Abstract
Background
Inotuzumab ozogamicin (InO) is an antibody‐drug conjugate used for adults with relapsed/refractory B‐cell precursor (BCP) acute lymphoblastic leukemia (ALL). The INotuzumab Ozogamicin trial to inVestigAte Tolerability and Efficacy (INO‐VATE) previously reported improved outcomes with InO versus standard‐of‐care (SoC) chemotherapy. This article reports the final INO‐VATE results (≥2 years of follow‐up) and additional analyses of patient characteristics associated with improved outcomes.
Methods
Between August 27, 2012, and January 4, 2015, this multicenter, parallel, open‐label, phase 3 trial randomized 326 adults with relapsed/refractory ALL to InO (n = 164) or SoC (n = 162); 307 received 1 or more doses of the study drug (164 in the InO arm and 143 in the SoC arm).
Results
The complete remission (CR)/complete remission with incomplete hematologic recovery (CRi) rate was higher with InO versus SoC (73.8% vs 30.9%; 1‐sided P < .0001), with consistent CR/CRi rates across patient subgroups. The median overall survival (OS) was 7.7 months with InO and 6.2 months with SoC, with 2‐year OS rates of 22.8% and 10.0%, respectively (overall hazard ratio, 0.75; 97.5% confidence interval [CI], 0.57‐0.99; 1‐sided P = .0105). The predictors of OS with InO were the best minimal residual disease status, baseline platelet count, duration of first remission, achievement of CR/CRi, and follow‐up hematopoietic stem cell transplantation (HSCT; all 2‐sided P values < .05). More InO arm patients proceeded directly to HSCT after achieving CR/CRi before any follow‐up induction therapy (39.6% [95% CI, 32.1%‐47.6%] vs 10.5% [6.2%‐16.3%]; 1‐sided P < .0001). The most frequent all‐grade and grade 3 or higher adverse events in both arms were hematologic. Veno‐occlusive disease (VOD)/sinusoidal obstruction syndrome (SOS) was more frequent with InO (23 of 164 [14.0%] vs 3 of 143 [2.1%]).
Conclusions
In patients with relapsed/refractory BCP ALL in INO‐VATE, InO was associated with a greater likelihood of CR/CRi across key patient subgroups, and it served as a bridge to HSCT. Potential VOD/SOS risk factors must be considered when InO treatment decisions are being made.
Keywords: acute lymphoblastic leukemia, adults, hematopoietic stem cell transplantation, hepatic veno‐occlusive disease, inotuzumab ozogamicin, remission induction
Short abstract
All key subgroups of patients with relapsed or refractory CD22‐positive B‐cell precursor acute lymphoblastic leukemia have the potential to benefit from inotuzumab ozogamicin in comparison with the standard of care (intensive chemotherapy). In the future, the combination of inotuzumab ozogamicin with other therapies may provide improved outcomes.
Introduction
The prognosis for adults with relapsed or refractory (R/R) B‐cell acute lymphoblastic leukemia (ALL) remains poor. Currently, the only curative option is allogeneic hematopoietic stem cell transplantation (HSCT), which can be attempted after hematologic remission. However, with standard chemotherapy induction, remission rates are 30% to 46% in the first salvage setting and 18% to 25% in the second salvage setting.1, 2, 3 With targeted agents such as blinatumomab and tisagenlecleucel, rates have improved4, 5; nevertheless, a significant need remains for improved treatment, and in this population, overall survival (OS) at 5 years is only 5% to 10%.1, 6
Inotuzumab ozogamicin (InO) is a recently introduced targeted therapy for adults with R/R CD22‐positive B‐cell precursor (BCP) ALL.7, 8 InO is an antibody‐drug conjugate composed of a humanized anti‐CD22 monoclonal antibody conjugated to the cytotoxic agent calicheamicin.9, 10 It binds with high affinity to CD22, a cell‐surface antigen expressed by >90% of B‐cell blasts in nearly all patients with B‐cell ALL.11, 12 The antibody‐drug conjugate is then rapidly internalized, and subsequent intracellular release of unconjugated calicheamicin leads to apoptosis via its binding to and cleavage of double‐stranded DNA.9, 10
In the INotuzumab Ozogamicin trial to inVestigAte Tolerability and Efficacy (INO‐VATE), InO monotherapy was evaluated against the standard of care (SoC; intensive chemotherapy) as first or second salvage therapy in adults with R/R BCP ALL.13 INO‐VATE was a phase 3, multinational, open‐label, randomized trial designed to analyze 2 primary endpoints at prespecified time points before trial completion. First, the proportion of patients achieving complete remission (CR) or complete remission with incomplete hematologic recovery (CRi) was analyzed among the initial 218 randomized participants (data cutoff, October 2, 2014). In the InO arm, a significantly higher proportion achieved CR/CRi (80.7% vs 29.4% with SoC); among those with CR/CRi, the rate of minimal residual disease (MRD) negativity was also higher (78.4% vs 28.1%), and more patients proceeded directly to HSCT (41% vs 11%).13 Second, survival was analyzed when a sufficient number of patients had been recruited and a sufficient number of events had been recorded (data cutoff, March 8, 2016; n = 326). The InO arm had longer progression‐free survival (PFS; hazard ratio [HR], 0.45; 97.5% confidence interval [CI], 0.34‐0.61; 2‐sided P < .001; median, 5.0 vs 1.8 months) and OS (HR, 0.77; 97.5% CI, 0.58‐1.03; 2‐sided P = .04; median, 7.7 vs 6.7 months), although the latter did not meet statistical significance criteria per the study design.13
After these primary analyses, the study continued until 2 years after the randomization of the last patient into the trial; thus, INO‐VATE was completed on January 4, 2017, and provided a minimum of 2 years’ follow‐up for each patient. Using the data available at this point, we report safety and efficacy outcomes as well as additional analyses of patient characteristics associated with improved outcomes.
Materials and Methods
Study Design and Participants
The study design and patient population of INO‐VATE (NCT01564784) have been published.13 Briefly, INO‐VATE was a phase 3, open‐label, randomized study conducted in North America, Europe, Asia, and Australia. Eligible patients were ≥18 years old, had a diagnosis of R/R CD22‐positive BCP ALL, and were scheduled to receive their first or second salvage treatment. Patients with Philadelphia chromosome–positive (Ph+) disease were eligible if treatment with 1 or more second‐generation BCR‐ABL tyrosine kinase inhibitors (TKIs) had failed, with the proportion capped at ~20% of the trial population.
The study protocol was approved by institutional review boards or independent ethics committees at each trial center, and the study was conducted according to the principles of the Declaration of Helsinki. All participants provided written informed consent.
Randomization, Stratification, and Masking
Patients were randomly allocated 1:1 to InO monotherapy or SoC, with randomization stratified for the duration of first remission (<12 or ≥12 months), salvage treatment setting (1 or 2), and age at randomization (<55 or ≥55 years), as previously described.14 Patients and investigators were not masked to the treatment allocation.
Procedures
Patients received intravenous InO or an SoC regimen of the investigator’s choice (Fig. 1). Consolidation therapy was not specified in the protocol, and this allowed investigators to use their own discretion; consolidation therapy data were not routinely collected. Patients could proceed to allogeneic HSCT at the treating investigator’s discretion and continued to be followed in the study for disease progression and survival.
Figure 1.
