles, type 2 diabetic participants. B, Mean glucose infusion rates during the procedure. Data are shown as means ± SD. *P < 0.05 by Bonferroni‐Dunn test
3.3. Counterregulatory hormones
For each group, levels of cortisol, growth hormone, epinephrine and norepinephrine measured before the clamping procedure were compared to levels measured during the procedure, when stable blood glucose values were reached. Hypoglycaemia significantly stimulated the release of the counterregulatory hormones epinephrine, norepinephrine, cortisol and growth hormone, whereas plasma levels of these hormones did not change in response to euglycaemia (Table 2). There were no major differences in these responses between subjects with type 2 diabetes and those without diabetes.
Table 2.
Counterregulatory hormone levels before and during the clamping procedure
| Healthy‐euglycaemia | Healthy‐hypoglycaemia | T2D‐hypoglycaemia | ||||
|---|---|---|---|---|---|---|
| Before | During | Before | During | Before | During | |
| Growth hormone (mE/L) | 8.5 ± 4.0 | 0.4 ± 0.2 | 19.1 ± 7.7 | 18.1 ± 7.3 | 3.1 ± 1.7 | 25.7 ± 9.4* |
| Cortisol (µmol/L) | 0.38 ± 0.04 | 0.25 ± 0.03 | 0.57 ± 0.18 | 0.53 ± 0.13 | 0.22 ± 0.02 | 0.47 ± 0.07* |
| Epinephrine (nmol/L) | 0.12 ± 0.02 | 0.11 ± 0.02 | 0.15 ± 0.04 | 1.15 ± 0.25* | 0.13 ± 0.03 | 1.69 ± 0.74* |
| Norepinephrine (nmol/L) | 1.16 ± 0.10 | 1.19 ± 0.17 | 0.94 ± 0.13 | 1.24 ± 0.10* | 1.28 ± 0.13 | 1.90 ± 0.26* |
Data are shown as mean ± SD
P < 0.05 vs before values by Wilcoxon signed rank test.
3.4. The effect of hypoglycaemia on AT [18F]FDG uptake
The distribution of [18F]FDG was altered under hypoglycaemic conditions compared to euglycaemic conditions in healthy obese individuals, with glucose uptake in visceral AT being significantly higher during hypoglycaemia (P = 0.004). Hypoglycaemia also caused a numerical increase in [18F]FDG uptake in abdominal subcutaneous AT and gluteofemoral subcutaneous AT, but this effect did not reach statistical significance (Figure 2A).
Figure 2.
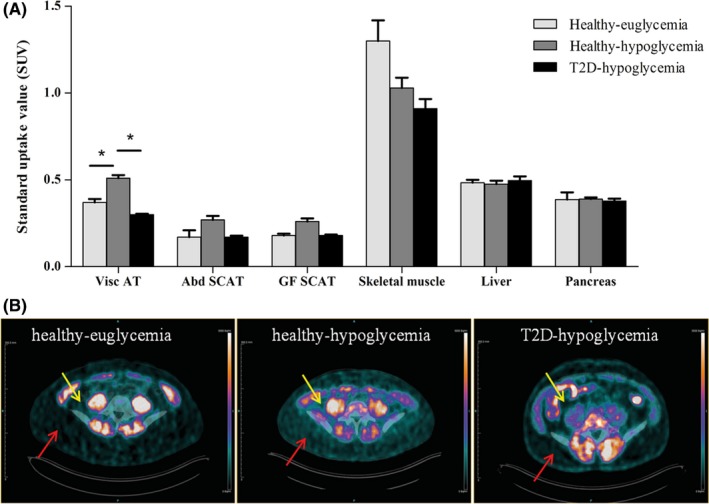
Uptake of 18F‐FDG in different AT depots and other organs (A) across the groups. Bars represent means ± SEM. *P < 0.05 by Bonferroni‐Dunn test. [18F]FDG uptake ratios between AT and skeletal muscle are 1:6.3 and 1:3.2 for subjects under euglycaemic and hypoglycaemic conditions, respectively. Representative PET images of healthy participants under euglycaemia and hypoglycaemia and type 2 diabetes patients under hypoglycaemia (B). Yellow arrows indicate visceral AT, and red arrows indicate abdominal subcutaneous AT
3.5. Comparison of [18F]FDG uptake in AT between healthy participants and patients with type 2 diabetes
Under hypoglycaemic conditions, patients with type 2 diabetes had a lower [18F]FDG uptake in visceral AT than the participants without diabetes (Figure 2A, P < 0.0001). Patients with type 2 diabetes and healthy participants showed no statistically significant difference in [18F]FDG uptake either in abdominal subcutaneous AT or in gluteofemoral subcutaneous AT (Figure 2A). The total AT volume as well as the AT volume in the different depots did not differ significantly between the groups (data not shown, but available upon request).
