Abstract
Background
Neuromodulation is a promising treatment modality for tinnitus, especially in chronic and severe cases. The auditory thalamus plays a key role in the pathophysiology of tinnitus, as it integrates and processes auditory and limbic information.
Objective
The effect of high frequency stimulation and low frequency stimulation of the medial geniculate bodies on tinnitus in a noise‐induced tinnitus rat model is assessed.
Materials and Methods
Presence of tinnitus was verified using the gap‐induced prepulse inhibition of the acoustic startle response paradigm. Hearing thresholds were determined before and after noise trauma with auditory brainstem responses. Anxiety‐related side‐effects were evaluated in the elevated zero maze and open field.
Results
Results show tinnitus development after noise exposure and preserved hearing thresholds of the ear that was protected from noise trauma. We found that high frequency stimulation of the medial geniculate bodies suppressed tinnitus. This effect maintained directly after stimulation when the stimulator was turned off. Low frequency stimulation did not have any effects on the gap:no‐gap ratio of the acoustic startle response.
Conclusion
High frequency stimulation of the MGB has a direct and residual suppressing effect on tinnitus in this animal model. Low frequency stimulation of the MGB did not inhibit tinnitus.
Keywords: Deep brain stimulation, medial geniculate body, neuromodulation, preclinical, tinnitus
Introduction
Hearing a sound in absence of an audible source is commonly defined as tinnitus. Currently, 2.4% of the general population suffer from the most severe form of tinnitus, which is often associated with sleeping disorders, anxiety and depression 1, 2. Even cases of suicide and euthanasia have been reported as a result of the disorder 3, 4. This chronic disorder has a high economic burden on society, illustrated by the substantial mean annual health care and productivity costs per patient in The Netherlands (€1544 and €3702, respectively) 5. Although collaborative efforts between multiple specialties have resulted in new therapeutic approaches, tinnitus treatment remains a challenge 6.
The hypothesis that tinnitus results from pathological increased neural activity in auditory brain structures, most commonly triggered by peripheral input loss, is now widely accepted 7. Several models that propose mechanisms leading to tinnitus have been described. Homeostatic plasticity might play an important role. Reduced auditory input results in an increased central neural gain to maintain mean firing rates 8, 9. Furthermore, breakdown of sensory gating at the level of the medial geniculate body (MGB) of the thalamus might occur 10, 11, 12. A reduced physiological inhibition could lead to an excitatory/inhibitory imbalance in auditory nuclei, resulting in a phantom auditory sensation 13, 14. In a computational model, stochastic resonance is proposed as an underlying mechanism in tinnitus development. Signals are lifted above threshold in order to let them be detected. To facilitate this, hyperactivity is crucial. Through short‐ and long‐term plasticity, this leads to a phantom sound 15.
The main neural correlates of tinnitus that are observed within the auditory pathway in human and animal studies are increased neural synchrony, tonotopic map changes and increased spontaneous firing 13, 16, 17. Specifically within the MGB, increased spontaneous firing and bursting, and tonotopic reorganization have been described in animals with cochlear damage due to sound exposure, lesioning or ototoxic agents 18, 19, 20. Besides structures of the auditory pathway, limbic areas play an important role in tinnitus pathophysiology and explain emotional and attentional symptoms of tinnitus 12.
Deep brain stimulation (DBS) has been proposed as a promising treatment option in severe, refractory tinnitus 21, 22. Several reports on the effect of DBS in tinnitus have already been reported in humans 23, 24, 25. However, these are case reports, case series and retrospective data. Preclinical studies have shown that DBS of the dorsal cochlear nucleus and inferior colliculus, both auditory structures, resulted in a reduction of tinnitus behavior in rats 26, 27. Of the structures in the hyperactive brain network in tinnitus, the MGB is especially promising as a target for DBS 22, 28. The MGB of the thalamus has an essential gating and shaping function of sensory information and plays a key role in the auditory pathway. At the level of the MGB, limbic and auditory information are integrated. Ascending inputs of the MGB project further to auditory cortices and limbic areas. Descending neurons from auditory and nonauditory cortices contribute to thalamo‐cortical loops and further connect to limbic areas, the reticular nucleus and project via the MGB down to the inferior colliculus and dorsal cochlear nucleus 29, 30. The MGB is part of the thalamus and anatomically accessible using stereotaxy. Furthermore, because the MGB connects the auditory pathway with limbic structures, DBS might alleviate tinnitus loudness as well as the distress accompanied by this phantom sound 22, 31. The exact working mechanism of DBS remains controversial 32, 33, 34. High frequency stimulation (HFS) may cause soma inhibition, in combination with axonal activation 35 and a general hypothesis is that HFS has a complete network effect 31, 36. Clinically, HFS mimics a lesioning effect 36, 37. Small patient studies have shown a positive effect of thalamic ablation on tinnitus 38. Therefore, we hypothesized that HFS of the MGB results in tinnitus suppression.
Here, we investigated the effects of both HFS and low frequency stimulation (LFS) of the MGB on tinnitus perception. To this aim, a rat model of noise‐induced tinnitus was used. Auditory brainstem responses (ABRs) were measured before and after noise trauma. Gap prepulse inhibition of the acoustic startle (GPIAS) response paradigm was used for tinnitus assessment. To test for undesired side‐effects, anxiety and general locomotor activity were evaluated with the open field (OF) and elevated zero maze (EZM).
Materials and Methods
Subjects
Eleven male Sprague Dawley rats (Charles River, Sulzfeld, Germany) were included, weighing approximately 350 g at time of surgery. All animals were housed individually in standard MakrolonTM cages (Central Animal Facility of Maastricht University, Maastricht, The Netherlands) to prevent damage to or luxation of the electrode construct. Animals were housed in an air‐ventilated room under a reversed 12/12 h light dark cycle with a constant room temperature of 20–22°C and a humidity of 60–70%. Standard laboratory chow and water was available ad libitum. All experiments were conducted within the dark period of the day. The experimental protocol was approved by the Animal Experiments and Ethics Committee of Maastricht University. A within subject design was used in order to reduce the error variance, thereby minimizing the number of animals needed.
Overview of Study and Experimental Design
All animals underwent surgery at the beginning of the experiment. Tinnitus was induced by unilateral noise exposure. ABRs were measured before and after noise exposure to estimate hearing levels. The GPIAS response paradigm was used to assess tinnitus perception during four main conditions: 1) baseline stimulation off; 2) baseline stimulation on (HFS); 3) post noise trauma stimulation off; 4) post noise trauma stimulation on, with three different stimulation paradigms. To control for possible confounding (order) effects, measurements were conducted following an incomplete counterbalanced measured design. A schematic overview of the experimental procedures and assessments is shown in Figure 1.
Figure 1.