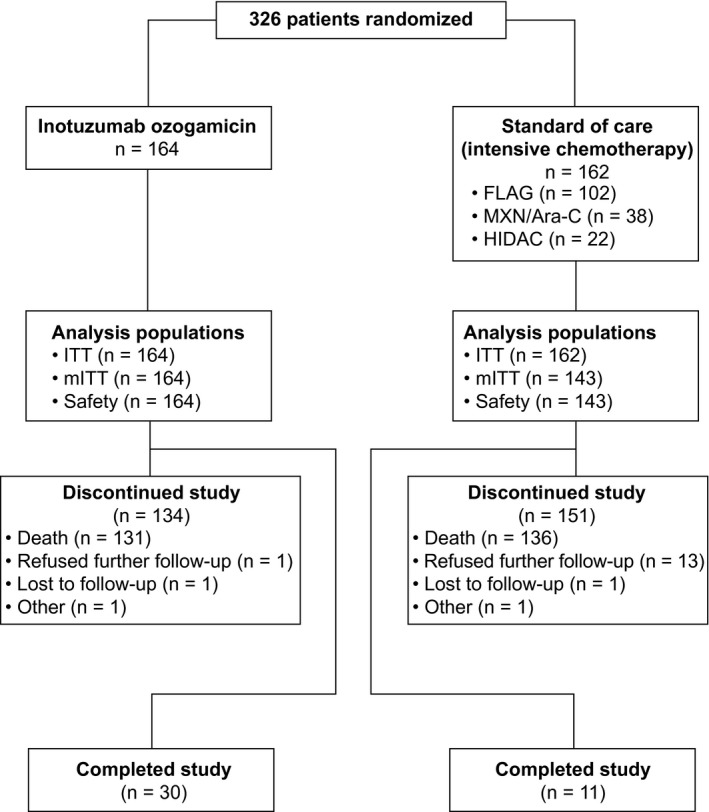
Trial profile. Completing the study was defined as completing follow‐up for 5 years from the individual patient’s randomization or for 2 years from randomization of the last patient (January 4, 2015), whichever occurred first. On the date of the last visit by the last patient to be randomized (January 4, 2017), all patients discontinued the study. In the InO arm, patients received InO intravenously at 1.8 mg/m2 per cycle, 0.8 mg/m2 on day 1 and 0.5 mg/m2 on days 8 and 15 of a 21‐ to 28‐day cycle, for a maximum of 6 cycles. Patients who achieved CR/CRi had their InO dose adjusted to 1.5 mg/m2 per cycle, with 0.5 mg/m2 administered on days 1, 8, and 15. Patients in the standard‐of‐care arm received a regimen of the investigator’s choice: 1) FLAG for up to four 28‐day cycles, which consisted of cytarabine at 2.0 g/m2/d on days 1 to 6, fludarabine at 30 mg/m2/d on days 2 to 6, and granulocyte colony‐stimulating factor at 5 μg/kg/d (or at the standard dose of the institute); 2) MXN/Ara‐C for up to four 15‐ to 20‐day cycles, which consisted of cytarabine at 200 mg/m2/d on days 1 to 7 and mitoxantrone at 12 mg/m2/d on days 1 to 3 (with dose reductions to 8 mg/m2 allowed on the basis of age, coexisting conditions, and previous anthracycline exposure); or 3) HIDAC for up to two 12‐dose cycles at 3.0 g/m2 every 12 hours (the dose could be reduced up to 1.5 g/m2 for patients aged 55 years or older and was reduced to 1.5 g/m2 for patients older than 60 years). CR indicates complete remission; CRi, complete remission with incomplete hematologic recovery; FLAG, fludarabine, cytarabine, and granulocyte colony‐stimulating factor; HIDAC, high‐dose cytarabine; InO, inotuzumab ozogamicin; ITT, intent‐to‐treat; mITT, modified intent‐to‐treat; MXN/Ara‐C, mitoxantrone and cytarabine.
Outcomes
Details of study endpoints and evaluation methods have been published.13 Two primary endpoints were prespecified: the CR/CRi rate (as assessed by the Endpoint Adjudication Committee) and OS.13 An analysis of outcomes at trial completion was prespecified and used outcome definitions as previously described, except that CR/CRi was not adjudicated but was based on the investigator’s assessment. In addition, we compared CR/CRi and OS by patient subgroups according to stratification factors and other patient characteristics. An exploratory analysis compared OS between treatment arms 2 and 3 years after randomization. Within treatment arms, a post hoc analysis compared characteristics of patients with OS ≥2 years and patients with OS <2 years (2 years is a clinically meaningful time point for OS but was also used for practical reasons because all patients had this length of follow‐up, which was defined by protocol specification for completing the trial). A further exploratory analysis of the restricted mean survival time (RMST) was used as an alternative approach to estimate the treatment effect when the OS data did not appear to satisfy the assumption of proportional hazards.15, 16, 17, 18 RMST measures average survival from time 0 to a specified time point (the truncation time) based on the maximum common follow‐up time, which was defined as the shorter maximum OS time for the 2 study arms.
We assessed efficacy in the intent‐to‐treat (ITT) population (all patients randomized) and the modified intent‐to‐treat (mITT) population (all randomized patients who started the treatment assigned according to the initial randomization). We assessed fatal events after HSCT in the ITT population and all other safety analyses in the safety population (all randomized patients who received 1 or more dose of the study drug). We assessed treatment‐emergent adverse events (AEs) across all cycles, as prespecified in the protocol; in addition, we assessed AEs by the maximum treatment cycle started. For AEs of any cause, treatment‐emergent was defined as commencing on or after day 1 of cycle 1 but within 42 days of the last dose; for AEs considered treatment‐related, treatment‐emergent was defined as commencing on or any time after day 1 of cycle 1. In addition, all veno‐occlusive disease (VOD)/sinusoidal obstruction syndrome (SOS) events within 2 years after randomization were considered as treatment‐emergent AEs, regardless of causality. VOD was assessed according to previously defined clinical criteria and diagnosed by the treating investigator.13 All potential VOD cases were reviewed by an independent hepatic events adjudication board, whose findings up to the March 2016 cutoff have been reported.19 Hepatotoxic AEs were defined as described previously.14 We coded AEs with the Medical Dictionary for Regulatory Activities (version 19.1), with toxicity graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (version 3.0).
Statistical Analysis
Sample size calculations have been published previously.13 Kaplan‐Meier methods were used to calculate the median survival times (and corresponding 95% and 97.5% CIs), for the prespecified analyses and for the exploratory post hoc analyses, and to plot curves for OS and PFS. Hazard ratios for treatment effects and corresponding 97.5% CIs and P values were calculated with Cox proportional hazards regression models and log‐rank tests, respectively. These were adjusted for the randomization stratification factors described previously, except for subgroup analyses, for which unstratified analyses are reported. SAS statistical software (version 9.1 or later) was used for all calculations.
Results
Patients and Treatments
INO‐VATE enrolled patients between August 27, 2012, and January 4, 2015. At the date of the last visit by the last patient (January 4, 2017), the ITT population included 326 patients (164 in the InO arm and 162 in the SoC arm; Fig. 1). Among these patients , 164 in the InO arm and 143 in the SoC arm received 1 or more doses of the study drug and were included in the mITT and safety populations. Overall, 41 patients completed the study (30 [18.3%] in the InO arm and 11 [6.8%] in the SoC arm); the most common reason for study discontinuation was patient death in both treatment arms (79.9% in the InO arm and 84.0% in the SoC arm). In addition, 18 patients were censored in OS analyses (3 [1.8%] in the InO arm and 15 [9.3%] in the SoC arm) because they were no longer followed for survival data. The median follow‐up duration for patients who completed the study or were censored for OS was 29.6 months (range, 1.7‐49.7 months).
In the safety population, the median treatment duration was 8.9 weeks for the InO arm (range, 0.1‐26.4 weeks; median number of cycles started, 3) and 0.9 weeks for the SoC arm (range, 0.1‐15.6 weeks; median number of cycles started, 1).
Efficacy
Hematologic response
In the ITT population, demographic and clinical characteristics were balanced between the treatment arms (Table 1). Compared with SoC, InO was associated with a significantly higher proportion of patients achieving CR/CRi, and this was consistent across all subgroups analyzed (Fig. 2). Among the patients who started treatment (the mITT population), the proportion with CR/CRi was higher with InO than SoC (121 of 164 [73.8%] vs 50 of 143 [35.0%]). For patients achieving CR/CRi, the duration of remission was significantly longer in the InO arm (HR, 0.62 [95% CI, 0.42‐0.91]; 1‐sided P = .0071; median, 5.4 months [95% CI, 4.2‐7.0 months] vs 4.2 months [95% CI, 2.7‐5.7 months]). Similarly, PFS was longer (HR, 0.45 [97.5% CI, 0.34‐0.60]; 1‐sided P < .0001; median, 5.0 months [95% CI, 3.9‐5.8 months] vs 1.7 months [95% CI, 1.4‐2.1 months]; Fig. 3).