3.6. Comparison of [18F]FDG uptake between different AT depots and other organs
Uptake of [18F]FDG was compared between the different AT depots. In all groups, [18F]FDG uptake in visceral AT was significantly higher than in subcutaneous AT. In healthy obese participants, visceral AT [18F]FDG uptake was approximately twice as high as abdominal subcutaneous AT and gluteofemoral subcutaneous AT tracer uptake, both during euglycaemia (P < 0.0001) and during hypoglycaemia (P < 0.003). In patients with type 2 diabetes, [18F]FDG in visceral AT was approximately 40% higher than in visceral subcutaneous AT and gluteofemoral subcutaneous AT (Figure 2A, P < 0.0001). Quantification of [18F]FDG accumulation in skeletal muscle, liver and pancreas did not reveal significant differences between the groups. However, because there was a numerically lower [18F]FDG uptake in skeletal muscle during hypoglycaemia, the tracer uptake ratio between AT and skeletal muscle was 1:6.3 versus 1:3.2 in subjects under euglycaemic and hypoglycaemic conditions, respectively (P = 0.018, Figure 2A).
4. DISCUSSION
The main finding of this study is the stimulatory effect of hypoglycaemia on the uptake of [18F]FDG by AT. This method thereby enables a more precise and specific analysis of glucose handling by both visceral and subcutaneous AT, which has been challenging so far because of the low uptake values in AT compared to skeletal muscle under nonhypoglycaemic conditions. By using this approach, we also showed that the uptake of glucose in visceral AT of patients with type 2 diabetes is about 40% lower than in BMI‐matched healthy participants (Figure 2), which suggests a specific impairment in visceral AT metabolism in patients with type 2 diabetes.
[18F]FDG PET provides a tissue‐specific, noninvasive method for whole‐body analysis of glucose handling. Previous studies evaluating AT glucose metabolism using [18F]FDG PET were performed either under fasting conditions without a clamping procedure or during a hyperinsulinaemic euglycaemic clamp.3, 4, 5, 6, 8, 10 Under these conditions, uptake values of [18F]FDG in AT are low, especially compared to uptake levels in skeletal muscle. Performing [18F]FDG PET during a hyperinsulinaemic hypoglycaemic clamp significantly increased the tracer uptake in visceral AT of healthy participants and also resulted in a numerical increase in [18F]FDG uptake in subcutaneous AT. Since tracer uptake was not raised in skeletal muscle during hypoglycaemia, the uptake ratio between AT and skeletal muscle increased, allowing better discrimination (Figure 2A). We therefore propose that this method may provide a new, attractive research tool that permits the comparison of glucose uptake in different AT depots and examination of glucose handling in patients with differences in metabolic health. This technique could potentially be valuable as a research tool to examine the underlying pathophysiology of patients at risk of developing type 2 diabetes.