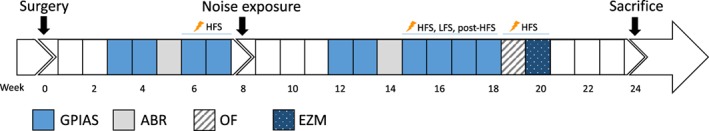
Timeline of the experimental procedures. ABR, auditory brainstem response recordings; EZM, elevated zero maze; GPIAS, gap‐prepulse inhibition of the acoustic startle reflex paradigm for tinnitus assessment; HFS, high frequency stimulation; LFS, low frequency stimulation; post‐HFS, stimulation off after 30 minutes of high frequency stimulation; OF, open field. [Color figure can be viewed at wileyonlinelibrary.com]
Surgical Procedure
General anesthesia was induced with Xylazin (10 mg/kg) and Ketamin (90 mg/kg) i.p. and maintained with Ketamin (60 mg/kg/h). Rats were mounted in a stereotactic frame (Model 51,653; Stoelting, Wood Dale, IL, USA) with a mouth clamp and blunt ear bars in order to prevent damage to the middle ears. DBS electrodes (coaxial gold‐coated with platinum‐iridium inner wire, shaft diameter of 250 µm, tip diameter of approximately 50 µm) were bilaterally implanted in the MGB (AP −5.7 mm, ML +/− 3.9 mm, and DV −6 mm; 39). The recovery period after surgery was two weeks. Details of the surgical procedure are described elsewhere 40.
In addition to the DBS stimulation electrodes, two Teflon‐coated stainless steel wire electrodes with an exposed tip were implanted for ABR measurements. The recording electrode was subcutaneously tunneled and placed behind the right mastoid bone, the reference electrode was located at the vertex and secured with a miniature anchoring screw. Permanent electrodes were used to minimize variability between measurements.
Deep Brain Stimulation
Rats were tested in the following conditions: 1) stimulation off, meaning attachment to the stimulation cable without electric stimulation; 2) HFS, which was HFS at 100 Hz, 60 µs pulse width, and 100 µA amplitude; 3) post‐HFS, same as paradigm 1 but testing was performed following 30 minutes of HFS; 4) LFS with 10 Hz, 60 µs pulse width, and 100 µA amplitude. HFS and LFS were applied continuously, from 15 minutes before until the end of the GPIAS response paradigm. Stimulation parameters were chosen based on results of previous DBS experiments 27, 41, 42. Stimulation was bipolar and monophasic pulses were used. The stimulation cable was connected to a constant‐current isolator (DLS 100; WPI, Berlin, Germany) which was connected to a stimulator (DS8000; WPI, Berlin, Germany).
Tinnitus Induction
All subjects were unilaterally exposed to a 16 kHz octave‐band noise at 115 dB for 90 minutes (Ultrasonic power amplifier and Ultrasonic Dynamic Speaker Vifa [Avisoft Bioacoustics, Berlin, Germany]), under general anesthesia (see protocol above). The contralateral ear was plugged with clay to prevent hearing loss. After acoustic overexposure, the subjects were not tested for three weeks.
Behavioral Testing
Behavioral testing was performed under different stimulation conditions. For GPIAS, subjects were tested for stimulation conditions 1 (stimulation off) and 2 (HFS) at baseline and for stimulation conditions 1 (stimulation off), 2 (HFS), 3 (post‐HFS), and 4 (LFS) after noise exposure. The EZM and OF were performed 10 and 11 weeks after noise trauma respectively. Since only HFS resulted in therapeutic effects, we evaluated the effects of only HFS in these behavioral tasks. Two stimulation conditions were randomly tested: stimulation off (attachment to the cable) and HFS (30 minutes of continuous HFS before and during testing). The two sessions took place on different days, separated by a two‐day washout period.
GPIAS Response Paradigm
Presence of tinnitus was assessed using the gap detection method 27, 43. The tests were performed in a sound‐attenuating chamber. Subjects were placed inside a cylinder made of vertical aluminum bars and polyethylene floor (diameter 17 cm, height 40 cm). The cylinder was placed on a piezo transducer which measured the startle force. A speaker (Ultrasonic Dynamic Speaker Vifa [Avisoft Bioacoustics, Berlin, Germany]) was mounted in the ceiling of the testing chamber, 50 cm above the animal. Sounds were amplified (Ultrasonic power amplifier [Avisoft Bioacoustics, Berlin, Germany]) and calibrated (Bruel & Kjaer 2231 decibel meter with a 4191 microphone). The stimulation cable was connected to a swivel in order to allow rotation of the cable.
Background signals in the startle chamber consisted of broadband noise (BBN), or narrow‐band noise of 10, 12, 16 or 20 kHz at 75 dB. The startle stimulus was a 20 msec long 115 dB peak equivalent sound pressure level (peSPL) BBN burst. In gap‐trials, a silent gap of 50 msec was inserted 100 msec prior to the startle stimulus. Each test condition consisted of 20 trials, half of them being gap‐trials, presented with a random variable stimulus interval of 20 ± 5 sec. The time to test one GPIAS background frequency was ±10 minutes. Prior to every session, each subject acclimatized for five minutes in the startle chamber, followed by ten startle‐trials in order to habituate the startle response. The time for one complete session, including all background frequencies, was approximately 60 minutes. The gap:no‐gap ratio was calculated for every test condition by dividing the amplitude of each gap startle by the corresponding mean of no‐gap startles of that test condition. Responses that contained too disturbing (moving) artifacts were excluded from analysis. For every condition, two GPIAS reflex testing sessions were performed, conducted on separate days. The mean gap:no‐gap ratio of the two sessions for every test condition was used for further analysis. All subjects underwent one complete session at the start of the experiment for habituation to the testing procedure, which was not used for analysis. To evaluate the effect of the stimulation paradigms “post‐HFS” and “LFS,” a shorter GPIAS reflex assessment protocol was used with only 10 and 16 kHz background sounds.
Elevated Zero Maze (EZM)
The EZM is a circular runway with a diameter of 98 cm and a path width of 10 cm, placed 70 cm above floor level and divided into two open and two enclosed parts with 50 cm high side walls. Rats were placed on one of the open parts and tracked for five minutes using the Ethovision tracking software (Ethovision; Noldus Information Technology, Wageningen, The Netherlands). Movements were recorded and the time spent in open and enclosed parts was calculated.
Open Field (OF)
The OF is a Plexiglas square arena of 100 × 100 cm with 40 cm high clear walls and a dark floor divided into a center of 70 × 70 cm with outer surrounding zones of 15 cm width. Subjects were placed in the center of the arena. During the ten minutes trial, the time spent in different zones and the total distance moved was recorded and calculated with Ethovision tracking software (Ethovision; Noldus Information Technology, Wageningen, The Netherlands).