Table 1.
Baseline Characteristics of Patients (Intent‐to‐Treat Population)
| Characteristic | InO (n = 164) | SoC (n = 162) |
|---|---|---|
| Age, median (range), y | 46.5 (18‐78) | 47.5 (18‐79) |
| Age, No. (%) | ||
| <55 y | 104 (63.4) | 103 (63.6) |
| ≥55 y | 60 (36.6) | 59 (36.4) |
| Male sex, No. (%) | 91 (55.5) | 102 (63.0) |
| Race, No. (%) | ||
| White | 112 (68.3) | 120 (74.1) |
| Black | 4 (2.4) | 3 (1.9) |
| Asian | 31 (18.9) | 24 (14.8) |
| Missing | 17 (10.4) | 15 (9.3) |
| ECOG performance status, No. (%) | ||
| 0 | 62 (37.8) | 61 (37.7) |
| 1 | 81 (49.4) | 80 (49.4) |
| 2 | 21 (12.8) | 20 (12.3) |
| Missing | 0 (0) | 1 (0.6) |
| Salvage status, No. (%) | ||
| 1 | 111 (67.7) | 102 (63.0) |
| 2 | 51 (31.1) | 59 (36.4) |
| Missing | 2 (1.2) | 1 (0.6) |
| Duration of first remission <12 mo, No. (%) | 96 (58.5) | 106 (65.4) |
| Response to previous induction regimen, No. (%) | ||
| Complete remission | 121 (73.8) | 112 (69.1) |
| Partial remission | 11 (6.7) | 10 (6.2) |
| Prior HSCT, No. (%) | 29 (17.7) | 32 (19.8) |
| WBC count, median (range), ×103 cells/mm3 | 4.1 (0.0‐47.4) | 4.0 (0.1‐68.8) |
| Peripheral blast count, median (range), cells/μL | 107.6 (0‐42,660) | 30 (0‐43,331) |
| No circulating blasts, No. (%) | 71 (43.3) | 74 (45.7) |
| Bone marrow blasts, No. (%) | ||
| <50% | 53 (32.3) | 48 (29.6) |
| ≥50% | 109 (66.5) | 113 (69.8) |
| Missing | 2 (1.2) | 1 (0.6) |
| CD22 expression on ALL blasts, No. (%) | ||
| ≥90% | 107 (65.2) | 93 (57.4) |
| ≥70 but <90% | 30 (18.3) | 18 (11.1) |
| <70% | 5 (3.0) | 18 (11.1) |
| Missing | 22 (13.4) | 33 (20.4) |
| Baseline cytogenetics, No. (%) | ||
| Normal | 46 (28.0) | 42 (25.9) |
| ≥20 metaphases analyzed | 35 (21.3) | 34 (21.0) |
| Ph+ | 22 (13.4) | 27 (16.7) |
| t(4;11) | 6 (3.7) | 8 (4.9) |
| Complex | 28 (17.1) | 22 (13.6) |
| Del (9p) | 2 (1.2) | 3 (1.9) |
| Hyperdiploidy | 7 (4.3) | 2 (1.2) |
| Other abnormalities | 33 (20.1) | 36 (22.2) |
| Unknown/missing | 20 (12.2) | 22 (13.6) |
Abbreviations: ALL, acute lymphoblastic leukemia; ECOG, Eastern Cooperative Oncology Group; HSCT, hematopoietic stem cell transplantation; InO, inotuzumab ozogamicin; Ph+, Philadelphia chromosome–positive; SoC, standard of care (intensive chemotherapy); WBC, white blood cell.
Figure 2.
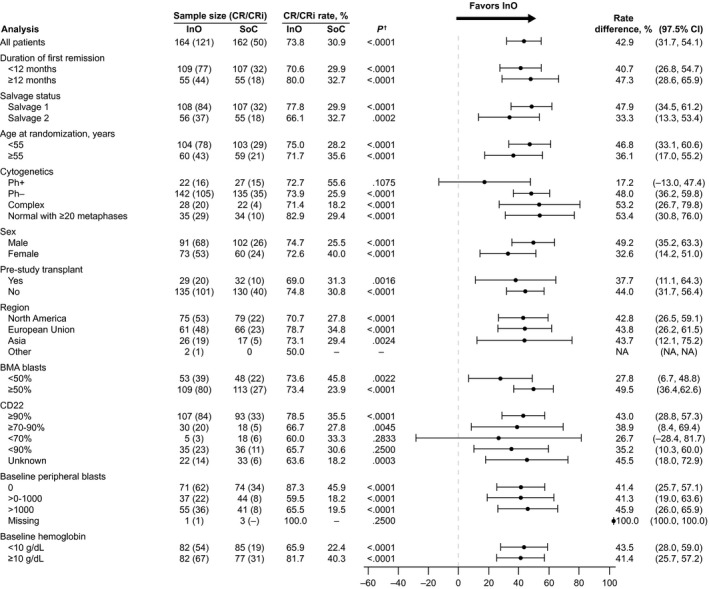
Stratified comparison of CR/CRi rates. Stratification factors (duration of first remission, salvage status, and age at randomization) were based on information provided for the interactive voice response system at randomization. †One‐sided P values. BMA indicates bone marrow aspirate; CI, confidence interval; CR, complete remission; CRi, complete remission with incomplete hematologic recovery; InO, inotuzumab ozogamicin; NA, not applicable; Ph–, Philadelphia chromosome–negative; Ph+, Philadelphia chromosome–positive; SoC, standard of care (intensive chemotherapy).
Figure 3.
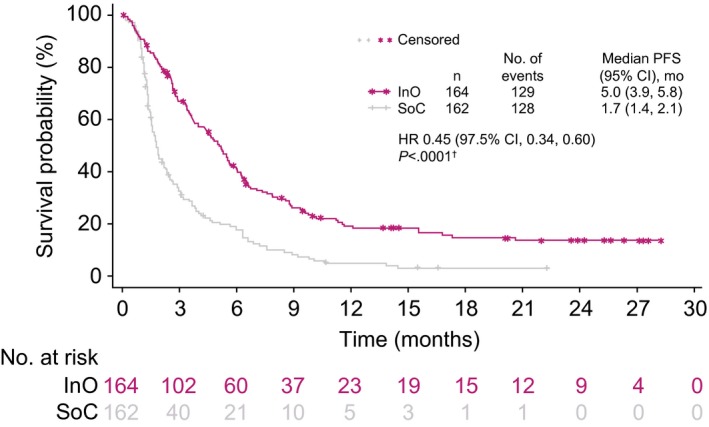
PFS. For INO‐VATE, PFS was defined as the time from randomization to the earliest of the following: disease progression (including objective progression, relapse after CR/CRi, or treatment discontinuation due to a global deterioration of health status), the start of new induction therapy or post‐therapy HSCT without the achievement of CR/CRi, or death from any cause. It was censored at the last valid disease assessment. †One‐sided log‐rank test. CI indicates confidence interval; CR, complete remission; CRi, complete remission with incomplete hematologic recovery; HR, hazard ratio; HSCT, hematopoietic stem cell transplantation; InO, inotuzumab ozogamicin; INO‐VATE, INotuzumab Ozogamicin trial to inVestigAte Tolerability and Efficacy; PFS, progression‐free survival; SoC, standard of care (intensive chemotherapy).
OS
In the ITT population, patients in the InO arm had a 25% lower risk of death than those in the SoC arm (HR, 0.75 [97.5% CI, 0.57‐0.99]; 1‐sided P = .0105; median OS, 7.7 months [95% CI, 6.0‐9.2 months] vs 6.2 months [95% CI, 4.7‐8.3 months]). The survival curves showed early separation, which increased from ~14 months onward, and an exploratory post hoc analysis at 2 and 3 years showed a larger OS rate difference between the treatment arms at later time points (Fig. 4), with 2‐year OS rates of 22.8% and 10.0% for the respective groups. For OS, an analysis of subgroups according to randomization stratification factors, baseline characteristics, and postbaseline characteristics showed that outcomes with InO were favorable in nearly all subgroups evaluated (Fig. 5). As expected, some subgroups showed generally improved OS in both arms (eg, patients younger than 55 years or those with a first duration of remission ≥12 months). When we looked at subgroups according to cytogenetics, the results for patients with normal cytogenetics, complex cytogenetics, and other abnormalities were consistent with the overall results. However, no difference in OS was observed in the Ph+ group, but when only Philadelphia chromosome–negative patients were considered, the HR was 0.68 (97.5% CI, 0.51‐0.92). We also examined OS in the subgroup of patients with t(4;11); however, the sample size was small (6 in the InO arm and 8 in the SoC arm), and this gave the analysis of OS a wide CI (median OS, 4.2 months for the InO arm vs 4.0 months for the SoC arm; unstratified HR, 1.42; 97.5% CI, 0.36‐5.62).