While providing interesting methodological opportunities, it is not fully clear what causes the stimulatory effect of hypoglycaemia on visceral [18F]FDG uptake. Catecholamines, which increase in response to hypoglycaemia, suppress insulin‐stimulated glucose uptake in skeletal muscle by activating protein kinase A through the stimulation of G‐protein coupled receptors.11, 12, 13 In contrast, while the counterregulatory response to hypoglycaemia was shown to increase lipolysis rates in AT, insulin signalling seems to remain intact.14 This could provide a possible explanation for the increased uptake ratio between AT and skeletal muscle observed in this study. Another contributing factor could be activation of the enzyme 5’adenosine monophosphate‐activated protein kinase (AMPK) in adipocytes. AMPK is activated by metabolic stresses that inhibit ATP production, like hypoglycaemia.15 This can in turn result in an increase of glucose uptake by adipocytes.16, 17, 18
By performing [18F]FDG PET during a hyperinsulinaemic hypoglycaemic clamp, we found a specific impairment in visceral AT metabolism in patients with type 2 diabetes compared to BMI‐matched, healthy participants. In subcutaneous AT depots, skeletal muscle, liver and pancreas, no significant differences in [18F]FDG uptake were found between healthy and type 2 diabetic participants (Figure 2A). Also, in line with previous literature,4, 5, 6, 8, 10 the uptake of glucose was found to be significantly higher in visceral AT than in subcutaneous AT (Figure 2A), which can be explained by greater abundance and metabolic activity of visceral AT adipocytes.10, 19 Because of the tissue specificity of the observed impairment in [18F]FDG uptake in this study, combined with the glucose handling capacity of visceral AT, we hypothesize that visceral AT glucose handling could play a role in the pathophysiology of type 2 diabetes. Following this pilot study, future investigations with larger patient numbers are key to further substantiate this hypothesis. Also, in this study, static PET scans were performed one hour after the injection of the radiopharmaceutical to quantify the tissue‐specific uptake of [18F]FDG. Future studies with dynamic PET could give insight into uptake kinetics and provide further information on systemic glucose metabolism and its role in type 2 diabetes.
The mechanism behind the observed impairment in visceral AT remains unclear. A possible explanation is a loss of visceral AT insulin sensitivity in type 2 diabetes, which is supported by clear mechanistic evidence for AT insulin resistance.7 However, differences in glucose uptake by AT between healthy and type 2 diabetic subjects have not been found during euglycaemia in previous studies.4, 8 Metabolic effects of counterregulatory hormones could also play a role. Cortisol and growth hormone, which were increased significantly in participants with type 2 diabetes during hypoglycaemia, while remaining stable in healthy participants (Table 2), are known to repress glucose uptake in AT.20, 21 However, basal levels of these hormones were also higher in healthy participants. Also, effects of these hormones would not become acutely apparent,22, 23 making their influence on the observed differences in this study less likely. Norepinephrine is known to increase the metabolic activity of adipose tissue via beta3‐adrenergic receptors. Levels of norepinephrine were slightly increased in participants with type 2 diabetes as compared to healthy participants. While this effect is generally described for brown adipose tissue, a deficiency in the beta3‐adrenergic pathway could potentially contribute to the observed impairment in [18F]FDG uptake in VAT of participants with type 2 diabetes in this study.
Limitations of this study that should be considered are the inequality in age and gender between the participants with type 2 diabetes and the healthy participants. However, it has been reported that age alone does not significantly influence insulin sensitivity.24 Furthermore, uptake of [18F]FDG in AT has been shown to increase with age,25 which would merely mask the decrease in [18F]FDG uptake in AT of type 2 diabetes patients observed in this study. Also, there is no report on any influence of gender on biodistribution of [18F]FDG. We therefore do not expect the inequality in age and gender between the groups to affect the validity of these results.
In conclusion, the present data indicate that [18F]FDG PET/CT performed during a hyperinsulinaemic hypoglycaemic clamp could be a promising new research tool to examine AT metabolism. By using this method, we here show that [18F]FDG uptake during hypoglycaemia is impaired in type 2 diabetes patients, specifically in visceral AT. This study thereby suggests a role for AT glucose handling in the pathophysiology of type 2 diabetes.