Auditory Brainstem Responses (ABR)
Subjects were tested under anesthesia (see protocol above) in a sound‐attenuating Faraday cage. Cables were connected to the sockets of the permanent electrodes on the head of the animal, the ground electrode was connected to the left front paw. One thousand 5‐msec tone bursts of 10, 12, 16, 20, 24, and 32 kHz and a cos2 rise and fall filter were created with Matlab and presented unilaterally with a frequency of 50 Hz at decreasing intensities from 100 to 0 dB peSPL with steps of 10 dB. The contralateral ear was plugged with clay. Auditory stimuli were calibrated (Bruel & Kjaer 2231 decibel meter with a 4191 microphone) and digitally triggered. ABRs were recorded in LabChart Pro 7 (ADInstruments, Castle Hill, Australia) and raw data were imported into Matlab. The evoked responses were amplified 1,000 times, band‐pass filtered (300–3000 Hz) and averaged. The auditory threshold was defined as the lowest decibel level (peSPL) of the stimuli that produced a distinctive ABR, in which at least two peaks (positive or negative) had to be clearly visible 27.
Tissue Collections and Electrode Verification
At the end of the experiments, all subjects received an overdose of pentobarbital (120–180 mg/kg, i.p.) followed by transcardial perfusion with Tyrode's buffer (0.1M) followed by fixative containing 4% paraformaldehyde, 15% picric acid, and 0.05% glutaraldehyde in 0.1M phosphate buffer (pH 7.6) at 4°C. Brains were removed and postfixed overnight in paraformaldehyde at 4°C and subsequently in 1% NaN3 at 4°C for long‐term storage. Brains embedded in 10% gelatin (Sigma‐Aldrich, Zwijndrecht, The Netherlands) were cut serially on a vibratome into 30 µm thick coronal sections. Sections containing the electrode trajectories were processed for a standard hematoxylin‐eosin staining to evaluate the locations of electrode tips.
Statistical Analyses
Normality was checked by the Shapiro–Wilk test and visual inspection of the outcome distributions using histograms and Q–Q plots. A two‐way repeated measures analysis of variance (ANOVA) was conducted to investigate the effect of the two‐leveled factor “noise trauma” (before and after noise trauma) and the two‐leveled factor “stimulation” (stimulation off and HFS) on GPIAS. Posthoc comparisons between different factor levels were made using two‐tailed paired samples t‐tests for all background frequencies. The effect of the four different stimulation paradigms (stimulation off, HFS, post‐HFS, and LFS) on GPIAS after noise trauma was analyzed with a one‐way repeated measures ANOVA. To assess hearing thresholds after noise trauma a one‐way repeated measures ANOVA was performed. Greenhouse‐Geisser adjustment was applied to correct against sphericity violations. Effects of HFS on OF and EZM outcomes were evaluated with two‐tailed paired samples t‐test. Where multiple comparisons were made, the Bonferroni adjusted p‐values are given. p‐values smaller than 0.05 were considered significant. All calculations were performed with SPSS (version 22.0 for Mac, SPSS, Chicago, IL, USA) and data are presented in mean ± standard error of the mean (SE).
Results
Electrode Localization
All electrode tips were located within the ventral part of the MGB. Exact coordinates of the electrode tips are illustrated in Figure 2. Besides the electrode tracts, no additional tissue damage due to electric stimulation was observed histologically.
Figure 2.
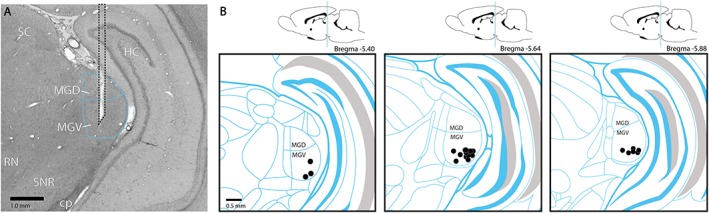
a. Representative example of an electrode trajectory in the medial geniculate body. b. Schematic representation of the electrode sites in the medial geniculate body. The symbol (•) indicates the locations of all electrode tips, shown schematically in one hemisphere. cp, cerebral peduncle; HC, hippocampus; MGD, dorsal part of the medial geniculate body; MGV, ventral part of the medial geniculate body; RN, red nucleus; SC, superior colliculus; SNR, reticular part of substantia nigra. [Color figure can be viewed at wileyonlinelibrary.com]
Auditory Brainstem Responses
The ABR thresholds (Fig. 3) were significantly higher after noise trauma compared to baseline in the ipsilateral (traumatized) side along all frequencies (t 9 = −5.25, p = 0.003, t 9 = −3.85, p = 0.008, t 9 = −9.49, p < 0.001, t 9 = −5.69, p < 0.001, t 9 = −6.82, p < 0.001, t 9 = −11.00, p < 0.001 for 10, 12, 16, 20, 24, and 32 kHz, respectively). Hearing thresholds on the contralateral side were not affected by noise exposure (p > 0.05 for all frequencies). ABR measurements were not successful in one rat due to hardware problems, this rat was therefore excluded from hearing threshold analysis.
Figure 3.
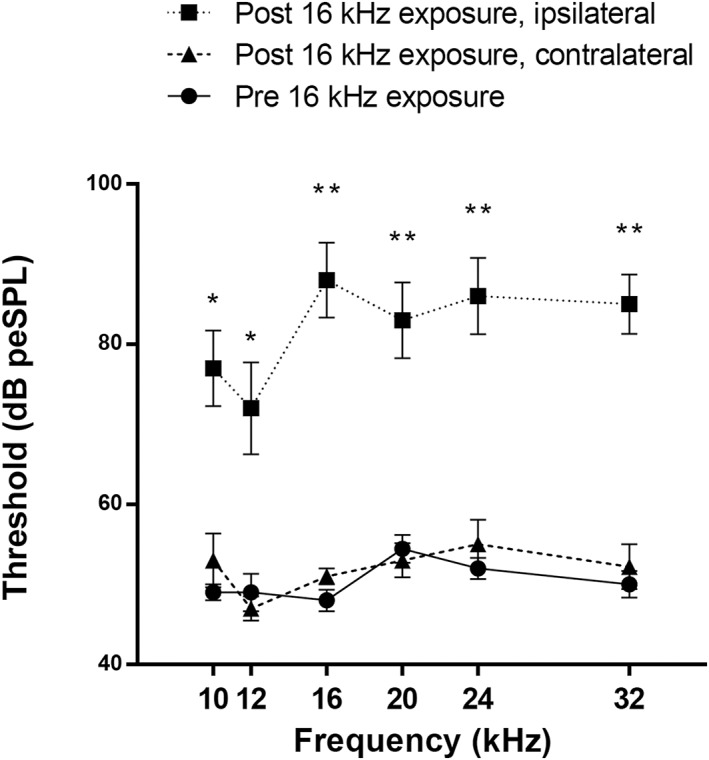
Auditory brainstem responses measured at baseline (round, solid) and after noise exposure in the traumatized ear (square, dotted) and the contralateral side (triangle, dashed). Thresholds are presented as means ± SE. * p < 0.05; ** p < 0.001.