Figure 4.
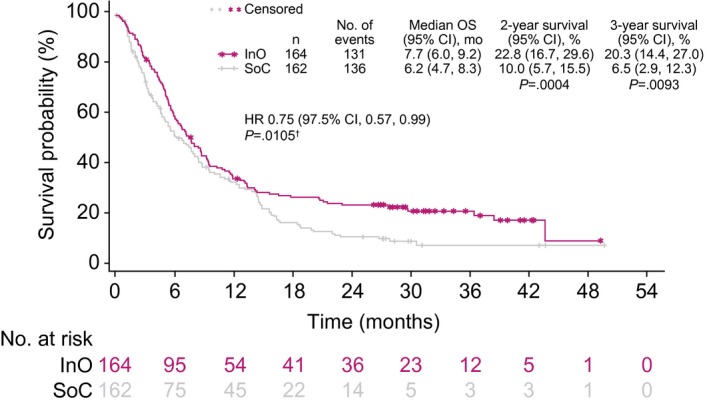
OS. For 2‐ and 3‐year survival, the 1‐sided P value was based on the chi‐square test or the Fisher exact test (if any cell count was <5). †One‐sided log‐rank test. CI indicates confidence interval; HR, hazard ratio; InO, inotuzumab ozogamicin; OS, overall survival; SoC, standard of care (intensive chemotherapy).
Figure 5.
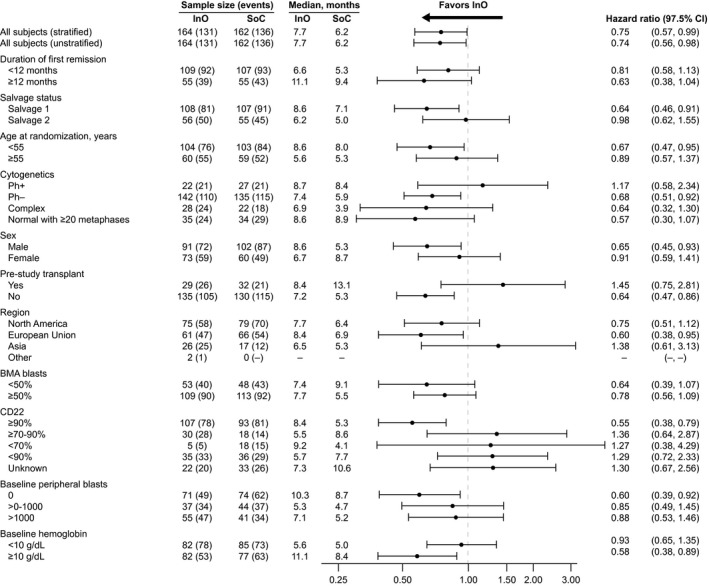
OS according to subgroups. BMA indicates bone marrow aspirate; CI, confidence interval; InO, inotuzumab ozogamicin; OS, overall survival; Ph–, Philadelphia chromosome–negative; Ph+, Philadelphia chromosome–positive; SoC, standard of care (intensive chemotherapy).
In addition, as the divergence of the survival curves indicated that the data might no longer fulfill the proportional hazards assumption (Fig. 4), an RMST analysis was undertaken with a truncation time of 49.3 months, the maximum follow‐up in the InO arm. In the ITT population, RMST was longer in the InO arm than the SoC arm (15.2 months [95% CI, 12.6‐17.8 months] vs 10.8 months [95% CI, 8.8‐12.9 months]; 1‐sided P = .0051).
Impact of subsequent salvage therapies on survival
Overall, patients randomized to the InO arm had a lower rate of subsequent induction/salvage therapy (56 [34.1%] vs 92 [56.8%] for SoC). In patients with CR/CRi, 1 or more subsequent salvage therapy was received by 35 of 121 patients (28.9%) in the InO arm and by 32 of 50 patients (64.0%) in the SoC arm, whereas among those who did not achieve CR/CRi, 21 of 43 (48.8%) and 60 of 112 (53.6%) in the respective arms received 1 or more salvage therapy. Supporting Table 1 gives a full list of subsequent salvage therapies. Overall, patients randomized to InO were less frequently treated with blinatumomab (10 [6.1%] vs 20 [12.3%]) and TKIs (3 [1.8%] vs 10 [6.2%]) as follow‐up induction/salvage therapy in comparison with those randomized to SoC. Two patients (1.2%) in each treatment arm received chimeric antigen receptor T‐cell (CAR‐T) therapy. Salvage therapy with InO was later used in 9 patients (5.6%) randomized to the SoC arm. Because subsequent therapy may have affected outcomes, an OS sensitivity analysis that censored patients at the time of their first subsequent salvage therapy with blinatumomab, TKIs, CAR‐T, or InO was undertaken to mitigate any potentially confounding influence of these therapies on OS. As shown in Figure 6, differences in the OS were preserved for the InO arm versus the SoC arm (HR, 0.71 [97.5% CI, 0.51‐0.97]; 1‐sided P = .0062), and at 2 years, 24% and 8%, respectively, were alive.
Figure 6.
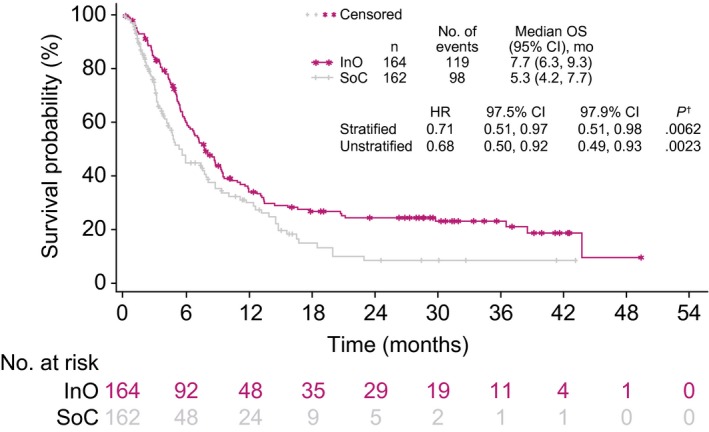
OS censored at the time of blinatumomab, a tyrosine kinase inhibitor, chimeric antigen receptor T‐cell therapy, or InO. †One‐sided log‐rank test. CI indicates confidence interval; HR, hazard ratio; InO, inotuzumab ozogamicin; OS, overall survival; SoC, standard of care (intensive chemotherapy).
Impact of HSCT on survival
According to transplant rates across the study, more patients proceeded to HSCT at any time after study treatment in the InO arm than the SoC arm (79 of 164 vs 36 of 162; 1‐sided P < .0001; Table 2). Most transplants were allogeneic (1 patient in the SoC arm had an autologous transplant). More patients in the InO arm proceeded directly to HSCT after study treatment but before starting any follow‐up induction therapy (70 of 164 vs 18 of 162; 1‐sided P < .0001), whereas 9 patients in the InO arm and 18 patients in the SoC arm proceeded to HSCT after additional treatment. Almost 4 times as many patients in the InO arm proceeded to HSCT after achieving CR/CRi before the start of any follow‐up induction therapy (65 of 164 [39.6%; 95% CI, 32.1%‐47.6%] vs 17 of 162 [10.5%; 95% CI, 6.2%‐16.3%]; 1‐sided P < .0001). Even when it was considered as a percentage of those who achieved CR/CRi, the proportion was much higher (65 of 121 [53.7%] vs 17 of 50 [34.0%]). Five patients in the InO arm and 1 in the SoC arm proceeded directly to HSCT without achieving CR/CRi. Among the patients who proceeded to HSCT, in both study arms, myeloablative conditioning therapy was more common than reduced‐intensity conditioning (67.1% and 32.9%, respectively, in the InO arm and 66.7% and 22.2%, respectively, in the SoC arm). Among InO‐treated patients achieving CR/CRi, OS was significantly improved for patients who underwent follow‐up HSCT in comparison with patients who achieved CR/CRi but did not proceed to HSCT (Fig. 7).