5. PRIOR PRESENTATION
Parts of this study were presented at.
The Annual Dutch Diabetes Research Meeting 2016, Oosterbeek, the Netherlands, 1‐2 December 2016.
The Gemeinsame Jahrestagung der Deutschen, Österreichischen und Schweizerischen Gesellschaften für Nuklearmedizin, Dresden, Germany, 26‐29 April 2017.
The Hot Topics in Molecular Imaging meeting of the European Society of Molecular Imaging in Les Houches, 21‐26 January 2018.
CONFLICT OF INTEREST
BEdG has received research support from Astra‐Zeneca and is on a scientific advisory board for Novo Nordisk.
AUTHOR CONTRIBUTIONS
MB HMR, LS, BEdG and MG designed the study with input from MJ, AA and L‐FdG‐O. MB recruited the participants. HMR performed the glucose clamps. MB and HMR collected the data. MB analysed the data. All authors discussed the results and implications and commented on the manuscript at all stages. BEdG and MG are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
ACKNOWLEDGEMENTS
The authors thank all the volunteers for their participation in this work. We thank M. van Riel, P. Kok, J. Butter, M Janssen and M.C. Attard for technical support during the PET/CT scans and D. Lobeek and T. Jansen for their help with collection of blood samples.
Boss M, Rooijackers HMM, Buitinga M, et al. PET imaging during hypoglycaemia to study adipose tissue metabolism. Eur J Clin Invest. 2019;49:e13120 10.1111/eci.13120
REFERENCES
- 1. Kodama S, Horikawa C, Fujihara K, et al. Quantitative relationship between body weight gain in adulthood and incident type 2 diabetes: a meta‐analysis. Obes Rev. 2014;15(3):202‐214. [DOI] [PubMed] [Google Scholar]
- 2. Ter Horst KW, Gilijamse PW, Ackermans MT, et al. Impaired insulin action in the liver, but not in adipose tissue or muscle, is a distinct metabolic feature of impaired fasting glucose in obese humans. Metabolism. 2016;65(5):757‐763. [DOI] [PubMed] [Google Scholar]
- 3. Dadson P, Landini L, Helmio M, et al. Effect of bariatric surgery on adipose tissue glucose metabolism in different depots in patients with or without type 2 diabetes. Diabetes Care. 2016;39(2):292‐299. [DOI] [PubMed] [Google Scholar]
- 4. Virtanen KA, Iozzo P, Hallsten K, et al. Increased fat mass compensates for insulin resistance in abdominal obesity and type 2 diabetes: a positron‐emitting tomography study. Diabetes. 2005;54(9):2720‐2726. [DOI] [PubMed] [Google Scholar]
- 5. Ng JM, Azuma K, Kelley C, et al. PET imaging reveals distinctive roles for different regional adipose tissue depots in systemic glucose metabolism in nonobese humans. Am J Physiol Endocrinol Metab. 2012;303(9):E1134‐1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Christen T, Sheikine Y, Rocha VZ, et al. Increased glucose uptake in visceral versus subcutaneous adipose tissue revealed by PET imaging. JACC Cardiovasc Imaging. 2010;3(8):843‐851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metabol. 2001;86(5):1930‐1935. [DOI] [PubMed] [Google Scholar]
- 8. Oliveira AL, Azevedo DC, Bredella MA, Stanley TL, Torriani M. Visceral and subcutaneous adipose tissue FDG uptake by PET/CT in metabolically healthy obese subjects. Obesity (Silver Spring, Md). 2015;23(2):286‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hofman MS, Hicks RJ. White fat, factitious hyperglycemia, and the role of FDG PET to enhance understanding of adipocyte metabolism. EJNMMI Res. 2011;1(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Virtanen KA, Lonnroth P, Parkkola R, et al. Glucose uptake and perfusion in subcutaneous and visceral adipose tissue during insulin stimulation in nonobese and obese humans. J Clin Endocrinol Metabol. 2002;87(8):3902‐3910. [DOI] [PubMed] [Google Scholar]