GPIAS Responses
Results of GPIAS response paradigm are shown in Figure 4. Significant main effects have been found for the factors stimulation and noise exposure at 16 kHz background sound (F 1,10 = 22.94, p = 0.001 and F 1,10 = 129.06, p < 0.001) and 20 kHz (F 1,10 = 9.35, p = 0.01 and F 1,10 = 5.36, p = 0.04). Significant interaction was found at BBN (F 1,10 = 7.21, p = 0.02) and 16 kHz background sound (F 1,10 = 76.86, p < 0.001). Since the power of the interaction testmight be insufficient, the simple effects were analyzed for all background sounds. Evaluation of these simple effects (Fig. 4a) showed increased gap:no‐gap ratios after noise exposure compared to baseline off‐stimulation at 16 kHz (mean difference 0.51[0.04], t 10 = −13.64, p < 0.001), indicating a reduced detection of the gap‐in‐noise at this frequency. After noise trauma, the gap:no‐gap ratios decreased during HFS in the 16 kHz background sound (mean difference −0.35[0.05], t 10 = 6.56, p < 0.001) and 20 kHz (−0.15[0.05], t 10 = 3.16, p = 0.04). HFS did not have a significant effect on the gap:no‐gap ratios in the baseline situation at any background frequency.
Figure 4.
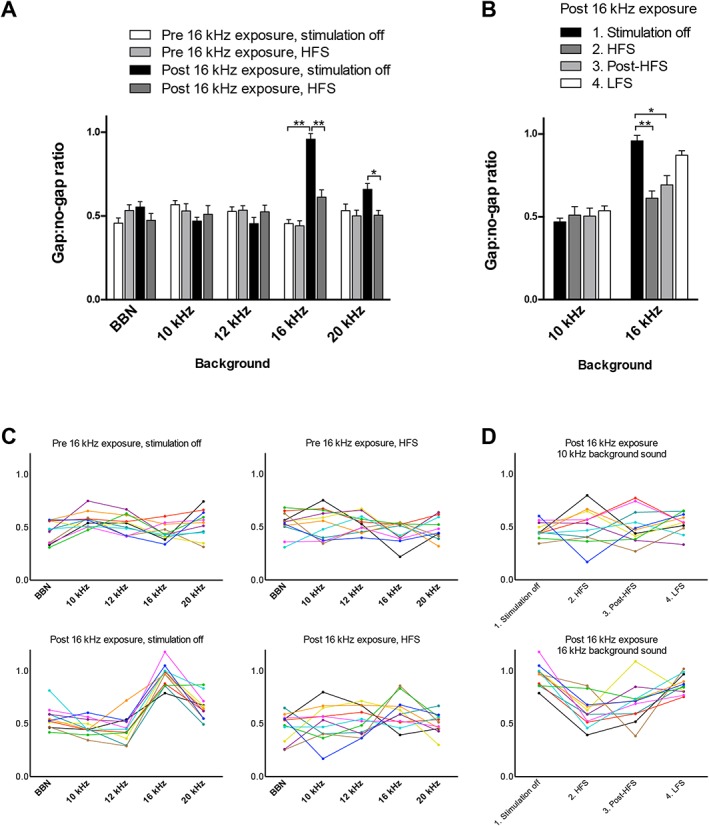
a. Gap‐prepulse inhibition of the acoustic startle reflex paradigm for tinnitus assessment before and after exposure to the 16 kHz tone, during stimulation off and HFS. Notice the reduced effect of the prepulse gap at 16 kHz background sound on the startle response after noise exposure. During HFS the effect of the prepulse gap was restored at 16 kHz and increased at 20 kHz. Gap:no‐gap ratios are presented as means ± SE. Presented significances are simple effects, * p < 0.05; ** p < 0.001. b. Gap‐prepulse of the acoustic startle reflex paradigm for tinnitus assessment at 10 and 16 kHz background sound after 16 kHz exposure. Four different stimulation paradigms were tested: 1) stimulation off; 2) HFS; 3) post‐HFS; 4) LFS. Compared to stimulation off, HFS and post‐HFS significantly increased the effect of the gap prepulse on the acoustic startle response. Gap:no‐gap ratios are presented as means ± SE. * p < 0.05; ** p < 0.001. c. Individual gap:no‐gap ratios before and after exposure to the 16 kHz tone, during stimulation off and HFS. Each colored line represents one subject. d. Individual gap:no‐gap ratios at 10 and 16 kHz background sound after 16 kHz exposure during the four different stimulation paradigms. Each colored line represents one subject. BBN, broadband noise; HFS, high frequency stimulation (100 Hz, 60 μs pulse width, and 100 μA amplitude); LFS, low frequency stimulation (10 Hz, 60 μs pulse width, and 100 μA amplitude); post‐HFS, DBS off after 30 minutes of high frequency stimulation; stimulation off, attached to cable without stimulation. [Color figure can be viewed at wileyonlinelibrary.com]
The effect of the three stimulation paradigms (HFS, post‐HPS, and LFS) compared to stimulation off after noise exposure is illustrated in Figure 4b. There is a significant main effect (F 2.258, 22.585 = 14.73, p < 0.001). The gap:no‐gap ratio significantly decreased at 16 kHz during HFS and post‐HFS (with mean differences of −0.35[0.05], p < 0.001) and −0.26[0.05], p = 0.002, respectively), but not during LFS (mean difference of −0.09[0.05], p = 0.095), indicating an increased detection of the gap during HFS and post‐HFS at this frequency. Stimulation did not have an effect at 10 kHz background sound.
Elevated Zero Maze and Open Field
HFS did not influence the duration spent in the enclosed arms (t 10 = −0.11, p = 0.91) or number of entries in the open arms (t 10 = −0.13, p = 0.90) of the EZM (Fig. 5a). There was neither an effect of HFS on the total distance moved (t 10 = 0.29, p = 0.78) or time spent in corners and/or walls (t 10 = 1.08, p = 0.31) in the OF (Fig. 5b).
Figure 5.