Table 2.
Proportion of Patients Proceeding to HSCT
| InO (n = 164) | SoC (n = 162) | P a | |||
|---|---|---|---|---|---|
| n/N | % (95% CI) | n/N | % (95% CI) | ||
| Proceeded to HSCT at any time after study treatment | 79/164 | 48.2 (40.3‐56.1) | 36/162 | 22.2 (16.1‐29.4) | <.0001 |
| Proceeded to HSCT after study treatment before start of any follow‐up induction therapy | 70/164 | 42.7 (35.0‐50.6) | 18/162 | 11.1 (6.7‐17.0) | <.0001 |
| After achieving CR/CRi, proceeded to HSCT before start of any follow‐up induction therapy | |||||
| Proportion of ITT population | 65/164 | 39.6 (32.1‐47.6) | 17/162 | 10.5 (6.2‐16.3) | <.0001 |
| Proportion of patients achieving CR/CRi | 65/121 | 53.7 (44.4‐62.8) | 17/50 | 34.0 (21.2‐48.8) | .0094 |
Abbreviations: CI, confidence interval; CR, complete remission; CRi, complete remission with incomplete hematologic recovery; HSCT, hematopoietic stem cell transplantation; InO, inotuzumab ozogamicin; ITT, intent‐to‐treat; SoC, standard of care (intensive chemotherapy).
One‐sided P value based on the chi‐square test or the Fisher exact test (if any cell count was <5).
Figure 7.
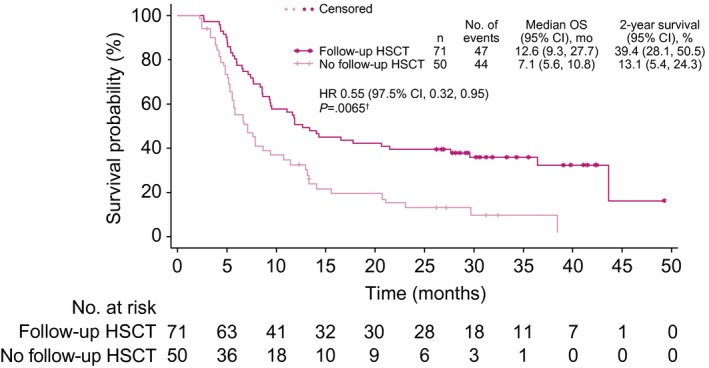
OS of InO‐treated patients who achieved CR/CRi according to follow‐up HSCT. The graph shows the time from randomization to the date of death due to any cause. Patients whose date of death could not be verified were censored at the date of last contact. †One‐sided log‐rank test. CI indicates confidence interval; CR, complete remission; CRi, complete remission with incomplete hematologic recovery; HR, hazard ratio; HSCT, hematopoietic stem cell transplantation; InO, inotuzumab ozogamicin; OS, overall survival.
Predictors of OS
In stepwise Cox regression modeling (a multivariate analysis including all patients with measurements for relevant variables), the factors associated with improved OS in the InO arm (n = 123) were as follows: best MRD status, baseline platelet count, baseline hemoglobin level, duration of first remission, achieving CR/CRi, and whether a patient underwent HSCT during follow‐up (all 2‐sided P values <.05; Table 3). In the SoC arm (n = 80), age, baseline platelets, prior HSCT, and follow‐up HSCT were associated with improved OS (Table 3). Other factors included in the model but not significantly associated with outcomes are listed in a footnote in Table 3.
Table 3.
Clinical Variables Predictive of Overall Survival (Intent‐to‐Treat Population)
| Variable | Subsets Analyzed | Hazard Ratio (95% CI) | 2‐Sided P |
|---|---|---|---|
| InO arm (n = 123) | |||
| Best MRD status | Positive (n = 36) | 1.99 (1.19‐3.31) | .0085 |
| Negative (n = 87) | |||
| Baseline platelets | <100 × 109/L (n = 85) | 1.65 (0.96‐2.86) | .0729 |
| ≥100 × 109/L (n = 38) | |||
| Baseline hemoglobin | <10 g/dL (n = 53) | 1.60 (1.01‐2.55) | .0463 |
| ≥10 g/dL (n = 70) | |||
| Duration of first remissiona | Continuous (n = 123) | 0.98 (0.97‐1.00) | .0157 |
| CR/CRib | Yes (n = 103) | 0.48 (0.26‐0.90) | .0214 |
| No (n = 20) | |||
| Follow‐up HSCT | Yes (n = 70) | 0.40 (0.26‐0.61) | <.0001 |
| No (n = 53) | |||
| SoC arm (n = 80) | |||
| Age | <55 y (n = 47) | 1.76 (1.04‐2.99) | .0363 |
| ≥55 y (n = 33) | |||
| Baseline platelets | <100 × 109/L (n = 44) | 1.70 (1.01‐2.85) | .0452 |
| ≥100 × 109/L (n = 36) | |||
| Prior HSCT | Yes (n = 17) | 0.28 (0.14‐0.56) | .0003 |
| No (n = 63) | |||
| Follow‐up HSCT | Yes (n = 23) | 0.15 (0.08‐0.31) | <.0001 |
| No (n = 57) |
Abbreviations: CI, confidence interval; CR, complete remission; CRi, complete remission with incomplete hematologic recovery; HSCT, hematopoietic stem cell transplantation; InO, inotuzumab ozogamicin; MRD, minimal residual disease; SoC, standard of care.
Only variables predictive of outcomes are shown in the table. The variables included in the stepwise Cox regression modeling were as follows: age (continuous), age (< or ≥55 years), sex, central CD22 (< or ≥90%), baseline hemoglobin (< or ≥10 g/dL), baseline platelets (< or ≥100 × 109/L), baseline absolute circulating blasts (< or ≥1 × 109/L), baseline percentage of bone marrow blasts (continuous), salvage status at randomization (2 or 1), presence of extramedullary disease (yes or no), prior HSCT (yes or no), primary refractory (yes or no), response to most recent prior regimen (CR or other), duration of first remission per case report form (continuous), dual alkylator during conditioning therapy (yes or no), donor for HSCT (alternate or matched related), best MRD status (positive or negative), CR/CRi per the investigator’s assessment (yes or no), CR/CRi at the last assessment before HSCT (yes or no), last platelet value recorded before transplantation (< or ≥100 × 109/L), and last bilirubin measurement recorded before transplantation (< or ≥ upper limit of normal).
According to the case report form.
According to the investigator’s assessment.
OS censored for HSCT
When patients in the OS analysis were censored at the HSCT date, OS was significantly longer in the InO arm than the SoC arm, with a 30% lower risk of death in the InO arm (stratified HR, 0.70 [97.5% CI, 0.50‐0.99]; 1‐sided P = .0095; median, 6.7 vs 5.5 months; Fig. 8). At 2 years, the survival probability was 11.7% in the InO arm and 2.6% in the SoC arm when data were censored for HSCT.
Figure 8.
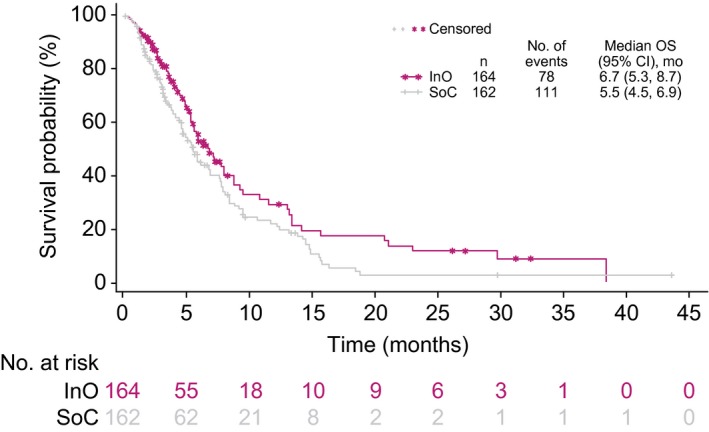
OS censored at the date of any HSCT. Patients were censored at the time of any HSCT, regardless of whether they were in continuous first complete remission. CI indicates confidence interval; HSCT, hematopoietic stem cell transplantation; InO, inotuzumab ozogamicin; OS, overall survival; SoC, standard of care (intensive chemotherapy).