- 11. Lynch GS, Ryall JG. Role of beta‐adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease. Physiol Rev. 2008;88(2):729‐767. [DOI] [PubMed] [Google Scholar]
- 12. Walker KS, Watt PW, Cohen P. Phosphorylation of the skeletal muscle glycogen‐targetting subunit of protein phosphatase 1 in response to adrenaline in vivo. FEBS Lett. 2000;466(1):121‐124. [DOI] [PubMed] [Google Scholar]
- 13. Voss TS, Vendelbo MH, Kampmann U, et al. Acute hypoglycemia in healthy humans impairs insulin stimulated glucose uptake and glycogen synthase in skeletal muscle; a randomized clinical study. Diabetes. 2017; 66(9):2483‐2494. [DOI] [PubMed] [Google Scholar]
- 14. Voss TS, Vendelbo MH, Kampmann U, et al. Effects of insulin‐induced hypoglycaemia on lipolysis rate, lipid oxidation and adipose tissue signalling in human volunteers: a randomised clinical study. Diabetologia. 2017;60(1):143‐152. [DOI] [PubMed] [Google Scholar]
- 15. Salt IP, Johnson G, Ashcroft SJ, Hardie DG. AMP‐activated protein kinase is activated by low glucose in cell lines derived from pancreatic beta cells, and may regulate insulin release. Biochem J. 1998;335(Pt 3):533‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holmes BF, Kurth‐Kraczek EJ, Winder WW. Chronic activation of 5'‐AMP‐activated protein kinase increases GLUT‐4, hexokinase, and glycogen in muscle. J Appl Physiol. 1999;87(5):1990‐1995. [DOI] [PubMed] [Google Scholar]
- 17. Kurth‐Kraczek EJ, Hirshman MF, Goodyear LJ, Winder WW. 5' AMP‐activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes. 1999;48(8):1667‐1671. [DOI] [PubMed] [Google Scholar]
- 18. Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP‐activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol. 1997;273(6 Pt 1):E1107‐1112. [DOI] [PubMed] [Google Scholar]
- 19. Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11(1):11‐18. [DOI] [PubMed] [Google Scholar]
- 20. Andrews RC, Walker BR. Glucocorticoids and insulin resistance: old hormones, new targets. Clin Sci. 1999;96(5):513–523. [DOI] [PubMed] [Google Scholar]
- 21. Vijayakumar A, Novosyadlyy R, Wu Y, Yakar S, LeRoith D. Biological effects of growth hormone on carbohydrate and lipid metabolism. Growth Horm IGF Res. 2010;20(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Djurhuus CB, Gravholt CH, Nielsen S, et al. Effects of cortisol on lipolysis and regional interstitial glycerol levels in humans. Am J Physiol Endocrinol Metab. 2002;283(1):E172‐177. [DOI] [PubMed] [Google Scholar]
- 23. Djurhuus CB, Gravholt CH, Nielsen S, Pedersen SB, Moller N, Schmitz O. Additive effects of cortisol and growth hormone on regional and systemic lipolysis in humans. Am J Physiol Endocrinol Metab. 2004;286(3):E488‐494. [DOI] [PubMed] [Google Scholar]
- 24. Ferrannini E, Vichi S, Beck‐Nielsen H, Laakso M, Paolisso G, Smith U. Insulin action and age. European Group for the Study of Insulin Resistance (EGIR). Diabetes. 1996;45(7):947‐953. [DOI] [PubMed] [Google Scholar]
- 25. Wehrli NE, Bural G, Houseni M, Alkhawaldeh K, Alavi A, Torigian DA. Determination of age‐related changes in structure and function of skin, adipose tissue, and skeletal muscle with computed tomography, magnetic resonance imaging, and positron emission tomography. Semin Nucl Med. 2007;37(3):195‐205. [DOI] [PubMed] [Google Scholar]