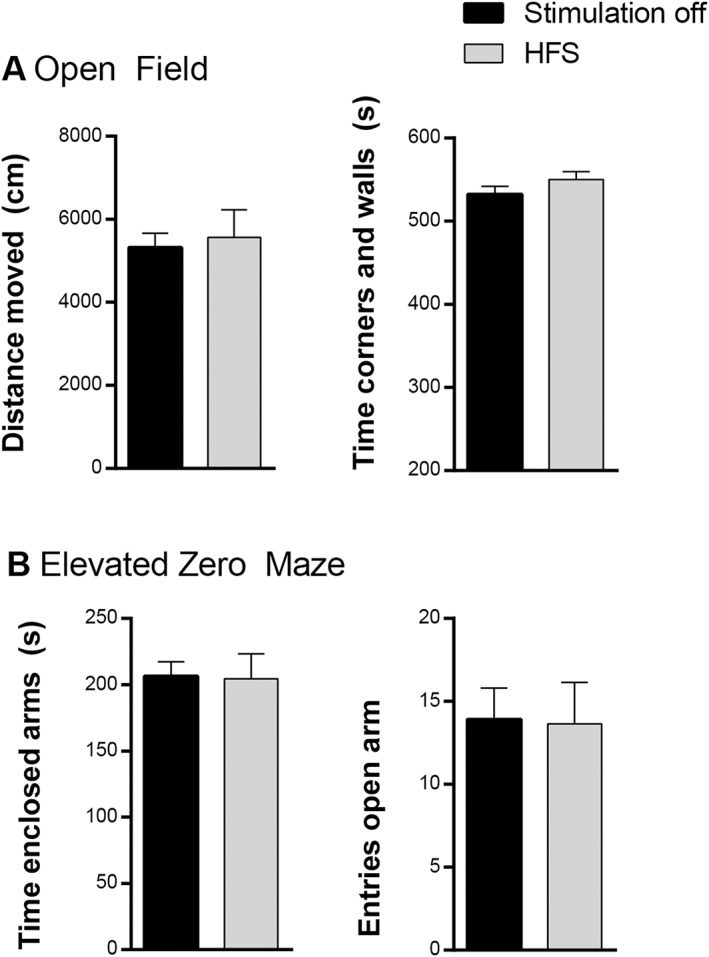
a. Number of entries in the open arm and time spent in the enclosed arms (in sec) of the elevated zero maze. b. Total distance moved (in cm) and time spent in corners and walls (in sec) in the open field. Data are presented as means ± SE. There were no significant differences between high frequency stimulation (HFS) and stimulation off.
Discussion
In this study, we showed that following noise exposure to induce tinnitus, the prepulse gap had a reduced effect on the startle reflex. Bilateral HFS of the MGB restored this effect. These results suggest that tinnitus can be suppressed with HFS of the MGB. This suppression was only seen when stimulating at a frequency of 100 Hz and not at a low frequency of 10 Hz. Moreover, no undesired anxiety‐ or locomotion‐related side‐effects were induced by HFS, assessed with the EZM and OF.
Tinnitus in the Acoustic Trauma Animal Model
We found increased gap:no‐gap ratios in the 16 kHz background sound, which can indicate the presence of tinnitus after unilateral noise trauma. This finding is in accordance with other studies in which a 16 kHz octave‐band noise also leads to a tinnitus pitch around 16 kHz 27, 50. In order to prevent loss of implanted electrodes during attachment of the animals to the stimulation cable, we tested all animals while they could freely move in the startle chamber. Startle amplitudes are greatly dependent on the location of the rat on the platform. In order to correct for the variability, group means of the ratios were used in our statistical analysis. All rats showed tinnitus‐like behavior, as can be seen in the individual plotted data. This is a surprising finding, since other studies did not show tinnitus‐like behavior in all animals 50, 51.
Tinnitus can be assessed in animals using interrogative models or reflexive models 44. Interrogative models rely on auditory perception and are therefore believed to mirror actual perception of tinnitus best. By making use of lick or lever pressing suppression or two‐choice operant conditioning, rats’ perception of certain sounds can be indicated. Training and motivation management is required, and these tests are generally not suitable for longitudinal tinnitus assessment. Reflexive models rely on unconditioned acoustic startle reflexes, which primarily depend on brainstem circuits 44, 45. The GPIAS reflex paradigm is nowadays the most commonly used method for tinnitus assessment in rodents 44, 46. The design of this test makes it possible to assess animals’ tinnitus overtime, which is especially an advantage in our repeated measures design. However, the results obtained by GPIAS test should be carefully interpreted. A relevant limitation of this study is that prepulse inhibition was not performed and absolute startle forces were not obtained. Therefore, it is unsure to what degree the results are influenced by existent hearing loss or hyperacusis in addition to tinnitus 46, 47, 48, 49.
In order to minimize the confounding effect of hearing loss, unilateral noise trauma was applied and hearing thresholds were assessed by ABRs before and after noise trauma. Loss of hearing was found after noise trauma at all frequencies in the traumatized ear, but not in the contralateral ear. While hearing loss was found in all frequency bands, the GPIAS reflex paradigm results only show specific increased gap:no‐gap ratios in the 16 kHz background frequency. This indicates that the confounding effect of hearing loss on the GPIAS response is nonessential. Hyperacusis is another potential confounder. Tinnitus and hyperacusis are coexistent in the majority of patients 52. Preclinical data suggest hyperacusis and tinnitus to be simultaneously induced after intense sound exposure in animals 50, 53. It is likely that etiologies are related 48, 53. However, evidence suggests important differences between the pathophysiological mechanisms in tinnitus and hyperacusis 9. Hyperacusis can increase acoustic startle response amplitudes for high‐intensity sounds (90 dB SPL or higher) and herewith possibly influence gap:no‐gap ratios 48. Although we believe specific 16 kHz increased ratios reflect tinnitus‐like behavior, it remains unsure to what extent possible confounders like hyperacusis influence the GPIAS 54.
In order to increase the statistical power, we chose to use a repeated measures design. The risk of order effects is minimized by applying the different stimulation paradigms randomly with substantial time between the conditions (at least one day between measurements). To minimize a time effect, we waited three weeks with further testing after tinnitus induction. For tinnitus induction, unilateral intense sound exposure was used as this is believed to induce chronic, irreversible symptoms, as opposed to systemic salicylates that have a reversible effect 44, 50, 55.
Tinnitus Suppression With HFS
Effects of HFS stimulation, but not of LFS on gap:no‐gap ratios were found after noise exposure. During HFS the gap:no‐gap ratios significantly decreased at the observed tinnitus pitch 16 kHz and also at 20 kHz frequency bands. Since no effect of HFS on the GPIAS was seen before noise trauma, the effect of stimulation on the GPIAS results is unlikely to be related to interference from electric stimulation.