Among patients who underwent follow‐up HSCT, 53 of 79 (67.1%) in the InO arm and 25 of 36 (69.4%) in the SoC arm died. The cause of death was not adjudicated, and investigators could list more than 1 cause on the case report form. In this subset of patients, active disease (relapse or disease progression) was recorded as the cause of death in 20 of 53 patients (37.7%) in the InO arm and in 17 of 36 patients (68.0%) in the SoC arm. Study treatment toxicity was reported as the cause in 6 of 53 patients (11.3%) in the InO arm (5 due to VOD and 1 due to multiorgan failure concomitant with grade 4 VOD/SOS) and in none in the SoC arm. Deaths due to other reasons were reported for 29 of 53 patients (54.7%) in the InO arm and for 10 of 25 patients (40.0%) in the SoC arm. The most frequent causes of death due to other reasons in the InO arm were infection (10 of 29 [34.5%]), respiratory disorders/failures (6 of 29 [20.7%]), toxicity from nonstudy ALL treatment (4 of 29 [13.8%]), cardiac disorders (4 of 29 [13.8%]), and graft‐versus‐host disease (4 of 29 [13.8%]). The most frequent causes of death due to other reasons in the SoC arm were infection (4 of 10 [40.0%]) and toxicity from ALL treatment other than the study drug (2 of 10 [20.0%]).The cause of death was recorded as unknown for 1 patient in the InO arm.
Patient characteristics associated with improved OS
When patients were categorized according to OS (< or ≥2 years), the baseline characteristics remained similar between the treatment arms (Supporting Table 2). Within the InO arm, 36 patients had OS ≥2 years, and 128 had OS <2 years. Patients with OS ≥2 years were more likely than patients with OS <2 years to be younger (median, 34.0 vs 49.0 years), white (88.9% vs 62.5%), and in the first salvage therapy setting (77.8% vs 64.8%). They were also more likely to have a baseline Eastern Cooperative Oncology Group performance status of 0 (61.1% vs 31.3%), a duration of first remission ≥12 months (61.1% vs 35.9%), a peripheral blast count of 0 cells/μL (66.7% vs 36.7%), bone marrow blasts <50% (41.7% vs 29.7%), and baseline CD22 leukemic blast positivity ≥90% (88.9% vs 58.6%). Among the 36 InO patients with a survival duration ≥2 years, 30 (83.3%) underwent HSCT during follow‐up.
Safety
In the safety population, patients in the InO arm more often had dose reductions due to AEs (3.0% vs 2.1% for SoC), temporary discontinuations due to AEs (43.9% vs 11.9%), and permanent discontinuations due to AEs (18.9% vs 7.7%). Although patients in the InO arm received more treatment cycles, rates of treatment‐emergent AEs were similar between the treatment arms (2023 and 2112 events in total for the respective groups), and most patients had 1 or more treatment‐emergent AEs (Supporting Table 3).
A serious AE was reported for 85 of the 164 InO patients (51.8%) and for 72 of the 143 SoC patients (50.3%; Table 4). In both treatment arms, the most frequent all‐grade and grade 3 or higher AEs were hematologic (Supporting Table 3), with the most frequent grade 3 or higher AEs in the InO arm being neutropenia (47%), thrombocytopenia (41%), leukopenia (27%), and febrile neutropenia (27%) and with the most frequent grade 3 or higher AEs in the SoC arm being thrombocytopenia (59%), febrile neutropenia (54%), neutropenia (44%), and anemia (44%). In comparison with the SoC arm, fewer patients in the InO arm received platelet transfusions (64.6% vs 97.2%; median transfusion duration, 7 days [range, 1‐51 days] vs 7 days [range, 1‐30 days]) and packed red blood cell transfusions (61.6% vs 96.5%; median transfusion duration, 3 days [range, 1‐28 days] vs 5 days [range, 1‐20 days]). In cycle 1, 124 of the 164 patients (75.6%) in the InO arm and 135 of the 143 patients (94.4%) in the SoC arm were hospitalized (median length of stay, 12 days [range, 1‐56 days] for InO vs 26 days [range, 1‐55 days] for SoC). As specified in the analysis plan, AEs were assessed across all cycles and unadjusted for the number of cycles; however, a post hoc analysis of the most frequent grade 3 or higher AEs by maximum cycle started showed that hematologic AEs were still the most frequent (Supporting Table 4).
Table 4.
Incidence of Treatment‐Emergent Serious Adverse Events
| Serious Adverse Event | InO (n = 164), No. (%) | SoC (n = 143), No. (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Any Grade | Grade ≥ 3 | Grade 3 | Grade 4 | Grade 5 | Any Grade | Grade ≥3 | Grade 3 | Grade 4 | Grade 5 | |
| Any | 85 (51.8) | 80 (48.8) | 37 (22.6) | 17 (10.4) | 26 (15.9) | 72 (50.3) | 71 (49.7) | 34 (23.8) | 21 (14.7) | 16 (11.2) |
| Febrile neutropenia | 19 (11.6) | 19 (11.6) | 16 (9.8) | 3 (1.8) | 0 (0) | 27 (18.9) | 27 (18.9) | 20 (14.0) | 7 (4.9) | 0 (0) |
| Veno‐occlusive liver disease | 23 (14.0) | 19 (11.6) | 8 (4.9) | 6 (3.7) | 5 (3.0) | 3 (2.1)a | 3 (2.1) | 3 (2.1) | 0 (0) | 0 (0) |
| Sepsis | 4 (2.4) | 4 (2.4) | 0 (0) | 2 (1.2) | 2 (1.2) | 10 (7.0) | 10 (7.0) | 1 (0.7) | 7 (4.9) | 2 (1.4) |
| Disease progression | 8 (4.9) | 8 (4.9) | 0 (0) | 0 (0) | 8 (4.9) | 5 (3.5) | 5 (3.5) | 0 (0) | 0 (0) | 5 (3.5) |
| Pneumonia | 10 (6.1) | 9 (5.5) | 5 (3.0) | 1 (0.6) | 3 (1.8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Respiratory failure | 2 (1.2) | 2 (1.2) | 1 (0.6) | 1 (0.6) | 0 (0) | 6 (4.2) | 6 (4.2) | 0 (0) | 3 (2.1) | 3 (2.1) |
| Pyrexia | 5 (3.0) | 2 (1.2) | 2 (1.2) | 0 (0) | 0 (0) | 3 (2.1) | 1 (0.7) | 0 (0) | 1 (0.7) | 0 (0) |
| Neutropenic sepsis | 3 (1.8) | 3 (1.8) | 1 (0.6) | 1 (0.6) | 1 (0.6) | 4 (2.8) | 4 (2.8) | 1 (0.7) | 3 (2.1) | 0 (0) |
| Septic shock | 3 (1.8) | 3 (1.8) | 1 (0.6) | 1 (0.6) | 1 (0.6) | 3 (2.1) | 3 (2.1) | 1 (0.7) | 1 (0.7) | 1 (0.7) |
| Fungal pneumonia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (2.1) | 3 (2.1) | 3 (2.1) | 0 (0) | 0 (0) |
| Hyperbilirubinemia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (2.1) | 3 (2.1) | 2 (1.4) | 1 (0.7) | 0 (0) |
| Subdural hematoma | 1 (0.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (2.1) | 3 (2.1) | 2 (1.4) | 1 (0.7) | 0 (0) |
| Hypotension | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (2.1) | 2 (1.4) | 0 (0) | 2 (1.4) | 0 (0) |
Abbreviations: InO, inotuzumab ozogamicin; SoC, standard of care (intensive chemotherapy).