The hypothesis that specifically HFS of the central auditory pathway might be able to inhibit tinnitus originates from previous studies in Parkinson's disease. The neural correlates associated with Parkinson's disease show similarities with those of tinnitus. In both preclinical and clinical studies, pathological bursting activity and hypersynchrony in the basal ganglia and specifically the subthalamic nucleus is seen in the parkinsonian state 56, 57. In animal models of tinnitus, similar pathological neurophysiological hallmarks have been found in the MGB and other nuclei within the auditory network 7, 13, 16, 58. Multiple working mechanisms of conventional DBS have been hypothesized, but a uniform theory is still lacking. Theories on changes induced by DBS at the synaptic, axonal, dendritic and neuronal soma exist. Moreover, complete network effects have been investigated 31. Conventional DBS at high frequencies leads to inactivation of the neuronal population around the electrode 59. A proposed mechanism for this inactivation is a depolarization block 60. Another mechanism that might play a role, is the stimulation‐induced release of GABA from presynaptic terminals 61. Besides this, the propagation of action potentials in antero‐, retrograde and passing fibers in the vicinity of the electrode is described, which may lead to metabolic and plastic changes in structures at distance and modulate network activity 62. Currently, disrupting signaling is key premise of most hypotheses on HFS mechanisms 32, 63. If HFS is minimal two times higher than the average firing rate of the target neurons, stimulation‐induced action potentials begin to take over and neurons lose the ability to transmit information 64. This “informational lesion” within a circuitopathy, such as described in tinnitus, could eliminate pathological oscillations and normalize neural firing patterns 65, 66, 67. This disruptive effect has not been described in LFS, instead, LFS has shown excitatory actions 68. These concepts are a reasonable but partial explanation why only HFS and not LFS of the MGB suppressed tinnitus in this study. It is an enormous challenge to unravel the complex question on mechanisms of DBS. It would be of interest to test HFS and LFS in the existing computational models on tinnitus development to further understand the possible working mechanism of DBS in tinnitus 8, 15.
Our results suggest a residual effect of HFS of the MGB on tinnitus suppression. After 30 minutes of HFS, the stimulator was switched off and GPIAS measurements were directly conducted (“post‐HFS”). The GPIAS measurement of 16 kHz was finished 10 minutes after electric stimulation was stopped. Mean gap:no‐gap ratios at 16 kHz background sound were significantly lower compared to no stimulation. Further experiments are needed to investigate possible mechanisms and the exact time the remaining effect lasts. Electrophysiological techniques could be used to investigate the changes in spontaneous bursting activity and oscillations within the auditory pathway after electric stimulation of the MGB. A residual effect of electric stimulation has been repeatedly described in other DBS as well as tinnitus studies 25, 69, 70.
Potential Side‐Effects of Stimulation
Limbic and auditory information is integrated at the level of the MGB 10, 71. Therefore, it can be hypothesized that MGB‐HFS could have adverse effects like anxiety. We, however, did not observe any anxiety‐related behavior during HFS with the stimulation parameters used in this study in two different behavioral paradigms. Theoretically, HFS of the MGB can have an effect on motor behavior, since motor nuclei of the thalamus are located adjacent to the MGB. We neither found any locomotion‐related side‐effects in the OF.
Hearing‐related side‐effects are an important concern when electrically stimulating the auditory system 72, 73. The effect of MGB‐HFS on hearing thresholds was not tested in this study. However, in one preclinical study, DBS of the inferior colliculus did not cause impaired hearing thresholds as estimated with sound induced prepulse inhibition 27. Generation of unwanted sounds is another potential side effect. This is illustrated by a case of sudden auditory illusions following a small hemorrhagic infarction in the MGB 74, which lasted for only ten minutes. GPIAS response measurements with HFS before noise trauma did not show significant changes in gap:no‐gap ratios, suggesting that HFS does not cause a continuous tinnitus percept at measured background frequencies. However, the GPIAS paradigm is not a validated tool to draw robust conclusions on this matter.
Conclusion
This study shows first evidence that MGB‐HFS suppresses tinnitus in rats. Altogether, our results suggest that specifically HFS (100 Hz) and not LFS (10 Hz) of the MGB suppresses tinnitus perception without inducing anxiety‐ and locomotion‐related side‐effects in an experimental rat model of tinnitus.
Authorship Statements
Gusta van Zwieten performed the experiments, analyzed the data, and wrote the manuscript. Jasper Smit, Ali Jahanshahi, Robert Stokroos, Yasin Temel, and Gusta van Zwieten designed the study. Milaine Roet assisted in executing experiments. A. Miranda Janssen provided statistical support. Marcus Janssen, Jasper Smit, Ali Jahanshahi, and Yasin Temel provided important intellectual input and critically evaluated versions of the manuscript. All authors have approved the final manuscript. Nobody who qualifies for authorship has been excluded from the list of authors.
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to http://www.wiley.com/WileyCDA/Section/id-301854.html
Source of financial support: This study is financially supported by a grant from the Dutch Heinsius‐Houbolt Foundation.
Conflict of Interest: There are no conflicts of interest to declare.
References
- 1. Axelsson A,Ringdahl A. Tinnitus: a study of its prevalence and characteristics. Br J Audiol 1989;23:53–62. [DOI] [PubMed] [Google Scholar]
- 2. McCormack A,Edmondson‐Jones M,Somerset S,Hall D. A systematic review of the reporting of tinnitus prevalence and severity. Hear Res 2016;337:70–79. [DOI] [PubMed] [Google Scholar]
- 3. Lewis JE,Stephens SD,McKenna L. Tinnitus and suicide. Clin Otolaryngol Allied Sci 1994;19:50–54. [DOI] [PubMed] [Google Scholar]
- 4. Altissimi G,Salviati M,Turchetta R et al. When alarm bells ring: emergency tinnitus. Eur Rev Med Pharmacol Sci 2016;20:2955–2973. [PubMed] [Google Scholar]
- 5. Maes IH,Cima RF,Vlaeyen JW,Anteunis LJ,Joore MA. Tinnitus: a cost study. Ear Hear 2013;34:508–514. [DOI] [PubMed] [Google Scholar]
- 6. Kreuzer PM,Vielsmeier V,Langguth B. Chronic tinnitus: an interdisciplinary challenge. Dtsch Arztebl Int 2013;110:278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eggermont JJ,Roberts LE. The neuroscience of tinnitus. Trends Neurosci 2004;27:676–682. [DOI] [PubMed] [Google Scholar]
- 8. Schaette R,McAlpine D. Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. JNeurosci 2011;31:13452–13457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Knipper M,van Dijk P,Nunes I,Ruttiger L,Zimmermann U. Advances in the neurobiology of hearing disorders: recent developments regarding the basis of tinnitus and hyperacusis. Prog Neurobiol 2013;111:17–33. [DOI] [PubMed] [Google Scholar]
- 10. Rauschecker JP,Leaver AM,Muhlau M. Tuning out the noise: limbic‐auditory interactions in tinnitus. Neuron 2010;66:819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vanneste S,Plazier M,der Loo E,de Heyning PV,Congedo M,De Ridder D. The neural correlates of tinnitus‐related distress. Neuroimage 2010;52:470–480. [DOI] [PubMed] [Google Scholar]
- 12. De Ridder D,Vanneste S,Weisz N et al. An integrative model of auditory phantom perception: tinnitus as a unified percept of interacting separable subnetworks. Neurosci Biobehav Rev 2014;44:16–32. [DOI] [PubMed] [Google Scholar]
- 13. Kaltenbach JA. Tinnitus: models and mechanisms. Hear Res 2011;276:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Ridder D,Vanneste S,Langguth B,Llinas R. Thalamocortical Dysrhythmia: a theoretical update in tinnitus. Front Neurol 2015;6:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krauss P,Tziridis K,Metzner C,Schilling A,Hoppe U,Schulze H. Stochastic resonance controlled upregulation of internal noise after hearing loss as a putative cause of tinnitus‐related neuronal hyperactivity. Front Neurosci 2016;10:597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roberts LE,Eggermont JJ,Caspary DM,Shore SE,Melcher JR,Kaltenbach JA. Ringing ears: the neuroscience of tinnitus. JNeurosci 2010;30:14972–14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Norena AJ,Eggermont JJ. Changes in spontaneous neural activity immediately after an acoustic trauma: implications for neural correlates of tinnitus. Hear Res 2003;183:137–153. [DOI] [PubMed] [Google Scholar]
- 18. Kalappa BI,Brozoski TJ,Turner JG,Caspary DM. Single unit hyperactivity and bursting in the auditory thalamus of awake rats directly correlates with behavioural evidence of tinnitus. JPhysiol 2014;592:5065–5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kamke MR,Brown M,Irvine DR. Plasticity in the tonotopic organization of the medial geniculate body in adult cats following restricted unilateral cochlear lesions. JComp Neurol 2003;459:355–367. [DOI] [PubMed] [Google Scholar]
- 20. Basta D,Gotze R,Groschel M et al. Bilateral changes of spontaneous activity within the central auditory pathway upon chronic unilateral intracochlear electrical stimulation. Otol Neurotol 2015;36:1759–1765. [DOI] [PubMed] [Google Scholar]
- 21. Yong‐bing S,Hal Martin W. Deep brain stimulation—a new treatment for tinnitus. Journal of Otology 2007;2:1–6. [Google Scholar]
- 22. van Zwieten G,Smit JV,Jahanshahi A,Temel Y,Stokroos RJ. Tinnitus: is there a place for brain stimulation? Surg Neurol Int 2016;7:S125–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheung SW,Larson PS. Tinnitus modulation by deep brain stimulation in locus of caudate neurons (area LC). Neuroscience 2010;169:1768–1778. [DOI] [PubMed] [Google Scholar]
- 24. Larson PS,Cheung SW. A stroke of silence: tinnitus suppression following placement of a deep brain stimulation electrode with infarction in area LC. JNeurosurg 2013;118:192–194. [DOI] [PubMed] [Google Scholar]
- 25. Shi Y,Burchiel KJ,Anderson VC,Martin WH. Deep brain stimulation effects in patients with tinnitus. Otolaryngol Head Neck Surg 2009;141:285–287. [DOI] [PubMed] [Google Scholar]
- 26. Luo H,Zhang X,Nation J,Pace E,Lepczyk L,Zhang J. Tinnitus suppression by electrical stimulation of the rat dorsal cochlear nucleus. Neurosci Lett 2012;522:16–20. [DOI] [PubMed] [Google Scholar]
- 27. Smit JV,Janssen ML,van Zwieten G,Jahanshahi A,Temel Y,Stokroos RJ. Deep brain stimulation of the inferior colliculus in the rodent suppresses tinnitus. Brain Res 2016; 1650:118–124. [DOI] [PubMed] [Google Scholar]
- 28. Smit JV,Janssen ML,Schulze H et al. Deep brain stimulation in tinnitus: current and future perspectives. Brain Res 2015;1608:51–65. [DOI] [PubMed] [Google Scholar]
- 29. Rees AP, A.R. The oxford handbook of auditory science, the auditory brain. Vol. 2 New York: Oxford University Press Inc; 2010. [Google Scholar]
- 30. Winer JA. Decoding the auditory corticofugal systems. Hear Res 2005;207:1–9. [DOI] [PubMed] [Google Scholar]
- 31. McIntyre CC,Hahn PJ. Network perspectives on the mechanisms of deep brain stimulation. Neurobiol Dis 2010;38:329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chiken S,Nambu A. Mechanism of deep brain stimulation: inhibition, excitation, or disruption? Neuroscientist 2015;22:313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dostrovsky JO,Lozano AM. Mechanisms of deep brain stimulation. Mov Disord 2002;17:S63–S68. [DOI] [PubMed] [Google Scholar]
- 34. Vitek JL. Mechanisms of deep brain stimulation: excitation or inhibition. Mov Disord 2002;17:S69–S72. [DOI] [PubMed] [Google Scholar]
- 35. McIntyre CC,Grill WM,Sherman DL,Thakor NV. Cellular effects of deep brain stimulation: model‐based analysis of activation and inhibition. JNeurophysiol 2004;91:1457–1469. [DOI] [PubMed] [Google Scholar]
- 36. Benabid AL,Pollak P,Gao D et al. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. JNeurosurg 1996;84:203–214. [DOI] [PubMed] [Google Scholar]
- 37. Benabid AL,Pollak P,Gross C et al. Acute and long‐term effects of subthalamic nucleus stimulation in Parkinson's disease. Stereotact Funct Neurosurg 1994;62:76–84. [DOI] [PubMed] [Google Scholar]
- 38. Jeanmonod D,Magnin M,Morel A. Chronic neurogenic pain and the medial thalamotomy. Schweiz Rundsch Med Prax 1994;83:702–707. [PubMed] [Google Scholar]
- 39. Paxinos GW,Watson C. The rat brain in stereotaxic coordinates: hard cover edition. New York: Academic press; 2006. [Google Scholar]
- 40. Tan S,Vlamings R,Lim L et al. Experimental deep brain stimulation in animal models. Neurosurgery 2010;67:1073; discussion1080. [DOI] [PubMed] [Google Scholar]
- 41. Lim LW,Janssen ML,Kocabicak E,Temel Y. The antidepressant effects of ventromedial prefrontal cortex stimulation is associated with neural activation in the medial part of the subthalamic nucleus. Behav Brain Res 2015;279:17–21. [DOI] [PubMed] [Google Scholar]