The data represent the safety population from the January 4, 2017, data cutoff. Serious adverse events with an incidence ≥2% in either of the treatment arms are shown. Adverse events were graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (version 3.0).
A clinical site visit conducted in July 2017 (after the clinical database had been locked) confirmed that a fourth case of veno‐occlusive liver disease/sinusoidal obstruction syndrome had occurred in a patient in the SoC arm. This case occurred in March 2013 (~3 months after the patient received the last dose of the study drug treatment), was not entered onto the case report form, and, therefore, is not included.
Hepatotoxicity
Hepatotoxicity AEs were more frequent in the InO arm (50.6% vs 36.4% in the SoC arm). VOD/SOS was reported during or after treatment (including after post‐HSCT follow‐up) in 23 of the 164 patients (14.0%) in the InO arm and in 3 of the 143 patients (2.1%) in the SoC arm. Fatal events in the ITT population that underwent transplantation are discussed previously. In the safety set, 79 of 164 patients and 35 of 143 patients proceeded to HSCT; among these patients, 18 of 79 InO patients (22.8%) developed VOD/SOS (including the 5 patients discussed earlier who had fatal events), whereas 3 of 35 SoC patients (8.6%) did. Of the 5 patients who experienced VOD/SOS during InO treatment but did not have an intervening HSCT, 2 had undergone HSCT before InO treatment, and 2 were older (74 and 76 years at enrollment). Among the 18 patients in the InO arm who experienced post‐HSCT VOD/SOS, 5 had also undergone at least 1 allogeneic HSCT before InO treatment. In the multivariate analysis (all P values 2‐sided), factors associated with increased VOD/SOS risk after HSCT in InO‐treated patients were conditioning regimens containing dual alkylators (odds ratio [OR], 8.61; P = .015), bilirubin levels at or above the upper limit of normal range at the last measurement before conditioning therapy (OR, 7.08; P = .011) or at the last measurement before follow‐up HSCT (OR, 15.31; P = .009), and prior HSCT (OR, 6.02; P = .032). In the univariate analysis, an age ≥55 years (OR, 3.25; 2‐sided P = .048) and the number of treatment cycles received (OR, 1.56; 2‐sided P = .048) were also identified as risk factors but did not remain significant in the multivariate analysis.
Deaths
Overall, 131 of the 164 patients (79.9%) in the InO arm and 126 of the 143 patients (88.1%) in the SoC arm died, with ALL being the most frequent cause in both arms and more frequent in the SoC arm (80 [48.8%] vs 100 [69.9%], respectively). A fatal study treatment toxicity was reported in 8 InO arm patients (4.9%; 5 due to VOD/SOS, 1 due to multiorgan failure concomitant with grade 4 VOD/SOS after poststudy HSCT, 1 due to acute respiratory distress syndrome [with grade 4 pneumonia ongoing at the time of death], and 1 due to pneumonia) and in 2 SoC arm patients (1.4%; 1 due to multiorgan failure with cytarabine and fludarabine treatment and 1 due to a pulmonary infection and respiratory failure). Of deaths not due to the disease under study or toxicity related to the study treatment, infections were the most common cause: they were listed for 23 of the 164 patients (14.0%) in the InO arm and for 13 of the 143 patients (9.1%) in the SoC arm.
Discussion
This analysis of the INO‐VATE trial contributes the longest follow‐up reported to date for this class of agents. Our findings can be considered only exploratory because the primary analysis has been previously reported,13 yet the length of follow‐up provides additional valuable information: we observed a statistically significant and clinically meaningful improvement in PFS with InO versus SoC as well as a 25% lower risk of death. Consistent with the PFS data, the OS improvement with InO was more pronounced at later time points (≥2 years) and after HSCT, which was mostly allogeneic. An exploratory RMST analysis confirmed that the observed difference was clinically meaningful and showed a significant gain in RMST of 4.32 months for InO. RMST is usually expected to yield more conservative estimates than hazard ratios and should be robust in our case because the method does not depend on proportional hazards or indeed any other assumptions to compare treatments.16
It is noteworthy that the median OS of 6.2 months in the SoC arm was longer than the expected median of 4.3 months, and this may have been due to the impact of subsequent salvage therapies administered to these patients, including InO (by compassionate access or by enrollment in a different clinical trial), blinatumomab, TKIs, and CAR‐T therapy. Because of the lower response rate in the SoC arm, patients often received subsequent induction/salvage therapy and were more likely to be treated with targeted agents. A post hoc sensitivity analysis exploring the possible impact of salvage therapies showed that OS was significantly longer in the InO arm than the SoC arm when the analysis was censored at the use of the first salvage therapy (HR, 0.71; P = .0062; median, 7.7 vs 5.3 months). Although this type of analysis removes subsequent relapses, it should mitigate the potentially confounding influence of salvage therapies, and the results appear to suggest that salvage therapies may have contributed to OS in the control arm. The reduced OS in the control arm in this censored analysis suggests that in the overall analysis of OS (uncensored for first salvage therapy subsequent to randomization), some SoC patients did benefit from use of these therapies (blinatumomab, TKIs, CAR‐T, or InO), and this possibly diluted the true benefit of InO versus SoC. This was further supported by an analysis of Ph+ patients, for whom TKI inhibitors are available, and it appeared that outcomes in this subgroup may have been affected by this salvage therapy (used in 3 patients in the InO arm vs 10 in the SoC arm). When Ph+ patients were excluded from the OS analysis, the difference between InO and SoC was greater, and this again suggested that the overall analyses overestimated the benefit of chemotherapy alone.
This study included a fairly wide range of patients, and analyses of OS and CR/CRi supported a benefit from InO treatment for most subgroups (eg, subgroups by the stratification factors of duration of first remission, salvage status, and age). No benefit was detected in those with the t(4;11) karyotype, although the analysis was limited by the low number of patients. Predictors of improved OS seemed to be those often associated with improved OS during ALL therapy. This was also true for the characteristics more often seen in the patients with OS ≥2 years in our study, such as younger age and being in the first salvage therapy setting.
Regardless of the treatment group, undergoing follow‐up HSCT was a significant predictor of OS. In INO‐VATE, HSCT was undertaken at the investigator’s discretion, and we must bear in mind that circumstances outside the trial’s control may have come into play because patients are selected for HSCT on the basis of factors such as age, comorbid disease, and donor availability. Nevertheless, it appeared that InO served as an effective bridge to HSCT: nearly 4 times as many InO‐treated patients proceeded directly to HSCT before the start of any follow‐up induction therapy. This must be considered a key result because of the improved long‐term survival associated with HSCT in R/R ALL.6, 20 Patients treated with InO who proceeded to HSCT had a median OS of 12.6 months versus 7.1 months for those who did not (overall HR, 0.55; P = .0065). Predictors of improved OS after HSCT included the patient’s MRD status, baseline platelet counts, duration of first remission, and whether the patient achieved CR/CRi during study therapy. However, after censoring for HSCT, the InO arm had significantly longer OS than the SoC arm (HR, 0.70; P = .0095; median, 6.7 vs 5.5 months), and this suggested that improved OS was not solely explained by more patients proceeding to HSCT.
In agreement with previous reports, the most frequent AEs in both treatment arms were hematologic. In comparison with the SoC arm, grade 3 or higher thrombocytopenia, anemia, and febrile neutropenia were all less frequent in the InO arm; in contrast, hepatic AEs such as VOD/SOS were more frequent. Of the 18 cases of VOD/SOS in patients treated with InO, 5 died, as has been previously reported in detail.14 Our analyses of factors increasing VOD risk were limited to some degree by small patient numbers; however, conditioning regimens containing dual alkylators, bilirubin levels greater than or equal to the upper limit of normal when tested before conditioning therapy or before follow‐up HSCT, and prior HSCT were associated with an increased risk of post‐HSCT VOD/SOS in the multivariate analysis, and an age ≥55 years and an increasing number of treatment cycles were associated with increased risk of post‐HSCT VOD/SOS in the univariate analysis. It is worth considering that, in this study, VOD was assessed by investigators, and a separate analysis of VOD events by an independent hepatic events adjudication board found that few of these events had a definite diagnosis.19 Nevertheless, as previously recognized,14 potential risk factors for the development of VOD/SOS must be carefully considered when one is making treatment decisions regarding InO, and procedures should be implemented to minimize the risk of post‐HSCT VOD/SOS, such as avoiding hepatotoxic conditioning agents.