- 42. Temel Y,Visser‐Vandewalle V,Aendekerk B et al. Acute and separate modulation of motor and cognitive performance in parkinsonian rats by bilateral stimulation of the subthalamic nucleus. Exp Neurol 2005;193:43–52. [DOI] [PubMed] [Google Scholar]
- 43. Turner JG,Brozoski TJ,Bauer CA et al. Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav Neurosci 2006;120:188–195. [DOI] [PubMed] [Google Scholar]
- 44. Brozoski TJ,Bauer CA. Animal models of tinnitus. Hear Res 2015;338: 88–97. [DOI] [PubMed] [Google Scholar]
- 45. Koch M. The neurobiology of startle. Prog Neurobiol 1999;59:107–128. [DOI] [PubMed] [Google Scholar]
- 46. Galazyuk A,Hebert S. Gap‐prepulse inhibition of the acoustic startle reflex (GPIAS) for tinnitus assessment: current status and future directions. Front Neurol 2015;6:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Heffner HHR. Behavioral tests for tinnitus in animals In: Eggermont JJ, Zheng FG, Popper AN, Fay RR, eds. Springer handbook of auditory research. New York: Springer, 2012. [Google Scholar]
- 48. Hayes SH,Radziwon KE,Stolzberg DJ,Salvi RJ. Behavioral models of tinnitus and hyperacusis in animals. Front Neurol 2014;5:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lobarinas E,Hayes SH,Allman BL. The gap‐startle paradigm for tinnitus screening in animal models: limitations and optimization. Hear Res 2013;295:150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Turner JG,Larsen D. Effects of noise exposure on development of tinnitus and hyperacusis: prevalence rates 12 months after exposure in middle‐aged rats. Hear Res 2016;334:30–36. [DOI] [PubMed] [Google Scholar]
- 51. Pace E,Zhang J. Noise‐induced tinnitus using individualized gap detection analysis and its relationship with hyperacusis, anxiety, and spatial cognition. PLoS One 2013;8:e75011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dauman R,Bouscau‐Faure F. Assessment and amelioration of hyperacusis in tinnitus patients. Acta Otolaryngol 2005;125:503–509. [DOI] [PubMed] [Google Scholar]
- 53. Chen G,Lee C,Sandridge SA,Butler HM,Manzoor NF,Kaltenbach JA. Behavioral evidence for possible simultaneous induction of hyperacusis and tinnitus following intense sound exposure. JAssoc Res Otolaryngol 2013;14:413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Eggermont JJ,Roberts LE. The neuroscience of tinnitus: understanding abnormal and normal auditory perception. Front Syst Neurosci 2012;6:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Eggermont JJ,Roberts LE. Tinnitus: animal models and findings in humans. Cell Tissue Res 2015;361:311–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Janssen ML,Zwartjes DG,Tan SK et al. Mild dopaminergic lesions are accompanied by robust changes in subthalamic nucleus activity. Neurosci Lett 2012;508:101–105. [DOI] [PubMed] [Google Scholar]
- 57. Bergman H,Wichmann T,Karmon B,DeLong MR. The primate subthalamic nucleus II. Neuronal activity in the MPTP model of parkinsonism. JNeurophysiol 1994;72:507–520. [DOI] [PubMed] [Google Scholar]
- 58. Basta D,Goetze R,Ernst A. Effects of salicylate application on the spontaneous activity in brain slices of the mouse cochlear nucleus, medial geniculate body and primary auditory cortex. Hear Res 2008;240:42–51. [DOI] [PubMed] [Google Scholar]
- 59. Lozano AM,Dostrovsky J,Chen R,Ashby P. Deep brain stimulation for Parkinson's disease: disrupting the disruption. Lancet Neurol 2002;1:225–231. [DOI] [PubMed] [Google Scholar]
- 60. Florence G,Sameshima K,Fonoff ET,Hamani C. Deep brain stimulation: more complex than the inhibition of cells and excitation of fibers. Neuroscientist 2016;22:332–345. [DOI] [PubMed] [Google Scholar]
- 61. Moser A,Gieselberg A,Ro B,Keller C,Qadri F. Deep brain stimulation: response to neuronal high frequency stimulation is mediated through GABA(A) receptor activation in rats. Neurosci Lett 2003;341:57–60. [DOI] [PubMed] [Google Scholar]
- 62. Hamani C,Temel Y. Deep brain stimulation for psychiatric disease: contributions and validity of animal models. Sci Transl Med 2012;4:142rv8. [DOI] [PubMed] [Google Scholar]
- 63. Grill WM,Snyder AN,Miocinovic S. Deep brain stimulation creates an informational lesion of the stimulated nucleus. Neuroreport 2004;15:1137–1140. [DOI] [PubMed] [Google Scholar]
- 64. McIntyre CC,Anderson RW. Deep brain stimulation mechanisms: the control of network activity via neurochemistry modulation. JNeurochem 2016;139: 338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. McConnell GC,So RQ,Hilliard JD,Lopomo P,Grill WM. Effective deep brain stimulation suppresses low‐frequency network oscillations in the basal ganglia by regularizing neural firing patterns. JNeurosci 2012;32:15657–15668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Whitmer D,de Solages C,Hill B,Yu H,Henderson JM,Bronte‐Stewart H. High frequency deep brain stimulation attenuates subthalamic and cortical rhythms in Parkinson's disease. Front Hum Neurosci 2012;6:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hamani C,Moro E. Neuromodulation: a more comprehensive concept beyond deep brain stimulation. Int Rev Neurobiol 2012;107:1–3. [DOI] [PubMed] [Google Scholar]
- 68. Nandi D,Jenkinson N,Stein J,Aziz T. The pedunculopontine nucleus in Parkinson's disease: primate studies. Br J Neurosurg 2008;22:S4–S8. [DOI] [PubMed] [Google Scholar]
- 69. Arts RA,George EL,Griessner A,Zierhofer C,Stokroos RJ. Tinnitus suppression by intracochlear electrical stimulation in single‐sided deafness: a prospective clinical trial – part I. Audiol Neurootol 2015;20:294–313. [DOI] [PubMed] [Google Scholar]
- 70. Cooper SE,McIntyre CC,Fernandez HH,Vitek JL. Association of deep brain stimulation washout effects with Parkinson disease duration. JAMA Neurol 2013;70:95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Barry KM,Paolini AG,Robertson D,Mulders WH. Modulation of medial geniculate nucleus neuronal activity by electrical stimulation of the nucleus accumbens. Neuroscience 2015;308:1–10. [DOI] [PubMed] [Google Scholar]
- 72. Lim HH,Lenarz T,Anderson DJ,Lenarz M. The auditory midbrain implant: effects of electrode location. Hear Res 2008;242:74–85. [DOI] [PubMed] [Google Scholar]
- 73. Hazell JW,Jastreboff PJ,Meerton LE,Conway MJ. Electrical tinnitus suppression: frequency dependence of effects. Audiology 1993;32:68–77. [DOI] [PubMed] [Google Scholar]
- 74. Fukutake T,Hattori T. Auditory illusions caused by a small lesion in the right medial geniculate body. Neurology 1998;51:1469–1471. [DOI] [PubMed] [Google Scholar]