In conclusion, these results confirm earlier findings from INO‐VATE that InO can be used to achieve high remission (CR/CRi) and MRD‐negative rates, serving as an effective bridge to HSCT, and it is associated with increased OS and PFS in patients with R/R BCP ALL in comparison with SoC.13 All key patient subgroups appeared to benefit from InO, and patient selection for therapy might, therefore, be focused instead on avoiding risk for VOD. On the basis of these results, the next rational step would be combining InO with chemotherapy, and pilot studies using such combinations in salvage and elderly frontline ALL are already showing promise.21, 22
Funding Support
This study was sponsored by Pfizer, Inc.
Conflict of Interest Disclosures
Hagop M. Kantarjian declares honoraria from AbbVie, Actinium, Agios, Amgen, ImmunoGen, Orsinex, Pfizer, and Takeda and research support from AbbVie, Agios, Amgen, ARIAD, Astex, Bristol‐Myers Squibb, Cyclacel, Daiichi‐Sankyo, ImmunoGen, Jazz Pharmaceuticals, Novartis, and Pfizer. Daniel J. DeAngelo declares honoraria from Amgen, ARIAD, Bristol‐Myers Squibb, Incyte, Novartis, and Pfizer and research support from ARIAD. Matthias Stelljes declares research support and honoraria from Pfizer. Michaela Liedtke declares honoraria from Pfizer. Wendy Stock declares honoraria from Agios, Astellas, Jazz Pharmaceuticals, and Kite. Nicola Gökbuget declares research support and honoraria from Amgen, Novartis, and Pfizer and honoraria from Celgene. Susan M. O’Brien declares honoraria from Pfizer. Elias Jabbour declares research support and consulting fees from AbbVie, Amgen, Pfizer, and Takeda. Tao Wang, Jane Liang White, Barbara Sleight, and Erik Vandendries are employees of and own stock in Pfizer. Anjali S. Advani declares consulting fees and honoraria from Pfizer.
Author Contributions
Hagop M. Kantarjian: Conceptualization and writing–review and editing. Daniel J. DeAngelo: Conceptualization and writing–review and editing. Matthias Stelljes: Conceptualization and writing–review and editing. Michaela Liedtke: Conceptualization and writing–review and editing. Wendy Stock: Conceptualization and writing–review and editing. Nicola Gökbuget: Conceptualization and writing–review and editing. Susan M. O’Brien: Conceptualization and writing–review and editing. Elias Jabbour: Conceptualization and writing–review and editing. Tao Wang: Conceptualization, formal analysis, and writing–review and editing. Jane Liang White: Conceptualization, formal analysis, and writing–review and editing. Barbara Sleight: Conceptualization and writing–review and editing. Erik Vandendries: Conceptualization, formal analysis, and writing–review and editing. Anjali S. Advani: Conceptualization and writing–review and editing.
Data Availability
Upon request and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de‐identified participant data from Pfizer‐sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices 1) for indications that have been approved in the United States and/or the European Union or 2) in programs that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de‐identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Supporting information
This study is registered at ClinicalTrials.gov (NCT01564784).
Medical writing support was provided by David Wateridge, PhD, and Geraldine Thompson (Engage Scientific Solutions, Horsham, United Kingdom) and was funded by Pfizer.
References
- 1. Tavernier E, Boiron JM, Huguet F, et al. Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA‐94 trial. Leukemia. 2007;21:1907‐1914. [DOI] [PubMed] [Google Scholar]
- 2. O’Brien S, Thomas D, Ravandi F, et al. Outcome of adults with acute lymphocytic leukemia after second salvage therapy. Cancer. 2008;113:3186‐3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gokbuget N, Stanze D, Beck J, et al. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood. 2012;120:2032‐2041. [DOI] [PubMed] [Google Scholar]
- 4. Kantarjian H, Stein A, Gokbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376:836‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B‐cell lymphoma. N Engl J Med. 2019;380:45‐56. [DOI] [PubMed] [Google Scholar]
- 6. Fielding AK, Richards SM, Chopra R, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109:944‐950. [DOI] [PubMed] [Google Scholar]
- 7. Yurkiewicz IR, Muffly L, Liedtke M. Inotuzumab ozogamicin: a CD22 mAb‐drug conjugate for adult relapsed or refractory B‐cell precursor acute lymphoblastic leukemia. Drug Des Devel Ther. 2018;12:2293‐2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DeAngelo DJ, Stock W, Stein AS, et al. Inotuzumab ozogamicin in adults with relapsed or refractory CD22‐positive acute lymphoblastic leukemia: a phase 1/2 study. Blood Adv. 2017;1:1167‐1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. DiJoseph JF, Armellino DC, Boghaert ER, et al. Antibody‐targeted chemotherapy with CMC‐544: a CD22‐targeted immunoconjugate of calicheamicin for the treatment of B‐lymphoid malignancies. Blood. 2004;103:1807‐1814. [DOI] [PubMed] [Google Scholar]
- 10. Shor B, Gerber HP, Sapra P. Preclinical and clinical development of inotuzumab‐ozogamicin in hematological malignancies. Mol Immunol. 2015;67:107‐116. [DOI] [PubMed] [Google Scholar]
- 11. Boue DR, LeBien TW. Expression and structure of CD22 in acute leukemia. Blood. 1988;71:1480‐1486. [PubMed] [Google Scholar]
- 12. Haso W, Lee DW, Shah NN, et al. Anti‐CD22–chimeric antigen receptors targeting B‐cell precursor acute lymphoblastic leukemia. Blood. 2013;121:1165‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375:740‐753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kantarjian HM, DeAngelo DJ, Advani AS, et al. Hepatic adverse event profile of inotuzumab ozogamicin in adult patients with relapsed or refractory acute lymphoblastic leukaemia: results from the open‐label, randomised, phase 3 INO‐VATE study. Lancet Haematol. 2017;4:e387‐e398. [DOI] [PubMed] [Google Scholar]
- 15. Royston P, Parmar MK. The use of restricted mean survival time to estimate the treatment effect in randomized clinical trials when the proportional hazards assumption is in doubt. Stat Med. 2011;30:2409‐2421. [DOI] [PubMed] [Google Scholar]
- 16. Trinquart L, Jacot J, Conner SC, Porcher R. Comparison of treatment effects measured by the hazard ratio and by the ratio of restricted mean survival times in oncology randomized controlled trials. J Clin Oncol. 2016;34:1813‐1819. [DOI] [PubMed] [Google Scholar]
- 17. Uno H, Claggett B, Tian L, et al. Moving beyond the hazard ratio in quantifying the between‐group difference in survival analysis. J Clin Oncol. 2014;32:2380‐2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Uno H, Wittes J, Fu H, et al. Alternatives to hazard ratios for comparing the efficacy or safety of therapies in noninferiority studies. Ann Intern Med. 2015;163:127‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McDonald GB, Freston JW, Boyer JL, DeLeve LD. Liver complications following treatment of hematologic malignancy with anti‐CD22–calicheamicin (inotuzumab ozogamicin). Hepatology. 2019;69:831‐844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oliansky DM, Larson RA, Weisdorf D, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of adult acute lymphoblastic leukemia: update of the 2006 evidence‐based review. Biol Blood Marrow Transplant. 2012;18:18‐36. [DOI] [PubMed] [Google Scholar]
- 21. Jabbour E, Pui CH, Kantarjian H. Progress and innovations in the management of adult acute lymphoblastic leukemia. JAMA Oncol. 2018;4:1413‐1420. [DOI] [PubMed] [Google Scholar]
- 22. Kantarjian H, Ravandi F, Short NJ, et al. Inotuzumab ozogamicin in combination with low‐intensity chemotherapy for older patients with Philadelphia chromosome–negative acute lymphoblastic leukaemia: a single‐arm, phase 2 study. Lancet Oncol. 2018;19:240‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon request and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de‐identified participant data from Pfizer‐sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices 1) for indications that have been approved in the United States and/or the European Union or 2) in programs that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de‐identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.


